Abstract
In a previous study, we demonstrated that humanized NOD/SCID/IL2Rγnull (hNOG) mice constructed with human hematopoietic stem cells (HSCs) allow efficient human immunodeficiency virus type 1 (HIV-1) infection. However, HIV-1 infection could be monitored for only 43 days in the animals due to their short life spans. By transplanting HSCs without any myeloablation methods, the mice successfully survived longer than 300 days with stable engraftment of human cells. The mice showed high viremia state for more than the 3 months examined, with systemic HIV-1 infection and gradual decrease of CD4+ T cells analogous to that in humans. These capacities of the hNOG mice are very attractive for modeling mechanisms of AIDS progression and therapeutic strategy.
One of the main problems in the field of human immunodeficiency virus type 1 (HIV-1) research is the lack of suitable small animal models for studying the virological and pathogenic aspects of human HIV-1 infection. To overcome the drawback that HIV-1 does not replicate in rodent cells, severe combined immunodeficiency (SCID) mice, engrafted with human peripheral blood mononuclear cells (hu-PBL-SCID) (16) or human fetal thymus and liver tissue [SCID-hu (Thy/Liv)] (18), have been used for the small animal models of HIV-1 infection. However, these mouse models fall short of accurately mirroring human HIV infection because of their short infection spans (17), limited infection of lymphoid tissues (15), and partial infection to coreceptor tropic HIVs (4, 10, 13).
Considering the significant advantages of developing a mouse model for HIV-1 infection, we previously introduced a novel HIV-1 mouse model using nonobese diabetic (NOD)/SCID/interleukin-2 receptor (IL-2R) gamma chain-knocked-out (NOG) mice (22). Multilineage human cells, including T, B, NK cells, monocytes/macrophages, and dendritic cells (DCs) differentiate in the mice when transplanted with human CD34+ hematopoietic stem cells (HSCs) (6, 9, 22). These mice show high levels of susceptibility to both CCR5 (R5)- and CXCR4 (X4)-tropic HIVs with intense plasma viral loads lasting for over 40 days (22). Thus, this mouse model may be valuable for the study of HIV-1 infection. However, a serious problem remains. The mice showed symptoms of a wasting condition and a hunched back 5 to 7 months after HSC transplantation, following which most of them died. This life span is not sufficient if we are to better understand HIV pathogenesis and to develop novel anti-HIV countermeasures, because more than 4 months posttransplantation is required for the development of human T cells before HIV-1 can be studied in mice.
In past studies for the construction of humanized mouse models using NOD/SCID, β2 microglobulin-deficient NOD/SCID (NOD/SCID/B2mnull) or NOG mice, the mice were subjected to total body irradiation or given drugs for HSC transplantation (6, 9, 11, 14, 21, 23). Since NOG mice do not develop any thymic lymphomas in contrast to NOD/SCID or NOD/SCID/B2mnull mice (3, 19), the irradiation might influence the reduction of their life spans. In this study, we therefore searched for optimal conditions for HSC transplantation and consequently found that in NOG mice, myeloablation procedures were not required for human cell generation. Importantly, these mice stably survived longer than 300 days after the HSC transplantation, which allowed further investigation of HIV-1 pathogenesis and progression to disease state in the animals.
NOG mice constructed with HSCs without myeloablation showed prolonged survival time and stable human cell generation.
