Abstract
The effect of glycoprotein K (gK) overexpression on herpes simplex virus type 1 (HSV-1) infection in two different strains of mice was evaluated using a recombinant HSV-1 virus that expresses two additional copies of the gK gene in place of the latency-associated transcript (LAT). This mutant virus (HSV-gK3) expressed higher levels of gK than either the wild-type McKrae virus or the parental dLAT2903 virus both in vitro (in cultured cells) and in vivo (in infected mouse corneas and trigeminal ganglia [TG] of BALB/c and C57BL/6 mice). gK transcripts were detected in the TG of both HSV-gK3-infected mouse strains on day 30 postinfection (p.i.), while gB transcripts were detected only in the TG of the HSV-gK3-infected C57BL/6 mice, a finding that suggests that increased gK levels promote chronic infection. C57BL/6 mice infected with HSV-gK3 also contained free virus in their TG on day 30 p.i. Both HSV-gK3-infected mouse strains had significantly higher corneal scarring (CS) than did McKrae-infected mice. T-cell depletion studies in C57BL/6 mice suggested that this CS enhancement in the HSV-gK3-infected mice was mediated by a CD8+ T-cell response. Taken together, these results strongly suggest that increased gK levels promote eye disease and chronic infection in infected mice.
Glycoprotein K (gK) is one of 11 known herpes simplex virus type 1 (HSV-1) glycoproteins (21, 28, 37). gK is thought to be an important determinant of virus-induced cell fusion, since single-amino-acid changes within gK cause extensive virus-induced cell fusion (3, 6, 34, 47). gK is also an important determinant of cytoplasmic virion envelopment, since viruses lacking gK fail to efficiently acquire a cytoplasmic envelope, which leads to a drastic defect in virion egress and spread (11, 29, 30). Similar to HSV-1 gK, the gKs of pseudorabies virus and varicella-zoster virus also play important roles in virion morphogenesis and egress (7, 32, 39).
We have previously demonstrated that the immunization of mice with gK, but not with any of the 10 other HSV-1 glycoproteins, results in an exacerbation of corneal scarring (CS) and herpetic dermatitis following ocular HSV-1 infection (13, 18). This exacerbated CS and facial dermatitis was due to the presence of CD8+ T cells in the cornea of ocularly infected mice (44). Furthermore, we have shown that the overexpression of gK in gK-transformed cells causes a collapse of the Golgi apparatus into the endoplasmic reticulum, which inhibits virion egress, glycoprotein transport, and virus-induced cell fusion (10).
To examine the pleiotropic effects associated with gK immunization or gK overexpression, we inserted the complete open reading frame (ORF) of the gK gene into both copies of the latency-associated transcript (LAT) gene (one in each viral long repeat). These ORFs were placed at the 5′ end of the LAT gene and are under the control of the LAT promoter. The LAT promoter was chosen for the overexpression of gK since it is the only viral promoter that sustains high levels of transcription during latent as well as primary infection (54). We designed this strategy to overcome the problems inherent in the temporary expression of various genes provided by immediate-early or human cytomegalovirus immediate-early promoter. This virus, named HSV-gK3, expressed elevated levels of gK transcripts both in vitro and in vivo compared with parental controls. Therefore, it can be used to examine the effect(s) of overexpression of gK on HSV-1 pathogenicity in mice.
In the present report, we describe several significant effects of elevated gK expression on HSV infection. First, despite displaying replication similar to that of wild-type (wt) virus in tissue culture, trigeminal ganglia (TG), and mouse tears and having a similar 50% lethal dose, HSV-gK3 infection resulted in more severe CS than wt virus infection. Second, the HSV-gK3 virus induced a chronic infection in the TG. Third, the level of CD8+ T cells in the corneas and TG was higher in HSV-gK3-infected mice than in wt virus-infected mice, while the level of CD4+ T cells was similar. Finally, in mice depleted of CD8+ T cells, infection with either HSV-gK3 or wt virus led to similar CS, suggesting that a CD8+ T-cell response is involved in the exacerbated CS detected in HSV-gK3-infected mice.
MATERIALS AND METHODS
Viruses, cells, and mice.
Triple-plaque-purified wt McKrae, dLAT2903, HSV-gK3, and HSV-gK3R viruses were used in this study. Rabbit skin (RS) cells (used for the preparation of virus stocks, the culturing of mouse tear films, and the determination of growth kinetics) were grown in Eagle's minimal essential medium supplemented with 5% fetal calf serum. The mice used were female 6-week-old inbred BALB/c and C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME).
Construction of the gK plasmid.
The parental virus for this construct was dLAT2903, a mutant of the HSV-1 strain McKrae in which the region of both LAT copies from −161 to +1667 relative to the LAT transcription start site (EcoRV-HpaI) was deleted (46). This LAT-null mutant is thus missing approximately 0.2 kb of the LAT promoter and 1.6 kb of the 5′ end of the primary 8.3-kb LAT transcript. To make the gK plasmid (pLAT-gK), the BamHI B fragment of McKrae was digested with SwaI-BamHI to produce a 5.5-kb DNA fragment including the region from −1041 to +4656 of HSV-1 LAT (46). A PacI linker was added to this 5.5-kb fragment, and the resulting construct was then ligated into the PacI site of a modified plasmid, pNEB193 (New England Biolabs), that lacks its internal BamHI site. The resulting plasmid was digested with StyI-HpaI to remove the 1.6-kb LAT fragment that corresponds to LAT nucleotides +76 to +1667. A BamHI linker was added, and the resulting plasmid was designated pLAT. This plasmid contains 880 bp upstream of the BamHI site and 2,989 bp downstream of the BamHI site. A plasmid containing the gK gene from the KOS HSV-1 strain was inserted into the BamHI site of pLAT (21). This gK insert contains the complete 338-amino-acid coding region of the gK gene plus 7 and 15 bp of noncoding 5′ and 3′ sequence, respectively. This plasmid, which contains the 1,036-bp gK gene bounded by 880- and 2,989-bp LAT fragments, was designated pLAT-gK.
Construction of HSV-gK3.
HSV-gK3 was generated by homologous recombination as previously described (46). Briefly, pLAT-gK was cotransfected with infectious dLAT2903 DNA by the calcium phosphate method. Viruses from the cotransfection were plated, and isolated plaques were picked and screened for the gK gene insertion using restriction digestion and Southern blot analysis. Selected plaques that contained the gK gene were plaque purified eight times and reanalyzed by restriction digestion and Southern blot analysis to verify that the gK gene was present in the LAT region. A single plaque was chosen for purification and was designated HSV-gK3. This virus contains the gK gene that is in the normal LAT location in the viral genome and is under control of the LAT promoter. Thus, in addition to the endogenous HSV-1 gK gene, this virus contains two additional copies of the gK gene under the LAT promoter (one in each viral long repeat).
HSV-gK3R, a rescued virus in which the inserted gK gene was removed and the original deletion of the LAT gene was restored, was generated by cotransfection and homologous recombination using infectious HSV-gK3 DNA and the pLAT plasmid. This virus behaves similarly to dLAT2903 and wt McKrae in terms of virus replication, survival, and eye diseases in most of the experiments performed in this study. Therefore, the wt McKrae virus was used as the negative control virus for these studies.
Southern analyses.
Briefly, viral DNA was digested with EcoRV/MluI; the restriction fragments were separated in a 0.9% agarose gel, transferred to Zeta paper, rinsed in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) for 5 min, and cross-linked to the membrane by UV light; and DNA-DNA hybridization was performed with 32P-labeled gK as previously described (21).
Virus replication in tissue culture.
RS cell monolayers at 70 to 80% confluence were infected with 10, 1, or 0.1 PFU/cell. Virus was harvested at various times by subjecting the cell monolayers to two freeze-thawing cycles. Virus titers were determined using standard plaque assays on RS cells as described previously (18).
Ocular infection.
Mice were infected ocularly with 2 μl of tissue culture media containing 2 × 105, 2 × 104, 2 × 103, or 2 × 102 PFU/eye of the HSV-1 strains McKrae, dLAT2903, HSV-gK3, or HSV-gK3R (18). In the surviving mice, the severity of blepharitis (on day 7 postinfection [p.i.]) and CS (on day 30 p.i.) was scored in a masked fashion using a scale from 0 to 4 as described previously (13, 17). Tear films were collected from 40 eyes on days 1 to 5 and 20 eyes on days 6 to 10 for each group as described previously (13). Each swab was placed in 1 ml of tissue culture medium, and the amount of virus in the medium was determined using a standard plaque assay on RS cells.
