Abstract
While recent studies have demonstrated that secondary CD8+ T cells develop into effector-memory cells, the impact of particular vaccine regimens on the elicitation of these cells remains poorly defined. In the present study we evaluated the effect of three different immunogens—recombinant vaccinia, recombinant adenovirus, and plasmid DNA—on the generation of memory cellular immune responses. We found that vectors that induce the rapid movement of CD8+ T cells into the memory compartment during a primary immune response also drive a rapid differentiation of these cells into effector-memory CD8+ T cells following a secondary immunization. In contrast, the functional profiles of both CD8+ and CD4+ T cells, assessed by measuring antigen-stimulated gamma interferon and interleukin-2 production, were not predominantly shaped by the boosting immunogen. We also demonstrated that the in vivo expression of antigen by recombinant vectors was brief following boosting immunization, suggesting that antigen persistence has a minimal impact on the differentiation of secondary CD8+ T cells. When used in heterologous or in homologous prime-boost combinations, these three vectors generated antigen-specific CD8+ T cells with different phenotypic profiles. Expression of the memory-associated molecule CD27 on effector CD8+ T cells decreased following heterologous but not homologous boosting, resulting in a phenotypic profile similar to that seen on primary CD8+ T cells. These data therefore suggest that the phenotype of secondary CD8+ T cells is determined predominantly by the boosting immunogen whereas the cytokine profile of these cells is shaped by both the priming and boosting immunogens.
As immunization to elicit cellular immune responses is being studied in the effort to develop a human immunodeficiency virus (HIV) vaccine, attention is focusing on the quality of CD8+ T cells generated by various vaccine modalities. Vaccine-elicited memory CD8+ T cells can persist in vivo for a long period of time before they increase in number and acquire effector function on reexposure to cognate antigen (24). Since the quality of vaccine-induced memory CD8+ T cells is likely to influence the expansion and functional changes that the CD8+ T cells undergo in the setting of viral or bacterial challenge, it will be important to understand the quality of memory CD8+ T cells elicited by different vaccine vectors.
There is no single cell surface molecule that identifies memory CD8+ T cells. Memory CD8+ T cells primarily traffic within lymphoid tissues and can be recognized by their expression of the lymph node-homing molecules CD62L and CCR7 (18). Expression of the interleukin-7 (IL-7) receptor α-chain molecule CD127 has also been used to identify long-lived memory cells (12). Huster et al. have suggested that murine CD8+ T cells can be divided into three major subsets based on their expression of the CD62L and CD127 molecules: effector, effector-memory, and central-memory cells (8). Studies have shown that the CD27 molecule is required for the generation and maintenance of long-term T-cell responses, suggesting that the expression of CD27 on CD8+ T cells can contribute to the identification of memory cells (5, 6). CD27 is a T-cell-costimulatory molecule that, following interaction with its ligand CD70, promotes survival of activated T cells by protecting them from apoptosis (6).
The majority of the studies delineating the differentiation of CD8+ T cells into memory cells have been performed on primary CD8+ T cells, and the nature of the CD8+ T cells generated following a secondary immunization is only now being evaluated (9, 14). The vaccine regimens being used for the induction of cell-mediated immune responses most often include both a prime and a boost immunization. The prime-boost regimens that are currently being favored employ heterologous immunogens, since these appear to generate the most robust transgene-specific responses (15, 20). It is not clear, however, how the particular vectors employed in prime-boost immunizations influence the character of the responding secondary T cells. The present study was initiated to evaluate the impact of boosting immunogens on the differentiation of secondary CD8+ T cells.
MATERIALS AND METHODS
Antibodies.
The antibodies used in this study were directly coupled to fluorescein isothiocyanate, phycoerythrin (PE), allophycocyanin, allophycocyanin-Cy7, peridinin chlorophyll protein-Cy5.5, Alexa Fluor 700, or PE-Cy7. The following monoclonal antibodies (MAbs) were used: anti-CD62L (MEL-14; eBiosciences), anti-CD107a (1D4B; BD Biosciences), anti-CD107b (ABL-93; BD Biosciences), anti-CD8α (53-6.7; BD Biosciences), anti-gamma interferon (IFN-γ) (XMG1.2; BD Biosciences), anti-IL-2 (JHS6-5H4; BD Biosciences), anti-CD127 (A7R34; eBioscience), anti-CD27 (LG.7F9; eBioscience), and anti-CD4 (GK1.5; BD Biosciences).
