Abstract
Skp2B, an F-box protein of unknown function, is frequently overexpressed in breast cancer. In order to determine the function of Skp2B and whether it has a role in breast cancer, we performed a two-hybrid screen and established transgenic mice expressing Skp2B in the mammary glands. We found that Skp2B interacts with the repressor of estrogen receptor activity (REA) and that overexpression of Skp2B leads to a reduction in REA levels. In the mammary glands of MMTV-Skp2B mice, REA levels are also low. Our results show that in virgin transgenic females, Skp2B induces lobuloalveolar development and differentiation of the mammary glands normally observed during pregnancy. As this phenotype is identical to what was observed for REA heterozygote mice, our observations suggest that the Skp2B-REA interaction is physiologically relevant. However, in contrast to REA+/− mice, MMTV-Skp2B mice develop mammary tumors, suggesting that Skp2B affects additional proteins. These results indicate that the observed expression of Skp2B in breast cancer does contribute to tumorigenesis at least in part by modulating the activity of the estrogen receptor.
Skp2 is an F-box protein that associates with the SCF complex designated the SCFSKP2 complex, which is involved in the ubiquitination of the cyclin-dependent kinase inhibitor p27 (5, 20). We previously reported the existence of a splice variant of Skp2 that we named Skp2B (7), and we will therefore refer to them herein as Skp2A and Skp2B, respectively. The C-terminal domain of Skp2A is encoded by exon 10 and is important for the stabilization of its interaction with the SCF complex (18) and binding to p27 (9). Exon 10 is however absent from Skp2B and replaced by exon 11, resulting in a different C-terminal domain (7). As a consequence, Skp2A and Skp2B have distinct substrate specificities, and Skp2B does not affect p27 (7, 17). Skp2B is frequently overexpressed in breast cancer (17); however, its function remains unknown.
The development of the mammary gland is dependent on the action of estrogen and progesterone (11). The majority of the development of the mammary gland takes place after birth (12). The mammary gland of newborn mice is composed of a rudimentary ductal tree connected to the nipple but otherwise is a duct-free fat pad. At puberty (∼3 weeks of age), ovarian hormones provoke ductal elongation characterized by the presence of specialized structures termed terminal end buds (TEBs) at the tips of the growing ducts (12). Once the growing ducts reach the end of the fat pad, the TEBs disappear. As the ducts extend, they also become progressively branched. The mature mammary gland remains dormant until the hormonal stimulus of pregnancy provokes lobuloalveolar development. After weaning, the alveolar epithelium undergoes a massive wave of apoptosis, provoking the involution of the mammary glands, which resume to an appearance similar to that of the pubertal ductal tree of a virgin animal.
The mammary glands of adult estrogen receptor (ER) knockout mice fail to undergo development and resemble those of newborn mice (6). The best-characterized function of the ER is as a transcriptional factor, which is highly regulated by binding to coactivators and corepressors. Among the corepressors, the repressor of ER activity (REA) was recently shown to have a profound effect on the development of the mammary gland (14, 15). REA heterozygote mice display accelerated invasion of the fat pad and development of the mammary gland during pregnancy, which is associated with an increase in the ER activity (15).
Here, we show that Skp2B interacts with REA and that Skp2B overexpression results in a decrease in REA levels. Further, we show that the mammary glands of MMTV-Skp2B transgenic mice display a phenotype closely related to that observed for REA heterozygote mice. Our data suggest that Skp2B represents a novel regulator of the ER and that Skp2B overexpression in primary breast cancer may play a significant role in breast cancer.
MATERIALS AND METHODS
Immunoprecipitation and immunoblot analysis.
Western blots and immunoprecipitations were performed as described previously (17), and membranes (PerkinElmer Life Sciences) were probed with the following antibodies: rabbit anti-REA (Upstate), mouse anti-FLAG (Sigma), mouse anti-Skp2 (Zymed,), mouse antitubulin antibody 12G10 (University of Iowa), mouse monoclonal anti-Myc antibody 9E10 to detect Myc-ubiquitin, and anti-mouse phospho-STAT5 (Upstate) along with rabbit anti-STAT5 (Upstate) and rabbit anti-IGFBP-2 and -4 (Upstate). Immunoblots were developed by ECL (Amersham Pharmacia Biotech).
Transfection and siRNA against Skp2B.
All transfections were performed using the Mirus reagents according to the manufacturer' instructions. For the inhibition of Skp2B expression, the sequence ACTATTAGTTGACAAAGAGCTGG, derived from the C-terminal domain of Skp2B, was annealed to its complementary sequence and cloned into the pSUPER-RNA1 system (VEC-PBS 0001/0002; Oligoengine) according to the manufacturer's instructions. The second small interfering RNA (siRNA) was derived from the sequence GGAUGCCCUCAAACAUACATT of the C-terminal domain of Skp2B and annealed to the reverse sequence UGUAUGUUUGAGGGCAUCCGA. The Skp2B-pSUPER plasmid was transfected using Mirus reagent, and 24 h following transfection, the medium was changed and the second siRNA transfected using RNAiFect reagent (QIAGEN) according to the manufacturer's instructions.
Ubiquitin ligation assay.
