Abstract
Tumor progression locus 2 (TPL-2) kinase is essential for Toll-like receptor 4 activation of the mitogen-activated protein kinase extracellular signal-regulated kinase (ERK) and for upregulation of the inflammatory cytokine tumor necrosis factor (TNF) in lipopolysaccharide (LPS)-stimulated macrophages. LPS activation of ERK requires TPL-2 release from associated NF-κB1 p105, which blocks TPL-2 access to its substrate, the ERK kinase MEK. Here we demonstrate that TPL-2 activity is also regulated independently of p105, since LPS stimulation was still needed for TPL-2-dependent activation of ERK in Nfkb1−/− macrophages. In wild-type macrophages, LPS induced the rapid phosphorylation of serine (S) 400 in the TPL-2 C-terminal tail. Mutation of this conserved residue to alanine (A) blocked the ability of retrovirally expressed TPL-2 to induce the activation of ERK in LPS-stimulated Nfkb1−/− macrophages. TPL-2S400A expression also failed to reconstitute LPS activation of ERK and induction of TNF in Map3k8−/− macrophages, which lack endogenous TPL-2. Consistently, the S400A mutation was found to block LPS stimulation of TPL-2 MEK kinase activity. Thus, induction of TPL-2 MEK kinase activity by LPS stimulation of macrophages requires TPL-2 phosphorylation on S400, in addition to its release from NF-κB1 p105. Oncogenic C-terminal truncations of TPL-2 that remove S400 could promote its transforming potential by eliminating this critical control step.
Tumor progression locus 2 (Tpl2) encodes a rat serine-threonine protein kinase that was originally cloned by virtue of its ability to induce T-cell lymphomas following insertional activation by Moloney leukemia virus (31). Tpl2 was subsequently identified as a site of proviral insertion in mouse mammary tumor virus-induced adenocarcinomas (16) and in two separate large-scale retroviral tagging screens for oncogenes able to promote lymphomagenesis (25, 26). Oncogenic activation always involves retroviral integration into the last intron of the Tpl2 gene, which results in the enhanced expression of an altered Tpl2 mRNA transcript encoding a protein that is truncated at its C terminus. A shortened form of the human homolog of Tpl2, known as Cot, also encoding a protein lacking a complete C terminus, was independently identified as a transforming gene for a human thyroid carcinoma cell line (8). The generation of transgenic mice expressing Tpl2 under the control of the Lck promoter confirmed that C-terminal truncation confers oncogenic activity to TPL-2 in T cells (7).
TPL-2 is a member of the mitogen-activated protein kinase kinase kinase (MAP 3-kinase) family of proteins (33) and is designated MAP 3-kinase 8. When overexpressed in cell lines, TPL-2 activates the extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 MAP kinase pathways, which results from the ability of TPL-2 to induce the phosphorylation and activation of the respective MAP 2-kinases (9, 32, 33). However, analyses of Map3k8−/− mice have indicated a more restricted role for TPL-2. Only activation of the MAP 2-kinases MEK-1 and -2, which phosphorylate and activate ERK-1 and -2 MAP kinases, is blocked in TPL-2-deficient macrophages following stimulation with lipopolysaccharide (LPS), lipopeptide, or tumor necrosis factor (TNF) (13, 15, 35), while activation of the p38 and JNK MAP kinase pathways is normal. Consistent with TPL-2 playing an important role in regulating gene expression during innate immune responses, LPS induction of TNF and cyclo-oxygenase 2 (COX-2) is dramatically reduced in TPL-2-deficient macrophages due to defective ERK activation (13, 14). Consequently, Map3k8−/− mice are resistant to endotoxin shock induced by LPS and d-galactosamine (13). Map3k8−/− B cells and macrophages also exhibit a specific defect in ERK activation when stimulated with CD40 antibodies (15), suggesting that TPL-2 is important in the physiological activation of ERK in adaptive as well as innate immune responses.
Interaction of TPL-2 with the C-terminal half of NF-κB1 p105 negatively regulates TPL-2 activation of the ERK MAP kinase cascade by preventing TPL-2 access to its substrate, the ERK kinase MEK (4, 6, 39). In unstimulated macrophages, TPL-2 is complexed with p105 (23) and TPL-2 phosphorylation of MEK is blocked (5, 39). LPS activation of the ERK MAP kinase cascade requires the release of TPL-2 from p105, which has been shown to occur as a consequence of p105 proteolysis by the proteasome (5, 38). This is triggered by p105 phosphorylation by the IκB kinase (IKK) complex, which is also critical for regulating the activation of NF-κB transcription factors (21). It has also been proposed that IKK-mediated phosphorylation of the TPL-2 activation loop on T290 may induce dissociation of p105 from TPL-2 independently of p105 proteolysis (10, 11). Both of these mechanisms imply that the IKK complex can directly regulate NF-κB activation and ERK activation in macrophages.
Multiple mechanisms are known to be important in regulating the activity of MAP 3-kinases (36). In the present study, the possible existence of p105-independent controls for regulating TPL-2 function was investigated. Evidence is presented to show that in addition to dissociation of TPL-2 from its inhibitor (p105), activation of ERK following LPS stimulation of macrophages requires phosphorylation of the TPL-2 C terminus on serine 400, which is needed to turn on the MEK kinase activity of TPL-2.
MATERIALS AND METHODS
cDNA constructs, antibodies, and recombinant proteins.
Myc epitope-tagged and untagged versions of TPL-2, TPL-2ΔC, and kinase-inactive TPL-2D270A have been described previously (4, 6). The v-AKT expression vector was kindly provided by Julian Downward (Cancer Research UK, London, United Kingdom). TPL-2S400A, Myc-TPL-2S400A, TPL-2S413A, and Myc-TPL-2S413A were generated by PCR and verified by DNA sequencing. For expression in 293 cells, the cDNAs were subcloned into the pcDNA3 vector (Invitrogen), and for stable expression in RAW264.7 cells, they were subcloned into the pMX-1 vector (Ingenius). Wild-type and mutant forms of TPL-2 cDNA were cloned into a pMSCV-based vector (30) and pCLSXN (Imgenex) for retroviral transduction of Nfkb1−/− and Map3k8−/− macrophages, respectively.
The antibodies used to immunoprecipitate and immunoblot Myc epitope-tagged TPL-2, NF-κB1 p105, and ABIN-2 have been described before (5, 23). Antibodies against MEK-1/2, phospho(S217/S221)-MEK-1/2 (activated MEK-1/2; phospho-MEK), p38, phospho(T180/Y182)-p38 (activated p38; phospho-p38), Akt, and phospho-S473-Akt (activated Akt; phospho-Akt) were purchased from Cell Signaling Technology. Antibodies to ERK-1 and ERK-2 were obtained from Santa Cruz Biotechnology, and phospho(T185/Y187)-ERK-1,2 (activated ERK; phospho-ERK) antibody was obtained from Biosource. Tubulin was detected with the TAT-1 α-tubulin monoclonal antibody (kindly provided by Keith Gull, University of Oxford, United Kingdom) and used as a loading control protein on immunoblots of total cell lysates. Glutathione S-transferase (GST)-MEKK207A was generously donated by Richard Marais (Cancer Research UK, London, United Kingdom). GST-p105497-968 and GST-ABIN-21-250 fusion proteins have been described previously (4, 23).
To generate the phospho-S400-TPL-2 antibody, a peptide was synthesized to correspond to residues 394 through 405 of murine TPL-2 in which S400 was phosphorylated. After high-pressure liquid chromatography purification, this phosphopeptide was coupled to keyhole limpet hemocyanin (Pierce) and injected into rabbits. Affinity purification of the resulting antibody was carried out as described previously (22).
Mouse strains and macrophages.