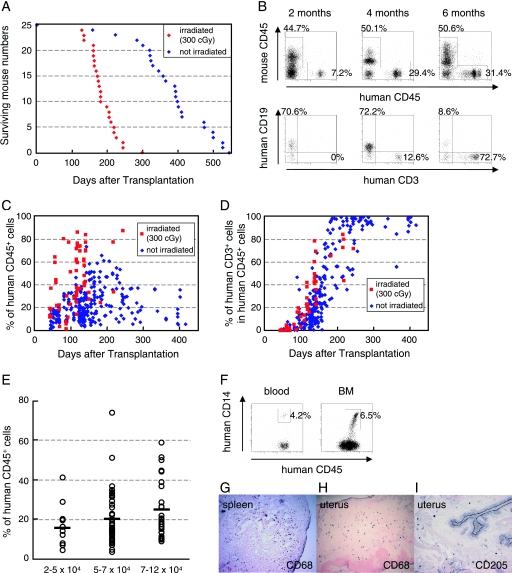
Six- to eight-week-old female NOG mice were obtained from the Central Institute for Experimental Animals (Kawasaki, Japan), and human cord blood-derived CD34+ HSCs (2 × 104 to 12 × 104 cells) were injected intravenously with or without irradiation. As shown in Fig. 1A, most of the mice that received 300 cGy irradiation were dead within 250 days posttransplantation (mean survival time, 188 days). In contrast, more than 80% of the mice with transplanted HSCs without irradiation survived over 300 days (mean survival time, 387 days). These mice were successfully engrafted with HSCs, resulting first in the generation of human CD19+ B cells and subsequently in the generation of human CD3+ T cells (Fig. 1B). Figure 1C and D show the percentages of human CD45+ leukocytes and human CD3+ T cells in peripheral blood at 40 to 413 days after HSC transplantation. Up to 74% of leukocytes in peripheral blood samples were reconstituted with human cells in nonirradiated mice (mean ± standard deviation, 22.8% ± 14.0%; n = 222), and this was maintained over 400 days after transplantation (Fig. 1C). Although higher levels of human cell reconstitution were observed in the irradiated mice (45.2% ± 23.9%; n = 65) (Fig. 1C), which may be due to reduction of absolute numbers of murine cells by destruction of their progenitor cells in bone marrow (BM), human CD3+ T cells developed with similar kinetics between the two groups (Fig. 1D). Figure 1E shows the engraftment efficiency of NOG mice transplanted with different numbers of HSCs without irradiation. More than 2 × 104 HSCs could be stably engrafted, and the levels of human cell reconstitution increased relative to the number of transplanted cells.
FIG. 1.
Human cell generation in hematopoietic stem cell-engrafted hNOG mice with or without myeloablation. (A) Life spans of NOG mice transplanted with human stem cells after receiving 300 cGy irradiation (n = 25) or not receiving irradiation (n = 25). (B) Representative flow cytometric profiles of the mice from 2 to 6 months after transplantation without irradiation. The ratio of human to murine CD45+ cells and that of human CD3+ cells to CD19+ cells are shown. Note that the mice generated human CD45+ leukocytes that eventually developed human CD19+ B cells first and then CD3+ T cells. (C and D) Percentages of human CD45+ cells (C) and CD3+ T cells in human CD45+ cells (D) in peripheral blood from 65 mice that received 300 cGy irradiation and 222 nonirradiated mice 40 to 413 days after transplantation. (E) Summary of engraftment levels in nonirradiated mice transplanted with 2 × 104 to 5 × 104 cells (n = 11), 5 × 104 to 7 × 104 cells (n = 53), or 7 × 104 to 12 × 104 (n = 30) human stem cells. Percentages of human CD45+ leukocytes in peripheral blood during 4 to 5 months after transplantation were shown. The horizontal black bars indicate the averages of the groups. (F to I) Flow cytometric analysis and immunohistochemical analysis of the expression of myelomonocytic markers in nonirradiated mice 4 months after transplantation. Human CD14+ monocytes/macrophages were recognized in peripheral blood and BM (F). A gate was set on the human CD45+ population. Human CD68+ macrophages and CD205+ DCs were also detected in spleen (G) and uterus (H and I). Visualization was performed with 5-bromo-4-chloro-3-indolylphosphate (BCIP). The original magnifications were ×100 (G and H) and ×200 (I).