HEK293T cells were transfected with hemagglutinin (HA)-tagged Roc1 and Cul1 overexpression plasmids alone (control) or together with a FLAG-tagged Skp2A (kindly provided by M. Pagano, NYU Medical Center Department of Experimental Pathology, New York, NY) or Skp2B overexpression plasmid. All transfections were performed using the FuGENE 6 system as described by the manufacturer (Boehringer Mannheim). Total protein lysates were prepared as described below, ultracentrifuged for 1 h at 37,000 rpm (L8-60 M Beckman ultracentrifuge, rotor SW80.1), and adjusted to 1 μg/μl. Overnight immunoprecipitation using M2 beads (Sigma) was performed using 300 and 1,800 μg control lysate, 300 μg FLAG-Skp2A-containing lysate, and 300, 900, and 1,800 μg FLAG-Skp2B-containing lysate. Beads were washed three times with lysate buffer containing 500 mM NaCl followed by two washes with low-salt buffer (25 mM Tris [pH 7.5], 1 mM EDTA, 10% glycerol, 0.01% NP-40, 100 mM NaCl, 1 mM dithiothreitol). Beads were then incubated under vigorous agitation in a total volume of 30 μl for 60 min at 37°C with 50 mM Tris (pH 7.4), 5 mM MgCl2, 0.6 mM dithiothreitol, 2 μM ATP, 300 pM 32P-labeled ubiquitin, 2 mM NaF, 10 nM okadaic acid, 2 pM ubiquitin-activating enzyme E1 and with or without 10 pM ubiquitin-conjugating enzyme E2. The enzymes E1 and E2 were prepared as described previously (21). One half of the samples were loaded onto a 4 to 20% gel (161-1105; Bio-Rad), and radioactive poly- and monoubiquitin were visualized by autoradiography. The other half of the samples were used for Western blotting using polyclonal anti-Skp2 antibody (Zymed) at a dilution of 1:1,000.
Two-hybrid screen.
The Skp2B C-terminal domain was cloned in the pACT2 plasmid and resulted in a fusion protein between the C-terminal domain of Skp2B and the activation domain of the GAL4 transcription factor. The screen was performed using Matchmaker two-hybrid system 2 according to the manufacturer's protocol (Clontech).
Luciferase reporter assay.
To measure the activity of the ER, MCF-7 and MCF-Skp2B cells were transfected with a plasmid where the ER-responsive element (ERE) is driving the expression of the firefly luciferase reporter and a plasmid constitutively expressing the Renilla luciferase. Twenty-four hours after transfection, the two luciferase activities were measured using a dual luciferase reporter assay system (Promega) according to the manufacturer's instructions. To calculate the relative ERE luciferase activity, the ratio of the ERE luciferase and Renilla luciferase activities was determined. All readings were performed in triplicate and in three separate experiments.
Generation of Skp2B transgenic mice.
The cDNA of human Skp2B was cloned into the EcoRI site of an expression plasmid containing the mouse mammary tumor virus long terminal repeat plus simian virus 40 intron and polyadenylation signals (13). The linearized MMTV-Skp2B plasmids were microinjected into FVB/n fertilized mouse oocytes, and transgenic founder mice were identified by Southern analysis of EcoRI digestion of tail genomic DNA.
RNA extraction and RT-PCR.
RNA was extracted from tissue powder by use of an RNeasy mini kit including RNase-free DNase (QIAGEN). One hundred nanograms of each sample was used in triplicate in a quantitative reverse transcription-PCR (RT-PCR) using a Quantitect SYBR green RT-PCR kit following the manufacturer's protocol (QIAGEN Science, MD). The Skp2B primers used have been described previously (17).
Immunohistochemistry and immunofluorescence.
Mammary epithelium was isolated from MMTV-Skp2B or nontransgenic littermates, fixed in 10% buffered formalin (Sigma-Aldrich), dehydrated, embedded in paraffin, and sectioned at 3.5 μm. For histology, sections were stained with hematoxylin and eosin (H&E). For immunohistochemical staining of Skp2B, tissue sections were treated as described previously (16). Immunofluorescence was also performed as described previously (17).
Whole-mount analysis.
Mammary epithelium was isolated from MMTV-Skp2B and nontransgenic littermates, spread onto glass slides, and fixed in Carnoy's fixative (6 parts 100% ethanol, 3 parts chloroform, 1 part glacial acetic acid) overnight. The glands were then transferred into 70% ethanol for 15 min at room temperature and gradually into sterile Milli-Q water before incubation in carmine aluminum stain overnight. Glands were stored in 70% ethanol in the dark at room temperature.
RESULTS
REA is ubiquitinated and binds to Skp2B.