Detection of infectious virus in TG.
BALB/c or C57BL/6 mice were infected ocularly with 2 × 105 PFU/eye of HSV-gK3, dLAT2903, or McKrae. On day 3, 5, or 6 p.i. for BALB/c mice or day 30 p.i. for C57BL/6 mice, the infected mice were euthanized, and the TG from each mouse were combined. The TG from each mouse were homogenized, and the debris was removed by centrifugation at 3,000 rpm for 10 min in a Beckman TA10 rotor. The viral titers in the supernatants of the TG from day 3, 5, or 6 p.i. and appearance of virus-induced cytopathic effects (CPE) in the supernatants of the TG from day 30 p.i. were measured on RS cells as described previously (20).
Depletion of T cells.
Each C57BL/6 mouse received intraperitoneal injections of 100 μg of purified GK1.5 (anti-CD4+) or 2.43 (anti-CD8+) antibodies (NCCC, Minneapolis, MN) in 100 μl of phosphate-buffered saline on days −4, −1, +2, and +5 relative to HSV-1 ocular infection. The efficiency of CD4+ and CD8+ T-cell depletion was monitored by fluorescence-activated cell sorter analysis. After the second depletion, more than 93% of CD4+ or CD8+ T cells were depleted from the spleens of the mice. Isotype-controlled mice were treated with anti-HLA-DR5 (clone SFR3-DR5) monoclonal antibody in 100 μl of PBS as described above, and this group was designated as the mock control. Due to the lack of survival of BALB/c mice following this procedure, the depletion studies were performed only in C57BL/6 mice.
RNA extraction and cDNA preparation.
Corneas and TG from ocularly infected BALB/c and C57BL/6 mice were collected on days 5 and 30 p.i. The tissues were immersed in the RNAlater RNA stabilization reagent and stored at −80°C until processing. The frozen tissue from each animal was processed for RNA extraction using TRI reagent (Molecular Research Center, Inc., Cincinnati, OH) and RNeasy column cleanup (QIAGEN, Inc., Valencia, CA). In the case of RNA extraction from virus-infected RS cells, the cells growing in six-well plates were infected with 10, 1, or 0.1 PFU/cell of the HSV-gK3, dLAT2903, or McKrae strain. The cells were harvested and washed 8 or 16 h p.i. For RNA preparation, frozen tissue or washed RS cells were resuspended in TRI reagent and homogenized, followed by the addition of chloroform and subsequent nucleic acid precipitation using isopropanol. The RNA was then treated with DNase I to degrade any contaminating genomic DNA followed by purification using a QIAGEN RNeasy column as described in the manufacturer's instructions. RNA yield was determined by spectroscopy (NanoDrop ND-1000; NanoDrop Technologies, Inc., Wilmington, DE). On average, the RNA yields from each mouse cornea and TG sample were 1.8 to 3.75 μg and 2.7 to 7.5 μg, respectively. Finally, 1,000 ng of total RNA was reverse transcribed using random hexamer primers and murine leukemia virus reverse transcriptase from the high-capacity cDNA reverse transcription (RT) kit from Applied Biosystems, Foster City, CA, in accordance with the manufacturer's recommendations.
The differences in expression levels of gK and gB in infected cells were evaluated using custom-made TaqMan gene expression primers described below for the in vivo studies. Relative copy numbers for the gK and gB genes were calculated using similar standard curves generated from the plasmids pGEM-gK1040 and pAc-gB1, respectively. Although glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is typically used as an internal loading control to normalize signal during quantitative real-time processing, variable GAPDH signals were detected in tissue culture cells infected with different PFU of each virus as was reported previously (43). Therefore, expression of the 18S rRNA gene was used for in vitro normalization. However, no variations in expression of GADPH were detected in the tissues collected from the infected animals. Thus, in all in vivo experiments we used GADPH for normalization of gB, gK, CD4, and CD8 transcripts.
TaqMan real-time PCR.
The expression levels of the CD4 and CD8 genes, along with the expression of the GAPDH and 18S rRNA genes (used as an endogenous loading controls), were evaluated using commercially available TaqMan gene expression assays (Applied Biosystems, Foster City, CA) with optimized primer and probe concentrations. Primer probe sets consisted of two unlabeled PCR primers and the 6-carboxyfluorescein (FAM) dye-labeled TaqMan MGB probe formulated into a single mixture. Additionally, all amplicons included an intron-exon junction to eliminate signal from genomic DNA contamination. The following assays were used: (i) CD4 ABI assay Mm00442754_m1 (amplicon length, 72 bp), (ii) CD8 (alpha chain) ABI assay Mn01182108_m1 (amplicon length, 67 bp), (iii) GAPDH ABI assay Mm999999.15_G1 (amplicon length, 107 bp), and (iv) 18S rRNA ABI assay Hs99999901_s1 (amplicon length, 187 bp).
Expression levels of HSV-1 gB and gK were also evaluated using custom-made TaqMan gene expression assays (Applied Biosystems, Foster City, CA). The gB primers and probe used were as follows: forward primer, 5′-AACGCGACGCACATCAAG-3′; reverse primer, 5′-CTGGTACGCGATCAGAAAGC-3′; and probe, 5′-FAM-CAGCCGCAGTACTACC-3′. The gK primers and probe used were as follows: forward primer, 5′-GGCCACCTACCTCTTGAACTAC-3′; reverse primer, 5′-CAGGCGGGTAATTTTCGTGTAG-3′; and probe, 5′-FAM-CAGGCCGCATCGTATC-3′. The amplicon lengths for gB and gK were 72 and 82 bp, respectively.
Quantitative real-time PCR was performed using an ABI Prism 7900HT sequence detection system (Applied Biosystems, Foster City, CA) in 384-well plates. Briefly, each 20-μl reaction mixture contained 2 μl of cDNA template, 1× TaqMan universal PCR master mix (Applied Biosystems), and 1× TaqMan gene expression assay (CD4, CD8, gB, gK, 18S rRNA, or GAPDH). The universal thermal cycling conditions used were as follows. After an initial 2 min at 50°C to induce AmpErase-UNG activity and 10 min at 95°C, the samples were cycled 40 times at 95°C for 15 s and 60°C for 1 min. Relative gene expression levels were normalized to the expression of the housekeeping gene coding for GAPDH (endogenous loading control). Real-time PCR was performed in triplicate for a given tissue sample from each animal in the group. The threshold cycle values, which represents the PCR cycle at which there is a noticeable increase in the reporter fluorescence above baseline, were determined using SDS 2.2 software. In each experiment, an estimated relative copy number of each target gene was calculated using standard curves generated from plasmids containing the gene of interest: pORF-9-mCD8-a (InvivoGen, San Diego, CA), pCMV-Sport1-CD4 (Open Biosystems, Huntsville, AL), pGem-gK (21), and pAc-gB1 (19). Briefly, each plasmid DNA template was serially diluted 10-fold such that 5 μl contained from 103 to 1011 copies of the desired gene and then was subjected to TaqMan PCR with the same set of primers as the test samples. By comparing the normalized threshold cycle of each sample to the threshold cycle of the standards, the copy number for each reaction was determined.
Statistical analysis.
Protective parameters were analyzed with the Student's t test and Fisher's exact test using Instat (GraphPad, San Diego, CA). Results were considered to be statistically significant if the P value was <0.05.
RESULTS
Structure of the HSV-gK3 virus.
We constructed a mutant derivative of HSV-1 strain McKrae that expresses two additional copies of the gK gene to examine the effects of extra doses of gK on HSV-1 pathogenesis. McKrae was used as the original parental virus. The genomic structure of the wt HSV-1 strain McKrae is shown schematically in Fig. 1A. The HSV-1 genome contains a unique long region (UL) and a unique short (US) region, both of which are flanked by inverted repeats (Fig. 1A; terminal and internal repeats long [TRL and IRL] and terminal and internal repeats short [TRS and IRS]). The location of the TATA box of the LAT promoter is indicated. The transcription start site of the primary 8.3-kb LAT RNA transcript is 28 nt downstream of the TATA box (54). The previously described LAT null mutant, dLAT2903 (Fig. 1B), was derived from the McKrae strain (46). It contains a 1.8-kb deletion in both copies of the LAT gene (one in each of the long repeats). This deletion encompasses 0.2 kb of the LAT promoter and the portion of the LAT gene that encodes the first 1.6 kb of the 8.3-kb primary LAT. The deleted region, designated “XXXXXX” (Fig. 1B), extends to LAT nt position +1667.
