Abstract
The Wnt signaling pathway is deregulated in over 90% of human colorectal cancers. β-Catenin, the central signal transducer of the Wnt pathway, can directly modulate gene expression by interacting with transcription factors of the TCF/LEF family. In the present study we investigate the role of Wnt signaling in the homeostasis of intestinal epithelium by using tissue-specific, inducible β-catenin gene ablation in adult mice. Block of Wnt/β-catenin signaling resulted in rapid loss of transient-amplifying cells and crypt structures. Importantly, intestinal stem cells were induced to terminally differentiate upon deletion of β-catenin, resulting in a complete block of intestinal homeostasis and fatal loss of intestinal function. Transcriptional profiling of mutant crypt mRNA isolated by laser capture microdissection confirmed those observations and allowed us to identify genes potentially responsible for the functional preservation of intestinal stem cells. Our data demonstrate an essential requirement of Wnt/β-catenin signaling for the maintenance of the intestinal epithelium in the adult organism. This challenges attempts to target aberrant Wnt signaling as a new therapeutic strategy to treat colorectal cancer.
The mucosa of the small intestine is composed of flask-shaped submucosal invaginations known as crypts of Lieberkühn and finger-like luminal protrusions termed villi. Crypts are composed of a mainly monoclonal, proliferative compartment, whereas villi are characterized by differentiated cells from various lineages and are polyclonal as they receive cells from multiple crypts (20). Crypts contain intestinal stem cells which currently cannot be identified morphologically or distinguished from other intestinal epithelial cells by any accepted set of markers. Intestinal stem cells are localized in the crypt above the Paneth cells based on long-term label-retaining assays (20). Each active stem cell appears to give rise to two distinct populations of transient-amplifying cells, one committed to producing absorptive enterocytes and the other committed to producing secretory cells of the goblet, Paneth, and endoenterocrine lineages (5, 20). Paneth cells are the only lineage that completes its differentiation at the crypt base; the members of the three other lineages finalize their differentiation as they migrate out of the crypt onto adjacent villi. The migration of these differentiated cells terminates at a cellular extrusion zone located near the villus tip. There, cells die by apoptosis and are shed into the intestinal lumen. This cellular cycle is completed within 3 to 5 days in mice (5, 20).
One of the major players involved in the establishment of tissue architecture during development and in homeostasis of a variety of adult tissues is the canonical Wnt/β-catenin signaling pathway (8). β-Catenin is an essential cytoplasmic signal transducer of this canonical Wnt pathway (2). In the absence of pathway stimulation by Wnt ligands, β-catenin is phosphorylated and targeted for degradation. The degradation complex responsible for β-catenin destabilization contains the tumor suppressor gene products axin or conductin and adenomatous polyposis coli (APC) as well as glycogen synthase kinase 3β and casein kinase I (CKI). Upon Wnt ligand binding to Frizzled and/or LRP transmembrane receptors, the cytoplasmic protein Disheveled is activated and blocks the action of the degradation complex. β-Catenin is then able to enter the nucleus and associate with TCF/LEF transcription factors, thus inducing transcriptional regulation of Wnt target genes (2). Colorectal cancers, the second most common human malignant tumor type, are by and large initiated by mutations that activate the Wnt signaling pathway (2). These tumors are characterized by truncating mutations in APC and axin, as well as mutations in the degradation-inducing phosphorylation sites in β-catenin, all leading to the formation of constitutive nuclear β-catenin/TCF complexes (8).
Several studies of Wnt signaling demonstrate its effect on stem cells from various tissues. In mouse epidermis, conditional ablation of the β-catenin gene blocked the differentiation of the bulge stem cells into follicular lineages (13). TCF3, a transcription factor of the Wnt signaling cascade, is preferentially expressed in the bulge stem cell compartment and has been suggested to maintain the stem cell pool (22). Activation of Wnt signaling in hematopoietic stem cells triggered increased self-renewal, whereas overexpression of axin, which can inactivate the pathway, led to a reduced reconstitution efficiency (31). In neural cells, inactivation of the Wnt pathway decreased the expansion of the progenitor compartment, whereas activation of the pathway increased that compartment (42). Several in vivo approaches based on inactivating and overactivating mutations of various pathway components were undertaken in order to elucidate the role of Wnt/β-catenin signaling in intestinal epithelium (14, 16, 17, 27, 35, 39, 40). During embryonic development, TCF4 was shown to be required to maintain the proliferative compartment of the intestinal epithelium, as gene ablation leads to neonatal epithelium entirely composed of differentiated, nondividing cells (16). In the adult, alterations in Wnt signaling also indicated an important function of the pathway in intestinal proliferation and Paneth cell differentiation (14, 16, 17, 27, 35, 39, 40). However, all these studies reported rather mild and only transient phenotypes, making it sometimes hard to distinguish whether the observed phenotypes were direct results of pathway inactivation or part of the recovery mechanism. Moreover, the role of Wnt signaling in the control of intestinal stem cells was not directly addressed.
In this study, we determine the immediate consequences of β-catenin loss in the intestinal epithelium of adult mice. Inactivation of β-catenin leads to a rapid loss of intestinal epithelial cells, starting with the loss of crypts that concurs with blocked proliferation and increased enterocytic differentiation. Importantly, intestinal stem cells are induced to terminally differentiate in the absence of Wnt signaling, resulting in fatal loss of intestinal function.
MATERIALS AND METHODS
Animal models.
The alleles for the null and the floxed β-catenin (13), the Wnt reporter mouse strain with the lacZ knock-in at the conductin locus (19), and the villin-creERT2 (9) have been described previously. β-Catenin ablation was induced by intraperitoneal injection of 1 mg of tamoxifen (Sigma) per 20 g of body weight into 10-week-old-mice, for 2 to 6 consecutive days before analysis, as employed by Bettess et al. (3). Both control (β-catenin+/lox-villin-creERT2) and inducible mutant (β-catenin−/lox-villin-creERT2) animals were injected according to the same dosage regimen. For example, a “day 3” experiment utilizes injection at 0 h, 24 h, and 48 h and sacrifice of the animal after 72 h. Since control animals displayed no phenotype compared to uninjected animals, we also refer to them as “wild type.” We routinely assessed the efficiency of β-catenin deletion by immunohistochemistry and observed efficient deletion in all intestinal epithelial cells starting at day 2 after induction. While all subsequent assays were performed after 1 to 5 days of deletion, we show only the time points when we first observed clear phenotypical changes. For short-term bromodeoxyuridine (BrdU) incorporation experiments, mice were injected with 1 mg of BrdU (Sigma) 2 h before sacrifice. For the BrdU migration assay, mice were injected once with 1 mg BrdU 2 or 3 days before sacrifice.
β-Galactosidase assay.
