Abstract
The initiation factor eukaryotic translation initiation factor 4E (eIF4E) plays a critical role in initiating translation of mRNAs, including those encoding oncogenic proteins. Therefore, eIF4E is considered a survival protein involved in cell cycle progression, cell transformation, and apoptotic resistance. Phosphorylation of eIF4E (usually at Ser209) increases its binding affinity for the cap of mRNA and may also favor its entry into initiation complexes. Mammalian target of rapamycin (mTOR) inhibitors suppress cap-dependent translation through inhibition of the phosphorylation of eIF4E-binding protein 1. Paradoxically, we have shown that inhibition of mTOR signaling increases eIF4E phosphorylation in human cancer cells. In this study, we focused on revealing the mechanism by which mTOR inhibition increases eIF4E phosphorylation. Silencing of either mTOR or raptor could mimic mTOR inhibitors’ effects to increase eIF4E phosphorylation. Moreover, knockdown of mTOR, but not rictor or p70S6K, abrogated rapamycin's ability to increase eIF4E phosphorylation. These results indicate that mTOR inhibitor-induced eIF4E phosphorylation is secondary to mTOR/raptor inhibition and independent of p70S6K. Importantly, mTOR inhibitors lost their ability to increase eIF4E phosphorylation only in cells where both Mnk1 and Mnk2 were knocked out, indicating that mTOR inhibitors increase eIF4E phosphorylation through a Mnk-dependent mechanism. Given that mTOR inhibitors failed to increase Mnk and eIF4E phosphorylation in phosphatidylinositol 3-kinase (PI3K)-deficient cells, we conclude that mTOR inhibition increases eIF4E phosphorylation through a PI3K-dependent and Mnk-mediated mechanism. In addition, we also suggest an effective therapeutic strategy for enhancing mTOR-targeted cancer therapy by cotargeting mTOR signaling and Mnk/eIF4E phosphorylation.
The initiation factor eukaryotic translation initiation factor 4E (eIF4E) plays a key regulatory role in initiating mRNA translation (17, 23). eIF4E binds to the mRNA cap structure and interacts with eIF4G, which is a large scaffold protein for assembly of eIF4E and eIF4A to form the eIF4F complex. Binding of eIF4E to eIF4G can be blocked by eIF4E-binding proteins (4E-BPs) such as 4E-BP1, which function through reversible association with eIF4E (2, 17). 4E-BP1 becomes phosphorylated in response to a large number of extracellular stimuli and consequently dissociates from eIF4E, facilitating formation of the eIF4F complex. In contrast, dephosphorylated 4E-BP1 associates strongly with eIF4E and inhibits cap-dependent translation initiation (2, 3). 4E-BP1 is known to be one of the best-characterized downstream effector proteins of mammalian target of rapamycin (mTOR) (3). Thus, mTOR phosphorylates 4E-BP1, leading to initiation of cap-dependent translation, whereas inhibition of mTOR by rapamycin inhibits 4E-BP1 phosphorylation, resulting in suppression of cap-dependent translation.
eIF4E is phosphorylated (usually at Ser209) in many systems in response to extracellular stimuli, including growth factors, hormones, and mitogens (10, 15, 16). Phosphorylation of eIF4E increases its affinity for the cap of mRNA and may also favor its entry into initiation complexes (10, 15, 16). Although the biological significance of eIF4E phosphorylation is not completely understood, a recent genetic study in Drosophila melanogaster has demonstrated that eIF4E phosphorylation is biologically significant and is essential for normal growth and development (9). The best candidate for eIF4E phosphorylation is the mitogen-activated protein kinase (MAPK)-activated protein kinase Mnk1, which physically associates with eIF4F and directly phosphorylates eIF4E at Ser 209. In addition to Mnk1, Mnk2 also phosphorylates eIF4E, albeit to a lesser extent. Both Mnk1 and Mnk2, but particularly Mnk1, are directly phosphorylated by ERK and p38 MAPKs (10, 15).
eIF4E is required for cell cycle progression, exhibits antiapoptotic activity, and, when overexpressed, transforms cells (2, 4, 11, 16), largely due to its critical role in initiating translation of mRNAs that encode cell cycle regulators or oncogenic proteins, such as cyclin D1, ornithine decarboxylase, c-Myc, hypoxia-inducible factor 1α, fibroblast growth factor, and vascular endothelial growth factor (2, 3, 11). Therefore, it is not surprising that elevated levels of eIF4E are found in a broad spectrum of transformed cells and human cancers, including lung cancers, and are often associated with aggressive, poorly differentiated tumors (3, 4).
The mTOR inhibitor rapamycin inhibits mTOR signaling, leading to suppression of 4E-BP1 phosphorylation, which promotes binding of 4E-BP1 to eIF4E, resulting in suppression of cap-dependent protein synthesis (3). Paradoxically, our previous work showed that rapamycin actually increases eIF4E phosphorylation while inhibiting 4E-BP1 phosphorylation (20). Moreover, our results suggest that rapamycin-induced eIF4E phosphorylation is phosphatidylinositol 3-kinase (PI3K) dependent but independent of ERK or p38 MAPK (20). In the current study, we further examined the modulation of eIF4E phosphorylation by mTOR inhibition in a broader setting and determined the involvement of PI3K and Mnks in mTOR inhibitor-induced eIF4E phosphorylation. Our results clearly demonstrate that rapamycin-induced eIF4E phosphorylation is mTOR inhibition dependent and is mediated by both Mnk1 and Mnk2. In addition, the possible clinical translation significance of these findings was also demonstrated.
MATERIALS AND METHODS
Reagents.