We further analyzed the development of human monocytes, macrophages, and DCs in the mice with transplanted HSCs without irradiation. Human CD14+ monocytes were detected in peripheral blood and BM using flow cytometry (Fig. 1F), and many human CD68+ macrophages were observed in various organs, including spleen (Fig. 1G), uterus (Fig. 1H), ovary, and lung (data not shown). Human CD205+ DCs were also detected in spleen (data not shown) and uterus (Fig. 1I). These observations were similar to those seen in irradiated mice as shown in our previous report (22). Thus, humanized NOG (hNOG) mice without any myeloablation procedures allowed sufficient development of human cells to study HIV-1 pathogenesis.
hNOG mice induced systemic and long-lasting HIV-1 infection with CD4+ T-cell depletion.
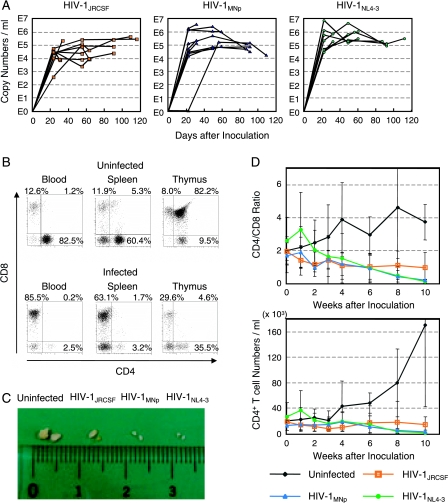
We prepared 29 stem cell-transplanted hNOG mice and inoculated them intravenously with a high dose of R5-tropic HIV-1JRCSF (65,000 50% tissue culture infective doses [TCID50]), X4-tropic HIV-1MNp (20,000 TCID50), or X4-tropic HIV-1NL4-3 (60,000 TCID50) at 122 to 150 days posttransplantation. Then, plasma viral RNA copy numbers were measured at successive time points. The mice showed marked, long-lasting viremia state for more than 3 months, reaching the highest levels of 3.0 × 105 copies/ml from HIV-1JRCSF-infected mice, 3.7 × 106 copies/ml from HIV-1MNp-infected mice, and 7.8 × 106 copies/ml from HIV-1NL4-3-infected mice (Fig. 2A). None of the mice weakened or died as a result of HIV-1 infection throughout the entire follow-up period.
FIG. 2.
Long-lasting viremia and CD4+ T-cell depletion in R5- and X4-tropic HIV-1-infected hNOG mice. (A) Viral copy numbers in plasma from 29 mice intravenously inoculated with R5-tropic HIV-1JRCSF (65,000 TCID50; n = 11), X4-tropic HIV-1MNp (20,000 TCID50; n = 10), and X4-tropic HIV-1NL4-3 (60,000 TCID50; n = 8). RNA viral copy numbers were measured using a real-time PCR quantification assay as previously described (22). (B) The percentages of CD4− CD8+ (top left), CD4+ CD8+ (top right), and CD4+ CD8− (bottom right) cells in blood, spleen, and thymus from a uninfected control mouse and a V-1NL4-3-infected mouse (32 days postinfection). These two mice were constructed with HSCs from the same cord blood donor, and sacrificed 181 and 169 days after transplantation, respectively. A gate was set on the human CD45+ population. (C) Comparison of the apparent size of mesenteric LN from uninfected mice or mice infected with HIV-1JRCSF (109 days postinfection), HIV-1MNp (109 days postinfection), or HIV-1NL4-3 (112 days postinfection). A uninfected control mouse was sacrificed 249 days after transplantation, and three HIV-1-infected mice were sacrificed 246, 246, and 249 days after transplantation. (D) Comparison of CD4/CD8 T-cell ratios and absolute CD4+ T-cell numbers in peripheral blood from uninfected control mice (n = 7), R5-tropic HIV-1JRCSF-infected mice (n = 7), X4-tropic HIV-1MNp-infected mice (n = 5), and X4-tropic HIV-1NL4-3-infected mice (n = 6). Results are expressed as means ± standard deviations (error bars).
