Abstract
Little is known about the fitness and virulence consequences of single-nucleotide substitutions in RNA viral genomes, and most information comes from the analysis of nonrandom sets of mutations with strong phenotypic effect or which have been assessed in vitro, with their relevance in vivo being unclear. Here we used site-directed mutagenesis to create a collection of 66 clones of Tobacco etch potyvirus, each carrying a different, randomly chosen, single-nucleotide substitution. Competition experiments between each mutant and the ancestral nonmutated clone were performed in planta to quantitatively assess the relative fitness of each mutant genotype. Among all mutations, 40.9% were lethal, and among the viable ones, 36.4% were significantly deleterious and 22.7% neutral. Not a single case of beneficial effects was observed within the level of resolution of our measures. On average, the fitness of a genotype carrying a deleterious but viable mutation was 49% smaller than that for its unmutated progenitor. Deleterious mutational effects conformed to a beta probability distribution. The virulence of a subset of viable mutants was assessed as the reduction in the number of viable seeds produced by infected plants. Mutational effects on virulence ranged between 17% reductions and 24.4% increases. Interestingly, the only mutations showing a significant effect on virulence were hypervirulent. Competitive fitness and virulence were uncorrelated traits.
A key property of RNA viruses is their error-prone replication (12). This is believed to confer on them the advantage of great adaptability (13). However, mutation has to be seen as a double-edged sword. At the one hand, it is the ultimate source of genetic variation and the raw material for selection to act upon; a genotype with a null mutation rate would be sentenced to extinction due to its inability to respond to environmental perturbations. On the other hand, mutations typically lead to reduced fitness (6, 10, 15) and are removed by purifying selection. It is generally assumed that mutation is a blind process, so that living beings cannot benefit from it without suffering its negative consequences. This is why the avoidance of the detrimental consequences of mutation may be as important for virus survival as the genesis of adaptive novelties (17). Surprisingly, very little is known about the distribution of mutational effects on viral fitness.
Elena and Moya (18) analyzed fitness data for vesicular stomatitis virus (VSV) clones serially transferred throughout bottlenecks (8, 15), finding that the probability density function (pdf) better fitting the data combined realizations from gamma and uniform pdfs (18). Lázaro et al. (30) explored the effect of random mutations on the long-term survival of foot-and-mouth disease virus clones subjected to continuous bottlenecks of size one. They found that the distribution of mutational effects was well described by a Weibull pdf whereas the distribution observed for large nonevolving populations was best described by a log-normal pdf (30). The first case would correspond to the combined effect of mutation and selection, whereas the second would correspond to mutation alone. In a recent similar study with Tobacco etch potyvirus (TEV), it has been shown that fitness fluctuations upon continuous bottlenecks of size one also fitted a Weibull pdf (10). However, these studies, although illustrative, suffer from problems: the number of mutations fixed per clone and its molecular nature were unknown, and the fraction of lethal mutations was undetectable. Therefore, inferences are possible only for the distribution of accumulated deleterious (but viable) effects. Additionally, sequence analysis has revealed the difficulty of unambiguously establishing the relationship between multiple mutations fixed and fitness (9, 37, 38). A straightforward way to avoid this problem is to create a collection of single-nucleotide-substitution mutants by site-directed mutagenesis with an infectious cDNA and then measure fitness for each member of the collection to infer the statistical properties of the distribution of mutational fitness effects. Following this approach, Sanjuán et al. (43) determined the distribution of random mutational effects for VSV in the artificial environment represented by in vitro cell cultures. The observed distribution was highly skewed and had a long, flat tail, conforming to a combination of log-normal and uniform pdfs, with 19% average mutational effect and up to 40% mutations being lethal.
The present study seeks to determine the distribution of effects of single-nucleotide substitutions on the fitness of the plant virus TEV. Fitness effects are estimated using one of the virus's natural hosts, Nicotiana tabacum, and hence the parameters describing the distribution (e.g., average mutational effects) are fully relevant for understanding the evolution of TEV natural populations. In addition, the virulence of some mutant genotypes is estimated and the relationship between competitive fitness and virulence explored.
MATERIALS AND METHODS
Detailed protocols are provided elsewhere (7); thus, here only a summary description for the more relevant ones is provided.
Site-directed mutagenesis.
The pTEV-7DA infectious clone (11), kindly provided by James C. Carrington (Oregon State University, Corvallis, OR), was used as the ancestor surrogated wild-type virus (accession DQ986288).
All 69 mutant genotypes used in this study were generated by site-directed mutagenesis using the Quikchange II XL kit (Stratagene) and following the indications of the manufacturer. Mutagenic primers were also designed according to Stratagene's recommendations. To minimize unwanted errors during the mutagenesis process, the kit incorporates the PfuUltra high-fidelity DNA polymerase. Both the site to be mutagenized and the nucleotide introduced were randomly chosen. The presence of the desired mutation was confirmed by sequencing. The infectivity and viability of the collection of mutants were confirmed by inoculating five N. tabacum L. cv. Xanthi plants with 5′-capped RNAs obtained in vitro as described below. In those cases where no infection was obtained, a second and even third experiment were performed, always starting with new transcription. For all viable cases, regardless of their fitness, the five plants were infected.
