Abstract
The unparalleled spread of highly pathogenic avian influenza A (HPAI) H5N1 viruses has resulted in devastating outbreaks in domestic poultry and sporadic human infections with a high fatality rate. To better understand the mechanism(s) of H5N1 virus pathogenesis and host responses in humans, we utilized a polarized human bronchial epithelial cell model that expresses both avian alpha-2,3- and human alpha-2,6-linked sialic acid receptors on the apical surface and supports productive replication of both H5N1 and H3N2 viruses. Using this model, we compared the abilities of selected 2004 HPAI H5N1 viruses isolated from humans and a recent human H3N2 virus to trigger the type I interferon (IFN) response. H5N1 viruses elicited significantly less IFN regulatory factor 3 (IRF3) nuclear translocation, as well as delayed and reduced production of IFN-β compared with the H3N2 virus. Furthermore, phosphorylation of Stat2 and induction of IFN-stimulated genes (ISGs), such as MX1, ISG15, IRF7, and retinoic acid-inducible gene I, were substantially delayed and reduced in cells infected with H5N1 viruses. We also observed that the highly virulent H5N1 virus replicated more efficiently and induced a weaker IFN response than the H5N1 virus that exhibited low virulence in mammals in an earlier study. Our data suggest that the H5N1 viruses tested, especially the virus with the high-pathogenicity phenotype, possess greater capability to attenuate the type I IFN response than the human H3N2 virus. The attenuation of this critical host innate immune defense may contribute to the virulence of H5N1 viruses observed in humans.
Since late 2003, highly pathogenic avian influenza A (HPAI) H5N1 viruses have swept through nine Asian countries, forcing the slaughter of over 150 million domestic poultry and creating an enormous economic burden for the region. More recently, HPAI H5N1 viruses have been detected in domestic and wild birds in Central Asia, the Middle East, Africa, and Europe, and migratory water fowl have been implicated in this latest geographic expansion (22). Primarily as a result of avian-to-human transmission, 306 laboratory-confirmed human cases of H5N1 virus infection in 12 countries have been reported to the World Health Organization as of 16 May 2007, highlighting the pandemic potential of H5N1 viruses and their growing impact on global public health (http://www.who.int/en/). Avian H5N1 influenza viruses were first recognized to be capable of causing human respiratory infection and disease in Hong Kong in 1997, when 18 documented human H5N1 infections with 6 fatalities were identified in conjunction with outbreaks of H5N1 disease among domestic poultry.
The underlying mechanism(s) of the heightened virulence of H5N1 viruses for humans remains largely unknown. Common symptoms among H5N1-infected human cases include fever, diarrhea, lymphopenia, and pneumonia with impairment of gas exchange; most patients developed acute respiratory distress syndrome (2, 44). Evidence is accumulating that extrapulmonary replication of H5N1 viruses may occur, at least in some individuals (5, 47). In patients infected with H5N1 virus, a high viral load and its induced exacerbated cytokine or chemokine responses have been shown to be associated with fatal cases (6, 34).
Animal models have generally recapitulated the severe and often fatal disease observed among human H5N1 virus cases since late 2003, and replication in systemic organs is more routinely associated with the high-virulence phenotype, particularly in small animals (9, 26, 36, 53). In ferrets, H5N1 viruses isolated from humans caused severe illness and replicated in respiratory and systemic organs, whereas human H3N2 viruses caused relatively mild respiratory symptoms and replication was primarily limited to the respiratory tract (53). Recent studies from this laboratory demonstrated that H5N1 viruses isolated from humans in 2004, in general, exhibited enhanced virulence in ferrets compared with the 1997 H5N1 strains (28). One exception was a virus, A/Thailand/SP/83/2004 (SP83), which caused milder illness and replicated less efficiently in respiratory organs in both ferrets and mice than the genetically related human isolate A/Thailand/16/2004 (Thai16) virus which was highly lethal and replicated systemically in mammals.
Infection with influenza virus, including H5N1 viruses, results in the activation of the type I interferon (IFN) responses (1, 16). The IFN signaling can be initiated by engagement of Toll-like receptors or retinoic acid-inducible gene I (RIG-I) in different cell types (39). In epithelial cells, RIG-I has been identified as a cytosolic receptor for double-stranded RNA, an intermediate during viral replication, to activate IFN transcription (19, 52). Recently, it has been suggested that RIG-I activated by genomic single-stranded RNA bearing 5′ phosphates is responsible for initiation of the IFN response to influenza virus infection (18, 35). Upon activation, RIG-I acts through the downstream adaptor IPS-1, resulting in activation of IFN regulatory factor 3 (IRF3), production of IFN, and eventually induction of IFN-stimulated genes (ISGs) with antiviral activity mediated through the JAK-STAT signaling pathway. However, influenza virus possesses the capability to escape this host antiviral action through the function of nonstructural protein 1 (NS1), which has been shown to inhibit the IFN response by interacting with RIG-I (12, 30, 33, 35), IRF3 (43), NF-κB (50), and Jun N-terminal kinase (27).
Since the airway epithelium is the primary site of influenza virus replication in humans, the aim of this study was to establish a human respiratory epithelial cell model to better understand the virulence of avian H5N1 viruses relative to that of contemporary human H3N2 viruses as well as virulence between H5N1 viruses demonstrating different phenotypes in mammals. Here we demonstrate that a polarized human bronchial epithelial cell line (Calu-3) supports efficient replication of both H5N1 and H3N2 viruses and provides a suitable in vitro human model for the study of host innate immune responses to influenza viruses. Compared with a recent human H3N2 virus, both H5N1 viruses, in particular the highly lethal Thai16 virus, induced significantly less IRF3 nuclear translocation, delayed and reduced IFN-β expression, substantially weaker JAK-STAT pathway activation, and reduced ISG expression. Our data suggest that HPAI H5N1 viruses are more effective than a contemporary human virus in attenuating type I IFN responses to escape the host innate immune response. Furthermore, the Thai16 virus, which exhibits heightened virulence in mammalian models, possesses a better capability to attenuate the host IFN response than the less virulent SP83 virus. These differences will contribute to our understanding of the virulence of H5N1 viruses observed in humans.
MATERIALS AND METHODS
Viruses.
Two HPAI H5N1 viruses isolated from fatal human cases in early 2004, Thai16 and SP83 (28), and a human H3N2 virus, A/Panama/2007/1999 (Pan99), were used in this study. Virus stocks were grown in the allantoic cavities of 10-day-old embryonated hen's eggs for 24 to 28 h at 37°C for H5N1 viruses or for 48 h at 33.5°C for the H3N2 virus. Allantoic fluids from multiple eggs were pooled, clarified by centrifugation, aliquoted, and stored at −70°C. Virus titers were determined by a plaque assay as described below. All research with avian H5N1 viruses was conducted under biosafety level 3 containment, including enhancements required by the U.S. Department of Agriculture and the Select Agent Program (http://www.cdc.gov/od/ohs/biosfty/bmbl5/bmbl5toc.htm).
Cell culture and viral infection.
The human bronchial epithelial cell line Calu-3 was originally derived from subbronchial epithelium of a lung adenocarcinoma (ATCC, Manassas, VA). Calu-3 cells were grown in Eagle's minimal essential medium (MEM) supplemented with 2 mM l-glutamine, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 1.5 g/liter sodium bicarbonate, and 10% fetal bovine serum. Calu-3 cells (5 × 105) were seeded onto Corning 24-mm-diameter semipermeable membrane inserts with a 0.4-μm pore size (Corning, NY) and cultured for 1 week with the addition of fresh culture medium every 2 to 3 days, after which the confluent monolayer achieved a stable transepithelial resistance of >1,000 Ω·cm2. Monolayers were washed and equilibrated for 10 min by addition of MEM supplemented with 0.3% bovine serum albumin (MEM-BSA). Virus was diluted in MEM-BSA and added to the apical surface of cells in a volume of 300 μl at a multiplicity of infection (MOI) ranging from 0.01 to 5. After a 1-hour incubation, monolayers were washed to remove nonadherent virus and 2 ml of MEM-BSA was added to both apical and basolateral reservoirs of cells and left for the duration of the experiment.
