Abstract
Herpes simplex virus type 2 (HSV-2) infects the genital mucosa and is one of the most common sexually transmitted viruses. Here we sequenced a segment comprising 3.5% of the HSV-2 genome, including genes coding for glycoproteins G, I, and E, from 27 clinical isolates from Tanzania, 10 isolates from Norway, and 10 isolates from Sweden. The sequence variation was low compared to that described for clinical HSV-1 isolates, with an overall similarity of 99.6% between the two most distant HSV-2 isolates. Phylogenetic analysis revealed a divergence into at least two genogroups arbitrarily designated A and B, supported by high bootstrap values and evolutionarily separated at the root. Genogroup A contained isolates collected in Tanzania, and genogroup B contained isolates collected in Tanzania and Scandinavia, implying that the genetic variability of HSV-2 is higher in Tanzania than in Scandinavia. Recombination network analysis and bootscan analysis revealed a complex pattern of phylogenetically conflicting informative sites in the sequence alignments. These signals were present in synonymous and nonsynonymous sites in all three genes and were not accumulated in specific regions, observations arguing against positive selection. Since the PHI test applied solely to synonymous sites revealed a high statistical probability of recombination, we suggest as a novel finding that homologous recombination is, as reported earlier for HSV-1 and varicella-zoster virus, a prominent feature in the evolution of HSV-2.
Herpes simplex virus type 2 (HSV-2) is classified together with HSV-1 and varicella-zoster virus (VZV) in the Alphaherpesvirinae subfamily of human herpesviruses, with the ability to infect and remain latent in the sensory ganglia (56). HSV-2 is sexually transmitted; it typically infects the genital mucosa and establishes a lifelong infection in the sacral ganglia. After a primary infection, HSV-2 may reactivate frequently, leading to widespread genital lesions. HSV-2 is the most common cause of genital ulcers globally, and a major problem, especially in developing countries, is that HSV-2 infection strongly facilitates the transmission of human immunodeficiency virus (HIV) (54, 59). HSV-2 may also induce acute and recurrent meningitis in adults and may occasionally infect neonates, with ensuing disseminated disease and/or encephalitis.
Herpesviruses are among the most extensively studied DNA viruses. The evolutionary relationships among the different herpesviruses infecting humans, reptiles, and other vertebrates, as well as invertebrates, have been described, and the speciation can be traced back 10 million to hundreds of millions of years ago (39). Based on DNA sequence data from clinical isolates, divergence into different genogroups has been described for human herpesviruses HSV-1 (2, 47, 55), VZV (45, 48, 50), Epstein-Barr virus (57), cytomegalovirus (7, 8), human herpesvirus 6 (HHV-6) (11, 20), HHV-7 (18), and HHV-8 (42). Information regarding the genetic variability of clinical HSV-2 isolates has been limited to date. This is the first evolutionary study based on HSV-2 DNA sequence data.
Recombination is a general molecular process that generates new combinations of genetic material (29). Similarly, viral recombination occurs when two viruses of different parental strains coinfect the same cell and interact during replication to generate progeny whose genomes consist of genetic segments obtained from both parental strains. Recombination has been shown to participate in the evolution of several alphaherpesviruses (65) as well as betaherpesviruses (21) and gammaherpesviruses (44). The underlying mechanisms are poorly understood but are associated with DNA replication (67) and different cell factors (14, 63). Several studies have shown that homologous recombination occurs frequently under experimental conditions (43, 58), and HSV-1 recombinants, for example, have been detected in vitro (3, 4, 24, 66) as well as in animal models (32, 69). Recombination has also been shown experimentally for several varicelloviruses, i.e., VZV, pseudorabies virus, bovine herpesvirus-1, and feline herpesvirus (12, 19, 22, 58). Furthermore, recombinants between bovine herpesvirus-1 mutants after coinoculation of calves by the natural route of infection have been demonstrated recently (58). Also, wild-type recombinants have recently been described for, e.g., HSV-1 (2, 47), VZV (45, 48, 50), and pseudorabies virus (9).
The HSV-2 genome contains approximately 155,000 nucleotides (nt) (13) and is related to that of HSV-1 with an overall nucleotide identity of approximately 50%. HSV DNA has two covalently linked segments consisting of the unique long (UL) and unique short (US) components. Recently, Norberg et al. sequenced the glycoprotein G (gG), gI, and gE genes, localized in the US segment, for 28 clinical HSV-1 isolates and defined three distinct genogroups by phylogenetic analysis (47). The clustering into different genogroups facilitated further analysis of the gene sequences, revealing that a substantial portion of the isolates presented evidence of homologous recombination. Thus, recombination is a mechanism used not only for repair of DNA damage but also to exchange genetic segments between different HSV-1 strains.
In the present study, we have sequenced and analyzed clinical HSV-2 isolates from Tanzania, from Bergen in Norway, and from Göteborg in Sweden. We have focused on the US4 gene, encoding HSV-2 gG (gG-2); the US7 gene, coding for gI; and the US8 gene, coding for gE. These genes were selected because the orthologous genes had been sequenced and analyzed for clinical HSV-1 isolates. Here we found, despite low overall genetic diversity, a divergence into at least two genogroups, designated A and B, and evidence of frequent recombination events.
MATERIALS AND METHODS
Clinical HSV-2 isolates.
In total, 47 clinical HSV-2 isolates were collected from patients with genital lesions attending sexually transmitted disease clinics in Bergen, Norway (10 isolates), Göteborg, Sweden (10 isolates), and Dar es Salaam, Tanzania (27 isolates). Samples were derived from indigenous people of each country. Sterile Dacron swabs were used to collect materials from the bases of either ulcers or vesicles, which were deliberately ruptured prior to specimen collection. The swabs were stored in a liquid virus transport medium. The Scandinavian samples were cultivated immediately, while the Tanzanian samples were stored at −80°C until cultivation in Bergen or Göteborg. Isolates from Tanzania and Bergen were confirmed as HSV-2 by using nested PCR targeting either the type-specific promoter region of the gD-2 gene or the coding sequences within the gG-2 gene, as described previously (1, 10). Sequences from the gG gene coding for the carboxy-terminal portion of the protein (approximately 1,000 nt) for the Swedish isolates (n = 10) as well as for the laboratory strain B4327UR have been published previously (30).
Virus stocks were prepared by infecting baby hamster kidney (BHK) cells or GMK-AH1 cells grown in Eagle's minimal essential medium supplemented with 2% calf serum and antibiotics. The clinical isolates were sequenced at a low passage number (<5). The laboratory strain B4327UR (27) was also sequenced and compared to HSV-2 HG52 (38), used as a reference. Strain B4327UR was passaged 10 times before sequencing at the laboratory in Göteborg.
PCR amplification and sequencing.
Two regions of the HSV-2 genome were amplified prior to sequencing. The US4 gene (encoding gG-2) was amplified as a 2,194-bp fragment spanning the region from 57 bp upstream of the start codon to 39 bp downstream of the termination codon (the positions refer to strain HG52). Several additional primers were used for sequencing purposes, and all primers have been published previously (31). Because the carboxy-terminal half of the gG-2 gene has been described previously for the Swedish isolates (30), only the gene segment coding for the amino-terminal secreted portion of gG-2 was sequenced. Amplification with the other set of primers, listed in Table 1, resulted in a 3,182-bp segment starting 57 bp upstream of the start codon of the US7 gene (encoding gI-2) and extending to 47 bp downstream of the stop codon for the US8 gene (encoding gE-2), thus including the noncoding sequence between the two genes.
