Abstract
Apoptosis is a potent host defense against microbes. Most viruses have adapted strategies to counteract this response. Herpes simplex virus (HSV) produces a balance between pro- and antiapoptotic processes during infection. When antiapoptotic signals become limiting, infected cells die through HSV-dependent apoptosis (HDAP). Oncogenic pathways were previously implicated in HDAP susceptibility. Here, we exploited our ability to selectively express all, one, or no oncogenes in the well-defined HeLa cell system to dissect the requirements for HDAP. Human papillomavirus E6 and E7 oncogene expression was inhibited by the E2 viral repressor. Sole expression of E6 mediated HDAP sensitization. Next, two known cellular targets of E6 were independently modulated. This demonstrated that E6 sensitizes HeLa cells to HDAP through hTERT and p53. Given the universality of the apoptotic antiviral response, p53 and telomerase regulation will likely be important for counteracting host defenses in many other viral infections.
A common cellular defense mechanism against viral invasion is the elimination of infected cells through apoptotic cell death (reviewed in reference 33). Accordingly, a wide array of virus families share the ability to modulate cellular apoptotic pathways (for examples, see references 38, 66, and 69). Members of each branch of the Herpesviridae family have been shown to possess apoptotic evasion strategies (reviewed in references 1, 12, and 45). One such herpesvirus, herpes simplex virus (HSV), sets up an intricate balance between pro- and antiapoptotic processes during virus propagation. When antiapoptotic pathways are suppressed, this balance is upset, and the cells die by apoptosis, which we refer to here as HSV-dependent apoptosis (HDAP). Maintenance of the apoptotic balance requires the viral ICP27 regulatory protein. ICP27 is expressed early in infection (reviewed in reference 51) and stimulates expression of the later sets of viral genes (53). Recombinant viruses lacking ICP27 fail to block proapoptotic signals (2) and do not produce progeny virions (53). We have used an apoptotic ICP27-null virus, vBSΔ27, to investigate the determinants of sensitivity to HDAP.
HSV infections can lead to disease as minor as a cold sore or as devastating as fatal encephalitis (reviewed in reference 51). Recently, the severity of certain herpetic diseases has been associated with apoptosis. For example, human corneal epithelial cells from patients with ocular HSV infections displayed more apoptosis than corneal cells from uninfected individuals (40). HSV-infected mice lacking the RNase L gene exhibited more severe HSV keratitis (HSK) than their wild-type littermates did, and this correlated with a decrease in apoptosis (73). Miles et al. reported a similar reverse correlation between apoptosis and HSK severity in human corneas (41). These findings suggest that apoptosis reduces the severity of HSK during HSV infection. HDAP is not solely restricted to ocular HSV disease, as others have reported that apoptosis plays a role in herpetic brain disease (49, 52). However, in this case apoptosis seems to be acting to facilitate brain disease progression. Therefore, it seems that differences in host response to HDAP may alter the outcome of herpesvirus infections. Previous studies have indicated that cancer cells exhibit an exquisite sensitivity to HDAP. For instance, almost all HeLa cells are apoptotic at 24 h following infection with vBSΔ27 (46). Similar apoptotic phenotypes were observed in cells derived from human colon, brain, and breast tumors (3, 47). In contrast, cells derived from nontumor tissue were quite resistant to this process (3, 46, 47). Together, these data implied that genes commonly altered during tumor formation play a role in the regulation of HDAP and may, in turn, contribute to HSV disease severity.
There could be many reasons why tumor cells are particularly sensitive to HDAP. We are able to directly test one of these possibilities, namely, that targets of human papillomavirus oncogenes contribute to this process. Our model system exploits the fact that continuous oncogene expression is essential for HeLa cells to maintain their tumorigenic properties (21, 35, 67). HeLa cells harbor integrated human papillomavirus type 18 (HPV18) genomes (59, 71) and express two viral oncogenes, E6 and E7. E6 and E7 of high-risk papillomaviruses like HPV18 and HPV16 are known to inactivate the p53 and p105Rb tumor suppressor proteins, respectively (16, 43, 57, 68). Additionally, E6 directly activates the catalytic component of telomerase, hTERT (32). These activities are essential for HPV to cause the postmitotic keratinocytes, which it infects, to enter the S phase of the cell cycle and replicate viral genomes. In a typical nontumor HPV infection, E6 and E7 expression is limited by the HPV E2 transcriptional repressor (Fig. 1A) (7, 15). However, in HeLa cells, the viral genome is integrated into the host genome such that the E2 open reading frame is disrupted (Fig. 1B) (59). The consequence of this is unrestrained oncogene expression and rampant proliferation of these tumor cells. Reconstituting HeLa cells with E2 using a simian virus 40 (SV40)-based recombinant virus (SV40/BPV-1) that expresses bovine papillomavirus type 1 (BPV-1) E2 inhibits E6 and E7 expression, reactivates p53 and p105Rb, inhibits hTERT activity, and represses cell growth (Fig. 1C) (21, 22, 27). In effect, the expression of E2 “untransforms” the HeLa cells. We used this system to investigate the importance of HPV18 oncogene expression in the HDAP of HeLa cells. Subsequently, we used HeLa-derived cell lines that were engineered either to express the HPV oncogenes or to alter their cellular targets in the presence of E2 to identify cellular determinants of HDAP (Fig. 1C and D).
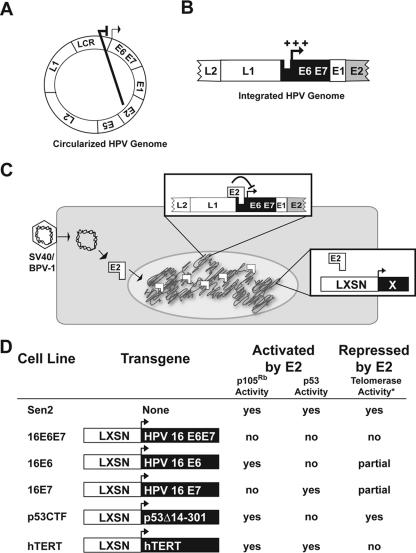
FIG. 1.
Description of model systems used in this study. (A and B) Schematic representations of the HPV genome as found in productively infected, normal cells (A) and tumor cells (HeLa) (B). During a productive infection in nontumor tissue, the HPV genome is found as an episome and expression of E6 and E7 is repressed by the viral E2 gene product. However, in cervical cancer cell lines, like the HeLa cells, the HPV genome is integrated into the host genome such that the E2 open reading frame is disrupted, allowing for overexpression (+++) of E6 and E7. (C) Experimental design. The Sen2/HeLa cells and derivatives were transduced with SV40/BPV-1, a recombinant virus based on SV40 that expresses BPV-1 E2. The E2 protein binds to the integrated HPV1 promoter sequences and represses expression of E6 and E7. Expression of genes from the LXSN transgene construct is unaffected by E2. “X” represents transgenes shown in panel D. (D) Properties of cell lines utilized in this study. A description of the transgene expressed by each cell line as well as the cellular pathways that are affected by E2 transduction are provided. The telomerase activities were measured previously (13, 25).
MATERIALS AND METHODS
Cells and viruses.
Sen2 cells are a clonally derived strain of HeLa cells previously described (22). 16E6E7, 16E6, 16E7, p53CTF, and hTERT cells are derivatives of Sen2 cells generated as previously reported (13, 20-22, 25, 27, 50). All other cells were obtained from the American Type Culture Collection (Rockville, MD). Sen2 cells and derivatives were propagated in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 0.1 mg/ml hygromycin (16E7), 0.5 mg/ml G418 (16E6, p53DN, and hTERT), or both (16E6E7). Vero 2.2 (gift of Saul Silverstein) cells are derivatives of Vero cells expressing ICP27 from its viral promoter (60). Vero 2.2 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 5% fetal bovine serum. HSV-1(KOS1.1) was the strain of wild-type HSV type 1 (HSV-1) used in this study. HSV-1(vBSΔ27) is an ICP27-null virus derived from HSV-1(KOS1.1) containing a replacement of the α27 gene with the Escherichia coli lacZ gene (61). This virus was propagated on Vero 2.2 cells, the titers of the virus were determined, and the virus was used to infect cells at a multiplicity of infection (MOI) of 10 as reported earlier (46). The SV40/BPV-1 recombinant virus expressing BPV-1 E2 was isolated, the titers of the virus were determined, and the recombinant virus was used to infect cells at an MOI of 20 as previously described (44). Unless noted otherwise, all cell culture reagents were obtained from Life Technologies and all biochemicals were from Sigma.