Whole-mount samples from small intestines were isolated; washed with phosphate-buffered saline (PBS); fixed at 4°C with 2.5% glutaraldehyde in PBS, pH 7.4, for 30 min; rinsed in PBS, and incubated in X-Gal solution (1 g/liter X-Gal [5-bromo-4-chloro-3-indolyl-β-d-galactoside] in PBS, pH 7.4, 5 mmol/liter of potassium ferrocyanide, 5 mmol/liter of potassium ferricyanide, 2 mmol/liter of MgCl2, and 0.2% Triton X-100) overnight in the dark at 37°C. Subsequently, tissue samples were washed with PBS and embedded in plastic (Technovit7100). Sections of 4 μm in thickness were counterstained with eosin, dehydrated through an ethanol series, and embedded in Entellan. Relative LacZ signal intensity was assessed as a percentage of maximal activity. Proliferation was assessed as the number of cells in metaphase/anaphase per position in the crypt. For both analyses, >100 crypts were counted.
Immunohistochemistry and in situ hybridizations.
Intestinal tissue for immunohistochemistry and hematoxylin and eosin (H&E) staining was cleaned with PBS, fixed in 4% formaldehyde, and embedded in paraffin. Sections of 4 μm in thickness were incubated with the following primary antibodies: β-catenin (BD Bioscience), BrdU (Sigma), p21 (BD Bioscience), active caspase 3 (Cell Signaling), CD44 (gift from Andreas Trumpp), lysozyme (DAKO), and FabpL (gift from Jeffrey Gordon). Envision+ (DakoCytomation) was used as a secondary reagent, and stainings were developed with diaminobenzidine. Hematoxylin was used for counterstaining. For in situ hybridization, sense and antisense riboprobes were synthesized from cDNA fragments of mSox4 (NM_009238.2, nucleotides 662 to 1984) and mDiap3 (NM_019670.1, nucleotides 727 to 3516) and hybridized as described previously (11).
Electron microscopy.
Intestinal tissue was washed in PBS and fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer at 4°C overnight. Tissue was cut into 1-mm3 cubes for fixing. Postfixation was done in 1% osmium tetroxide in 0.1 M cacodylate buffer for 1 h (2 ml of 4% of aqueous OsO4, 4 ml of 0.2 M buffer, and 2 ml H2O were mixed and used immediately). After rinsing in 0.1 M buffer and dehydration, tissue was put in propylene oxide (1.2 epoxy propane) twice for 15 min. Propylene oxide/epoxy resin mixture (50/50) was then applied twice for 1 h at 20°C and then overnight at 4°C. Tissues were embedded in freshly prepared resin and left to polymerize at 60°C for 72 h. Pictures were taken with a Philips CM 10 transmission electron microscope.
Tritiated TdR labeling of LRCs.
Three-week-old mice were injected twice daily with 25 μCi of [3H]thymidine ([3H]TdR) for four consecutive days. Three weeks later, animals were injected with tamoxifen for 2, 3, and 4 days, respectively, and sacrificed the following day. Intestines were washed with PBS and fixed in 4% formaldehyde. Sections of 4 μm in thickness were prepared for autoradiography (K5 emulsion; Ilford) and exposed for 15 days. The sections were counterstained with nuclear fast red. Label-retaining cells (LRCs) were counted in transverse sections of the intestine. The threshold for detecting LRCs was set at five or more grains per nucleus.
Transcriptional profiling.
Intestinal tissue was frozen in OCT, and sections of 10 μm in thickness were applied on membrane slides for laser capture microdissection (LCM; Molecular Machines and Industries, part no. 50102), stained with eosin, dehydrated, and dissected using an mCut laser microdissection system (Nikon Eclipse TE200). RNA was isolated using the Pico Pure Isolation Kit (Arcturus). After RNA quality control by agarose gel electrophoresis and Agilent Bioanalyzer analysis, amplification was performed using the MessageAmp II aRNA Kit (Ambion) and labeling of the amplified RNA using the IVT Affymetrix kit. Biotin-labeled cRNA was hybridized on 430v 2.0 mouse Affymetrix arrays. Normalization and signal estimation were performed by RMA in the R package using the RACE vignette developed by the DNA Array Facility, Lausanne, Switzerland. Statistical analysis was performed using the Bayes test, and only deregulated genes with changes above 1.5-fold and a P value threshold below 5% were considered for further analysis. Gene ontology and pathway analysis were performed using GenMAPP software.
Nucleotide sequence accession number.
The complete microarray data set is deposited in the GEO database (http://www.ncbi.nlm.nih.gov/geo) under accession number GSE8818.
RESULTS
Loss of Wnt/β-catenin signaling in the intestinal epithelium is lethal due to a rapid block of intestinal homeostasis.
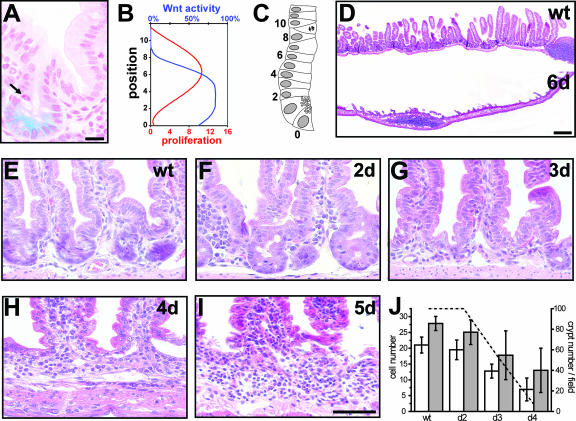
In order to visualize endogenous Wnt signaling activity in the intestine, we used a reporter mouse strain with a lacZ knock-in at the conductin locus (19). Conductin is a known, general target gene of Wnt signaling. The strongest Wnt activity is observed in the lower half of the intestinal crypt, in Paneth cells, and between cell positions 2 and 6 (Fig. 1A to C). This localization of Wnt activity is only partially overlapping with proliferating, transient-amplifying cells, since their peak distributions are situated between cell positions 6 and 8 as evidenced by the presence of mitotic figures (Fig. 1A to C). Thus, Wnt signaling activity is restricted to intestinal crypts and is strongest in the area where intestinal stem cells are located (cf. Fig. 5A) as opposed to rapidly proliferating transient-amplifying cells.
FIG. 1.