Nfkb1−/− mice (34) (from Jackson Laboratory, Bar Harbor, ME), Map3k8−/− mice (13), and wild-type C57BL/6 mice were bred in a specific-pathogen-free environment at the National Institute for Medical Research (London, United Kingdom). Bone marrow-derived macrophages (BMDMs) from these mice were prepared as described previously (37). Briefly, bone marrow cells were plated in complete BMDM medium (RPMI 1640 supplemented with 10% fetal bovine serum [FBS], 20% L-cell-conditioned medium, 2 mM glutamine, nonessential amino acids, 0.25 μM β-mercaptoethanol, 1 mM pyruvate, and antibiotics). On day 4 of culture, the cells were further supplemented with complete BMDM medium. After a total of 7 days of culture, the adherent macrophages were harvested and plated for experiments. More than 95% of the resulting cell populations were positive for the macrophage markers F4/80 and Mac-1, as judged by flow cytometric analysis (data not shown).
RAW264.7 cells were kindly provided by Lynn Williams (Kennedy Institute, Imperial College London, United Kingdom) and maintained in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) containing 10% FBS and antibiotics. For stable transfection, 5 × 107 RAW264.7 cells were electroporated with 10 μg of pMX-1 expression vector containing a cDNA insert encoding wild-type or mutant TPL-2. Transfected cells were cultured for a further 48 h and cloned by limiting dilution under neomycin selection (complete DMEM plus 1 mg/ml G418; Invitrogen). After 1 to 3 weeks, clones were expanded and tested for the expression of transfected proteins by immunoblotting. Positive clones were maintained in selection medium. Prior to use in experiments, the RAW264.7 cell lines were passaged once without G418.
Retrovirus infection of BMDMs.
Ecotropic TPL-2 recombinant retroviruses were produced by transfecting retroviral vectors into the Plat-E packaging cell line (27), using the GeneJuice transfection reagent (Merck Biosciences). Transfected cells (5 × 105 cells/well of a six-well plate; Nunc) were cultured at 37°C for 24 h in complete medium (DMEM plus 10% FBS and antibiotics). Two milliliters of fresh complete medium was added for the final 24 h, at which time culture supernatants were removed and filtered (pore size, 0.4 μm).
BMDMs generated from Nfkb1−/− and Map3k8−/− mice were prepared essentially as described above, with minor modifications. Cells were plated in complete BMDM medium at 1 × 106 cells/well of a six-well plate (2-ml culture volume; Sarstedt). Following 48 h of incubation, 200 μl of virus was added per well, and plates were centrifuged at 2,000 × g for 1 h. Cells were cultured for 3 h, 4 ml complete BMDM medium was added, and the cells were recultured for a further 4 days. Flow cytometric analyses confirmed that >95% of cells prepared in this way were F4/80 positive.
Protein analysis.
For analyses of cell lysates, BMDMs (3 × 106) or RAW264.7 cells (4 × 106) were plated in six-well dishes (Nunc). For experiments involving immunoprecipitation, BMDMs (8 × 106) and RAW 264.7 cells (10 × 106) were plated in 10-cm dishes (Nunc). After 18 h of culture, cells were stimulated with LPS (20 ng/ml; Salmonella enterica serovar Minnesota) (Alexis Biochemicals) for the times shown or left untreated. In the indicated experiments, cells were preincubated with 40 μM MG132 proteasome inhibitor (Biomol), 20 μM BMS-345541 (Calbiochem), 1 μM IKK2 inhibitor IV (Calbiochem), or dimethyl sulfoxide vehicle control for 30 min prior to LPS stimulation. Cells were washed once in phosphate-buffered saline prior to lysis in 1% NP-40-containing buffer A (50 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 20 mM NaF, 1 mM Na3VO4, 100 nM okadaic acid [Calbiochem], 2 mM Na4P2O7 plus a mixture of protease inhibitors [Roche Molecular Biochemicals]). Covalent coupling of antibodies to protein A-Sepharose (Amersham Biosciences) and immunoprecipitation and immunoblotting of proteins were carried out as described previously (19). p105 was precleared from BMDM lysates by immunodepletion twice with p105N antibody coupled to protein A-Sepharose (5). Complete removal of p105 was confirmed by immunoblotting of resulting lysates.
293T cells (3 × 105 to 5 × 105 cells per 60-mm-diameter Nunc dish) were transiently transfected using Lipofectamine (Life Technologies Inc.) and then cultured for a total of 24 h, as described previously (3). Cell lysates were prepared using buffer A containing 0.5% NP-40. For pull-down assays, 2 μg of recombinant protein was added to ultracentrifuged 293T cell lysate and incubated overnight with mixing. Fusion proteins were affinity isolated by the addition of 10 μl of glutathione-Sepharose 4B beads (Amersham Biosciences) and incubation for a further 30 min. Beads were then washed extensively in buffer A plus 0.5% NP-40, and isolated proteins were analyzed by immunoblotting.
Kinase assays.
293T cells (5 × 105 per 60-mm-diameter Nunc dish) were transiently transfected as described above, and lysates were prepared using kinase assay lysis buffer (buffer A containing 0.5% NP-40, 5 mM β-glycerophosphate, 1 mM dithiothreitol). Immunoprecipitation was carried out as described previously (19). Immunoprecipitates were washed four times in kinase assay lysis buffer, followed by two washes in kinase buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 5 mM β-glycerophosphate, 100 nM okadaic acid, 1 mM dithiothreitol, 0.1 mM sodium vanadate, 10 mM MgCl2, 1 mM EGTA, 0.03% Brij 35). Beads were then resuspended in 50 μl of kinase buffer plus 1 mM ATP. One microgram of GST-MEK1K207A and 1 μg of myelin basic protein (MBP; Sigma), plus 2.5 μCi of [γ-32P]ATP (Amersham Biosciences), were added to each reaction mixture. Reactions were carried out at room temperature for 15 min and terminated by adding 50 μl of 2× sodium dodecyl sulfate (SDS) sample buffer. Labeled TPL-2 and MBP were revealed by autoradiography after SDS-10% polyacrylamide gel electrophoresis (SDS-10% PAGE). Phosphorylation of GST-MEK1K207A was determined by immunoblotting of a replicate gel.
RAW264.7 cells (4 × 107) stably expressing Myc-TPL-2 or Myc-TPL-2S400A were stimulated with LPS (1 μg/ml) for 15 min or left unstimulated. Cell lysates were prepared using kinase lysis buffer, and immunoprecipitation with anti-Myc was carried out for 4 h. Immunoprecipitates were washed four times in kinase assay lysis buffer, followed by two washes in kinase buffer. Beads were then resuspended in 50 μl of kinase buffer plus 1 mM ATP and 1 μg of GST-MEK1K207A. Reaction mixtures were incubated at room temperature for 15 min and terminated by adding 50 μl of 2× SDS sample buffer. Phosphorylation of GST-MEK1K207A was assessed by immunoblotting. The results presented are representative of similar experiments with two independently isolated RAW264.7 cell clones expressing Myc-TPL-2 or Myc-TPL-2S400A.
ELISA.
Macrophages were plated at 5 × 105/ml in 250 μl of RPMI 1640 medium supplemented with 10% FBS and antibiotics in a 96-well plate (Nunc). After overnight culture, cells were stimulated with 10 ng/ml LPS for the indicated times. Levels of TNF in culture supernatants were determined using a commercial enzyme-linked immunosorbent assay (ELISA) kit (eBioscience) according to the manufacturer's instructions.
RESULTS
TPL-2 catalytic activity is regulated independently of NF-κB1 p105 binding.
