Abstract
Loss of heterozygosity (LOH), a causal event in tumorigenesis, frequently encompasses multiple genetic loci and whole chromosome arms. However, the mechanisms leading to such extensive LOH are poorly understood. We investigated the mechanisms of DNA double-strand break (DSB)-induced extensive LOH by screening for auxotrophic marker loss ∼25 kb distal to an HO endonuclease break site within a nonessential minichromosome in Schizosaccharomyces pombe. Extensive break-induced LOH was infrequent, resulting from large translocations through both allelic crossovers and break-induced replication. These events required the homologous recombination (HR) genes rad32+, rad50+, nbs1+, rhp51+, rad22+, rhp55+, rhp54+, and mus81+. Surprisingly, LOH was still observed in HR mutants, which resulted predominantly from de novo telomere addition at the break site. De novo telomere addition was most frequently observed in rad22Δ and rhp55Δ backgrounds, which disrupt HR following end resection. Further, levels of de novo telomere addition, while increased in ku70Δ rhp55Δ strains, were reduced in exo1Δ rhp55Δ and an rhp55Δ strain overexpressing rhp51. These findings support a model in which HR prevents de novo telomere addition at DSBs by competing for resected ends. Together, these results suggest that the mechanisms of break-induced LOH may be predicted from the functional status of the HR machinery.
Loss of heterozygosity (LOH), in which the second allele of a gene is lost, can lead to tumorigenesis through functional inactivation of tumor suppressor genes (17). LOH has been detected at specific tumor suppressor loci in familial cancers at high frequencies and is generally observed in all solid tumor types (20). In sporadic cancers, up to half of all chromosomes undergo LOH; however, not all of these regions are linked to tumor suppressor genes and arise as a consequence of general chromosomal instability (16). LOH often extends several megabases to encompass multiple genetic loci or even whole chromosome arms, thus leading to extensive loss of allele-specific information (20). A number of possible mechanisms responsible for LOH have been inferred from karyotype and polymorphic marker analyses in tumor cells and include chromosome loss, interstitial deletions, and mitotic recombination (13). However, despite LOH being the most common genetic alteration in human cancers, the molecular mechanisms underlying extensive LOH events are currently poorly understood.
A potential initiating event in LOH is a DNA double-strand break (DSB). These can arise either spontaneously, as a result of normal DNA metabolism, or through exposure to DNA-damaging agents, such as ionizing radiation. Compromising DSB repair can result in chromosomal loss or rearrangements, leading to cell death or genomic instability and tumorigenesis (15). Nonhomologous end joining (NHEJ) and homologous recombination (HR) are the two main pathways responsible for DSB repair in eukaryotes. During NHEJ, the broken ends are protected by the highly conserved Ku70-Ku80 heterodimer, which in turn recruits DNA-protein kinase catalytic subunit to form DNA-protein kinase. Following end processing, DNA-protein kinase facilitates XRCC4-ligase IV heterodimer binding and rejoining of the DSB ends (66). During HR, a sister chromatid or, less frequently, a homologous chromosome is utilized as a repair template (14). HR requires the evolutionarily conserved RAD52 epistasis group of genes, consisting of RAD51, RAD52, RAD54, RAD55, RAD57, MRE11, RAD50, and XRS2 in Saccharomyces cerevisiae (19). Homologs of these genes are required for HR in Schizosaccharomyces pombe (rhp51+, rad22+ and rti1+, rhp54+, rhp55+, rhp57+, rad32+, rad50+, and nbs1+, respectively) (4, 42, 61). HR is initiated by resection of the broken ends to generate single-stranded DNA (ssDNA) 3′ overhangs, facilitated by the trimeric Mre11-Rad50-Xrs2 (MRX) complex (equivalent to the Mre11-Rad50-Nbs1 [MRN] complex in fission yeast and mammalian cells) and Exo1 (25, 34, 59, 62). Replication protein A binds to ssDNA, where it is thought to facilitate Rad52 binding and strand invasion by removing secondary structures (54, 65). Replication protein A is displaced by Rad51 to form a nucleoprotein filament, a process facilitated by Rad52 and the heterodimeric Rad55-Rad57 complex (56, 57). Rad54 interacts with Rad51, stabilizing the nucleoprotein filament and stimulating strand invasion and displacement (D) loop formation (30, 44, 53). Extension of the 3′ end by DNA synthesis stimulates second-end capture through base pairing to the D loop, and upon ligation, a double Holliday junction structure can be formed (41). Such recombination intermediates may be resolved through diverse mechanisms in eukaryotes, including endonucleolytic cleavage by Mus81-Mms4 endonuclease (Mus81-Eme1 in S. pombe), which results in crossover products (1, 10, 40).
Break-induced LOH may result from a variety of mechanisms. Localized break-induced LOH can arise through both NHEJ and HR repair: During NHEJ, LOH can result through small insertions or deletions at, or near, the break site, while HR can cause localized LOH through gene conversion (33, 50, 63). Extensive break-induced LOH can also potentially arise through HR as a result of allelic crossovers between maternal and paternal chromatids during S/G2 phase, followed by cosegregation of a recombinant and a parental chromosome (13). Break-induced replication (BIR) is a recombination-dependent replication process initiated by a broken DNA end invading a homologous template that has the capacity to generate extensive LOH associated with nonreciprocal translocations (26). Chromosome “healing,” in which a broken chromosome end is stabilized through telomere addition (31), is associated with spontaneous or DSB-induced chromosomal truncations in a variety of organisms (29, 43) and may additionally lead to extensive LOH.
In this study, we sought to examine the mechanisms of extensive break-induced LOH using a fission yeast model system. We identify a role for HR in facilitating extensive LOH through translocations, arising through both allelic crossovers and BIR following DSB induction. Analysis of LOH in HR mutants led to the identification of a further role for HR in preventing break-induced extensive LOH through de novo telomere addition.
MATERIALS AND METHODS
Yeast strains, media, and genetic methods.
All S. pombe strains were cultured, manipulated, and stored as previously described (45). All strain genotypes and details of their construction are described in the supplemental material. Minichromosome loss per generation was calculated as previously described (52).
Site-specific DSB assay.
The DSB assay was performed as described previously (45). The percentage of ade+ G418r/HygBr his+ colonies and those cells undergoing gene conversion (ade+ G418s/HygBs his+ colonies) and LOH (ade+ G418s/HygBs his− or ade− G418s/HygBs his+ colonies) were calculated. To determine levels of break-induced minichromosome loss, background Ch16-MGH/Ch16-MHH (see below) loss (ade− G418s/HygBs his− colonies at 48 h without thiamine in blank-vector assays) was subtracted from the number of ade− G418s/HygBs his− colonies 48 h without thiamine. More than 1,000 colonies were scored for each time point (see Table S2 in the supplemental material), and each experiment was performed three times using three independently derived strains for all mutants tested. The proportion of LOH attributed to translocations (Chx), de novo telomere addition (“TEL+” in the figures), or uncharacterized mechanisms (“Other” in the figures) was calculated as a percentage of the overall level of DSB-induced LOH (Table 1) following pulsed-field gel electrophoresis (PFGE) and PCR analyses of ≥20 ade+ G418s/HygBs his− colonies (isolated from three individual strains from each genetic background) (Table 2; see also Table S1 in the supplemental material). Southern blots were performed as previously described (45).
TABLE 1.
DSB-induced marker loss in the wild type and HR and NHEJ mutantsa
| Genetic background | % ade+ G418s/Hygshis+ (GC) | % ade+ G418r/Hygrhis+ (EJ/SCR) | % ade− G418s/Hygshis− (Ch16 loss) | % ade+ G418s/Hygshis− (LOH)c | P value (relative to wild-type LOH) |
|---|---|---|---|---|---|
| Wild type | 49.7 ± 2.6 | 25.0 ± 1.4 | 23.7 ± 1.7 | 1.7 ± 0.3 | 1.000 |
| rad32Δ | 30.7 ± 2.0 | 25.6 ± 5.1 | 35.7 ± 5.8 | 0.6 ± 0.2 | 0.013 |
| rad50Δ | 18.3 ± 1.8 | 23.9 ± 0.6 | 49.7 ± 1.9 | 0.6 ± 0.2 | 0.011 |
| nbs1Δ | 15.6 ± 0.7 | 30.9 ± 2.6 | 43.6 ± 3.3 | 1.4 ± 0.2 | 0.441 |
| exo1Δ | 52.7 ± 1.0 | 33.1 ± 0.7 | 13.0 ± 0.8 | 1.1 ± 0.5 (0.09 ± 0.05) | 0.233 |
| rad22Δb | 0.0 ± 0.0 | 22.3 ± 1.1 | 68.8 ± 1.7 | 2.3 ± 0.6 | 0.453 |
| rhp55Δ | 2.9 ± 0.7 | 62.8 ± 9.8 | 30.5 ± 10.9 | 1.8 ± 1.1 | 0.936 |
| rhp51Δ | 1.0 ± 0.5 | 35.9 ± 2.9 | 57.0 ± 2.9 | 0.8 ± 0.3 | 0.058 |
| rhp54Δ | 2.8 ± 1.9 | 36.8 ± 3.3 | 32.6 ± 4.5 | 0.4 ± 0.2 | 0.004 |
| mus81Δ | 29.0 ± 2.9 | 28.1 ± 3.4 | 38.1 ± 4.4 | 0.2 ± 0.1 | 0.001 |
| ku70Δ | 53.4 ± 2.8 | 38.5 ± 2.1 | 6.0 ± 1.0 | 1.5 ± 0.1 (0.39 ± 0.14) | 0.613 |
| lig4Δ | 47.2 ± 3.6 | 39.1 ± 3.1 | 12.8 ± 0.5 | 0.7 ± 1.1 (0.19 ± 0.09) | 0.042 |
| ku70Δ rhp55Δ | 15.4 ± 0.9 | 46.7 ± 2.4 | 27.2 ± 2.1 | 7.6 ± 0.7 | 0.006 |
| lig4Δ rhp55Δ | 3.6 ± 1.3 | 58.8 ± 6.2 | 32.5 ± 5.7 | 2.3 ± 0.2 | 0.145 |
| exo1Δ rhp55Δ | 5.8 ± 2.4 | 54.4 ± 7.3 | 37.2 ± 5.8 | 1.0 ± 0.4 | 0.197 |
The levels of interchromosomal gene conversion (GC), end joining/sister chromatid recombination (EJ/SCR) minichromosome loss (Ch16 loss), and LOH are shown. Percentages were calculated as described in Materials and Methods. Standard errors of the mean are indicated.