Rapamycin and LY294002 were purchased from LKT Laboratories, Inc. (St. Paul, MN). RAD001 was provided by Novartis Pharmaceuticals Corporation (East Hanover, NJ). Wortmannin was purchased from Biomol (Plymouth Meeting, PA). The Mnk1 inhibitor 4-amino-5-(4-fluoroanilino)- pyrazolo[3,4-d]pyrimidine (CGP57380) and insulin-like growth factor 1 receptor (IGF-1R) inhibitor II [N-(2-methoxy-5-chlorophenyl)-N′-(2-methylquinolin- 4-yl)-urea] were purchased from EMD Chemical Inc./Calbiochem (San Diego, CA). These agents were dissolved in dimethyl sulfoxide at a concentration of 20 mM, and aliquots were stored at −80°C. Stock solutions were diluted to the desired final concentrations with growth medium just before use. Rabbit polyclonal antibodies against phospho-Akt (p-Akt) (Ser473), phospho-p70S6K (p-p70S6K) (Thr389), phospho-Mnk1 (Thr197/202), eIF4E, and phospho-eIF4E (p-eIF4E) (Ser209), respectively, were purchased from Cell Signaling Technology, Inc. (Beverly, MA). Rabbit polyclonal anti-insulin receptor substrate 1 (IRS-1) antibody was purchased from Upstate (Lake Placid, NY). Rabbit polyclonal anti-actin and mouse monoclonal antitubulin antibodies were purchased from Sigma Chemical Co. (St. Louis, MO). Rabbit polyclonal anti-glyceraldehyde-3-phosphate dehydrogenase antibody was purchased from Trevigen, Inc. (Gaithersburg, MD). Mnk1 and Mnk2 antibodies were generated as described previously (22). Adenoviruses (Ad) carrying control green fluorescent protein (GFP) (Ad-GFP) or a constitutively active p110*K227E mutant (Ad-p110*) (5) was provided by H. A. Franch (Emory University, Atlanta, GA).
Cell lines and cell culture.
The human lung cancer cell lines and breast cancer cell lines used in this study were purchased from the American Type Culture Collection (Manassas, VA). Glioblastoma U87MG and LN229 cell lines were provided by D. Durden (Emory University). The myeloma cell lines RPMI8266, OPM-1, and KMS18 were provided by J. Chen (Emory University). These cell lines were grown in monolayer culture in RPMI 1640 medium supplemented with glutamine and 5% fetal bovine serum (FBS) at 37°C in a humidified atmosphere consisting of 5% CO2 and 95% air. The colon cancer cell lines HCT116 p53+/+ and p53−/− were provided by B. Vogelstein (The Johns Hopkins Medical Institutions, Baltimore, MD). These cell lines were cultured in McCoy's 5A medium with 5% FBS. Wild-type (WT), Mnk1 knockout (1-KO), Mnk2 knockout (2-KO), and Mnk1/Mnk2 double-knockout (DKO) murine embryonic fibroblasts (MEFs) were described previously (22). WT and IRS-1−/− murine 3T3 fibroblasts (25) were obtained from C. R. Kahn (Joslin Diabetes Center and Harvard Medical School, Boston, MA). WT, p85α, and p85β DKO MEFs (p85-DKO) were provided by L. Cantley (Harvard Medical School, Boston, MA). These cell lines were cultured in Dulbecco's modified Eagle's medium with 5% FBS.
Gene knockdown by siRNA.
mTOR small interfering RNA (siRNA) (catalog no. 6381) was purchased from Cell Signaling Technology, Inc. p70S6K (catalog no. 1027217) and control (nonsilencing) (catalog no. 1022076) siRNAs were ordered from QIAGEN (Valencia, CA). Raptor siRNA that targets 5′-AAGGCTAGTCTGTTTCGAAAT-3′ was described previously (18) and was synthesized by QIAGEN. Stealth MNK1 siRNA that targets the sequence 5′-CCTTGCCAGGAAAGTTTGAAGATAT-3′ and stealth control siRNA that targets the sequence 5′-CCTACCAGGGAATTTAAGAGTGTAT-3′ were synthesized by Invitrogen (Carlsbad, CA). The IRS-1 siRNA/siAb assay kit, which contains IRS-1 and nonspecific control SMARTpool siRNAs, was purchased from Upstate. The transfection of siRNA was conducted in a 24- or 12-well plate using Lipofectamine 2000 (Invitrogen) following the manufacturer's instructions. Forty-eight hours after the transfection, the cells were treated with dimethyl sulfoxide, rapamycin, or RAD001 for the indicated times and then subjected to detection of the indicated proteins by Western blot analysis.
We also used lentiviral control (scramble), mTOR, and raptor and rictor short hairpin RNAs (shRNAs), which were described previously (18) and ordered from Addgene Inc. (Cambridge, MA). The viral package and production and cell infection were the same as described previously (18).
Western blot analysis.
The procedures for preparation of whole-cell protein lysates and for Western blotting were described previously (21). Whole-cell protein lysates (50 μg) were electrophoresed through 7.5%, 10%, or 12% denaturing polyacrylamide slab gels and transferred to an Immuno-Blot polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA) by electroblotting. The blots were probed or reprobed with the primary antibodies, and then antibody binding was detected using the SuperSignal West Pico Chemiluminescent Substrate (Pierce Biotechnology, Inc., Rockford, IL) according to the manufacturer's protocol.
PI3K activity assay.
Whole-cell protein lysates were prepared as described for Western blot analysis and then used for detection of PI3K activity as described previously (1).
Growth inhibition assays.
Growth inhibition was evaluated by determining the cell numbers in a 3-day monolayer culture using the sulforhodamine B assay and by counting colony formations on culture plates after a 10-day treatment, as described previously (20).
RESULTS
mTOR inhibitor-induced increase in eIF4E phosphorylation commonly occurs in various types of cancer cells.
Our previous work demonstrated that rapamycin increases eIF4E phosphorylation while inhibiting mTOR signaling, including inhibition of phosphorylation of p70S6K and 4E-BP1 in human lung cancer and other types of cancer cells (20). In this study, we further examined the effect of RAD001, another mTOR inhibitor being tested in clinical trials, on eIF4E phosphorylation in human lung cancer cells, as well as other types of cancer cell lines. RAD001 at concentrations of 1 nM or higher strikingly increased p-eIF4E levels, which could rapidly occur even at 5 min post-RAD001 incubation (see Fig. S1A in the supplemental material). By comparing the effects of RAD001 with those of rapamycin on eIF4E phosphorylation, we found that RAD001, with a potency comparable to that of rapamycin, effectively increased p-eIF4E levels in human lung cancer, colon cancer, breast cancer, glioblastoma, and myeloma cells (see Fig. S1B and C in the supplemental material). Taken together, the current results and our previous findings clearly indicate that increase of eIF4E phosphorylation is a common phenomenon during mTOR inhibition by mTOR inhibitors. Moreover, we found that both RAD001 and rapamycin induced a sustained increase in eIF4E phosphorylation up to 96 h after a single treatment (see Fig. S1D in the supplemental material), which was associated with sustained inhibition of mTOR signaling as measured by reduction of p-p70S6K levels (up to 96 h) (data not shown).
Rapamycin increases eIF4E phosphorylation independently of the IGF-1/IGF-1R/IRS-1 axis.
