Abstract
In Saccharomyces cerevisiae, replication through DNA lesions is promoted by Rad6-Rad18-dependent processes that include translesion synthesis by DNA polymerases η and ζ and a Rad5-Mms2-Ubc13-controlled postreplicational repair (PRR) pathway which repairs the discontinuities in the newly synthesized DNA that form opposite from DNA lesions on the template strand. Here, we examine the contributions of the RAD51, RAD52, and RAD54 genes and of the RAD50 and XRS2 genes to the PRR of UV-damaged DNA. We find that deletions of the RAD51, RAD52, and RAD54 genes impair the efficiency of PRR and that almost all of the PRR is inhibited in the absence of both Rad5 and Rad52. We suggest a role for the Rad5 pathway when the lesion is located on the leading strand template and for the Rad52 pathway when the lesion is located on the lagging strand template. We surmise that both of these pathways operate in a nonrecombinational manner, Rad5 by mediating replication fork regression and template switching via its DNA helicase activity and Rad52 via a synthesis-dependent strand annealing mode. In addition, our results suggest a role for the Rad50 and Xrs2 proteins and thereby for the MRX complex in promoting PRR via both the Rad5 and Rad52 pathways.
DNA lesions in the template strand block the progression of the replication fork. Genetic studies of the yeast Saccharomyces cerevisiae have indicated a crucial role for the Rad6-Rad18 ubiquitin conjugating enzyme complex (3, 4) in promoting replication through DNA lesions via three different pathways that include DNA polymerase η (Polη)- and Polζ-dependent translesion synthesis (TLS) and an Mms2-Ubc13-Rad5-dependent postreplicational repair (PRR) pathway which promotes the repair of discontinuities that form in the DNA synthesized from damaged templates (20, 33, 39, 51).
UV light induces the formation of cyclobutane pyrimidine dimers (CPDs) and (6-4) photoproducts in DNA. Both in yeast and in humans, Polη promotes error-free synthesis through the CPDs; consequently, inactivation of Polη confers an increase in the incidence of UV mutagenesis (21, 32, 43, 57) and causes a cancer-prone syndrome, the variant form of xeroderma pigmentosum, in humans (19, 30). Polζ promotes TLS through the UV lesions by extending from the nucleotides inserted opposite the CPD or the (6-4) photoproduct by another DNA Pol (17, 23). By contrast to Polη, which mediates error-free TLS through the CPDs, Polζ promotes error-prone TLS through the UV lesions (26-28).
Although the mechanism by which the Mms2-Ubc13-Rad5-dependent PRR pathway operates is not defined, it is likely to involve a transient template switching mechanism and a copy choice type of DNA synthesis wherein the newly synthesized daughter strand of the undamaged complementary sequence is used as a template for bypassing the lesion (5). In the Mms2-Ubc13-Rad5 complex, the Mms2-Ubc13 complex promotes the assembly of lysine 63-linked polyubiquitin chains (13) and Rad5 functions as an ubiquitin ligase (E3) for promoting Mms2-Ubc13-dependent ubiquitination (12, 54). In addition to the E3 activity, Rad5 has a DNA-dependent ATPase activity, characteristic of the SWI2/SNF2 family of ATPases to which it belongs (18, 22). Genetic studies of yeast have indicated that both the ATPase and E3 activities of Rad5 are indispensable for its role in PRR (9).
All the Rad6-Rad18 lesion bypass processes are dependent upon lysine 164 ubiquitination of PCNA. In DNA-damaged yeast cells, PCNA becomes monoubiquitinated at its lysine 164 residue via the action of Rad6-Rad18, and subsequently, this lysine residue is polyubiquitinated via a lysine 63-linked polyubiquitin chain by the Mms2-Ubc13-Rad5 enzyme complex (12). Genetic studies of yeast have indicated the requirement of PCNA monoubiquitination for Polη- and Polζ-dependent TLS and of PCNA polyubiquitination for Rad5-dependent PRR (10, 12, 44).
By contrast to inactivation of RAD6 and RAD18, which confers a large increase in UV sensitivity because of the inhibition of different Rad6-Rad18-dependent lesion bypass processes, mutations in the RAD52 epistasis group of genes confer only a modest increase in UV sensitivity (40). Instead, the genes of the RAD52 group play a crucial role in homology-dependent recombinational repair of double-strand DNA breaks that are induced upon exposure to ionizing radiation, such as X rays and γ rays; hence, inactivation of these genes confers a large increase in sensitivity to ionizing radiation (25, 47, 50). Rad51, Rad52, and Rad54 are three key proteins indispensable for recombinational repair. Rad51 forms an extended helical filament on single-stranded (ss) DNA (34, 46, 48). This nucleoprotein filament then invades the homologous region in duplex DNA, resulting in the formation of a three-stranded D loop. Rad52 and Rad54 stimulate the DNA pairing function of Rad51; Rad52 enhances the ability of Rad51 to displace RPA from ss DNA (42, 45), and Rad54 functionally cooperates with Rad51 at different steps of the pairing reaction. Rad54, a Swi2/Snf2 family DNA-dependent ATPase, physically interacts with Rad51; it stabilizes Rad51-ss DNA filaments and stimulates the DNA pairing reaction of Rad51 (31, 36, 41), and it also dissociates Rad51 from duplex DNA via its DNA translocase activity (1), thereby freeing up the primer end to initiate DNA synthesis.
Mutations in the RAD50, MRE11, and XRS2 genes also render cells highly sensitive to ionizing radiation and defective in double-strand break (dsb) repair (25, 50). In addition to a role in dsb repair by homologous recombination, these genes are required for dsb repair by nonhomologous end joining (25, 50). Mre11, Rad50, and Xrs2 exist in a stable complex, the MRX complex, in yeast cells (52, 55). Rad50 belongs to the structural maintenance of chromosome family of proteins, and like the other members of this family, it has a bipartite ATPase domain located at its amino and carboxy termini, which are separated by a long, antiparallel coiled-coil domain. The protein folds onto itself, and the two termini form a globular ATPase domain. Two molecules of Mre11 bind to two molecules of Rad50 in the vicinity of the globular ATPase domain. The globular head, formed by two molecules of Rad50 and two molecules of Mre11, contains two DNA binding sites in it, which could promote the alignment of the two DNA ends for dsb repair by nonhomologous end joining, and which could also mediate the alignment of DNA ends with the sister chromatid in recombination (2, 8, 14-16). Xrs2 binds the Rad50-Mre11 complex, and it helps target the MRX complex to DNA ends (53).