We have previously shown that the inhibitory effect of NF-κB1 p105 on TPL-2 phosphorylation of MEK correlates with the ability of p105 to block interaction of TPL-2 with MEK (5). These data suggest that TPL-2 must associate with MEK for efficient substrate phosphorylation, similar to the case for Raf MEK kinase (40). However, it remained unclear whether p105 binding also impairs the catalytic activity of TPL-2. To investigate this, 293 cells were transfected with vectors encoding TPL-2 plus hemagglutinin (HA)-p105 or with empty vector (EV). TPL-2 was then immunoprecipitated from cell lysates and tested in an in vitro kinase assay for its ability to autophosphorylate and also to phosphorylate MBP and a GST-MEKK207A fusion protein. The degrees of TPL-2 autophosphorylation and phosphorylation of MBP were not altered by interaction with p105 (Fig. 1a). However, phosphorylation of GST-MEKK207A (P-MEK) was blocked by p105, as expected (4). Thus, although p105 interaction inhibits the phosphorylation of GST-MEKK207A by TPL-2, it does not affect TPL-2 kinase activity. Together with previous studies, these results suggest that p105 functions as a competitive inhibitor which blocks TPL-2 binding to MEK but does not affect TPL-2 catalytic activity (2, 4).
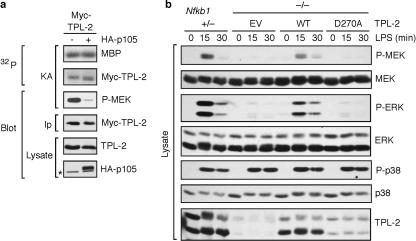
FIG. 1.
TPL-2 expression reconstitutes LPS activation of ERK in Nfkb1−/− macrophages. (a) TPL-2 was immunoprecipitated from lysates of 293T cells transiently cotransfected with vectors encoding Myc-TPL-2 plus HA-105 or EV (−). MBP and GST-MEKK207A were added to each immunoprecipitate, and in vitro kinase assays were performed. Phosphorylation of MBP and Myc-TPL-2 was determined by autoradiography. Immunoblotting was used to monitor GST-MEKK207A phosphorylation and the amount of Myc-TPL-2 in immunoprecipitates. Expression of Myc-TPL-2 and HA-p105 in lysates was determined by immunoblotting. (b) Macrophages generated from Nfkb1−/− mice were transduced with recombinant retroviruses encoding TPL-2 and TPL-2D270A or with EV. Cells were stimulated for the indicated times with LPS, and lysates were immunoblotted. Macrophages generated from Nfkb1+/− littermate mice were used as a positive control. The asterisk indicates the position of a background band.
Since the catalytic activity of other MAP 3-kinases, such as ASK-1 (36), is regulated by agonists, these results raised the possibility that TPL-2 signaling function might additionally be controlled by modulation of its catalytic activity independently of p105 binding. This was initially investigated using macrophages generated from Nfkb1−/− mice, which do not express p105. The stability of TPL-2 is dependent on complex formation with p105, and consequently, steady-state levels of TPL-2 are dramatically reduced and LPS activation of ERK is blocked in these cells (5, 39). Nfkb1−/− macrophages were infected with TPL-2 recombinant retrovirus to increase the steady-state concentration of TPL-2 in the absence of p105. This did not alter the basal levels of MEK and ERK phosphorylation (Fig. 1b). However, LPS-induced MEK and ERK phosphorylation was substantially increased relative to that in cells transduced with EV, to nearly the degree detected in Nfkb1+/− cells. These data indicate that transduced TPL-2 was not constitutively active in the absence of NF-κB1 p105 but still required LPS stimulation to promote activation of MEK and ERK. Transduction of Nfkb1−/− cells with TPL-2D270A recombinant retrovirus did not increase LPS induction of MEK and ERK phosphorylation compared with that in cells transduced with EV (Fig. 1b), suggesting that TPL-2 kinase activity was necessary for activation of MEK and ERK in Nfkb1−/− macrophages. However, this conclusion is tempered by the fact that TPL-2D270A was expressed at slightly lower levels, making direct comparison with wild-type TPL-2 difficult. Another kinase-inactive TPL-2 mutant, TPL-2K167M, is also produced at lower concentrations than wild-type TPL-2 in transfected 293 cells (10), suggesting that kinase activity may be required for efficient transient TPL-2 expression.
Alternative translational initiation on a second methionine (at residue 30) results in the expression of two TPL-2 isoforms, M1-TPL-2 and M30-TPL-2 (1). LPS stimulation of macrophages induces quantitative phosphorylation of M1-TPL-2, which is evident as a mobility shift by SDS-PAGE (5). Interestingly, when expressed in Nfkb1−/− macrophages, M1-TPL-2D270A did not shift its mobility after LPS stimulation, in contrast to wild-type TPL-2 (Fig. 1b). Similarly, the SDS-PAGE mobility of M1-TPL-2D270A was not changed following LPS stimulation of retrovirally transduced Map3k8−/− macrophages (13), which lack TPL-2 but express p105 (see Fig. 4a). These results demonstrate that the altered mobility of M1-TPL-2 in LPS-stimulated cells was due to TPL-2 autophosphorylation and suggest that LPS stimulation can activate the catalytic activity of M1-TPL-2 in the presence and absence of p105.
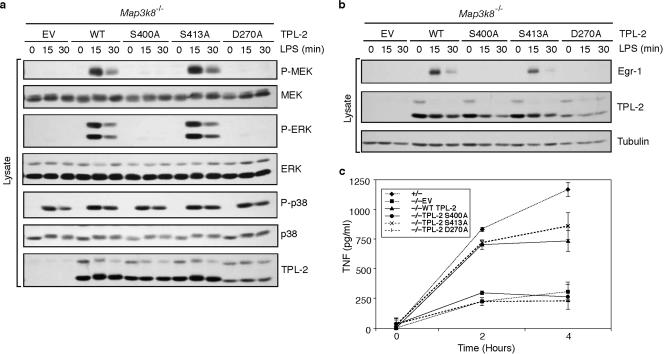
FIG. 4.
TPL-2S400A cannot reconstitute LPS activation of ERK phosphorylation and upregulation of Egr-1 and TNF in Map3k8−/− macrophages. (a and b) Macrophages generated from Map3k8−/− mice were transduced with recombinant retroviruses encoding TPL-2, TPL-2S400A, TPL-2S413A, TPL-2D270A, or EV. Cells were stimulated with LPS for the indicated times, and cell lysates were immunoblotted. (c) Map3k8−/− macrophages were transduced with the indicated recombinant retroviruses, and triplicate cultures were stimulated with LPS for 24 h. LPS-stimulated macrophages from Map3k8+/− littermate mice were used as controls. The mean concentration (± standard deviation) of TNF in culture supernatants was determined by ELISA.
Taken together, the observation that LPS stimulation induced both TPL-2 autophosphorylation and TPL-2-dependent activation of ERK in Nfkb1−/− macrophages implies that TPL-2 MEK kinase activity is regulated independently of NF-κB1 p105.
Serine 400 is essential for TPL-2 activation of ERK.