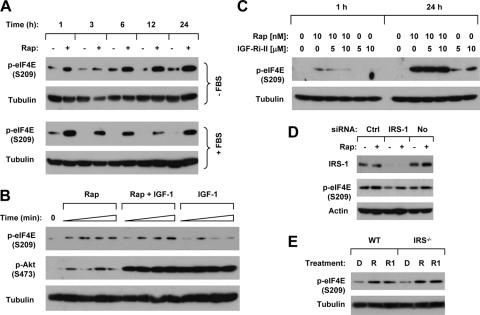
To determine whether the increase of eIF4E phosphorylation by rapamycin is dependent on the presence of growth factors, we examined the effect of rapamycin on eIF4E phosphorylation in the absence of serum. Specifically, the cells were serum starved for approximately 24 h and then exposed to rapamycin. As presented in Fig. 1A, rapamycin induced a quick and sustained increase in p-eIF4E levels, which was detected between 1 h and 24 h post-rapamycin treatment. Since the IGF-1/IGF-1R/IRS-1 axis is involved in mTOR-mediated feedback inhibition of Akt (14, 24), we determined whether this axis also plays a role in mTOR inhibition-mediated eIF4E phosphorylation. Therefore, we examined the effect of rapamycin on eIF4E phosphorylation in the presence of IGF-1. As shown in Fig. 1B, IGF-1 substantially increased Akt phosphorylation, but not eIF4E phosphorylation. The presence of IGF-1 did not alter the modulation of eIF4E phosphorylation by rapamycin. Collectively, these results suggest that rapamycin increases eIF4E phosphorylation independently of activation of a growth factor/receptor, particularly IGF-1 signaling. Moreover, we examined the effect of rapamycin in the presence of an IGF-1R inhibitor and found that rapamycin still increased eIF4E phosphorylation (Fig. 1C). This result further indicates that rapamycin induces eIF4E phosphorylation independently of IGF-1/IGF-1R signaling. We also analyzed the effects of rapamycin on eIF4E phosphorylation in cells where IRS-1 expression was knocked down or in IRS-1-deficient cells. As presented in Fig. 1D and E, rapamycin still increased eIF4E phosphorylation in cells where IRS-1 was silenced (Fig. 1D) or in IRS-1 KO cells (Fig. 1E). These findings indicate that mTOR inhibitors induce an IRS-1-independent eIF4E phosphorylation. Taking these data together, we conclude that mTOR inhibitors increase eIF4E phosphorylation independently of the IGF-1/IGF-1R/IRS-1 axis.
FIG. 1.
Rapamycin increases eIF4E phosphorylation under serum-free conditions (A), in the presence of IGF-1 (B) and an IGF-1R inhibitor (C), and in IRS-1-silenced (D) or -deficient (E) cells. (A) H157 cells were serum starved for 24 h and then treated with 10 nM rapamycin in the absence of FBS for the indicated times. In addition, H157 cells were cultured and treated with 10 nM rapamycin in medium containing 5% FBS for the given times. (B) H157 cells were serum starved for 20 h and then treated with 10 nM rapamycin alone, 1 ng/ml of IGF-1 alone, and their combination for 30, 60, 120, and 180 min. (C) A549 cells were pretreated with the given concentrations of IGF-1R inhibitor II (IGF-1Ri-II) for 30 min and then cotreated with 10 nM rapamycin (Rap) for the indicated times. (D) A549 cells were transfected with control (Ctrl) or IRS-1 siRNA. After 48 h, the cells were treated with 10 nM rapamycin (Rap) for 1 h before they were harvested for preparation of whole-cell protein lysates. In addition, untransfected cells (No) were included as a control. (E) WT and IRS−/− murine 3T3 fibroblasts were treated with 10 nM rapamycin (R) or RAD001 (R1) for 8 h. After the aforementioned treatments, the cells were subjected to preparation of whole-cell protein lysates and subsequent Western blot analysis.
Rapamycin increases eIF4E phosphorylation through inhibition of mTOR/raptor.
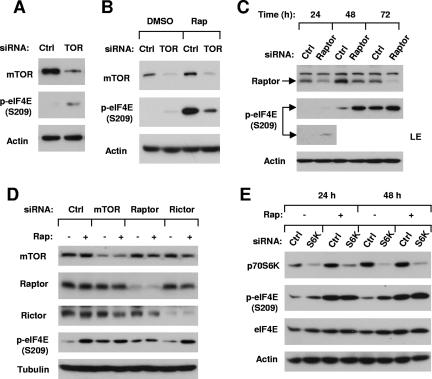
To determine whether rapamycin increases eIF4E phosphorylation through inhibition of mTOR signaling, we disrupted the function of mTOR or the mTOR/raptor complex by specifically knocking down the expression of mTOR or raptor using siRNA and then examined the impact on eIF4E phosphorylation. Transfection of mTOR siRNA into H157 cells decreased the levels of mTOR, indicating a successful reduction of mTOR expression. In mTOR-siRNA-transfected cells, p-eIF4E levels were increased (Fig. 2A). Consistently, we found that rapamycin increased p-eIF4E levels in control siRNA-transfected cells but only weakly elevated p-eIF4E levels in cells transfected with mTOR-siRNA (Fig. 2B). These results indicate that rapamycin-induced eIF4E phosphorylation is dependent on the presence of mTOR.
FIG. 2.
Effects of knockdown of mTOR (A, B, and D), raptor (C and D), rictor (D), or p70S6K (E) on basal levels of eIF4E phosphorylation and rapamycin-induced eIF4E phosphorylation. (A) H157 cells were transfected with control or mTOR siRNA. After 48 h, the cells were subjected to preparation of whole-cell protein lysates. (B) H157 cells were transfected with control or mTOR siRNA for 48 h. Before the cells were harvested for preparation of whole-cell protein lysates, the cells were treated with 10 nM rapamycin for 3 h. (C) H157 cells were transfected with control or raptor siRNA. After the indicated times, the cells were subjected to preparation of whole-cell protein lysates. (D) Calu-1 cells were infected once with lentivirus carrying control, mTOR, raptor, or rictor shRNA and then subjected to selection with 1 μg/ml puromycin for 10 days. The surviving cells were further cultured in puromycin-free medium. After another 10 days, the cells were seeded, treated with 10 nM rapamycin for 1 h, and then harvested for preparation of whole-cell protein lysates and subsequent Western blot analysis. (E) H157 cells were transfected with control or p70S6K siRNA for the indicated times. Before the cells were harvested at each time point for preparation of whole-cell protein lysates, the cells were treated with 10 nM rapamycin for 3 h. The indicated proteins in these experiments were detected by Western blot analysis. LE, longer exposure; Ctrl, control; TOR, mTOR; Rap, rapamycin.