Primary human bronchial epithelial (NHBE) cells were purchased from Cambrex Bio Science (Walkersville, MD) and grown in serum-free and hormone-supplemented bronchial epithelial growth medium according to the manufacturer's instructions. Cells of passage 3 were seeded onto semipermeable membrane inserts (Corning, NY) and cultured for 1 week with the addition of fresh culture medium every 2 days. Virus was diluted in bronchial epithelial growth medium-BSA and added to the apical surface of confluent monolayers in a volume of 300 μl at an MOI of 5. The monolayers were washed after 1 hour of incubation, and medium was replaced.
Plaque assay.
Madin-Darby canine kidney (MDCK) cells (ATCC, Manassas, VA) were grown in Dulbecco's MEM with 1 mM l-glutamine and 10% fetal bovine serum and seeded onto six-well plates. Confluent monolayers were washed and infected with serial 10-fold dilutions of virus. After 1 hour of incubation, the cells were washed, and an overlay consisting of a mixture of 1.6% agarose and double-strength L-15 medium (Cambrex Bio Science, Walkersville, MD) with (for H3N2 virus) or without (for H5N1 viruses) N-p-tosyl-l-phenylalanine chloromethyl ketone (TPCK)-treated trypsin (Sigma-Aldrich, St. Louis, MO) was added to the cells. After 48 h of incubation at 37°C, plaques were stained with 0.1% crystal violet and counted.
Immunofluorescence staining and microscopy.
Uninfected and influenza virus-infected Calu-3 cells were washed with phosphate-buffered saline (PBS) and fixed in 3.7% formaldehyde in PBS for 30 min. For detection of sialic acid residues on the surface of cells, apical monolayers were blocked with 3% BSA in PBS for 30 min and then sequentially incubated with either biotinylated Maackia amurensis lectin II (MAA) (20 μg/ml) or biotinylated Sambucus nigra lectin (SNA) (20 μg/ml) (Vector Laboratories, Burlingame, CA) for 1 hour, followed by the addition of fluorescein-conjugated avidin D or rhodamine-conjugated streptavidin (BD Biosciences, San Diego, CA). To confirm the specificity of lectin binding, monolayers were treated with 25 mU/ml of Vibrio cholerae neuraminidase (Roche, Indianapolis, IN) for 1 hour prior to fixation. To detect influenza A virus nucleoprotein (NP) antigen and IRF3, fixed cells were permeabilized with 0.5% Triton X-100 in PBS for 20 min and incubated with mouse anti-NP monoclonal antibody A-3 (49) and/or rabbit anti-IRF3 (Cell Signaling, MA) followed by rhodamine- or fluorescein isothiocyanate (FITC)-conjugated secondary antibody (BD Biosciences, San Diego, CA). All cells were stained with 4′,6′-diamidino-2-phenylindole (DAPI) to visualize nuclei. Finally, immunostained cells were mounted and examined under a Zeiss Axioskop 2 fluorescence microscope.
Detection of α-2,6- and α-2,3-linked sialic acid residues by flow cytometry.
Calu-3 cell monolayers grown on transwells were treated with trypsin, and the cell suspension was passed through a cell strainer (BD Biosciences, Bedford, MA) to obtain single cells. Cell suspensions were centrifuged at 1,000 × g for 10 min and washed twice with MEM and once with fluorescence-activated cell sorter washing buffer (PBS with 0.5% BSA). Twenty microliters of a 1:10 dilution of SNA-FITC or MAA-FITC (Vector Laboratories) was added to the cell pellet (5 × 105 cells), suspended, and incubated for 30 min at 4°C. Cells were washed, resuspended in fluorescence-activated cell sorter washing buffer, run through a FACSCalibur, and analyzed with CellQuest (BD Bioscience, San Jose, CA).
Transmission electron microscopy.
Polarized Calu-3 cells on transwell inserts were infected with three influenza viruses at an MOI of 5 at the apical surface. At 12 h postinfection (p.i.), cells were washed twice with PBS, fixed in 2.5% buffered glutaraldehyde, and gamma irradiated (2 × 106 rads). Specimens were postfixed in 1% buffered osmium tetroxide, stained in 4% uranyl acetate, dehydrated, and embedded in epoxy resin. Finally, ultrathin sections were cut and examined with an FEI Tecnai Spirit electron microscope.
Real-time quantitative reverse transcription-PCR (RT-PCR).
Total RNA from virus-infected or uninfected Calu-3 cells was extracted using the RNeasy minikit (QIAGEN, Carlsbad, CA), and 1.0 μg of total RNA was reverse transcribed with the TaqMan RT kit (Applied Biosystems, Foster City, CA). One microliter of the product was subjected to a SYBR green real-time PCR assay (Applied Biosystems, Foster City, CA). Reactions were performed in triplicates. All quantitations (threshold cycle [CT] values) were normalized to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) to generate ΔCT, and the difference between the ΔCT value of the sample and that of the reference (uninfected sample) was calculated as ΔΔCT. The relative level of gene expression was expressed as 2−ΔΔCT. Primer sequences for the genes of interest were designed using PrimerExpress (Applied Biosystems). The primer sequences are as followed: IFN-β forward primer, CTTACAGGTTACCTCCGAAACTGAA; IFN-β reverse primer, GGTTGAAGAATGCTTGAAGCAA; IRF7 forward primer, GAGAAGAGCCTGGTCCTGGT; IRF7 reverse primer, CTAGGTCACTCGGCACAG; RIG-I forward primer, GACTGGACGTGGCAAAACAA; RIG-I reverse primer, TTGAATGCATCCAATATACACTTCTG; Mx forward primer, ACACATGCTGAACATCACAGCTT; Mx reverse primer, ACACGGCACTCATGCTCCTAA; ISG15 forward primer, CCTGCTGGTGGTGGACAAAT; ISG15 reverse primer, TGCGGCCCTTGTTATTCC; GAPDH forward primer, GGGAAGGTGAAGGTCGGAGTC; GAPDH reverse primer, CAGCCTTGACGGTGCCATG.
SDS-polyacrylamide gel electrophoresis immunoblot analysis.
Virus-infected or uninfected Calu-3 cells were lysed in ice-cold lysis buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), and a protease inhibitor cocktail (Sigma, St. Louis, MO). Protein concentrations were determined using the Bradford reagent protocol (Bio-Rad, Hercules, CA). Equal amount of proteins were loaded on an SDS-polyacrylamide gel, electrophoresed, and blotted with the indicated antibodies according to the manufacturer's instructions. Immunoreactive proteins were detected using antibodies to phospho-STAT2 (Santa Cruz Biotechnology, Santa Cruz, CA) or anti-β-actin (Sigma, St. Louis, MO), followed by a horseradish peroxidase-conjugated secondary antibody (Cell Signaling, Beverly, MA).
Quantification of IFN-β production by ELISA.
Production of IFN-β in culture supernatants was measured using a human IFN-β enzyme-linked immunosorbent assay (ELISA) kit (PBL Biomedical Laboratories, Piscataway, NJ) according to the manufacturer's instructions. For each treatment, two sets of sample were collected, and each sample was tested in duplicate by ELISA.
Statistical analysis.
Student's t test was performed on virus titers and ELISA data.
RESULTS
Polarized human bronchial epithelial cells (Calu-3) express surface sialic acid receptors for both avian and human influenza A viruses.