FIG. 1.
Construction and structure of the HSV-gK3 mutant virus. (A) The top schematic diagram shows the HSV-1 McKrae genome in the prototypic orientation. TRL and IRL represent the terminal and internal (or inverted) long repeats, respectively, and TRS and IRS represent the terminal and internal (or inverted) short repeats, respectively. UL and US represent the long and short unique regions, respectively. The solid rectangle represents the very stable 2-kb LAT. The start site for LAT transcription is indicated by the arrow at +1. “TATA” designates the relative location of the LAT promoter TATA box 28 nt upstream of the start of transcription. (B) dLAT2903 has a deletion from LAT nt −161 to +1667 in both copies of LAT and makes no LAT RNA. (C) HSV-gK3 was constructed from dLAT2903 by homologous recombination between dLAT2903 DNA and a plasmid containing the complete LAT promoter and the entire structural gK gene [including its 3′ poly(A) signal] as described in Materials and Methods. (D) HSV-gK3 Southern blot. Subconfluent RS cell monolayers were infected with 10 PFU/cell of HSV-gK3 or McKrae for 16 h. Viral DNAs were isolated, and 5 μg of DNA from each virus was digested with EcoRI/EcoRV and hybridized to 32P-labeled HSV-1 gK (HSV-1 nt 112170 to 113193). As expected, only one fragment is seen in the McKrae lane. This band also is observed in HSV-gK3 and corresponds to HSV-1 nt 110094 to 118640 surrounding the naturally occurring gK (HSV-1 nt 112170 to 113193). In contrast to the McKrae lane, in the HSV-gK3 lane, in addition to the natural gK, there are two additional bands corresponding to two extra copies of the gK gene inserted within IRL (HSV-1 nt 110094 to 118640) and TRL (HSV-1 nt 13 to 7727) regions of dLAT2903. Both regions within the IRL and TRL have a deletion of approximately 1,600 bp of LAT.
HSV-gK3 was derived from the dLAT2903 strain by the insertion of the gK gene and restoration of the LAT promoter so that the inserted gK genes are under control of the endogenous LAT promoter (Fig. 1C [described in Materials and Methods]). The genomic structure of HSV-gK3 was confirmed by restriction enzyme analysis, Southern blotting (Fig. 1D), and partial sequencing (not shown). HSV-gK3 contains the entire sequence of the gK gene, including its polyadenylation signal, under the control of the LAT promoter. There is a noncoding region of 7 nt upstream of the first ATG. This is followed by the complete coding region of 1,014 nt and a 15-nt noncoding region downstream of the gK termination codon. Therefore, HSV-gK3 contains, in addition to its endogenous gK gene, two additional complete copies of gK, one in each viral long repeat. HSV-gK3 is identical to its LAT null mutant parent, dLAT2903, except that the LAT promoter has been restored to drive the expression of the inserted gK genes. dLAT2903 is identical to its wt parent, McKrae, in all parameters examined, and the HSV-gK3 rescued virus, HSV-gK3R, is identical to dLAT2903 in all parameters examined.
HSV-gK3 virus expresses higher levels of gK transcripts both in vitro and in vivo.
To investigate the relative expression of the gK gene in HSV-gK3 as compared to its parental dLAT2903 virus, confluent monolayers of RS cells were infected with HSV-gK3 or dLAT2903 at a multiplicity of 10 PFU/cell. Infected cells were collected 8 or 16 h p.i., and total RNA was isolated as described in Materials and Methods. TaqMan RT-PCR was performed on the isolated RNA to detect the levels of gK and gB transcripts relative to the levels of 18S rRNA transcripts. As expected, our results suggest that the level of gK transcripts in HSV-gK3-infected RS cells is significantly higher than in dLAT2903-infected RS cells at both 8 and 16 h p.i. (Fig. 2; P < 0.01). However, the levels of gB transcripts were similar between the two viruses (Fig. 2; P > 0.05). Similar results were obtained following infection with McKrae virus (data not shown). These results indicate that the two extra copies of gK in HSV-gK3 lead to increased expression of gK.
FIG. 2.
gK and gB transcriptions in infected RS cells. Subconfluent RS cell monolayers in quadruplicate were infected with 10 PFU/cell of HSV-gK3 or dLAT2903 for 8 and 16 h. Total RNA was isolated from these infected RS cells, and TaqMan RT-PCR was performed as described in Materials and Methods using gB- and gK-specific primers. The 18S rRNA expression was used to normalize the relative expression of gK and gB mRNAs in HSV-gK3- and dLAT2903-infected RS cells. PI, postinfection. Each point represents the mean ± standard error of the mean (n = 4).
We next wanted to determine if the HSV-gK3 virus expressed higher levels of gK in vivo (in mouse TG or corneas) using ocular infection in the mouse. Six-week-old female BALB/c or C57BL/6 mice were infected with 2 × 105 PFU/eye of HSV-gK3 or McKrae virus. It should be noted that C57BL/6 mice are less sensitive than BALB/c mice to infection with McKrae virus (44), but they can be sensitized to infection by immunization with gK (44). Corneas and TG from each infected mouse were collected 5 days post-ocular infection, and the total RNA was isolated as described in Materials and Methods. TaqMan RT-PCR was performed on the total RNA from the corneas and TG of individual mice to measure the levels of gK and gB mRNA per mouse cornea or TG. GAPDH mRNA levels in each samples were used as internal controls. In both BALB/c and C57BL/6 mice, TG from HSV-gK3-infected mice had more gK transcripts than the TG from wt McKrae-infected mice (Fig. 3A). In contrast, the levels of gB transcripts were similar in the TG of both BALB/c and C57BL/6 mice infected with either HSV-gK3 or McKrae (Fig. 3A). The corneas of BALB/c and C57BL/6 mice infected with HSV-gK3 also had more gK transcripts than the corneas of mice infected with McKrae, while the levels of gB mRNA were similar for both viruses (Fig. 3B). These results suggest that presence of the three copies of gK in HSV-gK3 virus results in higher gK transcription in HSV-gK3-infected cells both in vitro and in vivo.
FIG. 3.
Quantitation of gK and gB mRNAs in vivo. (A) gK and gB transcriptions in TG. BALB/c or C57BL/6 mice were ocularly infected with 2 × 105 PFU/eye of HSV-gK3 or McKrae virus. TG from individual mice were isolated 5 days p.i. (PI), and TaqMan RT-PCR was performed as described in Materials and Methods. GAPDH expression was used to normalize the relative expression of gK and gB mRNA in HSV-gK3- and McKrae-infected TGs. Each point represents the mean ± standard error of the mean from five mice. (B) gK and gB transcription in corneas. Individual mouse corneas infected as described above were harvested, and TaqMan RT-PCRs for gK and gB transcripts were performed as described above. Each point represents the mean ± standard error of the mean from 5 mice.
Replication of HSV-gK3 in tissue culture.
To determine if elevated gK levels affect viral replication, we measured viral yield following the infection of RS cells with 10 PFU/cell of HSV-gK3, dLAT2903, McKrae, or HSV-gK3R. The replications of each of these four viruses appeared to be similar (Fig. 4A). Additionally, no differences were detected in RS cells infected with HSV-gK3, dLAT2903, or McKrae at 1 or 0.1 PFU/cell (data not shown). Thus, the overexpression of gK in HSV-gK3 does not have a detectable effect on virus replication in tissue culture.
FIG. 4.