Here, we have examined the roles of the RAD51, RAD52, and RAD54 genes and of the RAD50 and XRS2 genes in PRR of DNA synthesized from UV-damaged templates. Although all these genes contribute to the PRR of UV damaged DNA in yeast cells, rather unexpectedly, they differ in their modus operandi. By contrast to Rad51, Rad52, and Rad54, which carry out PRR in a manner independent of the Rad5-mediated PRR, we find that Rad50 and Xrs2 contribute to PRR via both the Rad5- and Rad52-mediated PRR pathways.
MATERIALS AND METHODS
Strains.
For postreplication repair studies, yeast strains were treated with ethidium bromide to obtain [rho°] derivatives lacking mitochondrial DNA. The following yeast strains used in these studies are all derived from EMY74.7, MATa his3-Δ1 leu2-3,112 trp1Δ ura3-52: YR1-65, rad1Δ [rho°]; YR1-118, rad1Δ rad5Δ [rho°]; YR1-347, The rad1Δ rad50Δ [rho°]; YR1-315, rad1Δ rad51Δ [rho°]; YR1-231, rad1Δ rad52Δ [rho°]; YR1-318, rad1Δ rad54Δ [rho°]; YR1-350, rad1Δ xrs2Δ [rho°]; YR1-241, rad1Δ rad5Δ rad52Δ [rho°]; YR1-385, rad1Δ rad50Δ rad5Δ [rho°]; YR1-400, rad1Δ xrs2Δ rad5Δ [rho°]; YR1-356, rad1Δ rad50Δ rad52Δ [rho°]; and YR1-408, rad1Δ xrs2Δ rad52Δ [rho°].
UV irradiation and sedimentation in alkaline sucrose gradients.
For postreplication repair experiments, alkaline sucrose gradient sedimentation is used to determine the size of nuclear DNA synthesized in cells following UV irradiation. To avoid confusion between UV-damaged nuclear DNA synthesized after UV irradiation that has not been repaired and undamaged mitochondrial DNA, which could be similar in size, these experiments are best performed with [rho°] strains, which lack mitochondrial DNA. Although [rho°] strains are respiratory deficient in medium containing glucose as a carbon source, the growth of [rho°] cells and that of [rho+] cells are very similar.
Yeast cells were grown to logarithmic phase in synthetic complete medium lacking uracil but containing 5 μg of uridine/ml. When the cells reached a density of 0.5 × 107 to 1.0 × 107 cells per ml, they were UV irradiated at room temperature in the same growth medium in 150- by 20-mm petri dishes with constant stirring at a dose rate of 0.1 J/m2/s. To avoid photoreactivation, all operations after UV irradiation were performed in yellow light. Following UV irradiation, cells were labeled with radioisotope and incubated for various times, followed by conversion to spheroplasts. Briefly, after UV irradiation, cells collected by filtration were resuspended in fresh uridine medium at a density of 1 × 108 to 2 × 108 cells per ml. Pulse-labeling was achieved by the addition of 100 μCi of [3H]6′-uracil (20 to 25 Ci/mmol, 1 mCi/ml; Moravek Biochemicals and Radiochemicals, Brea, CA) to 1 ml of cells, followed by vigorous shaking for 15 min at 30°C. Cells were then washed, resuspended in synthetic complete medium containing 1.67 mg of uracil (high-uracil medium)/ml, and incubated for an additional period of 30 min or 6 h. Cells were converted to spheroplasts as described previously (9), and a 0.3-ml aliquot of the spheroplast suspension was layered directly onto a 0.2-ml lysing layer (0.79 M sorbitol, 0.66 M EDTA, 2.5% sarkosyl, 0.3 M NaCl) on top of a 15 to 30% (wt/vol) linear alkaline sucrose gradient made in 0.3 M NaOH, 0.7 M NaCl, 40 mM EDTA, 1% Sarkosyl (pH 12.5). Centrifugation was carried out in an SW41 rotor (Beckman) at 21,000 rpm for 15 h and 30 min at 4°C as described previously (9). Processing of samples was done as described previously (9).
UV sensitivity.
Cells grown to mid-logarithmic phase in yeast extract-peptone-dextrose medium were washed and sonicated to disperse clumps. Following sonication, they were pelleted by centrifugation and suspended in sterile distilled water to a density of 2 × 108 cells per ml. The cell suspensions were diluted, spread onto the surface of yeast extract-peptone-dextrose plates, and irradiated at a dose rate of 0.1 J/m2/s for doses of 10 J/m2 and below or at 1 J/m2/s for doses above 10 J/m2. Plates were incubated in the dark, and colonies were counted after 3 to 5 days.
RESULTS
Defective postreplication repair in the rad51Δ, rad52Δ, and rad54Δ mutants.