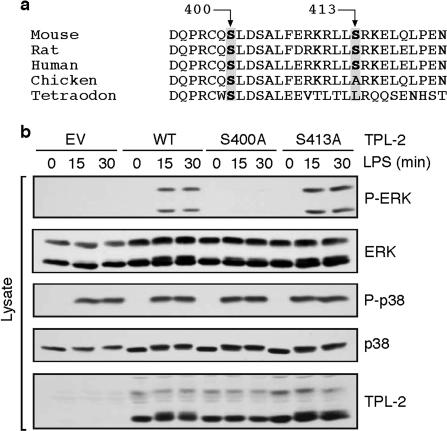
TPL-2 kinase activity is increased by removal of its C terminus, and the isolated TPL-2 C terminus inhibits the kinase activity of C-terminally truncated TPL-2 (7, 17). Since both of these effects occur in the apparent absence of p105 binding, this raised the possibility that p105-independent regulation of TPL-2 activity by LPS may involve modulation of its C terminus. Interestingly, overexpression of active Akt in Jurkat T cells has been shown to induce the phosphorylation of coexpressed TPL-2 on two sites in its C-terminal tail, namely, S400 and S413 (20). Comparison of TPL-2 protein sequences from humans, mice, rats, chickens, and Tetraodon indicated that S400 was completely conserved between these species, whereas the residue corresponding to S413 was not conserved in either chickens or Tetraodon (Fig. 2a). This suggests that S400, but not S413, may play an important role in regulating TPL-2 signaling function. Consistent with this hypothesis, S400, but not S413, is required for activation of NF-κB by overexpressed TPL-2 in Jurkat T cells (20). As described below, we investigated the possibility that phosphorylation of S400 and/or S413 of the TPL-2 C terminus is involved in p105-independent control of TPL-2 function in LPS-stimulated macrophages.
FIG. 2.
TPL-2 S400 is required for activation of ERK in LPS-stimulated Nfkb1−/− macrophages. (a) Amino acid alignment of the segment of the TPL-2 C terminus containing S400 and S413 from the indicated species. (b) Macrophages generated from Nfkb1−/− mice were transduced with recombinant retroviruses encoding TPL-2, TPL-2S400A, TPL-2S413A or EV. Cells were stimulated with LPS, and lysates were immunoblotted.
To investigate the potential role of phosphorylation of S400 and S413 in TPL-2 function, Nfkb1−/− macrophages were transduced with recombinant retroviruses encoding wild-type TPL-2, TPL-2S400A, or TPL-2S413A. LPS stimulation clearly induced MEK and ERK phosphorylation in cells expressing either wild-type TPL-2 or TPL-2S413A (Fig. 2b). However, LPS failed to induce MEK or ERK phosphorylation in TPL-2S400A-expressing cells, although p38 phosphorylation was stimulated to a similar degree to that in cells expressing wild-type TPL-2. Thus, S400, but not S413, of TPL-2 is required for LPS stimulation of the ERK signaling cascade in Nfkb1−/− macrophages.
LPS stimulation of macrophages induces TPL-2 serine 400 phosphorylation.
Although the previous experiments showed an essential role for S400 in TPL-2 function, it was important to demonstrate that this residue was actually phosphorylated after LPS stimulation of macrophages. An antibody was raised to a synthetic peptide equivalent to residues 394 to 405 of TPL-2 in which the residue corresponding to S400 was phosphorylated. Peptide ELISAs confirmed specific binding of the resulting antibody to the phospho-S400-TPL-2394-405 peptide in comparison with a nonphosphorylated TPL-2394-405 control peptide (data not shown). To investigate the potential phosphorylation of TPL-2 on S400, stably expressed Myc-TPL-2 was immunoprecipitated from transfected RAW264.7 macrophages and immunoblots were probed with the phospho-peptide antibody. This demonstrated that LPS stimulation promoted the rapid phosphorylation of Myc-TPL-2 on S400 (Fig. 3a). The phospho-S400-TPL-2 antibody did not detect any specific bands when anti-Myc immunoprecipitates from lysates of LPS-stimulated RAW264.7 cells stably expressing Myc-TPL-2S400A were immunoblotted (data not shown), verifying the specificity of the antibody. LPS stimulation also induced phosphorylation of S400 on kinase-inactive Myc-TPL-2D270A (Fig. 3a), suggesting that this residue was not autophosphorylated.
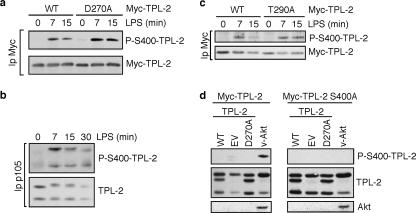
FIG. 3.
LPS stimulation of macrophages induces TPL-2 phosphorylation on S400. (a and c) RAW264.7 cells were stably transfected with vectors encoding Myc-TPL-2 (a and c), Myc-TPL-2D270A (a), and Myc-TPL-2T290A (c). Cells were stimulated with LPS, and anti-Myc immunoprecipitates of cell lysates were immunoblotted and probed for phospho-S-400-TPL-2 and total Myc-TPL-2. (b) Endogenous p105 was immunoprecipitated from lysates of MG132-pretreated wild-type macrophages stimulated with LPS. Immunoprecipitates were immunoblotted and probed for the indicated antigens. (d) 293 cells were cotransfected with vectors encoding Myc-TPL-2 or Myc-TPL-2S400A plus v-Akt, TPL-2, or EV. Cell lysates were immunoblotted for the indicated antigens.
To determine whether endogenous TPL-2 is phosphorylated on serine 400, TPL-2 was coimmunoprecipitated with p105 from lysates of wild-type primary macrophages by using a p105 antibody. Insufficient endogenous TPL-2 could be immunopurified directly by using available TPL-2 antibodies. Cells were pretreated with the proteasome inhibitor MG132 to block signal-induced proteolysis of TPL-2 and p105 (5). Immunoblotting with phospho-S400-TPL-2 antibody demonstrated that LPS stimulation induced the rapid phosphorylation of both M1- and M30-TPL-2 on S400 in primary macrophages (Fig. 3b). These data were consistent with the RAW264.7 cell experiments and also showed that TPL-2 could be phosphorylated on S400 while still associated with p105.
It has previously been reported that LPS stimulation promotes the phosphorylation of T290 in the kinase domain of TPL-2, which is required for TPL-2 kinase activity and may regulate its dissociation from p105 (10, 11). To investigate whether S400 phosphorylation is dependent on T290, RAW264.7 cells were stably transfected with Myc-TPL-2T290A. Immunoblotting of anti-Myc immunoprecipitates demonstrated that Myc-TPL-2T290A was phosphorylated on S400 to a similar degree to that of wild-type Myc-TPL-2 after LPS addition (Fig. 3c). Thus, LPS stimulation of TPL-2 S400 phosphorylation was not dependent on T290 phosphorylation or, by implication, activation of TPL-2 kinase activity. These data were consistent with our earlier conclusion that TPL-2 kinase activity was not required for LPS-induced phosphorylation of TPL-2 on S400 in RAW264.7 cells (Fig. 3a). It is not known whether T290 phosphorylation is regulated by prior S400 phosphorylation.
Activation loop phosphorylation of many MAP 3-kinases is triggered by their oligomerization after stimulation (36). Although overexpressed TPL-2 has been shown to oligomerize in 293T cells (11), endogenous TPL-2 was not detected in anti-Myc immunoprecipitates of Myc-TPL-2-transfected RAW264.7 cells after LPS stimulation, suggesting that TPL-2 does not undergo stimulus-induced oligomerization in macrophages (see Fig. 6d). To investigate the possibility that the serine 400 residues of TPL-2D270A and TPL-2T290A were phosphorylated in trans by endogenous TPL-2 in RAW264.7 cells, 293 cells were cotransfected with vectors encoding Myc-TPL-2 plus TPL-2 or EV. Previous experiments demonstrated that Myc-TPL-2 is constitutively active when overexpressed in 293 cells (4). No S400 phosphorylation of Myc-TPL-2 was detected when it was coexpressed with TPL-2 (Fig. 3d). However, coexpression with v-Akt induced Myc-TPL-2 S400 phosphorylation, as expected (20). These data indicate that Myc-TPL-2 S400 could not be phosphorylated by TPL-2, suggesting that S400 phosphorylation of Myc-TPL-2D270A and Myc-TPL-2T290A in LPS-stimulated RAW264.7 cells was not mediated by endogenous TPL-2.
FIG. 6.