Active Wnt signaling is required for crypt maintenance. (A to C) Wnt activity versus cell proliferation in the crypts. (A) Localization of Wnt activity using conductin-lacZ reporter mice. The arrow shows the mitotic figure. Bar, 20 μm. (B) Schematic representation of the localization of Wnt activity (blue; arbitrary units) compared to cell proliferation (red; cells in metaphase/anaphase) along the crypt-villus axis, from Paneth cells up to position 10 above Paneth cells in the crypt. (C) Schematic representation of an intestinal crypt. Numbers represent cell positions from Paneth cells at the bottom of the crypt corresponding to position 0 up to position 10 above them. A cell in metaphase/anaphase is represented at position 9. (D) H&E staining of intestinal sections at day 6 after β-catenin ablation. Note that the intestinal epithelium is largely lost in the mutants. Bar, 200 μm. (E to I) H&E staining of the crypt area. Mutants show progressive crypt loss starting from day 2 (F) after induction and leading to the establishment of an extended intervillus region by day 4 (H). Bar, 100 μm. (J) Quantification of morphological changes was performed from 200 crypt/villus units per sample and three mice per group. Left axis: white bars show numbers of epithelial cells per crypt and gray bars show total numbers of epithelial cells which are not part of a villus (intervillus region). Error bars depict standard deviations. Right axis: dashed line represents crypt number per field as a percentage. wt, wild type.
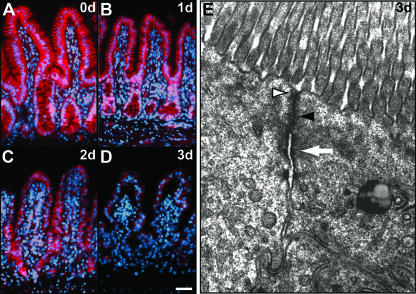
FIG. 5.
LRCs undergo differentiation in mutant mice. (A to C) Autoradiography of mouse intestine injected with tritiated TdR 20 days after labeling. In wild-type epithelium (A), LRCs (arrow) are found in the crypts above Paneth cells. In 4-day mutant epithelium (B), LRCs (arrow) are found in similar numbers but at aberrant positions. Bar, 25 μm. (C) Scheme of the localization pattern of LRCs in control (left) and mutants for which the frequency of LRCs in a certain position is depicted as black lines of different widths. Initially, LRCs in uninduced β-catenin−/lox mice (0d) are localized as in the control. Two days after β-catenin deletion, mutant LRCs are dispersed throughout the crypt area (data not shown), followed by an even distribution along the crypt-villus axis at day 4 (4d). We quantified 300 crypt-villus units for two mice per group. In both groups, about 20% of crypts (control) or crypt-villus units (mutant) were found to contain LRCs, which were scored as positive when retaining more than five grains per nucleus. (D and E) Counterstaining for the enterocytic differentiation marker FabpL demonstrates that LRCs (arrows) are differentiated in the mutant 4 days after β-catenin deletion (E), whereas they remain undifferentiated in the control (D). The picture gives a representative example; all LRCs in the mutants express FabpL at this time point. Bar, 25 μm. (F to I) In vivo intestinal epithelial cell migration assay. Proliferating cells were BrdU pulse-labeled immediately prior to tamoxifen injection, and subsequently migrating cells were localized by immunohistochemistry at day 2 (F and G) and day 3 (H to I) after tamoxifen injection in control (F and H) and mutant (G and I) epithelium. Bar, 50 μm. wt, wild type.
We generated a mouse model to study the role of Wnt/β-catenin signaling in intestinal homeostasis. This model uses a null and a floxed allele of the β-catenin gene (13) in combination with a tamoxifen-inducible variant of the cre recombinase (creERT2) expressed under the control of the intestinal villin promoter which allows spatial and temporal control of β-catenin deletion (9). In villin-creERT2 mice, all intestinal epithelial cells, including stem cells, undergo cre-mediated recombination as demonstrated by long-term follow-up analysis (9). Using this mouse system, we induced the ablation of the β-catenin gene in adult mice for various periods of time. This results in loss of all intestinal epithelial cells in mutant mice (villin-creERT2-β-catenin−/lox) within 6 days after induction (Fig. 1D), while control mice (villin-creERT2-β-catenin+/lox) show no phenotype and are henceforth referred to as “wild type.” Subsequently, mice had to be euthanized before they succumbed to the consequences of the loss of intestinal function. The first phenotypical changes are observed in the crypt area within 2 days after β-catenin deletion (Fig. 1E and F), when mutant crypts expand without apparent changes in cell number (Fig. 1J). Subsequently, number and cellularity of crypts decrease concomitantly with a change in crypt shape, giving rise to flattened intervillus regions at day 3 (Fig. 1G and 1J). After 4 days, crypts are virtually lost in response to β-catenin ablation (Fig. 1E to J). Villus size also progressively decreases and reaches about 50% of the control at day 4 post-β-catenin ablation.
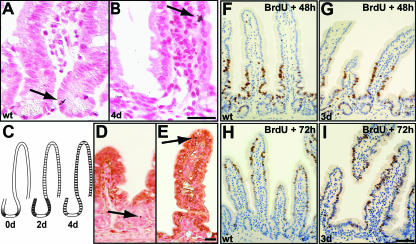
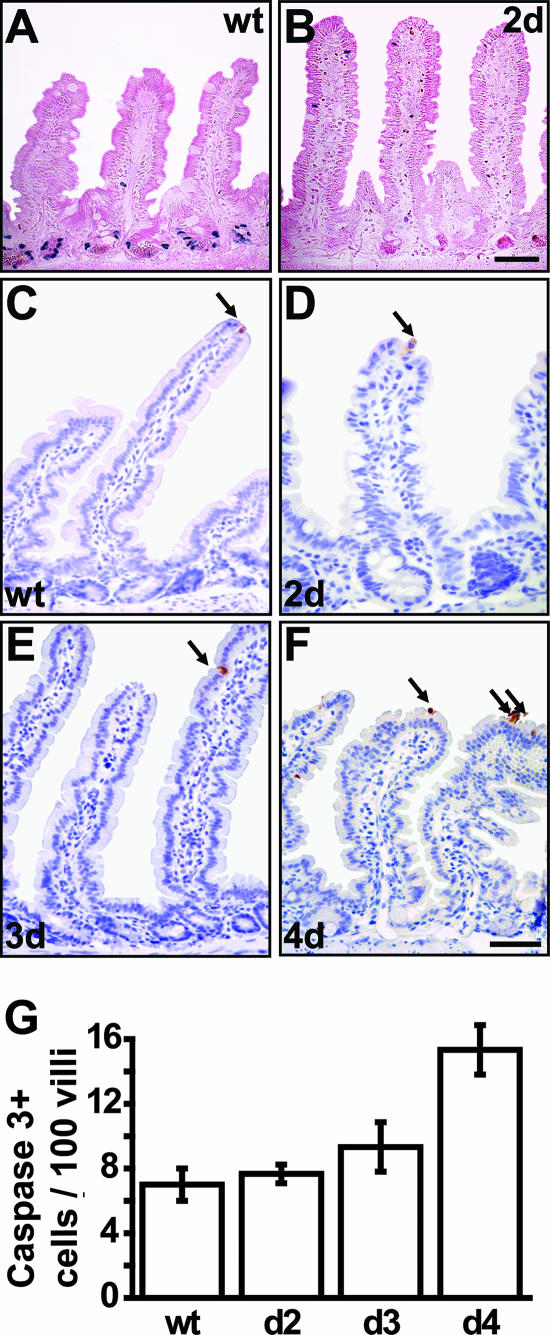
The observed changes in crypt morphology coincide with drastic changes in crypt cell proliferation. While 1 day after deletion mutant crypts display unaltered proliferation, a complete block of proliferation is observed after the second day of β-catenin deletion, as assessed by short-term BrdU incorporation (Fig. 2A and B) (see also the data in the supplemental material). Furthermore, known components of cell cycle control are found to be altered in β-catenin mutants. Expression of the Wnt target gene c-myc is decreased whereas the cell cycle inhibitor p21 is found to be increased in intervillus regions (data not shown). Increased apoptosis is not responsible for loss of crypts in β-catenin mutants as analyzed by immunohistochemistry for active caspase 3, a hallmark of apoptotic cell death (Fig. 2C to G). However, mutants show a minor increase of apoptosis at the tips of the villi 4 days after ablation (Fig. 2F and G). This late apoptotic event closely reflects the normal life span of these differentiated cells, which migrate up the villus, undergo apoptosis at the villus tip, and are shed into the lumen of the gut within 4 to 5 days in wild-type animals (cf. Fig. 2C).