Confirmation of the uniqueness of mutations.
This study strongly depends on whether a single mutation per genome was introduced during the above mutagenic PCR process or undesired mutations were introduced by the Pfu polymerase elsewhere. To assess the presence of undesired mutations in each clone, Surveyor mutation detection kit standard gel electrophoresis (transgenomic) was employed. This system uses the Surveyor DNA endonuclease S from celery to scan for known and unknown mutations and polymorphisms in heteroduplex DNA. In brief, the TEV genome was PCR amplified into three overlapping fragments of approximately 4 kb each using specific primers (available upon request) and PfuUltra. Each fragment being analyzed was then mixed with an equimolar amount of the same fragment from the unmutated TEV-7DA clone in a 10-μl reaction volume. Double-stranded DNA was denaturized at 95°C and then cooled to 85°C at 2°C/s and down to 25°C at 0.1°C/s in a Mastercycler ep gradient (Eppendorf). During this process, homo- and heteroduplex molecules would be formed. Next, 0.2 μl of Surveyor endonuclease and 0.2 μl endonuclease enhancer were added, and the reaction mixture was incubated for 20 min at 42°C. The reaction was stopped by adding 1 μl of stop solution. Fragments were resolved on a 1% agarose gel and stained with ethidium bromide. One nucleotide difference between the two DNA strands results in three bands, the homoduplex and the cut heteroduplex.
Only three clones (PC21, PC42, and PC87) showed an unexpected genome-wide band pattern. These clones were discarded from further studies, rendering a sample size of 66 valid mutant genotypes.
In vitro RNA transcription and infectivity.
Infectious plasmids were linearized with BglII (Fermentas) and transcribed into 5′-capped RNAs using the SP6 mMESSAGE mMACHINE kit (Ambion Inc). Transcripts were precipitated (1.5 volumes of diethyl pyrocarbonate (DEPC)-treated water, 1.5 volumes of 7.5 M LiCl, 50 mM EDTA), collected, and resuspended in DEPC-treated water (7). RNA integrity and quantity were assessed by gel electrophoresis and its concentration spectrophotometrically quantified (always in the range of 1.1 to 1.4 μg/μl) using a biophotometer (Eppendorf).
The infectivity of RNA transcripts was assessed for a set of four viral genotypes: unmutated TEV-7DA, the reference strain used in competition experiments, TEV-PC1 (7), and two randomly chosen mutants (PC63 and PC76). To this end, classic dose infectivity experiments were carried out (19). In brief, sets of five 4-week-old N. tabacum plants were inoculated by abrasion of the third true leaf with 5 μl of RNA obtained by serially diluting (from 1:1 to 1:50) the product of in vitro transcriptions. Plants were maintained in the greenhouse at 25°C with 16 h of light/day for 2 weeks. Frequencies of infected plants were transformed into probits and the median infectious dose computed by maximum-likelihood techniques from the probit dose-response curves (19). No differences among the regression slopes were observed among the four viral genotypes (χ2 = 6.937; 3 df; P = 0.074), indicating that the relationship between their infectivities is constant at any dilution in the range explored. The median infectious doses (with 95% confidence intervals) estimated for each genotype were 0.789 μg (0.310 to 1.955) for 7DA, 0.838 μg (0.318 to 2.133) for PC1, 0.588 μg (0.204 to 1.522) for PC63, and 0.645 μg (0.255 to 1.540) for PC76, respectively. Therefore, the infectivities of the different RNA transcripts were statistically indistinguishable, and thus, we can safely assume that mixing 1:1 RNAs from two independent transcriptions would be analogous to mixing equivalent numbers of infectious doses, as required for the competition assays described below.
Assaying relative fitness (W).
The fitness of every mutant was expressed relative to that of the common competitor, PC1 (7). This virus differs from TEV-7DA only in four contiguous synonymous substitutions in the conserved B domain of the NIb replicase gene, which do not significantly affect its fitness (7). These four nucleotide substitutions, however, allowed both competitors to be distinguished by real-time PCR using specific forward primers, a common TaqMan MGB-labeled probe, and a reverse primer (7).