Influenza viruses infect the human airway epithelium through binding to cell surface sialic acid receptors. We used polarized monolayers of Calu-3 cells, a continuous human bronchial epithelial cell line that possesses multiple features of in vivo airway epithelium and have been used as an in vitro model for respiratory virus infection and metabolic studies (7, 45). Confluent monolayers of Calu-3 cells grown on transwells established transepithelial resistance after 4 days of culture. After 1 week of culture, Calu-3 cells achieved a stable transepithelial resistance of >1,000 Ω·cm2, and tight junctions were detected by OZ-1 staining encircling the cells (data not shown). To estimate the susceptibility of polarized Calu-3 cells to influenza viruses, we first evaluated these cells for the presence of the known sialic acid receptors preferred by influenza virus. Both α-2,6- and α-2,3-linked sialic acid receptors, preferred by human and avian influenza viruses, respectively, were detected on the apical surface of polarized Calu-3 cells by lectin staining (Fig. 1A and B). Treatment of the apical surface of Calu-3 cells with neuraminidase abolished staining of either sialic acid species (Fig. 1A and B, insets). Flow cytometry determined that 98.5% and 99.7% of cells stained positive for α-2,6 (SNA-FITC)- and α-2,3 (MAA-FITC)-linked sialic acid receptors, respectively, although the relative intensity of staining for α-2,6-linked sialic acid was more heterogeneous (Fig. 1C and D).
FIG. 1.
Polarized human bronchial epithelial Calu-3 cells express both α-2,3- and α-2,6-linked sialic acid receptors. (A and B) Calu-3 cells grown on transwell inserts were fixed and stained for α-2,6-linked sialic acid residues (red) (A) or α-2,3-linked sialic acid (green) (B). The cells were stained with DAPI (blue) to detect cell nuclei. As shown in the insets, treatment with neuraminidase abolished sialic acid residue staining. (C and D) Flow cytometry analysis shows percentages of positive cells for α-2,6 (C)- and α-2,3 (D)-linked sialic acid residues on the Calu-3 cell surface. (E) Immunofluorescence detection of influenza virus NP in Calu-3 cells infected with influenza viruses. Calu-3 monolayers were infected apically with virus at an MOI of 1, stained for NP (red, top panels), and costained with DAPI (blue, bottom panels) at 10 h p.i.
To test the permissiveness of Calu-3 cells for human and avian influenza virus infection, polarized cell monolayers grown on transwell inserts were infected with Pan99, Thai16, and SP83 virus at an MOI of 1 and expression of influenza virus NP was evaluated after 10 h. As shown in Fig. 1E, NP expression indicated that 35 to 45% of cells were infected with each of the viruses at 10 h p.i., indicating that the Calu-3 cells were equally susceptible to infection by either H3N2 or H5N1 subtype virus. These results demonstrate that polarized Calu-3 cell monolayers express sialic acid receptors preferred by either avian H5N1 or human H3N2 viruses and are permissive for infection by either subtype.
Influenza viruses preferentially enter through and are released from the apical surface of polarized Calu-3 cells.
To validate the polarity of influenza virus infection, confluent Calu-3 monolayers were infected with Pan99 or Thai16 virus at an MOI of 5 from either the apical or basolateral surface and examined for the presence of influenza virus NP by immunostaining 10 h later. Infection at the apical surface resulted in 80% of cells in the monolayer staining positive for NP, whereas fewer than 2% of cells infected at the basolateral surface stained positive for NP. To evaluate the polarity of influenza virus release, Calu-3 cells were infected at an MOI of 0.01 and culture medium was collected from the apical and basolateral chambers at 24 h p.i. and examined for virus production. Titers ranging from 107.6 to 108.2 PFU/ml were detected in apical reservoir supernatants for each of the three viruses. In contrast, only trace amounts of each virus tested (<102.0 PFU/ml) were released into the basolateral reservoir. These results demonstrate that both human H3N2 and avian H5N1 viruses enter and release primarily from the apical surface of polarized Calu-3 cells.
Transmission electron microscopy confirmed that Calu-3 cells exhibited properties of airway epithelium and further demonstrated the preferential release of influenza virus from the apical surface of the polarized monolayer. As shown in Fig. 2A, tight junctions were evident between Calu-3 cells, and microvilli were present on apical surface of some cells, although no ciliated cells were observed. The presence of granular vesicles at the apical region of some cells is consistent with the features of mucin-secreting cells observed in Calu-3 (11, 25) or human bronchial epithelial cells, including NHBE cells, in other studies (Fig. 2A) (4, 21). Influenza virions, both filamentous and spherical particles, were detected at the apical surface of cells infected with each of the three viruses. Virions were seen budding from the cell surface and the tips of microvilli in cultures infected with Thai16 virus (Fig. 2B). Virus particles were rarely observed at the basolateral surface for any of the viruses tested.
FIG. 2.
Transmission electron microscopy demonstrates influenza virus release from the apical surface of polarized Calu-3 cells. Calu-3 cells were infected at the apical surface with virus at an MOI of 5. (A) Mock-infected cells, with the tight junction (arrow), the granular vesicles (arrowhead), and the membrane insert (*) indicated. (B). Thai16 virus-infected cells at 12 h p.i. Both spherical and filamentous virions develop by the positioning of the nucleocapsids at the cell surface (arrowhead). Bars, 1 μm (A) and 100 nm (B).
Avian H5N1 and human H3N2 viruses replicate productively in human bronchial epithelial cells in the absence of exogeneous trypsin.
In standard epithelial cells, such as MDCK cells, H3N2 viruses are dependent on extracellular proteases to support cleavage of the hemagglutinin for multiple-cycle infection. In contrast, we found that Pan99 virus replicated with similar kinetics and to similar titers in polarized Calu-3 cells, with or without the addition of exogeneous TPCK-trypsin (Fig. 3A), suggesting that polarized Calu-3 cells possess protease activity that can support H3N2 virus hemagglutinin cleavage. Next, we compared the replication kinetics of two HPAI H5N1 viruses (Thai16 and SP83) with that of the human H3N2 virus (Pan99). Polarized Calu-3 monolayers were infected with each virus at an MOI of 0.01, and supernatants were collected at intervals between 2 and 48 h p.i. As shown in Fig. 3B, Calu-3 monolayers supported productive infection and virus release for all three viruses. Infectious virus in culture supernatants was detected as early as 12 h p.i., with low titers of 101.5 to 103.0 PFU/ml. Thai16 and Pan99 viruses replicated to similar titers and with similar kinetics, whereas replication of SP83 virus was relatively slower and less efficient. When the replication kinetics of the two avian H5N1 viruses were compared, we found that Thai16 virus replicated more rapidly and reached significantly higher titers (P < 0.05) than SP83 virus at all time points from 16 to 48 h p.i. (Fig. 3B). Cytopathic effects and cell death were observed at 24 to 72 h p.i. in Calu-3 cells infected with each of the viruses at an MOI of 0.01. Cells infected with the H3N2 virus retained monolayer integrity, whereas monolayers infected with either avian H5N1 virus exhibited substantial cytopathic effects, with complete destruction of the cell monolayer by 48 h p.i. (Fig. 3C).
FIG. 3.

Kinetics of influenza virus replication in polarized human bronchial epithelial Calu-3 cells. One-week-old Calu-3 cells grown on transwells were infected apically with the indicated virus at an MOI of 0.01. Culture supernatants were collected at the indicated time points, and viral titers were determined by plaque assay. (A) Replication kinetics of Pan99 virus in Calu-3 cells with or without addition of TPCK-trypsin (0.3 μg/ml). (B) Replication kinetics of three influenza viruses, Pan99, Thai16, and SP83. Asterisks indicate statistically significant differences between SP83 and Thai16 viruses (P < 0.05). (C) Evaluation of the integrity of Calu-3 monolayers after virus infection. Calu-3 monolayers were infected with virus at an MOI of 0.01 and stained with DAPI 48 h later.