Microscopic analysis and monitoring of chromatin condensation.
The morphologies of infected cells were documented by phase-contrast and fluorescence microscopy using an Olympus IX70/IX-FLA inverted fluorescence microscope. Images were acquired with a Sony DKC-5000 digital photo camera linked to a PowerMac or Dell workstation and processed through Adobe Photoshop. For visualization of chromatin condensation in live cells, 5 μg/ml Hoechst 33258 (Sigma) was added to the medium and allowed to incubate at 37°C for 30 to 60 min. The percentage of nuclei containing condensed chromatin was determined by dividing the number of brightly stained, small nuclei by the total number of nuclei (uncondensed plus condensed) in a particular (×40) microscopic field. At least 100 nuclei were counted for each data point.
Immunoblotting.
Whole-cell protein extract was prepared using lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 1% deoxycholate, 0.1% sodium dodecyl sulfate) supplemented with 2 mM phenylmethylsulfonyl fluoride (freshly prepared stock), 1% Translysol, 0.1 mM l-1-chloro-3-(4-tosylamido)-4-phenyl-2-butanone (TPCK), 0.01 mM l-1-chlor-3-(4-tosylamido)-7-amino-2-heptanon-hydrochloride (TLCK), as previously reported (46). Protein concentrations were determined using a modified Bradford protein assay (Bio-Rad Laboratories). Samples (20 μg) of total protein were separated on 12 or 15% N,N′-diallyltartardiamide-acrylamide gels and electrically transferred to nitrocellulose. Membranes were incubated for 1 h at room temperature in blocking buffer (phosphate-buffered saline containing 5% nonfat dry milk) and incubated overnight at 4°C with primary antibody.
Monoclonal antibodies specific for ICP4 (Goodwin Institute for Cancer Research), ICP27 (Goodwin), gC (Goodwin), poly(ADP-ribose) polymerase (PARP) (PharMingin), procaspase 3 (BD Transduction), and p53 (PharMingin) and polyclonal antibodies specific for thymidine kinase (TK) and 45-kDa DNA fragmentation factor (DFF-45; Santa Cruz) were diluted at a concentration of 1:1,000 in Tris-buffered saline containing 0.1% Tween 20 (TBST) and 0.1% bovine serum albumin. After the membranes were washed in TBST, they were incubated with either anti-mouse or anti-rabbit antibodies conjugated to alkaline phosphatase (Southern Biotech) that was diluted in blocking buffer (1:1,000) for 1 h at room temperature. After the membranes were washed in TBST, immunoblots were developed in buffer containing 5-bromo-4-chloro-3-indolyl phosphate and 4-nitroblue tetrazolium chloride. Quantification of PARP cleavage was done using NIH Image as previously described (46).
RESULTS
The papillomavirus E2 protein represses HDAP in HeLa cells.
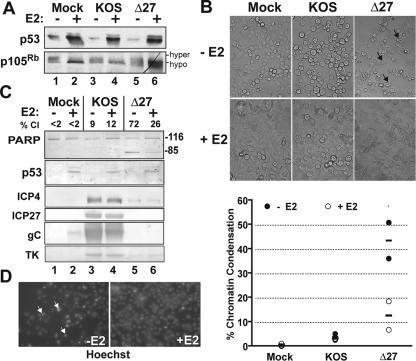
Since previous studies determined that HeLa cells are susceptible to HDAP (3, 46), we asked whether inhibition of HPV oncogenes through E2 expression would desensitize these cells. A clonally derived strain of HeLa cells, Sen2, was transduced with a BPV-1 E2-expressing recombinant virus (SV40/BPV-1) to repress the endogenous HPV18 oncogenes (as illustrated in Fig. 1C). At a time point (48 hours postinfection [hpi]) previously shown to be sufficient for HPV gene repression (27), the cells were analyzed for HDAP by infection with wild-type HSV strain KOS and the ICP27-null recombinant virus, vBSΔ27. Sen2 cells that were not transduced with SV40/BPV-1 recombinant virus were similarly infected with wild-type HSV strain KOS and vBSΔ27 viruses as controls. Twenty-four hours following HSV infection, assessment of apoptotic morphology and harvesting for death factor immunoblot analyses were performed.
Initially, we monitored the ability of E2 to inhibit HPV oncogenes during HSV infection. Because HPV18 E6 degrades p53 (57), we used p53 levels as a marker for E6 function during infection. Similarly, HPV18 E7's ability to destabilize hypophosphorylated p105Rb (10) was measured. In the absence of E2 expression, p53 accumulated to low levels (Fig. 2A, lanes 1, 3, and 5). The p53 levels were dramatically increased in cells transduced with SV40/BPV-1, confirming that E2 was inhibiting HPV E6 function in these cells (Fig. 2A, lanes 2, 4, and 6). While p105Rb was observed as a mixture of slower-migrating hyperphosphorylated and faster-migrating hypophosphorylated species in the absence of E2, the majority of p105Rb was hypophosphorylated with E2 expression (Fig. 2A, lanes 2, 4, and 6). This result confirmed that HPV E7 was being suppressed by E2. Importantly, HSV infection did not impair E2's ability to suppress either of the HPV oncogenes (Fig. 2A, compare lane 2 with lanes 4 and 6.)
FIG. 2.
E2 suppresses HDAP in HeLa cells. At 48 h postinfection with (+) or without (−) E2-expressing recombinant virus SV40/BPV-1 (MOI of 20), Sen2 cells were infected with HSV-1(KOS) or vBSΔ27 (Δ27) (MOI of 10). (A) Cellular tumor suppressors following E2 expression. Lysates from infected cells were immunoblotted for p53 and p105Rb. Hypophosphorylation (hypo) and hyperphosphorylation (hyper) states of p105Rb are indicated. (B) Cellular morphologies. Cells were visualized at 24 h following infection with KOS or Δ27 viruses. Black arrows denote examples of cells displaying membrane blebbing. Image magnification, ×40. (C) Immunoblots of death factor PARP, viral proteins (ICP4, ICP27, gC, and TK), and cell cycle regulatory protein p53. The positions of uncleaved 116,000-molecular-weight (116) and cleaved 85,000-molecular-weight (85) PARP products are indicated to the right of the blot. The percentage of PARP cleavage (% Cl) was calculated using NIH Image analysis as described in Materials and Methods. (D) Chromatin condensation. Representative fluorescent images (×40 magnification) of Hoechst-stained nuclei from Sen2 cells infected with vBSΔ27 in the presence (+E2) and absence (−E2) of SV40/BPV-1 transduction are shown beside the graph. White arrows denote examples of nuclei with condensed chromatin. The percentages of condensed chromatin from duplicate experiments were calculated as described in Materials and Methods. The mean (bar) and end points of the range (circles) are plotted.
We next assessed the effect of E2 on HSV replication and apoptosis. HSV strain KOS infection led to altered Sen2 cell morphology, such as cell rounding and loss of attachment to the substratum (Fig. 2B). Additionally, KOS-infected cells exhibited intense fluorescence around the periphery of the nuclei when stained with the fluorescent DNA dye, Hoechst, which is indicative of chromatin marginalization (data not shown). These morphological changes are characteristic of cytopathic effect (CPE) that typically accompanies productive HSV replication (46). CPE and the abundant accumulation of representative immediate-early (ICP4 and ICP27), early (TK), and late (gC) viral proteins occurred in Sen2 cells regardless of E2 expression status (Fig. 2C, lanes 3 and 4). Therefore, E2 does not influence HSV's ability to replicate.