TABLE 1.
Primers used for amplification and sequencinga
| Gene | Nucleotide positions | Type | Sequence |
|---|---|---|---|
| gI-2 | 8604-8620 | S | 5′-GCTGTCCCGACGATTAG |
| 8812-8832 | S | 5′-TGCGTGTTTTCGGGGAGCTTC | |
| 9111-9128 | S | 5′-GTCGGCAGCGCGACGAAC | |
| 9166-9148 | AS | 5′-TTGGCAGAGAGCGCCACCC | |
| 9375-9394 | S | 5′-ATAGCCCCGCCCAATTCCAC | |
| 9661-9680 | S | 5′-CGTCCACGACCATGCCTTCC | |
| 9715-9734 | S | 5′-CAGTCGTGCTGCTGTCCGTC | |
| 9719-9698 | AS | 5′-GACTGGACCTGGCTCCGATTCC | |
| gE-2 | 10058-10078 | S | 5′-CAGCTAGTCTCCGATCTGCCC |
| 10165-10148 | AS | 5′-CCGCCAGGCACGATACGA | |
| 10379-10400 | S | 5′-CGCTCGCCATAGCATACAGTCC | |
| 10454-10472 | S | 5′-GCGTAGCCGTGGTCAACGA | |
| 10578-10560 | AS | 5′-GACCAGAACCACCGACGCC | |
| 10740-10757 | S | 5′-CCACGTGCGCGGGGTAAC | |
| 10879-10897 | S | 5′-CGGTTTGACGTGCCGTCCT | |
| 10920-10900 | AS | 5′-GTAGATCCGCATATCGGCGCA | |
| 11006-10988 | AS | 5′-AGGCGGTACGCCCAGGAAC | |
| 11236-11254 | S | 5′-AACGCGGTGGTGGAACAGC | |
| 11418-11401 | AS | 5′-GCAGGTCATGCACGCCCA | |
| 11786-11766 | AS | 5′-TGGCAATCAGTTCATCGCCGA |
Nucleotide positions are given for the US region of strain HG52. Boldface indicates primers used for amplification; the rest were used for sequencing. S, sense; AS, antisense.
Each PCR was performed in a total volume of 50 μl using an automated GeneAmp system 2400 thermal cycler (Perkin-Elmer Corporation, Norwalk, CT). The reaction mixture contained 17.5 μl H2O, 1 μl each of the reverse and forward primers at a concentration of 1 pmol/μl, 0.5 μl Tfl DNA polymerase (Boule Nordic, Stockholm, Sweden), 25 μl buffer solution GN (Boule Nordic, Stockholm, Sweden), and 5 μl of diluted purified DNA extract. The incubation steps were as follows: initial denaturation at 96°C for 5 min; 30 cycles of denaturation at 95°C for 1 min, annealing of primers for 1 min at 60°C for US4 and 57°C for US7-US8, and elongation for 3 min at 68°C; and a final extension cycle of 68°C for 15 min. The sizes of amplified PCR products were analyzed by electrophoresis of 10-μl samples in 1% agarose gels stained with ethidium bromide. PCR products were purified using the QIAquick purification kit (Qiagen, Germany) according to the manufacturer's instructions.
Sets of overlapping primers as shown in Table 1 and the ABI Prism BigDye Terminator cycle sequencing ready reaction kit (Applied Biosystems) were used for sequencing. The reaction mixture contained 1 μl 5× sequencing buffer, 2 μl BigDye, 4.4 μl H2O, 1 μl PCR product, 1.6 μl primer at a concentration of 1 pmol/μl, and 10 μl deionized H2O in a total volume of 20 μl. Incubation was carried out according to the following program: 1 min at 96°C, followed by 25 cycles of 10 s at 96°C, 5 s at 50°C, and 4 min at 60°C. The reaction mixtures were then treated with a Sequencing Reaction Cleanup kit (Biomek 2000) according to the manufacturer's protocol. Both strands were sequenced in an ABI Prism 3700 DNA analyzer (Applied Biosystems). For the gG gene and the combined gI and gE genes, a minimum of two sequences were obtained in parallel experiments. Sequences were assembled using DNA Sequence Assembly software, version 3.7 (Applied Biosystems). The sequences were further analyzed by using the Staden sequence analysis package (61) and were compared to those of the reference strain HG52. No mixed-strain infections were detected.
Sequence analysis.
The sequences were easily aligned manually due to a high degree of similarity. To avoid interference by possible hypervariable repeat regions, all gaps in the alignment were analyzed separately and excluded prior to further analyses. The US4 gene and the US7-to-US8 segment were analyzed separately as well as concatenated.
To obtain information about evolutionary relationships among clinical isolates, sequences are traditionally analyzed by using algorithms for constructing bifurcating phylogenetic trees. However, recombination is a reticulate event, which may be difficult to detect. Because recombinants are the progeny of at least two parental strains, traditional phylogenetic trees are insufficient to represent the evolutionary history of, or evolutionary relationships among, isolates including recombinants. Since recombinants have been detected frequently for HSV-1 (47), the US4 and US7-to-US8 segments of the HSV-2 isolates were here analyzed first by using the SplitsTree program (25). In contrast to traditional bifurcating phylogenetic trees, SplitsTree constructs recombination networks, illustrating the evolutionary relationships among taxa in the presence of recombination. If recombination events have participated in the evolution of the isolates included, the sequence alignment will contain conflicting phylogenetic signals. These signals are utilized by SplitsTree to illustrate the evolutionary history of the isolates, including recombination events, as a non-tree-like recombination network. Thus, in such a network, a recombinant will have not one but several branches connected to the parental strains. That is, if a complex network represents the relationships between the isolates, it is likely that several recombination events have participated in their evolutionary history. In contrast, a recombination network with no recombinants included typically appears as a traditional bifurcating phylogenetic tree.
In an additional analysis, the US4 and US7-to-US8 segments were concatenated and analyzed by the SplitsTree program. Owing to a suspected high degree of recombination crossovers in this relatively long genomic segment, we first constructed a network based only on four randomly selected isolates. The analysis was then extended by randomly adding more isolates to the data set, one at a time, and new networks were constructed based on each data set. In total, nine networks including 4 to 12 isolates were constructed. To account for random sampling errors, this procedure was repeated several times with new isolates randomly added to the analysis. A network including all isolates, based solely on silent mutations in the concatenated US4 and US7-to-US8 segments, was constructed for purposes of comparison.
To further analyze the evolutionary history of HSV-2 regarding genetic divergence, isolates presenting conflicting signals (recombinant candidates), identified by using the SplitsTree program, were removed. Traditional phylogenetic trees were then constructed by using the maximum-likelihood method included in the Phylip package, version 3.66. To estimate the robustness of the trees, the calculations were based on 100 bootstrap replicates of each alignment. Phylogenetic trees including all isolates were constructed in parallel for purposes of comparison.
To validate the presence of recombination in HSV-2, the bootscan method included in the SimPlot program (35) was applied to the recombinant candidates. We used the bootscan method on isolates appearing in different clades in the phylogenetic trees based on US4 and US7-to-US8 segments, as well as on the recombinant candidates identified by using the SplitsTree program.