Avian H5N1 viruses induce a weaker type I IFN response in Calu-3 cells than that induced by a human H3N2 virus.
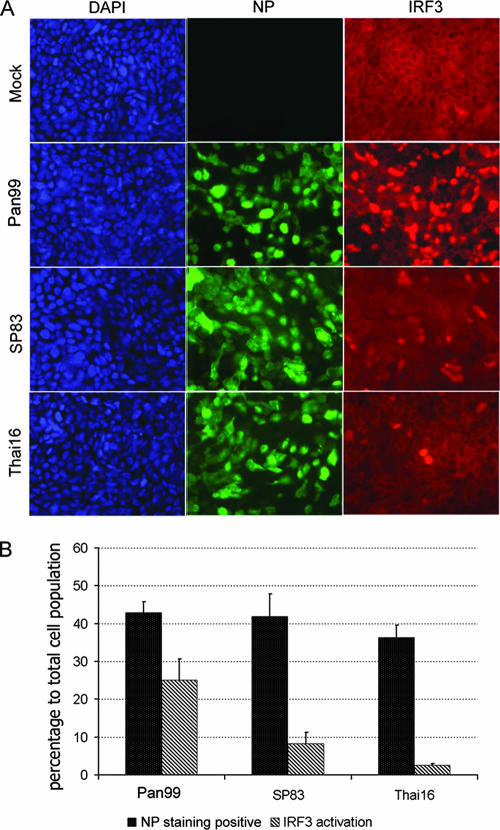
We next determined whether the avian H5N1 viruses differed from the human H3N2 virus in their ability to efficiently initiate type I IFN responses in Calu-3 cells during the first cycle of viral replication. Activation of IRF3, a transcription factor that regulates the expression of type I IFN, was detected by analyzing its nuclear translocation in infected cells. Cells were infected with influenza viruses at an MOI of 1, and cells were fixed at 8 h p.i. and stained to detect expression of influenza virus NP and distribution of IRF3 (Fig. 4A). For each virus infection, approximately 500 cells from four randomly chosen regions were examined. Approximately 35 to 45% of the total cell population was stained positive for influenza virus NP for each virus used; however, IRF3 nuclear translocation was not observed in all NP-positive cells. Markedly less IRF3 nuclear translocation of all cells was detected in cells infected with H5N1 viruses (8% for SP83 virus and 3% for Thai16 virus) than in cells infected with the H3N2 virus (25%) (Fig. 4B).
FIG. 4.
Influenza virus infection results in differential IRF3 nuclear translocation in Calu-3 cells. Polarized Calu-3 cells were infected with viruses at an MOI of 1. Cells were fixed and stained for influenza virus NP (green) and IRF3 (red) at 8 h p.i. (A) Immunofluorescent staining of virus-infected cells. (B) Assessment of rate of infection and IRF3 nuclear translocation in virus-infected cells. Error bars indicate standard deviations.
Next, we examined the transcription of IFN-β and activation of the JAK-STAT pathway in infected Calu-3 cells. Cells were infected with H5N1 and H3N2 viruses at an MOI of 1 or 3, and total RNA was isolated after 8 h and analyzed by RT-PCR for IFN-β mRNA expression. The human H3N2 virus induced substantially greater levels of IFN-β mRNA transcription than either avian H5N1 virus (Fig. 5A). Through binding to IFNAR1 and IFNAR2, IFN-β activates the JAK-STAT signaling pathway through phosphorylation of STAT1 and STAT2, resulting in the global induction of ISGs. To detect activation of the JAK-STAT pathway, Calu-3 cells were infected with each virus, and cell lysates were collected at 4, 5, and 6 h p.i. and examined for the phosphorylated form of STAT2 by Western blot analysis. Infection of Calu-3 cells with Pan99 virus resulted in strong phosphorylation of STAT2 as early as 4 h p.i., which increased up to 6 h p.i. In contrast, the two H5N1 avian viruses induced only weak phosphorylation of STAT2 at 6 h p.i. Among the H5N1 viruses, cells infected with SP83 virus exhibited slightly greater phosphorylation of STAT2 than cells infected with Thai16 virus (Fig. 5B).
FIG. 5.
Primary IFN response to influenza virus infection in Calu-3 cells. (A) Evaluation of IFN-β mRNA expression by RT-PCR. Total RNA was isolated from Calu-3 cells infected with virus at an MOI of 1 or 3 for 8 h. The data are presented as the relative fold gene induction and are representative of three independent replicates. (B) Assessment of activation of the JAK-STAT pathway induced by virus infection. Protein samples from the indicated treatments were harvested and examined by Western blotting for STAT2 phosphorylation. C, control.
To confirm that the Calu-3 cell IFN response to influenza viruses was similar to that detected in NHBE cells, we examined the transcription of IFN-β in NHBE cells infected with H5N1 and H3N2 viruses at an MOI of 5. As shown in Fig. 6A, NP was detected in approximately 60% of cells infected with each of the viruses at 8 h p.i. Total RNA was isolated at 5, 7, and 10 h p.i. and analyzed by RT-PCR for IFN-β mRNA expression. Similarly, the human H3N2 virus induced substantially greater levels of IFN-β mRNA transcription than either avian H5N1 virus (Fig. 6B), confirming the differential expression we had observed in Calu-3 cells. These results demonstrate that compared with a human H3N2 virus, avian H5N1 viruses fail to induce strong IFN-β transcription and activate the JAK-STAT signaling pathway during the first cycle of virus replication.
FIG. 6.
Transcription of the IFN-β gene in NHBE cells in response to influenza virus infection. Confluent NHBE cells grown on inserts were infected with virus at an MOI of 5. (A) Immunofluorescence detection of influenza virus NP protein in infected cells. (B) Examination of IFN-β expression by real-time RT-PCR.
To investigate further the IFN response elicited by these influenza viruses, we next examined the kinetics of IFN-β production and expression over a 24-h period. Calu-3 cells were infected with each of the three viruses at an MOI of 1, and the amounts of IFN-α and -β secreted into culture supernatants were measured by ELISA. IFN-α was not detected in supernatants from cells infected with any of the viruses (data not shown), but Calu-3 cells produced detectable levels of IFN-β in response to influenza virus infection (Fig. 7A). Pan99 virus induced rapid detectable IFN-β production as early as 8 h p.i. in infected cells. In contrast, IFN-β was not detected during the first 12 h p.i. in cells infected with either H5N1 virus, and by 16 h p.i. levels were still significantly lower than those elicited by the H3N2 virus infection. Interestingly, at later time points (20 and 24 h p.i.), SP83 virus-infected cells produced significantly more IFN-β than Thai16 virus-infected cells, reaching levels similar to those in H3N2 virus-infected cells. RT-PCR detection of IFN-β mRNA (Fig. 7B) demonstrated a similar pattern of IFN-β induction at transcription level. Pan99 virus induced IFN-β transcription as early as 4 h p.i., and the expression level was increased through 20 h p.i. The IFN-β expression induced by SP83 virus was delayed until 12 h p.i. and reached the highest levels of transcription at 16 and 20 p.i. Thai16 virus also induced IFN-β mRNA transcription at later time points; however, levels of IFN-β mRNA remained substantially lower than those induced by SP83 virus.
FIG. 7.