Replication of HSV-gK3 in vitro and in vivo. (A) Virus replication in RS cells. Subconfluent RS cell monolayers in triplicate from two separate experiments were infected with 10 PFU/cell of HSV-gK3, dLAT2903, McKrae, or HSV-gK3R as described in Materials and Methods. Total virus was harvested at the indicated times p.i. by two cycles of freeze-thawing. The amount of virus at each time point was determined by standard plaque assays on RS cells. Each point represents the mean ± standard error of the mean (n = 6). (B and C) Virus replication in mouse tears. BALB/c (B) or C57BL/6 (C) mice were ocularly infected with 2 × 105 PFU/eye of HSV-gK3, dLAT2903, or McKrae virus. Tear films were collected from days 1 to 10, and virus titers were determined by standard plaque assays. Each point represents the mean titers of 40 eyes from two separate experiments for days 1 to 5 and 20 eyes from two separate experiments for days 6 to 10. (D) Virus titers in TG. BALB/c mice were ocularly infected with 2 × 105 PFU/eye of HSV-gK3, dLAT2903, or McKrae virus as described above. Mice were euthanized on the indicated days, TG from each mouse were removed, combined, and homogenized, and virus titers were determined for the TG from each mouse. Each bar represents the mean ± standard error of the mean of the TG from 5, 15, and 5 mice for days 3, 5, and 6 p.i., respectively.
Replication of HSV-gK3 in mouse tears.
Viral replication during ocular infection was measured indirectly by determining the PFU in the tear films of infected mice. BALB/c or C57BL/6 mice were infected ocularly with 2 × 105 PFU/eye of HSV-gK3, dLAT2903, or McKrae (see Materials and Methods). Tear films were collected, and the amount of virus in those films was determined using plaque assays on RS cells. Throughout the course of the experiment, virus titers were similar in the tear films of C57BL/6 mice infected with HSV-gK3, dLAT2903, or McKrae (Fig. 4B; P > 0.1). In BALB/c mice, virus titers were similar in the tear films of mice infected with HSV-gK3, dLAT2903, or McKrae 1 to 3 days p.i. (Fig. 4C; P > 0.1). However, 4 to 6 days p.i., the tear films of dLAT2903- or McKrae-infected mice had significantly higher virus titers than did the tear films of HSV-gK3-infected mice (Fig. 4C; P < 0.01). By day 7 p.i., the three viruses produced similar low viral titers in BALB/c mice, and by day 8 p.i., no virus was detected in any eye for either BALB/c- or C57BL/6-infected mice (Fig. 4B and C).
Thus, it appears that the presence of two additional copies of gK significantly decreases the amount of HSV-1 found in the tear films of infected BALB/c mice 4 to 6 days p.i., but it has little effect on the amount of virus present in the tear films of infected C57BL/6 mice.
Virus replication in TG.
The results described above suggest that virus replication in the tears of BALB/c mice infected with HSV-gK3 was lower 4 to 6 days post-ocular infection than it was in the tears of mice infected with wt McKrae or parental dLAT2903. To determine if the lower virus titer correlated with virus replication in TG, BALB/c mice were ocularly infected with HSV-gK3, dLAT2903, or McKrae (as described in Materials and Methods). On days 3, 5, and 6 p.i., mice were sacrificed and TG were harvested for analysis of infectious virus as described in Materials and Methods. In contrast to the differences observed in viral titer between the three viruses in the eyes of BALB/c-infected mice, no significant differences were detected in the amount of virus recovered on day 3, 5, or 6 p.i. in the TG extracts of BALB/c mice infected with HSV-gK3, dLAT2903, or McKrae (Fig. 4D). Thus, HSV-gK3 appears to replicate similarly to wt McKrae or dLAT2903 in the TG of infected BALB/c mice during primary infection.
Virulence of HSV-gK3 in BALB/c and C57BL/6 mice.
To determine whether there is a difference in the virulence of the HSV-gK3 virus, the parental dLAT2903 virus, the wt McKrae virus, or the HSV-gK3R rescued virus, the ability of these viruses to cause a lethal infection in BALB/c mice (that are sensitive to HSV-1 infection) and C57BL/6 mice (that are resistant to HSV-1 infection) was evaluated. Groups of 5 to 50 BALB/c mice from one to four experiments were infected ocularly with 10-fold serial dilutions of HSV-gK3, wt McKrae, dLAT2903, or HSV-gK3R (as described in Materials and Methods). No significant differences were detected in BALB/c mice infected with different doses of HSV-gK3 compared with mice infected with McKrae, dLAT2903, or HSV-gK3R (Table 1; P > 0.05).
TABLE 1.
Mortality of BALB/c and C57BL/6 mice following ocular infection with HSV-gK3
| Virus | No. (%) of mice that died/no. infected with titer showna:
|
||||
|---|---|---|---|---|---|
| BALB/c
|
C57BL/6 (2 × 105) | ||||
| 2 × 102 | 2 × 103 | 2 × 104 | 2 × 105 | ||
| HSV-gK3 | 12/25 (48)b | 5/5 (100) | 5/5 (100) | 39/50 (78)b | 0/39 (0)b |
| dLAT2903 | 12/25 (48)b | 4/5 (80) | 5/5 (100) | 5/5 (100) | ND |
| McKrae | 2/5 (40) | 5/5 (100) | 5/5 (100) | 32/50 (64)b | 0/30 (0)b |
| HSV-gK3Rc | 2/5 (40) | 5/5 (100) | 5/5 (100) | 5/5 (100) | ND |
BALB/c or C57BL/6 mice were ocularly infected with the specified virus titers (PFU/eye), and mortality was determined 30 days after infection. ND, not done.
Data from four separate experiments.
HSV-gK3R rescued virus is a control virus with only the endogenous copy of gK (see Materials and Methods for details).
To determine the effect of HSV-gK3 infection on the mortality of C57BL/6 mice, groups of 39 and 30 mice from two separate experiments were infected ocularly with 2 × 105 PFU/eye of HSV-gK3 or McKrae, respectively (as described in Materials and Methods). None of the infected mice died following ocular infection (Table 1; P = 1). These results suggest that the pattern of neurovirulence in HSV-gK3-infected mice is similar to that of the dLAT2903, wt McKrae, and HSV-gK3R rescued viruses.
Effect of gK overexpression on eye diseases in infected mice.
To determine the effect of gK overexpression on eye disease, the severity of blepharitis (inflammation of the eyelid) and CS were evaluated following the ocular infection of BALB/c mice with 2 × 105 PFU/eye of either HSV-gK3 or wt McKrae virus. Blepharitis and CS were measured in the surviving mice 7 and 30 days, respectively, post-ocular infection. BALB/c mice infected with HSV-gK3 developed similar levels of blepharitis to mice infected with the wt McKrae virus, while CS was significantly higher in the mice infected with HSV-gK3 than in those infected with the McKrae virus (Fig. 5; BALB/c). Similar results were obtained following infection with parental dLAT2903 virus (data not shown).
FIG. 5.
Eye diseases in mice infected with HSV-gK3. BALB/c and C57BL/6 mice were ocularly infected with 2 × 105 PFU/eye of HSV-gK3 or McKrae virus and were used to determine blepharitis and CS. Blepharitis and CS in surviving mice were examined on days 7 and 30 p.i. as described in Materials and Methods. In the BALB/c mice, the blepharitis and CS scores represents the average ± standard error of the mean from 100 and 22 eyes, respectively, from HSV-gK3-infected mice and 100 and 36 eyes, respectively, from McKrae-infected mice. In C57BL/6 mice, the blepharitis and CS scores represent the average ± standard error of the mean from 78 eyes for HSV-gK3-infected mice and 60 eyes for McKrae-infected mice.
Previously, we have shown that C57BL/6 mice with detectable levels of preexisting gK antibody titers are more susceptible to virus-mediated eye diseases than naive mice (17). In the present study, we examined the effect of HSV-gK3 infection on eye diseases in C57BL/6 mice, which are refractory to HSV-1 infection. C57BL/6 mice were infected ocularly with 2 × 105 PFU/eye of HSV-gK3 or McKrae virus, and blepharitis and CS were measured as described above. Mice infected with HSV-gK3 displayed higher levels of blepharitis and CS than mice infected with the wt McKrae virus (Fig. 5; C57BL/6). Furthermore, approximately 50% of HSV-gK3-infected C57BL/6 mice developed severe facial dermatitis, while none of the McKrae-infected mice showed any sign of dermatitis (data not shown). Some of the surviving BALB/c mice infected with HSV-gK3 also showed higher levels of facial dermatitis than those infected with the wt McKrae virus (data not shown). Thus, the overexpression of gK exacerbates virus-induced CS in HSV-gK3-infected BALB/c and C57BL/6 mice.