Conflicting signals (i.e., phylogenetically incompatible sites) may be explained either by recombination or by parallel mutations (i.e., true homoplasies) caused by chance or by selection pressure on specific sites. Although evidence for parallel mutations is rarely detected, the pairwise homoplasy index (PHI) test (5) was applied to the sequence alignments in order to determine whether the conflicting signals detected were due to recombination or to parallel mutations. The PHI test is based on the principle that recombination results in fragmented genomes and that each fragment contains several phylogenetically informative sites. In the case of a finite level of recombination, distant loci tend to have a higher degree of incompatibility than adjacent sites. Thus, the presence of two or more nonconflicting informative sites near each other in the sequence alignment will increase the statistical probability of recombination. However, if the genetic distance between the parental strains is low, an insufficient number of informative sites may be present in each fragment, and the probability that the PHI test will falsely reject the hypothesis of recombination will increase.
Nucleotide sequence accession numbers.
The sequences obtained in this study were deposited in GenBank with accession numbers EU106374 to EU106469.
RESULTS
Sequence variability of HSV-2 isolates.
The coding sequence of US4 consists of 2,097 nt, and the US7 and US8 genes contain 1,119 and 1,638 nt, respectively (strain HG52). The noncoding region between the gI and gE genes contains 325 nt. Here, 47 clinical isolates and 2 laboratory strains were analyzed, in total approximately 260,000 nt. All 47 clinical isolates and the laboratory strains HG52 and B4327UR presented unique genetic patterns; none of the isolates were identical. In the gG-2 gene, 54 variable sites were detected in the alignment, 19 (35%) of which were silent and 35 (65%) of which were missense. In the gI gene, 23 variable sites were found, of which 10 (43%) were silent and 13 (57%) were missense. Finally, in the gE gene, 37 variable sites were detected, of which 16 (43%) were silent and 21 (57%) were missense. Thus, in total, 114 variable sites were detected in the alignment, of which 45 were silent (39%) and 69 (61%) were missense.
The similarity between the two most distant isolates was approximately 99.6%, indicating that the interstrain variability of clinical HSV-2 isolates is less than that described previously for HSV-1 based on the same gene segments (98%) (47). A deletion of 3 nt (CGT) was detected in US4 at nt 876 to 878, (positions refer to strain HG52) in nine isolates. In addition, a duplication of 3 nt (GGC) was detected in the US4 gene (nt 1284 to 1286) in five isolates. In US7, an insertion of nucleotides CCCGCG (isolate S_99-3322) or nucleotides CCCGCA (isolate T_64-3300) was detected at nt 706 to 711. Two variable regions were detected in the noncoding region located between US7 and US8; one run contained 6 to 16 cytosine residues, and the other run contained 5 to 14 guanine residues. In US8, one isolate (T_3034) presented an insertion of a single nucleotide (C) at position 584 and an insertion of 11 nt (CCCCCCCGACG) at position 612, leading to an insertion of four novel amino acids and a V→D shift at amino acid position 206. In the US8 alignment, we noted that the sequence from strain HG52, derived from GenBank, displayed a deletion of nucleotide G at position 542 compared to all the other isolates. In addition, at position 574, an extra nucleotide C was found, resulting in 12 altered amino acids from position 181 to 192. Strain HG52 was first received at the virology laboratory in Göteborg in 1981. This virus and HG52 from Bergen were sequenced and were found not to have these nucleotide changes.
SplitsTree recombination analysis.
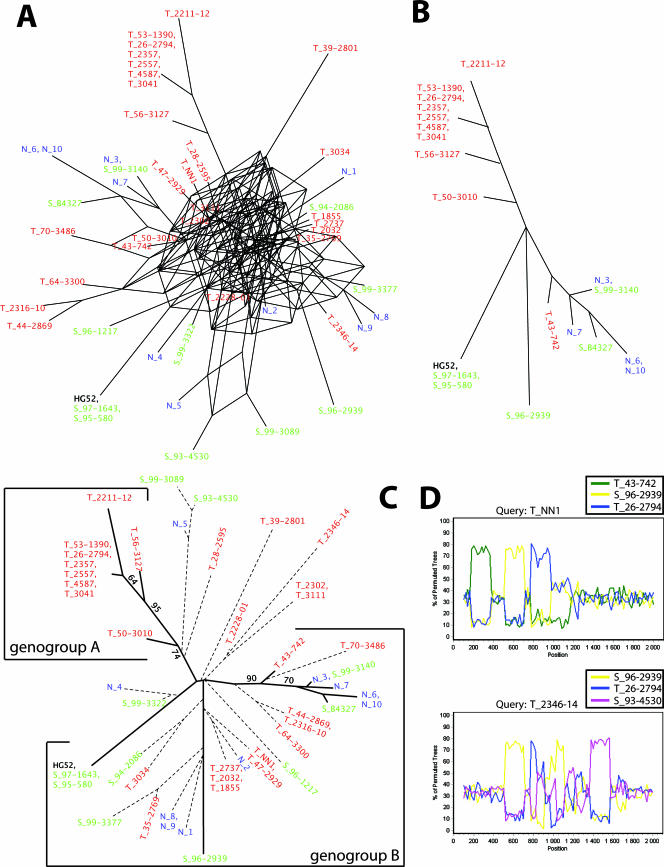
Separate recombination networks based on the US4 gene and based on the US7-to-US8 segment were constructed, including all isolates. The results show that both networks presented a reticulate topology, consistent with an evolutionary history involving recombination. The network based on US4 (Fig. 1A) was more complex than the network based on the US7-to-US8 segment (Fig. 2A), suggesting a higher rate of recombination crossovers in the US4 gene.
FIG. 1.
Sequence analysis of the US4 gene for 27 Tanzanian (red), 10 Swedish (green), and 10 Norwegian (blue) clinical HSV-2 isolates and the two laboratory strains HG52 and B4327. (A) Recombination networks, including all sequences, were first constructed by using the SplitsTree program. (B) The isolates inferring phylogenetically conflicting signals (recombinant candidates) were removed, and new phylogenetic networks were constructed based solely on nonrecombinants. (C) Trees based on all isolates using the maximum-likelihood algorithm. The recombinant isolates are connected with dotted branches and the nonrecombinant isolates with solid branches. The bootstrap values are derived from a consensus tree based on 100 bootstrap replicates, including solely nonrecombinant isolates. Only bootstrap values above 60 are shown. (D) Recombinant candidates were analyzed by using the bootscan method implemented in the SimPlot program. Results from analyses of the US4 gene segment in two isolates are shown.