Gene conversion levels in rad22Δ strains described here are significantly lower than those previously described (45), which were subsequently determined to include a suppressor mutation (9).
The values in parentheses represent the percentages of ade− G418s/Hygs his+ colonies identified for the mutant analyzed.
TABLE 2.
Mechanisms of break-induced LOH in the wild type and HR and NHEJ mutantsa
| Genetic background | % ade+ G418s/Hygshis− (LOH) | No. of ade+ G418s/Hygshis− colonies analyzed | % Allelic crossover/BIR (Chx) | % Telomere addition | % Other |
|---|---|---|---|---|---|
| Wild type | 1.7 ± 0.3 | 22 (100) | 86.4 (19/22) | 0.0 (0/20) (1.0 (1/100)) | 13.6 (3/22) |
| rad32Δ | 0.6 ± 0.2 | 21 | 0.0 (0/21) | 52.4 (11/21) | 47.6 (10/21) |
| rad50Δ | 0.6 ± 0.2 | 20 | 0.0 (0/20) | 30.0 (6/20) | 70.0 (14/20) |
| nbs1Δ | 1.4 ± 0.2 | 21 | 0.0 (0/20) | 81.0 (17/21) | 19.0 (4/21) |
| exo1Δ | 1.1 ± 0.5 | 22 | 13.6 (3/22) | 45.5 (10/22) | 40.9 (7/22) |
| rad22Δ | 2.3 ± 0.6 | 24 | 0.0 (0/24) | 75.0 (18/24) | 25.0 (6/24) |
| rhp55Δ | 1.8 ± 1.1 | 20 | 0.0 (0/20) | 80.0 (16/20) | 20.0 (4/20) |
| rhp51Δ | 0.8 ± 0.3 | 25 | 0.0 (0/25) | 80.0 (20/25) | 20.0 (5/25) |
| rhp54Δ | 0.4 ± 0.2 | 20 | 0.0 (0/20) | 90.0 (18/20) | 10.0 (2/20) |
| mus81Δ | 0.2 ± 0.1 | 22 | 0.0 (0/20) | 27.7 (6/22) | 72.3 (16/22) |
| ku70Δ | 1.5 ± 0.1 | 24 (100) | 29.2 (7/24) | 8.3 (2/24) (18.0 (18/100)) | 62.5 (15/24) |
| lig4Δ | 0.7 ± 1.1 | 20 (100) | 45.0 (9/20) | 10.0 (2/20) (5.0 (5/100)) | 45.0 (9/20) |
| ku70Δ rhp55Δ | 7.6 ± 0.7 | 22 | 0.0 (0/20) | 81.8 (18/22) | 18.2 (4/20) |
| lig4Δ rhp55Δ | 2.3 ± 0.2 | 24 | 0.0 (0/24) | 79.2 (19/24) | 20.8 (5/24) |
| exo1Δ rhp55Δ | 1.0 ± 0.4 | 20 | 0.0 (0/20) | 85.0 (17/20) | 15.0 (3/20) |
| rhp55Δ + pIRT3 | 1.1 ± 0.3 | 20 | 0.0 (0/20) | 85.0 (17/20) | 15.0 (3/20) |
| rhp55Δ + pIRT3-rhp51 | 0.5 ± 0.1 | 20 | 0.0 (0/20) | 70.0 (14/20) | 30.0 (6/20) |
LOH colonies (ade+ G418s/Hygs his−) from each strain were analyzed for the presence of Chx or de novo telomere addition. The percentages of ade+ G418s/Hygs his− cells undergoing these and “other” uncharacterized mechanisms of LOH are presented. A table detailing the PFGE profiles acquired from colonies which underwent “other” mechanisms of LOH can be seen in Table S3 in the supplemental material. The number of colonies undergoing distinct mechanisms of LOH for each mutant is shown in parentheses.
Pedigree analysis.
Cultures of TH1900 (see Table S1 in the supplemental material) were grown in log phase for 24 h in Edinburgh minimal medium plus uracil, histidine, and adenine with or without thiamine, and individual cells were placed onto a YE5S (yeast extract plus five supplements) plate and allowed to divide. The two daughter cells were separated (using an MSM System [Singer Instruments]), and colonies were scored for viability and marker loss by replica plating. ade+ G418s his− and sister colonies, where viable, were subjected to PFGE analysis to determine the presence of nonreciprocal translocations in one or both daughter cells.
PFGE.
The procedures used in this study for PFGE analysis have been described previously (45). For higher-resolution separation of Ch16, a 1.2% chromosomal grade agarose gel was used under the following conditions: 4 V/cm, 112° angle, with a switch time of 1 min. Samples were separated for 48 h in 1× Tris-acetate-EDTA at 14°C.
PCR assay for de novo telomere addition.
A colony PCR-based technique was used to detect and amplify telomeric sequence within 950 bp of the MATa break site on Ch16-MGH/Ch16-MHH. Test strains were inoculated into 50 μl of PCR mixture containing 75 mM Tris-HCl (pH 8.8), 20 mM (NH2)4SO2, 0.01% (vol/vol) Tween 20, 2 mM MgCl2, 0.2 mM deoxynucleoside triphosphates, 0.001 mM R1 primer (5′-GATTTAAACCTGGATTTGGGC-3′), 0.001 mM T1 (telomere-directed) primer (5′-CTGTAACCGTGTAACCGTAAC-3′), 2.5 units Thermoprime Plus DNA polymerase (Abgene, United Kingdom) and incubated at 94°C for 3 min; cycled 40 times at 94°C for 45 s, 58°C for 45 s, and 72°C for 4 min; and incubated at 72°C for 10 min. To test the specificities of PCR products, samples were digested with MfeI (New England Biosciences, United Kingdom), which yielded a 300-bp fragment in telomere-positive strains. PCR products generated from break-telomere junction amplifications were sequenced using suitable primers and the BigDye Terminator V3.1 Cycle Sequencing Kit (Applied Biosystems) according to the manufacturer's instructions.
RESULTS
Extensive LOH results from break-induced translocations in wild-type background cells.
To study the mechanisms of break-induced LOH, a strain was constructed in which a site-specific DSB could be generated within a nonessential minichromosome, thus preventing break-induced loss of viability. Ch16 is a highly stable 530-kb linear minichromosome derived from chromosome III (ChIII) (39) and includes ade6-M216, which, together with ade6-M210 on ChIII, results in an ade+ phenotype through heteroallelic complementation (23). This chromosomal element was previously modified through insertion of a MATa cut site with an adjacent kanMX6 gene encoding a G418 resistance marker (Ch16-MG) (45). In this study, Ch16-MG was adapted so that an additional marker (his3+) was integrated ∼25 kb distal to the MATa site (Ch16-MGH) (Fig. 1A), resulting in a strain that was heterozygous for ade6, G418r, and his3+ markers. Expression of HO endonuclease in this context generates a unique DSB at the MATa site within Ch16-MGH. To examine break-induced LOH in this background, HO endonuclease was expressed from a plasmid (pREP81X-HO) in the absence of thiamine, and colony phenotypes were recorded. Following DSB induction, 49.7% of the assayed colonies were ade+ G418s his+, having undergone DSB repair through gene conversion; 20.5% of the colonies were ade− G418s his− as a result of minichromosome loss through failed DSB repair; and 25.0% were ade+ G418r his+, consistent with error-free repair via end joining or possibly sister chromatid recombination (Table 1) (45). In addition, 1.7% of the colonies were ade+ G418s his−, indicating that they had undergone extensive LOH (Table 1). Comparable values were observed for a Ch16-MHH strain, constructed as described in the supplemental material (our unpublished data). Extensive LOH was break dependent, as his3+ and G418r marker loss was not observed following incubation for 48 h in the presence of thiamine or in strains transformed with blank vector (see Table S2 in the supplemental material).
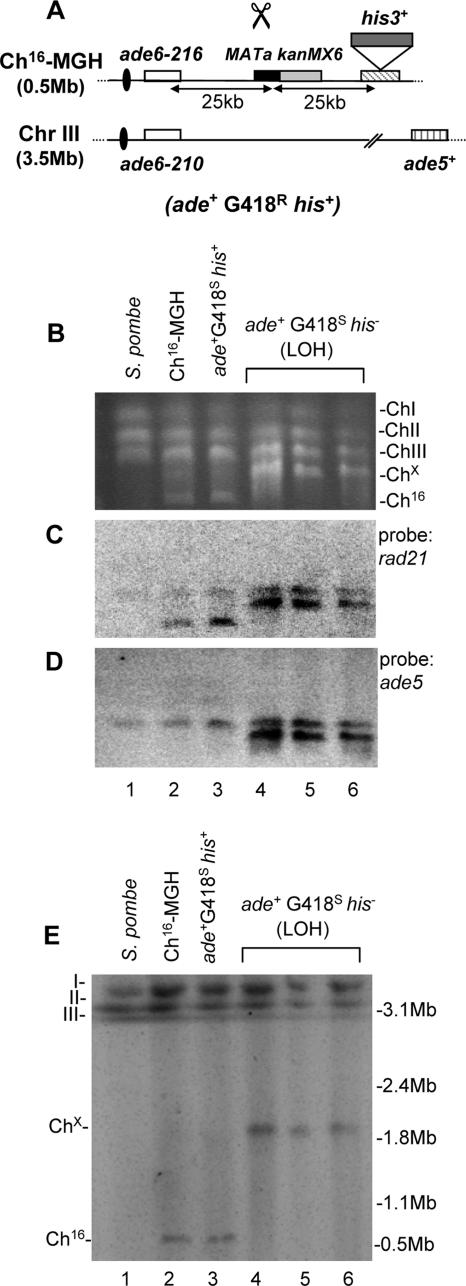
FIG. 1.
DSB-induced LOH results from large translocations in a wild-type background. (A) Schematic of the Ch16-MGH strain. Ch16-MGH, ChIII, centromeric regions (ovals), complementary heteroalleles (ade6-M216 and ade6-M210) (white), ade5+ (vertical stripes), and a MATa site (black) with an adjacent kanMX6 resistance marker (gray) are shown, as previously described (45). The his3+ marker (dark gray) was inserted ∼25 kb downstream of the MATa site. Derepression of pREP81X-HO (not shown) generates a DSB at the MATa target site (scissors). In Ch16-MHH, kanMX6 is replaced by hph. (B) PFGE analysis of chromosomal DNA from a wild-type S. pombe strain (lane 1), an “uncut” wild-type strain (TH1436) encoding Ch16-MGH (Ch16-MGH; lane 2), a strain encoding Ch16-MGH repaired by gene conversion following DSB induction (ade+ G418s his+; lane 3), and three individually isolated ade+ G418s his− colonies following DSB induction (lanes 4, 5, and 6). (C and D) Southern blot analysis of the PFGE shown in panel B probed with rad21 (C) and ade5 (D). (E) High-resolution PFGE analysis of colonies shown in panel B.