The vBSΔ27-infected Sen2 cells without E2 were smaller than KOS-infected Sen2 cells and displayed numerous membrane protrusions typical of cells undergoing membrane blebbing (Fig. 2B). In addition, the nuclei of these cells were smaller and exhibited more intense Hoechst staining than mock-infected cells (Fig. 2D). Such membrane blebbing and chromatin condensation are classic features of apoptotic cells (31). Next, we monitored the cleavage of PARP as an independent marker for apoptosis. PARP is cleaved from its 116,000-molecular-weight form into an 85,000-molecular-weight product by caspases activated during apoptosis. Without E2, vBSΔ27 infection induced 72% PARP cleavage (Fig. 2C, lane 5). Thus, the results from both the morphological and biochemical apoptotic assays indicated that Sen2 cells are susceptible to HDAP when E2 is absent.
However, E2-expressing Sen2 cells were more resistant to HDAP. First, E2 expression lowered the vBSΔ27-induced PARP cleavage from 72 to 26% (Fig. 2C, compare lane 6 with lane 5). Second, the number of vBSΔ27-infected Sen2 cells displaying membrane blebbing was reduced with E2 expression (Fig. 2B). Finally, E2 decreased the levels of chromatin condensation in vBSΔ27-infected cells from 36 to 7% and 51 to 19% in duplicate experiments (Fig. 2D). Since all three apoptotic markers were reduced by E2 expression, we conclude that E2 inhibits HDAP in Sen2 cells. It is of interest to note that even after suppression of endogenous E6 and E7, vBSΔ27 induced apoptosis in approximately 11% of the cells. This observation might suggest that in the absence of ICP27, HSV-infected cells are still susceptible to apoptosis by mechanisms which are presumably independent of papillomavirus oncogenes. Other HSV proteins besides ICP27, such as ICP4, have been implicated in suppressing apoptosis. Based on a detailed analysis of ICP27 mutant viruses (5) and ICP27 protein alone (4), we previously concluded that ICP27 indirectly blocks apoptosis as part of its regulatory functions and that ICP4 likely behaves in a similar manner (55). Thus, we predict that ICP4 mutant virus would have the same sensitivity pattern as vBSΔ27.
High-risk HPV E6 and E7 sensitize HeLa cells to HSV-dependent apoptosis.
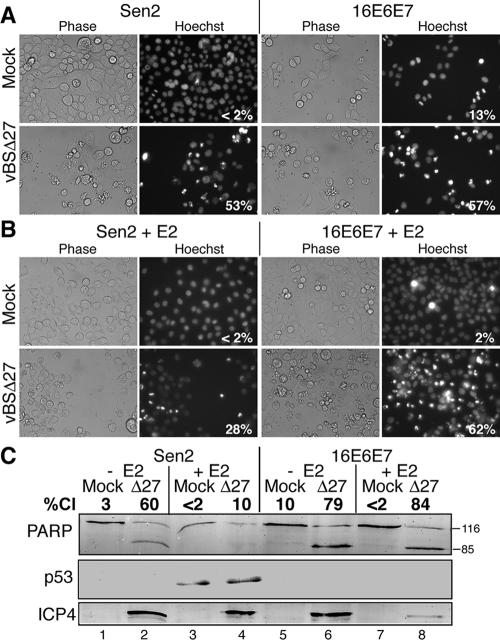
Because E2 has been reported to possess activities other than HPV transcriptional repression (14, 19, 54), the next experiment was designed to determine whether E2's effects on HDAP were due to suppression of HPV E6 and E7 gene expression. To do this, we used a Sen2-derived cell line which expresses HPV oncogenes in the presence of E2, 16E6E7 (Fig. 1D). Specifically, the 16E6E7 cells were generated by transducing Sen2 cells with a LXSN retrovirus expressing HPV16 E6 and E7 (13). Importantly, in the 16E6E7 cells, oncogene expression is driven from the Moloney murine leukemia virus (MMLV) promoter-enhancer sequences which are not inhibited by E2. This allows for coexpression of E2, E6, and E7 in the Sen2 cells. We transduced the 16E6E7 and parental Sen2 cells with SV40/BPV-1. Forty-eight hours later, the E2-transduced and nontransduced controls were infected with vBSΔ27, and apoptosis was measured via morphological (Fig. 3A and B) and biochemical (Fig. 3C) assays.
FIG. 3.
E6 and E7 repression is responsible for the E2-mediated suppression of HDAP in HeLa cells. Sen2 and a Sen2-derived cell line that constitutively expresses HPV16 E6 and E7 (16E6E7) were infected with (+) or without (−) E2-expressing recombinant virus SV40/BPV-1 (MOI of 20). Forty-eight hours later, cells were mock infected or infected with vBSΔ27 (Δ27) (MOI of 10). Hoechst DNA dye was added to the medium at a concentration of 5 μg/ml 1 h prior to visualization of condensed chromatin. (A and B) Cellular morphologies. Cells were visualized at 24 h following infection with Δ27 virus using phase-contrast and fluorescence (Hoechst) microscopy. The numbers within the Hoechst panels reflect the percentage of nuclei with condensed chromatin. Image magnification, ×40. (C) Immunoblots of death factor PARP, viral proteins (ICP4, ICP27, gC, and TK), and cell cycle regulatory protein p53. The positions of uncleaved 116,000-molecular-weight (116) and cleaved 85,000-molecular-weight (85) PARP products are indicated to the right of the blot. The percentage of PARP cleavage (%Cl) was calculated using NIH Image analysis as described in Materials and Methods.
The 16E6E7 and Sen2 cells infected with vBSΔ27 accumulated similar levels of the viral ICP4 protein in the absence of E2 (Fig. 3C, lanes 2 and 6), demonstrating their similar susceptibility to infection. Both cell lines displayed detectable ICP4 protein levels following infection with vBSΔ27 in the presence of E2 (Fig. 3C, lanes 4 and 8). The level of ICP4 was somewhat lower in the E2-expressing 16E6E7 cells than in the other cells. The reason for this is unknown at this time; however, it could be a result of the high level of cell death observed under these conditions (see below). In all, these results demonstrate that vBSΔ27 is capable of infecting the 16E6E7 cells regardless of E2 status.
The levels of p53 were increased in the Sen2 cells following E2 transduction (Fig. 3C, lanes 3 and 4), consistent with previous experiments (Fig. 2). However, no p53 was detected in the 16E6E7 cells regardless of E2 status, demonstrating that the HPV16 E6 expressed from the MMLV promoter was effectively degrading cellular p53 in the 16E6E7 cells (Fig. 3C, lanes 5 to 8).
In the absence of E2 (Fig. 3A), vBSΔ27-infected Sen2 and 16E6E7 cells displayed similar levels of chromatin condensation (53% and 57%, respectively) and PARP cleavage (60% and 79%, respectively). E2 expression (Fig. 3B) reduced membrane blebbing and decreased chromatin condensation in the vBSΔ27-infected Sen2 cells from 53% to 28%. However, no such reductions were evident in vBSΔ27-infected 16E6E7 cells (compare 57% with 62%). Similarly, E2 failed to reduce the percentage of PARP cleavage (compare 84% with 79%) in the vBSΔ27-infected 16E6E7 cells (Fig. 3C, lanes 6 and 8). Consistent with the results in Fig. 2C, E2 suppressed cleavage of PARP in vBSΔ27-infected Sen2 cells (compare lane 2 and 4). These results indicate that the expression of E6 and E7 can reverse the antiapoptotic activities of E2 in the Sen2 cells. Therefore, E2 inhibits HDAP in Sen2 cells by reducing the expression of the HPV oncogenes. We conclude from these studies that HPV E6 and E7 sensitize cells to HDAP.
HPV E6 expression is sufficient to sensitize HeLa cells to HSV-dependent apoptosis.