Vectors.
The codon-optimized HIV-1 HXB2 env or the firefly luciferase gene was cloned into the VRC vector. The recombinant replication-defective adenovirus (rAd) human serotype 5 containing the HIV-1 HXB2 env or the firefly luciferase gene and the recombinant vaccinia virus (rVac) expressing the gp160 protein were generously provided by Gary Nabel, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, NIH. The vaccinia vector expressing the firefly luciferase gene was provided by David Bartlett (University of Pennsylvania).
Mice and immunization.
Six- to eight-week-old female BALB/c mice were purchased from Charles River Laboratories (Wilmington, MA) and maintained under specific-pathogen-free conditions. Research on mice was approved by the Beth Israel Deaconess Institutional Animal Care and Use Committee. Groups of mice were immunized either intraperitoneally with rVac-gp160 (2 × 107 PFU) or intramuscularly with rAd-gp140 (2 × 107 particles) or DNA-gp120 (50 μg of DNA in a 100-μl total injection volume; 50 μl was delivered into each quadriceps muscle). Ten weeks after the first immunization, mice were boosted with both homologous and heterologous combinations of immunogens using the previously mentioned vectors via the same route and with the same quantity as described for the priming immunization. To measure luciferase expression following immunization, an identical immunization strategy was employed using rVac-Luc (2 × 107 PFU), rAd-Luc (2 × 107 particles), or DNA-Luc (50 μg).
Phenotypic T-lymphocyte analyses.
Tetrameric H-2Dd complexes folded with the gp120 p18 epitope peptide (RGPGRAFVTI) (23) were prepared as previously described (21). Blood was collected from individual mice in RPMI 1640 medium containing 40 U of heparin per ml, and peripheral blood mononuclear cells were isolated using Lympholyte-M (Cedarlane). Cells were washed with phosphate-buffered saline (PBS) containing 2% fetal bovine serum (FBS) and stained for 15 min at room temperature with PE-conjugated H-2Dd/p18 tetramer. The cells were then stained with anti-CD8α, anti-CD62L, anti-CD127, and anti-CD27 for an additional 15 min at room temperature; they were then washed once and fixed with PBS containing 2% paraformaldehyde. Samples were collected on an LSR II instrument (BD Biosciences) and analyzed using the FlowJo software (Tree Star).
Splenocyte stimulation and intracellular cytokine staining.
Splenocytes were harvested from individual mice, and red blood cells were lysed by using ACK buffer (0.15 M NH4Cl, 1 mM KHO3, and 0.1 mM disodium EDTA). The cells were then washed with PBS-2% FBS, counted, and stained with PE-conjugated H-2Dd/p18 tetramer. The cells were then resuspended (4 × 106 cells per tube) in RPMI 1640 medium (Cellgro, Herndon, VA) supplemented with 10% FBS, 25 mM HEPES, 2 mM l-glutamine, 20 U of penicillin per ml, 20 μg of streptomycin per ml, 1 mM sodium pyruvate, and 0.1 mM nonessential amino acids. For CD8+ T-cell stimulation, cells were incubated with Golgi Plug (2 μl/ml), anti-CD28 (2 μg/ml), anti-CD49d (2 μg/ml), anti-CD107a (10 μl/ml), anti-CD107b (5 μl/ml), and p18 peptide (2 μg/ml). For CD4+ T-cell stimulation, instead of the p18 peptide, the cells were incubated with 2 μg of Env peptide pool per ml. The pool consisted of 47 overlapping 15-mer peptides spanning the HIV-1 IIIB gp120 protein (Centralized Facility for AIDS Reagents, Potters Bar, United Kingdom), and each peptide was present at a concentration of 2 μg/ml. Unstimulated cells were incubated with all the above reagents except for the peptides. As a positive control, splenocytes were incubated with phorbol myristate acetate (2 μg/ml) and ionomycin (10 μg/ml) and Golgi Plug. The cells were incubated at 37°C for 6 h and then washed with PBS-2% FBS and stained with PE-conjugated H-2Dd/p18 tetramer for 15 min, followed by antibodies specific for cell surface molecules for an additional 15 min. Permeabilization was performed overnight with Cytofix/Cytoperm solution (BD Biosciences). Cells were washed with 1× Perm/Wash buffer (BD Biosciences) and then stained with anti-cytokine MAb. After an additional washing step with 1× Perm/Wash buffer, the cells were fixed in 2% formaldehyde-PBS. Samples were collected on an LSR II instrument (BD Biosciences) and analyzed using FlowJo software (Tree Star).