In a similar fashion, silencing of raptor expression using raptor siRNA increased p-eIF4E levels as well, starting at 24 h posttransfection and sustained to 72 h (Fig. 2C and D). Rapamycin substantially increased p-eIF4E levels in control siRNA-transfected cells but only minimally in raptor siRNA-transfected cells (Fig. 2D). In contrast, silencing of rictor, a key component of mTOR complex 2, neither altered the basal levels of p-eIF4E nor prevented rapamycin from increasing eIF4E phosphorylation (Fig. 2D). Thus, these results indicate that disruption of mTOR/raptor signaling, but not mTOR/rictor, increases eIF4E phosphorylation.
It is known that mTOR inhibitors suppress the phosphorylation of p70S6K due to inhibition of mTOR/raptor activity. To determine whether eIF4E phosphorylation induced by mTOR inhibitors is secondary to p70S6K inhibition, we also determined the impact of silencing of p70S6K on rapamycin-induced eIF4E phosphorylation. As shown in Fig. 2E, transfection of p70S6K substantially reduced p70S6K levels at either 24 h or 48 h posttransfection. Rapamycin increased p-eIF4E in both control siRNA- and p70S6K-transfected cells with comparable potencies, indicating that rapamycin increases eIF4E phosphorylation regardless of p70SK expression. Collectively, these results demonstrate that rapamycin increases eIF4E independently of p70S6K inhibition.
Rapamycin increases eIF4E phosphorylation independently of Akt activation.
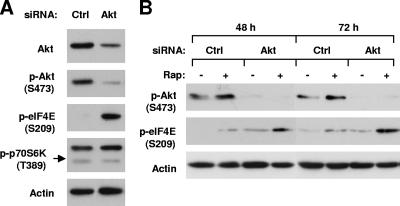
Our previous work showed that rapamycin increases the phosphorylation of both Akt and eIF4E (20). To determine whether eIF4E phosphorylation induced by rapamycin is associated with the increase in Akt phosphorylation, we examined the effect of rapamycin on eIF4E phosphorylation when Akt expression was silenced using Akt siRNA. As shown in Fig. 3A, inhibition of Akt (phosphorylation) by silencing its expression using Akt siRNA actually increased eIF4E phosphorylation while minimally decreasing p-p70S6K levels. In Akt siRNA-transfected cells, where Akt phosphorylation is inhibited, the increase in eIF4E phosphorylation by rapamycin was further enhanced (Fig. 3B). Taken together, these results indicate that Akt actually exerts a negative effect on eIF4E phosphorylation. Therefore, it is unlikely that rapamycin increases eIF4E phosphorylation secondary to Akt activation.
FIG. 3.
Effects of Akt knockdown on eIF4E phosphorylation (A) or rapamycin-induced eIF4E phosphorylation (B). (A) H157 cells were transfected with control (Ctrl) or Akt siRNA. After 48 h, the cells were subjected to preparation of whole-cell protein lysates. (B) H157 cells were transfected with control (Ctrl) or Akt siRNA for the indicated times. Before the cells were harvested for preparation of whole-cell protein lysates, the cells were treated with 10 nM rapamycin (Rap) for 3 h. The indicated proteins in these experiments were detected by Western blot analysis.
mTOR inhibitors induce PI3K-dependent eIF4E phosphorylation.
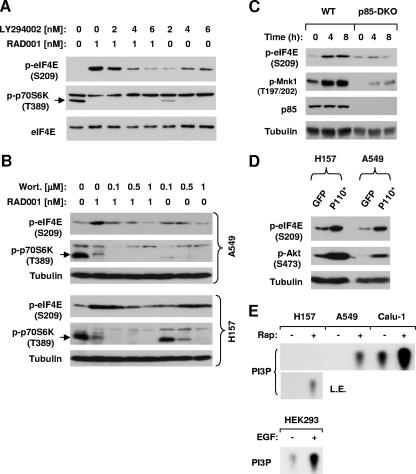
We previously suggested that rapamycin increases eIF4E phosphorylation through a PI3K-dependent mechanism because the PI3K inhibitor LY294002 blocked rapamycin-induced eIF4E phosphorylation (20). To further demonstrate the role of PI3K in mTOR inhibitor-induced eIF4E phosphorylation, we examined the effect of the presence of LY294002 or wortmannin on RAD001-induced eIF4E phosphorylation. As shown in Fig. 4A and B, RAD001 increased p-eIF4E levels. This effect was abrogated in the presence of LY294002 (2 to 6 μM) or wortmannin (100 to 1000 nM), suggesting that RAD001-induced eIF4E phosphorylation is also PI3K dependent. Moreover, we examined the effects of rapamycin on eIF4E phosphorylation in PI3K (p85α and p85β DKO)-deficient MEFs (p85-DKO). We found that rapamycin increased p-eIF4E levels at both 4 h and 8 h posttreatment in WT MEFs but not in p85-DKO MEFs (Fig. 4C), indicating that intact p85 or PI3K is essential for rapamycin to increase eIF4E phosphorylation. To further demonstrate the relationship between PI3K and eIF4E phosphorylation, we activated PI3K by expressing the constitutively active p110 catalytic subunit of PI3K through Ad infection and then examined its effect on eIF4E phosphorylation. As presented in Fig. 4D, infection of both H157 and A549 cells with Ad-p110* resulted in increased levels of p-eIF4E, in addition to increased levels of p-Akt, an indicator of PI3K activation. Thus, it is conceivable that PI3K indeed regulates eIF4E phosphorylation.
FIG. 4.
mTOR inhibitors induce PI3K-dependent eIF4E phosphorylation. (A and B) Modulation of eIF4E phosphorylation by RAD001 in the presence of the PI3K inhibitor LY294002 (A) or wortmannin (Wort.) (B). H157 (A) or H157 and A549 (B) cells were treated with 1 nM RAD001 in the absence and presence of the indicated concentrations of LY194002 (A) or wortmannin (B) for 3 h. (C) Impact of p85 deficiency on rapamycin-induced eIF4E phosphorylation. The WT and p85-DKO MEFs were treated with 10 nM rapamycin for the indicated times. (D) Effects of expressing a constitutively active p110 catalytic subunit of PI3K on eIF4E phosphorylation. The indicated cancer cell lines were plated in six-well cell culture plates and then infected with Ad-GFP or Ad-p110* for 24 h. (E) Effects of rapamycin on PI3K activity. The indicated cancer cell lines were treated with 10 nM rapamycin (Rap) for 1 h. After the aforementioned treatments, the cell lines were subjected to preparation of whole-cell protein lysates and subsequent Western blot analysis for detection of the proteins as presented (A to D) or PI3K activity assay (E). L.E., longer exposure; EGF, epidermal growth factor.