To investigate the mechanism of break-induced LOH in Ch16-MGH, the chromosomes of 22 ade+ G418s his− colonies were examined by PFGE. No size changes were detected in the endogenous chromosomes of these colonies (Fig. 1B, compare lanes 4 to 6 with lanes 1 and 2). However, the majority of ade+ G418s his− colonies lacked a 530-kb band corresponding to Ch16-MGH that is present in uncut ade+ G418r his+ (Ch16-MGH) and gene conversion (ade+ G418s his+) strains but instead possessed a significantly larger chromosomal element, termed Chx (Fig. 1B, compare lanes 4 to 6 with lanes 2 and 3). PFGE analysis revealed the presence of Chx in 86% of the individually isolated ade+ G418s his− colonies (Table 2). Chx sizes were equivalent in all colonies tested. As these cells were ade+, this suggested that they carried both ade6-M216 and ade6-M210 alleles and that Chx was derived from Ch16-MGH. Consistent with this, Southern blot analysis indicated that the rad21 allele present on Ch16-MGH in the uncut ade+ G418r his+ parental strain was present on Chx from ade+ G418s his− colonies (Fig. 1C, compare lane 2 with lanes 4 to 6). Since Ch16-MGH is homologous to ChIII, it was possible that a recombination event between Ch16-MGH and ChIII resulted in the formation of Chx through duplication of the distal arm of ChIII. Consistent with this, Southern blot analysis revealed ade5+ (located 1 Mb distal to rad21+ on ChIII but absent from Ch16-MGH) to be present on Chx in ade+ G418s his− cells (Fig. 1D, compare lane 2 with lanes 4 to 6). A recombination event between the 400-kb centromere-proximal arm of Ch16-MGH and the 1.6-Mb centromere-distal arm of ChIII would be expected to generate a chromosomal element of 2 Mb. This was confirmed through analysis of ade+ G418s his− colonies under high-resolution PFGE conditions (Fig. 1E, lanes 4 to 6). Together, these findings strongly suggest that Chx resulted from recombination between the 400-kb centromere-proximal arm of Ch16-MGH and the distal 1.6-Mb arm of ChIII.
Break-induced translocations arise from allelic crossovers and BIR.
Two possible recombination-based mechanisms could account for such a large translocation associated with extensive break-induced LOH (see Fig. S1 in the supplemental material). In the first, Chx could have resulted from an allelic crossover in late S/G2 phase of the cell cycle, in which DSB repair of one of the Ch16 chromatids by gene conversion is associated with a crossover with ChIII. Such an event would result in one viable ade+ G418s his− daughter cell, containing chromosomes ChIII and Chx (Ch16::ChIII), and one nonviable ade+ G418s his+ daughter cell containing chromosomes Ch16 and ChIII::Ch16 but lacking the essential distal arm of ChIII. Alternatively, DSB induction in Ch16-MGH could have resulted in BIR of the distal arm of the homologous ChIII, thus generating Chx accompanied by loss of the distal arm of Ch16-MGH. Two viable daughter cells would result from such a repair event, with one or both being ade+ G418s his−, depending on whether one or both broken sister chromatids had undergone BIR. To distinguish between these possibilities, pedigree analysis was performed in which daughter cells were separated following break induction, their marker loss was determined, and the chromosomes of ade+ G418s his− colonies and their sisters were examined by PFGE. Following separation of a total of 869 such cells, 22 break-induced ade+ G418s his− colonies were identified that carried Chx (Table 3). The sisters of 15 of these colonies were nonviable, consistent with Chx arising through break-induced allelic crossovers between Ch16-MGH and ChIII chromatids. The sisters of the remaining seven LOH colonies were viable, indicating that at least one of the two broken chromatids had undergone BIR using ChIII as a template. Analysis of the seven sisters of these ade+ G418s his− cells suggested that the second broken chromatid either had also undergone BIR, had been repaired by gene conversion (with or without a crossover), or had failed to be repaired (Table 3).
TABLE 3.
Pedigree analysis of ade+ G418s his− colonies carrying Chx
| Daughter 1
|
Daughter 2
|
Total no. | ||
|---|---|---|---|---|
| Phenotype | Genotype | Phenotype | Genotype | |
| ade+ G418shis− | Chx | Nonviable | 15 | |
| ade+ G418shis− | Chx | ade+ G418shis− | Chx | 2 |
| ade+ G418shis− | Chx | ade− G418shis− | Chx | 1 |
| ade+ G418shis− | Chx | ade+ G418shis+ | Ch16 | 1 |
| ade+ G418shis− | Chx | ade− G418shis− | Absent | 1 |
| ade+ G418shis− | Chx | ade+ G418shis+ | Crossover | 1 |
| ade+ G418shis− | Chx | ade− G418shis− | Ch16 + other | 1 |
Analysis for sisters of ade+ G418s his− colonies carrying Chx is shown. A total of 869 daughter cells were segregated following HO induction in strain TH1900 on YE5S plates. Marker loss was determined for the colonies formed (phenotype). ade+ G418s his− colonies and sister colonies were subject to PFGE analysis, and the chromosomal organization was determined (genotype).
Allelic crossovers and BIR require HR.
Both allelic crossovers and BIR are predicted to require HR. To test this, break-induced LOH was assessed in an rhp51Δ background in which HR is disrupted (37). Gene conversion levels were significantly reduced in this strain (1.0%), and instead, a high degree of minichromosome loss was observed following break induction (57.0%) (Table 1), consistent with our previous study (45). Surprisingly, break-induced LOH was still observed in an rhp51Δ background, despite the loss of HR (0.8%) (Table 1). However, analysis of 25 individual rhp51Δ ade+ G418s his− colonies, obtained from three independent strains, by PFGE revealed that none exhibited Chx (Fig. 2A and Table 2). Instead, 23 of these colonies contained a band of a size similar to that of Ch16-MGH. Thus, break-induced translocations occurring via allelic crossovers or BIR required rhp51+. Further analysis of marker loss in rad22Δ, rhp55Δ, and rhp54Δ backgrounds revealed low levels of gene conversion in these HR mutants, as expected (Table 1) (45). Levels of break-induced LOH were higher in the rad22Δ background than that observed in the wild type (2.3%) (Table 1), while in contrast, levels of break-induced LOH were significantly lower in an rhp54Δ background than in the wild type (0.4%; P = 0.004) (Table 1). As with rhp51Δ, Chx was not observed in any rad22Δ, rhp55Δ, or rhp54Δ ade+ G418s his− colonies analyzed by PFGE (Table 2).
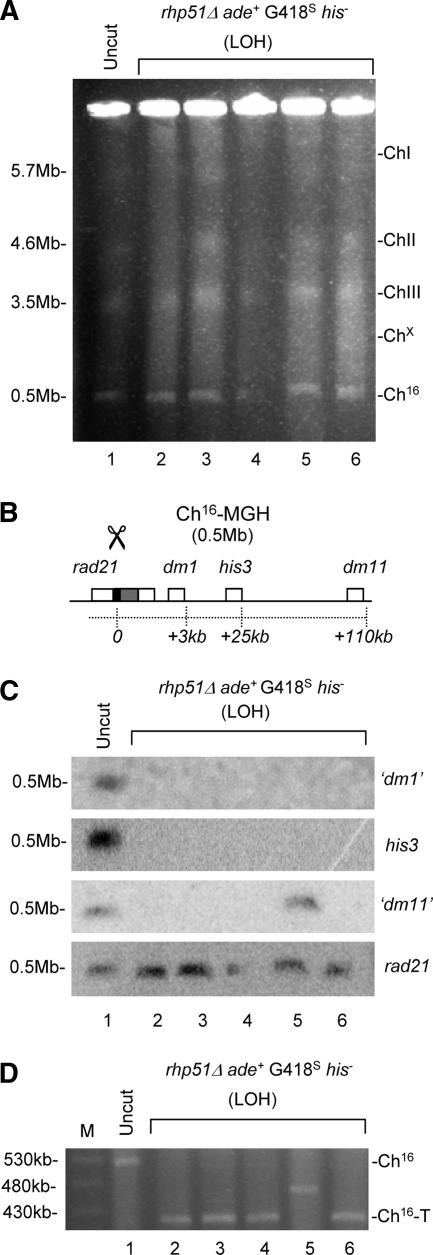
FIG. 2.
DSB-induced LOH in rhp51Δ cells results from Ch16 truncations. (A) PFGE analysis of chromosomal DNA from a parental rhp51Δ Ch16-MGH strain (lane 1) and individual rhp51Δ ade+ G418s his− strains isolated following DSB induction (lanes 2 to 6). (B) Schematic of the distal arm of Ch16-MGH indicating distances of markers from the break site (scissors). (C) Southern blot analysis of the gel in panel A probed for distal marker “dm1” (SPCC338.14), his3, “dm11” (SPCC61.05), and rad21 (as indicated). (D) High-resolution PFGE analysis of the strains described above. Size markers are shown (M).
Mus81 functions as a branched-molecule endonuclease in resolving HR intermediates (11). To determine whether there was a correlation between break-induced LOH levels and the stage at which HR repair was disrupted, LOH was examined following DSB induction in a mus81Δ background. Break-induced gene conversion in this strain was substantially reduced (29.0%), with a concomitant increase in minichromosome loss (38.1%) compared to that of the wild type (Table 1), thus indicating a role for Mus81 in efficient HR repair. The level of LOH was also significantly reduced in mus81Δ compared to that of the wild-type background (0.2%; P = 0.001) (Table 1), consistent with disruption of later-acting HR genes exhibiting reduced levels of LOH compared to those of earlier-acting HR genes. Further PFGE analysis of mus81Δ ade+ G418s his− colonies revealed Chx to be absent (Table 2) and instead a band of a size similar to that of Ch16-MGH to be present in the majority of colonies analyzed. Together, these results identify a requirement for rad22+, rhp55+, rhp51+, rhp54+, and mus81+ in Chx formation, indicating that translocations resulting from either allelic crossovers or BIR require HR in S. pombe.
Allelic crossovers and BIR require the MRN complex.