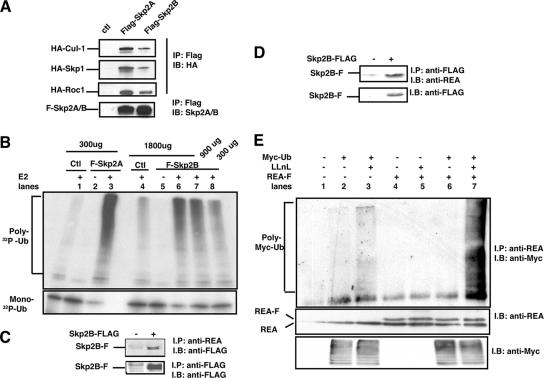
As Skp2A associates with an ubiquitin ligase composed of cul-1, skp1, and roc-1, we first tested whether Skp2B can also associate with these proteins. Following transfection in HEK293T cells, we found that Skp2B did coimmunoprecipitate with cul1, skp1, and roc-1, although less strongly than did Skp2A (Fig. 1A). We then assessed the ubiquitin ligase activity associated with Skp2B, again using Skp2A as a positive control. HEK293T cells were transfected with cul-1 and either Flag-tagged Skp2A or Flag-tagged Skp2B. After 48 h, the SCFSkp2A or SCFSkp2B complex was immunoprecipitated using anti-Flag antibody, and the resulting beads were used in an in vitro ubiquitination assay where 32P-labeled ubiquitin and recombinant ubiquitin-conjugating enzyme (E2) are added to the reaction. As expected, in the absence of Skp2A or Skp2B (Fig. 1B, lanes 1 and 4) and in the absence of E2 (Fig. 1B, lanes 2 and 5), no 32P-polyubiquitin chain formation was detected. In the presence of Skp2A and E2, however (lane 3), the formation of 32P-polyubiquitin chain was detected and correlated to a decrease in 32P-monoubiquitin (Fig. 1B, bottom), therefore confirming the ubiquitin ligase activity of the SCFSkp2A complex. Such activity was also associated with the SCFSkp2B complex (Fig. 1B, lanes 6 to 8); however, using 300 μg of proteins for the immunoprecipitation of the SCF complex (lane 8) led to an ubiquitin ligase activity that was ∼10-fold weaker than that observed with Skp2A (lane 3). The detection of the ubiquitin ligase activity increased with increasing amounts of proteins used for the immunoprecipitation (Fig. 1B, lanes 6 and 7). We therefore conclude that SCFSkp2B is also associated with an ubiquitin ligase activity, although its activity is weaker than that of SCFSkp2A.
FIG. 1.
Skp2B interacts with REA. (A) HEK293T cells were transfected with the indicated plasmids, FLAG-Skp2A and FLAG-Skp2B were immunoprecipitated using FLAG antibody, and their association with either HA-Cul-1, HA-Skp1, or HA-Roc1 was analyzed using anti-HA antibody. ctl, control. (B) HEK293T cells were transfected with either FLAG-Skp2A or FLAG-Skp2B along with Cul-1 and Roc1 followed by anti-FLAG immunoprecipitation. The resulting immunoprecipitations were then used in an in vitro ubiquitination assay using 32P-labeled ubiquitin (32P-Ub). Top, polyubiquitin chain formation; bottom, level of monoubiquitin. Extracts from HEK293T cells transfected with Cul-1 and Roc1 alone were used as controls. (C) Coimmunoprecipitation of endogenous REA with FLAG-Skp2B, where REA was immunoprecipitated (IP) and levels of FLAG-Skp2B were determined by immunoblotting (IB). (D) Coimmunoprecipitation of endogenous REA with FLAG-Skp2B, where FLAG-Skp2B was immunoprecipitated and levels of REA were determined by immunoblotting. (E) HEK293T cells were transfected with the indicated plasmids, and REA was immunoprecipitated followed by immunoblotting using anti-Myc antibody to detect ubiquitinated proteins.
In order to identify potential substrates of Skp2B, we performed a two-hybrid screen using the C-terminal domain that is unique to Skp2B and is not present in Skp2A as a bait. This screen identified REA as an interacting partner of Skp2B, and the two-hybrid interaction was confirmed by coimmunoprecipitation (Fig. 1C and D). The interaction between REA and Skp2B raised the possibility that REA is a substrate for ubiquitination. To test this possibility, HEK293T cells were transfected with Myc-tagged ubiquitin and REA, and the presence of polyubiquitinated REA was tested by immunoprecipitation in the presence or absence of the proteasome inhibitor LLnL. We found that in the presence of the proteasome inhibitor LLnL, polyubiquitination of endogenous REA was detectable (Fig. 1E, lane 3), and transfection of REA increased the detection of polyubiquitinated REA (Fig. 1E, lane 7). Therefore, these results identify REA as a potential substrate for degradation by an Skp2B-associated ubiquitin ligase.
Skp2B reduces REA levels in MCF-7 cells and stimulates ER transcriptional activity.
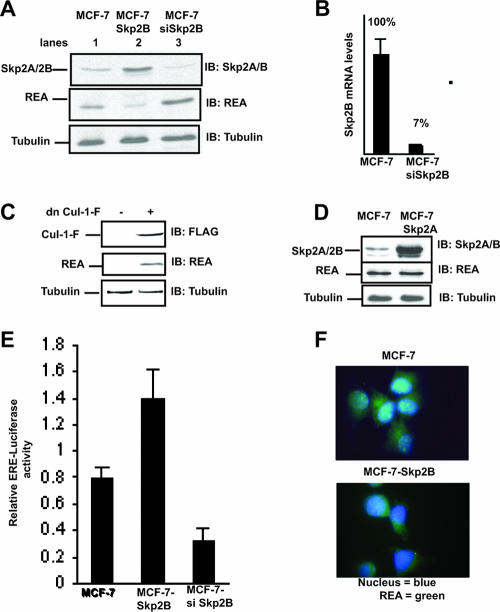
Since REA is a repressor of the ER, we then established stable clones overexpressing Skp2B in the ER-positive breast cancer cell line MCF-7. The resulting increase in Skp2B levels (Fig. 2A, lane 2, top) was associated with a decrease in REA levels (Fig. 2A, lane 2, middle). As we previously reported that Skp2B is higher in MCF-7 cells than in the nontumorigenic breast cell line 184B5 (17), we also established siRNA against Skp2B to determine the consequence of Skp2B inhibition on REA levels. By use of a combination of two siRNA sequences mapping to the unique C-terminal domain of Skp2B, Skp2B expression was inhibited by 93% (Fig. 2B), and such inhibition resulted in an elevation in REA levels (Fig. 2A, lane 2). We further tested the effect of the SCFSkp2B complex on REA levels by use of dominant negative cul-1. In MCF-Skp2B cells, inhibition of cul-1 by transfection of dominant negative cul-1 rescued REA levels (Fig. 2C), further supporting the role of this complex in REA degradation. As a control, we also established a cell line overexpressing Skp2A in MCF-7 cells. While Skp2A was expressed at much higher levels (Fig. 2D, top) than Skp2B (Fig. 2A, top) as we expected, since Skp2A is more stable than Skp2B (17), the overexpression of Skp2A nevertheless failed to affect REA levels (Fig. 2D, middle). This result is consistent with the fact that REA was isolated using the C-terminal domain that is unique to Skp2B as the bait in the two-hybrid screen.