Transcripts from each competitor were mixed 1:1 and diluted with a 1:10 volume of inoculation buffer (100 mg/ml Carborundum, 50 mM K2HPO4). Five 4-week-old N. tabacum plants were inoculated by abrasion with 5 μl of inoculum applied to the third true leaf. Plants were maintained in the greenhouse at 25°C with 16 h/day of light. One week after inoculation, whole plants, with the inoculated leaf discarded, were collected and 3 ml of extraction buffer (0.35 M glycin, 50 mM NaOH, 0.35 M NaCl, 35 mM EDTA, 14 mM 2-mercaptoethanol) per gram of tissue added. Whole plants rather than single systemically infected leaves were sampled to avoid the random effects associated with bottleneck colonization of different leaves by different viral subpopulations. After homogenization, the homogenate was briefly centrifuged and 500 μl from the supernatant mixed with an equal volume of phenol-chloroform-isoamylic alcohol and further centrifuged. Forty microliters of the upper phase was taken, mixed with an equal volume of 7.5 M LiCl-50 mM EDTA, and incubated for ≥2 h at −20°C. After a wash with 70% ethanol, the pellet was finally suspended in 30 μl of DEPC-treated water. Afterwards, RNAs from both competitors in the mixture were reverse transcribed using MultiScribe reverse transcriptase (Applied Biosystems International). The reaction was primed by using a specific antisense oligonucleotide, enabling detection exclusively of the positive (genomic) strands. cDNAs were further amplified and quantified by real-time PCR using TaqMan Universal PCR master mix (Applied Biosystems International), following the indications of the manufacturer, in two independent reactions, each containing a specific forward primer, as described in reference 7. Amplification and quantification were done using an ABI Prism 7000 sequence detection system (Applied Biosystems International).
Fitness was computed according to the equation W = [R(t)/R(0)]1/t, where R(0) and R(t) represent the ratios of mutant-to-TEV-PC1 densities in the inoculation mixture and at t days postinoculation, respectively. Five replicates were run per competition experiment. Competitions were run in two random blocks. On each block, a control competition (with the same number of replicates) between the ancestral TEV-7DA and TEV-PC1 was included. The corresponding estimate for TEV-7DA, W7DA, was used to normalize the Wi of ith mutant included on the same block, hence removing any undesired block effect. Fitness effects for the ith genotype, si, were computed as follows: si=1 − Wi/W7DA.
Quantification of virulence (V).
A subset of 16 viable mutants and TEV-7DA were used to estimate their virulence. To do so, five plants were inoculated with each viral genotype. Plants were placed in the greenhouse and maintained until fruits maturated. At this point, all fruits produced were collected and seeds extracted. Seeds were maintained for between 3 and 16 days (median, 3 days) at 4°C for vernalization. The germination frequency (g) was estimated by placing 50 seeds per plant on a germination agar plate (8 g/liter agarose, 10 g/liter sucrose, 1% Murashige and Skoog salts) and incubating them for 4 days at 25°C. The time of vernalization did not affect g (partial correlation coefficient controlling for the genotype of virus infecting the plant source, r = 0.126; 85 df; P = 0.247). Enumerating all of the seeds produced by a tobacco plant is tiresome. Instead, the dry weight of the total seeds produced was measured after 48 h at 37°C. The relationship between the dry weight and the seed number was linear in all cases studied. The regression slopes estimated for plants infected with a set of viral genotypes were homogeneous (analysis of covariance, P = 0.455; average, 63.3 ± 0.2 μg/seed). However, the slope was significantly smaller for noninfected plants (analysis of covariance, P < 0.001; 56.9 ± 0.2 μg/seed), reflecting that infected plants produced 11.25% heavier seeds. Therefore, the total number of seeds produced, n, was inferred from the dry weight by interpolation in the corresponding regression lines. After these computations, it turned out that healthy plants produced on average 3.43-fold more seeds than infected ones. Here we adopted an ecological definition of virulence, that is, the fitness reduction experienced by an infected plant relative to the average fitness of healthy plants. Plant fitness can be estimated as the lifetime amount of viable seeds produced (F = n × g). Therefore, the virulence of the ith viral genotype can be computed as follows: Vi=1 − Fi/F̄, which takes Vi values in the range of ≥0 and ≤1.
Statistical analyses.
Statistical analyses were done using SPSS software, version 14. To determine whether any given mutation had a significant effect on fitness, we performed a bootstrap significance test to compare the n fitness values obtained for the mutant clone with the m values obtained for the unmutated progenitor TEV-7DA. We employed the bootstrap because it does not assume equal variances; the sampling variance for W tends to increase at low values because the final sample typically contains fewer individuals of the inferior competitor. Specifically, for each mutant, we generated 1,000 bootstrap samples (with replacement) from the observed data, with each bootstrap sample including n and m values for the mutant and TEV-7DA, respectively. For each bootstrap sample, we then calculated the ratio of the mutant's W value to that for TEV-7DA. We excluded the most extreme 2.5% of bootstrap samples in each tail, and we judged the mutation to have had a significant effect if the value 1.0 was outside the resulting 95% confidence interval. Furthermore, since the null hypothesis of nonneutral fitness effects was to be evaluated multiple times, the overall type I error did not have to be held constant but was allowed to vary, case by case, according to the false-discovery-rate method (2).