To test whether the MRN complex was required for Chx formation, break-induced LOH was assessed in mutants in which the MRN gene rad32+, rad50+, or nbs1+ was deleted. Gene conversion was reduced, and minichromosome loss was increased in rad32Δ, rad50Δ, and nbs1Δ backgrounds compared to that of wild-type cells, consistent with a role for the MRN complex in efficient interchromosomal HR (Table 1) (3). Levels of gene conversion were also significantly lower in nbs1Δ (15.6%) than in rad32Δ (30.7%; P = 0.011), but not rad50Δ (18.3%; P = 0.258). Levels of break-induced LOH were also significantly reduced in rad32Δ (0.6%; P = 0.013) and rad50Δ (0.6%; P = 0.011) compared to wild-type Ch16-MHH (Table 1). However, the level of break-induced LOH in nbs1Δ cells (1.4%) (Table 1), while significantly higher than in rad32Δ (0.6%; P = 0.034) and rad50Δ (0.6%; P = 0.032) cells, was similar to that observed in the wild type (1.7%). Subsequent PFGE analysis of 20 or more individual ade+ G418s/Hygs his− colonies isolated from all three backgrounds failed to identify any Chx associated with LOH (Table 2), indicating a requirement for the MRN complex in both allelic crossovers and BIR in S. pombe.
HR prevents de novo telomere addition at break sites.
To investigate the mechanisms of break-induced LOH in HR mutants, chromosomal DNA from five rhp51Δ ade+ G418s his− colonies was separated by PFGE and probed for sequences situated directly proximal (rad21), 3 kb distal (“dm1”), 25 kb distal (his3), and 110 kb distal (“dm11”) to the MATa site on Ch16-MGH (Fig. 2B). Surprisingly, probes distal to the break site failed to anneal to Ch16 bands in four of the rhp51Δ ade+ G418s his− colonies tested (Fig. 2C, compare lanes 2 to 6 with lane 1 in “dm1,” his3, and “dm11”). In contrast, the proximal rad21 probe annealed to all Ch16-size bands observed in these cells (Fig. 2C, lanes 2 to 6). The presence of “dm11” in one rhp51Δ ade+ G418s his− colony (Fig. 2C, lane 5, “dm11”) was suggestive of an interstitial deletion. Together, these results are consistent with extensive LOH arising through truncation of the minichromosome at or near the break site following HO induction in the majority of cases examined. Such a break-induced truncation should result in shortening of the minichromosome from ∼530 kb (the size of “uncut” Ch16-MGH) to ∼400 kb. This was confirmed by PFGE analysis using higher-resolution conditions (Fig. 2D, compare lane 1 with lanes 2, 3, 4, and 6). These novel chromosomal elements were termed Ch16-T (truncated).
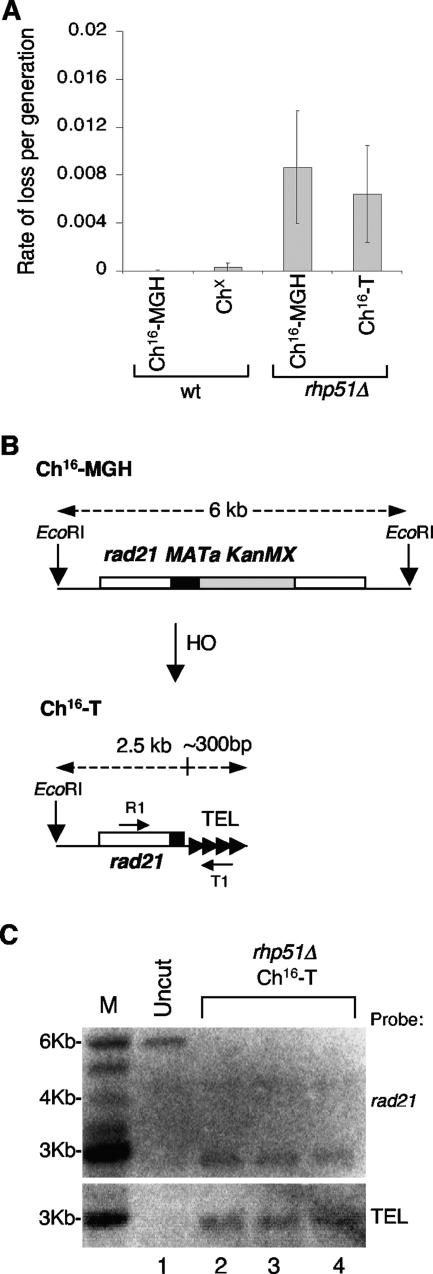
The minichromosome stability of Ch16-T was found to be equivalent to that of uncut Ch16-MGH (Fig. 3A), suggesting that Ch16-T could have been stabilized by telomere addition at or near the break site. This was confirmed by Southern blot analysis, which indicated that a 6-kb EcoRI fragment encoding the MATa target site in the uncut control was truncated (∼2.8 kb) in three individual rhp51Δ ade+ G418s his− colonies possessing Ch16-T, consistent with Ch16 being truncated at or near the MATa break site (Fig. 3C, top). Moreover, a telomere-specific probe was able to hybridize to these truncated EcoRI fragments, indicating the presence of telomeric sequence at or near the MATa break site in these strains (Fig. 3B and C, lanes 2 to 4). To directly demonstrate the addition of a telomere at the break site in rhp51Δ ade+ G418s his− cells, PCR amplification was performed using a primer specific to rad21 proximal to the break site and another predicted to anneal specifically to S. pombe telomere repeat sequence (Fig. 3B). Specific PCR products were obtained for 20 of 25 individually isolated rhp51Δ ade+ G418s his− colonies obtained from three independent strains, consistent with LOH resulting through telomere addition at the break site in 80% of these colonies (Table 2). Sequence analysis of PCR products from randomly selected rhp51Δ ade+ G418s his− strains indicated the presence of telomeric repeats (G2-5TTACA0-1) (54) added directly to, or near, the MATa break site in each case (Table 4). These findings indicated that extensive LOH resulted from de novo telomere addition in the majority of rhp51Δ colonies, which corresponded with minichromosome truncation in all cases (our unpublished observations). From the Southern blot analysis, the length of the telomeric repeat added to the break site was estimated to be ∼300 bp (Fig. 3B and C, lanes 2 to 4).
FIG. 3.
Break-induced LOH results from de novo telomere addition in rhp51Δ cells. (A) Analysis of loss per generation of Ch16-MGH, Chx, and Ch16-T minichromosomes in wild-type (wt) or rhp51Δ background. The error bars indicate standard errors of the mean. (B) Schematic of EcoRI fragments derived from “uncut” Ch16-MGH and Ch16-T. rad21+ (white), MATa (black), kanMX6 (gray), and telomere (TEL) sequences with R1 and T1 PCR primer binding sites are shown. (C) Southern blot analysis of EcoRI-digested chromosomal DNA from an “uncut” rhp51Δ Ch16-MGH strain (Uncut; lane 1) and three individual rhp51Δ ade+ G418s his− colonies containing Ch16-T (rhp51Δ Ch16-T; lanes 2 to 4) probed for rad21 (top) and telomeric sequence (bottom). Size markers are shown (M).
TABLE 4.
Breakpoint telomere junctions in HR mutantsa
| Genetic background | PCR product | Sequence |
|---|---|---|
| ↓ | ||
| MATa | 1 | agtcgggtttttcttttagtttcagctttccgcaacaaaaattttataaaccctggttttg |
| rad32Δ | 1 | agtcgggtttttcttttagtttcaGGTTACGGTTACGGTTACGGTTACAGGGTTACAGGGG |
| 2 | agtcgggtttttcttttagtttcaGCTTACGGTTACAGGGGTTACGGTTACAGGGTTACGG | |
| 3 | agtcgggtttttcttttagtttcagctttccgcaacaGGTTACAGGTTACAGGGGTTACGG | |
| 4 | agtcgggtttttcttttagtttcagctttccGGTTACAGGGGTTACGGTTACAGGGTTACA | |
| 5 | agtcgggtttttcttttagtttcagctttccgcaacGGTTACAGGTTACGGTTACAGGTTA | |
| rad50Δ | 1 | agtcgggtttttcttttagtttcagctttccgcGGTTACAGGGTTACAGGTTACAGGTTAC |
| 2 | agtcgggtttttcttttagtttcagctttccgGGTTACAGGGTTACGGTTACAGTTACGGT | |
| 3 | agtcgggtttttcttttagtttcagctttccGGTTACAGGTTACAGGTTACGGTTACAGGG | |
| 4 | agtcgggtttttcttttagtttcaGGTTACAGGTTACGGTTACGGTTACACGGTTACAGAG | |
| 5 | agtcgggtttttcttttagtttcGGTTACACGGTTACGGTTACGGTTACAGGTTACAGGGT | |
| nbs1Δ | 1 | agtcgggtttttcttttagtttcagctttccgcaacaGTTACAGGTTACAGGTTACAGGTT |
| 2 | agtcgggtttttcttttagtttcagctttccgcaacaCGGTTACACGGTTACACGGTTACA | |
| 3 | agtcgggtttttcttttagtttcagctttccgcaacaGTTACACGGTTACAGGTTACAGGT | |
| 4 | agtcgggtttttcttttagtttcagctttccgcaacaCGGTTACAGGTTACAGGTTACAGG | |
| 5 | agtcgggtttttcttttagtttcagctttccgcGGTTACAGGTTACACGGTTACAGGGTTA | |
| exo1Δ | 1 | agtcgggtttttettttagtttcagGGGTTACAGGTTACAGGTTACGGTTACGGTTAGGTT |
| 2 | agtcgggtttttcttttagtttcagctttccgcaacaGGGGTTACGGTTACAGGGGTTACG | |
| 3 | agtcgggtttttcttttagtttcagctttccgcaacGGTTACAGGTTACGGGTTACAGGGGTT | |
| 4 | agtcgggtttttcttttagtttcagctttccgdaacaGGGGTTACGGTTACAGGGTTACGG | |
| rad22Δ | 1 | agtcgggtttttcttttagtttGGTTACAGGTTACCGGGTTTACGGTTACAGGGGTTACGG |
| 2 | agtcgggtttttcttttagttCGGTTACAGGTTACAGGGGTTACGGTTACAGGGTACAGGG | |
| 3 | agtcgggtttttcttttagtttcagctttccgcaacGGTTACAGGGGTTACGGTTACAGGT | |
| 4 | agtcgggtttttcttttagtttcagctttccgcaacGGTTACAGTTACAGGGTTACAGGTTA | |
| 5 | agtcgggtttttcttttagtttcagctttccGGTTACAGTTACAGGGTTACGGTTACAGGTT | |
| rhp55Δ | 1 | agtcgggtttttcttttagtttcagctttccgcaacaGTTACGGTTACAGGGTTACACGGT |
| 2 | agtcgggtttttcttttagtttcagctttccgcaacaGTTACGGTTACAGGGGTTACGGTT | |
| 3 | agtcgggtttttcttttagtttcagctttccgcaacGGTTACAGGTTACAGGTTACAGGGT | |
| 4 | agtcgggtttttcttttagtttcagctttccGGTTACACGGTTACAGGTTACAGGGGGTAC | |
| 5 | agtcgggtttttcttttagtttCACGGTTACACGGTTACAGGTTACGGTTACAGGTTACGG | |
| rhp51Δ | 1 | agtcgggtttttcttttagtttcagctttccgcaacaGGTTACAGGTTACAGGTTACAGGT |
| 2 | agtcgggtttttcttttagtttcagctttccgcaacGGTTACAGGGTACGGTTACAGGGTT | |
| 3 | agtcgggtttttcttttagtttcagctttccgcaacGGTTACAGGTTACAGGTTACAGGGT | |
| 4 | agtcgggtttttcttttagtttcagctttccGGTTACAGGGTTACAGGGGGTTACGGTTAC | |
| 5 | agtcgggtttttcttttagtttcagctttccGGTTACAGGTTACAGGTTACGGTTACAGGG | |
| rhp54Δ | 1 | agtcgggtttttcttttagtttcagctttccgcaacaGGGTTACGGTTACACGGTACAGGG |
| 2 | agtcgggtttttcttttagtttcagctttccgcaacaGGGTTACGGTTACAGGTTACAGGG | |
| 3 | agtcgggtttttcttttagtttcagctttccgcaacGGTTACGGTTACGGTTACGGTTACG | |
| 4 | agtcgggtttttcttttagtttcagctttccgcaacGGTTACAGGGTTACGGTTACAGGGT | |
| 5 | agtcgggtttttcttttaCGGTTACAGGGTACGGTTACGGTTACAGTTACGGTTACAGGGG | |
| mus81Δ | 1 | agtcgggtttttcttttagtttcagctttccgcaacaGGGTTACAGGTTACAGTTACAGGG |
| 2 | agtcgggtttttcttttagtttcagctttccgcaacaCGGTTACAGGTTACAGGTTACGGT |
Examples of breakpoint telomere junctions in HR mutants are listed. Sequences of PCR products acquired from individual TEL+ strains from each background are shown; 61 bp of MATa are depicted, together with the HO endonuclease cleavage site (arrow). The telomeric repeat sequence (G1-5TTACA0-1) is in uppercase letters.