Bioimaging of luciferase protein expression.
Bioimaging of vectors expressing firefly luciferase was done using the In Vivo Imaging System 110 (IVIS-110) distributed by Xenogen (Alameda, CA). Mice were anesthetized with ketamine-xylazine and injected intraperitoneally with 100 μl of an isotonic salt solution containing 30 mg/ml d-luciferin (Xenogen). Fifteen minutes after luciferin injection, photonic emissions were measured using an IVIS-110 charge-coupled-device camera. Luciferase quantification was done using Living Image software to identify and measure regions of interest.
Statistical analysis.
Data were expressed as means ± standard error of the means. Statistical tests were performed using one-way analysis of variance or a Student's t test, and a P value of <0.05 was considered significant.
RESULTS
Kinetics of CD8+ T-cell responses following homologous and heterologous prime-boost immunization.
rVac, replication-defective rAd, and plasmid DNA expressing various antigens have been used in many vaccine studies for elicitation of antigen-specific CD8+ T-cell responses. We sought to assess the magnitudes and kinetics of the CD8+ T-cell responses induced by these vectors against a common antigen, the p18 immunodominant epitope of the HIV-1 envelope protein. Mice were immunized with the three vectors using the optimal route of immunization for each vector. Priming mice with rVac or rAd generated similar percentages of p18-specific CD8+ T cells (∼8%) that declined to a steady-state level of 4% (Fig. 1). Immunization with plasmid DNA generated a lower magnitude p18-specific CD8+ T-cell response (∼1%), and this cell population declined gradually over time to 0.4%. We next boosted the mice with both homologous and heterologous vaccine constructs. As expected, boosting the mice generated higher percentages of p18-specific CD8+ T cells than were seen after the priming immunization. Boosting the mice with rVac in heterologous prime-boost vector combinations resulted in considerably larger p18-specific CD8+ T-cell responses than were generated using rAd as a boosting immunogen. Interestingly, the magnitudes of the p18-specific CD8+ T-cell responses elicited in rAd-primed mice following heterologous boosting immunization were considerably lower than the levels found after homologous prime-boost immunization with rAd. These data therefore indicate that both the nature of the vector and the order in which it is delivered in the setting of a prime-boost immunization shape the magnitude of the antigen-specific CD8+ T cells.
FIG. 1.
Kinetics of HIV-1 Env-specific CD8+ T cells elicited after prime-boost immunization with different recombinant vectors. BALB/c mice were primed with rVac (2 × 107 PFU), rAd (2 × 107 particles), or plasmid DNA (50 μg) expressing the HIV-1 envelope protein. Ten weeks after the first immunization, mice were boosted with homologous or heterologous combinations of immunogens using the previously mentioned vectors and the same quantity and route of administration used for the priming immunizations; p18-specific CD8+ T cells in the peripheral blood of individual mice were quantitated with an H-2Dd/p18 tetramer. Data are presented as the percentages of CD8+ T cells that bind tetramer and represent the means of five mice per group ± SE.
rVac, rAd, and plasmid DNA induce memory CD8+ T-cell subpopulations with different kinetics.
Antigen-specific CD8+ T cells can be divided into three functionally distinct cell subpopulations on the basis of their expression of cell surface molecules: effector (CD62Llo CD127lo), effector-memory (CD62Llo CD127hi), and central-memory (CD62Lhi CD127hi) cells (Fig. 2). Using this phenotyping paradigm, we next evaluated the differentiation of the different memory CD8+ T-cell subpopulations in the immunized mice. Mice primed with rVac generated a large population of effector p18-specific CD8+ T cells at the time of the peak immune response that differentiated rapidly to effector-memory, and to a lesser extent, to central-memory cells (Fig. 3). A high-frequency effector p18-specific CD8+ T-cell response was also detected immediately following immunization with rAd. However, these cells differentiated slowly to effector-memory and central-memory CD8+ T cells. Immunization of mice with plasmid DNA generated a small p18-specific CD8+ T-cell response. Interestingly, the differentiation of these cells into effector-memory CD8+ T cells was comparable to that seen in the rAd-immunized mice, but their differentiation to central-memory cells was similar to that observed after immunization with rVac. Thus, each vector induced a different distribution of memory p18-specific CD8+ T-cell subpopulations following the priming immunization.