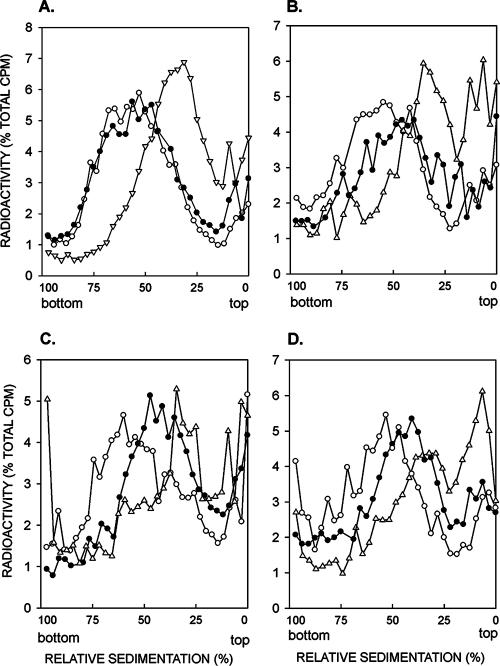
To determine the effects of the rad51Δ, rad52Δ, and rad54Δ mutations on PRR, we examined the sizes of newly synthesized DNA in rad1Δ rad51Δ, rad1Δ rad52Δ, and rad1Δ rad54Δ mutant strains. Because of the absence of nucleotide excision repair in the rad1Δ strain, UV lesions are not removed from DNA and replication of such lesion-containing DNA templates becomes highly dependent upon the various lesion bypass processes. As we have shown previously (9), and the results are presented here for comparison (Fig. 1A), when rad1Δ cells are UV irradiated at 3.5 J/m2 and the size of newly synthesized DNA is examined by pulse-labeling of DNA with [3H]uracil for 15 min, followed by a chase for 30 min, the DNA sediments toward the top of the alkaline sucrose gradient, indicating the presence of discontinuities in the newly synthesized DNA (Fig. 1A). By contrast, in unirradiated rad1Δ cells, the size of newly synthesized DNA following the 15-min pulse and 30-min chase period becomes the same as that in uniformly labeled cells. Incubation of rad1Δ cells for 6 h following the 15-min pulse after UV irradiation results in the formation of normal-size DNA, indicating that postreplicative gap filling processes have restored normal size to DNA synthesized from the UV-damaged templates (Fig. 1A). In the rad1Δ rad51Δ, rad1Δ rad52Δ, and rad1Δ rad54Δ cells, however, we find that normal-size DNA is not reconstituted in cells UV irradiated at 3.5 J/m2 and then given a 15-min pulse followed by a 6-h recovery period, and the efficiency of PRR is reduced to about the same extent in all three mutant strains (Fig. 1B, C, and D).
FIG. 1.
Requirement of RAD51, RAD52, and RAD54 genes for postreplication repair of UV-damaged DNA. Sedimentation in alkaline sucrose gradients of nuclear DNA from cells incubated for different periods following UV irradiation with 3.5 J/m2. The rad1Δ (A), rad1Δ rad51Δ (B), rad1Δ rad52Δ (C), and rad1Δ rad54Δ (D) strains were UV irradiated at 3.5 J/m2 and then pulse-labeled with [3H]uracil for 15 min, followed by a 30-min (Δ) or 6-h (•) chase in high-uracil medium. Synthesis of normal-size DNA from unirradiated cells pulse-labeled with [3H]uracil for 15 min was followed by a 6-h chase (○). The data for the rad1Δ strain in panel A shown here for comparison are taken from reference 9.
Rad5 and Rad52 control alternate pathways of PRR.
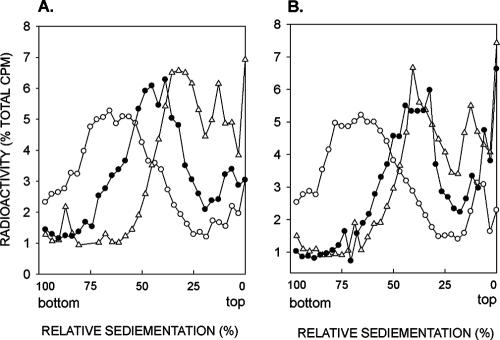
Since an increase in UV sensitivity is observed when deletion mutations of the genes in the RAD52 group are combined with the rad6Δ or rad18Δ mutations, RAD52 and the other genes of this group are presumed to function in PRR via a pathway that operates independently of Rad6-Rad18-mediated lesion bypass pathways. Because Rad6-Rad18-dependent PRR is subsumed via the Rad5-Mms2-Ubc13 action (9, 51), we examined whether the introduction of the rad5Δ mutation enhances the PRR defect of the rad52Δ mutation. As shown in Fig. 2, the rad1Δ rad5Δ rad52Δ strain displays a much higher level of PRR defect than the rad1Δ rad5Δ strain or the rad1Δ rad52Δ strain (compare Fig. 1 and 2). In fact, compared to the intermediate level of repair capacity that remains in the rad1Δ rad5Δ or the rad1Δ rad52Δ strain, there is little, if any, evidence of repair in the rad1Δ rad5Δ rad52Δ strain. From these observations, we infer that PRR in yeast cells is effected via two pathways, a Rad6-Rad18 and Rad5-Mms2-Ubc13-dependent pathway and a Rad52-dependent pathway, and these two pathways function independently of one another for promoting replication through UV lesions.
FIG. 2.
RAD5 and RAD52 control alternate pathways for postreplication repair of UV-damaged DNA. Sedimentation in alkaline sucrose gradients of nuclear DNA from cells incubated for different periods following UV irradiation with 3.5 J/m2. The rad1Δ rad5Δ (A) and rad1Δ rad5Δ rad52Δ (B) strains were UV irradiated at 3.5 J/m2 and then pulse-labeled with [3H]uracil for 15 min, followed by a 30-min (Δ) or 6-h (•) chase in high-uracil medium. Synthesis of normal-size DNA from unirradiated cells pulse-labeled with [3H]uracil for 15 min was followed by a 6-h chase (○). The data for the rad1Δ rad5Δ strain in panel A shown here for comparison are taken from reference 9.
Impaired PRR in rad50Δ and xrs2Δ mutants.
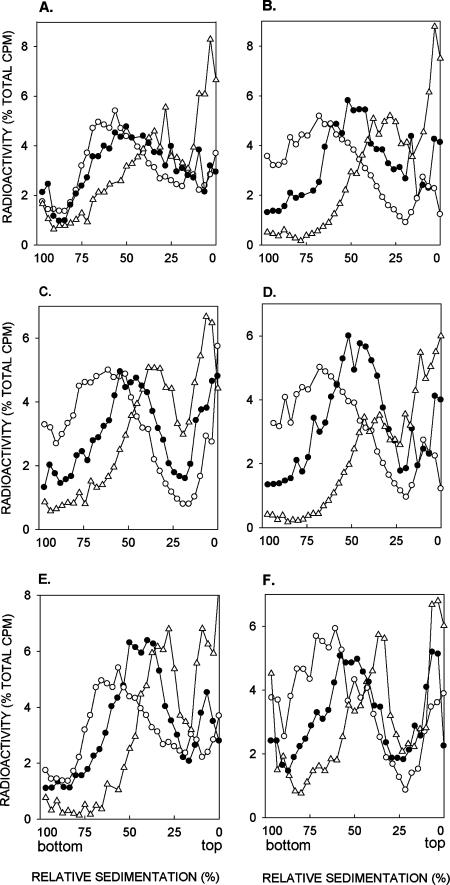
Since the RAD50, MRE11, and XRS2 genes function in the repair of dsb's by homologous recombination that is mediated by the proteins encoded by the RAD52 group genes, we determined whether the MRX complex contributes also to efficient PRR and whether it carries out its role via the Rad52-dependent PRR pathway. To examine this, the rad50Δ and xrs2Δ mutations were combined with the rad1Δ mutation and the sizes of newly synthesized DNA examined in the double mutants following UV irradiation. As shown in Fig. 3A and B, normal-size DNA is not reconstituted in the rad1Δ rad50Δ and rad1Δ xrs2Δ strains that were UV irradiated at 3.5 J/m2 and then given a 15-min pulse followed by a 6-h recovery period. Although the efficiency of PRR is affected in both of the mutant strains, in different experiments we have consistently observed a higher level of PRR defect in the rad1Δ xrs2Δ strain than in the rad1Δ rad50Δ strain (Fig. 3A and B).