Production of IFN-β in influenza virus-infected Calu-3 cells. Polarized Calu-3 cells were infected with virus at an MOI of 1. (A) Evaluation of IFN-β production by ELISA. Supernatants from infected Calu-3 cells, collected at the designated times, were analyzed by ELISA. Three sets of samples from independent treatments were tested in duplicate. Single asterisks indicate statistically significant differences between human H3N2 virus and avian viruses (P < 0.05), and double asterisks indicate statistically significant differences between two avian viruses (P < 0.05). Error bars indicate standard deviations. (B) Analysis of IFN-β expression in virus-infected Calu-3 cells by RT-PCR. The data are presented as the relative fold gene induction and are representative of three independent replicates. (C) Activation of the JAK-STAT pathway during influenza virus infection. Phosphorylation of STAT2 is shown in the top panel and β-actin in the bottom panel in the Western blot. C, control.
We next examined activation of JAK-STAT over a 24-h period of infection. Infection of cells with Pan99 virus strongly induced phosphorylation of STAT2, by 7 h p.i (Fig. 7C). In contrast, activation of JAK-STAT in Calu-3 cells infected with either avian H5N1 virus was delayed, and it increased at later time points (16 to 20 h p.i.), when virus was undergoing a second round of replication. Interestingly, longer and stronger STAT2 phosphorylation was observed in cells infected with SP83 virus than in those infected with Thai16 virus, which correlated with the differences in IFN-β production between the two viruses. These results confirm that H5N1 viruses, in particular the highly virulent Thai16 virus, induced significantly delayed and lower levels of IFN-β than the human H3N2 virus.
H5N1 viruses induce a weaker and later ISG response than the H3N2 virus.
The antiviral activity of the type I IFN response is mediated by various ISGs, such the genes for as MxA GTPase and the ubiquitin-like ISG15 protein. Transcription of these two genes was induced in influenza virus-infected Calu-3 cells (Fig. 8). The earliest induction of these ISGs was detected at 8 h p.i. in cells infected with Pan99 virus, and the transcription levels remained higher than those detected in cells infected with H5N1 viruses. Consistent with observation of IFN-β induction in cells infected with H5N1 virus, transcription levels for both MxA and ISG15 were greater in cells infected with SP83 virus than in cells infected with Thai16 virus.
FIG. 8.
Expression of ISGs in infected Calu-3 cells. Polarized Calu-3 cells were infected with virus at an MOI of 1, and total RNA was isolated and examined with real-time PCR. Expression analysis of four genes, encoding myxovirus (influenza virus) resistance 1 (Mx1), IFN-induced protein IFI-15K (ISG15), RIG-I, and IRF7, is shown. Expression of the viral M1 gene also was determined; at 8 h p.i. expression of M1 in SP83-infected cells was lower than that for the Thai16 and Pan99 viruses (data not shown). The data are presented as the relative fold gene induction and are representative of three independent replicates.
Expression of the RIG-I and IRF7 genes, which are critical for the IFN response (17, 35, 52), was also examined using RT-PCR in infected cells. Although RIG-I was induced by all three influenza viruses by 8 h p.i. (Fig. 8), expression of RIG-I in cells infected with Pan99 virus was substantially higher than that detected in cells infected with either avian H5N1 virus at all time points tested. Likewise, IRF7 transcription was detected by 8 h p.i. and levels were initially higher in cells infected with Pan99 virus than in those infected with either H5N1 virus (Fig. 8). However by 16 h p.i., the level of IRF7 mRNA in cells infected with SP83 virus was comparable to that detected in cells infected with Pan99 virus. In contrast, transcription of IRF7 in cells infected with Thai16 virus remained low at all time points tested. In summary, compared with the human H3N2 virus, avian H5N1 viruses, and particularly Thai16 virus, exhibited reduced up-regulation of two ISG proteins as well as RIG-I and IRF7. These results are consistent with the observed difference in IFN-β expression and production induced by these viruses in Calu-3 cells.
Pretreatment with human IFN-β results in a delay in viral replication.
Previous studies have suggested that some avian H5N1 viruses are resistant to the effects of type I IFN when evaluated in a porcine epithelial cell monolayer (38). To evaluate the effect of IFN-β pretreatment on the replication of the avian H5N1 viruses and human H3N2 virus in human respiratory epithelial cell monolayers, Calu-3 cells were pretreated with 200 U/ml human IFN-β for 24 h, washed to remove the IFN-β, and infected with viruses at an MOI of 0.01. IFN-β pretreatment of cells resulted in a reduction of virus replication ranging from 2 to 3 logs in the first 24 h p.i., but by 36 h p.i., titers of all three viruses in IFN-β pretreated cells were equivalent to that detected in cultures that had not been treated with IFN-β (Fig. 9). Similar results were obtained when 100 U/ml human IFN-β was used to treat cells before infection (data not shown). These results suggest that although H5N1 viruses elicit an attenuated type I IFN response in comparison to that elicited by the H3N2 virus, both subtype viruses were equally sensitive to the effects of human IFN-β in human respiratory epithelial cells.
FIG. 9.
Pretreatment with IFN-β results in delay and reduction of viral replication. Calu-3 cells were pretreated with 200 U/ml of human IFN-β (Sigma, St. Louis, MO) for 24 h and were then infected with viruses at an MOI of 0.01. Supernatants were collected at the indicated time points, and virus titers were measured by plaque assay. Error bars indicate standard deviations.
DISCUSSION
Fatal human infections with avian H5N1 influenza viruses are characterized by the presence of a high viral load in the respiratory tract and levels of plasma cytokines and chemokines that are significantly higher than those detected in patients infected with human influenza virus strains (6). These findings suggest that avian H5N1 virus interactions with the host immune response may differ from those of seasonal human influenza viruses. Here we describe the use of polarized human bronchial epithelial cells (Calu-3) as an in vitro human airway epithelium model to evaluate interactions between influenza A virus and the human host. The presence of both α-2,6- and α-2,3-linked sialic acid receptors on the apical surface of polarized Calu-3 cells, together with the ability to support productive human virus replication in the absence of exogenous proteases, enables the efficient replication of both avian H5N1 and human H3N2 viruses and the direct comparison of the kinetics and magnitude of the respective type I IFN responses induced by these viruses. Using this model, we observed that avian H5N1 viruses isolated from humans in 2004 induced a delayed and weaker IFN-β response and reduced ISG expression compared with a contemporary human H3N2 virus. In contrast, H5N1 and H3N2 viruses were equally sensitive to the effects of IFN-β in pretreated cells. This result suggests that HPAI H5N1 viruses have the capacity to moderate and/or delay the human type I IFN response, which may, in part, contribute to their virulence in humans.
Human airway epithelium is the primary site for infection and replication of influenza A viruses. While both α-2,6- and α-2,3-linked sialic acid receptors have been detected in human primary airway cells in vitro and ex vivo, knowledge of their relative distribution in vivo and their detailed composition in the human respiratory tract remains incomplete (29, 40). H5N1 viruses were shown to bind to human lower respiratory tract epithelium in preference to upper airway tracheal epithelium (40, 48). However, more recently Nicholls et al. have reported that H5N1 virus infection of and replication in ex vivo cultures of human nasopharyngeal tissues were comparable to those seen with a human H1N1 virus (31), but the specific receptor recognized by the avian virus was not identified. Taken together with the high viral load detected in pharyngeal respiratory specimens collected from H5N1 virus-infected persons, these data suggest that avian H5N1 viruses may have a broader tropism for the human respiratory tract than previously reported (6, 40, 48). Human bronchial epithelial Calu-3 cells form monolayers with tight junctions in culture and maintain many properties of human primary bronchial epithelial cells, including secretory granule content and production of secretory component (8). In fact, NHBE cells, which expressed both α-2,6- and α-2,3-linked sialic acid receptors (data not shown), supported avian H5N1 virus as well as human H3N2 virus infection and type I IFN responses similar to those demonstrated for Calu-3 cells in the present study. However, compared to primary cell cultures, Calu-3 cells provide a more convenient and reproducible model for extended influenza virus-host interaction studies. In addition, these cells have been widely used as a model for drug delivery and recently for the study of the reconstructed 1918 Spanish influenza pandemic virus and severe acute respiratory syndrome coronavirus (8, 45, 46).