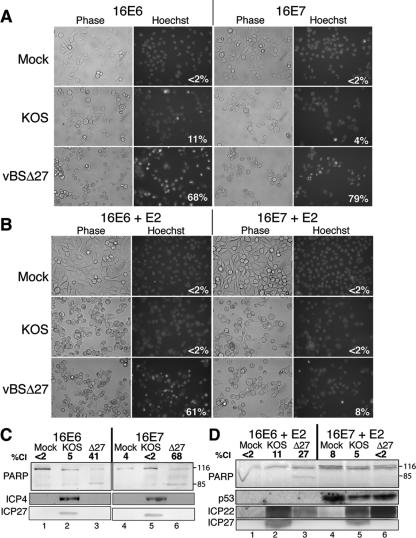
Both E6 and E7 have been implicated in apoptosis sensitization in other systems (reviewed in reference 18). For example, E6 sensitized human foreskin keratinocytes to apoptosis induced by several chemotherapeutic agents (36). Similarly, E7 has been shown to enhance apoptosis induced by tumor necrosis factor plus cycloheximide in immortalized keratinocytes (6, 64). Therefore, it was possible that either E6 or E7 or both could be responsible for conferring susceptibility to HDAP. To distinguish between these possibilities, we used cell systems that allowed us to selectively express either E6 or E7 alone in Sen2 cells. It was not feasible to singly inhibit E6 and E7 transcription from the integrated HPV genome because they are generated by differential splicing of a common pre-mRNA molecule (58). Instead, we used Sen2-derived cells that stably express either HPV16 E6 (16E6 cells) or E7 (16E7 cells) from the MMLV promoter. Similar to the 16E6E7 cells (Fig. 3), the 16E6 and 16E7 cell lines were generated through transduction with a LXSN retrovirus expressing HPV16 E6 or E7, respectively (Fig. 1D) (13). Because expression from the MMLV promoter is not affected by E2, this allowed us to concurrently (i) use E2 to repress expression of the HPV18 oncogenes from the integrated genome and (ii) exogenously express single HPV16 oncogenes from the integrated LXSN transgene. 16E6 and 16E7 cells were transduced with SV40/BPV-1 prior to infection with KOS or vBSΔ27. The cells were monitored for chromatin condensation and viral protein accumulation and p53 levels at 24 h after HSV infection (Fig. 4).
FIG. 4.
Expression of HPV E6 facilitates HDAP in HeLa cells. At 48 h posttransduction with E2-expressing recombinant virus SV40/BPV-1 (MOI of 20), 16E6 and 16E7 cells were infected with wild-type HSV-1 (KOS) or vBSΔ27 (Δ27) (MOI of 10). Hoechst DNA dye was added to the medium 1 h prior to imaging to allow visualization of chromatin. Images shown in panel A were captured at 24 hpi with HSV. The percentages inside each the Hoechst panels represent the percentage of nuclei containing condensed chromatin. (C and D) Immunoblots of death factor PARP, viral proteins (ICP4, ICP27, gC, and TK), and cell cycle regulatory protein p53. The positions of uncleaved 116,000-molecular-weight (116) and cleaved 85,000-molecular-weight (85) PARP products are indicated to the right of the immunoblots. The percentage of PARP cleavage (%Cl) was determined using NIH Image densitometry analysis of the scanned immunoblot.
As expected, E2 expression increased the p53 levels in 16E7 cells (Fig. 4D, compare lanes 1 to 3 with lanes 4 to 6). However, p53 levels were undetectable in the 16E6 cells even in the presence of E2. This was not surprising due to E6's ability to destabilize p53. The vBSΔ27-infected 16E6 and 16E7 cells displayed similar levels of chromatin condensation (68% and 79%, respectively) and PARP cleavage (41% and 68%, respectively) in the absence of E2 (Fig. 4A). However, 16E6 and 16E7 cells displayed a dramatically different response to vBSΔ27 infection in the presence of E2 (Fig. 4B). The vBSΔ27-infected 16E7 cells that expressed E2 exhibited almost 10-fold-lower levels of chromatin condensation than 16E7 cells that did not express E2 (compare 8% with 79%). Additionally, E2 expression reduced vBSΔ27-induced PARP cleavage from 68% to undetectable levels in the 16E7 cells (Fig. 4D, lane 6). In contrast, vBSΔ27-infected 16E6 cells exhibited a similar level of chromatin condensation (Fig. 4A and B) regardless of E2 expression status (68% and 61%). Substantial PARP cleavage was evident in the vBSΔ27-infected 16E6 cells transduced with the E2-expressing virus (Fig. 4D, lane 3). On this basis, we conclude that E6 alone is sufficient for HDAP susceptibility in Sen2 cells. It is important to note that since both Sen2 and 16E6 cells are induced to senesce by E2 (13), resistance to HDAP is not merely a measure of cell proliferation. The next step was to investigate the mechanism whereby E6 sensitized these cells.
HPV E6 sensitizes HeLa cells to HSV-dependent apoptosis through p53 inactivation and hTERT induction.
E6 is a multifunctional protein known to affect the activity of over a dozen cellular proteins (reviewed in reference 42). The ability to destabilize the tumor suppressor protein p53 was the first function attributed to E6 (57). E6 binds in a tertiary complex with p53 and the cellular ubiquitin ligase E6-associated protein (26). This leads to polyubiquination of p53 and its eventual degradation through a proteosome-mediated pathway (56). In this way, E6 inactivates p53. In unstressed cells, p53 is maintained at very low levels due to its short half-life (reviewed in reference 23). However, upon stress induction, p53 is stabilized and accumulates inside the nuclei of cells where it transcriptionally activates numerous p53-responsive genes including those involved in apoptosis (reviewed in reference 72). An earlier study from our laboratory showed the presence of a slower-migrating form of p53 in cells which were susceptible to HDAP, but not in those that were resistant (3). Although the identity of the altered form of p53 has not yet been established, it is possible that differences in p53 function could explain the variations in response to HDAP in those cell lines. This result along with p53's known roles in apoptosis regulation led us to examine the importance of p53 function during HDAP.
E6 also possesses a number of p53-independent activities, including the ability to activate telomerase by inducing expression of the catalytic component of the enzyme, hTERT (32). Telomerase is the enzyme that replicates chromosome telomeres. Telomerase is found at high levels during embryogenesis and in tumors, but it is maintained at relatively low levels in the majority of somatic tissues. Telomerase activation has been shown to repress apoptosis induced by a variety of agents (reviewed in reference 65). Therefore, we also investigated whether HPV E6's effects on hTERT contributed to HDAP sensitization.
Sen2 cells that stably express either a dominant-negative mutant of p53 (p53CTF) or hTERT under the control of the MMLV promoter were utilized (Fig. 1D) (20, 25) to determine the roles of these genes in HDAP. The hTERT-expressing cells display elevated telomerase activity and greatly extended telomeres (25). Sen2 and 16E6 cells were similarly treated as controls for these experiments. At 48 h prior to infection with KOS or vBSΔ27, all cell lines were transduced with SV40/BPV-1. Non-E2-expressing cells were also infected with HSV as controls. Staurosporine (STS) was used as an additional apoptotic stimulus. Cells were visualized by phase-contrast microscopy (Fig. 5) and fluorescence microscopy (Fig. 6), and whole-cell extracts were immunoblotted for PARP, p53, and ICP4 (Fig. 7).
FIG. 5.
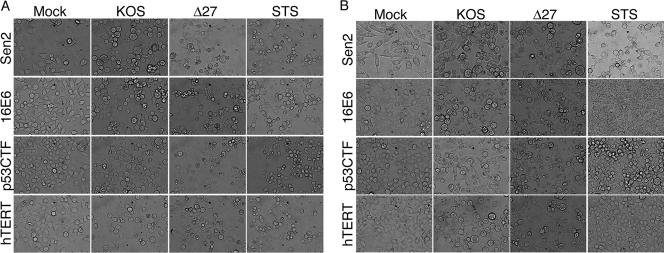
Cellular morphology showing that either inactivation of p53 or induction of hTERT renders HeLa cells sensitive to HDAP. Sen2, 16E6, and Sen2 cells constitutively expressing a dominant-negative C-terminal mutant of p53 (p53CTF) or the catalytic component of human telomerase (hTERT) from the MMTV promoter were treated with E2-expressing recombinant virus SV40/BPV-1 (MOI of 20) (B) or without E2-expressing SV40/BPV-1 (A). At 48 h posttransduction, cells were infected with wild-type HSV (KOS) or vBSΔ27 (Δ27) (MOI of 10). Staurosporine (STS) was used as a proapoptotic control. Phase-contrast microscopy images captured at 24 hpi with HSV are shown. Magnification, ×40.
FIG. 6.