FIG. 2.
Skp2B but not Skp2A overexpression leads to a reduction in REA and activation of the ER. (A) Western analysis of the levels of REA and Skp2B in MCF-7 cells, MCF-7-Skp2B cells, and MCF-7 cells transfected with siRNA against Skp2B (siSkp2B). (B) The reduction of Skp2B mRNA following the transfection of siRNA against SKP2B was determined by quantitative RT-PCR. (C) MCF-Skp2B cells were transfected with FLAG-tagged dominant negative cul-1 (dn Cul-1-F), and the levels of dn-Cul-1-F and REA were determined by immunoblotting (IB). (D) Immunoblot analysis of the levels of REA and Skp2A in MCF-7 and MCF7-Skp2A cells. (E) ER activity was monitored using an ERE luciferase reporter transfected in MCF-7, MCF-7-Skp2B, and MCF-7 cells cotransfected with siRNA Skp2B. (F) Immunofluorescence of REA in MCF-7 and MCF-7-Skp2B cells.
As REA represses the transcriptional activity of the ER (14), the reduction in REA levels following Skp2B overexpression is predicted to lead to an increase in the activity of the ER. To test this possibility, MCF-7 and MCF-Skp2B cells were transfected with a plasmid in which the expression of the firefly luciferase reporter is under the control of an ERE and a constitutive Renilla luciferase reporter, which acts as an internal control for transfection efficiency. The luciferase activities of both reporters were measured, and the ratio between the two activities was set as the relative ERE luciferase activity. Consistent with the role of REA on the ER, we found that the transcriptional activity of the ER was increased in MCF-Skp2B cells compared to what was seen for MCF-7 (Fig. 2E). In contrast, the inhibition of Skp2B expression in MCF-7 cells led to a reduction in the activity of the ER (Fig. 2E).
We next determined the localization of REA and found that while REA is present in both the nucleus and the cytoplasm in MCF-7 cells (Fig. 2F), upon overexpression of Skp2B, REA staining was restricted to the cytoplasm (Fig. 2F), indicating that only the nuclear levels of REA are affected in Skp2B-overexpressing cells. These results identify Skp2B as a novel repressor of the nuclear pool of REA and therefore as an indirect activator of the ER.
Expression of Skp2B in the mammary glands of transgenic mice reduces REA levels.
We previously reported that Skp2B is overexpressed in breast cancer (17). To determine whether its overexpression may contribute to breast cancer, we established transgenic mice expressing Skp2B under the control of the mouse mammary tumor virus promoter. We obtained four separate founders that were found to express Skp2B at various levels (Fig. 3A) as determined by quantitative RT-PCR using primers that are specific for Skp2B and do not recognize Skp2A, as they map to the unique C-terminal sequence of Skp2B (17). The expression was also determined by immunohistochemistry on mammary gland tissue sections (Fig. 3B), which revealed that the expression of Skp2B is restricted to the epithelial compartment of the mammary glands. We then determined the levels of REA in the mammary glands of three independent transgenic mice and found that when the ratio between REA and tubulin is calculated, the levels of REA were reduced in average by ∼50% compared to that for the wild type (Fig. 3C). Therefore, this reduction in the levels of REA was also observed in vivo.
FIG. 3.
Skp2B expression in the mammary glands reduces REA levels. (A) Four separate MMTV-Skp2B founders were used for breeding, and the mammary gland of one female of the second generation from each line was tested for Skp2B expression by quantitative RT-PCR using Skp2B-specific primers. WT, wild type. (B) The expression of Skp2B was tested by immunohistochemistry on mammary gland sections of a wild-type female (left) and a female from the Skp2B-4 transgenic line (right) by use of anti-Skp2 antibody. (C) Western analysis of the levels of REA in the mammary glands of a wild-type mouse and of three MMTV-Skp2B transgenic mice.
Skp2B induces precocious invasion of the fat pad and side branching in the mammary glands of mice during early puberty.