For the purpose of describing the distribution of mutational effects on fitness, each mutant was treated as an independent observation. The fit of the observed distribution to alternative pdf models was performed by least-squares nonlinear regression. The models chosen share the basic feature that mutations with small effects are more common than mutations with larger effects. The bayesian information criterion (BIC) was used to compare the log likelihood of the models (27). The model that better explains the observations, while requiring the lower number of parameters, is the one with the lower BIC. Akaike's weights (BICw) can be used to assess the probability that a given model is the best one for the observed data given the candidate set of models (27).
RESULTS
Assessing the proportion of deleterious, neutral, and beneficial mutations.
Table S1 in the supplemental material collects relevant information for each of the 66 mutants analyzed, including genomic location, type of substitution, and W value (with 95% bootstrap confidence limits). Successful infections were produced by 39 of the mutants. The fitness of each infectious mutant was compared with that of ancestral TEV-7DA using the bootstrap procedure described above. After the stringent false-discovery-rate procedure was applied, 27 mutations were lethal (W = 0), 24 were significantly deleterious (W < 1), and 15 had no significant fitness effect (W = 1). Not a single instance of beneficial mutation (W > 1) was observed at the 95% confidence limit. However, if confidence intervals are computed at 90%, PC19 becomes significantly beneficial. Nonetheless, caution must be used in accepting this case as truly beneficial, since it corresponds to a synonymous change in the HC-Pro gene. On average, the effect associated with a random mutation is very strong (s̄ = 0.490 ± 0.080), mostly driven by the large fraction of mutations with lethal effect (W = 0). When only viable deleterious mutations are considered, the average fitness effect is still large (s̄d = 0.490 ± 0.043).
Dealing with the existence of lethal mutations.
Lethal mutations and failed inoculation experiments produce the same apparent result: an absence of viral replication in the plant. We failed to infect plants after three trials of the transcription-inoculation experiment for 27 mutants. To rule out the possibility of these mutations not being lethal but of having failed inoculation experiments, we proceeded as follows. First, we estimated our rate of failure to produce an infection when starting the experiment with an a priori viable virus as follows. We ran 20 independent experiments with the nonmutated ancestor TEV-7DA, starting with different pTEV-7DA-transformed Escherichia coli colonies, followed by plasmid preparation, digestion, in vitro transcription, and RNA precipitation and finishing up with plant inoculation. All 20 plants were successfully infected. The number of successful experiments in a series of k trials follows a binomial distribution with parameter p. According to the adjusted Wald method (1), the best estimator for the binomial parameter is as follows: p̂ = 0.955, with 95% confidence limits of p̂ being ≥0.859 and ≤1. Therefore, in the worst possible scenario, our probability of failing an inoculation experiment would be 1 − 0.859 = 0.141. By using this figure, the likelihood of not inducing a single infection by recurrent experimental failure after inoculating the first set of five plants is as follows: 0.1415 = 5.57 × 10−5. This figure becomes vanishingly small as the number of trials increases up to 3 (1.73 × 10−13). In a set of 66 mutants, hence, we expect much less than 1 case (66 × 1.73 × 10−13 = 1.14 × 10−11) to be erroneously assigned to the category of lethal mutations.
One more piece of evidence supports the lethality of these mutations. Among these 27 putative lethals, only one corresponds to a synonymous change (PC74, in the NIa-Pro gene), a distribution that significantly departs from randomness (Fisher's exact test, P = 0.021).
In conclusion, we are confident that the cases classified as lethal mutations are not due to failed inoculation experiments.
Distribution of deleterious but nonlethal fitness effects (W values of >0 and <1).
The average fitness effect for the 31 nonlethal mutations (see Table S1 in the supplemental material) with a W value of <1 (not necessarily significant) was as follows: s̄d=0.411 ± 0.043.
For theoretical reasons (22), it is interesting to determine which of many competing statistical models better describes the distribution of mutational effects on fitness. Table S2 in the supplemental material shows the statistics describing the fitting of several pdf models to the deleterious fitness effects. In general, all the compared distributions share an exponential-like shape but differ in how much probability mass lies in the tail or near the median. All eight models significantly fitted the data (for all cases, P < 0.001), although they differed in the amount of variability in s that was explained (R2 in Table S2 in the supplemental material). The beta pdf was the best-fitting model, with the largest Akaike weight (93.9%) and explaining 97.5% of variability in s. Figure 1 shows the fit of the beta cdf to the data. A beta distribution is characterized by two shape parameters, α and β. The expected value of the beta distribution is as follows: E(s) = α/(α + β) = 0.403, in good agreement with the observed average value (0.411).
FIG. 1.
Fit of the beta distribution to the observed cdf for mutational fitness effects.
Whether the beta distribution looks like a dome, exponential-like, or U shaped depends on the relative values of α and β. In general, when α is <β, as it is the present case (see Table S2 in the supplemental material), the probability mass tends to be hunchbacked around small values but with a long tail that quickly approaches zero. In other words, the greater variance is contributed by infrequent observations of large effect.
Effects of deleterious mutations on virulence.