Effect of gK overexpression on CD4 and CD8 transcription in TG and corneas of ocularly infected mice.
Both CD4+ and CD8+ T cells have been implicated in HSV-1-induced eye diseases (1, 2, 23, 38, 42, 53). Since the overexpression of gK also induces eye disease in C57BL/6 mice, we next determined whether gK overexpression altered T-cell differentiation in HSV-1-infected TG and corneas. CD4 and CD8 mRNA levels were estimated in the corneas and TG following ocular infection with 2 × 105 PFU/eye of either the HSV-gK3 or the wt McKrae virus. The corneas and TG from five mice per group were collected on days 5 and 30 post-ocular infection, and TaqMan RT-PCR for CD4 and CD8 was performed on the total RNA from the corneas or TG of individual mice (as described in Materials and Methods). On day 5 p.i., the level of CD8 transcript in the corneas of BALB/c mice infected with HSV-gK3 was higher than that in the corneas of BALB/c mice infected with the wt McKrae virus (Fig. 6A). Similar results were found using C57BL/6 mice (Fig. 6A). In contrast, in the TG, the patterns of CD4 and CD8 transcript levels on day 5 p.i. were similar for both viruses in both BALB/c mice and C57BL/6 mice (Fig. 6B).
FIG. 6.
Estimation of CD4 and CD8 copy numbers in corneas and TG of infected mice. BALB/c or C57BL/6 mice were ocularly infected with 2 × 105 PFU/eye of HSV-gK3 or McKrae virus. Corneas and TG from individual mice were isolated on day 5 or 30 p.i. (PI), and TaqMan RT-PCR was performed on total RNA isolated from those tissues as described in Materials and Methods. GAPDH was used as an endogenous control to normalize the relative expression of CD4 and CD8 mRNA in HSV-gK3- and McKrae-infected corneas and TG. Each point represents the mean ± standard error of the mean from 5 mice. (A) CD4 and CD8 mRNAs in mouse corneas on day 5 p.i. (B) CD4 and CD8 mRNAs in mouse TG on day 5 p.i. (C) CD4 and CD8 mRNAs in mouse corneas on day 30 p.i. (D) CD4 and CD8 mRNAs in mouse TG on day 30 p.i.
On day 30 p.i., no significant differences were detected for CD4 transcript levels in the corneas (Fig. 6C) or TG (Fig. 6D) of BALB/c or C57BL/6 mice infected with either HSV-gK3 or wt McKrae. However, the levels of CD8 transcripts were significantly higher in the corneas of mice infected with the HSV-gK3 virus than in mice infected with the wt McKrae virus (Fig. 6C). Similarly, the level of CD8 transcript was higher in the TG of HSV-gK3-infected BALB/c and C57BL/6 mice than in mice infected with McKrae (Fig. 6D), although these differences were not statistically significant. Thus, there is a correlation between higher CD8 transcript levels in the corneas of infected mice (at days 5 and 30 p.i.) and more severe eye disease, especially in HSV-gK3-infected mice.
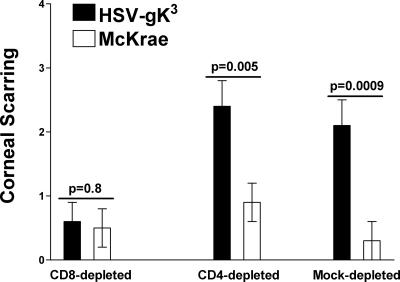
Effect of CD4+ and CD8+ T-cell depletion on CS following HSV-gK3 infection.
The RT-PCR analyses described above suggest that the level of CD8 transcripts in the corneas of HSV-gK3-infected mice, especially in C57BL/6 mice, correlates with the severity of CS. To examine the potential role CD4+ and CD8+ T cells play in HSV-1-induced eye disease, we used antibodies to specifically deplete these subclasses of T cells from C57BL/6 mice (as described in Materials and Methods). Irrespective of the virus that was used for ocular infection, 9 of 10 (90%) CD8+-depleted mice, 8 of 10 (80%) CD4+-depleted mice, and 10 of 10 (100%) mock-depleted mice survived the ocular infection (not shown). CS in surviving mice was determined 30 days after ocular infection. As expected, the amount of CS was decreased in the CD8+-depleted mice infected with HSV-gK3, while it was increased in CD8+-depleted mice infected with McKrae (Fig. 7; CD8 depleted). These differences between CD8+-depleted mice infected with HSV-gK3 or McKrae were not statistically significant (Fig. 7; P = 0.8). In contrast, depletion of CD4+ T cells significantly increased CS in mice infected with HSV-gK3 compared with mice infected with McKrae (Fig. 7; P = 0.005), while mock depletion had no effect on CS in infected mice (Fig. 7; P = 0.0009). Thus, these results suggest that, in C57BL/6 mice ocularly infected with HSV-gK3, increased CS is dependent on the presence of CD8+ but not CD4+ T cells.
FIG. 7.
CS in C57BL/6 mice is CD8+ T-cell dependent. Ten C57BL/6 mice were depleted of their CD4+ or CD8+ T cells prior to ocular infection with HSV-gK3 or McKrae virus as described in Materials and Methods. Mock control mice were depleted with irrelevant monoclonal antibody. CS was measured 30 days after ocular infection in surviving mice. For each bar, CS (y axis) represents the average of the scarring from 16 eyes (CD4+ depleted), 18 eyes (CD8+ depleted), or 20 eyes (mock depleted). The error bars indicate the standard error of the mean.
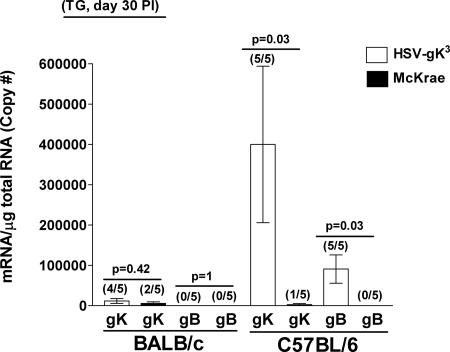
Detection of gK transcript during the latent state of infection.
During HSV-1 neuronal latency, only one viral gene is consistently expressed at high levels (8, 48). This viral gene is called the LAT gene (12, 48, 52). The LAT gene has a powerful promoter that is active in most cell types (31, 36), and the two additional copies of gK in the HSV-gK3 virus are expressed from this promoter. We next wanted to determine whether gK was expressed in a latent HSV-gK3 infection. Thus, the surviving BALB/c or C57BL/6 mice described in Table 1 and ocularly infected with 2 × 105 PFU/eye of HSV-gK3 or McKrae were euthanized and corneas and TG from individual mouse were isolated. TaqMan RT-PCR was performed on the total RNA isolated from these tissues, and the levels of gK and gB transcripts from each virus were determined. In the BALB/c mice, gK transcription was detected in four of the five mice infected with HSV-gK3 and in two of the five mice infected with McKrae (Fig. 8). However, no gB transcript was detected in BALB/c mice infected with either the HSV-gK3 or McKrae virus (Fig. 8), an expected result given that gB is not expressed during latency. C57BL/6 mice infected with HSV-gK3 had detectable levels of gK transcripts in all five of the TG tested, while the mice infected with the wt McKrae virus had detectable levels of gK transcripts in only one of the five TG tested (Fig. 8). In contrast to the absence of the gB transcript in the TG of BALB/c mice infected with HSV-gK3, 100% (five of five) of TG from C57BL/6 mice infected with HSV-gK3 expressed gB transcript (Fig. 8). No gB transcript was detected in the TG of C57BL/6 mice infected with the wt McKrae virus (Fig. 8). Neither gK nor gB transcripts were detected in the corneas nor brains of these mice on day 30 p.i. (data not shown).
FIG. 8.
Detection of gK and gB transcripts in the TG during latency. BALB/c or C57BL/6 mice were ocularly infected with 2 × 105 PFU/eye of HSV-gK3 or McKrae virus. TG from individual mice were isolated 30 days p.i. (PI), and TaqMan RT-PCR was performed as described in Materials and Methods. GAPDH was used as an endogenous control to normalize the relative expression of gK and gB mRNAs in HSV-gK3-and McKrae-infected TG. Each point represents the mean ± standard error of the mean from 5 mice. The numbers in parentheses on top of each bar indicate the number of positive mouse TG per total mouse TG tested for each transcript.