FIG. 2.
Gating strategies used for the phenotypic analysis. p18-specific CD8+ T cells were identified by gating on the CD8+ T cells in the lymphocyte population that bind the H-2Dd/p18 tetramer. These cells were divided into effector CD62Llo CD127lo (E), effector-memory CD62Llo CD127hi (EM), and central-memory CD62Lhi CD127hi (CM) cell subsets using gates based on the relevant “fluorescence minus one” (FMO) controls (A). We then measured the expression of the CD27 surface molecule on these E, EM, and CM cell populations (B).
FIG. 3.
Differentiation of p18-specific CD8+ T cells into effector, effector-memory, and central-memory subsets. Mice were primed with rVac (2 × 107 PFU), rAd (2 × 107 particles), or plasmid DNA (50 μg) expressing the HIV-1 envelope protein. Ten weeks after the first immunization, mice were boosted with homologous or heterologous combinations of immunogens using the previously mentioned vectors and the same quantity and route of administration used for the priming immunizations. Data are presented as the percentage of effector (E), effector-memory (EM), or central-memory (CM) p18-specific CD8+ T cells and represent the means of five mice per group ± SE.
We then assessed whether a boosting immunization with these three vectors would result in a different distribution of memory subpopulations in secondary p18-specific CD8+ T cells. In contrast to what was observed following the priming immunization, the secondary p18-specific CD8+ T cells differentiated mainly to effector and effector-memory cells following the boosting immunization. However, each vector induced CD8+ T cells with a unique differentiation profile. When rVac was used to boost the mice, these cells underwent a rapid decrease in effector and an acquisition of effector-memory phenotypic markers (Fig. 3). In mice primed and boosted with plasmid DNA, the p18-specific CD8+ T cells differentiated to an effector-memory phenotype at an intermediate rate. The generation of effector-memory cells was slowest in mice boosted with rAd. Thus, our findings indicate that while secondary CD8+ T cells differentiate mainly to effector-memory cells, the kinetics of this differentiation is shaped by the secondary immunogen. The priming vector seems to have minimal impact on this differentiation process. In addition, the impact of a particular vector on the differentiation of the secondary CD8+ T cells can be predicted based on how this vector modifies the primary differentiation of the CD8+ T cells.
Kinetics of expression of CD27 differ on effector, effector-memory, and central-memory CD8+ T-cell subsets.
Several studies have demonstrated an association between the expression of the costimulatory molecule CD27 on antigen-specific CD8+ T cells and the generation and long-term maintenance of that immune cell population. We therefore assessed the expression of CD27 on the effector, effector-memory, and central-memory populations of the vaccine-elicited p18-specific CD8+ T cells (Fig. 2B). After a priming immunization with each of the vectors, all the central-memory p18-specific CD8+ T cells always expressed the CD27 molecule, whereas the expression of CD27 by the effector and effector-memory p18-specific CD8+ T cells declined over time (Fig. 4). Moreover, the level of expression of CD27 on these effector and effector-memory cell populations varied significantly between the different groups of vector-immunized mice. Interestingly, despite these variations in the expression level of CD27, the rate of decline of CD27 expression on the effector-memory cells in each group of immunized mice was comparable to the rate of decline of CD27 expression on the effector cell population. These data are consistent with the evidence that, following priming immunization, each vector generates p18-specific CD8+ T cells with a unique differentiation profile. In addition, the parallel rate of decline of CD27 expression on the effector and effector-memory cells seen following immunization with each vector suggests that the rate of maturation of the p18-specific CD8+ T cells is constant but unique for each vector.
FIG. 4.
Expression of CD27 on effector, effector-memory, and central-memory subsets of p18-specific CD8+ T cells. Mice were primed with rVac (2 × 107 PFU), rAd (2 × 107 particles), or plasmid DNA (50 μg) expressing the HIV-1 envelope protein. Ten weeks after the first immunization, mice were boosted with homologous or heterologous combinations of immunogens using the previously mentioned vectors and the same quantity and route of administration used for the priming immunizations. Data are presented as the percentage of CD27 expression on effector (E), effector-memory (EM), or central-memory (CM) p18-specific CD8+ T cells and represent the means of five mice per group ± SE.