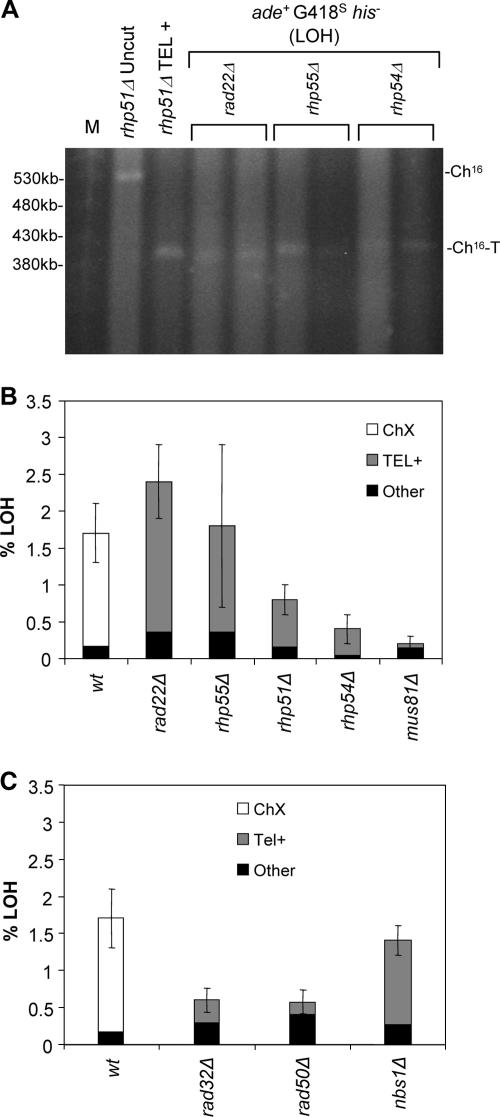
To further examine the relationship between HR and de novo telomere addition, 20 or more break-induced ade+ G418s his− colonies from rad22Δ, rhp55Δ, rhp54Δ, and mus81Δ backgrounds were analyzed. High-resolution PFGE analysis revealed Ch16 to be truncated to ∼400 kb in the majority of ade+ G418s his− colonies from rad22Δ, rhp55Δ, and rhp54Δ backgrounds, consistent with LOH resulting from de novo telomere addition (Fig. 4A). PCR and sequence analysis confirmed the presence of telomeric DNA at or near the break sites in more than 75% of the individually isolated ade+ G418s his− colonies from rad22Δ, rhp55Δ, and rhp54Δ backgrounds (Table 4). In contrast, 28% of mus81Δ ade+ Hygs his− colonies gave rise to telomere-specific PCR products (Fig. 4B). These analyses identified an increased level of LOH through de novo telomere addition in these mutants, which was most strikingly observed in rad22Δ and rhp55Δ backgrounds (approximately 100- and 85-fold, respectively) compared to the wild type, where only one of 100 individual ade+ G418s his− colonies had arisen from de novo telomere addition (Fig. 4B) (Table 2). Together, these results identify a role for HR in preventing extensive LOH arising through de novo telomere addition.
FIG. 4.
HR prevents de novo telomere addition at break sites. (A) PFGE analysis of chromosomal DNA from “uncut” rhp51Δ Ch16-MGH (rhp51Δ Uncut), rhp51Δ ade+ G418s his− (rhp51Δ TEL+ve) carrying Ch16-T, and two individual DSB-induced ade+ G418s his− strains from rad22Δ, rhp55Δ, and rhp54Δ backgrounds. (B) Analysis of the frequency of break-induced LOH mechanisms in HR mutants. wt, wild type. (C) Analysis of the frequency of break-induced LOH mechanisms in MRN mutants. The percentages of ade+ G418s his− strains exhibiting Chx (white), telomere addition (gray), or “other” (black), in which neither Chx nor de novo telomere addition was observed, are shown for each background (see Materials and Methods). Error bars (standard errors of the mean) for LOH values are depicted.
Analysis of break-telomere junctions from all HR mutants indicated that telomeres were added preferentially after CA, AC, or CC dinucleotides, with over half occurring within 1 bp of the cleaved MATa 5′-AACA-3′ overhang (Table 4). Telomeres from all mutants were initiated with the S. pombe telomeric consensus sequence G1-5TTACA0-1 (55), although subtle variations from this were observed in subsequent repeats (Table 4).
End resection facilitates de novo telomere addition in HR mutants.
As levels of de novo telomere addition were highest in rad22Δ and rhp55Δ strains, which disrupt HR after resection (19), this suggested that such processing might facilitate de novo telomere addition at break sites. To further examine the role of resection in de novo telomere addition, the levels of break-induced LOH were assessed in MRN deletion backgrounds, in which resection is predicted to be compromised (12, 60). PCR analysis of 20 or more break-induced ade+ G418s/Hygs his− colonies from rad32Δ, rad50Δ, and nbs1Δ backgrounds indicated that levels of extensive LOH through de novo telomere addition were reduced in rad32Δ and rad50Δ compared to that observed in rad22Δ and rhp55Δ backgrounds (Fig. 4B and C) (Table 2). In contrast, break-induced LOH in nbs1Δ ade+ G418s his− colonies resulted predominantly from de novo telomere addition and thus appears distinct from that in rad32Δ and rad50Δ backgrounds (Fig. 4C).
A redundant role for Exo1 with the MRN complex has been identified in resecting DSBs (24, 25), but not telomeres (58). DSB induction in an exo1Δ background led to levels of gene conversion comparable to that seen in wild-type Ch16-MGH cells, consistent with the proposed redundancy of Exo1 with MRN and/or other exonucleases (Table 1). Levels of LOH were slightly reduced in an exo1Δ background compared to the wild type (Table 1), and a low frequency of ade− G418s his+ colonies was also observed (see Table S2 in the supplemental material). Subsequent PFGE analysis of the 1.1% of exo1Δ ade+ G418s his− colonies that had undergone break-induced LOH revealed a substantial reduction in Chx formation compared to the wild type (Table 2 and Fig. 5A). PCR analysis of these colonies indicated that levels of de novo telomere addition were also reduced in an exo1Δ background compared to that of either rhp55Δ or rad22Δ cells (Fig. 4B and 5A and Table 2). To further test the role of Exo1-dependent resection in de novo telomere addition in an HR-deficient background, LOH levels were examined in an exo1Δ rhp55Δ strain. The level of break-induced LOH was reduced in this background (1.0%) compared to that of rhp55Δ (1.8%) and was similar to that observed in an exo1Δ background (1.1%). Further analysis indicated that LOH levels resulting from de novo telomere addition in exo1Δ rhp55Δ cells were substantially reduced compared to those of rhp55Δ (Fig. 5A).
FIG. 5.
HR competes with de novo telomere addition for resected ends. Analysis of the frequency of break-induced LOH mechanisms in rhp55Δ, exo1Δ, and exo1Δ rhp55Δ backgrounds (A); ku70Δ and lig4Δ backgrounds (B); ku70Δ rhp55Δ and lig4Δ rhp55Δ backgrounds (C); and rhp55Δ+pIRT3 and rhp55Δ+pIRT3-rhp51 strains (D). The percentages of LOH colonies exhibiting Chx (white), telomere addition (gray), or “other” (including ade− G418s his+ colonies; black) are shown for each background. Error bars (standard errors of the mean) for LOH values are shown. wt, wild type.