FIG. 2.
Cell proliferation and death as a result of β-catenin ablation in the intestinal epithelium. (A and B) Complete loss of cell proliferation as assessed by short-time BrdU incorporation 2 days after β-catenin ablation. (C to F) Immunostaining for active caspase 3 (arrows) shows no increase in apoptotic features in mutant crypts up to day 3 (E). However, a moderate increase is observed at day 4 in mutant villus tips (F). Bars, 50 μm. (G) Quantification of apoptosis expressed as number of caspase 3-positive cells per 100 villi. For quantification, 200 villi were counted per mouse and three mice per group. wt, wild type.
Since β-catenin has an important role in the maintenance of cell-to-cell adherens junctions (2), crypt loss could be due to a defect in cell adhesion. β-Catenin protein is still found at cell membranes 2 days after cre-mediated recombination (Fig. 3A to D), indicating that defects in adhesion cannot account for early phenotypes such as the block in proliferation. In addition, we confirmed by electron microscopy that junctional complexes are not affected 3 days after loss of β-catenin (Fig. 3E). The presence of normal adherens junctions, desmosomes, and tight junctions in the mutants at a time point when the β-catenin protein is already missing at the cell membrane (cf. Fig. 3D) strongly suggests that the loss of intestinal epithelial cells is not due to a disruption of cell adhesion but rather due to a process involving β-catenin's role in canonical Wnt signaling.
FIG. 3.
Loss of intestinal epithelial cells is due to canonical Wnt signaling. (A to D) Immunofluorescence time course for β-catenin protein expression reveals its presence in adherens junctions up to day 2 (C) after induction. Bar, 50 μm. (E) Electron micrograph of a mouse intestinal crypt region. Junctional complexes such as tight junctions (white arrowhead), adherens junctions (black arrowhead), and desmosomes (white arrow) remain unaffected 3 days after β-catenin deletion. Magnification, ×23,000.
Impact on intestinal lineage differentiation upon β-catenin ablation.
An event that could be causal for the loss of crypts in the β-catenin mutants is premature differentiation. Immunohistochemical analysis of CD44 expression, a marker of crypt progenitor cells, reveals progressive loss of CD44-positive cells in crypts and the newly formed intervillus regions of mutant mice (Fig. 4A to C). Instead, these regions are found to express markers of enterocytic differentiation. In the wild-type mouse intestine, alkaline phosphatase enzymatic activity is characteristic for villus cells and absent from the crypt region; in contrast, alkaline phosphatase activity is abundant in the intervillus region of the 4-day β-catenin mutants (Fig. 4D and E).
FIG. 4.
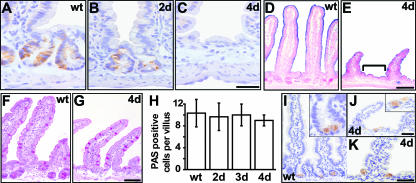
Impact on differentiation upon β-catenin ablation in intestinal epithelium. (A to C) Immunostaining for CD44 reveals progressive loss of the crypt phenotype in mutants (B and C) between day 2 and day 4. Bar, 10 μm. (D and E) The enterocytic differentiation marker alkaline phosphatase (as detected by blue nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate staining) is absent from wild-type (D) crypts but is present in mutant (E) intervillus regions (bracket). (F to H) PAS staining for goblet cells reveals unchanged numbers. The graph shows quantification of goblet cell numbers expressed as numbers of PAS stain-positive cells per villus (H). Two hundred villi were counted per mouse and three mice per group. (I to K) Immunostaining for lysozyme shows Paneth cell localization in wild-type crypts (I) and in mutants (J and K). Insets show higher magnifications for panels I and J, respectively. Bar, 20 μm. wt, wild type.
Goblet cells do not seem to be affected in β-catenin mutants, as analyzed by periodic acid-Schiff (PAS) staining (Fig. 4F to H). However, given the relatively long life span of goblet cells and the rapid death of mutant mice, late effects on goblet cell differentiation might have been missed. Paneth cells show aberrant morphology, as lysozyme-containing vesicles are enlarged in size and reduced in number, and cells become mislocalized upon β-catenin deletion throughout the intervillus region (Fig. 4I to K). Again, as Paneth cells have a long life span, late effects on their function or differentiation could not be studied.
Differentiation of stem cells upon β-catenin ablation in intestinal epithelium.
Loss of progenitor fates suggested a possible defect of intestinal stem cell function upon deletion of Wnt/β-catenin signaling. Most tissue-specific stem cells divide infrequently, and this characteristic can be employed to identify these cells in situ. After saturated labeling of all dividing cells by tritiated [3H]TdR, cells which divide infrequently will retain the label over long periods of time (so-called LRCs), while cells which cycle more often will lose the label. We injected 3-week-old mice twice daily with tritiated TdR for four consecutive days. Three weeks later we induced the ablation of β-catenin for 2 to 4 days. As expected, wild-type mouse intestines contain label-retaining stem cells in about every fifth crypt at the previously reported cell position 2, immediately above the Paneth cells (Fig. 5A) (29). In the β-catenin deletion mutants, total LRC numbers are similar to those in the wild type; however, their distribution becomes aberrant. Observation of over 500 crypt-villus units revealed that LRCs disperse throughout the crypt area and are occasionally found in the villi within 2 days after deletion of β-catenin. Subsequently, LRCs become evenly distributed throughout the crypt-villus axis within 4 days (Fig. 5B and C). In order to evaluate whether cell migration of the intestinal epithelium is affected by inactivation of β-catenin, we pulse-labeled control and mutant mice with BrdU shortly before tamoxifen injection for 2 to 3 days. We thereby labeled a cohort of progenitor cell progeny which would differentiate and migrate towards the villus tip within the next few days. BrdU immunohistochemistry revealed that the overall cell migration rate remains unchanged in mutant mice (Fig. 5F to I). We therefore assessed next whether the dispersed LRCs might be migrating, differentiated cells. We performed dual stainings in order to detect LRCs by autoradiography and differentiated enterocytes by immunohistochemistry for the enterocyte marker FabpL. In 4-day mutants, we detected all LRCs colabeled with FabpL (Fig. 5E, n = 30), in contrast to control animals, where LRCs are restricted to crypts and do not express FabpL (Fig. 5D). This strongly suggests that β-catenin deletion causes forced differentiation of stem cells into the enterocytic lineage. This process of differentiation supports the loss of crypts and contributes to the block of proliferation.