All the mice were sacrificed within 4 months postinfection, and the percentages of CD4+ and CD8+ cells in lymphoid tissues were analyzed by flow cytometry. In a representative HIV-1-infected mouse, as shown in Fig. 2B, CD4/CD8 ratios in blood and spleen significantly decreased with apparent loss of CD4+ CD8+ double positive thymocytes. The size of lymphoid tissues, such as thymus and lymph node (LN), in the HIV-1-infected mice was very small compared with uninfected mice (Fig. 2C), suggesting that they shrank as a result of HIV-1 infection. Table 1 illustrates the overall profile of CD4/CD8 ratios in blood and spleen and the percentages of CD4+ CD8+ thymocytes from the 29 HIV-1-infected mice. Most of the mice, both R5- and X4-tropic and HIV-1 infected, had reduced CD4/CD8 ratios in blood and spleen compared with uninfected control mice. On the other hand, a reduction of CD4+ CD8+ thymocytes was observed especially in X4-tropic HIV-1-infected mice, which seemed to correlate with the predominant expression of CXCR4 on the thymocytes as we previously described (22). Interestingly, two mice that were infected with HIV-1MNp (mouse identification number 5 and 8) maintained their high percentages of CD4+ CD8+ thymocytes in spite of significant CD4/CD8 decline in their blood and spleen, suggesting no direct relationship between thymic T-cell depletion and CD4/CD8 decrease in peripheral blood or spleen by HIV-1 infection.
TABLE 1.
CD4/CD8 ratios in peripheral blood and spleen and CD4+ CD8+ cells in thymus of groups of uninfected and HIV-1-infected micea
| Group and mouse identification no. | No. of days after inoculation | CD4/CD8 ratio
|
% of CD4+ CD8+ cells in thymus | No. of RNA viral copies/ml | |
|---|---|---|---|---|---|
| Blood | Spleen | ||||
| Uninfected control group (n = 15) | 2.92 ± 1.68 | 2.78 ± 1.46 | 67.8 ± 20.5 | ||
| HIV-1JRCSF-infected group | |||||
| 1 | 30 | 1.86 | 0.88 | 77.1 | 9,078 |
| 2 | 30 | 0.46 | 0.53 | 12.5 | 7,703 |
| 3 | 33 | 2.61 | 2.17 | 85.7 | 223,020 |
| 4 | 63 | 0.17 | 0.27 | 25.5 | 9,965 |
| 5 | 63 | 0.36 | 0.44 | 27.2 | 8,734 |
| 6 | 63 | 0.18 | 0.88 | 69.6 | 42,198 |
| 7 | 90 | 0.03 | 0.37 | 82.5 | 24,441 |
| 8 | 90 | 0.30 | 0.79 | 84.6 | 24,454 |
| 9 | 90 | 1.77 | 1.55 | 56.9 | 80,636 |
| 10 | 109 | 0.20 | 0.17 | 43.4 | 470,392 |
| 11 | 116 | 0.09 | 0.78 | 11.8 | 299,080 |
| HIV-1MNp-infected group | |||||
| 1 | 31 | 0.82 | 0.44 | 34.6 | 3,709,520 |
| 2 | 31 | 1.02 | 0.61 | 90.2 | 219,971 |
| 3 | 31 | 1.64 | 1.57 | 78.2 | 135,592 |
| 4 | 59 | 0.21 | 0.38 | 35.4 | 78,848 |
| 5 | 59 | 0.10 | 0.07 | 77.0 | 1,039,716 |
| 6 | 87 | 0.20 | 0.40 | 0.5 | 49,080 |
| 7 | 87 | 0.19 | 0.08 | 11.7 | 121,817 |
| 8 | 91 | 0.04 | 0.04 | 82.9 | 30,706 |
| 9 | 91 | 0.28 | 0.10 | 1.2 | 7,407 |
| 10 | 109 | 0.00 | 0.21 | 2.8 | 17,310 |
| HIV-1NL4-3-infected group | |||||
| 1 | 32 | 1.01 | 0.81 | 64.5 | 195,375 |
| 2 | 32 | 0.03 | 0.05 | 4.6 | 770,721 |
| 3 | 60 | 0.21 | 0.13 | 3.9 | 1,108,003 |
| 4 | 60 | 0.14 | NDb | ND | 328,375 |
| 5 | 87 | 0.03 | 0.04 | 1.0 | 201,207 |
| 6 | 92 | 0.03 | 0.17 | 11.1 | 90,831 |
| 7 | 92 | 0.03 | 0.03 | 1.4 | 135,514 |
| 8 | 112 | 0.30 | 0.23 | 0.2 | 325,202 |
Twenty-nine mice inoculated with R5-tropic HIV-1JRCSF (n = 11), X4-tropic HIV-1MNp (n = 10), or X4-tropic HIV-1NL4-3 (n = 8) were sacrificed 161 to 249 days after HSC transplantation. Fifteen uninfected control mice were sacrificed 174 to 249 days after transplantation, and results for the control group are expressed as means ± standard deviations.