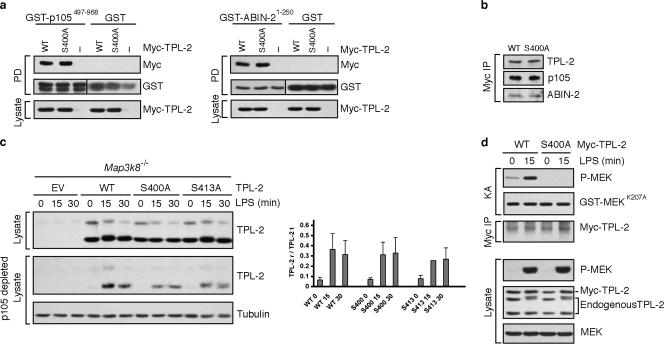
S400 is required for LPS activation of TPL-2. (a) Lysates were prepared from 293 cells transfected with vectors encoding TPL-2 or TPL-2S400A. Pull-downs (PD) from cell lysates were carried out with GST-p105468-967, GST-ABIN-21-250, or GST. Interaction with TPL-2 or TPL-2S400A was determined by immunoblotting. (b) Myc-TPL-2 and Myc-TPL-2S400A were immunoprecipitated from lysates of stably transfected RAW264.7 cells by using a Myc antibody. Coimmunoprecipitating p105 and ABIN-2 were revealed by immunoblotting. (c) Map3k8−/− macrophages were transduced with the indicated recombinant retroviruses and stimulated with LPS. Cell lysates were depleted of p105 by immunoprecipitation with p105 antibody and then immunoblotted. Blots from three similar experiments were quantified by laser scanning densitometry, and data are presented graphically as ratios of released TPL-2 (TPL-2 r) to total TPL-2 (TPL-2 t) (means ± standard errors of the means). No significant difference was found (using Student's t test) between LPS-induced release of wild-type TPL-2 and TPL-2S400A from p105 at 15 min (P = 0.522) or 30 min (P = 0.48). (d) Myc-TPL-2 or Myc-TPL-2S400A was immunoprecipitated from stably transfected RAW264.7 cells that were stimulated for 15 min with LPS or left unstimulated. Immunoprecipitates were assayed in vitro for the ability to induce phosphorylation of GST-MEKK207A by immunoblotting.
Together, the experiments in this section support the hypothesis that transphosphorylation on S400 in the C terminus of TPL-2 is required to promote the downstream activation of ERK in LPS-stimulated macrophages. Furthermore, since S400 was necessary for TPL-2 function in Nfkb1−/− cells, the regulatory role of S400 phosphorylation was independent of p105 (5, 38).
Serine 400 of TPL-2 is essential for LPS upregulation of Egr-1 and TNF in macrophages.
The above experiments demonstrated that TPL-2 was phosphorylated on S400 after LPS stimulation and suggested that S400 phosphorylation was critical for TPL-2 activation of ERK in p105-deficient macrophages. To confirm the physiological significance of these data, it was important to determine whether S400 was important for TPL-2 function in cells that do express p105. To investigate this, macrophages from Map3k8−/− mice (13) were transduced with recombinant retroviruses encoding wild-type and mutant TPL-2 proteins. Expression of TPL-2S400A in TPL-2-deficient macrophages failed to reconstitute LPS activation of ERK, similar to expression of kinase-inactive TPL-2D270A (Fig. 4a). In contrast, ERK was clearly activated in LPS-stimulated Map3k8−/− cells expressing either wild-type TPL-2 or TPL-2S413A. Thus, S400 of TPL-2 was necessary for LPS stimulation of ERK in macrophages that contain p105 protein.
Activation of the TPL-2/ERK signaling pathway is essential for LPS upregulation of the transcription factor Egr-1 and the proinflammatory cytokine TNF in macrophages (13, 39). Consistent with the critical role of S400 in TPL-2 activation of ERK, retroviral transduction of Map3k8−/− macrophages with TPL-2S400A failed to reconstitute LPS upregulation of either Egr-1 (Fig. 4b) or TNF (Fig. 4c). In contrast, LPS stimulation clearly induced the expression of both of these proteins to similar amounts in Map3k8−/− cells that were transduced with either TPL-2 or TPL-2S413A (Fig. 4b and c). TPL-2D270A also failed to reconstitute LPS upregulation of Egr-1 (Fig. 4b) and TNF (Fig. 4c) in Map3k8−/− macrophages. LPS induction of two genes known to be regulated by the TPL-2/ERK signaling pathway in macrophages (13, 39) was therefore dependent on S400 and the kinase activity of TPL-2.
TPL-2 phosphorylation on serine 400 is independent of Akt and IKK activity.
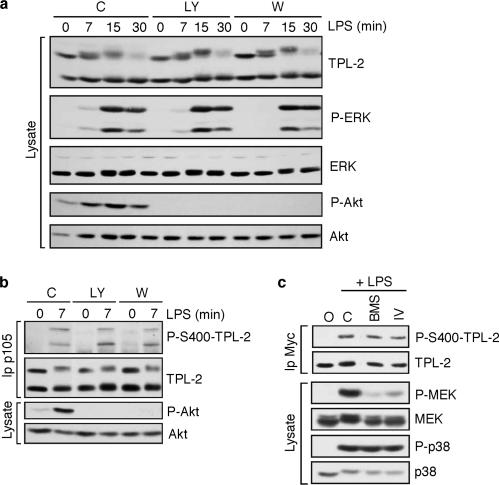
Serine 400 was originally identified as a phosphorylation site on TPL-2 that was phosphorylated by overexpressed Akt in Jurkat T cells (20). Although LPS stimulation is known to activate the phosphatidylinositol 3-kinase/Akt pathway in macrophages (18, 29), it remained unclear whether TPL-2 S400 is a physiological target of endogenous Akt in LPS-stimulated macrophages. To investigate this possibility, wild-type macrophages were separately pretreated with the phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 prior to stimulation with LPS. Both inhibitors completely blocked LPS activation of Akt, as revealed by immunoblotting of cell lysates with a phospho-Akt antibody (Fig. 5a). However, LPS stimulation of ERK phosphorylation was unaffected. Since TPL-2 is essential for LPS induction of ERK phosphorylation (13), this indicated that Akt was not required for TPL-2 activation in macrophages. Consistently, LPS induction of TPL-2 S400 phosphorylation was also unaffected by wortmannin or LY294002 pretreatment (Fig. 5b). Together, these results ruled out Akt as the kinase that phosphorylates S400 of TPL-2 after LPS stimulation of macrophages.
FIG. 5.
LPS stimulates TPL-2 S400 phosphorylation independently of Akt and IKK. (a and b) Wild-type macrophages (C57/BL6) were pretreated with wortmannin, LY294002, or vehicle control (C) and then stimulated with LPS. (a) Lysates were immunoblotted. (b) p105 antibody immunoprecipitates were immunoblotted. (c) RAW264.7 cells stably expressing Myc-TPL-2 were pretreated with BMS-345541, IKK2 inhibitor IV, or vehicle control prior to stimulation with LPS. Lysates were immunoprecipitated with Myc antibody, and immunoprecipitates were immunoblotted. The efficacy of the IKK inhibitors was confirmed by immunoblotting of cell lysates and probing for phospho-MEK (P-MEK).
Based on experiments with an IKK2 inhibitor, it has been suggested that IKK activity is required for phosphorylation of TPL-2 on T290 and for consequent activation of TPL-2 kinase activity (10). To investigate whether IKK activity is required for TPL-2 phosphorylation on S400, RAW264.7 macrophages stably expressing wild-type Myc-TPL-2 were separately pretreated with the IKK2 inhibitors BMS-345541 and IKK2 inhibitor IV. Cells were then stimulated with LPS, and S400 phosphorylation was assayed by anti-Myc immunoprecipitation followed by immunoblotting with phospho-S400-TPL-2 antibody. LPS-induced phosphorylation of TPL-2 on S400 was similar in cells treated with the IKK2 inhibitors to that in cells treated with vehicle control (Fig. 5c). However, both inhibitors dramatically reduced LPS activation of MEK phosphorylation, which is dependent on IKK activity (4, 38), confirming their biological activity (Fig. 5c). Therefore, LPS-induced phosphorylation of TPL-2 on S400 was not mediated by the IKK complex.