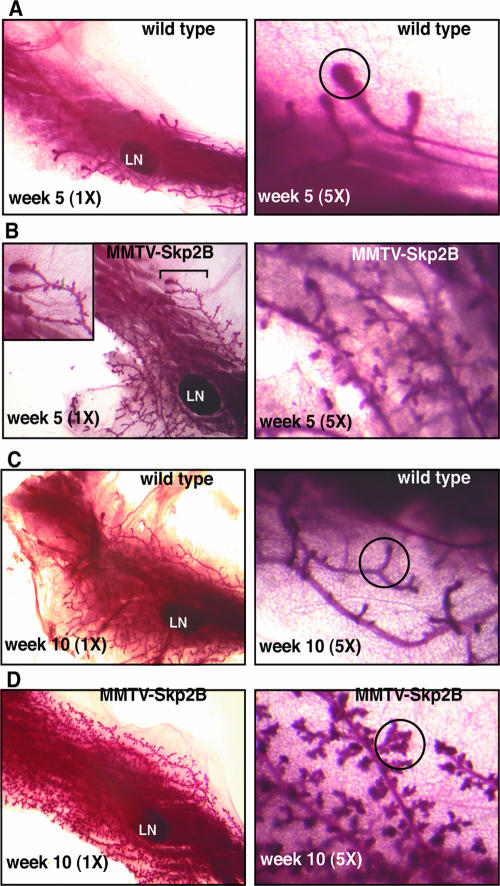
The 50% reduction in REA levels observed for REA heterozygote mice has been reported to lead to an accelerated invasion of the fat pad during puberty (15). To determine the effect of MMTV-Skp2B expression on the early mammary gland development, we analyzed whole-mount preparations of mammary glands from age-matched virgin transgenic and nontransgenic littermates. Differences between transgenic and nontransgenic animals could be observed at an early stage of puberty (Fig. 4). At 5 weeks of age, ductal elongation was detectable by the presence of TEB and extended from the nipple to the lymph nodes in wild-type females. Further, the ducts displayed a smooth surface (Fig. 4A). In transgenic animals, however, ductal growth had extended well beyond the lymph node, indicating an accelerated invasion of the fat pad. In addition, at higher magnification, a large number of small lobular protuberances were evident, indicating a precocious side branching along the ducts (Fig. 4B). At 10 weeks of age, while the surface of ducts from the mammary glands of wild-type animals remained smooth and had small and equally distributed side branches (Fig. 4C), in MMTV-Skp2B mice, the mammary glands also displayed an increase in side branching as well as the presence of lobules (Fig. 4D), indicating an abnormal lobuloalveolar development in virgin mice.
FIG. 4.
Expression of Skp2B accelerates ductal invasion of the fat pad and promotes lobuloalveolar development. (A) Mammary gland from a wild-type female at week 5 was stained by whole-mount staining and visualized at magnifications of ×1 and ×5. LN indicates the position of the lymph node, while a TEB is indicated by the circle. (B) Mammary gland from an MMTV-Skp2B female at week 5 was stained by whole-mount staining and visualized at magnifications of ×1 and ×5. (C) Mammary gland from a wild-type female at week 10 was stained by whole-mount staining and visualized at magnifications of ×1 and ×5. A site of side branching is indicated by the circle. (D) Mammary gland from an MMTV-Skp2B female at week 10 was stained by whole-mount staining and visualized at magnifications of ×1 and ×5. Lobules are indicated by the circle. In each case, one representative gland is shown.
Skp2B induces mammary gland differentiation in adult virgin mice.
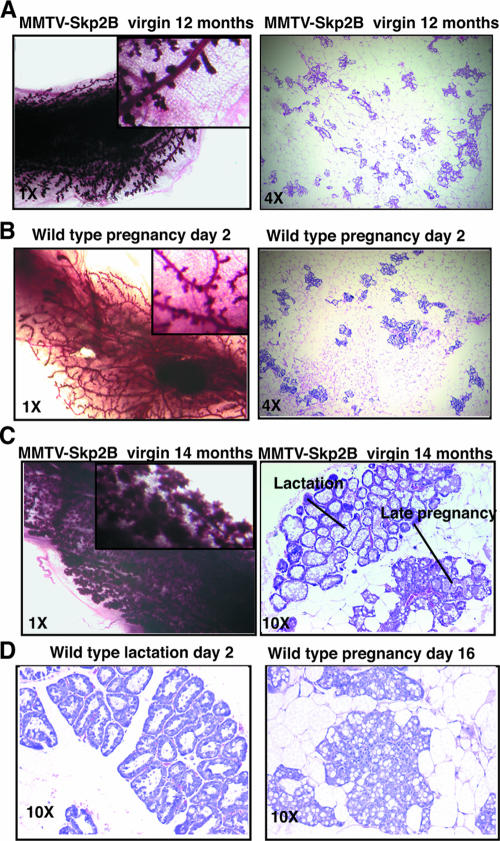
The lobuloalveolar development of the mammary glands was also observed for older virgin females (Fig. 5) and closely resembled the mammary glands associated with early pregnancy in wild-type mice. To directly compare these phenotypes, mammary glands from virgin transgenic females and those from wild-type pregnant females were analyzed. The distributions and numbers of epithelial cells were similar between pregnant wild-type and virgin transgenic animals, although the ducts appeared more dilated in MMTV-Skp2B transgenic mice (compare Fig. 5A and B). Whole-mount analysis using other transgenic females revealed that their mammary glands resemble those of wild-type females during late pregnancy or lactation (Fig. 5C). This observation was confirmed by histological analysis, which revealed an area of epithelial differentiation characteristic of lactating mammary gland and an area characteristic of late pregnancy within the same gland (Fig. 5C and D). Therefore, as observed for REA+/− mice, MMTV-Skp2B transgenic mice show an accelerated lobuloalveolar development of the mammary glands; however, while REA+/− mice must become pregnant to exhibit this phenotype, it is observed for virgin MMTV-Skp2B mice. This observation indicates a more severe phenotype in MMTV-Skp2B mice.
FIG. 5.