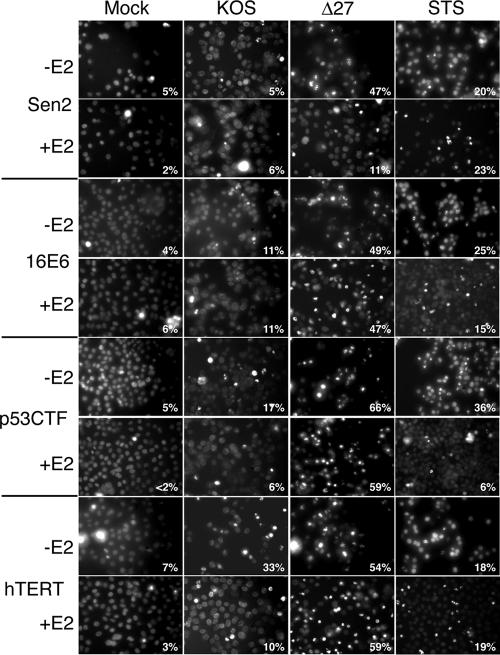
Nuclear morphology showing that either inactivation of p53 or induction of hTERT renders HeLa cells sensitive to HDAP. At 48 h posttransduction with E2-expressing recombinant virus SV40/BPV-1 (MOI of 20), Sen2 cells, 16E6 cells, Sen2 and p53CTF cells, and Sen2 and hTERT cells were infected with wild-type HSV (KOS) or vBSΔ27 (Δ27) (MOI of 10). Cells infected with virus expressing E2 (+E2) and virus that did not express E2 (−E2) are shown. STS was used as a proapoptotic control. Hoechst DNA dye was added to the medium at 1 h prior to imaging to allow visualization of chromatin. Images shown were captured by fluorescence microscopy at 24 hpi with HSV-1. The percentage inside each frame is the percentage of cells containing condensed chromatin.
FIG. 7.
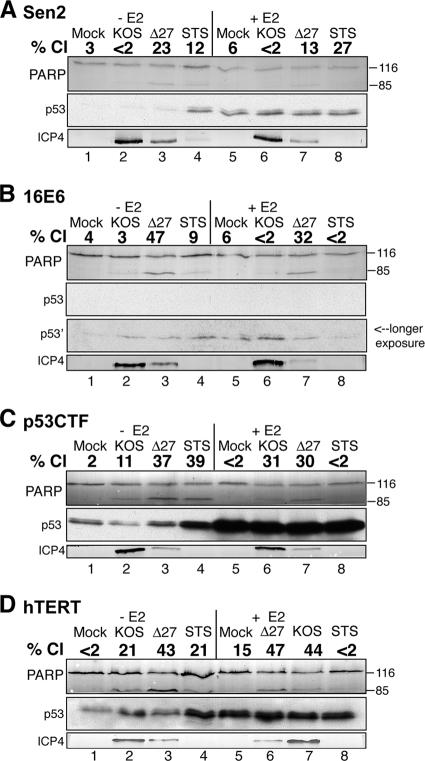
Biochemical assay results showing that either inactivation of p53 or induction of hTERT renders HeLa cells sensitive to HDAP. At 48 h posttransduction with E2-expressing recombinant virus SV40/BPV-1 (MOI of 20), Sen2 (A), 16E6 (B), p53CTF (C), and hTERT (D) cells were infected with wild-type HSV (KOS) or vBSΔ27 (Δ27) (MOI of 10). Cells infected with virus expressing E2 (+E2) and virus that did not express E2 (−E2) are shown. At 24 hpi with HSV, the cells were harvested, and infected-cell extracts were immunoblotted for PARP, ICP4, ICP27, gC, and TK, and p53. STS was used as a proapoptotic control. The positions of uncleaved 116,000-molecular-weight (116) and cleaved 85,000-molecular-weight (85) PARP products are indicated to the right of the immunoblots. The percentage of PARP cleavage (% Cl) was calculated using NIH Image analysis as described in Materials and Methods.
To determine the role of p53 inactivation in HDAP sensitization, we compared the sensitivities of the p53CTF and 16E6 cells to HDAP. Initially, we used CPE and viral protein accumulation as markers of viral replication efficiency. Cellular (Fig. 5) and nuclear (Fig. 6) morphologies indicative of CPE were evident in p53CTF cells infected with KOS. Additionally, KOS-infected p53CTF cells expressed ICP4 at a level equivalent to that of similarly treated Sen2 and 16E6 cells, which confirmed their ability to support viral replication (Fig. 7, compare lanes 2 and 6 in panels C, A, and B).
The p53 levels were also analyzed as a marker of E2 function during infection. As expected, p53 accumulated in the E2-expressing Sen2 and hTERT cells, but not in the 16E6 cells (Fig. 7). Consistent with previous studies, E2 caused an accumulation of wild-type p53 in the p53CTF cells (Fig. 7C, lanes 5 to 8) due to loss of p53-mediated transactivation of the mdm2 gene, which encodes a protein that destabilizes p53 (25). It is important to note, however, that this accumulation is not accompanied by an increase in p53 activity in the p53CTF cells (25). The vBSΔ27-infected p53CTF cells displayed abundant membrane blebbing (Fig. 5A), 66% condensed chromatin (Fig. 6), and 37% PARP cleavage (Fig. 7C, lane 3) in the absence of E2. Comparing these data with the apoptosis observed in controls led us to conclude that the p53CTF, 16E6, and Sen2 cells display similar levels of sensitivity to HDAP without E2 expression. E2 suppressed vBSΔ27-induced apoptosis in Sen2, but not in the 16E6 cells, consistent with previous experiments (Fig. 1 and 3). As in the 16E6 cells, E2 expression failed to reduce the levels of membrane blebbing (Fig. 5B), chromatin condensation (59% [Fig. 6]), or PARP cleavage (30% [Fig. 7C, lane 7]) in the vBSΔ27-infected p53CTF cells. From these results, we conclude that p53 inactivation sensitizes HeLa cells to HDAP.
The hTERT cells were also susceptible to HSV infection as evidenced by CPE (Fig. 5 and 6) and ICP4 production (Fig. 7D, lane 3). Interestingly, KOS-infected hTERT cells displayed higher levels of chromatin condensation (33%) and PARP cleavage (21%) than other similarly treated Sen2 derivatives. The significance of this finding is unclear at this time. Nevertheless, vBSΔ27 infection led to more chromatin condensation (54%) and PARP cleavage (43%) than KOS infection in these cells, demonstrating that the hTERT cells are highly sensitive to HDAP. This high level of apoptosis induction by vBSΔ27 was still evident in the presence of E2 (59% chromatin condensation and 47% PARP cleavage [Fig. 6 and 7]). Therefore, hTERT overexpression also contributes to the sensitivity of HeLa cells to HDAP. Taken together, these results indicate that E6 can sensitize HeLa cells to HDAP both through p53 inactivation and hTERT stimulation. Our previous findings that E2-expressing 16E7 cells were highly resistant to HDAP (Fig. 4) and earlier studies showing that E2 partially inhibits telomerase activity in both 16E6 and 16E7 cells (13) raises at least two intriguing points. First, partial repression of hTERT may not be sufficient to render cells resistant to HDAP. Alternatively, additional, yet undefined, factors may also play a role in the HDAP sensitization process.
Determinants of STS-induced apoptosis differ from those of HSV-dependent apoptosis.
It is of interest to note that the pattern of STS-induced apoptosis differed from that of HDAP. STS induced p53 expression (Fig. 7) and chromatin condensation (Fig. 6) in the parental and the three Sen2-derived cell lines tested. However, dramatic PARP cleavage was evident only in the STS-treated p53CTF cells (Fig. 7C, lane 4). E2 did not repress STS-induced chromatin condensation in Sen2 and hTERT cell lines (Fig. 6). In contrast, E2 reduced the levels of STS-induced chromatin condensation in the p53CTF cells from 36% to 6% (Fig. 6) and PARP cleavage from 39% to less than 2% (Fig. 7C, lanes 4 and 8). A partial repression of chromatin condensation was apparent in the 16E6 cells (Fig. 6). One implication for this result is that E2 may suppress STS-induced apoptosis only under conditions where p53 is inactivated. Importantly, this result demonstrates that the susceptibility to STS-induced apoptosis is regulated by a mechanism distinct from that regulating susceptibility to HDAP.