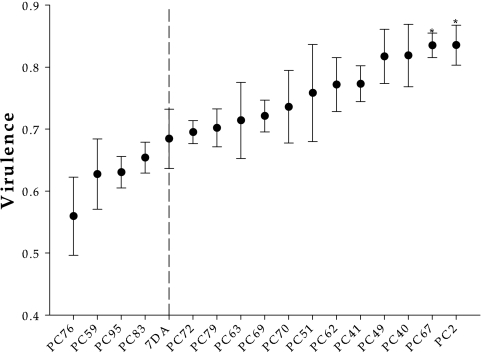
Figure 2 shows the distribution of mutational effects on virulence. The virulence of TEV-7DA was as follows: V7DA = 0.684 ± 0.048, which indicates that the fitness of infected plants was significantly less than that of noninfected plants (Mann-Whitney U test, one-tailed P = 0.014). All 17 mutant genotypes also significantly affected the fitness of the host plant (in all cases, one-tailed P is ≤0.014). Virulence values for the mutant genotypes ranged between 0.560 (TEV-PC76) and 0.836 (TEV-PC2), with an average value of 0.728 ± 0.020. As illustrated in Fig. 2, heterogeneity exists between the V values for all 17 genotypes (including TEV-7DA), as indicated by a nonparametric Kruskal-Wallis test (H = 35.504; 16 df; P = 0.003). The total variance for V was partitioned into its genetic (σG2) and random (σe2) components. The corresponding maximum-likelihood estimators were as follows: σG2̂ = (3.828 ± 0.004) × 10−3 and σe2̂ = (9.295 ± 0.003) × 10−3, respectively. Therefore, the broad-sense heritability for V, that is, the percentage of phenotypic variation that is genetically determined, was only 29.17%.
FIG. 2.
Distribution of mutational effects on virulence. Genotypes are ordered according to the magnitude of their virulence. A dashed line has been drawn to indicate the location of the ancestral TEV-7DA in the distribution. Error bars represent ± standard errors of means. Asterisks indicate the cases that were significantly different from TEV-7DA.
The virulence of each mutant was compared with the V7DA value. Only two cases were significantly different from that of the ancestor (Fig. 2): PC2 (Mann-Whitney test, P = 0.032) and PC67 (P = 0.029). Interestingly, both mutants were more virulent than the ancestral virus.
It is commonly assumed for most theoretical models of parasite within-host population dynamics that V is directly proportional to the pathogen's rate of proliferation whereas the transmission rate is directly proportional to the within-host density (4, 16, 32). If this is true, then selection would favor parasites with intermediate rates of within-host replication and virulence (31). To test whether this prediction holds for TEV, we sought an association between W and V values obtained for the 18 genotypes in hand. Since W is a value relative to fitness of TEV-7DA, for the sake of this analysis, V has also been normalized by using V7DA. Figure 3 shows the lack of significant association between these two variables (Spearman's ρ = 0.0074; 15 df; one-tailed P = 0.489). However, among deleterious mutants (W < 1), virulence is not randomly distributed, since 11 out of 14 cases had a virulence greater than that of the wild-type TEV-7DA (binomial test, P = 0.029). In other words, a mutation impairing viral fitness would likely produce a more virulent virus.
FIG. 3.
Relationship between relative fitness and relative virulence. Dashed axes are drawn as a reference to the values of the ancestral TEV-7DA.
DISCUSSION
This work represents a first study of the distribution of mutational effects on fitness and virulence for a plant RNA virus using explicit single-nucleotide substitutions and measuring both variables in a biologically meaningful context, i.e., the natural host plant. On average, mutations were deleterious, even when lethals were ignored, and with an average effect of 49.0%. Given the structural compactness and functional nonredundancy of RNA viruses, it is not unexpected that most mutations will either be lethal or have a strong deleterious fitness effect. This result is in good agreement with previous observations. In a conceptually and methodologically similar experiment with VSV (43), it was found that the distribution of random deleterious mutational effects was highly skewed and had a long heavy tail, with an s̄d value of 0.19 and up to 40% mutations being lethal. However, the average effect estimated from mutation accumulation experiments using different genotypes of VSV was larger (s̄d = 0.34) (18). Finally, using a phylogenetic approach, it has recently been shown that most amino acid variation observed in natural populations is deleterious and is transiting towards elimination by purifying selection (40).
Estimating the rate of deleterious mutations.
In a recent mutation accumulation experiment done with TEV, the rate of fitness decline was estimated to be about 0.052 ± 0.008 per day (10). The rate of fitness decline in a mutation accumulation experiment equals Us̄d (33), where U is the genomic deleterious mutation rate. Here we have estimated the average mutational effect, excluding lethal mutations, to be as follows: s̄d = 0.490 ± 0.043. Combining these two figures, it is possible to estimate the deleterious mutation rate as follows: U = 0.106 ± 0.026. This figure is in the same ballpark as recent estimates of the deleterious genomic mutation rate obtained for VSV (0.052 to 0.223) (23) and Tobacco mosaic virus (0.10 to 0.13) (34), which have jeopardized the commonly accepted idea of U values of ≥1 for RNA viruses (14).