FIG. 3.
Involvement of RAD50 and XRS2 in postreplication repair of UV-damaged DNA. Sedimentation in alkaline sucrose gradients of nuclear DNA from cells incubated for different periods following UV irradiation with 3.5 J/m2. The rad1Δ rad50Δ (A), rad1Δ xrs2Δ (B), rad1Δ rad50Δ rad5Δ (C), rad1Δ xrs2Δ rad5Δ (D), rad1Δ rad50Δ rad52Δ (E), and rad1Δ xrs2Δ rad52Δ (F) strains were UV irradiated at 3.5 J/m2 and then pulse-labeled with [3H]uracil for 15 min, followed by a 30-min (Δ) or 6-h (•) chase in high-uracil medium. Synthesis of normal-size DNA from unirradiated cells pulse-labeled with [3H]uracil for 15 min was followed by a 6-h chase (○).
To examine the possibility that Rad50 and Xrs2 function in the Rad52-dependent PRR pathway, we examined the efficiency of PRR in the rad1Δ rad50Δ rad52Δ and rad1Δ xrs2Δ rad52Δ strains. As expected, the PRR deficiency in these strains was no greater than that in the rad1Δ rad52Δ strain, thus suggesting a role for Rad50 and Xrs2 in the Rad52-dependent PRR pathway (compare Fig. 3E and F with Fig. 1C).
To verify that the role of Rad50 and Xrs2 was restricted to the Rad52-dependent pathway and that they did not contribute to Rad5-dependent PRR, we examined the PRR efficiency of the rad1Δ rad50Δ rad5Δ and rad1Δ xrs2Δ rad5Δ mutant strains. However, surprisingly, the PRR defect in these mutant strains was no greater than that observed in the rad1Δ rad5Δ strain (compare Fig. 3C and D with Fig. 2A). These observations lead us to conclude that the role of Rad50 and Xrs2 in PRR is not restricted just to the Rad52-dependent pathway but contributes also to Rad5-dependent PRR.
Epistasis relationship of the rad50Δ and xrs2Δ mutations with the rad5Δ and rad52Δ mutations.
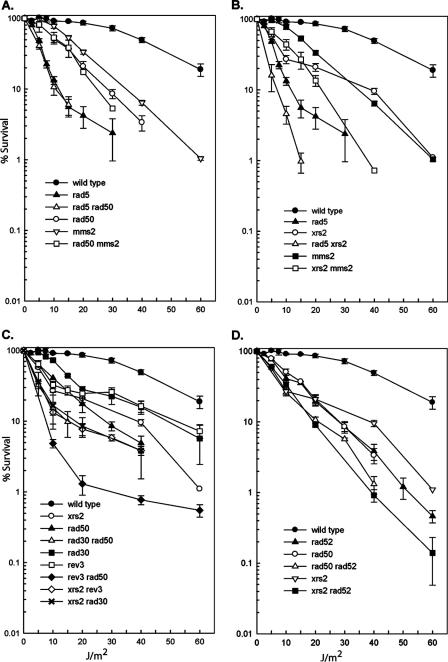
Although the RAD50, MRE11, and XRS2 genes function in the repair of dsb's by homologous recombination in collaboration with the RAD52 group of genes, and mutations in the genes encoding the MRX complex and in the genes belonging to the RAD52 group display epistasis for γ-ray sensitivity, our observation that Rad50 and Xrs2 contribute to PRR mediated by both the Rad5- and Rad52-dependent pathways has necessitated a reexamination of the epistasis relationships among these genes for repairing UV damage. As shown in Fig. 4A, the rad5Δ rad50Δ strain displays the same UV sensitivity as the rad5Δ strain and the UV sensitivity of the mms2Δ rad50Δ strain resembles the UV sensitivity profile of the rad50Δ strain. Thus, an epistatic relationship is indicated among the rad5, mms2, and rad50 mutations. Since the UV sensitivity of the rad50Δ rad52Δ strain is about the same as that of the rad50Δ or rad52Δ strains (Fig. 4D), an epistatic relationship is also indicated between the rad50 and rad52 genes. For the xrs2 mutation, however, we observe increases in the UV sensitivities of the xrs2Δ rad5Δ and xrs2Δ mms2Δ double mutants compared to the UV sensitivity of the more sensitive single-mutant strain (Fig. 4B), and the xrs2Δ rad52Δ double mutant also exhibits a higher level of UV sensitivity than either of the single mutants (Fig. 4D).
FIG. 4.
Epistasis relationships of rad50 and xrs2 with rad5 and rad52. Survival after UV irradiation of wild-type strain EMY74.7 and its isogenic derivatives. Survival curves represent an average for at least three different experiments for each strain. (A) Epistasis of rad50Δ with mms2Δ and with rad5Δ; (B) lack of epistasis of xrs2Δ with rad5Δ and with mms2Δ; (C) increased UV sensitivity of the rad50Δ and xrs2Δ mutations when combined with the rev3Δ or rad30Δ mutations; (D) epistasis of rad50Δ with the rad52Δ mutation and the lack of epistasis between the xrs2Δ and rad52Δ mutations. Error bars represent standard errors.