Previous studies have shown a role for Ku70-mediated end protection in preventing resection at DSBs (22, 58). To test whether DSB end protection functioned to prevent telomere addition at the MATa cut site, levels of break-induced LOH were assessed in a ku70Δ background. DSB-induced LOH in a ku70Δ background was comparable to wild-type levels (1.9%), the majority being ade+ G418s his− colonies, although a small proportion of ade− G418s his+ colonies were also observed (Table 1). Subsequent PFGE and PCR analyses of ku70Δ ade+ G418s his− LOH colonies revealed a substantial decrease in Chx formation and an increase in the levels of de novo telomere addition (18/100 ade+ G418s his− colonies) (Table 2) compared to that observed in a wild-type background (Fig. 5B). In a lig4Δ background, the level of break-induced LOH was significantly reduced compared to that of wild-type cells (0.9%) (Table 1), with some ade− G418s his+ colonies also being observed. PFGE analysis of the lig4Δ ade+ G418s his− colonies revealed a decrease in Chx formation compared to that of the wild type (Table 2). However, the levels of de novo telomere addition in this background were comparable to wild-type levels (Table 2). These data identify roles for both ku70+ and lig4+ in LOH translocations and further suggest a specific role for Ku70 in preventing de novo telomere addition. The ku70Δ and lig4Δ strains exhibited an increase in ade+ G418r his+ colonies following break induction (Table 1) (45). This was unexpected, as NHEJ is abrogated in these backgrounds. To investigate these populations further, the MATa sites from 20 individual ade+ G418r his+ ku70Δ or lig4Δ colonies were subjected to sequence analysis. However, no insertions, deletions, or point mutations across the MATa site were observed (our unpublished data).
To further investigate the role of ku70+ in the absence of HR, DSB-induced LOH was also examined in a ku70Δ rhp55Δ background. The rhp55Δ and ku70Δ rhp55Δ strains exhibited a high proportion of ade+ G418r his+ colonies following break induction (Table 1) (45); however, sequencing the MATa sites of 20 individual ade+ G418r his+ rhp55Δ and rhp55Δ ku70Δ colonies failed to identify any mutations (our unpublished data). DSB-induced LOH in a ku70Δ rhp55Δ background was significantly increased (7.6%; P = 0.014) compared to that of rhp55Δ (1.8%) (Table 1). Subsequent PFGE and PCR analysis of ku70Δ rhp55Δ ade+ G418s his− colonies revealed no Chx formation but a significant increase in the levels of de novo telomere addition compared to that observed in an rhp55Δ background (Fig. 5C and Table 2). In contrast, DSB-induced LOH in a lig4Δ rhp55Δ background (2.3%) was similar to that of rhp55Δ (1.8%) (Table 1), and PFGE and PCR analyses of lig4Δ rhp55Δ ade+ G418s his− colonies revealed no Chx formation and no significant increase in the levels of de novo telomere addition compared to that observed in an rhp55Δ background (Fig. 5B and Table 2). Together, these results suggest that Ku70-mediated end protection, and not NHEJ per se, functions to prevent break-induced de novo telomere addition. The above-mentioned data strongly suggested that both HR and de novo telomere addition were facilitated by end resection. These findings raised the possibility that HR prevented de novo telomere addition through competition for resected ends. To test this further, rhp51 was overexpressed in an rhp55Δ background to determine whether promoting HR in this context resulted in reduced levels of de novo telomere addition. Overexpression of rhp51 on a plasmid (pIRT3-rhp51) in an rhp55Δ background resulted in increased levels of gene conversion and a reduction in LOH through de novo telomere addition compared to vector alone (pIRT3) (Table 5 and Fig. 5D). These findings strongly suggest a role for HR in preventing de novo telomere addition by competing for resected ends.
TABLE 5.
Effect of rhp51 overexpression on DSB-induced marker loss in rhp55Δa
| Genetic background | % ade+ G418s/Hygshis+ (GC) | % ade+ G418r/Hygrhis+ (EJ/SCR) | % ade− G418s/Hygshis− (Ch16 loss) | % ade+ G418r/Hygrhis− (LOH) | P value (relative to rhp55Δ LOH) |
|---|---|---|---|---|---|
| rhp55Δ + pIRT3 | 3.1 ± 1.1 | 46.2 ± 3.5 | 45.3 ± 4.6 | 1.1 ± 0.3 | 1.000 |
| rhp55Δ + pIRT3-rhp51 | 12.2 ± 0.2 | 71.5 ± 0.4 | 15.4 ± 0.3 | 0.5 ± 0.1 | 0.166 |
Levels of interchromosomal gene conversion (GC), end joining/sister chromatid recombination (EJ/SCR), minichromosome loss (Ch16 loss), and LOH were calculated as described in Materials and Methods.
DISCUSSION
We have examined the mechanisms by which DSBs can generate extensive LOH in fission yeast. We found break-induced LOH to be associated predominantly with large translocations, consistent with extensive homozygosis across a whole chromosomal arm, resulting from both allelic crossovers and BIR. Analysis of the genetic requirements for such extensive LOH identified a key role for HR in facilitating such translocations. Surprisingly, break-induced extensive LOH was still observed in HR mutants but was found to arise through de novo telomere addition at or near the break site. Thus, in contrast to that of wild-type cells, DSB induction in HR mutants led to a distinct hemizygous LOH profile in which a whole chromosomal arm was lost. These data identify dual roles for HR in break-induced LOH in both facilitating translocations and preventing de novo telomere addition in fission yeast.
Analysis of the role of recombination in break-induced LOH identified a requirement for the MRN complex, encoded by rad32+, rad50+, and nbs1+, together with the HR genes rhp51+, rad22+, rhp55+, rhp54+, and mus81+ in the formation of break-induced translocations through either allelic crossovers or BIR. Deletion of the HR genes rhp51+, rad22+, rhp55+, and rhp54+ was found to abrogate both gene conversion and translocations. As homologs of these genes in S. cerevisiae have been shown to function during the strand invasion step of HR (19), these findings strongly suggest this step is common to gene conversion (with or without crossovers) and BIR. In contrast, deletion of components of the MRN complex abrogated translocations while permitting gene conversion, albeit at reduced levels. Thus, pathways leading to gene conversion and translocations are genetically distinguishable. One hypothesis to explain these findings is that the MRN complex promotes gene conversion through the classical DNA DSB repair model (41), thus promoting crossovers and possibly BIR. In the absence of the MRN complex, gene conversion may proceed through synthesis-dependent strand annealing, which is expected to prevent crossovers and possibly BIR as a result of displacement of the invading strand. A requirement for rad22+, rhp55+, rhp51+, and rhp54+ in Chx formation is consistent with the genetic requirements for RAD51-dependent BIR in S. cerevisiae (6, 27). Our data also suggest that the MRN complex is required for Rhp51-dependent BIR in S. pombe. Intriguingly, in S. cerevisiae, while RAD50 is required for efficient RAD51-dependent BIR and is essential for RAD51-independent BIR, following introduction of an HO break in a chromosomal context (27, 48), RAD50 was found not to be required for BIR in either RAD51 or rad51 strains following analysis of BIR utilizing a chromosome fragmentation assay (6).
The finding that mus81+ is required for efficient DSB repair and for generating allelic crossovers and BIR during vegetative growth is consistent with a role for Mus81-Eme1 endonuclease in processing recombination intermediates resulting in meiotic crossovers (5, 40). Moreover, a role for Mus81 in BIR conforms with a proposed role in resolving D loops at stalled or collapsed replication forks, thus facilitating replication restart following strand invasion of a homologous template (9). The fact that gene conversion is still observed in a mus81Δ background is also consistent with a redundant role for Mus81 with Rqh1/TopIII in HR resolution (19). A reduction in the levels of Chx was also observed in both ku70Δ and lig4Δ backgrounds. These findings are consistent with observations made in S. cerevisiae (38) and may suggest a role for NHEJ in either allelic crossovers or BIR.
We found break-induced LOH was directionally biased toward ade+ G418s his− colonies in all strains. Such directional bias may result from several causes. First, ade− G418s his+ colonies arising from single allelic crossovers between Ch16 and ChIII would be nonviable, as such cells would lack the distal 1.6-Mb arm of ChIII. Secondly, BIR initiated through strand invasion by the distal arm of Ch16, encoding the his3+ marker, may be compromised by the presence of ∼2 kb of nonhomologous kanMX6 sequence directly adjacent to the MATa break site on this arm. Thirdly, generating ade− G418s his+ colonies by BIR would also necessitate BIR through the centromere, which in S. cerevisiae can block unscheduled DNA replication (35). A small proportion of ade− G418s his+ colonies were observed in exo1Δ, ku70Δ, and lig4Δ backgrounds, which may have arisen through long-tract gene conversion.
Importantly, our data identify an additional role for HR in preventing de novo telomere addition at a break site, most probably through competition for resected ssDNA ends (Fig. 6). Consistent with this is the observation that levels of LOH and de novo telomere addition were most frequently found in rad22Δ or rhp55Δ mutants that disrupt HR after resection. In contrast, DSB induction in early-acting HR mutants, including rad32Δ, rad50Δ, and exo1Δ, in which DSB resection is compromised, resulted in substantially reduced levels of both LOH and de novo telomere addition compared to that observed in rad22Δ cells. Similarly, DSB induction in rhp54Δ and mus81Δ backgrounds, which disrupt HR at later stages in which resected ssDNA ends are presumably sequestered through either Rhp51 nucleoprotein filament or heteroduplex formation, also resulted in significantly reduced levels of LOH and de novo telomere addition compared to that observed in rad22Δ or rhp55Δ mutants. Further, we found that levels of de novo telomere addition were reduced in exo1Δ rhp55Δ double mutants compared to that of rhp55Δ, consistent with Exo1-dependent end resection facilitating de novo telomere addition in the absence of competing HR. The fact that de novo telomere addition was still observed in the exo1Δ rhp55Δ double mutant presumably reflects the redundant role of Exo1 with the MRN complex in resecting DSBs (24, 25). Moreover, levels of de novo telomere addition were significantly increased in ku70Δ rhp55Δ compared to that of rhp55Δ, consistent with elevated resection resulting from loss of Ku-dependent end protection, facilitating de novo telomere addition in the absence of competing HR. These findings strongly suggest that resected single-stranded 3′ overhangs are preferred substrates for de novo telomere addition at DSBs, as has been observed at telomeres (21). Finally, we found that promoting HR by overexpressing rhp51 in an rhp55Δ background resulted in increased gene conversion and reduced levels of de novo telomere addition, in accordance with a role for HR in preventing de novo telomere addition at DSBs through competition for resected ssDNA ends. This model extends previous observations of de novo telomere addition in rad52Δ and other HR mutants in response to HO-induced breaks or spontaneous damage in S. cerevisiae (2, 8, 18, 38). We speculate that resected ends facilitate recruitment of factors required for de novo telomere addition. Consistent with this, a requirement for resected ends as substrates for de novo telomere addition in S. cerevisiae is implied by the fact that break-telomere junctions were frequently located several kilobases upstream of an HO break site in a rad52 background and by data demonstrating that resection is required for telomere elongation at “seed” sequences proximal to an HO break site (7, 18, 28, 47). It has recently been suggested that broken chromosomes might undergo telomere addition after recruitment to subnuclear compartments that maintain telomeres through telomerase but exclude DNA repair activities (43). It would be of interest to determine whether a subpopulation of resected ends are recruited to such compartments in the absence of HR. Thus, de novo telomere addition may be employed as a mutagenic survival pathway to rescue broken chromosomes with single-stranded ends that cannot be otherwise repaired. A role for HR in maintaining telomeres in the absence of telomerase is now well documented (32). The finding that de novo telomere addition can maintain broken chromosomes in the absence of HR strongly suggests a more general functional interplay between these two processes.