Comparison of present results with those reported for VSV.
Since they represent similar studies, it is worth comparing the present data with those reported for VSV (43). For VSV the fraction of lethal mutations was 40%, which is almost identical to our estimate for TEV, 40.9%. The fraction of deleterious mutations was larger for TEV (29.2% for VSV versus 36.4% for TEV), and the average deleterious effect for VSV, 19%, is much smaller than the figure here reported for TEV, 49%. Regarding neutral mutations, more mutations (27.1%) were neutral for VSV than for TEV (22.7%). Therefore, the picture that pops up from this comparison is that TEV suffers more strongly from the pernicious effect of viable deleterious mutations than VSV. Two possible explanations can be put forward to explain these differences. The first is almost straightforward: VSV experiments were done in a more permissive environment, cell cultures, than TEV experiments, which were carried out using a natural host that imposes all kind of strong selective pressures on the virus to avoid its systemic movement. The second, more tantalizing explanation is that the two viruses differ in their intrinsic robustness against mutational effects. What differences in genome architecture and content may generate a difference in mutational robustness? The two viruses have similar genome lengths (11,161 nucleotides for VSV and 9,539 nucleotides for TEV) but different polarities. A major difference is that VSV encodes five proteins whereas TEV encodes a single polyprotein that has the ability to self-process into 10 final products. In the case of VSV, mutations affecting one gene would not necessarily influence the other gene products. By contrast, in the case of TEV, mutations in one gene may affect the polyprotein proteolysis and thus alter the production of other products.
Evolution of mutational robustness in RNA viruses.
Recent work has considered the robustness of genomes against deleterious mutation (46). However, the small and compacted nature of viral RNA genomes implies that there is little opportunity for redundancy to evolve, so robustness is expected to be generally low. Instead, as a consequence of this lack of genetic and functional redundancy, a hallmark of viral genomes is the large effect of deleterious mutations and the dominance of positive epistasis among mutated loci (17). Increasing evidences for the large s̄d value for RNA viruses have already been discussed above. Regarding positive epistasis, several studies have reported a dominance of this kind of genetic interaction in RNA viruses, such as bacteriophage φ6 (5), VSV (44), human immunodeficiency virus type 1 (3), Rous sarcoma virus (41), TEV (10), and the viroids (42). Individual mutational hypersensitivity, paradoxically, may create robustness at the population level. The efficiency of natural selection in purging deleterious alleles from a population depends on the product Ns̄d, where N stands for population size. The efficiency of natural selection increases at a large N, and N reaches enormous values even within a single infected host. A large N acts in synergy with a large s̄d, making selection remarkably efficient and keeping the average population fitness high (29). Therefore, individual genomes may be very sensitive to mutation, but this makes the population as a whole robust.
Relationship between fitness and virulence.
Most theoretical models seeking to explain the evolution of pathogen virulence assume that it is a side effect of within-host replication and accumulation, that is, its within-host replicative fitness (4, 16, 32). Despite the obvious interest of this question, data directly testing the existence of the predicted correlation are, at least, scarce, and the molecular basis of virulence is poorly understood. To give some light to this question, we tested the virulence of a subset of TEV mutant genotypes. However, we failed to detect the predicted positive correlation. Two reasons can explain our failure. First, our virulence estimates are too noisy for making reliable statistical inferences. In this sense, it is worth noting that most of the observed phenotypic variance (70.8%) was not explained by genetic differences among clones but was attributable to stochastic effects. By contrast, fitness is determined with much more precision, and 96.5% of observed variation is due to differences among genotypes (7).
A second explanation for the lack of positive correlation between virulence and within-host fitness may be that indeed it does not exist and many other factors influencing the progression of viral infection and how it affects the host's fitness would explain virulence. In particular, virulence would not depend on within-host replication if the extent of damage is not proportional to the amount of viral particles, as in the case of a hypersensitive response after plant infection (35), if expressing the systemic acquired resistance pathway is costly for the plant, as has been reported for Arabidopsis thaliana (24), or if allocating resources to defense detracts from vegetative growth or reproductive effort (25). Examples exist of uncorrelated changes in plant virus fitness and virulence. For instance, it has been shown that when Barley stripe mosaic virus evolved by serial horizontal transfers, its virulence increased with no concomitant increase in the viral load (45). Despite having radically different levels of virulence, necrogenic and nonnecrogenic variants of Cucumber mosaic virus did not differ in their accumulation levels in tomato (20, 21). Finally, in a recent study, a lack of correlation between fitness and virulence was also reported for foot-and-mouth disease virus (26). Altogether, these findings support the hypothesis that virulence should not necessarily be a direct consequence of within-host replication efficiency.