Serine 400 does not regulate TPL-2 release from p105.
We have previously shown that the TPL-2 C terminus is involved in the interaction of TPL-2 with both p105 and ABIN-2 in the TPL-2/p105/ABIN-2 ternary complex in macrophages (5, 23). To determine whether S400 regulated the TPL-2 association with these proteins, we tested the ability of wild-type TPL-2 and TPL-2S400A to interact with GST-p105497-968 and GST-ABIN-21-250 (4, 23). In pull-down assays with transfected 293 cell lysates, both fusion proteins interacted to a similar degree with TPL-2 and TPL-2S400A, whereas no interaction was detected using GST alone (Fig. 6a). Consistently, the interaction of Myc-TPL-2 with endogenous p105 and ABIN-2 in stably transfected RAW264.7 cells was also unaffected by the TPL-2 S400A mutation (Fig. 6b). S400 was therefore not required for binding of TPL-2 to either p105 or ABIN-2.
The release of TPL-2 from p105 is an essential step in MEK activation by TPL-2 in LPS-stimulated macrophages and has been shown to occur as a consequence of IKK-induced p105 proteolysis by the proteasome (4, 38). It has also been proposed that TPL-2 phosphorylation on T290 can induce its release from p105 independently of p105 proteolysis (10, 11). To investigate whether phosphorylation of S400 affects the release of TPL-2 from p105, Map3k8−/− macrophages were retrovirally transduced with wild-type and mutant TPL-2 molecules and stimulated with LPS. To monitor TPL-2 release from p105, cell lysates were depleted of p105 by immunoprecipitation with p105 antibody, and the resulting p105-free lysates were immunoblotted for TPL-2 (5). LPS induced the release of TPL-2, TPL-2S400A, and TPL-2S413A from p105 to similar degrees (Fig. 6c). TPL-2 S400 therefore did not regulate LPS-induced release of TPL-2 from p105.
Serine 400 phosphorylation is required for LPS activation of TPL-2 MEK kinase activity.
The inability of TPL-2S400A to reconstitute LPS activation of ERK in both Nfkb1−/− and Map3k8−/− macrophages raised the possibility that S400 was required for LPS activation of TPL-2 catalytic activity. To investigate this, Myc-TPL-2 and Myc-TPL-2S400A were immunoprecipitated from stably transfected RAW264.7 cells and tested for the ability to phosphorylate GST-MEKK207A in vitro. LPS stimulation induced a clear activation of wild-type TPL-2 (Fig. 6d), as expected (5). However, no detectable MEK kinase activity was associated with Myc-TPL-2S400A in the absence or presence of LPS stimulation. LPS induction of endogenous MEK phosphorylation was similar in cells expressing Myc-TPL-2 and Myc-TPL-2S400A (Fig. 6d, lower panels), suggesting that Myc-TPL-2S400A did not act as a dominant-negative mutant. When expressed stably in RAW264.7 cells, Myc-TPL-2S400E also had no detectable MEK kinase activity, with or without LPS stimulation (data not shown). S400 was therefore required for LPS to stimulate the catalytic activity of TPL-2, and the introduction of a negatively charged amino acid at this residue did not mimic S400 phosphorylation.
DISCUSSION
TPL-2 is negatively regulated by NF-κB1 p105, and dissociation from this inhibitor is required for TPL-2 activation of the ERK MAP kinase pathway in LPS-stimulated macrophages (4, 5, 38, 39). In the present study, it was demonstrated that release from p105 was not sufficient for TPL-2 to induce ERK activation, as LPS stimulation was still required to promote TPL-2-dependent activation of ERK in p105-deficient macrophages. This implied the existence of a regulatory step in the activation of TPL-2 in addition to its dissociation from p105 (5, 38). LPS stimulation was found to induce the rapid phosphorylation of TPL-2 on S400 in its C-terminal tail, which was essential for TPL-2 activation since TPL-2S400A failed to reconstitute LPS stimulation of ERK in either Nfkb1−/− or Map3k8−/− macrophages. Consistently, in vitro kinase assays demonstrated that S400 was required for LPS activation of TPL-2 MEK kinase activity. Therefore, phosphorylation of S400 defines a second regulatory step essential for LPS activation of TPL-2 MEK kinase activity in macrophages. IKK-induced p105 proteolysis is required for TNF activation of TPL-2, similar to that by LPS (38). It will clearly be interesting in future studies to determine whether TPL-2 S400 phosphorylation is necessary for TNF activation of the TPL-2/ERK pathway in macrophages and whether this TPL-2 modification is also needed to couple TPL-2 to the activation of ERK, JNK, and NF-κB in TNF-stimulated fibroblasts (12).
Binding to p105 was shown to block phosphorylation of GST-MEKK207A without inhibiting the catalytic activity of TPL-2, as evidenced by TPL-2 autophosphorylation and MBP phosphorylation. This is consistent with our previous data demonstrating that p105 specifically inhibits TPL-2 activation of MEK by preventing TPL-2-MEK association (4). Furthermore, LPS stimulation appears to activate the catalytic activity of TPL-2 when complexed with p105, as indicated by the autophosphorylation mobility shift of p105-associated M1-TPL-2 (5). This raises the interesting possibility that TPL-2 may have physiological substrates in addition to MEK, which it can phosphorylate when bound to its inhibitor, p105. Indeed, it was recently shown that TPL-2 phosphorylates associated p105 in LPS-stimulated macrophages (2), although the functional consequences of this activity are presently unclear.
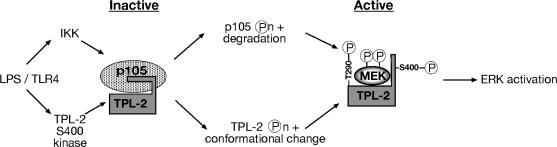
Activation loop phosphorylation is the most common mechanism for regulating kinase activity (28), allowing the activation segment to position optimally for substrate binding. Indeed, phosphorylation of T290 in the C-terminal anchor (P+1 loop) of the TPL-2 activation loop has been implicated in TPL-2 activation (10, 24, 36). In addition to facilitating substrate access, T290 phosphorylation appears to control the release of TPL-2 from p105 independently of p105 proteolysis (10, 11), suggesting that phosphorylation of this site may alter binding of TPL-2 to its inhibitor, p105. S400 mutation did not affect the association of TPL-2 with p105 or its release from p105 after LPS stimulation, implying that S400 phosphorylation controls TPL-2 signaling function independently of p105. Consistent with this hypothesis, S400 is essential for TPL-2 activation of ERK in p105-deficient macrophages. Earlier work indicated that the C-terminal tail of TPL-2, which encompasses S400, forms intermolecular interactions with its kinase domain and inhibits kinase activity (7). These findings raise the possibility that S400 phosphorylation may prevent or alter the binding of TPL-2 to its inhibitory C-terminal tail. Thus, it is possible that S400 phosphorylation induces an activating conformational change in the C terminus of TPL-2 that is necessary for TPL-2 activation following LPS stimulation of macrophages (Fig. 7).
FIG. 7.