The epistatic relationship of the rad50Δ mutation with both the rad5Δ and rad52Δ mutations and the somewhat increased UV sensitivity of the xrs2Δ mutation with both the rad5Δ and rad52Δ mutations raised the possibility that as a structural element, the MRX complex not only affects the efficiency of PRR mediated by both the Rad5- and Rad52-dependent pathways but also contributes to TLS mediated by Pols η and ζ. For this reason, we examined the UV sensitivities of the rad50Δ and xrs2Δ mutations in combination with the rad30Δ or the rev3Δ mutation. Such double mutants, however, exhibit higher levels of UV sensitivity than the corresponding single mutants (Fig. 4C), which suggests that Rad50 and Xrs2 do not contribute to TLS by Pols η and ζ.
DISCUSSION
Here, we show that RAD51, RAD52, and RAD54, which are indispensable for mediating dsb repair by homologous recombination, contribute also to the repair of discontinuities that form in the newly synthesized DNA in UV-irradiated yeast cells. The results with the rad52Δ mutation validate the observations that we reported previously for the rad52-1 point mutation (38). Our observation that the PRR of UV-damaged DNA is inhibited to a much greater extent in the rad1Δ rad5Δ rad52Δ mutant than in the rad1Δ rad5Δ or the rad1Δ rad52Δ strain indicates that Rad5 and Rad52 promote PRR via alternate pathways.
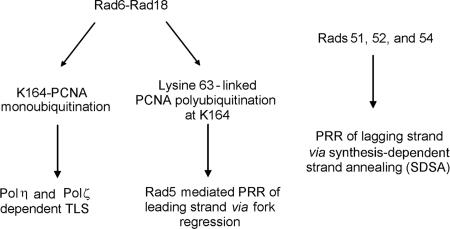
Replication through UV-induced DNA lesions in yeast cells thus is mediated by Rad6-Rad18-dependent processes and by a Rad52-dependent pathway which acts independently of Rad6 and Rad18 (Fig. 5). The existence of alternate PRR pathways raises a number of questions: how do the PRR pathways controlled by Rad6-Rad18 and by the Rad52 group of proteins differ? Do they compete for bypassing DNA lesions, and does the action of each pathway extend to repairing the gaps opposite lesions in both the leading and lagging strand templates, or are the two pathways confined to act specifically on one of the two DNA strands? Although our study yields no definitive answers to these questions, the available data allow us to make certain observations that are discussed below.
FIG. 5.
Rad6-Rad18-dependent and Rad51-, Rad52-, and Rad54-dependent pathways for replication of UV-damaged DNA in yeast. It is proposed (see text for details) that Rad5-mediated PRR is restricted to the leading strand and that Rad proteins 51, 52, and 54, which are also likely to involve the other proteins that function with this group, such as Rad55 and Rad57, promote lesion bypass on the lagging strand. Further, it is suggested that both of the PRR pathways utilize nonrecombinational means that involve fork regression and template switching mediated by Rad5 (5) and the SDSA pathway, in which the Rad51-coated ss nucleoprotein filament formed on the strand with the 3′-OH terminus from the gapped region on the lagging strand invades the duplex on the leading strand side, and this is followed by D-loop formation, synthesis, and reannealing reactions (25, 47).
In the Mms2-Ubc13-Rad5-controlled PRR pathway, the Mms2-Ubc13 complex promotes the polyubiquitination of PCNA, and Rad5 acts as the ubiquitin ligase. In addition, Rad5 has a DNA helicase activity capable of performing replication fork regression (5), a reaction that was envisaged in a model for damage bypass by template switching proposed over 30 years ago (11). The requirement of Rad5, however, is not restricted just to PRR, as it contributes also to TLS (9). By contrast, all the available genetic evidence is consistent with the involvement of the Mms2-Ubc13 complex in PRR only (51). Whereas a combination of mutations in genes that function in competing pathways results in a synergistic enhancement in sensitivity to DNA-damaging agents and in repair deficiency, a combination of mutations in genes that affect different but noncompeting pathways results in an additive increase in sensitivity to DNA-damaging agents and in repair deficiency. Because the increased UV sensitivity of the mms2Δ rad52Δ double mutant over that of the corresponding single mutants approximates an additive rather than a synergistic relationship (51), and because the PRR defect of the rad1Δ rad5Δ rad52Δ strain compared to the PRR defect in the rad1Δ rad5Δ or rad1Δ rad52Δ strain is indicative of an additive relationship (compare Fig. 1 and 2), we suggest that the Rad5- and Rad52-dependent PRR pathways act predominantly in a noncompeting manner.
Genetic experiments in which rad1Δ yeast strains harboring the rad18Δ, rad5Δ, or rad52Δ mutation were transformed with a plasmid that carried a single (6-4) TT photoproduct in each DNA strand and that were 28 bp apart have indicated the requirement of Rad18, Rad5, and Rad52 for replication through this DNA lesion (58). Since a (6-4) TT photoproduct presents a strong block to synthesis by the TLS Pols, TLS makes only a small contribution (∼4%) to replication through this lesion. Whereas Rad18 was responsible for the replication of ∼70% of the plasmids and Rad5 was involved in ∼60% of the replication events, Rad52 accounted for ∼45% of the replication events (58). The nearly equivalent contribution of Rad5 and Rad52 for promoting replication through the (6-4) TT photoproduct suggested in this study is also in accord with the idea that Rad5 and Rad52 lesion bypass pathways do not compete. The inference that Rad5 and Rad52 modulate PRR via noncompeting pathways suggests that their action is confined to one of the two template strands. For reasons that we elaborate upon below, we suggest a role for the Rad5 pathway in promoting replication when the lesion is on the leading strand template and for the Rad52 pathway in promoting replication when the lesion is on the lagging strand template (Fig. 5).
Analyses of replication products from human cell extracts replicating simian virus 40-derived plasmids (49) have indicated that a site-specific T-T dimer when placed in the template of the leading strand of newly synthesized DNA blocks the synthesis on the leading strand, but synthesis on the lagging strand continues. When the T-T dimer is placed in the template for the lagging strand, however, there is little inhibition of replication fork progression and a small gap is left in the lagging strand, which presumably results from the inhibition of Okazaki fragment completion (49). An uncoupling of leading strand synthesis from lagging strand synthesis similar to that observed in human cells has also been suggested to occur in UV-irradiated yeast cells when the lesion is on the leading strand template (29). Overall, the various studies with mammalian and yeast cells (6, 7, 29, 49) have pointed to the existence of fundamental differences in the effects a lesion has on replication fork progression when it is located on the leading versus the lagging strand template.