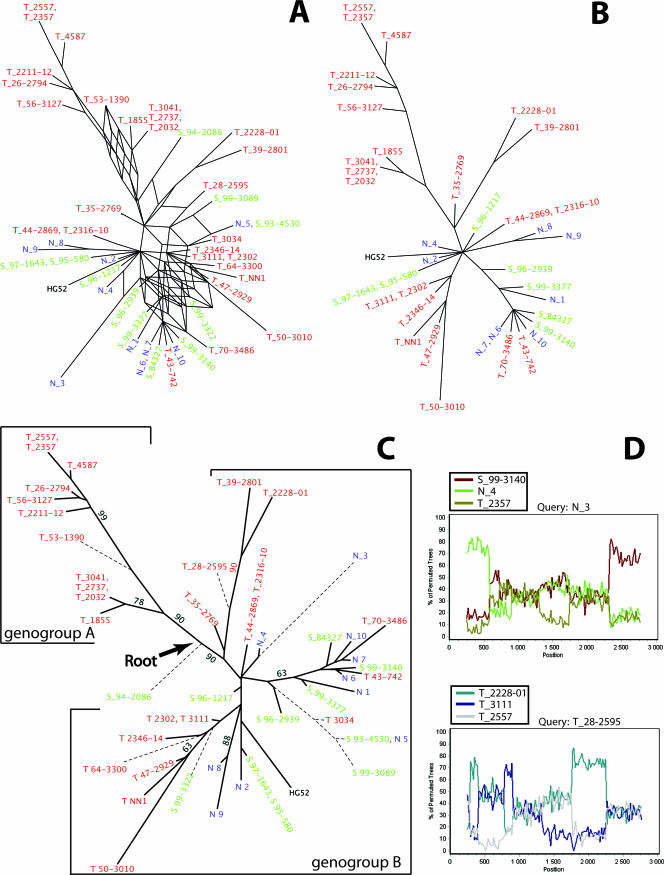
FIG. 2.
Sequence analysis of the US7-to-US8 segment. See the legend to Fig. 1 for details.
Isolates inferring conflicting phylogenetic signals were identified and excluded from further analyses, and new recombination networks were constructed to illustrate the evolutionary history without the presence of recombination (Fig. 1B and 2B). These networks did not present a reticulate topology, and the isolates were divided into at least two genogroups. To evaluate these topologies further, the nonrecombinants were analyzed by using the maximum-likelihood method (see below).
Network analysis was also performed on the concatenated US4 and US7-to-US8 segments by including an increasing number of isolates. First, a network was constructed based on four randomly selected isolates, followed by networks constructed by randomly adding new isolates, one at a time. The majority of the networks presented reticulate topologies, consistent with recombination. Furthermore, the complexity of the networks increased drastically as more isolates were included in the analysis, and networks including 12 isolates were too complex to reveal specific evolutionary relationships among the isolates (data not shown). The network based solely on silent mutations in the concatenated US4 and US7-to-US8 segments also presented a reticulate topology (Fig. 3), a finding that further supports recombination rather than parallel mutations caused by selection pressure.
FIG. 3.
Recombination network based solely on silent mutations. The network includes all isolates and is based on the concatenated genes US4, US7, and US8. The Tanzanian, Swedish, and Norwegian isolates are shown in red, green, and blue, respectively.
Phylogenetic tree analysis.
To refine the results of the SplitsTree analysis of nonrecombinants shown in Fig. 1B and 2B, the maximum-likelihood method was applied to 100 bootstrap replicates. Two trees including all isolates were also constructed, one based on US4 (Fig. 1C) and one based on the US7-to-US8 segment (Fig. 2C). Owing to the conflicting phylogenetic signals inferred by the recombinant candidates, which would falsely lower the bootstrap support for each genogroup, the bootstrap values shown in the trees were derived from the consensus trees based solely on the nonrecombinant isolates.
The nonrecombinant isolates in the trees shown in Fig. 1C and 2C diverge into at least two genogroups, arbitrarily designated genogroups A and B, supported by high bootstrap values. The topologies of the two trees are similar, and genogroup A contains only isolates collected in Tanzania, while genogroup B contains isolates collected in Tanzania as well as in Scandinavia. Although genogroup B may be further divided into subgenogroups, more sequences would have to be included in order to confirm such subdivisions.
The orthologous gI and gE genes in the HSV-1 genome present high similarity to the corresponding HSV-2 genes (∼80%). In contrast, the gG-1 gene has a large internal deletion and includes only 717 nt (strain 17), in comparison to 2,097 nt described for the HSV-2 strain HG52. Thus, only the gI and gE genes from HSV-1 could be used as an outgroup to establish a reliable root, which was localized between the two HSV-2 genogroups, A and B (Fig. 2C). The tree based on US4 includes 20 nonrecombinant isolates and 29 recombinant candidates. Nine nonrecombinants cluster to genogroup A and 11 to genogroup B. The tree based on the US7-to-US8 segment includes 39 nonrecombinant isolates and 10 recombinant candidates. Ten nonrecombinants cluster to genogroup A and 29 to genogroup B. Furthermore, both phylogenetic trees, the tree based on US4 and that based on the US7-to-US8 region, showed a star-like topology, an appearance that remained when the recombinant candidates were excluded from analysis.
In an additional analysis, the gap regions in the sequence alignment were compared to the topology of the most parsimonious tree as well as the maximum-likelihood tree. In the first gap region in US4, the deletion of the triplet CTG was shared among isolates N_3, N_6, N_7, N_10, T_43-742, T_70-3486, S_99-3140, S_96-1217, and S_B4327, which support the subgenogroup divergence within genogroup B (Fig. 1C and 2C). In contrast, in the second gap region in US4, only isolates HG52, S_99-3140, T_64-3300, S_97-1643, and and S_95-580 displayed an insertion of the nucleotides GGC, a finding that does not support the subgenogroup divergence within genogroup B. Thus, the two gap regions in US4 are phylogenetically conflicting. Furthermore, the repeat regions in the noncoding region between US7 and US8 showed no correlation to the topology of the most parsimonious tree or the maximum-likelihood tree, suggesting hypervariability and/or recombination.
Bootscan and the PHI test.
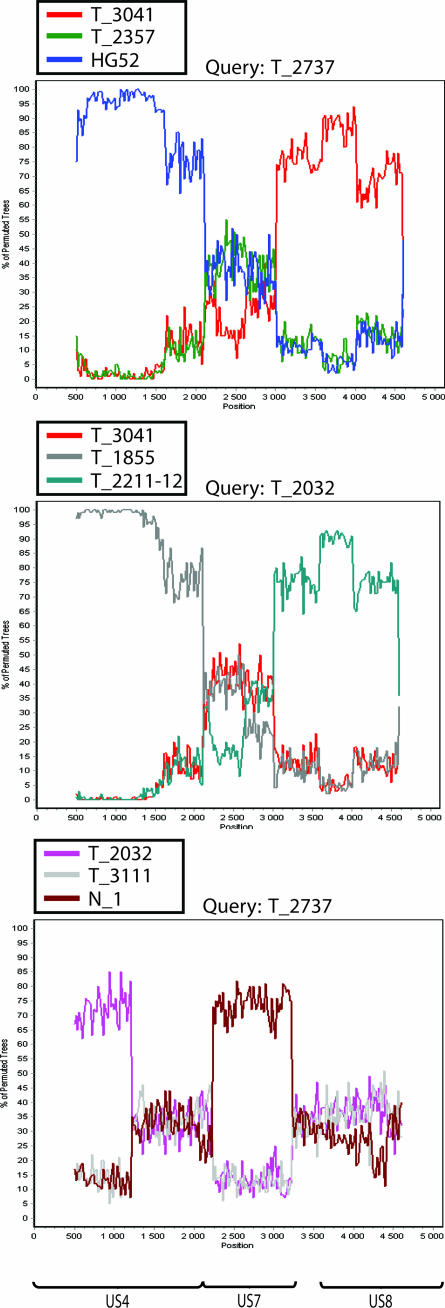
All three genomic regions—US4, the US7-to-US8 segment, and the concatenated US4 and US7-to-US8 segments—were analyzed by the bootscan method. Recombinant candidates were compared to each other as well as to nonrecombinants. In these analyses, variable degrees of segmentation were detected, findings that further support the idea that the isolates have been involved in recombination events. The results obtained from analyzing US4 in two isolates are shown in Fig. 1D, and those from analysis of the US7-to-US8 segment in two other isolates are shown in Fig. 2D. Bootscan analysis of three isolates based on the complete concatenated region is shown in Fig. 4.