Schematic diagram of the mechanism of TPL-2 activation in LPS-stimulated macrophages. In unstimulated macrophages, TPL-2 is held in an inactive complex with p105. LPS stimulation induces p105 phosphorylation by the IKK complex, which targets p105 for degradation by the proteasome and frees TPL-2 to interact with and phosphorylate its substrate, MEK. TPL-2 activation also requires phosphorylation of T290 in the TPL-2 kinase domain and phosphorylation of S400 in the TPL-2 C terminus, both by unknown TPL-2 kinases. S400 phosphorylation may facilitate TPL-2 activation by relieving an inhibitory intramolecular interaction between TPL-2 and its C terminus (7).
S400 is within the C-terminal tail, which must be removed to activate TPL-2 oncogenic potential (7). C-terminal truncation alters two aspects of TPL-2 kinase function that are important in stimulating its transforming activity. TPL-2 associates with its inhibitor, p105, via two distinct interactions, with the TPL-2 C terminus binding to a region encompassing residues 497 to 534 of p105 and the TPL-2 kinase domain interacting with the p105 death domain (4). The inhibitory effect of the p105 death domain on TPL-2 phosphorylation of MEK requires simultaneous interaction of the TPL-2 C terminus with p105. Consequently, the MEK kinase activity of oncogenic TPL-2ΔC, which lacks the C-terminal 70 amino acids, is refractory to inhibition by p105 (4). S400 is not necessary for the interaction of TPL-2 with p105. However, independent of p105 binding, deletion of the C terminus increases the specific kinase activity of TPL-2 by removing the autoinhibitory interaction between the C terminus and the rest of the kinase (7, 17). The present study indicates that S400 phosphorylation is required for physiological activation of TPL-2 kinase activity. By removing S400, C-terminal truncation obviates the need for phosphorylation of this site for activating TPL-2 signaling function, eliminating an essential control step.
S400 was initially shown to be a site on TPL-2 that was phosphorylated by cotransfected Akt in Jurkat T cells (20). The present study confirmed that activated Akt could phosphorylate TPL-2 S400 in cotransfected 293T cells. However, S400 of endogenous TPL-2 was not phosphorylated by endogenous Akt after LPS stimulation of macrophages, and consistently, that Akt activity was not required for LPS induction of ERK phosphorylation in this cell type. It is possible that TPL-2 S400 is phosphorylated by Akt in T cells following stimulation with the appropriate agonist. However, it is also conceivable that TPL-2 S400 phosphorylation by Akt in Jurkat T cells and 293T cells is the artifactual consequence of the high catalytic activity of transfected Akt (20) and the accessibility of TPL-2 S400 to kinases. The present study also ruled out that TPL-2 S400 was phosphorylated by TPL-2 itself or transphosphorylated by the IKK complex. Clearly, the identification of the physiological TPL-2 S400 kinase is an important area of future research, which may suggest novel approaches for the pharmacological inhibition of the TPL-2/ERK signaling pathway in autoimmune diseases.
Acknowledgments
We thank Alain Israel for reagents used in this study. We are also grateful to Ben Seddon and Antony Symons (Division of Immune Cell Biology, NIMR) for critical readings of the manuscript and to NIMR Biological Services, NIMR Photographics, and other members of the Ley laboratory for their support during the course of this work.
Work in the laboratory of S.C.L. was supported by the UK Medical Research Council and the Association for International Cancer Research (project grant 03-297 to A.K.). Work on the regulation of TPL-2 kinase in the P.N.T. laboratory was supported by NIH grant RO1CA095431.
Footnotes
Published ahead of print on 20 August 2007.
REFERENCES
- 1.Aoki, M., F. Hamada, T. Sugimoto, S. Sumida, T. Akiyama, and K. Toyoshima. 1993. The human cot proto-oncogene encodes two protein serine/threonine kinases with different transforming activities by alternative initiation of translation. J. Biol. Chem. 268: 22723-22732. [PubMed] [Google Scholar]
- 2.Babu, G. R., W. Jin, L. Norman, M. Waterfield, M. Chang, X. Wu, M. Zhang, and S. C. Sun. 2006. Phosphorylation of NF-κB1/p105 by oncoprotein kinase Tpl2: implications for a novel mechanism of Tpl2 regulation. Biochim. Biophys. Acta 1763: 174-181. [DOI] [PubMed] [Google Scholar]
- 3.Beinke, S., M. P. Belich, and S. C. Ley. 2002. The death domain of NF-κB1 p105 is essential for signal-induced p105 proteolysis. J. Biol. Chem. 277: 24162-24168. [DOI] [PubMed] [Google Scholar]
- 4.Beinke, S., J. Deka, V. Lang, M. P. Belich, P. A. Walker, S. Howell, S. J. Smerdon, S. J. Gamblin, and S. C. Ley. 2003. NF-κB p105 negatively regulates TPL-2 MEK kinase activity. Mol. Cell. Biol. 23: 4739-4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beinke, S., M. J. Robinson, A. Salmeron, M. Hugunin, H. Allen, and S. C. Ley. 2004. Lipopolysaccharide activation of the TPL-2/MEK/extracellular signal-regulated kinase mitogen-activated protein kinase cascade is regulated by IκB kinase-induced proteolysis of NF-κB1 p105. Mol. Cell. Biol. 24: 9658-9667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belich, M. P., A. Salmeron, L. H. Johnston, and S. C. Ley. 1999. TPL-2 kinase regulates the proteolysis of the NF-κB inhibitory protein NF-κB1 p105. Nature 397: 363-368. [DOI] [PubMed] [Google Scholar]
- 7.Ceci, J. D., C. P. Patriotis, C. Tsatsanis, A. M. Makris, R. Kovatch, D. A. Swing, N. A. Jenkins, P. N. Tsichlis, and N. G. Copeland. 1997. TPL-2 is an oncogenic kinase that is activated by carboxy-terminal truncation. Genes Dev. 11: 688-700. [DOI] [PubMed] [Google Scholar]
- 8.Chan, A. M.-L., M. Chedid, E. S. McGovern, N. C. Popescu, T. Miki, and S. A. Aaronson. 1993. Expression cDNA cloning of a serine kinase transforming gene. Oncogene 8: 1329-1333. [PubMed] [Google Scholar]
- 9.Chiariello, M., M. J. Marinissen, and J. S. Gutkind. 2000. Multiple mitogen-activated protein kinase signaling pathways connect the Cot oncoprotein to the c-Jun promoter and to cellular transformation. Mol. Cell. Biol. 20: 1747-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho, J., M. Melnick, G. P. Solidakis, and P. N. Tsichlis. 2005. Tpl2 (tumor progression locus 2) phosphorylation at Thr290 is induced by lipopolysaccharide via an IκB kinase-β-dependent pathway and is required for Tpl2 activation by external signals. J. Biol. Chem. 280: 20442-20448. [DOI] [PubMed] [Google Scholar]
- 11.Cho, J., and P. N. Tsichlis. 2005. Phosphorylation at T290 regulates Tpl2 binding to NF-κB1/p105 and Tpl2 activation and degradation by lipopolysaccharide. Proc. Natl. Acad. Sci. USA 102: 2350-2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das, S., J. Cho, I. Lambertz, M. A. Kelliher, A. G. Eliopoulos, K. Du, and P. N. Tsichlis. 2005. Tpl2/Cot signals activate ERK, JNK and NF-κB in a cell-type and stimulus-specific manner. J. Biol. Chem. 280: 23748-23757. [DOI] [PubMed] [Google Scholar]