Transcriptional changes underlying the loss of the intestinal progenitor compartment.
In order to understand the molecular mechanisms of crypt loss as a result of β-catenin deletion in the intestinal epithelium, we performed transcriptional profiling of intestinal crypts. We isolated crypt cells by LCM from wild-type and mutant mouse epithelium 2 days after induction of β-catenin deletion. After RNA isolation and assessment of RNA quality, RNA was amplified, and Affymetrix analysis of differential gene expression was performed (a complete list of differentially expressed genes is found in the supplemental material as Table S1).
Overall, the expression profile matches the phenotype observed by histological analysis. Loss of Wnt signaling activity is reflected by downregulation of β-catenin itself and of the Wnt target genes conductin and c-myc. It is further reflected by the upregulation of p21, which is transcriptionally repressed by c-myc (37). Indeed, in vitro transfection of colorectal cells with a dominant-negative form of TCF resulted in decreased c-myc, increased p21, and subsequent G1 cell cycle arrest and increased differentiation (37), which is consistent with our results. In line with this, other key genes of cell cycle control (i.e., ccnd2 and ccna2) are downregulated in our transcriptional profiling analysis, as are other known target genes of Wnt signaling such as ephB2 and ephB3. Disruption of these ephrin receptors has been linked to aberrant cell intermingling and mislocalization of Paneth cells (1) in accordance with our immunohistochemical analysis of Paneth cell localization (cf. Fig. 4I to K). Upregulated genes include the enterocytic differentiation marker carbonic anhydrase 4, concurring with our analysis of enhanced terminal differentiation. Together, these results support our conclusion that stem cells and transient-amplifying cells are arrested in the cell cycle and are forced into enterocytic differentiation as a result of β-catenin deletion (cf. Fig. 5E).
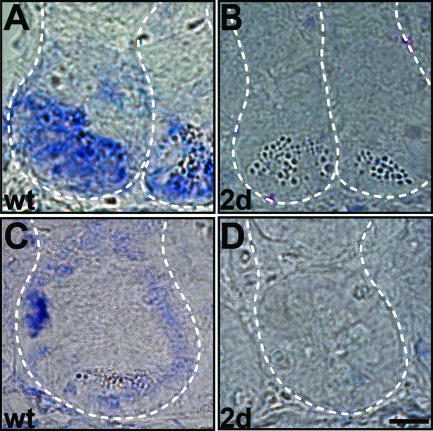
Recently, CD133 has been used as a marker to enrich for cancer stem cells responsible for the formation of human colorectal tumors (24, 32). However, markers which can unambiguously identify normal intestinal stem cells or intestinal cancer stem cells have not been identified. We reasoned that our list of genes functionally implicated in intestinal stem cell maintenance might contain such markers, and we therefore compared our list of genes with genes found to be deregulated in intestinal tumors as well as in other stem cell populations. In detail, we compared our gene list to transcriptional profiles from murine intestinal tumors of PTEN-deficient mice and APC (Min/+) and protein kinase Cα-deficient APC (Min/+) mice (12, 18, 25, 26, 30). We identified common genes that were upregulated in those arrays and downregulated in our β-catenin deletion arrays. Next, we compared the obtained gene list with transcriptomes of over 90 human colon adenomas and adenocarcinomas and narrowed the list down to genes that are also upregulated in at least 20% of those human arrays (4, 10, 23, 43). In order to focus on stemness-related genes, we next determined the overlap with genes that have been shown to be characteristic for various stem cell populations (15, 33). Numerous cell cycle-associated genes are deregulated both in our studies and in those used for comparison, due to either the slow cycling nature of stem cells or the elevated proliferation in tumors. We therefore excluded known cell cycle-related genes from our gene list, based on transcriptional profiling by Whitfield et al. (38) and Cho et al. (7) and on the Celera and Stanford online databases. Common deregulated genes matching all of the criteria described above are summarized in Table 1, which therefore lists candidates for intestinal stem and cancer stem cell markers. For six of these genes, stem cell-specific expression has been described independently based on analyses of human adenomas and in situ hybridization of normal intestines (36). We confirmed stem cell expression by in situ hybridization for several others such as Sox4, which is also expressed by Paneth cells, and Diap3 (Fig. 6A and C; also data not shown). Importantly, we find expression of both genes to be strongly repressed in mutant mouse crypts in agreement with the loss of the stem cell phenotype after loss of β-catenin signaling (Fig. 6B and D).
TABLE 1.
Downregulated genes in β-catenin-knockout crypts which overlap with genes characteristic for other stem cell populations and intestinal tumorsa
| Gene symbol | Gene name | Change in expression (fold) |
|---|---|---|
| Ankrd10 | Ankyrin repeat domain 10 | 0.54 |
| Apex1* | Apurinic/apyrimidinic endonuclease 1 | 0.44 |
| Ascl2* | Achaete-scute complex homolog-like 2 (Drosophila) | 0.40 |
| Bcl7c | B-cell CLL/lymphoma 7C | 0.64 |
| Diap3 | Diaphanous homolog 3 (Drosophila) | 0.58 |
| Gemin4* | Gem (nuclear organelle)-associated protein 4 | 0.53 |
| Hat1 | Histone aminotransferase 1 | 0.58 |
| Hdac2 | Histone deacetylase 2 | 0.63 |
| Ifitm2 | Interferon-induced transmembrane protein 2 | 0.40 |
| Impdh2 | Inosine 5′-phosphate dehydrogenase 2 | 0.49 |
| Msi2 | Musashi homolog 2 (Drosophila) | 0.54 |
| Narg1 | NMDA receptor-regulated gene 1 | 0.51 |
| Neurog3 | Neurogenin 3 | 0.40 |
| Notch2 | Notch gene homolog 2 (Drosophila) | 0.64 |
| Rasa3 | RAS p21 protein activator 3 | 0.64 |
| Rgs12 | Regulator of G-protein signaling 12 | 0.66 |
| Rhobtb3* | Rho-related BTB domain-containing 3 | 0.53 |
| Rnf138 | Ring finger protein 138 | 0.63 |
| Rwdd3 | RWD domain-containing 3 | 0.65 |
| Smarca5 | SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily a, member 5 | 0.30 |
| Smyd2 | SET and MYND domain-containing 2 | 0.55 |
| Smyd5 | SET and MYND domain-containing 5 | 0.63 |
| Sos2 | Son of sevenless homolog 2 (Drosophila) | 0.66 |
| Sox4* | SRY-box-containing gene 4 | 0.41 |
| Tiam1 | T-cell lymphoma invasion and metastasis 1 | 0.66 |
| Tox | Thymocyte selection-associated HMG box gene | 0.52 |
| Wdr12* | WD repeat domain 12 | 0.59 |
| Zfp62 | Zinc finger protein 62 | 0.59 |
| Zfp148 | Zinc finger protein 148 | 0.61 |
| Zfp191 | Zinc finger protein 191 | 0.42 |
| Zfp277 | Zinc finger protein 277 | 0.56 |
Details for the selection can be found in the text. These represent potential intestinal stem and cancer stem cell markers. Asterisks indicate genes whose stem cell-specific expression was independently demonstrated by Van der Flier et al. (36).