Expression of Skp2B in virgin females promotes a pregnancy phenotype for the mammary glands. (A) Whole mount and H&E stained-section of the mammary glands from a 12-month-old MMTV-Skp2B transgenic female. (B) Whole mount and H&E-stained section of the mammary glands from a 2-day-pregnant wild-type female. (C) Whole mount and H&E-stained section of the mammary glands from a 14-month-old MMTV-Skp2B transgenic female. (D) H&E-stained sections of the mammary glands from a wild-type female that had been nursing her pups for 2 days after delivery and from a 16-day-pregnant female. Pictures were taken at magnifications of ×1, ×4, and ×10 as indicated.
Cyst formation in the mammary glands of MMTV-Skp2B females.
In addition to the pregnancy and lactation phenotypes, in a number of transgenic females the most pronounced alteration was the presence of extremely dilated ducts filled with secretion. In some cases, such cysts were observed in several ducts in the absence of an area of pregnancy or lactation morphology (Fig. 6A, left), while in others, gross cysts were adjacent to a pregnancy-like/hyperplastic area (Fig. 6A, right). To test whether these phenotypes are associated with molecular events that normally take place only during pregnancy and lactation, we next analyzed the mammary glands from two wild-type and three transgenic females that display either cyst alone, cyst and pregnancy, or pregnancy and lactation without cyst morphology for their levels of STAT5 and IGFBP-2 and -4. STAT5 phosphorylation is a key event during lactation, while the levels of IGFBP are reduced specifically during the short time window of late pregnancy and lactation and resume during involution (1, 2). We found that STAT5 was activated and that both IGFBP-2 and -4 were reduced in transgenic females compared to what was seen for wild-type mice (Fig. 6B). Therefore, the pattern of expression of these proteins in virgin transgenic females mimics the pattern of expression of these proteins normally observed during late pregnancy and lactation.
FIG. 6.
Cyst formation and deregulation of STAT5 and IGFBPs in virgin MMTV-Skp2B females. (A) H&E staining of mammary gland from 5-month-old and 9-month-old virgin MMTV-Skp2B females. (B) The mammary glands from three transgenic females were selected based on the phenotypes indicated. The levels of STAT5, phospho-STAT5, and IGFBP-2 and -4 were determined by Western blotting (immunoblotting [IB]) and compared to the levels observed for wild-type females. Morphology abbreviations: C, cyst alone; C+P, cyst and pregnancy; P+L, pregnancy and lactation without cyst.
Skp2B induces hyperplasia and tumor formation.
We next monitored the incidence of tumor formation in MMTV-Skp2B mice. We found that the rate of palpable tumors was less than 10% and that tumors appeared only in mice that were more than 1 year old. The histologies of mammary tumors were diverse and included squamous cell carcinoma (Fig. 7A), a glandular pattern with a secretory component with and without lymphocytic infiltrate (Fig. 7B), and a high-grade adenocarcinoma with an undifferentiated pattern consisting of densely packed epithelial cells with necrotic areas (Fig. 7C).
FIG. 7.
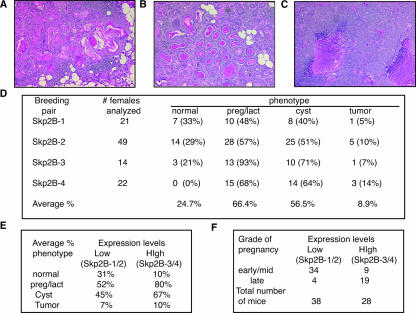
MMTV-Skp2B mice develop mammary carcinoma. (A) H&E staining of mammary gland from a virgin MMTV-Skp2B female with squamous cell carcinoma. (B) H&E staining of mammary gland from a virgin MMTV-Skp2B female with glandular carcinoma that displays a secretory component. (C) H&E staining of mammary gland from a virgin MMTV-Skp2B female with high-grade adenocarcinoma with a necrotic area. (D) Summary of the phenotypes observed for the mammary glands of MMTV-Skp2B transgenic mice. The ages of the mice analyzed ranged from 3 to 16 months for each transgenic group. (E) The average frequencies of each phenotype in transgenic lines that expressed low levels of Skp2B (Skp2B-1/2) and transgenic lanes that expressed high levels of Skp2B (Skp2B-3/4) are shown. preg/lact, pregnant/lactating. (F) The numbers of mice that expressed low levels of Skp2B (Skp2B-1/2) and high levels of Skp2B (Skp2B-3/4) were subclassified according to the severity of the pregnancy phenotype.
A summary of the various phenotypes observed is shown in Fig. 7D. On average, the pregnancy/lactation morphology was the most frequent phenotype and was observed for 66.4% of all glands analyzed. Cyst formation was also frequently observed (56.5%), but tumor formation was less frequent (8.9%). Since the phenotypes were observed for all four different founder lines, we conclude that the phenotypes are not linked to a particular integration site of the transgene. However, when the lines were subdivided into two groups relative to low (Skp2B-1 and -2) and high (Skp2B-3 and -4) expression levels, we found that mice with low expression levels were more frequently normal (31%) than mice that had high expression levels (10%) (Fig. 7E). Further, the mice with low expression levels showed the pregnancy (52%) and cyst (45%) phenotypes less frequently than mice expressing high levels of Skp2B (80% and 67%, respectively) (Fig. 7E). The rate of tumor formation also indicated a tendency of being less frequent for mice expressing low levels, although the difference was smaller. However, as the number of mice expressing low levels of the transgene was larger and included a larger number of old mice, these factors are likely to affect this observation. The correlation between the severity of the phenotype and the expression levels also became more apparent when the grade of pregnancy was further classified as either early/mid-pregnancy or late pregnancy (Fig. 7F). The majority of mice from the low-expression group showed an early/mid-pregnancy grade, while in the high-expression group, the majority of mice with the pregnancy phenotype were classified as late pregnancy (Fig. 7F). We conclude that while even only modest expression of Skp2B can lead to the formation of the various phenotypes, the severity of the phenotype correlates with the level of expression.