The Rad5 and Rad52 PRR pathways differ in that the former pathway has the requirement for PCNA polyubiquitination whereas the latter pathway requires no such PCNA modification. This raises the possibility that when the lesion is on the leading strand template, PCNA polyubiquitination modulates the uncoupling of leading strand synthesis from lagging strand synthesis. In that case, the Rad5 pathway could act primarily when leading strand synthesis is blocked. The role of the Rad52 PRR pathway would then be relegated to the lagging strand.
The various genetic and biochemical observations with Rad5 have suggested a role for Rad5 in mediating error-free lesion bypass by transient template switching, wherein its DNA helicase activity, which is highly specialized for replication fork regression, could promote template switching and a copy choice type of DNA synthesis, in which the lesion on the leading strand is bypassed by template switching using the newly synthesized lagging strand as the template that would be formed upon fork regression (5). Although the proteins encoded by the RAD52 group of genes are required for recombinational repair of dsb's, we consider it highly probable that the gap left opposite the DNA lesion on the lagging strand is filled in by the action of the Rad52 group proteins via a nonrecombinational pathway like that depicted in the synthesis-dependent strand annealing (SDSA) model, wherein the Rad51-coated ss nucleoprotein filament invades the DNA duplex on the leading strand side and the gap on the lagging strand is then filled in by DNA synthesis using the newly synthesized leading strand as the template (see references 25 and 47 for details of the SDSA model). The reason for suggesting a nonrecombinational mode of PRR for the Rad52-dependent pathway is that recombination is normally suppressed in yeast cells and it is only in the absence of Srs2 DNA helicase (24, 40, 56) or in the absence of Siz1-mediated PCNA sumoylation (10, 35, 37) that the recombinational repair pathway becomes activated.
The involvement of Rad50 and Xrs2 in PRR and our observation that the PRR defect of rad1Δ rad50Δ or rad1Δ xrs2Δ mutants is not increased in the presence of rad5Δ or rad52Δ mutations might suggest that the MRX complex contributes to both the Rad5- and Rad52-dependent PRR pathways, and it raises the possibility that the MRX complex plays a structural role in aligning the sister chromatids into close proximity, and thereby, this complex contributes to both of these pathways.
Acknowledgments
This work was supported by National Institutes of Health grant CA107650.
Footnotes
Published ahead of print on 4 September 2007.
REFERENCES
- 1.Amitani, I., R. J. Baskin, and S. C. Kowalczykowski. 2006. Visualization of Rad52, a chromatin remodeling protein, translocating on single DNA molecules. Mol. Cell 23: 143-148. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, D. E., K. M. Trujillo, P. Sung, and H. P. Erickson. 2001. Structure of the Rad50-Mre11 DNA repair complex from Saccharomyces cerevisiae by electron microscopy. J. Biol. Chem. 276: 37027-37033. [DOI] [PubMed] [Google Scholar]
- 3.Bailly, V., J. Lamb, P. Sung, S. Prakash, and L. Prakash. 1994. Specific complex formation between yeast RAD6 and RAD18 proteins: a potential mechanism for targeting RAD6 ubiquitin-conjugating activity to DNA damage sites. Genes Dev. 8: 811-820. [DOI] [PubMed] [Google Scholar]
- 4.Bailly, V., S. Lauder, S. Prakash, and L. Prakash. 1997. Yeast DNA repair proteins Rad6 and Rad18 form a heterodimer that has ubiquitin conjugating, DNA binding, and ATP hydrolytic activities. J. Biol. Chem. 272: 23360-23365. [DOI] [PubMed] [Google Scholar]
- 5.Blastyak, A., L. Pinter, I. Unk, L. Prakash, S. Prakash, and L. Haracksa. Yeast Rad5 protein required for postreplication repair has a DNA helicase activity specific for replication fork regression. Mol. Cell, in press. [DOI] [PMC free article] [PubMed]
- 6.Cordeiro-Stone, M., A. M. Makhov, L. S. Zaritskaya, and J. D. Griffith. 1999. Analysis of DNA replication forks encountering a pyrmidine dimer in the template to the leading strand. J. Mol. Biol. 289: 1207-1218. [DOI] [PubMed] [Google Scholar]
- 7.Cordeiro-Stone, M., L. S. Zaritskaya, L. K. Price, and W. K. Kaufmann. 1997. Replication fork bypass of a pyrimidine dimer blocking leading strand DNA synthesis. J. Biol. Chem. 272: 13945-13954. [DOI] [PubMed] [Google Scholar]
- 8.de Jager, M., J. van Noort, D. C. van Gent, C. Dekker, R. Kanaar, and C. Wyman. 2001. Human Rad50/Mre11 is a flexible complex that can tether DNA ends. Mol. Cell 8: 1129-1135. [DOI] [PubMed] [Google Scholar]
- 9.Gangavarapu, V., L. Haracska, I. Unk, R. E. Johnson, S. Prakash, and L. Prakash. 2006. Mms2-Ubc13-dependent and -independent roles of Rad5 ubiquitin ligase in postreplication repair and translesion DNA synthesis in Saccharomyces cerevisiae. Mol. Cell. Biol. 26: 7783-7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haracska, L., C. A. Torres-Ramos, R. E. Johnson, S. Prakash, and L. Prakash. 2004. Opposing effects of ubiquitin conjugation and SUMO modification of PCNA on replicational bypass of DNA lesions in Saccharomyces cerevisiae. Mol. Cell. Biol. 24: 4267-4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins, N. P., K. Kato, and B. Strauss. 1976. A model for replication repair in mammalian cells. J. Mol. Biol. 101: 417-425. [DOI] [PubMed] [Google Scholar]