We next evaluated the expression of the CD27 molecule on the different memory CD8+ T-cell subsets following a boosting immunization. While the expression of CD27 decreased over time both on the effector and effector-memory cells in the primed mice, the expression of CD27 increased on the effector-memory cells and remained constant on the effector cell population in the mice boosted with the homologous vectors. We also observed differences in the expression kinetics of CD27 between the groups of immunized mice, with higher levels of CD27 on secondary p18-specific CD8+ T cells in rVac-boosted animals (Fig. 4). Although both homologous and heterologous prime-boost immunization induced similar patterns of differentiation of p18-specific CD8+ T cells to effector and effector-memory cells based on expression of CD62L and CD127, the expression profile of CD27 on the secondary CD8+ T cells was different using these two immunization strategies. Expression of CD27 on the secondary effector CD8+ T-cell subsets decreased over time in mice boosted with a heterologous but not homologous vector. Moreover, we observed a parallel acquisition of CD27 expression by the secondary effector-memory and central-memory CD8+ T-cell populations, an expression pattern that was not seen following priming or a homologous prime-boost immunization.
These data therefore demonstrate that the CD8+ T cells elicited by the boosting immunogen have different memory qualities than the cells generated by the same immunogen when it is used as a priming vector. We also show that even as a boosting immunogen, each vector induced memory CD8+ T cells with a unique differentiation profile. Importantly, the analysis of CD27 expression on the different memory subsets reveals that homologous and heterologous prime-boost immunizations generate secondary CD8+ T cells that differ in their state of differentiation.
Kinetics of transgene expression in vivo by rVac, rAd, and plasmid DNA expressing the luciferase protein.
The unique biology of each vector might influence the level and duration of transgene expression. Because the persistence of antigen may affect the maturation of the CD8+ T cells into memory cells, we evaluated the expression profiles of the luciferase protein in mice primed and then boosted with rVac, rAd, and plasmid DNA expressing the luciferase gene. We first analyzed the level of antigen expression following the priming immunization. Intraperitoneal inoculation of mice with rVac resulted in high-level expression of the luciferase protein, which peaked at day 4 and was gone by day 10 (Fig. 5A and B). Immunization with plasmid DNA also generated high levels of luciferase expression, but the peak of expression was observed on day 14. The luciferase expression following DNA immunization was localized to the injection site in the muscles and persisted for more than 80 days after priming (data not shown). Mice immunized with rAd developed the lowest luciferase expression, peaking on day 7 and persisting until approximately 40 days after injection (data not shown).
FIG. 5.
In vivo expression of the luciferase protein by rVac, rAd, and DNA plasmid. Mice were immunized with rVac (2 × 107 PFU), rAd (2 × 107 particles), and DNA (50 μg) expressing the luciferase protein, and ten weeks later they were boosted with homologous or heterologous combinations of immunogens using the noted vectors and the same quantity and route of administration used for the priming immunizations. The levels of luciferase expression were measured over time in the immunized mice using IVIS. (A) Representative images of luciferase expression in the mice following priming immunization. (B) The mean values of the amount of luciferase expressed by groups of four or five mice ± SE following the priming immunization or following homologous or heterologous prime-boost immunizations using the different vectors. The dotted line represents the level of background luminescence. RLU, relative light units.
We next asked how the immune response generated by each vector following the priming immunization affects transgene expression after boosting. Boosting the mice with the same vector used for the priming immunization resulted in low expression levels of luciferase that were no longer detectable 2 weeks after boosting (Fig. 5B). Nevertheless, after both the priming and homologous boosting immunizations, expression of luciferase was highest in plasmid DNA-immunized mice, moderate in rVac-immunized mice, and lowest in rAd-immunized mice. Boosting DNA-primed mice with rVac only minimally affected the kinetics of luciferase expression by rVac (Fig. 5B) but significantly shortened the duration of luciferase expression in mice boosted with rAd. Priming with rAd moderately reduced the expression of luciferase following boosting immunization with rVac; however, expression of luciferase by rAd was almost completely abrogated in mice primed with rVac. These findings demonstrate that immune responses generated by priming with a recombinant vector modulate the expression of the transgene of a recombinant vector following a boosting immunization.
Functional analysis of the CD8+ and CD4+ T cells generated by rVac, rAd, and plasmid DNA immunogens.