To summarize, gK transcription is detected in the TG of both BALB/c and C57BL/6 mice infected with HSV-gK3 and in 20 to 40% of mice infected with the wt McKrae virus. These results indicate that, as expected, gK is being expressed from the LAT promoter during latency. Finally, the presence of gB transcripts in the TG of C57BL/6 mice may suggest a chronic infection in addition to possible latent infection.
HSV-gK3 induces a chronic infection in the TG of infected mice.
The analyses described above revealed the presence of gB mRNA in the TG of HSV-gK3-infected mice but not in the TG of wt McKrae-infected C57BL/6 mice. During HSV neuronal latency, there is an absence of viral proteins and infectious virus and LAT is the only gene product consistently detected in abundance (51). The detection of the gK mRNA was not unexpected since the two additional copies of gK are under the control of the LAT promoter. However, the detection of gB mRNA in the TG of infected mice 30 days p.i. suggests that HSV-gK3 may have established a chronic infection in addition to a possible latent infection. To determine if presence of gB mRNA in the TG of HSV-gK3-infected C57BL/6 mice was a result of the lack of viral clearance, C57BL/6 mice were infected ocularly with 2 × 105 PFU/eye of either HSV-gK3 or McKrae and their TG were analyzed for the presence of infection virus. Thirty days post-ocular infection, a time at which latency has been established in wt mice and no infectious virus can be detected in the TG (5, 15), the surviving mice were euthanized. TG from individual mice were homogenized, and the extracts were plated on indicator (RS) cells to look for the presence of infectious virus (as described in Materials and Methods). All TG (10/10 [100%]) from the C57BL/6 mice infected with the HSV-gK3 virus contained infectious HSV-1 particles. In contrast, no infectious virus (0/10 [0%]) was detected in the TG of C57BL/6 mice infected with the wt McKrae virus (P < 0.0001). Thus, when infected with virus carrying two additional copies of gK, the mouse immune system is unable to efficiently clear infectious HSV-1 virus from the TGs.
DISCUSSION
We demonstrated previously that the immunization of mice with gK, but not with any of the other known HSV-1 glycoproteins, results in an exacerbation of CS and facial dermatitis following ocular HSV-1 infection (13, 18). The exacerbated CS is independent of mouse or virus strains (17). Vaccination with gK also appears to block viral clearance from the trigeminal ganglia, which results in a chronic, productive infection (15). We also found that the exacerbation of CS in the gK-immunized mice was due to the presence of CD8+ T cells in the cornea of ocularly infected mice (44). The depletion of CD8+ T cells, but not the depletion of CD4+ T cells or macrophages, reduced the severity of the eye disease to the level detected in mock-immunized or ocularly infected mice. Of many different protein antigens tested in conjunction with Freund's adjuvant, gK was the only antigen that induced a CD8+ T-cell response in the cornea of ocularly infected mice (44). To help define the effect of gK on HSV-1 pathogenesis, we constructed a recombinant HSV-1 virus that expresses two additional copies of the HSV-1 gK gene, each under the control of the LAT promoter. This virus provided a useful tool for studying how the overexpression of gK affects HSV-1-induced eye disease.
The viral titers of HSV-gK3 in RS cells, BALB/c TG, and C57BL/6 tears as well as the 50% lethal doses and duration of virus clearance were similar to those of the parental and control viruses. Despite having lower virus titers in the tears of BALB/c mice, HSV-gK3-infected mice had more severe CS than mice infected with the wt McKrae virus. These results demonstrate that the exacerbated CS in HSV-gK3-infected BALB/c or C57BL/6 mice was independent of virus titers in the eyes or TG.
Different mouse strains have differential susceptibilities to HSV-1 infection. C57BL/6 (H-2b) mice are more resistant to HSV-1 infection, and even the highly virulent wt McKrae strain does not produce CS in these mice (16). In contrast, BALB/c (H-2d) mice are very susceptible to ocular infection with the McKrae strain. Exacerbation of CS in the C57BL/6 mice infected with HSV-gK3 was surprising, but the result was consistent with our previous finding that immunization with gK followed by infection with McKrae (or with the avirulent HSV-1 KOS virus) leads to CS in both BALB/c and C57BL/6 mice (17).
The immune response leading to HSV-1-induced CS is cell mediated (14, 24, 42), and macrophages, CD4+ T cells, and CD8+ T cells have been implicated in this response (1, 2, 23, 38, 42, 53). The results presented here show that, following ocular infection, CD8+ T-cell transcripts in the eyes and TG of HSV-gK3-infected mice were higher than in McKrae-infected mice. In addition, the depletion of CD8+ T cells, but not the depletion of CD4+ T cells, eliminated the exacerbated CS observed in HSV-gK3-infected mice. These results suggest that CD8+ T cells are involved in the exacerbation of CS in these mice, a result consistent with the finding that CD8+ T cells play a major role in HSV-1-mediated corneal pathogenesis (1, 25, 26, 49). Furthermore, we have previously shown that the CS exacerbation in gK-immunized mice correlates with CD8+ T cells, but not with CD4+ T cells or macrophages (44). Recently, it was shown that CD8+ T cells mediate transient herpes stromal keratitis in CD4-deficient mice (33), and that CD8+ T cells are also actively involved in autoimmune diseases (27, 41, 45, 50). However, our results contradict previous reports that CD4+ T cells play a major role in HSV-1-induced eye disease (4). Although it is unlikely, the possible effect of CD4+ T-cell depletion on CD25+ regulatory T cells cannot be ruled out as reason for our observed contradiction. However, our preliminary results have shown that these regulatory T cells are not detectable in the corneas of infected mice (data not shown). Previously, we have shown that the overexpression of gK in gK-transformed cells collapses the Golgi apparatus into the endoplasmic reticulum (10). Thus, the disruption of the Golgi structure by the overexpression of gK in the HSV-gK3 virus may also damage cellular structure of the eye that may result in the exacerbation of CS in infected mice.
During HSV neuronal latency, there is an absence of viral proteins and infectious virus, and LAT is the only gene product consistently detected in abundance (51). Detection of the gK transcript in the TG of HSV-gK3 latently infected mice is not unexpected, given that the two additional copies of gK are under control of the LAT promoter. However, gK transcripts were also detected in some mice infected with the wt McKrae virus. These results suggest that gK is transcribed in the TG of latently infected mice. The low level of expression of gK in some of the infected mice could explain our recently published data showing that individuals with a history of ocular HSV-1 infection had higher anti-gK antibody titers than did HSV-1-seropositive individuals with no history of ocular infection (40). In support of this idea, analysis of latently infected TG sections revealed abundant expression of viral transcripts, viral protein, and viral DNA replication in about 1 neuron per 10 TG tested (9). However, no infectious virus was detected in any of these TG.
In this study, we also detected gB transcripts 30 days p.i. in the TG of C57BL/6 mice, indicating that a chronic infection can be established by HSV-gK3 (but not in BALB/c mice, an effect that may be due to differential responses of these two mouse strains to virus infection). The TG, specifically the nuclei of neuron bodies, are known to be the site of latent, not chronic, infection following the HSV-1 ocular challenge of mice (5, 51). When mouse TG are removed at autopsy and the explant is cocultivated in tissue culture media with indicator cells, latent viruses reactivate and can be observed via CPE on the indicator cell monolayer (15, 22). In contrast, when cell-free lysates of latently infected TG are plated on indicator cells, CPE is not observed (15). This indicates that there is no infectious virus present in the TG and confirms that reactivation from latency by cocultivation requires that explant of intact neurons (5, 15, 22). However, in this study we have shown that the HSV-gK3-mediated ocular infection of mice resulted in a chronic infection in the TG. Although we repeatedly found infectious virus in the TG of gK3 “latently” infected mice, we did not detect infectious virus in the brains or cornea of the same mice. The reason for this is currently not understood, but it suggests that this virus in the TG does not efficiently spread to and/or replicate in the brain or the cornea at this time. Similar to HSV-gK3 virus, we have previously shown that ocular infection of AβO/O mice immunized with baculovirus-expressed gK also resulted in a chronic HSV-1 infection in those mice (15). Finally, during latency, the levels of CD8+ T-cell transcripts in the TG of mice infected with HSV-gK3 or McKrae were higher than the levels of CD4+ T-cell transcripts, especially in HSV-gK3-infected mice. Previously, it was shown that CD8+ T cells could block HSV-1 reactivation from latency in sensory neurons without destroying the infected neurons (35). However, in HSV-gK3-infected C57BL/6 mice, the presence of high levels of CD8+ T-cell transcripts in the TG did not correlate with the quiescence of virus during the latent stage of HSV-1 infection.