FIG. 4.
Results from the bootscan analysis of three recombinant candidates based on concatenations of the US4 and US7-to US8 segments.
The PHI test for detecting the presence of recombination was applied to each data set analyzed. A statistical probability of recombination (0.008 < P < 0.042) was found for each analysis of the concatenated genomic segments consisting of US4 and the US7-to-US8 region when isolates were randomly added to the analysis, i.e., for all data sets including 4 to 12 isolates. A statistical probability of recombination (P = 0.015) was also found when the concatenated US4 and US7-to-US8 segments were analyzed based on silent mutations, including all isolates. In contrast, no statistical probability of recombination was found for the same data set when all mutations, both silent and missense, were included. Missense mutations are nucleotide changes that lead to amino acid shifts and hence may alter the functionality of the protein. Missense mutations therefore have a higher probability of being influenced by a selection pressure. Similarly, no statistical probability of recombination was found when the PHI test was applied to the US4 or the US7-to-US8 genomic region separately, including all sequences.
DISCUSSION
In this study we analyzed gene segments localized in the US region, which comprises 3.5% of the complete genome, for 47 clinical HSV-2 isolates and 2 laboratory strains. Although the divergence of the HSV-2 sequences into two genogroups was not as distinct as that for the different genogroups described for HSV-1 (47), the division was supported by high bootstrap values. Furthermore, genogroups A and B were separated at the root in the tree based on the US7-to-US8 segment, which further supports this divergence. Isolates collected in Tanzania clustered to both genogroups A and B. In contrast, isolates collected in Norway or Sweden all clustered to genogroup B. Although the isolates were collected from relatively few sources, these results imply that genetic variability is higher in Tanzania than in Scandinavia. Such a distribution corresponds well with what is known for other viruses, bacteria, and animals. For example, it has been demonstrated that another sexually transmitted virus, human papillomavirus type 16 (HPV-16), exists in different genetic variants (23). As demonstrated here for HSV-2, the genetic variability of HPV-16 was higher in Africa than in the rest of the world, and both the African and European HPV-16 variants were found in Tanzania, while only the European variant was found in Europe. Similarly, all HIV genotypes described have been detected in Africa (49). Furthermore, the genetic diversity of Helicobacter pylori, as well as that of the human population, has been demonstrated to be higher in Africa than in the rest of the world (33). The most common explanation of these observations is the origin of humans in Africa (40, 41). Several studies, based on global genetic analysis, suggest a single origin somewhere in Africa (34, 51-53). The migration out of Africa caused a loss of genetic divergence due to several founder events and bottlenecks, and the genetic variability of the human population decreases as the distance from Africa increases (34, 51, 52). Since HSV-2 is likely to have coevolved with the human host, the higher genetic variability detected in Tanzania than in Sweden or Norway may be explained by the same founder effects and bottlenecks that influenced the human population during its migration out of Africa.
The genetic variability of the HSV-2 isolates was significantly lower than that previously described for HSV-1 (47). The underlying reason for this observation is currently unknown, but an explanation might be that HSV-2 has historically been a smaller population than HSV-1. Since small populations are more sensitive to genetic drift and fixation, the divergence of HSV-2 may have been restricted. In contrast, large populations are less sensitive to random sampling errors, which may have allowed HSV-1 to diverge at an earlier stage than HSV-2, implying that the most recent ancestor for the HSV-1 isolates investigated previously is older than that for the HSV-2 isolates investigated here. Furthermore, other factors, such as different transmission routes, may also have influenced the population genetics of HSV-1 and HSV-2, exerting different selection pressures, bottlenecks, and founder effects on the two populations. In addition, more-frequent recombination events in the HSV-2 population during evolution may have decreased genetic diversity. Taken together, the lower genetic variability for HSV-2 may not necessarily be explained by a higher mutation rate for HSV-1 than for HSV-2, but by differences in variables affecting the population genetics of HSV-1 and HSV-2.
By constructing recombination networks and using the bootscan method, we showed that the sequence alignment of the clinical HSV-2 isolates contained a substantial number of phylogenetically conflicting signals. We suggest that the majority of these conflicting signals are results of homologous recombination. However, in addition to recombination, phylogenetically conflicting signals may also be explained by parallel mutations (i.e., true homoplasies), either randomly introduced into the genome or caused by selection pressure on specific, functionally important amino acids. While conflicting signals caused by selection pressure are usually found accumulated at specific sites or epitopes, the conflicting signals described here for the HSV-2 alignment were present in all three genes investigated, US4, US7, and US8, and had not accumulated in certain regions, which argues against an overt selection pressure. Furthermore, the recombination network based solely on silent mutations also presented a reticulate topology, which may not be explained by selection pressure. Owing to the close homology of the sequences, the probability of such frequent conflicting signals arising by chance is considered extremely low. In addition, the gap regions were also phylogenetically conflicting in that the number of repeated triplets or nucleotides for each isolate was not entirely reflected by the topology of the most parsimonious tree including all isolates. When the PHI test was applied solely to silent mutations, a high statistical probability of recombination was found. The fact that the PHI test failed to prove a statistical probability of the presence of recombination in the sequence alignments including all isolates and nucleotide substitutions may not necessarily reject the hypothesis of recombination. When more isolates are included in the analysis, the number of crossovers will increase in the alignment if the frequency of recombination is high, and hence, the distances between the crossovers will decrease. Since the average genetic distance between the isolates is smaller than 0.4%, the probability that two or more phylogenetically informative sites will be identified between each pair of crossovers is low. Thus, it is likely that the number of such sites in the sequence alignment analyzed here is insufficient to be utilized for calculation of the statistical probability of recombination by the PHI test. Furthermore, it has been shown that the probability of the PHI test falsely rejecting the hypothesis of recombination increases if the evolutionary history includes exponential population growth (5).
Conflicting phylogenetic signals may also be explained by random introduction of nucleotide substitutions by Taq polymerase during the PCR. Although we cannot entirely exclude this possibility for all nucleotide substitutions described, this explanation is unlikely. First, all sequences were generated from two strands in both directions. Second, most isolates have been resequenced from new PCRs, giving identical results. Third, to achieve a Taq polymerase artifact, the error must be introduced early in the amplification reaction, where the original sample contains a low number of DNA copies. To avoid this possibility, the PCRs were initiated with a high number of DNA copies (>106 genome copies) measured by real-time PCR (46). Finally, although some nucleotide substitutions are specific for single isolates, most substitutions are shared among two or more isolates, a feature that would have been unlikely if the substitutions were introduced randomly by the Taq polymerase. In addition, since the isolates were sequenced after a low number of passages in cell culture (<5), and it has been found that multiple passages of HSV-2 in cell cultures do not alter the DNA sequences (30, 64), it seems unlikely that the substitutions described here were cell culture artifacts.