FIG. 6.
Rapid downregulation of stem cell markers upon β-catenin ablation. (A and B) Sox4 is located in the bottom part of intestinal wild-type crypts (A) and is strongly repressed in β-catenin mutants at day 2 (B). (C and D) Diap3 is expressed at the stem cell location in wild-type crypts (C) and is strongly reduced in β-catenin mutants (D). Dashed lines mark crypt limits. Bar, 10 μm. wt, wild type.
DISCUSSION
Homeostasis of the intestinal epithelium is dependent on the balance between cell proliferation, cell cycle arrest, migration, differentiation, and cell death. By inactivating β-catenin in the intestinal epithelium of adult mice, we demonstrate that Wnt/β-catenin signaling is a key player in many of those cellular processes. Indeed, β-catenin deletion induces rapid phenotypic alterations in the progenitor compartment of the crypt and blocks proliferation. Continuous crypt-to-villus migration results in crypt disappearance as more and more differentiated cells enter the villus. Due to the short life span of enterocytes, overall cell numbers gradually decrease and the intestinal epithelium is lost within 6 days of β-catenin ablation. Importantly, we demonstrate that Wnt signaling is required to maintain stemness and the undifferentiated state of intestinal stem cells. Based on our analysis of the localization of Wnt signaling activity in the intestinal crypt, we suggest that higher levels of Wnt signaling are required for maintaining stemness than for preventing cell cycle arrest. Differentiation of stem cells in response to ablation of Wnt signaling might be responsible for the absence of repair and recovery mechanisms, which otherwise occur rapidly after damage to the intestinal epithelium. This lack of repair results in intestinal failure and lethality.
Previous approaches to studying the role of Wnt signaling in the intestinal epithelium included knocking down or overexpressing different components of the signaling cascade. Overall, these studies induced only minor, transient phenotypes, making it difficult to distinguish consequences of altered Wnt signaling from regeneration mechanisms. Moreover, previous studies did not address the fate of stem cells following Wnt/β-catenin ablation. Two groups reported overexpression of the secreted Wnt inhibitor Dickkopf1 (Dkk1). In one study, Kuhnert et al. infected mice with adenoviruses expressing Dkk1 (17). Infrequent loss of crypts accompanied by diminished proliferation was observed after 1 week of viral administration. However, decreased Dkk1 expression at later points was followed by epithelial regeneration. In a second study, Pinto et al. generated transgenic mice expressing Dkk1 under the control of the villin promoter (27). Those mice displayed a mild phenotype with reduced epithelial proliferation, partial crypt loss, and increased enterocytic differentiation. In contrast to our mutants, those mice survived, probably due to an incomplete block of Wnt signaling and scattered areas of unaffected crypts due to mosaic expression of the transgene (27). An approach of conditional ablation of β-catenin was undertaken by Ireland et al., using an Ah promoter-driven cre recombinase (14). Again, this induced only a transient (24-h) deletion of β-catenin, and complete regeneration was observed within a few days. Partial reduction in the number of crypt cells was followed by recovery from increased proliferation of wild-type cells, which makes comparisons with our results difficult. Several other studies used a complementary approach, i.e., overactivation of the Wnt/β-catenin pathway (35, 39, 40). As expected, these experiments induced phenotypes opposite from those in our gene ablation model: β-catenin-overexpressing crypts showed increased proliferation, causing crypt expansion and decreased enterocytic differentiation (35). Based on our novel results, we suggest that this phenotype might reflect an expansion of the intestinal stem cell pool in these mutant animals.
c-Myc is a known target of the Wnt pathway in vitro (8, 37) and was recently confirmed as a critical downstream signal controlling intestinal proliferation in vivo (34). Several groups conditionally deleted c-myc in the intestinal epithelium (3, 21, 34). Surprisingly, in all those studies intestinal function was only transiently perturbed since c-myc-negative crypts were replaced by escaper cells through a crypt fission process, which is reminiscent of postirradiation repair (6). In agreement with our data for an essential requirement of Wnt/β-catenin signaling in progenitor cell proliferation, this repopulation mechanism involved increased Wnt/β-catenin activity. However, the preservation of repair mechanisms in these mutants indicates that c-myc is most likely not one of the essential Wnt targets to maintain intestinal stem cell function.
With the aim of identifying novel markers of intestinal stem and cancer stem cells, we performed transcriptional profiling followed by extensive comparisons to other stem cell populations and intestinal tumor studies. This allowed identification of novel marker candidates. In a recent complementary approach, genes upregulated in human intestinal tumors were compared to genes repressed upon a block of Wnt signaling in human colorectal cancer cell lines (36). We note significant overlap between our murine gene list and this human gene list, which together appear to define the set of common intestinal Wnt targets in human and mouse. Importantly, numerous genes that are shown by this study to be specifically expressed in intestinal stem cells, such as apex1, ascl2, gemin4, rhobtb3, sox4, and wdr12, are also deregulated in our arrays. Stem cell-specific expression is also demonstrated in both studies for several genes encoding members of the zinc finger family. Furthermore, we identify additional murine stem cell markers such as msi2, which belongs to the same family as the suggested marker msi1 (28), and in particular diaphanous3 (diap3). Interestingly, the diaphanous gene family has been identified to affect germ cell formation in Drosophila melanogaster and humans and has been implicated in regulating microtubule attachment to kinetochores (41).
Activating mutations of the Wnt/β-catenin pathway are well-known genetic alterations in premalignant lesions in the intestine (8). This introduced the concept that aberrant Wnt/β-catenin signaling initiates the transformation process. Based on these results, various efforts have been initiated to identify small-molecule drugs to be used for colorectal cancer therapy which function as inhibitors of Wnt signal transduction. While clinical trials with these inhibitors have not been published, our results challenge this approach and predict serious side effects of such drugs.