Calu-3 cells provide a useful translational human system to identify factors that contribute to the observed differences in virulence among H5N1 viruses in animal models (20, 28, 32, 53). Thai16 virus replicated to higher titers in the ferret respiratory tract and was highly lethal for animals compared with the substantially less virulent SP83 virus (28). Likewise, HPAI H5N1 Thai16 virus replicated to significantly higher titers than SP83 virus in Calu-3 cells. Amino acid sequence comparison of the Thai16 and SP83 viruses revealed differences at 16 amino acid residues in seven gene products. A total of seven substitutions distinguished the polymerase gene complex of Thai16 virus from that of SP83 virus, including the E627K substitution in PB2 (28). The E627K substitution in PB2 was previously shown to convert a nonlethal H5N1 influenza virus into a lethal virus in the BALB/c mouse model and to enhance the replication efficiency of an H5N1 virus in murine lung epithelial cell cultures (13, 41). More recently, it has been suggested that adaptive changes in the polymerase complex (PB1-PB2-PA) rather than the single amino acid at residue 627 of PB2 may play a role in H5N1 virulence in ferrets (10, 37). Reverse genetics and mutagenesis studies will help to further characterize the role of genetic differences in polymerase complex genes in H5N1 virus replication in the human airway epithelial cell model. In addition to the higher replication efficiency, lower levels of IFN-β and higher levels of tumor necrosis factor alpha (data not shown) expression and production were detected in Calu-3 cells infected with Thai16 virus compared with SP83 virus. Furthermore, differential expression of RIG-I, IRF7, and ISGs (MX1 and ISG15) were observed in cells infected with these two viruses. There is a single amino acid difference (V128I) between the NS1 proteins of these two viruses (28). Studies are currently under way to determine if this substitution plays any role in the differences in IFN induction noted in this study.
The type I IFN response plays an important role in the early control of virus replication and spread, as well as in the induction of adaptive T- and B-cell responses (3, 14). In the present study, two H5N1 influenza viruses initiated a weaker and delayed type I IFN response in polarized Calu-3 cells compared with a human H3N2 virus. Two key players in the IFN response, RIG-I and IRF7, were induced at much lower levels by H5N1 influenza viruses than by the human H3N2 virus. With a higher level of RIG-I expression, H3N2-infected cells may be able to initiate a more robust IFN response than H5N1 virus-infected cells.
Previous studies demonstrated that some H5N1 viruses isolated from humans in 1997 and 2004 induced significantly higher levels of type I IFN, evaluated with real-time RT-PCR, in cultured primary human alveolar and bronchial epithelial cells compared with a human H1N1 virus (1). In the present study, we chose to use an H3N2 virus as a representative human strain, because H3N2 viruses generally result in greater excess deaths during seasonal epidemics (42) and generally exhibit a greater level of virulence than H1N1 viruses (51). The influenza virus NS1 acts as an IFN antagonist (24). However, Hayman et al. recently demonstrated that although the NS1 proteins from different strains all effectively block Sendai virus-induced IFN production, the wild-type strains possessing these NS1 proteins have different capabilities to induce the IFN response in infected cells (15, 16). These findings suggest that in addition to properties conferred by the NS1 protein, other determinants/modes of action of the influenza virus may contribute to the variation in type I IFN induction. Furthermore, the ability to induce IFN, and indeed to inhibit the antiviral effects of IFN, may not necessary correlate with viral replication (15). Although the H3N2 virus tested here induced a significantly stronger IFN-β response than either H5N1 virus, the replication efficiency of the human strain in Calu-3 cells was not compromised. On the other hand, among viruses possessing comparable replication efficiencies, such as the H3N2 and Thai16 H5N1 viruses, an attenuated IFN response may contribute to virulence.
In contrast to earlier observations with the porcine host that H5N1 viruses isolated in 1997 were resistant to the antiviral response mediated by IFN (38), the H5N1 viruses tested here, like the H3N2 virus, were sensitive to the antiviral effects of human IFN-β. All viruses tested exhibited a significant reduction in virus replication during the first 24 h of infection of Calu-3 cells pretreated with IFN-β. Recently, Hayman et al. demonstrated that replication of avian viruses, as well as human influenza viruses, was enhanced in human cell lines deficient in the IFN response (15). These results, together with those presented here, strongly suggest that the human IFN response is a powerful host defense mechanism against influenza virus infection, not only for influenza viruses adapted to the human host but also for avian influenza virus strains, including H5N1 viruses.
The ability of HPAI H5N1 viruses to induce unusually high levels of cytokines and chemokines has been associated with the high lethality of H5N1 virus infection in humans. Similarly, the reconstructed 1918 influenza pandemic virus strain was recently shown to induce dysregulated immune responses in nonhuman primates, characterized by a highly up-regulated proinflammatory cytokine response but a reduced type I IFN response, compared with a contemporary human H1N1 virus strain (23). We propose that Calu-3 cells are a useful model to compare human host responses to infection with human and avian influenza virus subtypes and to identify phenotypic signature associated with enhanced virulence of influenza viruses in humans. Our results suggest that the attenuation of the host's innate antiviral response may be one factor that contributes to the virulence of H5N1 viruses in humans.
Acknowledgments
We thank Zhu Guo for RT-PCR primers and Sanjay Garg and Julie Plowden for help with flow cytometry analysis.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Published ahead of print on 12 September 2007.