DISCUSSION
We initiated this study to test the potential role of Skp2B expression in breast cancer. We previously reported that Skp2B is expressed at very low levels in normal cells but is found frequently in breast cancers. This observation raises the possibility that Skp2B is a tumor-specific isoform. The observation that Skp2B expression could not be detected at any of the six time points we tested during mammary gland development in wild-type females suggests that Skp2B is not normally expressed in this tissue (data not shown). This situation is not unique to Skp2B, since Wnt-1 and Wnt-3, which are well-described oncogenes of the mammary gland, are also not expressed in the mammary glands of wild-type mice (3, 8). Further, our data suggest that Skp2B expression even at relatively low levels may be relevant to disease progression.
Using a two-hybrid screen, we identified REA as a putative substrate of Skp2B-mediated ubiquitination. In support of this notion, REA was found to be ubiquitinated and its levels were found to be reduced by Skp2B expression but not by Skp2A. However, endogenous Skp2B is present at very low levels in HEK293T cells, while REA ubiquitination is easily detected in this cell line; these results argue that REA is normally ubiquitinated by another ligase. This situation is also not unique to Skp2B, since while p53 is normally ubiquitinated by mdm2, in cervical cancer, the virally derived oncoprotein E6 utilizes the cellular ubiquitin ligase E6-AP to mediate p53 degradation (10). Likewise, we hypothesize that in the abnormal situation where Skp2B is expressed at higher levels, REA may only then become a substrate of an SCFSkp2B complex. In addition, we observed that the reduction in REA is restricted to the nuclear pool of REA. Since binding to F-box proteins requires prior phosphorylation of their substrates, we hypothesize that nuclear import of REA may require phosphorylation and explain how Skp2B in the cytoplasm affects the nuclear pool of REA by targeting the phosphorylated form of REA, therefore reducing the nuclear staining specifically. Further studies aimed at understanding the mechanism regulating the nuclear import and export of REA will be required to test this possibility.
The relevance of the interaction between Skp2B and REA is strongly supported by the observations first that the transcriptional activity of the ER is increased in MCF-7-Skp2B cells and second that the expression of Skp2B in the mammary glands of transgenic mice mimics the phenotype observed for the REA heterozygous mice (15). As the 50% reduction in REA levels observed for Skp2B transgenic mice is equivalent to that observed for REA heterozygous mice and that this level of reduction was shown to be sufficient to induce the activity of the ER in vivo (15), the decrease of REA level observed for MMTV-Skp2B mice is likely to participate in the phenotype observed. However, since REA+/− mice show an accelerated proliferation of the mammary gland only in association with pregnancy, while this effect was observed for virgin MMTV-Skp2B mice, the more severe phenotype observed for MMTV-Skp2B suggests that Skp2B affects other proteins in addition to REA. Notably, MMTV-Skp2B mice develop cysts and tumors, while REA heterozygotes do not (15). In support of an additional function of Skp2B in tumor progression that is unrelated to the ER, we previously reported that Skp2B is overexpressed in the ER-negative cell line HS578T (17), and in a study that did not distinguish between Skp2A and Skp2B but recorded both nuclear and cytoplasmic staining, Skp2A/B were found to be overexpressed in both ER-positive and ER-negative breast cancers (19).
Despite the fact that clinically detectable cysts are not considered premalignant lesions, the presence of cysts is nevertheless considered a marker predicting an increased risk of breast cancer (4). Further, gross cyst is one of the most frequent lesions in the human breast. Therefore, our results indicate that the expression of Skp2B results in a number of benign and malignant lesions of the mammary gland which can be linked at least partially to the downregulation of REA. The additional targets of Skp2B await further characterization.
Acknowledgments
We thank Liliana Ossowski, who provided us with invaluable comments during the course of this work. We also thank Shabnam Jaffer, who provided us with her pathological expertise, and Ramla Benmaamar for Fig. 1D.
We dedicate this work to the memory of Rafael Mira-Lopez, who was a fine mammary gland biologist and a wonderful man.
All authors declare that they have no commercial affiliation and no conflict of interest related to this publication. This work was supported by NIH RO1 grant no. CA109482 to D.G. and by the Samuel Waxman Cancer Research Foundation.
Footnotes
Published ahead of print on 4 September 2007.