Differentiation into memory cells should also be associated with the generation of specific patterns of effector molecules by the antigen-specific CD8+ T cells. We therefore first assessed the functions of the Env-specific CD8+ T cells elicited by the vectors when they were used as priming immunogens. Mice were immunized with rVac, rAd, or plasmid DNA expressing the HIV envelope protein, and both at the time of the peak immune response (days 7, 12, and 14, respectively) and 10 weeks following that first immunization (preboost), splenocytes were exposed to the p18 peptide for 6 h, stained with selected MAbs, and analyzed by flow cytometry. The p18-specific CD8+ T cells elicited by all these vectors produced IFN-γ, with the highest production by cells of the rVac-immunized mice (Fig. 6A). Ten weeks after the priming immunization, the frequency of p18-specific CD8+ T cells producing IFN-γ increased in rVac- and plasmid DNA-immunized mice to levels significantly higher than those in mice immunized with rAd. The p18-specific CD8+ T cells elicited by all these vaccine modalities produced IL-2 at the time of the peak immune response. However, as was observed with IFN-γ, over time higher frequencies of p18-specific CD8+ T cells producing IL-2 were seen in mice inoculated with rVac and plasmid DNA than in rAd-immunized mice. In contrast to IFN-γ and IL-2 production, expression of the degranulation-associated molecules CD107a and CD107b by p18-specific CD8+ T cells was comparable in all groups of vaccinated mice.
FIG. 6.
Functional analysis of p18-specific CD8+ T cells elicited after priming by different recombinant vaccine vectors. Mice were immunized with rVac (2 × 107 PFU), rAd (2 × 107 particles), or plasmid DNA (50 μg) expressing the HIV-1 envelope protein. Splenocytes were harvested on day 7 (rVac), day 12 (rAd), or day 14 (plasmid DNA) after the priming immunization or 10 weeks later (preboost). The splenocytes were then cultured for 6 h in the presence of medium alone (Med.) or p18 peptide (2 μg/ml) (A) or cultured for 6 h in the presence of a pool of 47 overlapping peptides spanning the HIV-1 IIIB gp120 protein (2 μg/ml) (B). Data are presented as the percentages of tetramer-positive CD8+ T cells or Env peptide-reacting CD4+ T cells staining positively for IFN-γ, IL-2, or CD107a and CD107b and represent the means of five mice per group ± SE. *, P < 0.001 (production of cytokines by splenocytes of rVac- and plasmid DNA-immunized mice compared to cytokines produced after immunization with rAd); #, P < 0.001 (production of cytokines by splenocytes collected 10 weeks postimmunization compared to cytokines measured at the time of the peak immune response).
To assess the Env-specific CD4+ T-cell responses generated by the different vaccine modalities, we exposed splenocytes of the immunized mice to a pool of 47 overlapping peptides spanning the HIV-1 gp120 protein, permeabilized the cells, and then stained the cells with MAbs. At the time of the peak immune responses, only the rVac- and plasmid DNA-immunized mice had Env-specific CD4+ T cells that produced IFN-γ and IL-2 (Fig. 6B). Ten weeks later, however, while the Env-specific CD4+ T cells from mice immunized with all three vectors produced IFN-γ, only cells from rVac-immunized mice were able to produce IL-2. These functional data are consistent with the phenotypic analysis of the vaccine-elicited p18-specific CD8+ T cells showing the rapid generation of memory cells in rVac- and plasmid DNA-immunized mice. Our findings also indicate that immunization with rVac induced sustained production of IL-2 by the CD4+ T cells.
Functional analysis of vaccine-elicited Env-specific CD4+ and CD8+ T-cell responses following heterologous and homologous immunizations.
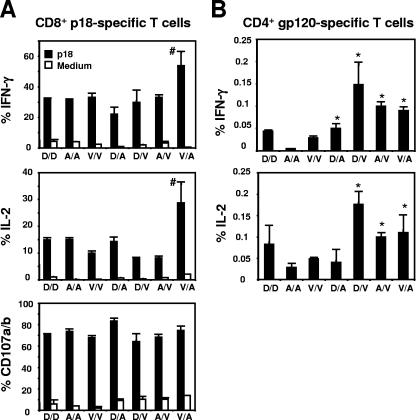
We next sought to assess the functional profile of the Env-specific T cells elicited by the recombinant vectors when they were used as boosting immunogens. We limited our analysis to rVac and rAd since plasmid DNA is used for priming rather than boosting an immune response. Splenocytes were collected at the time of peak immune responses, exposed to the p18 peptide for 6 h, stained with selected MAbs, and analyzed by flow cytometry. Production of IFN-γ by the secondary CD8+ T cells was seen following homologous and heterologous immunization with either rVac or rAd (Fig. 7A). Interestingly, production of IFN-γ by p18-specific CD8+ T cells was considerably higher in mice that were primed with rVac and boosted with rAd (rVac/rAd). This was also observed for IL-2 production by p18-specific CD8+ T cells. In fact, production of IL-2 by those cells was higher when the mice were boosted with rAd than with rVac, after both homologous and heterologous immunization. No significant differences were found in CD107a and CD107b expression on epitope-specific CD8+ T cells between the groups of immunized mice.