FIG. 6.
Model depicting dual roles for HR in extensive break-induced LOH. HR promotes allelic crossovers and BIR, while additionally preventing de novo telomere addition through competition for resected ends. See the text for details.
Sequence analysis of break-telomere junctions suggested that telomeres were added preferentially after CA, AC, or CC dinucleotides and telomeric sequences were initiated with the S. pombe telomere consensus sequence, G1-4TTACA0-1 (55). Although such addition occurred predominantly at or near the MATa cleavage site, this might be expected given that the preferred sites of addition occur within the cleaved 5′-AACA-3′ overhang. In contrast, sequence analysis of over 500 break-telomere junctions obtained from spontaneous events in budding yeast revealed that the majority of telomeres were added preferentially after GT, TG, and GG dinucleotides and started with a distinct initiating sequence (GTGTGGGTGTG) (46). A similar result has also been observed at low frequency following HO break induction in rad52Δ S. cerevisiae strains, although such addition was found to require telomere-like (T2G4)13 sequences (18). These data are consistent with telomeres being added to short sequences of chromosomal DNA with homology to the 3′ end of the telomerase guide RNA, TLC1, but this domain is not used as a template during the synthesis of subsequent heterogeneous telomeric repeat sequences (46). Our data exhibit similar patterns, with telomeres almost always beginning with the same permutation of G1-5TTACA0-1, although the initiating sequence and subsequent repeats show less heterogeneity than has been observed in S. cerevisiae. This suggests that de novo telomere addition may occur by a similar mechanism in S. pombe, although synthesis utilizing the as-yet-unidentified RNA template in fission yeast may be more processive.
Since the stable truncated minichromosomes do not encode telomere-associated sequences at the break site, these sequences are clearly dispensable for chromosomal stability during mitosis in S. pombe, consistent with previous observations (29, 55). Together, these data suggest that de novo telomere addition has the potential to occur widely throughout the S. pombe genome. The finding that de novo telomere addition is observed in MRN mutants in our assay appears consistent with recent findings indicating that the MRX complex is not absolutely required for de novo telomere addition in S. cerevisiae (49). Surprisingly, we found levels of de novo telomere addition to be significantly increased in nbs1Δ compared to rad32Δ or rad50Δ backgrounds. Given that MRN deletion mutants show similar defects in intra-S phase checkpoint activation (4), perhaps these differences reflect a greater role for Rad32 and Rad50 in resection and/or telomere addition than Nbs1 in fission yeast. A recent in vitro study using telomeric DNA has suggested that Nbs1 plays a greater role than Mre11 in promoting strand invasion in nuclear extracts from primary human fibroblasts (64). These findings are consistent with the observed difference in gene conversion between rad32Δ and nbs1Δ in our in vivo assay system in S. pombe. Perhaps a reduction in strand invasion in nbs1Δ increases the amount of ssDNA substrate available for de novo telomere addition compared to that of a rad32Δ background. Since rad50Δ and nbs1Δ strains have similar defects in gene conversion, the reduction in telomere addition in rad50Δ cells may be due to a distinct mechanism.
The observation that de novo telomere addition was facilitated in the absence of Ku70 appears to contrast with findings in S. cerevisiae in which a Ku80-TLC1 interaction was found to promote de novo telomere addition (51). These differences may reflect distinct mechanisms of chromosome healing between these two evolutionarily divergent yeasts. It is possible, however, that redundant mechanisms of telomerase recruitment can facilitate de novo telomere addition when such repair occurs at low levels.
Our data identify a role for DSBs in promoting extensive LOH in S. pombe through allelic recombination and BIR in wild-type cells or through chromosome healing in HR mutants. As extensive LOH caused by chromosomal rearrangements is less likely to result in cell death than that resulting from chromosome loss, we predict these mechanisms of LOH are likely to be more pernicious, thus contributing to genetic disease, including tumorigenesis in higher eukaryotes. Although evidence for BIR in genetic disease awaits formal demonstration, allelic recombination has previously been identified as a mechanism leading to LOH at tumor suppressor loci in human cancer (13) and is significantly stimulated by DSB induction (36). Moreover, de novo telomere addition has been associated with genetic diseases, including α-thalassemia and mental retardation (67, 68). Our data suggest that determining the functional status of the HR machinery may be predictive of the types of extensive break-induced LOH that may arise and thus may contribute to understanding the etiology of genetic disease.
Supplementary Material
Acknowledgments
We thank the laboratories of Nick Boddy, Tony Carr, Stefania Francesconi, Chris Norbury, Paul Russell, Masaru Ueno, and Matthew Whitby for strains and reagents.
This research was funded by the Medical Research Council. B.-Y.W. was funded by the Agency for Science Technology and Research, Singapore.
Footnotes
Published ahead of print on 27 August 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Boddy, M. N., P. H. Gaillard, W. H. McDonald, P. Shanahan, J. R. Yates III, and P. Russell. 2001. Mus81-Eme1 are essential components of a Holliday junction resolvase. Cell 107: 537-548. [DOI] [PubMed] [Google Scholar]
- 2.Bosco, G., and J. E. Haber. 1998. Chromosome break-induced DNA replication leads to nonreciprocal translocations and telomere capture. Genetics 150: 1037-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bressan, D. A., B. K. Baxter, and J. H. Petrini. 1999. The Mre11-Rad50-Xrs2 protein complex facilitates homologous recombination-based double-strand break repair in Saccharomyces cerevisiae. Mol. Cell. Biol. 19: 7681-7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chahwan, C., T. M. Nakamura, S. Sivakumar, P. Russell, and N. Rhind. 2003. The fission yeast Rad32 (Mre11)-Rad50-Nbs1 complex is required for the S-phase DNA damage checkpoint. Mol. Cell. Biol. 23: 6564-6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cromie, G. A., R. W. Hyppa, A. F. Taylor, K. Zakharyevich, N. Hunter, and G. R. Smith. 2006. Single Holliday junctions are intermediates of meiotic recombination. Cell 127: 1167-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis, A. P., and L. S. Symington. 2004. RAD51-dependent break-induced replication in yeast. Mol. Cell. Biol. 24: 2344-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diede, S. J., and D. E. Gottschling. 2001. Exonuclease activity is required for sequence addition and Cdc13p loading at a de novo telomere. Curr. Biol. 11: 1336-1340. [DOI] [PubMed] [Google Scholar]
- 8.Diede, S. J., and D. E. Gottschling. 1999. Telomerase-mediated telomere addition in vivo requires DNA primase and DNA polymerases alpha and delta. Cell 99: 723-733. [DOI] [PubMed] [Google Scholar]
- 9.Doe, C. L., F. Osman, J. Dixon, and M. C. Whitby. 2004. DNA repair by a Rad22-Mus81-dependent pathway that is independent of Rhp51. Nucleic Acids Res. 32: 5570-5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaillard, P. H., E. Noguchi, P. Shanahan, and P. Russell. 2003. The endogenous Mus81-Eme1 complex resolves Holliday junctions by a nick and counternick mechanism. Mol. Cell 12: 747-759. [DOI] [PubMed] [Google Scholar]
- 11.Hollingsworth, N. M., and S. J. Brill. 2004. The Mus81 solution to resolution: generating meiotic crossovers without Holliday junctions. Genes Dev. 18: 117-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ivanov, E. L., N. Sugawara, C. I. White, F. Fabre, and J. E. Haber. 1994. Mutations in XRS2 and RAD50 delay but do not prevent mating-type switching in Saccharomyces cerevisiae. Mol. Cell. Biol. 14: 3414-3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jasin, M. 2000. LOH and mitotic recombination, p. 191-209. In M. Ehrlich (ed.), DNA alterations in cancer. Eaton Publishing, Natick, MA.
- 14.Kadyk, L. C., and L. H. Hartwell. 1992. Sister chromatids are preferred over homologs as substrates for recombinational repair in Saccharomyces cerevisiae. Genetics 132: 387-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khanna, K. K., and S. P. Jackson. 2001. DNA double-strand breaks: signaling, repair and the cancer connection. Nat. Genet. 27: 247-254. [DOI] [PubMed] [Google Scholar]
- 16.Kinzler, K., and B. Vogelstein. 1997. Introduction to cancer genetics. In C. R. Scriver, A. L. Beaudet, W. S. Sly, and D. Valle (ed.), The metabolic and molecular bases of inherited disease, 7th ed. McGraw-Hill, New York.