Since nucleotide substitutions are rare events for herpesviruses compared to many RNA viruses, recombination may act as a powerful and essential driving force of evolution. For example, it has been shown experimentally that two avirulent herpes simplex viruses may generate lethal recombinants in vivo (26). Recombination can break down associations between deleterious and beneficial mutations at different loci (negative disequilibrium). A genome can also collect beneficial mutations from several other genomes, which is advantageous when different individuals in a population carry different beneficial mutations (16). A consequence is that recombination can increase the additive genetic variance and, by Fisher's fundamental theorem for natural selection, can increase the rate of adaptation (15, 17). In addition, all organisms randomly introduce harmful mutations into their genomes, and a recent study has demonstrated that recombination is a powerful mechanism for the deletion of such mutations (28). Whether HSV-2 uses recombination for adaptation is unknown.
Star phylogenies may typically be explained either by an evolutionary history with frequent recombination or by an exponentially growing population. This pattern of evolution has been described for several RNA viruses, such as HIV (36, 62), hepatitis C viruses (68), Puumala hantaviruses (60), and enteroviruses (37). In addition, HPV-16 (23), HPV-44, HPV-55, and HPV-68 (6) present a tree topology similar to that described here, i.e., two dichotomous clusters and a star-like appearance of the variants within the clusters. Due to the suggested high frequency of recombinants described here for HSV-2, it is likely that the star phylogeny of the trees including all isolates is caused, at least partly, by recombination. However, since the trees based solely on nonrecombinants also presented star-like topologies, two possibilities can be considered: (i) some of the nonrecombinants are in fact recombinants, which we failed to classify, or (ii) the HSV-2 population has expanded exponentially. The latter suggestion corresponds well with what we know of the HSV-2 host, i.e., the human population. Nevertheless, the conservation of the genome and the complex pattern of recombination crossovers complicate the predictions of which isolates are recombinants. Hence, it may be possible that some of the isolates classified here as recombinants are nonrecombinants, and vice versa. On the other hand, the phylogenetic trees based solely on nonrecombinants presented similar topologies for both the US4 region and the US7-to-US8 region. These results not only increase the support for the evolutionary history illustrated by the two trees, i.e., the divergence into genogroups A and B and the subgroups within genogroup B, but also decrease the probability that the isolates classified here as nonrecombinants are recombinants.
In conclusion, based on clinical data from HSV-2 isolates collected in Scandinavia and Tanzania, the results presented here demonstrate a divergence into at least two genogroups. By applying different algorithms, it was also possible to perform a novel and thorough analysis of recombination of clinical HSV-2 isolates. The results of this study suggest that, as has been described for HSV-1 and VZV, recombination is a prominent feature of the evolution of HSV-2 as well. To increase the understanding of the genetic variability and divergence of HSV-2, it would be of interest to analyze isolates from other regions of Africa, as well as from the rest of the world. Possible biological implications of genetic variability for tissue tropism, symptomatic versus asymptomatic transmission, vaccine development, and different clinical manifestations of HSV-2 infection need to be addressed further.
Acknowledgments
We thank Anette Roth and Kjerstin Jakobsen for skillful technical assistance, Gro Njølstad for collecting the Norwegian isolates, and Jan Albert for valuable discussions. We also thank two anonymous reviewers for useful comments on the manuscript.
Financial support was received from the Swedish International Development Agency, the Swedish Research Council, the ALF Foundation at Sahlgren's Hospital, the Swedish Society for Medical Research, and the Western Norway Regional Health Authority.
Footnotes
Published ahead of print on 19 September 2007.
REFERENCES
- 1.Aurelius, E., B. Johansson, B. Skoldenberg, and M. Forsgren. 1993. Encephalitis in immunocompetent patients due to herpes simplex virus type 1 or 2 as determined by type-specific polymerase chain reaction and antibody assays of cerebrospinal fluid. J. Med. Virol. 39:179-186. [DOI] [PubMed] [Google Scholar]
- 2.Bowden, R., H. Sakaoka, P. Donnelly, and R. Ward. 2004. High recombination rate in herpes simplex virus type 1 natural populations suggests significant co-infection. Infect. Genet. Evol. 4:115-123. [DOI] [PubMed] [Google Scholar]
- 3.Brown, S. M., and D. A. Ritchie. 1975. Genetic studies with herpes simplex virus type 1. Analysis of mixed plaque-forming virus and its bearing on genetic recombination. Virology 64:32-42. [DOI] [PubMed] [Google Scholar]
- 4.Brown, S. M., J. H. Subak-Sharpe, J. Harland, and A. R. MacLean. 1992. Analysis of intrastrain recombination in herpes simplex virus type 1 strain 17 and herpes simplex virus type 2 strain HG52 using restriction endonuclease sites as unselected markers and temperature-sensitive lesions as selected markers. J. Gen. Virol. 73:293-301. [DOI] [PubMed] [Google Scholar]
- 5.Bruen, T. C., H. Philippe, and D. Bryant. 2006. A simple and robust statistical test for detecting the presence of recombination. Genetics 172:2665-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calleja-Macias, I. E., M. Kalantari, B. Allan, A. L. Williamson, L. P. Chung, R. J. Collins, R. E. Zuna, S. T. Dunn, R. Ortiz-Lopez, H. A. Barrera-Saldana, H. A. Cubie, K. Cuschieri, L. L. Villa, and H. U. Bernard. 2005. Papillomavirus subtypes are natural and old taxa: phylogeny of human papillomavirus types 44 and 55 and 68a and -b. J. Virol. 79:6565-6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou, S. 1992. Comparative analysis of sequence variation in gp116 and gp55 components of glycoprotein B of human cytomegalovirus. Virology 188:388-390. [DOI] [PubMed] [Google Scholar]
- 8.Chou, S. W., and K. M. Dennison. 1991. Analysis of interstrain variation in cytomegalovirus glycoprotein B sequences encoding neutralization-related epitopes. J. Infect. Dis. 163:1229-1234. [DOI] [PubMed] [Google Scholar]
- 9.Christensen, L. S., and B. Lomniczi. 1993. High frequency intergenomic recombination of suid herpesvirus 1 (SHV-1, Aujeszky's disease virus). Arch. Virol. 132:37-50. [DOI] [PubMed] [Google Scholar]
- 10.Cinque, P., L. Vago, H. Dahl, M. Brytting, M. R. Terreni, C. Fornara, S. Racca, A. Castagna, A. D. Monforte, B. Wahren, A. Lazzarin, and A. Linde. 1996. Polymerase chain reaction on cerebrospinal fluid for diagnosis of virus-associated opportunistic diseases of the central nervous system in HIV-infected patients. AIDS 10:951-958. [DOI] [PubMed] [Google Scholar]
- 11.Clark, D. A. 2000. Human herpesvirus 6. Rev. Med. Virol. 10:155-173. [DOI] [PubMed] [Google Scholar]
- 12.Dohner, D. E., S. G. Adams, and L. D. Gelb. 1988. Recombination in tissue culture between varicella-zoster virus strains. J. Med. Virol. 24:329-341. [DOI] [PubMed] [Google Scholar]
- 13.Dolan, A., F. E. Jamieson, C. Cunningham, B. C. Barnett, and D. J. McGeoch. 1998. The genome sequence of herpes simplex virus type 2. J. Virol. 72:2010-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dutch, R. E., V. Bianchi, and I. R. Lehman. 1995. Herpes simplex virus type 1 DNA replication is specifically required for high-frequency homologous recombination between repeated sequences. J. Virol. 69:3084-3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards, A. W. 1994. The fundamental theorem of natural selection. Biol. Rev. Camb. Philos. Soc. 69:443-474. [DOI] [PubMed] [Google Scholar]
- 16.Felsenstein, J., and S. Yokoyama. 1976. The evolutionary advantage of recombination. II. Individual selection for recombination. Genetics 83:845-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher, R. A. 1930. The genetical theory of natural selection. Oxford University Press, Oxford, United Kingdom.