ND, not determined because of a lack of cells.
In one mouse from each R5- and X4-tropic HIV-infected group, HIV-1 proviral DNA copy numbers in various organs were measured by real-time PCR assay (Table 2). High HIV DNA copy numbers were detected in the spleen, BM, and LN of the R5-tropic HIV-1-infected mouse and in the thymus, spleen, and LN of the X4-tropic HIV-1-infected mice. In addition, HIV DNA copies were detectable in various other organs, including the lung, liver, ovary, and uterus. The fact that many human CD68+ macrophages, the source of HIV-1 throughout the body (7, 8), were recognized in these organs (22) (Fig. 1H) may help explain the susceptibility of these organs to HIV-1.
TABLE 2.
Comparison of DNA proviral copies in various organs from HIV-1-infected micea
| Organ | No. of HIV-1 DNA copies/100 ng DNA in mice infected withb:
|
||
|---|---|---|---|
| HIV-1JRCSF | HIV-1MNp | HIV-1NL4-3 | |
| Peripheral blood | 60 | 6 | UD |
| Spleen | 793 | 1,143 | 2,115 |
| Bone marrow | 2,432 | 656 | 584 |
| Thymus | 23 | 2,074 | 17,374 |
| Lymph node | 2,103 | 942 | 2,115 |
| Lung | 239 | 145 | 177 |
| Liver | 74 | 49 | 12 |
| Small intestine | ND | 6 | 9 |
| Ovary | 24 | 122 | 10 |
| Uterus | 14 | 5 | 16 |
| Rectum | UD | 16 | 11 |
| Heart | 9 | UD | UD |
| Skin | UD | UD | 138 |
| Brain | UD | 2 | UD |
| Eyeball | 3 | 25 | UD |
Viral DNA was extracted from various organs of mice infected with HIV-1JRCSF (33 days postinfection), HIV-1MNp (59 days postinfection), and HIV-1NL4-3 (60 days postinfection). Determination of HIV-1 DNA copy numbers was performed by real-time PCR assay as previously described (22).
UD, undetected; ND, not done.
To further investigate the progression of CD4+ T-cell depletion by HIV-1 infection, 25 mice 120 to 151 days after HSC transplantation were randomly separated into groups of uninfected control mice (n = 7), HIV-1JRCSF-inoculated mice (n = 7), HIV-1MNp-inoculated mice (n = 5), and HIV-1NL4-3-inoculated mice (n = 6), and then CD4/CD8 ratios and absolute CD4+ T-cell numbers in peripheral blood were monitored at regular intervals. X4-tropic HIV-infected mice showed gradual decreases of their CD4/CD8 ratios and CD4+ T-cell numbers, which eventually resulted in an almost complete depletion from peripheral blood (Fig. 2D). While CD4+ T-cell depletion was also seen in R5-tropic HIV-infected mice, this was less prominent compared with X4-tropic HIV-1-infected mice (Fig. 2D). This pattern of R5- versus X4-tropic HIV-1 infection seems to correlate with the general observation that the emergence of X4-tropic HIVs accelerates CD4+ T-cell decline and disease progression in HIV patients (12, 20).