REFERENCES
- 1.Chan, M. C., C. Y. Cheung, W. H. Chui, S. W. Tsao, J. M. Nicholls, Y. O. Chan, R. W. Chan, H. T. Long, L. L. Poon, Y. Guan, and J. S. Peiris. 2005. Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells. Respir. Res. 6:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chotpitayasunondh, T., K. Ungchusak, W. Hanshaoworakul, S. Chunsuthiwat, P. Sawanpanyalert, R. Kijphati, S. Lochindarat, P. Srisan, P. Suwan, Y. Osotthanakorn, T. Anantasetagoon, S. Kanjanawasri, S. Tanupattarachai, J. Weerakul, R. Chaiwirattana, M. Maneerattanaporn, R. Poolsavathitikool, K. Chokephaibulkit, A. Apisarnthanarak, and S. F. Dowell. 2005. Human disease from influenza A (H5N1), Thailand, 2004. Emerg. Infect. Dis. 11:201-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coro, E. S., W. L. Chang, and N. Baumgarth. 2006. Type I IFN receptor signals directly stimulate local B cells early following influenza virus infection. J. Immunol. 176:4343-4351. [DOI] [PubMed] [Google Scholar]
- 4.Davies, J. R., S. Kirkham, N. Svitacheva, D. J. Thornton, and I. Carlstedt. 2007. MUC16 is produced in tracheal surface epithelium and submucosal glands and is present in secretions from normal human airway and cultured bronchial epithelial cells. Int. J. Biochem. Cell. Biol. 39:1943-1954. [DOI] [PubMed] [Google Scholar]
- 5.de Jong, M. D., V. C. Bach, T. Q. Phan, M. H. Vo, T. T. Tran, B. H. Nguyen, M. Beld, T. P. Le, H. K. Truong, V. V. Nguyen, T. H. Tran, Q. H. Do, and J. Farrar. 2005. Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma. N. Engl. J. Med. 352:686-691. [DOI] [PubMed] [Google Scholar]
- 6.de Jong, M. D., C. P. Simmons, T. T. Thanh, V. M. Hien, G. J. Smith, T. N. Chau, D. M. Hoang, N. V. Chau, T. H. Khanh, V. C. Dong, P. T. Qui, B. V. Cam, Q. Ha do, Y. Guan, J. S. Peiris, N. T. Chinh, T. T. Hien, and J. Farrar. 2006. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 12:1203-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Florea, B. I., M. L. Cassara, H. E. Junginger, and G. Borchard. 2003. Drug transport and metabolism characteristics of the human airway epithelial cell line Calu-3. J. Control Release 87:131-138. [DOI] [PubMed] [Google Scholar]
- 8.Forbes, I. I. 2000. Human airway epithelial cell lines for in vitro drug transport and metabolism studies. Pharm. Sci. Technol. Today 3:18-27. [DOI] [PubMed] [Google Scholar]
- 9.Gao, P., S. Watanabe, T. Ito, H. Goto, K. Wells, M. McGregor, A. J. Cooley, and Y. Kawaoka. 1999. Biological heterogeneity, including systemic replication in mice, of H5N1 influenza A virus isolates from humans in Hong Kong. J. Virol. 73:3184-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Govorkova, E. A., J. E. Rehg, S. Krauss, H. L. Yen, Y. Guan, M. Peiris, T. D. Nguyen, T. H. Hanh, P. Puthavathana, H. T. Long, C. Buranathai, W. Lim, R. G. Webster, and E. Hoffmann. 2005. Lethality to ferrets of H5N1 influenza viruses isolated from humans and poultry in 2004. J. Virol. 79:2191-2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gruenert, D. C., W. E. Finkbeiner, and J. H. Widdicombe. 1995. Culture and transformation of human airway epithelial cells. Am. J. Physiol. 268:L347-L360. [DOI] [PubMed] [Google Scholar]
- 12.Guo, Z., L. M. Chen, H. Zeng, J. A. Gomez, J. Plowden, T. Fujita, J. M. Katz, R. O. Donis, and S. Sambhara. 2007. NS1 protein of influenza A virus inhibits the function of intracytoplasmic pathogen sensor, RIG-I. Am. J. Respir. Cell Mol. Biol. 36:263-269. [DOI] [PubMed] [Google Scholar]
- 13.Hatta, M., P. Gao, P. Halfmann, and Y. Kawaoka. 2001. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science 293:1840-1842. [DOI] [PubMed] [Google Scholar]
- 14.Havenar-Daughton, C., G. A. Kolumam, and K. Murali-Krishna. 2006. The direct action of type I IFN on CD4 T cells is critical for sustaining clonal expansion in response to a viral but not a bacterial infection. J. Immunol. 176:3315-3319. [DOI] [PubMed] [Google Scholar]
- 15.Hayman, A., S. Comely, A. Lackenby, L. C. Hartgroves, S. Goodbourn, J. W. McCauley, and W. S. Barclay. 2007. NS1 proteins of avian influenza A viruses can act as antagonists of the human alpha/beta interferon response. J. Virol. 81:2318-2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayman, A., S. Comely, A. Lackenby, S. Murphy, J. McCauley, S. Goodbourn, and W. Barclay. 2006. Variation in the ability of human influenza A viruses to induce and inhibit the IFN-beta pathway. Virology 347:52-64. [DOI] [PubMed] [Google Scholar]
- 17.Honda, K., H. Yanai, H. Negishi, M. Asagiri, M. Sato, T. Mizutani, N. Shimada, Y. Ohba, A. Takaoka, N. Yoshida, and T. Taniguchi. 2005. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434:772-777. [DOI] [PubMed] [Google Scholar]
- 18.Hornung, V., J. Ellegast, S. Kim, K. Brzozka, A. Jung, H. Kato, H. Poeck, S. Akira, K. K. Conzelmann, M. Schlee, S. Endres, and G. Hartmann. 2006. 5′-Triphosphate RNA is the ligand for RIG-I. Science 314:994-997. [DOI] [PubMed] [Google Scholar]
- 19.Imaizumi, T., M. Hatakeyama, K. Yamashita, A. Ishikawa, H. Yoshida, K. Satoh, K. Taima, F. Mori, and K. Wakabayashi. 2005. Double-stranded RNA induces the synthesis of retinoic acid-inducible gene-I in vascular endothelial cells. Endothelium 12:133-137. [DOI] [PubMed] [Google Scholar]
- 20.Kandun, I. N., H. Wibisono, E. R. Sedyaningsih, Yusharmen, W. Hadisoedarsuno, W. Purba, H. Santoso, C. Septiawati, E. Tresnaningsih, B. Heriyanto, D. Yuwono, S. Harun, S. Soeroso, S. Giriputra, P. J. Blair, A. Jeremijenko, H. Kosasih, S. D. Putnam, G. Samaan, M. Silitonga, K. H. Chan, L. L. Poon, W. Lim, A. Klimov, S. Lindstrom, Y. Guan, R. Donis, J. Katz, N. Cox, M. Peiris, and T. M. Uyeki. 2006. Three Indonesian clusters of H5N1 virus infection in 2005. N. Engl. J. Med. 355:2186-2194. [DOI] [PubMed] [Google Scholar]
- 21.Kemp, P. A., R. A. Sugar, and A. D. Jackson. 2004. Nucleotide-mediated mucin secretion from differentiated human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 31:446-455. [DOI] [PubMed] [Google Scholar]
- 22.Kilpatrick, A. M., A. A. Chmura, D. W. Gibbons, R. C. Fleischer, P. P. Marra, and P. Daszak. 2006. Predicting the global spread of H5N1 avian influenza. Proc. Natl. Acad. Sci. USA 103:19368-19373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobasa, D., S. M. Jones, K. Shinya, J. C. Kash, J. Copps, H. Ebihara, Y. Hatta, J. H. Kim, P. Halfmann, M. Hatta, F. Feldmann, J. B. Alimonti, L. Fernando, Y. Li, M. G. Katze, H. Feldmann, and Y. Kawaoka. 2007. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature 445:319-323. [DOI] [PubMed] [Google Scholar]