- 18.Franti, M., J. T. Aubin, L. Poirel, A. Gautheret-Dejean, D. Candotti, J. M. Huraux, and H. Agut. 1998. Definition and distribution analysis of glycoprotein B gene alleles of human herpesvirus 7. J. Virol. 72:8725-8730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujita, K., K. Maeda, N. Yokoyama, T. Miyazawa, C. Kai, and T. Mikami. 1998. In vitro recombination of feline herpesvirus type 1. Arch. Virol. 143:25-34. [DOI] [PubMed] [Google Scholar]
- 20.Gompels, U. A., D. R. Carrigan, A. L. Carss, and J. Arno. 1993. Two groups of human herpesvirus 6 identified by sequence analyses of laboratory strains and variants from Hodgkin's lymphoma and bone marrow transplant patients. J. Gen. Virol. 74:613-622. [DOI] [PubMed] [Google Scholar]
- 21.Haberland, M., U. Meyer-Konig, and F. T. Hufert. 1999. Variation within the glycoprotein B gene of human cytomegalovirus is due to homologous recombination. J. Gen. Virol. 80:1495-1500. [DOI] [PubMed] [Google Scholar]
- 22.Henderson, L. M., J. B. Katz, G. A. Erickson, and J. E. Mayfield. 1990. In vivo and in vitro genetic recombination between conventional and gene-deleted vaccine strains of pseudorabies virus. Am. J. Vet. Res. 51:1656-1662. [PubMed] [Google Scholar]
- 23.Ho, L., S. Y. Chan, R. D. Burk, B. C. Das, K. Fujinaga, J. P. Icenogle, T. Kahn, N. Kiviat, W. Lancaster, P. Mavromara-Nazos, et al. 1993. The genetic drift of human papillomavirus type 16 is a means of reconstructing prehistoric viral spread and the movement of ancient human populations. J. Virol. 67:6413-6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Honess, R. W., A. Buchan, I. W. Halliburton, and D. H. Watson. 1980. Recombination and linkage between structural and regulatory genes of herpes simplex virus type 1: study of the functional organization of the genome. J. Virol. 34:716-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huson, D. H. 1998. SplitsTree: analyzing and visualizing evolutionary data. Bioinformatics 14:68-73. [DOI] [PubMed] [Google Scholar]
- 26.Javier, R. T., F. Sedarati, and J. G. Stevens. 1986. Two avirulent herpes simplex viruses generate lethal recombinants in vivo. Science 234:746-748. [DOI] [PubMed] [Google Scholar]
- 27.Jeansson, S., and L. Molin. 1974. On the occurrence of genital herpes simplex virus infection. Clinical and virological findings and relation to gonorrhoea. Acta Derm. Venereol. 54:479-485. [PubMed] [Google Scholar]
- 28.Keightley, P. D., and S. P. Otto. 2006. Interference among deleterious mutations favours sex and recombination in finite populations. Nature 443: 89-92. [DOI] [PubMed] [Google Scholar]
- 29.Leach, D. R. F. 1996. Genetic recombination. Blackwell Science, Oxford, United Kingdom.
- 30.Liljeqvist, J.-Å., B. Svennerholm, and T. Bergström. 2000. Conservation of type-specific B-cell epitopes of glycoprotein G in clinical herpes simplex virus type 2 isolates. J. Clin. Microbiol. 38:4517-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liljeqvist, J.-Å., B. Svennerholm, and T. Bergström. 1999. Herpes simplex virus type 2 glycoprotein G-negative clinical isolates are generated by single frameshift mutations. J. Virol. 73:9796-9802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lingen, M., F. Hengerer, and D. Falke. 1997. Mixed vaginal infections of BALB/c mice with low virulent herpes simplex type 1 strains result in restoration of virulence properties: vaginitis/vulvitis and neuroinvasiveness. Med. Microbiol. Immunol. 185:217-222. [DOI] [PubMed] [Google Scholar]
- 33.Linz, B., F. Balloux, Y. Moodley, A. Manica, H. Liu, P. Roumagnac, D. Falush, C. Stamer, F. Prugnolle, S. W. van der Merwe, Y. Yamaoka, D. Y. Graham, E. Perez-Trallero, T. Wadstrom, S. Suerbaum, and M. Achtman. 2007. An African origin for the intimate association between humans and Helicobacter pylori. Nature 445:915-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu, H., F. Prugnolle, A. Manica, and F. Balloux. 2006. A geographically explicit genetic model of worldwide human-settlement history. Am. J. Hum. Genet. 79:230-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lole, K. S., R. C. Bollinger, R. S. Paranjape, D. Gadkari, S. S. Kulkarni, N. G. Novak, R. Ingersoll, H. W. Sheppard, and S. C. Ray. 1999. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J. Virol. 73:152-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Louwagie, J., F. E. McCutchan, M. Peeters, T. P. Brennan, E. Sanders-Buell, G. A. Eddy, G. van der Groen, K. Fransen, G. M. Gershy-Damet, R. Deleys, et al. 1993. Phylogenetic analysis of gag genes from 70 international HIV-1 isolates provides evidence for multiple genotypes. AIDS 7:769-780. [DOI] [PubMed] [Google Scholar]
- 37.Lukashev, A. N., V. A. Lashkevich, O. E. Ivanova, G. A. Koroleva, A. E. Hinkkanen, and J. Ilonen. 2005. Recombination in circulating human enterovirus B: independent evolution of structural and non-structural genome regions. J. Gen. Virol. 86:3281-3290. [DOI] [PubMed] [Google Scholar]
- 38.McGeoch, D. J., H. W. M. Moss, D. McNab, and M. C. Frame. 1987. DNA sequence and genetic content of the HindIII l region in the short unique component of the herpes simplex virus type 2 genome: identification of the gene encoding glycoprotein G, and evolutionary comparisons. J. Gen. Virol. 68:19-38. [DOI] [PubMed] [Google Scholar]
- 39.McGeoch, D. J., F. J. Rixon, and A. J. Davison. 2006. Topics in herpesvirus genomics and evolution. Virus Res. 117:90-104. [DOI] [PubMed] [Google Scholar]
- 40.Mellars, P. 2006. Going east: new genetic and archaeological perspectives on the modern human colonization of Eurasia. Science 313:796-800. [DOI] [PubMed] [Google Scholar]
- 41.Mellars, P. 2006. Why did modern human populations disperse from Africa ca. 60,000 years ago? A new model. Proc. Natl. Acad. Sci. USA 103:9381-9386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meng, Y. X., T. J. Spira, G. J. Bhat, C. J. Birch, J. D. Druce, B. R. Edlin, R. Edwards, C. Gunthel, R. Newton, F. R. Stamey, C. Wood, and P. E. Pellett. 1999. Individuals from North America, Australasia, and Africa are infected with four different genotypes of human herpesvirus 8. Virology 261:106-119. [DOI] [PubMed] [Google Scholar]