- 12.Hoege, C., B. Pfander, G.-L. Moldovan, G. Pyrowolakis, and S. Jentsch. 2002. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature 419: 135-141. [DOI] [PubMed] [Google Scholar]
- 13.Hofmann, R. M., and C. M. Pickart. 1999. Noncanonical MMS2-encoded ubiquitin-conjugating enzyme functions in assembly of novel polyubiquitin chains for DNA repair. Cell 96: 645-653. [DOI] [PubMed] [Google Scholar]
- 14.Hopfner, K. P., L. Craig, G. Moncalian, R. A. Zinkel, T. Usui, B. A. K. Owen, A. B. Henderson, J. L. Bodmer, C. T. McMurray, J. P. Carney, J. H. J. Petrini, and J. A. Tainer. 2002. The Rad50 zinc-hook is a structure joining Mre11 complexes in DNA recombination and repair. Nature 418: 562-566. [DOI] [PubMed] [Google Scholar]
- 15.Hopfner, K. P., A. Karcher, L. Craig, T. T. Woo, J. P. Carney, and J. A. Tainer. 2001. Structural biochemistry and interaction architecture of the DNA double-strand break repair Mre11 nuclease and Rad50-ATPase. Cell 105: 473-485. [DOI] [PubMed] [Google Scholar]
- 16.Hopfner, K. P., C. D. Putnam, and J. A. Tainer. 2002. DNA double-strand break repair from head to tail. Cur. Opin. Struct. Biol. 12: 115-122. [DOI] [PubMed] [Google Scholar]
- 17.Johnson, R. E., L. Haracska, S. Prakash, and L. Prakash. 2001. Role of DNA polymerase η in the bypass of a (6-4) TT photoproduct. Mol. Cell. Biol. 21: 3558-3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson, R. E., S. T. Henderson, T. D. Petes, S. Prakash, M. Bankmann, and L. Prakash. 1992. Saccharomyces cerevisiae RAD5-encoded DNA repair protein contains DNA helicase and zinc-binding sequence motifs and affects the stability of simple repetitive sequences in the genome. Mol. Cell. Biol. 12: 3807-3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson, R. E., C. M. Kondratick, S. Prakash, and L. Prakash. 1999. hRAD30 mutations in the variant form of xeroderma pigmentosum. Science 285: 263-265. [DOI] [PubMed] [Google Scholar]
- 20.Johnson, R. E., S. Prakash, and L. Prakash. 1999. Efficient bypass of a thymine-thymine dimer by yeast DNA polymerase, Polη. Science 283: 1001-1004. [DOI] [PubMed] [Google Scholar]
- 21.Johnson, R. E., S. Prakash, and L. Prakash. 1999. Requirement of DNA polymerase activity of yeast Rad30 protein for its biological function. J. Biol. Chem. 274: 15975-15977. [DOI] [PubMed] [Google Scholar]
- 22.Johnson, R. E., S. Prakash, and L. Prakash. 1994. Yeast DNA repair protein RAD5 that promotes instability of simple repetitive sequences is a DNA-dependent ATPase. J. Biol. Chem. 269: 28259-28262. [PubMed] [Google Scholar]
- 23.Johnson, R. E., M. T. Washington, L. Haracska, S. Prakash, and L. Prakash. 2000. Eukaryotic polymerases ι and ζ act sequentially to bypass DNA lesions. Nature 406: 1015-1019. [DOI] [PubMed] [Google Scholar]
- 24.Krejci, L., S. Van Komen, Y. Li, J. Villemain, M. S. Reddy, H. Klein, T. Ellenberger, and P. Sung. 2003. DNA helicase Srs2 disrupts the Rad51 presynaptic filament. Nature 423: 305-309. [DOI] [PubMed] [Google Scholar]
- 25.Krogh, B. O., and L. S. Symington. 2004. Recombination proteins in yeast. Annu. Rev. Genet. 38: 233-271. [DOI] [PubMed] [Google Scholar]
- 26.Lawrence, C. W., and R. B. Christensen. 1979. Ultraviolet-induced reversion of cyc1 alleles in radiation-sensitive strains of yeast. III. rev3 mutant strains. Genetics 92: 397-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawrence, C. W., P. E. Nisson, and R. B. Christensen. 1985. UV and chemical mutagenesis in rev7 mutants of yeast. Mol. Gen. Genet. 200: 86-91. [DOI] [PubMed] [Google Scholar]
- 28.Lawrence, C. W., T. O'Brien, and J. Bond. 1984. UV-induced reversion of his4 frameshift mutations in rad6, rev1, and rev3 mutants of yeast. Mol. Gen. Genet. 195: 487-490. [DOI] [PubMed] [Google Scholar]
- 29.Lopes, M., M. Foiani, and J. M. Sogo. 2006. Multiple mechanisms control chromosome integrity after replication fork uncoupling and restart at irreparable UV lesions. Mol. Cell 21: 15-27. [DOI] [PubMed] [Google Scholar]
- 30.Masutani, C., R. Kusumoto, A. Yamada, N. Dohmae, M. Yokoi, M. Yuasa, M. Araki, S. Iwai, K. Takio, and F. Hanaoka. 1999. The XPV (xeroderma pigmentosum variant) gene encodes human DNA polymerase η. Nature 399: 700-704. [DOI] [PubMed] [Google Scholar]
- 31.Mazin, A. V., C. J. Bomarth, J. A. Solinger, W. D. Heyer, and S. C. Kowalczykowski. 2000. Rad54 protein is targeted to pairing loci by the Rad51 nucleoprotein filament. Mol. Cell 6: 583-592. [DOI] [PubMed] [Google Scholar]
- 32.McDonald, J. P., A. S. Levine, and R. Woodgate. 1997. The Saccharomyces cerevisiae RAD30 gene, a homologue of Escherichia coli dinB and umuC, is DNA damage inducible and functions in a novel error-free postreplication repair mechanism. Genetics 147: 1557-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson, J. R., C. W. Lawrence, and D. C. Hinkle. 1996. Thymine-thymine dimer bypass by yeast DNA polymerase ζ. Science 272: 1646-1649. [DOI] [PubMed] [Google Scholar]
- 34.Ogawa, T., X. Yu, A. Shinohara, and E. H. Egelman. 1993. Similarity of the yeast RAD51 filament to the bacterial RecA filament. Science 259: 1896-1899. [DOI] [PubMed] [Google Scholar]