In this study, we successfully prolonged the life span of hNOG mice by improving the HSC transplantation method and further clarified characteristics of HIV-1 infection in the mice including the following: (i) high levels of viremia lasting over 3 months, (ii) CD4+ T-cell depletion in peripheral blood and spleen regardless of thymic T-cell loss, (iii) systemic HIV-1 infection not only in lymphoid tissues but also in various other organs, and (iv) a different rate of CD4+ T-cell depletion for R5- versus X4-tropic HIV-1 strains. Recently, several studies on HIV-1 infection in Rag2−/− γc−/− mice, transplanted with HSCs at birth, have also been reported (1, 2, 5, 24). The mice showed high susceptibility to both R5- and X4-tropic HIVs and long-term viremia with CD4+ T-cell depletion, which is partly similar to our present results. However, the efficiency of human cell generation in Rag2−/− γc−/− mice strongly depends on the dose of irradiation, and levels of chimerism in mice are not stable even receiving 550 to 750 cGy irradiation, which does eventually induces reduction of their life spans (5). In contrast, very stable engraftment of HSCs and subsequent human cell generation were noted in our hNOG mice even without any myeloablation procedures. Their long life spans and long-term human cell reconstitution allowed persistent HIV-1 infections mirroring HIV-1 infections in humans. Thus, this hNOG mouse system is a very useful tool as an advanced mouse model for the study of AIDS progression and long-term evaluation of new anti-HIV-1 drugs.
Acknowledgments
We thank Tomohiro Morio, Ken Watanabe, and Eiko Ogata of Tokyo Medical and Dental University for their helpful comments and skillful technical support. We are also grateful to Yukari Sasaki and Kazuhiro Takimoto of the National Institute of Infectious Diseases and Teruaki Tanaka and Junichi Fujita of the Nihon University School of Medicine for their management of animals. Human umbilical cord blood samples were obtained from the Tokyo Cord Blood Bank of the Nihon University School of Medicine.
This work was supported by a grant from the Ministry of Education, Culture, Sports, Science, and Technology to promote open research for young academics and specialists.
Footnotes
Published ahead of print on 19 September 2007.
REFERENCES
- 1.Baenziger, S., R. Tussiwand, E. Schlaepfer, L. Mazzucchelli, M. Heikenwalder, M. O. Kurrer, S. Behnke, J. Frey, A. Oxenius, H. Joller, A. Aguzzi, M. G. Manz, and R. F. Speck. 2006. Disseminated and sustained HIV infection in CD34+ cord blood cell-transplanted Rag2−/− γc−/− mice. Proc. Natl. Acad. Sci. USA 103:15951-15956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berges, B. K., W. H. Wheat, B. E. Palmer, E. Connick, and R. Akkina. 2006. HIV-1 infection and CD4 T cell depletion in the humanized Rag2−/− γc−/− (RAG-hu) mouse model. Retrovirology 3:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christianson, S. W., D. L. Greiner, R. A. Hesselton, J. H. Leif, E. J. Wagar, I. B. Schweitzer, T. V. Rajan, B. Gott, D. C. Roopenian, and L. D. Shultz. 1997. Enhanced human CD4+ T cell engraftment in beta2-microglobulin-deficient NOD-scid mice. J. Immunol. 158:3578-3586. [PubMed] [Google Scholar]