In summary, the results presented here support the hypothesis that overexpression of gK from the LAT promoter increases CS in mice.
The pathogenic role of gK in HSV-1-induced CS appears to be mediated by CD8+ T cells. However, the effect of similar overexpression of a different HSV-1 glycoprotein on CS in mice remains to be determined. Finally, these results suggest that viral gK may play a role in immune evasion.
Acknowledgments
This work was supported by NIH grant EY13615 and the Skirball Program in Molecular Ophthalmology to H.G.
Footnotes
Published ahead of print on 26 September 2007.
REFERENCES
- 1.Banerjee, K., S. Deshpande, M. Zheng, U. Kumaraguru, S. P. Schoenberger, and B. T. Rouse. 2002. Herpetic stromal keratitis in the absence of viral antigen recognition. Cell Immunol. 219:108-118. [DOI] [PubMed] [Google Scholar]
- 2.Berra, A., A. Rodriguez, A. Heiligenhaus, B. Pazos, N. Van Rooijen, and C. S. Foster. 1994. The role of macrophages in the pathogenesis of HSV-1 induced chorioretinitis in BALB/c mice. Investig. Ophthalmol. Vis. Sci. 35:2990-2998. [PubMed] [Google Scholar]
- 3.Bond, V. C., and S. Person. 1984. Fine structure physical map locations of alterations that affect cell fusion in herpes simplex virus type 1. Virology 132:368-376. [DOI] [PubMed] [Google Scholar]
- 4.Brandt, C. R. 2005. The role of viral and host genes in corneal infection with herpes simplex virus type 1. Exp. Eye Res. 80:607-621. [DOI] [PubMed] [Google Scholar]
- 5.Cook, M. L., V. B. Bastone, and J. G. Stevens. 1974. Evidence that neurons harbor latent herpes simplex virus. Infect. Immun. 9:946-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Debroy, C., N. Pederson, and S. Person. 1985. Nucleotide sequence of a herpes simplex virus type 1 gene that causes cell fusion. Virology 145:36-48. [DOI] [PubMed] [Google Scholar]
- 7.Dietz, P., B. G. Klupp, W. Fuchs, B. Köllner, E. Weiland, and T. C. Mettenleiter. 2000. Pseudorabies virus glycoprotein K requires the UL20 gene product for processing. J. Virol. 74:5083-5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dobson, A. T., T. P. Margolis, F. Sedarati, J. G. Stevens, and L. T. Feldman. 1990. A latent, nonpathogenic HSV-1-derived vector stably expresses beta-galactosidase in mouse neurons. Neuron 5:353-360. [DOI] [PubMed] [Google Scholar]
- 9.Feldman, L. T., A. R. Ellison, C. C. Voytek, L. Yang, P. Krause, and T. P. Margolis. 2002. Spontaneous molecular reactivation of herpes simplex virus type 1 latency in mice. Proc. Natl. Acad. Sci. USA 99:978-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster, T. P., X. Alvarez, and K. G. Kousoulas. 2003. Plasma membrane topology of syncytial domains of herpes simplex virus type 1 glycoprotein K (gK): the UL20 protein enables cell surface localization of gK but not gK-mediated cell-to-cell fusion. J. Virol. 77:499-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster, T. P., and K. G. Kousoulas. 1999. Genetic analysis of the role of herpes simplex virus type 1 glycoprotein K in infectious virus production and egress. J. Virol. 73:8457-8468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraser, N. W., T. M. Block, and J. G. Spivack. 1992. The latency-associated transcripts of herpes simplex virus: RNA in search of function. Virology 191:1-8. [DOI] [PubMed] [Google Scholar]
- 13.Ghiasi, H., S. Bahri, A. B. Nesburn, and S. L. Wechsler. 1995. Protection against herpes simplex virus-induced eye disease after vaccination with seven individually expressed herpes simplex virus 1 glycoproteins. Investig. Ophthalmol. Vis. Sci. 36:1352-1360. [PubMed] [Google Scholar]
- 14.Ghiasi, H., S. Cai, A. B. Nesburn, and S. L. Wechsler. 1997. MHC-II but not MHC-I responses are required for vaccine-induced protection against ocular challenge with HSV-1. Curr. Eye Res. 16:1152-1158. [DOI] [PubMed] [Google Scholar]
- 15.Ghiasi, H., S. Cai, A. B. Nesburn, and S. L. Wechsler. 1996. Vaccination with herpes simplex virus type 1 glycoprotein K impairs clearance of virus from the trigeminal ganglia resulting in chronic infection. Virology 224:330-333. [DOI] [PubMed] [Google Scholar]
- 16.Ghiasi, H., S. Cai, G. C. Perng, A. B. Nesburn, and S. L. Wechsler. 2000. The role of natural killer cells in protection of mice against death and corneal scarring following ocular HSV-1 infection. Antivir. Res. 45:33-45. [DOI] [PubMed] [Google Scholar]
- 17.Ghiasi, H., S. Cai, S. Slanina, A. B. Nesburn, and S. L. Wechsler. 1997. Nonneutralizing antibody against the glycoprotein K of herpes simplex virus type-1 exacerbates herpes simplex virus type-1-induced corneal scarring in various virus-mouse strain combinations. Investig. Ophthalmol. Vis. Sci. 38:1213-1221. [PubMed] [Google Scholar]
- 18.Ghiasi, H., R. Kaiwar, A. B. Nesburn, S. Slanina, and S. L. Wechsler. 1994. Expression of seven herpes simplex virus type 1 glycoproteins (gB, gC, gD, gE, gG, gH, and gI): comparative protection against lethal challenge in mice. J. Virol. 68:2118-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghiasi, H., R. Kaiwar, A. B. Nesburn, and S. L. Wechsler. 1992. Expression of herpes simplex virus type 1 glycoprotein B in insect cells. Initial analysis of its biochemical and immunological properties. Virus Res. 22:25-39. [DOI] [PubMed] [Google Scholar]
- 20.Ghiasi, H., G. C. Pemg, F. M. Hofman, S. Cai, A. B. Nesburn, and S. L. Wechsler. 1999. Specific and nonspecific immune stimulation of MHC-II-deficient mice results in chronic HSV-1 infection of the trigeminal ganglia following ocular challenge. Virology 258:208-216. [DOI] [PubMed] [Google Scholar]
- 21.Ghiasi, H., S. Slanina, A. B. Nesburn, and S. L. Wechsler. 1994. Characterization of baculovirus-expressed herpes simplex virus type 1 glycoprotein K. J. Virol. 68:2347-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon, Y. J., and D. L. Rock. 1984. Co-cultivation versus blot hybridization for the detection of trigeminal ganglionic latency following corneal inoculation with HSV-1 strains of varying TK expression and pathogenicity. Curr. Eye Res. 3:1097-1100. [DOI] [PubMed] [Google Scholar]
- 23.Hendricks, R. L., R. J. Epstein, and T. Tumpey. 1989. The effect of cellular immune tolerance to HSV-1 antigens on the immunopathology of HSV-1 keratitis. Investig. Ophthalmol. Vis. Sci. 30:105-115. [PubMed] [Google Scholar]
- 24.Hendricks, R. L., M. S. Tao, and J. C. Glorioso. 1989. Alterations in the antigenic structure of two major HSV-1 glycoproteins, gC and gB, influence immune regulation and susceptibility to murine herpes keratitis. J. Immunol. 142:263-269. [PubMed] [Google Scholar]
- 25.Hendricks, R. L., and T. M. Tumpey. 1991. Concurrent regeneration of T lymphocytes and susceptibility to HSV-1 corneal stromal disease. Curr. Eye Res. 10:47-53. [DOI] [PubMed] [Google Scholar]
- 26.Hendricks, R. L., and T. M. Tumpey. 1990. Contribution of virus and immune factors to herpes simplex virus type I-induced corneal pathology. Investig. Ophthalmol. Vis. Sci. 31:1929-1939. [PubMed] [Google Scholar]