- 17.Knudson, A. G. 1993. Antioncogenes and human cancer. Proc. Natl. Acad. Sci. USA 90: 10914-10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kramer, K. M., and J. E. Haber. 1993. New telomeres in yeast are initiated with a highly selected subset of TG1-3 repeats. Genes Dev. 7: 2345-2356. [DOI] [PubMed] [Google Scholar]
- 19.Krogh, B. O., and L. S. Symington. 2004. Recombination proteins in yeast. Annu. Rev. Genet. 38: 233-271. [DOI] [PubMed] [Google Scholar]
- 20.Lasko, D., W. Cavenee, and M. Nordenskjold. 1991. Loss of constitutional heterozygosity in human cancer. Annu. Rev. Genet. 25: 281-314. [DOI] [PubMed] [Google Scholar]
- 21.Lee, M. S., R. C. Gallagher, J. Bradley, and E. H. Blackburn. 1993. In vivo and in vitro studies of telomeres and telomerase. Cold Spring Harbor Symp. Quant. Biol. 58: 707-718. [DOI] [PubMed] [Google Scholar]
- 22.Lee, S. E., J. K. Moore, A. Holmes, K. Umezu, R. D. Kolodner, and J. E. Haber. 1998. Saccharomyces Ku70, Mre11/Rad50 and RPA proteins regulate adaptation to G2/M arrest after DNA damage. Cell 94: 399-409. [DOI] [PubMed] [Google Scholar]
- 23.Leupold, U., and H. Gutz. 1964. Genetic fine structure in Schizosaccharomyces pombe. Proc. XIth Intl. Congr. Genet. 2: 31-35. [Google Scholar]
- 24.Lewis, L. K., and M. A. Resnick. 2000. Tying up loose ends: non-homologous end-joining in Saccharomyces cerevisiae. Mutat. Res. 451: 71-89. [DOI] [PubMed] [Google Scholar]
- 25.Llorente, B., and L. S. Symington. 2004. The Mre11 nuclease is not required for 5′ to 3′ resection at multiple HO-induced double-strand breaks. Mol. Cell. Biol. 24: 9682-9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malkova, A., E. L. Ivanov, and J. E. Haber. 1996. Double-strand break repair in the absence of RAD51 in yeast: a possible role for break-induced DNA replication. Proc. Natl. Acad. Sci. USA 93: 7131-7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malkova, A., M. L. Naylor, M. Yamaguchi, G. Ira, and J. E. Haber. 2005. RAD51-dependent break-induced replication differs in kinetics and checkpoint responses from RAD51-mediated gene conversion. Mol. Cell. Biol. 25: 933-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mangahas, J. L., M. K. Alexander, L. L. Sandell, and V. A. Zakian. 2001. Repair of chromosome ends after telomere loss in Saccharomyces. Mol. Biol. Cell 12: 4078-4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsumoto, T., K. Fukui, O. Niwa, N. Sugawara, J. W. Szostak, and M. Yanagida. 1987. Identification of healed terminal DNA fragments in linear minichromosomes of Schizosaccharomyces pombe. Mol. Cell. Biol. 7: 4424-4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazin, A. V., A. A. Alexeev, and S. C. Kowalczykowski. 2003. A novel function of Rad54 protein. Stabilization of the Rad51 nucleoprotein filament. J. Biol. Chem. 278: 14029-14036. [DOI] [PubMed] [Google Scholar]
- 31.McClintock, B. 1941. The stability of broken ends of chromosomes of Zea mays. Genetics 23: 234-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McEachern, M. J., and J. E. Haber. 2006. Break-induced replication and recombinational telomere elongation in yeast. Annu. Rev. Biochem. 75: 111-135. [DOI] [PubMed] [Google Scholar]
- 33.Moore, J. K., and J. E. Haber. 1996. Cell cycle and genetic requirements of two pathways of nonhomologous end-joining repair of double-strand breaks in Saccharomyces cerevisiae. Mol. Cell. Biol. 16: 2164-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreau, S., J. R. Ferguson, and L. S. Symington. 1999. The nuclease activity of Mre11 is required for meiosis but not for mating type switching, end joining, or telomere maintenance. Mol. Cell. Biol. 19: 556-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morrow, D. M., C. Connelly, and P. Hieter. 1997. “Break copy” duplication: a model for chromosome fragment formation in Saccharomyces cerevisiae. Genetics 147: 371-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moynahan, M. E., and M. Jasin. 1997. Loss of heterozygosity induced by a chromosomal double-strand break. Proc. Natl. Acad. Sci. USA 94: 8988-8993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muris, D. F., K. Vreeken, A. M. Carr, B. C. Broughton, A. R. Lehmann, P. H. Lohman, and A. Pastink. 1993. Cloning the RAD51 homologue of Schizosaccharomyces pombe. Nucleic Acids Res. 21: 4586-4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Myung, K., C. Chen, and R. D. Kolodner. 2001. Multiple pathways cooperate in the suppression of genome instability in Saccharomyces cerevisiae. Nature 411: 1073-1076. [DOI] [PubMed] [Google Scholar]
- 39.Niwa, O., T. Matsumoto, and M. Yanagida. 1986. Construction of a minichromosome by deletion and its mitotic and meiotic behaviour in fission yeast. Mol. Gen. Genet. 203: 397-405. [Google Scholar]
- 40.Osman, F., J. Dixon, C. L. Doe, and M. C. Whitby. 2003. Generating crossovers by resolution of nicked Holliday junctions: a role for Mus81-Eme1 in meiosis. Mol. Cell 12: 761-774. [DOI] [PubMed] [Google Scholar]
- 41.Paques, F., and J. E. Haber. 1999. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 63: 349-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pastink, A., J. C. Eeken, and P. H. Lohman. 2001. Genomic integrity and the repair of double-strand DNA breaks. Mutat. Res. 480-481: 37-50. [DOI] [PubMed] [Google Scholar]
- 43.Pennaneach, V., C. D. Putnam, and R. D. Kolodner. 2006. Chromosome healing by de novo telomere addition in Saccharomyces cerevisiae. Mol. Microbiol. 59: 1357-1368. [DOI] [PubMed] [Google Scholar]
- 44.Petukhova, G., S. Stratton, and P. Sung. 1998. Catalysis of homologous DNA pairing by yeast Rad51 and Rad54 proteins. Nature 393: 91-94. [DOI] [PubMed] [Google Scholar]
- 45.Prudden, J., J. S. Evans, S. P. Hussey, B. Deans, P. O'Neill, J. Thacker, and T. Humphrey. 2003. Pathway utilization in response to a site-specific DNA double-strand break in fission yeast. EMBO J. 22: 1419-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Putnam, C. D., V. Pennaneach, and R. D. Kolodner. 2004. Chromosome healing through terminal deletions generated by de novo telomere additions in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 101: 13262-13267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schulz, V. P., and V. A. Zakian. 1994. The Saccharomyces PIF1 DNA helicase inhibits telomere elongation and de novo telomere formation. Cell 76: 145-155. [DOI] [PubMed] [Google Scholar]
- 48.Signon, L., A. Malkova, M. L. Naylor, H. Klein, and J. E. Haber. 2001. Genetic requirements for RAD51- and RAD54-independent break-induced replication repair of a chromosomal double-strand break. Mol. Cell. Biol. 21: 2048-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith, S., A. Gupta, R. D. Kolodner, and K. Myung. 2005. Suppression of gross chromosomal rearrangements by the multiple functions of the Mre11-Rad50-Xrs2 complex in Saccharomyces cerevisiae. DNA Repair 4: 606-617. [DOI] [PubMed] [Google Scholar]
- 50.Stark, J. M., and M. Jasin. 2003. Extensive loss of heterozygosity is suppressed during homologous repair of chromosomal breaks. Mol. Cell. Biol. 23: 733-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stellwagen, A. E., Z. W. Haimberger, J. R. Veatch, and D. E. Gottschling. 2003. Ku interacts with telomerase RNA to promote telomere addition at native and broken chromosome ends. Genes Dev. 17: 2384-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stewart, E., C. R. Chapman, F. Al-Khodairy, A. M. Carr, and T. Enoch. 1997. rqh1+, a fission yeast gene related to the Bloom's and Werner's syndrome genes, is required for reversible S phase arrest. EMBO J. 16: 2682-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sugawara, N., X. Wang, and J. E. Haber. 2003. In vivo roles of Rad52, Rad54, and Rad55 proteins in Rad51-mediated recombination. Mol. Cell 12: 209-219. [DOI] [PubMed] [Google Scholar]
- 54.Sugiyama, T., J. H. New, and S. C. Kowalczykowski. 1998. DNA annealing by RAD52 protein is stimulated by specific interaction with the complex of replication protein A and single-stranded DNA. Proc. Natl. Acad. Sci. USA 95: 6049-6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suguwara, N. F. 1988. DNA sequences at the telomeres of the fission yeast S. pombe. Ph.D. thesis. Harvard University, Cambridge, MA.
- 56.Sung, P. 1997. Function of yeast Rad52 protein as a mediator between replication protein A and the Rad51 recombinase. J. Biol. Chem. 272: 28194-28197. [DOI] [PubMed] [Google Scholar]
- 57.Sung, P. 1997. Yeast Rad55 and Rad57 proteins form a heterodimer that functions with replication protein A to promote DNA strand exchange by Rad51 recombinase. Genes Dev. 11: 1111-1121. [DOI] [PubMed] [Google Scholar]
- 58.Tomita, K., A. Matsuura, T. Caspari, A. M. Carr, Y. Akamatsu, H. Iwasaki, K. Mizuno, K. Ohta, M. Uritani, T. Ushimaru, K. Yoshinaga, and M. Ueno. 2003. Competition between the Rad50 complex and the Ku heterodimer reveals a role for Exo1 in processing double-strand breaks but not telomeres. Mol. Cell. Biol. 23: 5186-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trujillo, K. M., and P. Sung. 2001. DNA structure-specific nuclease activities in the Saccharomyces cerevisiae Rad50*Mre11 complex. J. Biol. Chem. 276: 35458-35464. [DOI] [PubMed] [Google Scholar]
- 60.Tsubouchi, H., and H. Ogawa. 1998. A novel Mre11 mutation impairs processing of double-strand breaks of DNA during both mitosis and meiosis. Mol. Cell. Biol. 18: 260-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ueno, M., T. Nakazaki, Y. Akamatsu, K. Watanabe, K. Tomita, H. D. Lindsay, H. Shinagawa, and H. Iwasaki. 2003. Molecular characterization of the Schizosaccharomyces pombe nbs1+ gene involved in DNA repair and telomere maintenance. Mol. Cell. Biol. 23: 6553-6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Usui, T., T. Ohta, H. Oshiumi, J. Tomizawa, H. Ogawa, and T. Ogawa. 1998. Complex formation and functional versatility of Mre11 of budding yeast in recombination. Cell 95: 705-716. [DOI] [PubMed] [Google Scholar]
- 63.Varga, T., and P. D. Aplan. 2005. Chromosomal aberrations induced by double strand DNA breaks. DNA Repair 4: 1038-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Verdun, R. E., and J. Karlseder. 2006. The DNA damage machinery and homologous recombination pathway act consecutively to protect human telomeres. Cell 127: 709-720. [DOI] [PubMed] [Google Scholar]
- 65.Wang, X., and J. E. Haber. 2004. Role of Saccharomyces single-stranded DNA-binding protein RPA in the strand invasion step of double-strand break repair. PLoS Biol. 2: e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weterings, E., and D. C. van Gent. 2004. The mechanism of non-homologous end-joining: a synopsis of synapsis. DNA Repair 3: 1425-1435. [DOI] [PubMed] [Google Scholar]
- 67.Wilkie, A. O., J. Lamb, P. C. Harris, R. D. Finney, and D. R. Higgs. 1990. A truncated human chromosome 16 associated with alpha thalassaemia is stabilized by addition of telomeric repeat (TTAGGG)n. Nature 346: 868-871. [DOI] [PubMed] [Google Scholar]
- 68.Wong, A. C., Y. Ning, J. Flint, K. Clark, J. P. Dumanski, D. H. Ledbetter, and H. E. McDermid. 1997. Molecular characterization of a 130-kb terminal microdeletion at 22q in a child with mild mental retardation. Am. J. Hum. Genet. 60: 113-120. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.