- 35.Papouli, E., S. Chen, A. A. Davies, D. Huttner, L. Krejci, P. Sung, and H. D. Ulrich. 2005. Crosstalk between SUMO and ubiquitin on PCNA is mediated by recruitment of the helicase Srs2p. Mol. Cell 19: 123-133. [DOI] [PubMed] [Google Scholar]
- 36.Petukhova, G., S. Stratton, and P. Sung. 1998. Catalysis of homologous DNA pairing by yeast Rad51 and Rad54 proteins. Nature 393: 91-94. [DOI] [PubMed] [Google Scholar]
- 37.Pfander, B., G.-L. Moldovan, M. Sacher, C. Hoege, and S. Jentsch. 2005. SUMO-modified PCNA recruits Srs2 to prevent recombination during S phase. Nature 436: 428-433. [DOI] [PubMed] [Google Scholar]
- 38.Prakash, L. 1981. Characterization of postreplication repair in Saccharomyces cerevisiae and effects of rad6, rad18, rev3 and rad52 mutations. Mol. Gen. Genet. 184: 471-478. [DOI] [PubMed] [Google Scholar]
- 39.Prakash, S., R. E. Johnson, and L. Prakash. 2005. Eukaryotic translesion synthesis DNA polymerases: specificity of structure and function. Annu. Rev. Biochem. 74: 317-353. [DOI] [PubMed] [Google Scholar]
- 40.Schiestl, R. H., S. Prakash, and L. Prakash. 1990. The SRS2 suppressor of rad6 mutations of Saccharomyces cerevisiae acts by channeling DNA lesions into the RAD52 DNA repair pathway. Genetics 124: 817-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sigurdsson, S., S. Van Komen, G. Petukhova, and P. Sung. 2002. Homologus DNA pairing by human recombination factors Rad51 and Rad54. J. Biol. Chem. 277: 42790-42794. [DOI] [PubMed] [Google Scholar]
- 42.Song, B., and P. Sung. 2000. Functional interactions among yeast Rad51 recombinase, Rad52 mediator, and replication protein A in DNA strand exchange. J. Biol. Chem. 275: 15895-15904. [DOI] [PubMed] [Google Scholar]
- 43.Stary, A., P. Kannouche, A. R. Lehmann, and A. Sarasin. 2003. Role of DNA polymerase η in the UV mutation spectrum in human cells. J. Biol. Chem. 278: 18767-18775. [DOI] [PubMed] [Google Scholar]
- 44.Stelter, P., and H. D. Ulrich. 2003. Control of spontaneous and damage-induced mutagenesis by SUMO and ubiquitin conjugation. Nature 425: 188-191. [DOI] [PubMed] [Google Scholar]
- 45.Sugiyama, T., and S. C. Kowalczykowski. 2002. Rad52 protein associates with replication protein A (RPA)-single-stranded DNA to accelerate Rad51-mediated displacement of RPA and presynaptic complex formation. J. Biol. Chem. 277: 31663-31672. [DOI] [PubMed] [Google Scholar]
- 46.Sung, P. 1994. Catalysis of ATP-dependent homologous DNA pairing and strand exchange by yeast RAD51 protein. Science 265: 1241-1243. [DOI] [PubMed] [Google Scholar]
- 47.Sung, P., and H. Klein. 2006. Mechanism of homologous recombination: mediators and helicases take on regulatory functions. Nat. Rev. Mol. Cell Biol. 7: 739-750. [DOI] [PubMed] [Google Scholar]
- 48.Sung, P., and D. L. Robberson. 1995. DNA strand exchange mediated by a RAD51-ssDNA nucleoprotein filament with polarity opposite to that of RecA. Cell 82: 453-461. [DOI] [PubMed] [Google Scholar]
- 49.Svoboda, D. L., and J.-M. H. Vos. 1995. Differential replication of a single, UV-induced lesion in the leading or lagging strand by a human cell extract: fork uncoupling or gap formation. Proc. Natl. Acad. Sci. USA 92: 11975-11979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Symington, L. S. 2002. Role of RAD52 epistasis group genes in homologous recombination and double-strand break repair. Microbiol. Mol. Biol. Rev. 66: 630-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torres-Ramos, C., S. Prakash, and L. Prakash. 2002. Requirement of RAD5 and MMS2 for postreplication repair of UV-damaged DNA in Saccharomyces cerevisiae. Mol. Cell. Biol. 22: 2419-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trujillo, K., and P. Sung. 2001. DNA structure-specific nuclease activities in the Saccharomyces cerevisiae Rad50/Mre11 complex. J. Biol. Chem. 276: 35458-35464. [DOI] [PubMed] [Google Scholar]
- 53.Trujillo, K. M., D. H. Roh, L. Chen, S. van Komen, A. E. Tomkinson, and P. Sung. 2003. Yeast Xrs2 binds DNA and helps target Rad50 and Mre11 to DNA ends. J. Biol. Chem. 278: 48957-48964. [DOI] [PubMed] [Google Scholar]
- 54.Ulrich, H. D., and S. Jentsch. 2000. Two RING finger proteins mediate cooperation between ubiquitin-conjugating enzymes in DNA repair. EMBO J. 19: 3388-3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Usui, T., T. Ohta, H. Oshiumi, J.-I. Tomizawa, H. Ogawa, and T. Ogawa. 1998. Complex formation and functional versatility of Mre11 of budding yeast in recombination. Cell 95: 705-716. [DOI] [PubMed] [Google Scholar]
- 56.Veaute, X., J. Jeusset, C. Soustelle, S. C. Kowalczykowski, E. Le Cam, and F. Fabre. 2003. The Srs2 helicase prevents recombination by disrupting Rad51 nucleoprotein filaments. Nature 423: 309-312. [DOI] [PubMed] [Google Scholar]
- 57.Yu, S.-L., R. E. Johnson, S. Prakash, and L. Prakash. 2001. Requirement of DNA polymerase η for error-free bypass of UV-induced CC and TC photoproducts. Mol. Cell. Biol. 21: 185-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang, H., and C. W. Lawrence. 2005. The error-free component of the RAD6/RAD18 DNA damage tolerance pathway of budding yeast employs sister-strand recombination. Proc. Natl. Acad. Sci. USA 102: 15954-15959. [DOI] [PMC free article] [PubMed] [Google Scholar]