Given the dependence of mTOR inhibition-induced eIF4E phosphorylation on PI3K, we then examined whether inhibition of mTOR with rapamycin actually increased PI3K activity in our cell systems. As shown in Fig. 4E, rapamycin, which like epidermal growth factor is known to activate PI3K, increased PI3K activity as indicated by increased amounts of phosphatidylinositol 3-phosphate in the three tested lung cancer cell lines. We conclude that mTOR inhibitors, such as rapamycin, activate PI3K, leading to an increase in eIF4E phosphorylation.
We noted that LY294002 (4 and 6 μM) or wortmannin (0.5 and 1 μM) alone at relatively high concentrations that inhibited p70S6K phosphorylation increased p-eIF4E levels as well in H157 cells (Fig. 4A and B). However, we did not find that wortmannin alone increased p-eIF4E levels in A549 cells (Fig. 4B). Thus, whether the increase in eIF4E phosphorylation by a PI3K inhibitor in certain cancer cell lines is due to inhibition of mTOR signaling needs to be investigated further.
mTOR inhibitors increase eIF4E phosphorylation through an Mnk-mediated mechanism.
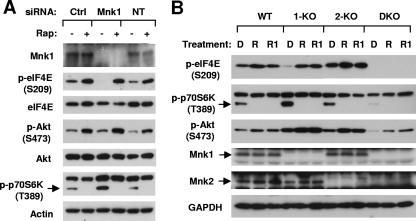
It is well known that Mnks, particularly Mnk1, regulate eIF4E activation through its phosphorylation at Ser209 (10, 22). Rapamycin increases eIF4E phosphorylation at Ser209 (20), as did rapamycin and RAD001, as has been demonstrated (see Fig. S1 in the supplemental material). Therefore, we determined whether rapamycin increased eIF4E phosphorylation through a Mnk-dependent mechanism. To this end, we knocked down Mnk1 expression and then analyzed its impact on rapamycin-induced eIF4E phosphorylation. As shown in Fig. 5A, knockdown of Mnk1 expression decreased basal levels of p-eIF4E but could not prevent its phosphorylation being increased by rapamycin treatment. Modulation of p-Akt and p-p70S6K were not affected by Mnk1 silencing. This result clearly suggests that Mnk1 silencing is not sufficient to prevent eIF4E phosphorylation by rapamycin. It is possible that both Mnk1 and Mnk2 are responsible for rapamycin-induced eIF4E phosphorylation. Loss of Mnk1 function can be compensated for by Mnk2. As a result, knockdown of either gene's expression may not be sufficient to abrogate rapamycin-induced increase in eIF4E phosphorylation. To test this possibility, we examined the effects of mTOR inhibitors on eIF4E phosphorylation in WT, 1-KO, 2-KO, and Mnk1/Mnk2 DKO MEFs. Both rapamycin and RAD001 increased eIF4E phosphorylation in WT, 1-KO, and 2-KO MEFs but not in Mnk1/Mnk2 DKO MEFs. In fact, we did not detect basal levels of p-eIF4E in Mnk1/Mnk2 DKO MEFs. In contrast, p-p70S6K levels were inhibited and p-Akt levels were increased in all of the cell lines (Fig. 5B), indicating that Mnk deficiency does not affect mTOR inhibitor-induced inhibition of p70S6K phosphorylation and increase in Akt phosphorylation. Collectively, these results indicate that mTOR inhibitors increase eIF4E phosphorylation through a Mnk-dependent mechanism that involves both Mnk1 and Mnk2. We noted that the basal levels of p-p70S6K and p-Akt in Mnk1/Mnk2 DKO MEFs were lower than those in WT, 1-KO, and 2-KO MEFs (Fig. 5B). Whether Mnks are linked to regulation of the basal levels of p-Akt and p-p70S6K needs to be investigated in the future.
FIG. 5.
mTOR inhibitors increase eIF4E phosphorylation through a Mnk-dependent mechanism. (A) Knockdown of Mnk1 does not abrogate rapamycin-induced eIF4E phosphorylation. H157 cells that were not transfected (NT) or were transfected with control (Ctrl) or Mnk1 siRNA for 48 h were treated with 10 nM rapamycin (Rap). After 3 h, the cells were subjected to preparation of whole-cell protein lysates. (B) Modulation of eIF4E phosphorylation by mTOR inhibitors in WT, 1-KO, 2-KO, and Mnk1/Mnk2 DKO cells. The indicated MEF lines were treated with 10 nM rapamycin (R) or RAD001 (R1) for 3 h and then subjected to preparation of whole-cell protein lysates. The indicated proteins in these experiments were detected by Western blot analysis.
Rapamycin increases Mnk phosphorylation in a PI3K-dependent manner.
Given the involvement of both PI3K and Mnks in rapamycin-induced eIF4E phosphorylation, we were interested in understanding the relationship between PI3K and Mnk activation caused by rapamycin treatment. Thus, we examined whether Mnks function downstream of PI3K by examining the effect of rapamycin on Mnk phosphorylation in p85-DKO cells. As presented in Fig. 4C, the basal levels of p-Mnk were lower in p85-DKO cells than in WT MEFs. p-Mnk levels were strongly increased in WT MEFs but only minimally in p85-DKO cells. These results suggest that inhibition of mTOR by rapamycin results in PI3K-dependent Mnk activation, leading to an increase in eIF4E phosphorylation.
Cotargeting mTOR and Mnk/eIF4E signaling augments inhibition of the growth of cancer cells.