Supplementary Material
Acknowledgments
We are grateful to S. Duboux and J. Bamat for technical assistance; A. Trumpp, M. Bettess, and J. Gordon for antibodies; O. Hagenbuchle and S. Wicker for Affymetrix gene chip hybridization; F. Naef and F. Parisi for bioinformatical analysis; M. Bacac for advice with LCM; and A. D. Durham for proofreading.
T.F. and J.H. were supported in part by the Swiss League against Cancer (OCS 01838-02-2006), the SNF (3100AO-104209), and the Swiss NCCR in Molecular Oncology.
Footnotes
Published ahead of print on 4 September 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Batlle, E., J. T. Henderson, H. Beghtel, M. M. van den Born, E. Sancho, G. Huls, J. Meeldijk, J. Robertson, M. van de Wetering, T. Pawson, and H. Clevers. 2002. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell 111: 251-263. [DOI] [PubMed] [Google Scholar]
- 2.Behrens, J., and B. Lustig. 2004. The Wnt connection to tumorigenesis. Int. J. Dev. Biol. 48: 477-487. [DOI] [PubMed] [Google Scholar]
- 3.Bettess, M. D., N. Dubois, M. J. Murphy, C. Dubey, C. Roger, S. Robine, and A. Trumpp. 2005. c-Myc is required for the formation of intestinal crypts but dispensable for homeostasis of the adult intestinal epithelium. Mol. Cell. Biol. 25: 7868-7878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bianchini, M., E. Levy, C. Zucchini, V. Pinski, C. Macagno, P. De Sanctis, L. Valvassori, P. Carinci, and J. Mordoh. 2006. Comparative study of gene expression by cDNA microarray in human colorectal cancer tissues and normal mucosa. Int. J. Oncol. 29: 83-94. [PubMed] [Google Scholar]
- 5.Bjerknes, M., and H. Cheng. 1999. Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology 116: 7-14. [DOI] [PubMed] [Google Scholar]
- 6.Booth, D., J. D. Haley, A. M. Bruskin, and C. S. Potten. 2000. Transforming growth factor-B3 protects murine small intestinal crypt stem cells and animal survival after irradiation, possibly by reducing stem-cell cycling. Int. J. Cancer 86: 53-59. [DOI] [PubMed] [Google Scholar]
- 7.Cho, R. J., M. Huang, M. J. Campbell, H. Dong, L. Steinmetz, L. Sapinoso, G. Hampton, S. J. Elledge, R. W. Davis, and D. J. Lockhart. 2001. Transcriptional regulation and function during the human cell cycle. Nat. Genet. 27: 48-54. [DOI] [PubMed] [Google Scholar]
- 8.Clevers, H. 2006. Wnt/beta-catenin signaling in development and disease. Cell 127: 469-480. [DOI] [PubMed] [Google Scholar]
- 9.el Marjou, F., K. P. Janssen, B. H. Chang, M. Li, V. Hindie, L. Chan, D. Louvard, P. Chambon, D. Metzger, and S. Robine. 2004. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 39: 186-193. [DOI] [PubMed] [Google Scholar]
- 10.Gardina, P. J., T. A. Clark, B. Shimada, M. K. Staples, Q. Yang, J. Veitch, A. Schweitzer, T. Awad, C. Sugnet, S. Dee, C. Davies, A. Williams, and Y. Turpaz. 2006. Alternative splicing and differential gene expression in colon cancer detected by a whole genome exon array. BMC Genomics 7: 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gregorieff, A., D. Pinto, H. Begthel, O. Destree, M. Kielman, and H. Clevers. 2005. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology 129: 626-638. [DOI] [PubMed] [Google Scholar]
- 12.He, X. C., T. Yin, J. C. Grindley, Q. Tian, T. Sato, W. A. Tao, R. Dirisina, K. S. Porter-Westpfahl, M. Hembree, T. Johnson, L. M. Wiedemann, T. A. Barrett, L. Hood, H. Wu, and L. Li. 2007. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat. Genet. 39: 189-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huelsken, J., R. Vogel, B. Erdmann, G. Cotsarelis, and W. Birchmeier. 2001. β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 105: 533-545. [DOI] [PubMed] [Google Scholar]
- 14.Ireland, H., R. Kemp, C. Houghton, L. Howard, A. R. Clarke, O. J. Sansom, and D. J. Winton. 2004. Inducible Cre-mediated control of gene expression in the murine gastrointestinal tract: effect of loss of beta-catenin. Gastroenterology 126: 1236-1246. [DOI] [PubMed] [Google Scholar]
- 15.Kim, C. G., J. J. Lee, D. Y. Jung, J. Jeon, H. S. Heo, H. C. Kang, J. H. Shin, Y. S. Cho, K. J. Cha, C. G. Kim, B. R. Do, K. S. Kim, and H. S. Kim. 2006. Profiling of differentially expressed genes in human stem cells by cDNA microarray. Mol. Cells 21: 343-355. [PubMed] [Google Scholar]
- 16.Korinek, V., N. Barker, P. Moerer, E. van Donselaar, G. Huls, P. J. Peters, and H. Clevers. 1998. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat. Genet. 19: 379-383. [DOI] [PubMed] [Google Scholar]
- 17.Kuhnert, F., C. R. Davis, H. T. Wang, P. Chu, M. Lee, J. Yuan, R. Nusse, and C. J. Kuo. 2004. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc. Natl. Acad. Sci. USA 101: 266-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leclerc, D., L. Deng, J. Trasler, and R. Rozen. 2004. ApcMin/+ mouse model of colon cancer: gene expression profiling in tumors. J. Cell. Biochem. 93: 1242-1254. [DOI] [PubMed] [Google Scholar]
- 19.Lustig, B., B. Jerchow, M. Sachs, S. Weiler, T. Pietsch, U. Karsten, M. van de Wetering, H. Clevers, P. M. Schlag, W. Birchmeier, and J. Behrens. 2002. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol. Cell. Biol. 22: 1184-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshman, E., C. Booth, and C. S. Potten. 2002. The intestinal epithelial stem cell. Bioessays 24: 91-98. [DOI] [PubMed] [Google Scholar]
- 21.Muncan, V., O. J. Sansom, L. Tertoolen, T. J. Phesse, H. Begthel, E. Sancho, A. M. Cole, A. Gregorieff, I. Moreno de Alboran, H. Clevers, and A. R. Clarke. 2006. Rapid loss of intestinal crypts upon conditional deletion of the Wnt/Tcf-4 target gene c-Myc. Mol. Cell. Biol. 26: 8418-8426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen, H., M. Rendl, and E. Fuchs. 2006. Tcf3 governs stem cell features and represses cell fate determination in skin. Cell 127: 171-183. [DOI] [PubMed] [Google Scholar]