- 4.Fais, S., C. Lapenta, S. M. Santini, M. Spada, S. Parlato, M. Logozzi, P. Rizza, and F. Belardelli. 1999. Human immunodeficiency virus type 1 strains R5 and X4 induce different pathogenic effects in hu-PBL-SCID mice, depending on the state of activation/differentiation of human target cells at the time of primary infection. J. Virol. 73:6453-6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorantla, S., H. Sneller, L. Walters, J. G. Sharp, S. J. Pirruccello, J. T. West, C. Wood, S. Dewhurst, H. E. Gendelman, and L. Poluektova. 2007. Human immunodeficiency virus type 1 pathobiology studied in humanized BALB/c-Rag2−/−γc−/− mice. J. Virol. 81:2700-2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hiramatsu, H., R. Nishikomori, T. Heike, M. Ito, K. Kobayashi, K. Katamura, and T. Nakahata. 2003. Complete reconstitution of human lymphocytes from cord blood CD34+ cells using the NOD/SCID/γcnull mice model. Blood 102:873-880. [DOI] [PubMed] [Google Scholar]
- 7.Igarashi, T., C. R. Brown, Y. Endo, A. Buckler-White, R. Plishka, N. Bischofberger, V. Hirsch, and M. A. Martin. 2001. Macrophage are the principal reservoir and sustain high virus loads in rhesus macaques after the depletion of CD4+ T cells by a highly pathogenic simian immunodeficiency virus/HIV type 1 chimera (SHIV): implications for HIV-1 infections of humans. Proc. Natl. Acad. Sci. USA 98:658-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Igarashi, T., O. K. Donau, H. Imamichi, M. J. Dumaurier, R. Sadjadpour, R. J. Plishka, A. Buckler-White, C. Buckler, A. F. Suffredini, H. C. Lane, J. P. Moore, and M. A. Martin. 2003. Macrophage-tropic simian/human immunodeficiency virus chimeras use CXCR4, not CCR5, for infections of rhesus macaque peripheral blood mononuclear cells and alveolar macrophages. J. Virol. 77:13042-13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito, M., H. Hiramatsu, K. Kobayashi, K. Suzue, M. Kawahata, K. Hioki, Y. Ueyama, Y. Koyanagi, K. Sugamura, K. Tsuji, T. Heike, and T. Nakahata. 2002. NOD/SCIDγcnull mouse: an excellent recipient mouse model for engraftment of human cells. Blood 100:3175-3182. [DOI] [PubMed] [Google Scholar]
- 10.Kaneshima, H., L. Su, M. L. Bonyhadi, R. I. Connor, D. D. Ho, and J. M. McCune. 1994. Rapid-high, syncytium-inducing isolates of human immunodeficiency virus type 1 induce cytopathicity in the human thymus of the SCID-hu mouse. J. Virol. 68:8188-8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kollet, O., A. Peled, T. Byk, H. Ben-Hur, D. Greiner, L. Shultz, and T. Lapidot. 2000. β2 Microglobulin-deficient (B2mnull) NOD/SCID mice are excellent recipients for studying human stem cell function. Blood 95:3102-3105. [PubMed] [Google Scholar]
- 12.Koot, M., I. P. Keet, A. H. Vos, R. E. de Goede, M. T. Roos, R. A. Coutinho, F. Miedema, P. T. Schellekens, and M. Tersmette. 1993. Prognostic value of HIV-1 syncytium-inducing phenotype for rate of CD4+ cell depletion and progression to AIDS. Ann. Intern. Med. 118:681-688. [DOI] [PubMed] [Google Scholar]