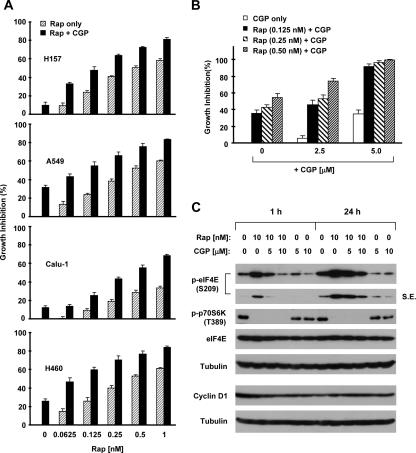
Given that the increase in eIF4E phosphorylation might counteract rapamycin's inhibitory effect on cap-dependent translation initiation, we hypothesized that cotargeting mTOR and Mnk/eIF4E signaling would result in augmented growth-inhibitory effects in cancer cells. Thus, we examined the effects of rapamycin combined with the Mnk1 inhibitor CGP57380 on the growth of several lung cancer cell lines. Although CGP57380 was originally identified as a Mnk1 inhibitor, this agent in fact also inhibits Mnk2-mediated eIF4E phosphorylation (8). As presented in Fig. 6A, in a 3-day assay, the combination of rapamycin and CGP57380 was more potent than each single agent alone in inhibiting the growth of four human lung cancer cell lines, which was either additive or synergistic. Moreover, we confirmed the effect of this combination in a long-term colony formation assay. Compared with the effects of rapamycin alone, the addition of CGP57380 enhanced rapamycin's ability to inhibit colony formation and growth (Fig. 6B). To demonstrate whether CGP57380 indeed blocked rapamycin-induced eIF4E phosphorylation, we further treated cells with rapamycin in the absence and presence of CGP57380 and analyzed eIF4E phosphorylation in these cells by Western blotting. As shown in Fig. 6C, the presence of CGP57380 inhibited rapamycin-induced eIF4E phosphorylation, particularly at an early time (e.g., 1 h), whereas inhibition of p70S6K phosphorylation by rapamycin was not affected. Thus, these data clearly show that CGP57380 abrogates rapamycin-induced eIF4E phosphorylation without interfering with rapamycin's inhibitory effect on p70S6K phosphorylation. We noted that rapamycin alone did not apparently reduce the levels of cyclin D1, a cap-dependent protein known to be regulated by mTOR signaling. However, the cotreatment with rapamycin and CGP57380 did reduce cyclin D1 levels at early times (e.g., 1 h), at which rapamycin-induced eIF4E phosphorylation was substantially inhibited by CGP57380 (Fig. 6C).
FIG. 6.
Inhibition of Mnk-dependent eIF4E phosphorylation by a Mnk1 inhibitor (C) augments rapamycin-mediated growth inhibition (A and B) in human lung cancer cells. (A) The individual cell lines, as indicated, were seeded in 96-well plates. On the next day, they were treated with the indicated concentrations of rapamycin (Rap) alone, 2.5 μM CGP57380 (CGP) alone, and their combination. After 3 days, the plates were subjected to determination of cell numbers using the sulforhodamine B assay. The bars are means of four replicate determinations plus standard deviations. (B) H460 cells at a density of ∼ 250 cells per well were seeded in 12-well plates. On the second day, the cells were treated with the indicated concentrations of rapamycin (Rap) alone, CGP57380 (CGP) alone, and their combination. The same treatments were repeated every 3 days. After 10 days, the plates were stained for the formation of cell colonies with crystal violet. The colonies in each well were counted. The bars are means of three replicate determinations plus standard deviations. (C) H157 cells were pretreated with the indicated concentrations of CGP57380 (CGP) for 30 min and then cotreated with 10 nM rapamycin (Rap) for 1 h or 24 h. The cells were then subjected to preparation of whole-cell protein lysates for detection of the indicated proteins using Western blotting.
DISCUSSION
Our previous study showed that rapamycin increases eIF4E phosphorylation in human lung cancer cells (20). In the current study, we further show that, in addition to rapamycin, the clinically tested mTOR inhibitor RAD001 also increases eIF4E phosphorylation in various types of cancer cells, including lung cancer cells. Collectively, these findings provide compelling evidence that mTOR inhibitors increase the phosphorylation of eIF4E, a critical survival protein, while inhibiting mTOR signaling. Moreover, disruption of mTOR/raptor function by knocking down the expression of mTOR or raptor mimicked rapamycin's effect of increasing eIF4E phosphorylation. In cells where mTOR expression was substantially knocked down, the effect of rapamycin on the increase of eIF4E phosphorylation was attenuated. Thus, we conclude that mTOR inhibitors increase eIF4E phosphorylation secondary to inhibition of mTOR/raptor signaling. This is further supported by our finding that knockdown of rictor did not alter the basal levels of p-eIF4E and prevented rapamycin from increasing eIF4E phosphorylation.
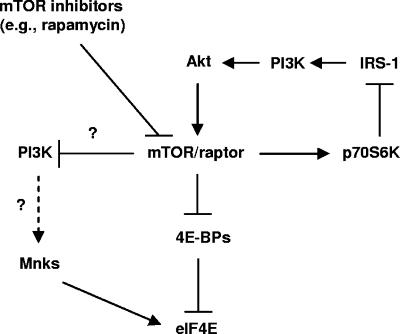
Our previous work clearly demonstrated that rapamycin increases eIF4E phosphorylation while inhibiting 4E-BP1 phosphorylation (20). eIF4E function is known to be negatively regulated by its reversible association with the 4E-BPs, including 4E-BP1. Hyperphosphorylated 4E-BPs dissociate from eIF4E, leading to eIF4E activation, whereas hypophosphorylated 4E-BPs associate strongly with eIF4E and inhibit its phosphorylation and activity (15). Because eIF4E, when bound to 4E-BP1, is no longer a substrate for Mnks (15), it is unlikely that eIF4E phosphorylation induced by an mTOR inhibitor is secondary to inhibition of 4E-BP1 phosphorylation. Moreover, we found that knockdown of p70S6K expression using p70S6K siRNA did not affect rapamycin's effect on the increase of eIF4E phosphorylation, suggesting that rapamycin-induced eIF4E phosphorylation is not secondary to inhibition of p70S6K phosphorylation. Taken together, we suggest that eIF4E phosphorylation is likely a parallel event to inhibition of phosphorylation of p70S6K and 4E-BP1 during mTOR inhibition (Fig. 7).
FIG. 7.
Schema for PI3K-dependent and Mnk-mediated eIF4E phosphorylation during mTOR inhibition in human cancer cells. In the literature, it has been suggested that mTOR/raptor exerts feedback inhibition of PI3K/Akt via p70S6K-mediated phosphorylation and degradation of IRS-1. Our results in this study suggest that mTOR/raptor can also negatively regulate PI3K activity through an unknown mechanism independently of p70S6K/IRS-1. Moreover, we suggest that PI3K may regulate Mnk activity. Thus, we propose that inhibition of mTOR/raptor with an mTOR inhibitor (e.g., rapamycin) can increase PI3K activity independently of p70S6K/IRS-1, resulting in a Mnk-mediated increase in eIF4E phosphorylation.