- 13.Dumitru, C. D., J. D. Ceci, C. Tsatsanis, D. Kontoyiannis, K. Stamatakis, J.-H. Lin, C. Patriotis, N. A. Jenkins, N. G. Copeland, G. Kollias, and P. N. Tsichlis. 2000. TNFα induction by LPS is regulated post-transcriptionally via a TPL2/ERK-dependent pathway. Cell 103: 1071-1083. [DOI] [PubMed] [Google Scholar]
- 14.Eliopoulos, A. G., C. D. Dumitru, C.-C. Wang, J. Cho, and P. N. Tsichlis. 2002. Induction of COX-2 by LPS in macrophages is regulated by TPL2-dependent CREB activation signals. EMBO J. 21: 4831-4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eliopoulos, A. G., C.-C. Wang, C. D. Dumitru, and P. N. Tsichlis. 2003. TPL-2 transduces CD40 and TNF signals that activate ERK and regulates IgE induction by CD40. EMBO J. 22: 3855-3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erny, K. M., J. Peli, J.-F. Lambert, V. Muller, and H. Diggelmann. 1996. Involvement of the TPL-2/COT oncogene in MMTV tumorigenesis. Oncogene 13: 2015-2020. [PubMed] [Google Scholar]
- 17.Gandara, M. L., P. Lopez, R. Hernando, J. G. Castano, and S. Alemany. 2003. The COOH-terminal domain of wild-type Cot regulates its stability and kinase-specific activity. Mol. Cell. Biol. 23: 7377-7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guha, M., and N. Mackman. 2002. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J. Biol. Chem. 277: 32124-32132. [DOI] [PubMed] [Google Scholar]
- 19.Kabouridis, P. S., A. I. Magee, and S. C. Ley. 1997. S-acylation of LCK protein tyrosine kinase is essential for its signalling function in T lymphocytes. EMBO J. 16: 4983-4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kane, L. P., M. N. Mollenauer, Z. Xu, C. W. Turck, and A. Weiss. 2002. Akt-dependent phosphorylation specifically regulates Cot induction of NF-κB-dependent transcription. Mol. Cell. Biol. 22: 5962-5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karin, M., and Y. Ben-Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18: 621-663. [DOI] [PubMed] [Google Scholar]
- 22.Lang, V., J. Janzen, G. Z. Fischer, Y. Soneji, S. Beinke, A. Salmeron, H. Allen, R. T. Hay, Y. Ben-Neriah, and S. C. Ley. 2003. βTrCP-mediated proteolysis of NF-κB1 p105 requires phosphorylation of p105 serines 927 and 932. Mol. Cell. Biol. 23: 402-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lang, V., A. Symons, S. J. Watton, J. Janzen, Y. Soneji, S. Beinke, S. Howell, and S. C. Ley. 2004. ABIN-2 forms a ternary complex with TPL-2 and NF-κB1 p105 and is essential for TPL-2 protein stability. Mol. Cell. Biol. 24: 5235-5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luciano, B. S., S. Hsu, P. L. Channavajhala, L.-L. Lin, and J. W. Cuozzo. 2004. Phosphorylation of threonine 290 in the activation loop of Tpl2/Cot is necessary but not sufficient for kinase activity. J. Biol. Chem. 279: 52117-52123. [DOI] [PubMed] [Google Scholar]
- 25.Lund, A. H., G. Turner, A. Trubetskoy, E. Verhoeven, E. Wientjens, D. Hulsman, R. Russell, R. A. DePinho, J. Lenz, and M. van Lohuizen. 2002. Genome-wide retroviral insertional tagging of genes involved in cancer in Cdkn2a-deficient mice. Nat. Gene 32: 160-165. [DOI] [PubMed] [Google Scholar]
- 26.Mikkers, H., J. Allen, P. Knipscheer, L. Romeyn, A. Hart, E. Vink, and A. Berns. 2002. High throughput retroviral tagging to identify components of specific signaling pathways in cancer. Nat. Gene 32: 153-159. [DOI] [PubMed] [Google Scholar]
- 27.Morita, S., T. Kojima, and T. Kitamura. 2000. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 7: 1063-1066. [DOI] [PubMed] [Google Scholar]
- 28.Nolen, B., S. Taylor, and G. Ghosh. 2004. Regulation of protein kinases: controlling activity through activation segment conformation. Mol. Cell 15: 661-675. [DOI] [PubMed] [Google Scholar]
- 29.Ojaniemi, M., V. Glumoff, K. Harju, M. Liljeroos, K. Vuori, and M. Hallman. 2003. Phosphatidylinositol 3-kinase is involved in Toll-like receptor 4-mediated cytokine expression in mouse macrophages. Eur. J. Immunol. 33: 597-605. [DOI] [PubMed] [Google Scholar]
- 30.Papoutsopoulou, S., A. Symons, T. Tharmalingham, M. P. Belich, F. Kaiser, D. Kioussis, A. O'Garra, V. Tybulewicz, and S. C. Ley. 2006. ABIN-2 is required for optimal activation of the TPL-2/ERK MAP kinase pathway in innate immune responses. Nat. Immunol. 7: 606-615. [DOI] [PubMed] [Google Scholar]
- 31.Patriotis, C., A. Makris, S. E. Bear, and P. N. Tsichlis. 1993. Tumor progression locus 2 (Tpl-2) encodes a protein kinase involved in the progression of rodent T cell lymphomas and in T cell activation. Proc. Natl. Acad. Sci. USA 90: 2251-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patriotis, C., A. Makris, J. Chernoff, and P. N. Tsichlis. 1994. Tpl-2 acts in concert with Ras and Raf-1 to activate mitogen-activated protein kinase. Proc. Natl. Acad. Sci. USA 91: 9755-9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salmeron, A., T. B. Ahmad, G. W. Carlile, D. Pappin, R. P. Narsimhan, and S. C. Ley. 1996. Activation of MEK-1 and SEK-1 by Tpl-2 proto-oncoprotein, a novel MAP kinase kinase kinase. EMBO J. 15: 817-826. [PMC free article] [PubMed] [Google Scholar]
- 34.Sha, W. C., H.-C. Lious, E. I. Tuomanen, and D. Baltimore. 1995. Targeted disruption of the p50 subunit of NF-κB leads to multifocal defects in immune responses. Cell 80: 321-330. [DOI] [PubMed] [Google Scholar]
- 35.Sugimoto, K., M. Ohata, J. Miyoshi, H. Ishizaki, N. Tsuboi, A. Masuda, Y. Yoshikai, M. Takamoto, K. Sugane, S. Matsuo, Y. Shimada, and T. Matsuguchi. 2004. A serine/threonine kinase, Cot/Tpl2, modulates bacterial DNA-induced IL-12 production and Th cell differentiation. J. Clin. Investig. 114: 857-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Symons, A., S. Beinke, and S. C. Ley. 2006. MAP kinase kinase kinases and innate immunity. Trends Immunol. 27: 40-48. [DOI] [PubMed] [Google Scholar]
- 37.Warren, M. K., and S. N. Vogel. 1985. Bone marrow-derived macrophages: development and regulation of differentiation markers by colony-stimulating factor and interferons. J. Immunol. 134: 982-989. [PubMed] [Google Scholar]
- 38.Waterfield, M., W. Jin, W. Reiley, M. Y. Zhang, and S.-C. Sun. 2004. IKKβ is an essential component of the TPL-2 signaling pathway. Mol. Cell. Biol. 24: 6040-6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waterfield, M. R., M. Zhang, L. P. Norman, and S.-C. Sun. 2003. NF-κB1/p105 regulates lipopolysaccharide-stimulated MAP kinase signaling by governing the stability and function of the TPL-2 kinase. Mol. Cell 11: 685-694. [DOI] [PubMed] [Google Scholar]
- 40.Yeung, K. T., P. Janosch, B. McFerran, D. W. Rose, H. Mischak, J. M. Sedivy, and W. Kolch. 2000. Mechanism of suppression of the Raf/MEK/extracellular signal-regulated kinase pathway by the Raf kinase inhibitor protein. Mol. Cell. Biol. 20: 3079-3085. [DOI] [PMC free article] [PubMed] [Google Scholar]