- 24.Kochs, G., A. Garcia-Sastre, and L. Martinez-Sobrido. 2007. Multiple anti-interferon actions of the influenza A virus NS1 protein. J. Virol. 81:7011-7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kreda, S. M., S. F. Okada, C. A. van Heusden, W. O'Neal, S. Gabriel, L. Abdullah, C. W. Davis, R. C. Boucher, and E. R. Lazarowski. 2007. Coordinated release of nucleotides and mucin from human airway epithelial Calu-3 cells. J. Physiol. 584:245-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu, X., T. M. Tumpey, T. Morken, S. R. Zaki, N. J. Cox, and J. M. Katz. 1999. A mouse model for the evaluation of pathogenesis and immunity to influenza A (H5N1) viruses isolated from humans. J. Virol. 73:5903-5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ludwig, S., X. Wang, C. Ehrhardt, H. Zheng, N. Donelan, O. Planz, S. Pleschka, A. Garcia-Sastre, G. Heins, and T. Wolff. 2002. The influenza A virus NS1 protein inhibits activation of Jun N-terminal kinase and AP-1 transcription factors. J. Virol. 76:11166-11171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maines, T. R., X. H. Lu, S. M. Erb, L. Edwards, J. Guarner, P. W. Greer, D. C. Nguyen, K. J. Szretter, L. M. Chen, P. Thawatsupha, M. Chittaganpitch, S. Waicharoen, D. T. Nguyen, T. Nguyen, H. H. Nguyen, J. H. Kim, L. T. Hoang, C. Kang, L. S. Phuong, W. Lim, S. Zaki, R. O. Donis, N. J. Cox, J. M. Katz, and T. M. Tumpey. 2005. Avian influenza (H5N1) viruses isolated from humans in Asia in 2004 exhibit increased virulence in mammals. J. Virol. 79:11788-11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matrosovich, M. N., T. Y. Matrosovich, T. Gray, N. A. Roberts, and H. D. Klenk. 2004. Human and avian influenza viruses target different cell types in cultures of human airway epithelium. Proc. Natl. Acad. Sci. USA 101:4620-4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mibayashi, M., L. Martinez-Sobrido, Y. M. Loo, W. B. Cardenas, M. Gale, Jr., and A. Garcia-Sastre. 2007. Inhibition of retinoic acid-inducible gene I-mediated induction of beta interferon by the NS1 protein of influenza A virus. J. Virol. 81:514-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicholls, J. M., M. C. Chan, W. Y. Chan, H. K. Wong, C. Y. Cheung, D. L. Kwong, M. P. Wong, W. H. Chui, L. L. Poon, S. W. Tsao, Y. Guan, and J. S. Peiris. 2007. Tropism of avian influenza A (H5N1) in the upper and lower respiratory tract. Nat. Med. 13:147-149. [DOI] [PubMed] [Google Scholar]
- 32.Oner, A. F., A. Bay, S. Arslan, H. Akdeniz, H. A. Sahin, Y. Cesur, S. Epcacan, N. Yilmaz, I. Deger, B. Kizilyildiz, H. Karsen, and M. Ceyhan. 2006. Avian influenza A (H5N1) infection in eastern Turkey in 2006. N. Engl. J. Med. 355:2179-2185. [DOI] [PubMed] [Google Scholar]
- 33.Opitz, B., A. Rejaibi, B. Dauber, J. Eckhard, M. Vinzing, B. Schmeck, S. Hippenstiel, N. Suttorp, and T. Wolff. 2007. IFNbeta induction by influenza A virus is mediated by RIG-I which is regulated by the viral NS1 protein. Cell. Microbiol. 9:930-938. [DOI] [PubMed] [Google Scholar]
- 34.Peiris, J. S., W. C. Yu, C. W. Leung, C. Y. Cheung, W. F. Ng, J. M. Nicholls, T. K. Ng, K. H. Chan, S. T. Lai, W. L. Lim, K. Y. Yuen, and Y. Guan. 2004. Re-emergence of fatal human influenza A subtype H5N1 disease. Lancet 363:617-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pichlmair, A., O. Schulz, C. P. Tan, T. I. Naslund, P. Liljestrom, F. Weber, and C. Reis e Sousa. 2006. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 314:997-1001. [DOI] [PubMed] [Google Scholar]
- 36.Rimmelzwaan, G. F., D. van Riel, M. Baars, T. M. Bestebroer, G. van Amerongen, R. A. Fouchier, A. D. Osterhaus, and T. Kuiken. 2006. Influenza A virus (H5N1) infection in cats causes systemic disease with potential novel routes of virus spread within and between hosts. Am. J. Pathol. 168:176-183, 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salomon, R., J. Franks, E. A. Govorkova, N. A. Ilyushina, H. L. Yen, D. J. Hulse-Post, J. Humberd, M. Trichet, J. E. Rehg, R. J. Webby, R. G. Webster, and E. Hoffmann. 2006. The polymerase complex genes contribute to the high virulence of the human H5N1 influenza virus isolate A/Vietnam/1203/04. J Exp. Med. 203:689-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seo, S. H., E. Hoffmann, and R. G. Webster. 2004. The NS1 gene of H5N1 influenza viruses circumvents the host anti-viral cytokine responses. Virus Res. 103:107-113. [DOI] [PubMed] [Google Scholar]
- 39.Seth, R. B., L. Sun, and Z. J. Chen. 2006. Antiviral innate immunity pathways. Cell Res. 16:141-147. [DOI] [PubMed] [Google Scholar]
- 40.Shinya, K., M. Ebina, S. Yamada, M. Ono, N. Kasai, and Y. Kawaoka. 2006. Avian flu: influenza virus receptors in the human airway. Nature 440:435-436. [DOI] [PubMed] [Google Scholar]
- 41.Shinya, K., S. Hamm, M. Hatta, H. Ito, T. Ito, and Y. Kawaoka. 2004. PB2 amino acid at position 627 affects replicative efficiency, but not cell tropism, of Hong Kong H5N1 influenza A viruses in mice. Virology 320:258-266. [DOI] [PubMed] [Google Scholar]
- 42.Simonsen, L., M. J. Clarke, G. D. Williamson, D. F. Stroup, N. H. Arden, and L. B. Schonberger. 1997. The impact of influenza epidemics on mortality: introducing a severity index. Am. J. Public Health 87:1944-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Talon, J., C. M. Horvath, R. Polley, C. F. Basler, T. Muster, P. Palese, and A. Garcia-Sastre. 2000. Activation of interferon regulatory factor 3 is inhibited by the influenza A virus NS1 protein. J. Virol. 74:7989-7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tran, T. H., T. L. Nguyen, T. D. Nguyen, T. S. Luong, P. M. Pham, V. C. Nguyen, T. S. Pham, C. D. Vo, T. Q. Le, T. T. Ngo, B. K. Dao, P. P. Le, T. T. Nguyen, T. L. Hoang, V. T. Cao, T. G. Le, D. T. Nguyen, H. N. Le, K. T. Nguyen, H. S. Le, V. T. Le, D. Christiane, T. T. Tran, J. Menno de, C. Schultsz, P. Cheng, W. Lim, P. Horby, and J. Farrar. 2004. Avian influenza A (H5N1) in 10 patients in Vietnam. N. Engl. J. Med. 350:1179-1188. [DOI] [PubMed] [Google Scholar]
- 45.Tseng, C. T., J. Tseng, L. Perrone, M. Worthy, V. Popov, and C. J. Peters. 2005. Apical entry and release of severe acute respiratory syndrome-associated coronavirus in polarized Calu-3 lung epithelial cells. J. Virol. 79:9470-9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tumpey, T. M., C. F. Basler, P. V. Aguilar, H. Zeng, A. Solorzano, D. E. Swayne, N. J. Cox, J. M. Katz, J. K. Taubenberger, P. Palese, and A. Garcia-Sastre. 2005. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science 310:77-80. [DOI] [PubMed] [Google Scholar]
- 47.Uiprasertkul, M., P. Puthavathana, K. Sangsiriwut, P. Pooruk, K. Srisook, M. Peiris, J. M. Nicholls, K. Chokephaibulkit, N. Vanprapar, and P. Auewarakul. 2005. Influenza A H5N1 replication sites in humans. Emerg. Infect. Dis. 11:1036-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Riel, D., V. J. Munster, E. de Wit, G. F. Rimmelzwaan, R. A. Fouchier, A. D. Osterhaus, and T. Kuiken. 2006. H5N1 virus attachment to lower respiratory tract. Science 312:399. [DOI] [PubMed] [Google Scholar]
- 49.Walls, H. H., M. W. Harmon, J. J. Slagle, C. Stocksdale, and A. P. Kendal. 1986. Characterization and evaluation of monoclonal antibodies developed for typing influenza A and influenza B viruses. J. Clin. Microbiol. 23:240-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang, X., M. Li, H. Zheng, T. Muster, P. Palese, A. A. Beg, and A. Garcia-Sastre. 2000. Influenza A virus NS1 protein prevents activation of NF-κB and induction of alpha/beta interferon. J. Virol. 74:11566-11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wright, P. F., J. Thompson, and D. T. Karzon. 1980. Differing virulence of H1N1 and H3N2 influenza strains. Am. J. Epidemiol. 112:814-819. [DOI] [PubMed] [Google Scholar]
- 52.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730-737. [DOI] [PubMed] [Google Scholar]
- 53.Zitzow, L. A., T. Rowe, T. Morken, W. J. Shieh, S. Zaki, and J. M. Katz. 2002. Pathogenesis of avian influenza A (H5N1) viruses in ferrets. J. Virol. 76:4420-4429. [DOI] [PMC free article] [PubMed] [Google Scholar]