DISCUSSION
HDAP has been associated with viral pathogenesis in humans, as well as in animal model systems. Certain tumor cells are exquisitely sensitive to HDAP. Although there may have been many reasons for this susceptibility, we directly tested the hypothesis that cellular targets of viral oncogenes contribute to HDAP. The goal of this study was to utilize a well-defined cell system to investigate the molecular determinants of apoptotic cell killing by HSV.
We found that inactivating p53 through either constitutive expression of HPV16 E6 or the overexpression of a dominant-negative mutant p53 protein, p53CTF, sensitizes otherwise resistant HeLa cells to HDAP. This result implies that p53 acts to inhibit apoptosis during HSV infection. Two previous studies have investigated the ability of HSV to modulate the p53 pathway. In the first study, the viral ICP0 protein was found to be capable of ubiquitinating and degrading p53 in vitro as well as in murine cells and the U2OS human carcinoma cells (9). However, a subsequent report from the same group suggests that the destabilizing effects ICP0 has on p53 are outweighed by other factors during a complete viral infection (8). In the latter study, p53 levels were found to be stabilized during wild-type HSV infection of primary human fibroblasts. We have recently observed a similar p53 stabilization in primary mammary epithelial cells infected with wild-type HSV (M. L. Nguyen and J. A. Blaho, unpublished results). In light of the role of p53 in HDAP in HeLa cells, it seems that p53 regulation may be a critical step in the resistance of primary human epithelial cells to apoptosis during infection.
Although p53 activation is generally perceived to contribute to apoptosis induction in many cells, there are now several examples showing that it can also act in an antiapoptotic manner (reviewed in reference 72). The antiapoptotic effects of p53 have been thought to be largely due to p53 inducing cell cycle arrest rather than directly inhibiting apoptosis. Consistent with a role for p53-mediated cell cycle inhibition in HDAP suppression, perturbations in the cell cycle have been reported during HSV infection. Specifically, HSV infection of asynchronous cell populations increases the percentage of cells in the G0/G1 phase of the cell cycle and decreases the rate of cellular DNA synthesis (17, 62, 63). Additionally, HSV infection has been reported to block the progression of synchronous HEL cell cycle progression (17). Multiple groups have linked this cell cycle perturbation to the pocket protein family of transcription factors (17, 28, 48, 62, 63); however, a role for p53 in this process has not been ruled out. Another link between cell cycle control and apoptosis during HSV infection can be drawn from the viral factors involved. The viral ICP0 protein has been reported to be responsible for HSV cell cycle inhibitory activities (24, 30, 37, 63). Interestingly, we have previously shown that the gene that encodes ICP0, α0, is responsible for triggering HDAP (55). Further research to determine whether these two functions of ICP0 are linked is planned.
The second HDAP susceptibility factor identified in this study was hTERT. To our knowledge, this is the first example of hTERT being associated with an increased sensitivity to apoptosis. Many questions regarding the mechanism of hTERT's sensitization function remain. Perhaps the apoptotic sensitization by hTERT occurs indirectly due to the increased telomere length that accompanies high levels of telomerase activity. Alternatively, it is possible that the hTERT protein directly alters apoptotic pathways through some previously undescribed mechanism. The observed elevation of apoptotic markers in wild-type HSV-infected cells supports such a model. Studies in which hTERT is expressed acutely are needed to provide insight into these issues. Interestingly, p53 has been proposed as a negative regulator of hTERT gene expression (29, 70). Therefore, it is possible that p53 inactivation may be sensitizing cells to this process by relieving transcriptional repression of hTERT. Since hTERT is activated in almost all tumor cells and such cells are highly susceptible to HDAP (47), our findings suggest a dominant role for hTERT in the process. This might explain our earlier observations that certain p53-positive tumor cells are also sensitive to HDAP (47). Studies currently under way testing whether inactivation of p53 alone or activation of Tert alone would confer sensitivity of otherwise normal human epithelial cells to HDAP will address whether either or both are sufficient.
While either p53 inactivation or hTERT expression was sufficient to sensitize HeLa cells to HDAP, we realize that the HeLa cells are highly aneuploid (11, 39) and thus possess multiple genetic changes besides HPV genome integration. Therefore, it remains possible that p53 inactivation and hTERT expression may be insufficient to sensitize cells outside the context of HeLa cells. However, in our preliminary analyses we have found that mammary epithelial cells immortalized through the expression of hTERT are sensitive to HDAP, while primary mammary epithelial cultures are resistant, suggesting that hTERT expression alone may be sufficient for sensitizing cells (Nguyen and Blaho, unpublished). Further investigations that directly place our retroviral constructs (Fig. 1D) into nontumor cells are currently under way to clarify this issue.
Together, the data presented here and in our previous publications demonstrate that there are three distinct responses to HDAP. With few exceptions, fully transformed tumor cells are exquisitely sensitive to this death stimulus (3, 34, 47). In contrast, cells derived from normal tissue are resistant to HDAP, and immortalized but nontransformed cell lines display an intermediate susceptibility (3, 46, 47). Our ability to restore resistance to HDAP by HPV oncogene repression indicates that the tumorigenesis-related changes that confer susceptibility to HDAP are reversible and provides a system to identify these changes. Here, we provide evidence that two cellular pathways commonly altered during tumorigenesis mediate susceptibility to HDAP. Given the importance of apoptosis on the HSV life cycle, this implies that p53 and telomerase pathways are likely to impact HSV replication and pathogenesis. Because a wide array of viruses modulate apoptosis as part of their natural life cycle, it is likely that such p53 and telomerase regulation are also important for efficient replication of viruses other than HSV.
Acknowledgments
We thank Elise Morton (Mount Sinai School of Medicine) for expert technical cell culture assistance.
These studies were supported in part by grants from the PHS (AI48582 to J.A.B. and CA16038 to D.D.). M.L.N. was supported in part by PHS institutional research training awards (AI07647 and CA088796). R.M.K. was supported in part by an undergraduate research fellowship from the Howard Hughes Medical Institute to Manhattan College, Riverdale, NY.
Footnotes
Published ahead of print on 12 September 2007.