- 27.Huseby, E. S., D. Liggitt, T. Brabb, B. Schnabel, C. Ohlen, and J. Goverman. 2001. A pathogenic role for myelin-specific CD8+ T cells in a model for multiple sclerosis. J. Exp. Med. 194:669-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutchinson, L., K. Goldsmith, D. Snoddy, H. Ghosh, F. L. Graham, and D. C. Johnson. 1992. Identification and characterization of a novel herpes simplex virus glycoprotein, gK, involved in cell fusion. J. Virol. 66:5603-5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hutchinson, L., and D. C. Johnson. 1995. Herpes simplex virus glycoprotein K promotes egress of virus particles. J. Virol. 69:5401-5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hutchinson, L., C. Roop-Beauchamp, and D. C. Johnson. 1995. Herpes simplex virus glycoprotein K is known to influence fusion of infected cells, yet is not on the cell surface. J. Virol. 69:4556-4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Javier, R. T., J. G. Stevens, V. B. Dissette, and E. K. Wagner. 1988. A herpes simplex virus transcript abundant in latently infected neurons is dispensable for establishment of the latent state. Virology 166:254-257. [DOI] [PubMed] [Google Scholar]
- 32.Klupp, B. G., J. Baumeister, P. Dietz, H. Granzow, and T. C. Mettenleiter. 1998. Pseudorabies virus glycoprotein gK is a virion structural component involved in virus release but is not required for entry. J. Virol. 72:1949-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lepisto, A. J., G. M. Frank, M. Xu, P. M. Stuart, and R. L. Hendricks. 2006. CD8 T cells mediate transient herpes stromal keratitis in CD4-deficient mice. Investig. Ophthalmol. Vis. Sci. 47:3400-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Little, S. P., and P. A. Schaffer. 1981. Expression of the syncytial (syn) phenotype in HSV-1, strain KOS: genetic and phenotypic studies of mutants in two syn loci. Virology 112:686-702. [DOI] [PubMed] [Google Scholar]
- 35.Liu, T., K. M. Khanna, X. Chen, D. J. Fink, and R. L. Hendricks. 2000. CD8+ T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J. Exp. Med. 191:1459-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Margolis, T. P., F. Sedarati, A. T. Dobson, L. T. Feldman, and J. G. Stevens. 1992. Pathways of viral gene expression during acute neuronal infection with HSV-1. Virology 189:150-160. [DOI] [PubMed] [Google Scholar]
- 37.McGeoch, D. J., M. A. Dalrymple, A. J. Davison, A. Dolan, M. C. Frame, D. McNab, L. J. Perry, J. E. Scott, and P. Taylor. 1988. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J. Gen. Virol. 69:1531-1574. [DOI] [PubMed] [Google Scholar]
- 38.Metcalf, J. F., and R. W. Reichert. 1979. Histological and electron microscopic studies of experimental herpetic keratitis in the rabbit. Investig. Ophthalmol. Vis. Sci. 18:1123-1138. [PubMed] [Google Scholar]
- 39.Mo, C., J. Suen, M. Sommer, and A. Arvin. 1999. Characterization of varicella-zoster virus glycoprotein K (open reading frame 5) and its role in virus growth. J. Virol. 73:4197-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mott, K. R., Y. Osorio, E. Maguen, A. B. Nesburn, A. E. Wittek, S. Cai, S. Chattopadhyay, and H. Ghiasi. 2007. Role of anti-glycoproteins D (anti-gD) and K (anti-gK) IgGs in pathology of herpes stromal keratitis in humans. Investig. Ophthalmol. Vis. Sci. 48:2185-2193. [DOI] [PubMed] [Google Scholar]
- 41.Neumann, H., I. M. Medana, J. Bauer, and H. Lassmann. 2002. Cytotoxic T lymphocytes in autoimmune and degenerative CNS diseases. Trends Neurosci. 25:313-319. [DOI] [PubMed] [Google Scholar]
- 42.Newell, C. K., S. Martin, D. Sendele, C. M. Mercadal, and B. T. Rouse. 1989. Herpes simplex virus-induced stromal keratitis: role of T-lymphocyte subsets in immunopathology. J. Virol. 63:769-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nystrom, K., M. Biller, A. Grahn, M. Lindh, G. Larson, and S. Olofsson. 2004. Real time PCR for monitoring regulation of host gene expression in herpes simplex virus type 1-infected human diploid cells. J. Virol. Methods 118:83-94. [DOI] [PubMed] [Google Scholar]
- 44.Osorio, Y., S. Cai, F. M. Hofman, D. J. Brown, and H. Ghiasi. 2004. Involvement of CD8+ T cells in exacerbation of corneal scarring in mice. Curr. Eye Res. 29:145-151. [DOI] [PubMed] [Google Scholar]
- 45.Osorio, Y., S. F. La Point, S. Nusinowitz, F. M. Hofman, and H. Ghiasi. 2005. CD8+-dependent CNS demyelination following ocular infection of mice with a recombinant HSV-1 expressing murine IL-2. Exp. Neurol. 193:1-18. [DOI] [PubMed] [Google Scholar]
- 46.Perng, G.-C., E. C. Dunkel, P. A. Geary, S. M. Slanina, H. Ghiasi, R. Kaiwar, A. B. Nesburn, and S. L. Wechsler. 1994. The latency-associated transcript gene of herpes simplex virus type 1 (HSV-1) is required for efficient in vivo spontaneous reactivation of HSV-1 from latency. J. Virol. 68:8045-8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pogue-Geile, K. L., and P. G. Spear. 1987. The single base pair substitution responsible for the Syn phenotype of herpes simplex virus type 1, strain MP. Virology 157:67-74. [DOI] [PubMed] [Google Scholar]
- 48.Rock, D. L., A. B. Nesburn, H. Ghiasi, J. Ong, T. L. Lewis, J. R. Lokensgard, and S. L. Wechsler. 1987. Detection of latency-related viral RNAs in trigeminal ganglia of rabbits latently infected with herpes simplex virus type 1. J. Virol. 61:3820-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimeld, C., J. L. Whiteland, S. M. Nicholls, D. L. Easty, and T. J. Hill. 1996. Immune cell infiltration in corneas of mice with recurrent herpes simplex virus disease. J. Gen. Virol. 77:977-985. [DOI] [PubMed] [Google Scholar]
- 50.Sun, D., J. N. Whitaker, Z. Huang, D. Liu, C. Coleclough, H. Wekerle, and C. S. Raine. 2001. Myelin antigen-specific CD8+ T cells are encephalitogenic and produce severe disease in C57BL/6 mice. J. Immunol. 166:7579-7587. [DOI] [PubMed] [Google Scholar]
- 51.Wechsler, S. L., A. B. Nesburn, R. Watson, S. Slanina, and H. Ghiasi. 1988. Fine mapping of the major latency-related RNA of herpes simplex virus type 1 in humans. J. Gen. Virol. 69:3101-3106. [DOI] [PubMed] [Google Scholar]
- 52.Wechsler, S. L., A. B. Nesburn, R. Watson, S. M. Slanina, and H. Ghiasi. 1988. Fine mapping of the latency-related gene of herpes simplex virus type 1: alternative splicing produces distinct latency-related RNAs containing open reading frames. J. Virol. 62:4051-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yan, X. T., T. M. Tumpey, S. L. Kunkel, J. E. Oakes, and R. N. Lausch. 1998. Role of MIP-2 in neutrophil migration and tissue injury in the herpes simplex virus-1-infected cornea. Investig. Ophthalmol. Vis. Sci. 39:1854-1862. [PubMed] [Google Scholar]
- 54.Zwaagstra, J. C., H. Ghiasi, A. B. Nesburn, and S. L. Wechsler. 1991. Identification of a major regulatory sequence in the latency associated transcript (LAT) promoter of herpes simplex virus type 1 (HSV-1). Virology 182:287-297. [DOI] [PubMed] [Google Scholar]