- 23.Notterman, D. A., U. Alon, A. J. Sierk, and A. J. Levine. 2001. Transcriptional gene expression profiles of colorectal adenoma, adenocarcinoma, and normal tissue examined by oligonucleotide arrays. Cancer Res. 61: 3124-3130. [PubMed] [Google Scholar]
- 24.O'Brien, C. A., A. Pollett, S. Gallinger, and J. E. Dick. 2007. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 445: 106-110. [DOI] [PubMed] [Google Scholar]
- 25.Oster, H., and M. Leitges. 2006. Protein kinase C alpha but not PKCzeta suppresses intestinal tumor formation in ApcMin/+ mice. Cancer Res. 66: 6955-6963. [DOI] [PubMed] [Google Scholar]
- 26.Paoni, N. F., M. W. Feldman, L. S. Gutierrez, V. A. Ploplis, and F. J. Castellino. 2003. Transcriptional profiling of the transition from normal intestinal epithelia to adenomas and carcinomas in the APCMin/+ mouse. Physiol. Genomics 15: 228-235. [DOI] [PubMed] [Google Scholar]
- 27.Pinto, D., A. Gregorief, H. Begthel, and H. Clevers. 2003. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 17: 1709-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Potten, C. S., C. Booth, G. L. Tudor, D. Booth, G. Brady, P. Hurley, G. Ashton, R. Clarke, S. Sakakibara, and H. Okano. 2003. Identification of a putative intestinal stem cell and early lineage marker; musashi-1. Differentiation 71: 28-41. [DOI] [PubMed] [Google Scholar]
- 29.Potten, C. S., G. Owen, and D. Booth. 2002. Intestinal stem cells protect their genome by selective segregation of template DNA strands. J. Cell Sci. 115: 2381-2388. [DOI] [PubMed] [Google Scholar]
- 30.Reichling, T., K. H. Goss, D. J. Carson, R. W. Holdcraft, C. Ley-Ebert, D. Witte, B. J. Aronow, and J. Groden. 2005. Transcriptional profiles of intestinal tumors in Apc(Min) mice are unique from those of embryonic intestine and identify novel gene targets dysregulated in human colorectal tumors. Cancer Res. 65: 166-176. [PubMed] [Google Scholar]
- 31.Reya, T., A. W. Duncan, L. Ailles, J. Domen, D. C. Scherer, K. Willert, L. Hintz, R. Nusse, and I. L. Weissman. 2003. A role for wnt signalling in self-renewal of hematopoietic stem cells. Nature 423: 409-414. [DOI] [PubMed] [Google Scholar]
- 32.Ricci-Vitiani, L., D. G. Lombardi, E. Pilozzi, M. Biffoni, M. Todaro, C. Peschle, and R. De Maria. 2007. Identification and expansion of human colon-cancer-initiating cells. Nature 445: 111-115. [DOI] [PubMed] [Google Scholar]
- 33.Rochon, C., V. Frouin, S. Bortoli, K. Giraud-Triboult, V. Duverger, P. Vaigot, C. Petat, P. Fouchet, B. Lassalle, O. Alibert, X. Gidrol, and G. Pietu. 2006. Comparison of gene expression pattern in SP cell populations from four tissues to define common “stemness functions.” Exp. Cell Res. 312: 2074-2082. [DOI] [PubMed] [Google Scholar]
- 34.Sansom, O. J., V. S. Meniel, V. Muncan, T. J. Phesse, J. A. Wilkins, K. R. Reed, J. K. Vass, D. Athineos, H. Clevers, and A. R. Clarke. 2007. Myc deletion rescues Apc deficiency in the small intestine. Nature 446: 676-679. [DOI] [PubMed] [Google Scholar]
- 35.Sansom, O. J., K. R. Reed, A. J. Hayes, H. Ireland, H. Brinkmann, I. P. Newton, E. Batlle, P. Simon-Assmann, H. Clevers, I. S. Nathke, A. R. Clarke, and D. J. Winton. 2004. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 18: 1385-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van der Flier, L. G., J. Sabates-Bellver, I. Oving, A. Haegebarth, M. De Palo, M. Anti, M. E. van Gijn, S. Suijkerbuijk, M. van de Wetering, G. Marra, and H. Clevers. 2007. The intestinal Wnt/TCF signature. Gastroenterology 132: 628-632. [DOI] [PubMed] [Google Scholar]
- 37.van de Wetering, M., E. Sancho, C. Verweij, W. de Lau, I. Oving, A. Hurlstone, K. van der Horn, E. Batlle, D. Coudreuse, A. P. Haramis, M. Tjon-Pon-Fong, P. Moerer, M. van den Born, G. Soete, S. Pals, M. Eilers, R. Medema, and H. Clevers. 2002. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111: 241-250. [DOI] [PubMed] [Google Scholar]
- 38.Whitfield, M. L., G. Sherlock, A. J. Saldanha, J. I. Murray, C. A. Ball, K. E. Alexander, J. C. Matese, C. M. Perou, M. M. Hurt, P. O. Brown, and D. Botstein. 2002. Identification of genes periodically expressed in the human cell cycle and their expression in tumors. Mol. Biol. Cell 13: 1977-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong, M. H., J. Huelsken, W. Birchmeier, and J. I. Gordon. 2002. Selection of multipotent stem cells during morphogenesis of small intestinal crypts of Lieberkuhn is perturbed by stimulation of Lef-1/β-catenin signaling. J. Biol. Chem. 277: 15843-15850. [DOI] [PubMed] [Google Scholar]
- 40.Wong, M. H., B. Rubinfeld, and J. I. Gordon. 1998. Effects of forced expression of an NH2-terminal truncated beta-catenin on mouse intestinal epithelial homeostasis. J. Cell Biol. 141: 765-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yasuda, S., F. Oceguera-Yanez, T. Kato, M. Okamoto, S. Yonemura, Y. Terada, T. Ishizaki, and S. Narumiya. 2004. Cdc42 and mDia3 regulate microtubule attachment to kinetochores. Nature 428: 767-771. [DOI] [PubMed] [Google Scholar]
- 42.Zechner, D., Y. Fujita, J. Huelsken, T. Muller, I. Walther, M. M. Taketo, E. B. Crenshaw III, W. Birchmeier, and C. Birchmeier. 2003. β-Catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev. Biol. 258: 406-418. [DOI] [PubMed] [Google Scholar]
- 43.Zhang, J., A. Moseley, A. G. Jegga, A. Gupta, D. P. Witte, M. Sartor, M. Medvedovic, S. S. Williams, C. Ley-Ebert, L. M. Coolen, G. Egnaczyk, M. B. Genter, M. Lehman, J. Lingrel, J. Maggio, L. Parysek, R. Walsh, M. Xu, and B. J. Aronow. 2004. Neural system-enriched gene expression: relationship to biological pathways and neurological diseases. Physiol. Genomics 18: 167-183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.