REFERENCES
- 1.Andoniou, C. E., and M. A. Degli-Esposti. 2006. Insights into the mechanisms of CMV-mediated interference with cellular apoptosis. Immunol. Cell Biol. 84:99-106. [DOI] [PubMed] [Google Scholar]
- 2.Aubert, M., and J. A. Blaho. 1999. The herpes simplex virus type 1 regulatory protein ICP27 is required for the prevention of apoptosis in infected human cells. J. Virol. 73:2803-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aubert, M., and J. A. Blaho. 2003. Viral oncoapoptosis of human tumor cells. Gene Ther. 10:1437-1445. [DOI] [PubMed] [Google Scholar]
- 4.Aubert, M., L. E. Pomeranz, and J. A. Blaho. 2007. Herpes simplex virus blocks apoptosis by precluding mitochondrial cytochrome c release independent of caspase activation in infected human epithelial cells. Apoptosis 12:19-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aubert, M., S. A. Rice, and J. A. Blaho. 2001. Accumulation of herpes simplex type 1 early and leaky-late proteins correlates with the prevention of apoptosis in infected human HEp-2 cells. J. Virol. 75:1013-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basile, J. R., V. Zacny, and K. Munger. 2001. The cytokines tumor necrosis factor-alpha (TNF-α) and TNF-related apoptosis-inducing ligand differentially modulate proliferation and apoptotic pathways in human keratinocytes expressing the human papillomavirus-16 E7 oncoprotein. J. Biol. Chem. 276:22522-22528. [DOI] [PubMed] [Google Scholar]
- 7.Bernard, B. A., C. Bailly, M. C. Lenoir, M. Darmon, F. Thierry, and M. Yaniv. 1989. The human papillomavirus type 18 (HPV18) E2 gene product is a repressor of the HPV18 regulatory region in human keratinocytes. J. Virol. 63:4317-4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boutell, C., and R. D. Everett. 2004. Herpes simplex virus type 1 infection induces the stabilization of p53 in a USP7- and ATM-independent manner. J. Virol. 78:8068-8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boutell, C., and R. D. Everett. 2003. The herpes simplex virus type 1 (HSV-1) regulatory protein ICP0 interacts with and ubiquitinates p53. J. Biol. Chem. 278:36596-36602. [DOI] [PubMed] [Google Scholar]
- 10.Boyer, S. N., D. E. Wazer, and V. Band. 1996. E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Cancer Res. 56:4620-4624. [PubMed] [Google Scholar]
- 11.Chen, T. R. 1988. Re-evaluation of HeLa, HeLa S3, and HEp-2 karyotypes. Cytogenet. Cell Genet. 48:19-24. [DOI] [PubMed] [Google Scholar]
- 12.Clemens, M. J. 2006. Epstein-Barr virus: inhibition of apoptosis as a mechanism of cell transformation. Int. J. Biochem. Cell Biol. 38:164-169. [DOI] [PubMed] [Google Scholar]
- 13.DeFilippis, R. A., E. C. Goodwin, L. Wu, and D. DiMaio. 2003. Endogenous human papillomavirus E6 and E7 proteins differentially regulate proliferation, senescence, and apoptosis in HeLa cervical carcinoma cells. J. Virol. 77:1551-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desaintes, C., C. Demeret, S. Goyat, M. Yaniv, and F. Thierry. 1997. Expression of the papillomavirus E2 protein in HeLa cells leads to apoptosis. EMBO J. 16:504-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durst, M., A. Kleinheinz, M. Hotz, and L. Gissmann. 1985. The physical state of human papillomavirus type 16 DNA in benign and malignant genital tumours. J. Gen. Virol. 66:1515-1522. [DOI] [PubMed] [Google Scholar]
- 16.Dyson, N., P. M. Howley, K. Munger, and E. Harlow. 1989. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 243:934-937. [DOI] [PubMed] [Google Scholar]
- 17.Ehmann, G. L., T. I. McLean, and S. L. Bachenheimer. 2000. Herpes simplex virus type 1 infection imposes a G1/S block in asynchronously growing cells and prevents G1 entry in quiescent cells. Virology 267:335-349. [DOI] [PubMed] [Google Scholar]
- 18.Finzer, P., A. Aguilar-Lemarroy, and F. Rosl. 2002. The role of human papillomavirus oncoproteins E6 and E7 in apoptosis. Cancer Lett. 188:15-24. [DOI] [PubMed] [Google Scholar]
- 19.Frattini, M. G., S. D. Hurst, H. B. Lim, S. Swaminathan, and L. A. Laimins. 1997. Abrogation of a mitotic checkpoint by E2 proteins from oncogenic human papillomaviruses correlates with increased turnover of the p53 tumor suppressor protein. EMBO J. 16:318-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodwin, E. C., and D. DiMaio. 2001. Induced senescence in HeLa cervical carcinoma cells containing elevated telomerase activity and extended telomeres. Cell Growth Differ. 12:525-534. [PubMed] [Google Scholar]
- 21.Goodwin, E. C., and D. DiMaio. 2000. Repression of human papillomavirus oncogenes in HeLa cervical carcinoma cells causes the orderly reactivation of dormant tumor suppressor pathways. Proc. Natl. Acad. Sci. USA 97:12513-12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodwin, E. C., E. Yang, C. J. Lee, H. W. Lee, D. DiMaio, and E. S. Hwang. 2000. Rapid induction of senescence in human cervical carcinoma cells. Proc. Natl. Acad. Sci. USA 97:10978-10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris, S. L., and A. J. Levine. 2005. The p53 pathway: positive and negative feedback loops. Oncogene 24:2899-2908. [DOI] [PubMed] [Google Scholar]
- 24.Hobbs, W. E., II, and N. A. DeLuca. 1999. Perturbation of cell cycle progression and cellular gene expression as a function of herpes simplex virus ICP0. J. Virol. 73:8245-8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horner, S. M., R. A. DeFilippis, L. Manuelidis, and D. DiMaio. 2004. Repression of the human papillomavirus E6 gene initiates p53-dependent, telomerase-independent senescence and apoptosis in HeLa cervical carcinoma cells. J. Virol. 78:4063-4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huibregtse, J. M., M. Scheffner, and P. M. Howley. 1991. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus types 16 or 18. EMBO J. 10:4129-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang, E. S., D. J. Riese II, J. Settleman, L. A. Nilson, J. Honig, S. Flynn, and D. DiMaio. 1993. Inhibition of cervical carcinoma cell line proliferation by the introduction of a bovine papillomavirus regulatory gene. J. Virol. 67:3720-3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones, D. L., D. A. Thompson, and K. Munger. 1997. Destabilization of the RB tumor suppressor protein and stabilization of p53 contribute to HPV type 16 E7-induced apoptosis. Virology 239:97-107. [DOI] [PubMed] [Google Scholar]
- 29.Kanaya, T., S. Kyo, K. Hamada, M. Takakura, Y. Kitagawa, H. Harada, and M. Inoue. 2000. Adenoviral expression of p53 represses telomerase activity through down-regulation of human telomerase reverse transcriptase transcription. Clin. Cancer Res. 6:1239-1247. [PubMed] [Google Scholar]
- 30.Kawaguchi, Y., C. Van Sant, and B. Roizman. 1997. Herpes simplex virus 1 alpha regulatory protein ICP0 interacts with and stabilizes the cell cycle regulator cyclin D3. J. Virol. 71:7328-7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kerr, J. F., A. H. Wyllie, and A. R. Currie. 1972. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 26:239-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klingelhutz, A. J., S. A. Foster, and J. K. McDougall. 1996. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature 380:79-82. [DOI] [PubMed] [Google Scholar]
- 33.Koyama, A. H., A. Adachi, and H. Irie. 2003. Physiological significance of apoptosis during animal virus infection. Int. Rev. Immunol. 22:341-359. [DOI] [PubMed] [Google Scholar]
- 34.Kraft, R. M., M. L. Nguyen, X. H. Yang, A. D. Thor, and J. A. Blaho. 2006. Caspase 3 activation during herpes simplex virus 1 infection. Virus Res. 120:163-175. [DOI] [PubMed] [Google Scholar]
- 35.Lee, C. J., E. J. Suh, H. T. Kang, J. S. Im, S. J. Um, J. S. Park, and E. S. Hwang. 2002. Induction of senescence-like state and suppression of telomerase activity through inhibition of HPV E6/E7 gene expression in cells immortalized by HPV16 DNA. Exp. Cell Res. 277:173-182. [DOI] [PubMed] [Google Scholar]
- 36.Liu, Y., A. McKalip, and B. Herman. 2000. Human papillomavirus type 16 E6 and HPV-16 E6/E7 sensitize human keratinocytes to apoptosis induced by chemotherapeutic agents: roles of p53 and caspase activation. J. Cell. Biochem. 78:334-349. [PubMed] [Google Scholar]