It is somehow striking that most mutations with deleterious fitness effects appear to be associated with increased virulence, and in two cases (Fig. 2, PC2 and PC67), virulence even was significantly greater than that of TEV-7DA. Again, this observation may either be a spurious consequence of noise measurements and/or reduced sample size or reflect a biological property. If selection may have favored intermediate virulence levels (31), then a priori one should expect mutations to be as likely to reduce virulence as to increase it. Therefore, the observed results are compatible only with selection favoring a low level of virulence for TEV, so most mutations can simply increase it. This interesting possibility needs to be further explored.
Supplementary Material
Acknowledgments
We thank the laboratory members and J. A. Daròs for help, comments, and fruitful discussions. The constructive comments from three reviewers are welcomed.
This work was supported by grants from the Spanish MEC-FEDER (BFU2005-23720-E/BMC and BFU2006-14819-C02-01/BMC), the Generalitat Valenciana (ACOMP07/263), and the EMBO Young Investigator Program to S.F.E.
Footnotes
Published ahead of print on 26 September 2007.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Agresti, A., and B. A. Coull. 1998. Approximate is better than “exact” for interval estimation of binomial proportions. Am. Stat. 52:119-126. [Google Scholar]
- 2.Benjamini, Y., and Y. Hochberg. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57:289-300. [Google Scholar]
- 3.Bonhoeffer, S., C. Chappey, N. T. Parkin, I. M. Whitcomb, and C. I. Petropoulos. 2004. Evidence for positive epistasis in HIV-1. Science 306:1547-1550. [DOI] [PubMed] [Google Scholar]
- 4.Brown, N. F., M. E. Wickham, B. K. Coombes, and B. B. Finaly. 2006. Crossing the line: selection and evolution of virulence traits. PLoS Pathog. 2:e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burch, C. L., and L. Chao. 2004. Epistasis and its relationship to canalization in the RNA virus φ6. Genetics 167:559-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chao, L. 1990. Fitness of RNA virus decreased by Muller's ratchet. Nature 348:454-455. [DOI] [PubMed] [Google Scholar]
- 7.Carrasco, P., J. A. Daròs, P. Agudelo-Romero, and S. F. Elena. 2007. A real-time RT-PCR assay for quantifying the fitness of Tobacco etch virus in competition experiments. J. Virol. Methods 139:181-188. [DOI] [PubMed] [Google Scholar]
- 8.Clarke, D. K., E. A. Duarte, A. Moya, S. F. Elena, E. Domingo, and J. J. Holland. 1993. Genetic bottleneck and population passages cause profound fitness differences in RNA viruses. J. Virol. 67:222-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuevas, J. M., S. F. Elena, and A. Moya. 2002. Molecular basis of adaptive convergence in experimental populations of RNA viruses. Genetics 162:533-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de la Iglesia. F., and S. F. Elena. 2007. Fitness declines in Tobacco etch virus upon serial bottleneck passages. J. Virol. 81:4941-4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dolja, V. V., H. J. McBride, and J. C. Carrington. 1992. Tagging of plant potyvirus replication and movement by insertion of beta-glucuronidase into the viral polyprotein. Proc. Natl. Acad. Sci. USA 89:10208-10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domingo, E., and J. J. Holland. 1997. RNA virus mutations and fitness for survival. Annu. Rev. Microbiol. 51:151-178. [DOI] [PubMed] [Google Scholar]
- 13.Domingo, E. 2000. Viruses at the edge of adaptation. Virology 270:251-253. [DOI] [PubMed] [Google Scholar]
- 14.Drake, J. W., and J. J. Holland. 1999. Mutation rates among RNA viruses. Proc. Natl. Acad. Sci. USA 96:13910-13913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duarte, E. A., D. K. Clarke, A. Moya, E. Domingo, and J. J. Holland. 1992. Rapid fitness losses in mammalian RNA virus clones due to Muller's ratchet. Proc. Natl. Acad. Sci. USA 89:6015-6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ebert, D. 1998. Experimental evolution of parasites. Science 282:1432-1435. [DOI] [PubMed] [Google Scholar]
- 17.Elena, S. F., P. Carrasco, J. A. Daròs, and R. Sanjuán. 2006. Mechanisms of genetic robustness in RNA viruses. EMBO Rep. 7:168-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elena, S. F., and A. Moya. 1999. Rate of deleterious mutation and the distribution of its effects on fitness in Vesicular stomatitis virus. J. Evol. Biol. 12:1078-1088. [Google Scholar]
- 19.Ercolani, G. L. 1984. Infectivity titration with bacterial plant pathogens. Annu. Rev. Phytopathol. 22:35-52. [Google Scholar]
- 20.Escriu, F., A. Fraile, and F. García-Arenal. 2000. Evolution of virulence in natural populations of the satellite RNA of Cucumber mosaic virus. Phytopathology 90:480-485. [DOI] [PubMed] [Google Scholar]
- 21.Escriu, F., A. Fraile, and F. García-Arenal. 2003. The evolution of virulence in a plant virus. Evolution 57:755-765. [DOI] [PubMed] [Google Scholar]
- 22.Eyre-Walker, A., and P. D. Keightley. 2007. The distribution of fitness effects of new mutations. Nat. Rev. Genet. 8:610-618. [DOI] [PubMed] [Google Scholar]
- 23.Furió, V., A. Moya, and R. Sanjuán. 2005. The cost of replication fidelity in an RNA virus. Proc. Natl. Acad. Sci. USA 102:10233-10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heidel, A. J., J. D. Clarke, J. Antonovics, and X. Dong. 2004. Fitness costs of mutations affecting the systemic acquired resistance pathway in Arabidopsis thaliana. Genetics 168:2197-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heil, M. 2001. The ecological concept of costs of induced systemic resistance (ISR). Eur. J. Plant Pathol. 107:137-146. [Google Scholar]
- 26.Herrera, M., J. García-Arriaza, N. Pariente, C. Escarmís, and E. Domingo. 2007. Molecular basis for a lack of correlation between viral fitness and cell killing capacity. PLoS Pathog. 3:e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson, J. B., and K. S. Omland. 2004. Model selection in ecology and evolution. Trends Ecol. Evol. 19:101-108. [DOI] [PubMed] [Google Scholar]
- 28.Reference deleted.