In addition to eIF4E phosphorylation, Akt phosphorylation is also increased by mTOR inhibitors, as demonstrated previously (14, 20), albeit in a cell-type-specific response (19). We found that inhibition of Akt actually increased eIF4E phosphorylation and enhanced rapamycin's effect on the increase of eIF4E phosphorylation. These results suggest that Akt negatively regulates eIF4E phosphorylation. Thus, we also conclude that eIF4E phosphorylation during mTOR inhibition is unlikely to be due to activation of Akt.
We previously suggested that rapamycin increases eIF4E phosphorylation through a PI3K-mediated mechanism using a PI3K inhibitor (20). In the current study, we further showed that the PI3K inhibitor LY294002 or wortmannin blocked eIF4E phosphorylation by RAD001, a derivative of rapamycin. Moreover, in PI3K-deficient MEFs, rapamycin lost its activity in increasing eIF4E phosphorylation. Thus, these results clearly indicate that mTOR inhibitors indeed increase eIF4E phosphorylation in a PI3K-dependent fashion. This notion is further supported by our finding that activation of PI3K by enforcing expression of constitutively active p110 elevated p-eIF4E levels (Fig. 4).
It has been suggested that mTOR activation (e.g., by insulin) initiates a feedback inhibition of PI3K/Akt through p70S6K activation and its subsequent phosphorylation and degradation of IRS-1 (Fig. 7). Rapamycin suppresses p70S6K and thus may relieve this negative-feedback inhibition of Akt (6, 12). In our current study, we have demonstrated that rapamycin-induced eIF4E phosphorylation is independent of p70S6K and IRS-1 (Fig. 1), although rapamycin does activate PI3K (Fig. 4E) and induce PI3K-mediated eIF4E phosphorylation (Fig. 4A to D). Thus, these findings argue that mTOR inhibition may activate PI3K signaling through another unknown mechanism(s), which is currently under investigation in our laboratory.
It is known that Mnks, particularly Mnk1, phosphorylate eIF4E at Ser209 (15, 16). In our study, we found that knockdown or KO of Mnk1 was not sufficient to abrogate mTOR inhibitors’ effects on eIF4E phosphorylation. Also, KO of Mnk2 was not able to abrogate mTOR inhibitors’ effects on eIF4E phosphorylation. eIF4E phosphorylation by mTOR inhibitors was abolished only when both Mnk1 and Mnk2 were knocked out. Thus, we conclude that both Mnk1 and Mnk2 are responsible for mTOR inhibitor-induced eIF4E phosphorylation. The question remained how mTOR inhibition increases Mnk activity. Mnks are known to be activated by ERK and p38 MAPKs (10, 15). However, our preliminary results suggest that rapamycin increases eIF4E phosphorylation independently of ERK and p38 because a MEK inhibitor or a p38 inhibitor failed to inhibit rapamycin-induced eIF4E phosphorylation (20). Interestingly, our previous work (20) and current results demonstrate that rapamycin, as well as RAD001, induced a PI3K-dependent phosphorylation of eIF4E because these mTOR inhibitors failed to increase eIF4E phosphorylation in the presence of the PI3K inhibitor LY294002 or wortmannin or in PI3K (p85)-deficient cells. Moreover, PI3K (p85) deficiency attenuated rapamycin's ability to increase Mnk1 phosphorylation, an event that activates Mnk1. Thus, we suggest that mTOR inhibition by mTOR inhibitors induced PI3K-dependent activation of Mnks, leading to an increase in eIF4E phosphorylation (Fig. 7). To the best of our knowledge, this is the first demonstration that links PI3K to modulation of Mnks.
mTOR inhibitors suppress 4E-BP1 phosphorylation, which presumably inhibits eIF4E phosphorylation or activity, leading to inhibition of cap-dependent translation (3). Paradoxically, our results clearly show that mTOR inhibitors also increase eIF4E phosphorylation while still inhibiting 4E-BP1 phosphorylation. Since eIF4E phosphorylation by mTOR inhibitors occurs very rapidly, which almost parallels inhibition of 4E-BP1 phosphorylation (20), we assume that an increase in eIF4E phosphorylation by mTOR inhibitors will counteract their effects on inhibition of cap-dependent translation. Thus, the net effect of an mTOR inhibitor on cap-dependent translation depends on whether the mTOR inhibitor-induced suppression of 4E-BP1 phosphorylation overrides its effect on eIF4E phosphorylation. Indeed, we found that rapamycin did reduce cyclin D levels in human prostate cancer cells while having minimal effects on downregulation of cyclin D1 in several lung cancer cell lines (our unpublished preliminary data). It needs to be pointed out that several other studies have also shown that eIF4E phosphorylation may not necessarily be involved in the assembly of the eIF4F complex or general protein synthesis (7, 8, 13, 22). Nonetheless, the impact of mTOR inhibition-induced eIF4E phosphorylation on cap-dependent translation, particularly in cancer cells, needs further investigation.
eIF4E phosphorylation or activation is associated with cell proliferation, transformation, tumor progression, and apoptosis resistance. Therefore, an increase in eIF4E phosphorylation during mTOR inhibition may counteract mTOR inhibitors’ growth-inhibitory effects on cancer cells. Since mTOR inhibitor-induced eIF4E phosphorylation is PI3K and Mnk dependent, one strategy to improve an mTOR inhibitor's efficacy against cancer, particularly those with high levels of eIF4E, is to prevent eIF4E phosphorylation by combining an mTOR inhibitor with a PI3K inhibitor or a Mnk inhibitor. Our previous work indeed showed that the combination of rapamycin and LY294002 exhibited synergistic growth-inhibitory effects in human lung cancer cells (20). In this study, we have further demonstrated that the combination of rapamycin and the Mnk1 inhibitor CGP57380 (which inhibits Mnk2, as well) also exerts augmented growth-inhibitory effects on a panel of human lung cancer cells (Fig. 7). Thus, our findings in this study are of translational significance from a clinical cancer treatment point of view using mTOR inhibitors.
Supplementary Material
Acknowledgments
We are grateful to D. Durden, J. Chen, B. Vogelstein, C. R. Kahn, and L. Cantley for kindly providing some cell lines used in this work. We also thank X. Liu in our laboratory for his help with siRNA designs and advice and H. Elrod for editing of the manuscript.