- 37.Lomonte, P., and R. D. Everett. 1999. Herpes simplex virus type 1 immediate-early protein Vmw110 inhibits progression of cells through mitosis and from G1 into S phase of the cell cycle. J. Virol. 73:9456-9467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ludwig, S., S. Pleschka, O. Planz, and T. Wolff. 2006. Ringing the alarm bells: signalling and apoptosis in influenza virus infected cells. Cell. Microbiol. 8:375-386. [DOI] [PubMed] [Google Scholar]
- 39.Macville, M., E. Schrock, H. Padilla-Nash, C. Keck, B. M. Ghadimi, D. Zimonjic, N. Popescu, and T. Ried. 1999. Comprehensive and definitive molecular cytogenetic characterization of HeLa cells by spectral karyotyping. Cancer Res. 59:141-150. [PubMed] [Google Scholar]
- 40.Miles, D., S. Athmanathan, A. Thakur, and M. Willcox. 2003. A novel apoptotic interaction between HSV-1 and human corneal epithelial cells. Curr. Eye Res. 26:165-174. [DOI] [PubMed] [Google Scholar]
- 41.Miles, D. H., M. D. Willcox, and S. Athmanathan. 2004. Ocular and neuronal cell apoptosis during HSV-1 infection: a review. Curr. Eye Res. 29:79-90. [DOI] [PubMed] [Google Scholar]
- 42.Münger, K., A. Baldwin, K. M. Edwards, H. Hayakawa, C. L. Nguyen, M. Owens, M. Grace, and K. Huh. 2004. Mechanisms of human papillomavirus-induced oncogenesis. J. Virol. 78:11451-11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Munger, K., B. A. Werness, N. Dyson, W. C. Phelps, E. Harlow, and P. M. Howley. 1989. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 8:4099-4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naeger, L. K., E. C. Goodwin, E. S. Hwang, R. A. DeFilippis, H. Zhang, and D. DiMaio. 1999. Bovine papillomavirus E2 protein activates a complex growth-inhibitory program in p53-negative HT-3 cervical carcinoma cells that includes repression of cyclin A and cdc25A phosphatase genes and accumulation of hypophosphorylated retinoblastoma protein. Cell Growth Differ. 10:413-422. [PubMed] [Google Scholar]
- 45.Nguyen, M. L., and J. A. Blaho. 2007. Apoptosis during herpes simplex virus infection. Adv. Virus Res. 69:67-97. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen, M. L., R. M. Kraft, and J. A. Blaho. 2005. African green monkey kidney Vero cells require de novo protein synthesis for efficient herpes simplex virus 1-dependent apoptosis. Virology 336:274-290. [DOI] [PubMed] [Google Scholar]
- 47.Nguyen, M. L., R. M. Kraft, and J. A. Blaho. 2007. Susceptibility of cancer cells to herpes simplex virus-dependent apoptosis. J. Gen. Virol. 88:1866-1875. [DOI] [PubMed] [Google Scholar]
- 48.Olgiate, J., G. L. Ehmann, S. Vidyarthi, M. J. Hilton, and S. L. Bachenheimer. 1999. Herpes simplex virus induces intracellular redistribution of E2F4 and accumulation of E2F pocket protein complexes. Virology 258:257-270. [DOI] [PubMed] [Google Scholar]
- 49.Perkins, D., K. A. Gyure, E. F. Pereira, and L. Aurelian. 2003. Herpes simplex virus type 1-induced encephalitis has an apoptotic component associated with activation of c-Jun N-terminal kinase. J. Neurovirol. 9:101-111. [DOI] [PubMed] [Google Scholar]
- 50.Psyrri, A., R. A. DeFilippis, A. P. Edwards, K. E. Yates, L. Manuelidis, and D. DiMaio. 2004. Role of the retinoblastoma pathway in senescence triggered by repression of the human papillomavirus E7 protein in cervical carcinoma cells. Cancer Res. 64:3079-3086. [DOI] [PubMed] [Google Scholar]
- 51.Roizman, B., D. M. Knipe, and R. J. Whitley. 2007. Herpes simplex viruses, p. 2502-2601. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 52.Sabri, F., F. Granath, A. Hjalmarsson, E. Aurelius, and B. Skoldenberg. 2006. Modulation of sFas indicates apoptosis in human herpes simplex encephalitis. J. Neuroimmunol. 171:171-176. [DOI] [PubMed] [Google Scholar]
- 53.Sacks, W. R., C. C. Greene, D. P. Aschman, and P. A. Schaffer. 1985. Herpes simplex virus type 1 ICP27 is an essential regulatory protein. J. Virol. 55:796-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanchez-Perez, A. M., S. Soriano, A. R. Clarke, and K. Gaston. 1997. Disruption of the human papillomavirus type 16 E2 gene protects cervical carcinoma cells from E2F-induced apoptosis. J. Gen. Virol. 78:3009-3018. [DOI] [PubMed] [Google Scholar]
- 55.Sanfilippo, C. M., and J. A. Blaho. 2006. ICP0 gene expression is a herpes simplex virus type 1 apoptotic trigger. J. Virol. 80:6810-6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scheffner, M., J. M. Huibregtse, R. D. Vierstra, and P. M. Howley. 1993. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 75:495-505. [DOI] [PubMed] [Google Scholar]
- 57.Scheffner, M., B. A. Werness, J. M. Huibregtse, A. J. Levine, and P. M. Howley. 1990. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 63:1129-1136. [DOI] [PubMed] [Google Scholar]
- 58.Schneider-Gadicke, A., and E. Schwarz. 1986. Different human cervical carcinoma cell lines show similar transcription patterns of human papillomavirus type 18 early genes. EMBO J. 5:2285-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwarz, E., U. K. Freese, L. Gissmann, W. Mayer, B. Roggenbuck, A. Stremlau, and H. zur Hausen. 1985. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature 314:111-114. [DOI] [PubMed] [Google Scholar]
- 60.Sekulovich, R. E., K. Leary, and R. M. Sandri-Goldin. 1988. The herpes simplex virus type 1 α protein ICP27 can act as a trans-repressor or a trans-activator in combination with ICP4 and ICP0. J. Virol. 62:4510-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soliman, T. M., R. M. Sandri-Goldin, and S. J. Silverstein. 1997. Shuttling of the herpes simplex virus type 1 regulatory protein ICP27 between the nucleus and cytoplasm mediates the expression of late proteins. J. Virol. 71:9188-9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Song, B., J. J. Liu, K. C. Yeh, and D. M. Knipe. 2000. Herpes simplex virus infection blocks events in the G1 phase of the cell cycle. Virology 267:326-334. [DOI] [PubMed] [Google Scholar]
- 63.Song, B., K. C. Yeh, J. Liu, and D. M. Knipe. 2001. Herpes simplex virus gene products required for viral inhibition of expression of G1-phase functions. Virology 290:320-328. [DOI] [PubMed] [Google Scholar]
- 64.Stoppler, H., M. C. Stoppler, E. Johnson, C. M. Simbulan-Rosenthal, M. E. Smulson, S. Iyer, D. S. Rosenthal, and R. Schlegel. 1998. The E7 protein of human papillomavirus type 16 sensitizes primary human keratinocytes to apoptosis. Oncogene 17:1207-1214. [DOI] [PubMed] [Google Scholar]
- 65.Sung, Y. H., Y. S. Choi, C. Cheong, and H. W. Lee. 2005. The pleiotropy of telomerase against cell death. Mol. Cells 19:303-309. [PubMed] [Google Scholar]
- 66.Taylor, J. M., and M. Barry. 2006. Near death experiences: poxvirus regulation of apoptotic death. Virology 344:139-150. [DOI] [PubMed] [Google Scholar]
- 67.Wells, S. I., D. A. Francis, A. Y. Karpova, J. J. Dowhanick, J. D. Benson, and P. M. Howley. 2000. Papillomavirus E2 induces senescence in HPV-positive cells via pRB- and p21CIP-dependent pathways. EMBO J. 19:5762-5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Werness, B. A., A. J. Levine, and P. M. Howley. 1990. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 248:76-79. [DOI] [PubMed] [Google Scholar]
- 69.White, E. 2001. Regulation of the cell cycle and apoptosis by the oncogenes of adenovirus. Oncogene 20:7836-7846. [DOI] [PubMed] [Google Scholar]
- 70.Xu, D., Q. Wang, A. Gruber, M. Bjorkholm, Z. Chen, A. Zaid, G. Selivanova, C. Peterson, K. G. Wiman, and P. Pisa. 2000. Downregulation of telomerase reverse transcriptase mRNA expression by wild type p53 in human tumor cells. Oncogene 19:5123-5133. [DOI] [PubMed] [Google Scholar]
- 71.Yee, C., I. Krishnan-Hewlett, C. C. Baker, R. Schlegel, and P. M. Howley. 1985. Presence and expression of human papillomavirus sequences in human cervical carcinoma cell lines. Am. J. Pathol. 119:361-366. [PMC free article] [PubMed] [Google Scholar]
- 72.Yu, J., and L. Zhang. 2005. The transcriptional targets of p53 in apoptosis control. Biochem. Biophys. Res. Commun. 331:851-858. [DOI] [PubMed] [Google Scholar]
- 73.Zheng, X., R. H. Silverman, A. Zhou, T. Goto, B. S. Kwon, H. E. Kaufman, and J. M. Hill. 2001. Increased severity of HSV-1 keratitis and mortality in mice lacking the 2-5A-dependent RNase L gene. Investig. Ophthalmol. Vis. Sci. 42:120-126. [PubMed] [Google Scholar]