- 29.Krakauer, D. C., and J. B. Plotkin. 2002. Redundancy, antiredundancy, and the robustness of genomes. Proc. Natl. Acad. Sci. USA 99:1405-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lázaro, E., C. Escarmís, J. Pérez-Mercader, S. C. Manrubia, and E. Domingo. 2003. Resistance of virus to extinction on bottleneck passages: study of a decaying and fluctuating pattern of fitness loss. Proc. Natl. Acad. Sci. USA 100:10830-10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lenski, R. E., and R. M. May. 1994. The evolution of virulence in parasites and pathogens: reconciliation between two competing hypothesis. J. Theor. Biol. 169:253-265. [DOI] [PubMed] [Google Scholar]
- 32.Levin, B. R. 1996. The evolution and maintenance of virulence in microparasites. Emerg. Infect. Dis. 2:93-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lynch, M., and W. Gabriel. 1990. Mutation load and the survival of small populations. Evolution 44:1725-1737. [DOI] [PubMed] [Google Scholar]
- 34.Malpica, J. M., A. Fraile, I. Moreno, C. I. Obies, J. W. Drake, and F. García-Arenal. 2002. The rate and character of spontaneous mutation in an RNA virus. Genetics 162:1505-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morel, J. B., and J. L. Dangl. 1997. The hypersensitive response and the induction of cell death in plants. Cell Death Differ. 4:671-683. [DOI] [PubMed] [Google Scholar]
- 36.Reference deleted.
- 37.Novella, I. S., C. L. Hershey, C. Escarmis, E. Domingo, and J. J. Holland. 1999. Lack of evolutionary stasis during alternating replication of an arbovirus in insect and mammalian cells. J. Mol. Biol. 287:459-465. [DOI] [PubMed] [Google Scholar]
- 38.Novella, I. S., and B. E. Ebendick-Corpus. 2004. Molecular basis of fitness loss and fitness recovery in Vesicular stomatitis virus. J. Mol. Biol. 342:1423-1430. [DOI] [PubMed] [Google Scholar]
- 39.Reference deleted.
- 40.Pybus, O. G., A. Rambaut, R. Belshaw, R. P. Freckleton, A. J. Drummond, and E. C. Holmes. 2007. Phylogenetic evidence for deleterious mutation load in RNA viruses and its contribution to viral evolution. Mol. Biol. Evol. 24:845-852. [DOI] [PubMed] [Google Scholar]
- 41.Sanjuán, R. 2006. Quantifying antagonistic epistasis in a multifunctional RNA secondary structure of the Rous sarcoma virus. J. Gen. Virol. 87:1595-1602. [DOI] [PubMed] [Google Scholar]
- 42.Sanjuán, R., J. Forment, and S. F. Elena. 2006. In silico predicted robustness of viroid RNA secondary structures. II. Interactions between mutation pairs. Mol. Biol. Evol. 23:2123-2130. [DOI] [PubMed] [Google Scholar]
- 43.Sanjuán, R., A. Moya, and S. F. Elena. 2004. The distribution of fitness effects caused by single-nucleotide substitutions in an RNA virus. Proc. Natl. Acad. Sci. USA 101:8396-8401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanjuán, R., A. Moya, and S. F. Elena. 2004. The contribution of epistasis to the architecture of fitness in an RNA virus. Proc. Natl. Acad. Sci. USA 101:15376-15379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stewart, A. D., J. M. Logsdon, Jr., and S. E. Kelly. 2005. An empirical study of the evolution of virulence under both horizontal and vertical transmission. Evolution 59:730-739. [PubMed] [Google Scholar]
- 46.Wagner, A. 2005. Robustness and evolvability in living systems. Princeton University Press, Princeton, NJ.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.