- 13.Koyanagi, Y., Y. Tanaka, J. Kira, M. Ito, K. Hioki, N. Misawa, Y. Kawano, K. Yamasaki, R. Tanaka, Y. Suzuki, Y. Ueyama, E. Terada, T. Tanaka, M. Miyasaka, T. Kobayashi, Y. Kumazawa, and N. Yamamoto. 1997. Primary human immunodeficiency virus type 1 viremia and central nervous system invasion in a novel hu-PBL-immunodeficient mouse strain. J. Virol. 71:2417-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsumura, T., Y. Kametani, K. Ando, Y. Hirano, I. Katano, R. Ito, M. Shiina, H. Tsukamoto, Y. Saito, Y. Tokuda, S. Kato, M. Ito, K. Motoyoshi, and S. Habu. 2003. Functional CD5+ B cells develop predominantly in the spleen of NOD/SCID/γcnull (NOG) mice transplanted either with human umbilical cord blood, bone marrow, or mobilized peripheral blood CD34+ cells. Exp. Hematol. 31:789-797. [DOI] [PubMed] [Google Scholar]
- 15.McCune, J., H. Kaneshima, J. Krowka, R. Namikawa, H. Outzen, B. Peault, L. Rabin, C. C. Shih, E. Yee, M. Lieberman, I. Weissman, and L. Shultz. 1991. The SCID-hu mouse: a small animal model for HIV infection and pathogenesis. Annu. Rev. Immunol. 9:399-429. [DOI] [PubMed] [Google Scholar]
- 16.Mosier, D. E., R. J. Gulizia, S. M. Baird, D. B. Wilson, D. H. Spector, and S. A. Spector. 1991. Human immunodeficiency virus infection of human-PBL-SCID mice. Science 251:791-794. [DOI] [PubMed] [Google Scholar]
- 17.Mosier, D. E., R. J. Gulizia, P. D. MacIsaac, B. E. Torbett, and J. A. Levy. 1993. Rapid loss of CD4+ T cells in human-PBL-SCID mice by noncytopathic HIV isolates. Science 260:689-692. [DOI] [PubMed] [Google Scholar]
- 18.Namikawa, R., H. Kaneshima, M. Lieberman, I. L. Weissman, and J. M. McCune. 1988. Infection of the SCID-hu mouse by HIV-1. Science 242:1684-1686. [DOI] [PubMed] [Google Scholar]
- 19.Shultz, L. D., P. A. Schweitzer, S. W. Christianson, B. Gott, I. B. Schweitzer, B. Tennent, S. McKenna, L. Mobraaten, T. V. Rajan, D. L. Greiner, et al. 1995. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J. Immunol. 154:180-191. [PubMed] [Google Scholar]
- 20.Tersmette, M., R. A. Gruters, F. de Wolf, R. E. de Goede, J. M. Lange, P. T. Schellekens, J. Goudsmit, H. G. Huisman, and F. Miedema. 1989. Evidence for a role of virulent human immunodeficiency virus (HIV) variants in the pathogenesis of acquired immunodeficiency syndrome: studies on sequential HIV isolates. J. Virol. 63:2118-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ueda, T., H. Yoshino, K. Kobayashi, M. Kawahata, Y. Ebihara, M. Ito, S. Asano, T. Nakahata, and K. Tsuji. 2000. Hematopoietic repopulating ability of cord blood CD34+ cells in NOD/Shi-scid mice. Stem Cells 18:204-213. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe, S., K. Terashima, S. Ohta, S. Horibata, M. Yajima, Y. Shiozawa, M. Z. Dewan, Z. Yu, M. Ito, T. Morio, N. Shimizu, M. Honda, and N. Yamamoto. 2007. Hematopoietic stem cell-engrafted NOD/SCID/IL2Rγnull mice develop human lymphoid systems and induce long-lasting HIV-1 infection with specific humoral immune responses. Blood 109:212-218. [DOI] [PubMed] [Google Scholar]
- 23.Yahata, T., K. Ando, Y. Nakamura, Y. Ueyama, K. Shimamura, N. Tamaoki, S. Kato, and T. Hotta. 2002. Functional human T lymphocyte development from cord blood CD34+ cells in nonobese diabetic/Shi-scid, IL-2 receptor γ null mice. J. Immunol. 169:204-209. [DOI] [PubMed] [Google Scholar]
- 24.Zhang, L., G. I. Kovalev, and L. Su. 2007. HIV-1 infection and pathogenesis in a novel humanized mouse model. Blood 109:2978-2981. [DOI] [PMC free article] [PubMed] [Google Scholar]