This study was supported by the Georgia Cancer Coalition Distinguished Cancer Scholar award (to S.-Y.S.), National Institute of Health grant RO1 CA118450-01 (to S.-Y.S.), and Department of Defense grant IMPACT W81XWH-05-0027 (Project 5 to F.R.K. and S.-Y.S.). S.-Y. S. and F.R.K. are Georgia Cancer Coalition Distinguished Cancer Scholars.
Footnotes
Published ahead of print on 27 August 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Ahn, J. Y., R. Rong, X. Liu, and K. Ye. 2004. PIKE/nuclear PI 3-kinase signaling mediates the antiapoptotic actions of NGF in the nucleus. EMBO J. 23: 3995-4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjornsti, M. A., and P. J. Houghton. 2004. Lost in translation: dysregulation of cap-dependent translation and cancer. Cancer Cell 5: 519-523. [DOI] [PubMed] [Google Scholar]
- 3.Bjornsti, M. A., and P. J. Houghton. 2004. The TOR pathway: a target for cancer therapy. Nat. Rev. Cancer 4: 335-348. [DOI] [PubMed] [Google Scholar]
- 4.De Benedetti, A., and A. L. Harris. 1999. eIF4E expression in tumors: its possible role in progression of malignancies. Int. J. Biochem. Cell. Biol. 31: 59-72. [DOI] [PubMed] [Google Scholar]
- 5.Franch, H. A., X. Wang, S. Sooparb, N. S. Brown, and J. Du. 2002. Phosphatidylinositol 3-kinase activity is required for epidermal growth factor to suppress proteolysis. J. Am. Soc. Nephrol. 13: 903-909. [DOI] [PubMed] [Google Scholar]
- 6.Harrington, L. S., G. M. Findlay, and R. F. Lamb. 2005. Restraining PI3K: mTOR signalling goes back to the membrane. Trends Biochem. Sci. 30: 35-42. [DOI] [PubMed] [Google Scholar]
- 7.Herbert, T. P., G. R. Kilhams, I. H. Batty, and C. G. Proud. 2000. Distinct signalling pathways mediate insulin and phorbol ester-stimulated eukaryotic initiation factor 4F assembly and protein synthesis in HEK 293 cells. J. Biol. Chem. 275: 11249-11256. [DOI] [PubMed] [Google Scholar]
- 8.Knauf, U., C. Tschopp, and H. Gram. 2001. Negative regulation of protein translation by mitogen-activated protein kinase-interacting kinases 1 and 2. Mol. Cell. Biol. 21: 5500-5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lachance, P. E., M. Miron, B. Raught, N. Sonenberg, and P. Lasko. 2002. Phosphorylation of eukaryotic translation initiation factor 4E is critical for growth. Mol. Cell. Biol. 22: 1656-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahalingam, M., and J. A. Cooper. 2001. Phosphorylation of mammalian eIF4E by Mnk1 and Mnk2: tantalizing prospects for a role in translation. Prog. Mol. Subcell. Biol. 27: 132-142. [PubMed] [Google Scholar]
- 11.Mamane, Y., E. Petroulakis, L. Rong, K. Yoshida, L. W. Ler, and N. Sonenberg. 2004. eIF4E—from translation to transformation. Oncogene 23: 3172-3179. [DOI] [PubMed] [Google Scholar]
- 12.Manning, B. D. 2004. Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis. J. Cell Biol. 167: 399-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morley, S. J., and S. Naegele. 2002. Phosphorylation of eukaryotic initiation factor (eIF) 4E is not required for de novo protein synthesis following recovery from hypertonic stress in human kidney cells. J. Biol. Chem. 277: 32855-32859. [DOI] [PubMed] [Google Scholar]
- 14.O'Reilly, K. E., F. Rojo, Q. B. She, D. Solit, G. B. Mills, D. Smith, H. Lane, F. Hofmann, D. J. Hicklin, D. L. Ludwig, J. Baselga, and N. Rosen. 2006. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 66: 1500-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pyronnet, S. 2000. Phosphorylation of the cap-binding protein eIF4E by the MAPK-activated protein kinase Mnk1. Biochem. Pharmacol. 60: 1237-1243. [DOI] [PubMed] [Google Scholar]
- 16.Raught, B., and A. C. Gingras. 1999. eIF4E activity is regulated at multiple levels. Int. J. Biochem. Cell. Biol. 31: 43-57. [DOI] [PubMed] [Google Scholar]
- 17.Richter, J. D., and N. Sonenberg. 2005. Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature 433: 477-480. [DOI] [PubMed] [Google Scholar]
- 18.Sarbassov, D. D., D. A. Guertin, S. M. Ali, and D. M. Sabatini. 2005. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307: 1098-1101. [DOI] [PubMed] [Google Scholar]
- 19.Sarbassov, D. D., S. M. Ali, S. Sengupta, J. H. Sheen, P. P. Hsu, A. F. Bagley, A. L. Markhard, and D. M. Sabatini. 2006. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell 22: 159-168. [DOI] [PubMed] [Google Scholar]
- 20.Sun, S. Y., L. M. Rosenberg, X. Wang, Z. Zhou, P. Yue, H. Fu, and F. R. Khuri. 2005. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res 65: 7052-7058. [DOI] [PubMed] [Google Scholar]
- 21.Sun, S. Y., P. Yue, G. S. Wu, W. S. El-Deiry, B. Shroot, W. K. Hong, and R. Lotan. 1999. Mechanisms of apoptosis induced by the synthetic retinoid CD437 in human non-small cell lung carcinoma cells. Oncogene 18: 2357-2365. [DOI] [PubMed] [Google Scholar]
- 22.Ueda, T., R. Watanabe-Fukunaga, H. Fukuyama, S. Nagata, and R. Fukunaga. 2004. Mnk2 and Mnk1 are essential for constitutive and inducible phosphorylation of eukaryotic initiation factor 4E but not for cell growth or development. Mol. Cell. Biol. 24: 6539-6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von der Haar, T., J. D. Gross, G. Wagner, and J. E. McCarthy. 2004. The mRNA cap-binding protein eIF4E in post-transcriptional gene expression. Nat. Struct. Mol. Biol. 11: 503-511. [DOI] [PubMed] [Google Scholar]
- 24.Wan, X., B. Harkavy, N. Shen, P. Grohar, and L. J. Helman. 2006. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene 26: 1932-1940. [DOI] [PubMed] [Google Scholar]
- 25.Winnay, J. N., J. C. Bruning, D. J. Burks, and C. R. Kahn. 2000. Gab-1-mediated IGF-1 signaling in IRS-1-deficient 3T3 fibroblasts. J. Biol. Chem. 275: 10545-10550. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.