REFERENCES
- 1.Allar, M. A., and T. L. Wood. 2004. Expression of the insulin-like growth factor binding proteins during postnatal development of the murine mammary gland. Endocrinology 145: 2467-2477. [DOI] [PubMed] [Google Scholar]
- 2.Boutinaud, M., J. H. Shand, M. A. Park, K. Phillips, J. Beattie, D. J. Flint, and G. J. Allan. 2004. A quantitative RT-PCR study of the mRNA expression profile of the IGF axis during mammary gland development. J. Mol. Endocrinol. 33: 195-207. [DOI] [PubMed] [Google Scholar]
- 3.Brennan, K. R., and A. M. Brown. 2004. Wnt proteins in mammary development and cancer. J. Mammary Gland Biol. Neoplasia 9: 119-131. [DOI] [PubMed] [Google Scholar]
- 4.Bruzzi, P., L. Dogliotti, C. Naldoni, L. Bucchi, M. Costantini, A. Cicognani, M. Torta, G. F. Buzzi, and A. Angeli. 1997. Cohort study of association of risk of breast cancer with cyst type in women with gross cystic disease of the breast. BMJ 314: 925-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carrano, A. C., E. Eytan, A. Hershko, and M. Pagano. 1999. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat. Cell Biol. 1: 193-199. [DOI] [PubMed] [Google Scholar]
- 6.Couse, J. F., and K. S. Korach. 1999. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr. Rev. 20: 358-417. [DOI] [PubMed] [Google Scholar]
- 7.Ganiatsas, S., R. Dow, A. Thompson, B. Schulman, and D. Germain. 2001. A splice variant of Skp2 is retained in the cytoplasm and fails to direct cyclin D1 ubiquitination in the uterine cancer cell line SK-UT. Oncogene 20: 3641-3650. [DOI] [PubMed] [Google Scholar]
- 8.Gavin, B. J., and A. P. McMahon. 1992. Differential regulation of the Wnt gene family during pregnancy and lactation suggests a role in postnatal development of the mammary gland. Mol. Cell. Biol. 12: 2418-2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hao, B., N. Zheng, B. A. Schulman, G. Wu, J. J. Miller, M. Pagano, and N. P. Pavletich. 2005. Structural basis of the Cks1-dependent recognition of p27(Kip1) by the SCF(Skp2) ubiquitin ligase. Mol. Cell 20: 9-19. [DOI] [PubMed] [Google Scholar]
- 10.Hengstermann, A., L. K. Linares, A. Ciechanover, N. J. Whitaker, and M. Scheffner. 2001. Complete switch from Mdm2 to human papillomavirus E6-mediated degradation of p53 in cervical cancer cells. Proc. Natl. Acad. Sci. USA 98: 1218-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hennighausen, L., and G. W. Robinson. 2001. Signaling pathways in mammary gland development. Dev. Cell 1: 467-475. [DOI] [PubMed] [Google Scholar]
- 12.Hennighausen, L., and G. W. Robinson. 1998. Think globally, act locally: the making of a mouse mammary gland. Genes Dev. 12: 449-455. [DOI] [PubMed] [Google Scholar]
- 13.Huang, A. L., M. C. Ostrowski, D. Berard, and G. L. Hager. 1981. Glucocorticoid regulation of the Ha-MuSV p21 gene conferred by sequences from mouse mammary tumor virus. Cell 27: 245-255. [DOI] [PubMed] [Google Scholar]
- 14.Montano, M. M., K. Ekena, R. Delage-Mourroux, W. Chang, P. Martini, and B. S. Katzenellenbogen. 1999. An estrogen receptor-selective coregulator that potentiates the effectiveness of antiestrogens and represses the activity of estrogens. Proc. Natl. Acad. Sci. USA 96: 6947-6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mussi, P., L. Liao, S. E. Park, P. Ciana, A. Maggi, B. S. Katzenellenbogen, J. Xu, and B. W. O'Malley. 2006. Haploinsufficiency of the corepressor of estrogen receptor activity (REA) enhances estrogen receptor function in the mammary gland. Proc. Natl. Acad. Sci. USA 103: 16716-16721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pirkmaier, A., R. Dow, S. Ganiatsas, P. Waring, K. Warren, A. Thompson, J. Hendley, and D. Germain. 2003. Alternative mammary oncogenic pathways are induced by D-type cyclins; MMTV-cyclin D3 transgenic mice develop squamous cell carcinoma. Oncogene 22: 4425-4433. [DOI] [PubMed] [Google Scholar]
- 17.Radke, S., A. Pirkmaier, and D. Germain. 2005. Differential expression of the F-box proteins Skp2 and Skp2B in breast cancer. Oncogene 24: 3448-3458. [DOI] [PubMed] [Google Scholar]
- 18.Schulman, B. A., A. C. Carrano, P. D. Jeffrey, Z. Bowen, E. R. Kinnucan, M. S. Finnin, S. J. Elledge, J. W. Harper, M. Pagano, and N. P. Pavletich. 2000. Insights into SCF ubiquitin ligases from the structure of the Skp1-Skp2 complex. Nature 408: 381-386. [DOI] [PubMed] [Google Scholar]
- 19.Signoretti, S., L. Di Marcotullio, A. Richardson, S. Ramaswamy, B. Isaac, M. Rue, F. Monti, M. Loda, and M. Pagano. 2002. Oncogenic role of the ubiquitin ligase subunit Skp2 in human breast cancer. J. Clin. Investig. 110: 633-641. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Sutterluty, H., E. Chatelain, A. Marti, C. Wirbelauer, M. Senften, U. Muller, and W. Krek. 1999. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat. Cell Biol. 1: 207-214. [DOI] [PubMed] [Google Scholar]
- 21.Tan, P., S. Y. Fuchs, A. Chen, K. Wu, C. Gomez, Z. Ronai, and Z. Q. Pan. 1999. Recruitment of a ROC1-CUL1 ubiquitin ligase by Skp1 and HOS to catalyze the ubiquitination of I kappa B alpha. Mol. Cell 3: 527-533. [DOI] [PubMed] [Google Scholar]