- 43.Meurens, F., G. M. Keil, B. Muylkens, S. Gogev, F. Schynts, S. Negro, L. Wiggers, and E. Thiry. 2004. Interspecific recombination between two ruminant alphaherpesviruses, bovine herpesviruses 1 and 5. J. Virol. 78:9828-9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Midgley, R. S., N. W. Blake, Q. Y. Yao, D. Croom-Carter, S. T. Cheung, S. F. Leung, A. T. Chan, P. J. Johnson, D. Huang, A. B. Rickinson, and S. P. Lee. 2000. Novel intertypic recombinants of Epstein-Barr virus in the Chinese population. J. Virol. 74:1544-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muir, W. B., R. Nichols, and J. Breuer. 2002. Phylogenetic analysis of varicella-zoster virus: evidence of intercontinental spread of genotypes and recombination. J. Virol. 76:1971-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Namvar, L., S. Olofsson, T. Bergström, and M. Lindh. 2005. Detection and typing of herpes simplex virus (HSV) in mucocutaneous samples by TaqMan PCR targeting a gB segment homologous for HSV types 1 and 2. J. Clin. Microbiol. 43:2058-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Norberg, P., T. Bergström, E. Rekabdar, M. Lindh, and J.-A. Liljeqvist. 2004. Phylogenetic analysis of clinical herpes simplex virus type 1 isolates identified three genetic groups and recombinant viruses. J. Virol. 78:10755-10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Norberg, P., J. A. Liljeqvist, T. Bergstrom, S. Sammons, D. S. Schmid, and V. N. Loparev. 2006. Complete-genome phylogenetic approach to varicella-zoster virus evolution: genetic divergence and evidence for recombination. J. Virol. 80:9569-9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Papathanasopoulos, M. A., G. M. Hunt, and C. T. Tiemessen. 2003. Evolution and diversity of HIV-1 in Africa—a review. Virus Genes 26:151-163. [DOI] [PubMed] [Google Scholar]
- 50.Peters, G. A., S. D. Tyler, C. Grose, A. Severini, M. J. Gray, C. Upton, and G. A. Tipples. 2006. A full-genome phylogenetic analysis of varicella-zoster virus reveals a novel origin of replication-based genotyping scheme and evidence of recombination between major circulating clades. J. Virol. 80:9850-9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prugnolle, F., A. Manica, and F. Balloux. 2005. Geography predicts neutral genetic diversity of human populations. Curr. Biol. 15:R159-R160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramachandran, S., O. Deshpande, C. C. Roseman, N. A. Rosenberg, M. W. Feldman, and L. L. Cavalli-Sforza. 2005. Support from the relationship of genetic and geographic distance in human populations for a serial founder effect originating in Africa. Proc. Natl. Acad. Sci. USA 102:15942-15947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ray, N., M. Currat, P. Berthier, and L. Excoffier. 2005. Recovering the geographic origin of early modern humans by realistic and spatially explicit simulations. Genome Res. 15:1161-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rebbapragada, A., C. Wachihi, C. Pettengell, S. Sunderji, S. Huibner, W. Jaoko, B. Ball, K. Fowke, T. Mazzulli, F. A. Plummer, and R. Kaul. 2007. Negative mucosal synergy between herpes simplex type 2 and HIV in the female genital tract. AIDS 21:589-598. [DOI] [PubMed] [Google Scholar]
- 55.Rekabdar, E., P. Tunback, J. A. Liljeqvist, M. Lindh, and T. Bergstrom. 2002. Dichotomy of glycoprotein G gene in herpes simplex virus type 1 isolates. J. Clin. Microbiol. 40:3245-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roizman, B. 1996. Herpesviridae, p. 2221-2230. In B. N. Fields, D. M. Knipe, P. M. Howley, et al. (ed.), Fields virology. Lippincott-Raven, Philadelphia, PA.
- 57.Sample, J., L. Young, B. Martin, T. Chatman, E. Kieff, and A. Rickinson. 1990. Epstein-Barr virus types 1 and 2 differ in their EBNA-3A, EBNA-3B, and EBNA-3C genes. J. Virol. 64:4084-4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schynts, F., F. Meurens, B. Detry, A. Vanderplasschen, and E. Thiry. 2003. Rise and survival of bovine herpesvirus 1 recombinants after primary infection and reactivation from latency. J. Virol. 77:12535-12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Serwadda, D., R. H. Gray, N. K. Sewankambo, F. Wabwire-Mangen, M. Z. Chen, T. C. Quinn, T. Lutalo, N. Kiwanuka, G. Kigozi, F. Nalugoda, M. P. Meehan, R. Ashley Morrow, and M. J. Wawer. 2003. Human immunodeficiency virus acquisition associated with genital ulcer disease and herpes simplex virus type 2 infection: a nested case-control study in Rakai, Uganda. J. Infect. Dis. 188:1492-1497. [DOI] [PubMed] [Google Scholar]
- 60.Sironen, T., A. Vaheri, and A. Plyusnin. 2001. Molecular evolution of Puumala hantavirus. J. Virol. 75:11803-11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Staden, R. 1994. Staden: introduction. Methods Mol. Biol. 25:9-26. [DOI] [PubMed] [Google Scholar]
- 62.Strunnikova, N., S. C. Ray, R. A. Livingston, E. Rubalcaba, and R. P. Viscidi. 1995. Convergent evolution within the V3 loop domain of human immunodeficiency virus type 1 in association with disease progression. J. Virol. 69:7548-7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taylor, T. J., and D. M. Knipe. 2004. Proteomics of herpes simplex virus replication compartments: association of cellular DNA replication, repair, recombination, and chromatin remodeling proteins with ICP8. J. Virol. 78:5856-5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Terhune, S. S., K. T. Coleman, R. Sekulovich, R. L. Burke, and P. G. Spear. 1998. Limited variability of glycoprotein gene sequences and neutralizing targets in herpes simplex virus type 2 isolates and stability on passage in cell culture. J. Infect. Dis. 178:8-15. [DOI] [PubMed] [Google Scholar]
- 65.Thiry, E., F. Meurens, B. Muylkens, M. McVoy, S. Gogev, J. Thiry, A. Vanderplasschen, A. Epstein, G. Keil, and F. Schynts. 2005. Recombination in alphaherpesviruses. Rev. Med. Virol. 15:89-103. [DOI] [PubMed] [Google Scholar]
- 66.Umene, K. 1985. Intermolecular recombination of the herpes simplex virus type 1 genome analysed using two strains differing in restriction enzyme cleavage sites. J. Gen. Virol. 66:2659-2670. [DOI] [PubMed] [Google Scholar]
- 67.Umene, K. 1999. Mechanism and application of genetic recombination in herpesviruses. Rev. Med. Virol. 9:171-182. [DOI] [PubMed] [Google Scholar]
- 68.Wang, H., T. Bian, S. J. Merrill, and D. D. Eckels. 2002. Sequence variation in the gene encoding the nonstructural 3 protein of hepatitis C virus: evidence for immune selection. J. Mol. Evol. 54:465-473. [DOI] [PubMed] [Google Scholar]
- 69.Wildy, P. 1955. Recombination with herpes simplex virus. J. Gen. Microbiol. 13:346-360. [DOI] [PubMed] [Google Scholar]