FIG. 7.
Analysis of Env-specific CD8+ and CD4+ T-cell function after homologous or heterologous boosting immunization. Groups of mice were primed with rVac (2 × 107 PFU), rAd (2 × 107 particles), or DNA (50 μg) (V, A, and D, respectively) and 10 weeks later were boosted with both homologous and heterologous combinations of immunogens using the noted vectors and the same quantity and route of administration used for the priming immunization. Splenocytes were harvested after the boosting immunization and cultured for 6 h in the presence of medium alone (Med.) or p18 peptide (2 μg/ml) (A), or cultured for 6 h in the presence of a pool of 47 overlapping peptides spanning the HIV-1 IIIB gp120 protein (2 μg/ml) (B). Data are presented as the percentages of tetramer-positive CD8+ or Env peptide-reacting CD4+ T cells staining positively for IFN-γ, IL-2, or CD107a and CD107b and represent the means of five mice per group ± SE. *, P < 0.001 (production of cytokines by CD4+ T cells following heterologous versus homologous prime-boost with rVac or rAd); #, P < 0.01 (production of cytokines by CD8+ T cells generated after rVac/rAd immunization compared to the other groups).
We next analyzed the Env-specific CD4+ T-cell responses in these mice. The splenocytes were exposed to a pool of 47 overlapping peptides spanning the HIV-1 gp120 protein for 6 h and then stained with MAbs specific for various cytokines. Mice that received heterologous boost with rVac or rAd generated a higher frequency of IFN-γ and IL-2 responses than mice receiving homologous boosting immunizations (Fig. 7B). However, in contrast to the robust production of IL-2 and IFN-γ by Env-specific CD4+ T cells in the DNA/rVac-immunized mice, the production of cytokines by the CD4+ T cells was relatively low in the DNA/rAd-immunized mice. These findings demonstrate that although heterologous immunization enhanced the quantity of antigen-specific immune responses, the type and sequence of the vectors used in this immunization had significant impact on the function of the elicited CD8+ and CD4+ T cells.
DISCUSSION
In the present study we investigated the kinetics of differentiation of secondary CD8+ T cells following heterologous and homologous prime-boost immunization using the vectors that have proven most efficient to date in eliciting cellular immune responses. The rationale for this work was to employ vectors that elicit different memory CD8+ T-cell responses following priming immunization and to evaluate how the choice of vector impacts vaccine-elicited secondary immune responses. While both the priming and boosting vectors influenced the magnitude of the secondary CD8+ T-cell response, the ultimate differentiation of these cells into memory cells was exclusively shaped by the second immunogen. In addition, the vector administered second also influenced the rate of differentiation of secondary CD8+ T cells into effector-memory cells.
The variation observed in the differentiation of the primary CD8+ T cells generated by each of the three recombinant vector immunogens may in part be explained by the kinetics of transgene expression in the immunized mice. Antigen load influences the stimulatory signals provided to T cells by antigen-presenting cells and subsequently influences the magnitude of the elicited immune response. In addition, the duration of antigen expression was found to modulate the rate of differentiation of memory CD8+ T cells, as this process begins only after most of the antigen is cleared (1). Consistent with this paradigm, rVac expressed its transgene for the shortest period of time following the priming immunization and induced memory T cells most rapidly. However, the present study suggests that the levels of antigen expression after the boosting immunization have a negligible impact on the differentiation of secondary CD8+ T cells, since the duration of antigen expression by all the vectors was brief and since differences in the rate of differentiation of the secondary CD8+ T cells were still observed. The finding that rVac induces a more rapid differentiation of secondary memory CD8+ T cells than rAd suggests that it might be possible to predict how a particular immunogen will influence the secondary immune response by evaluating how it shapes a primary T-cell response.
Other factors also appear to influence the evolution of cellular immune memory, since the plasmid DNA expressed antigen longer than rAd following the priming immunization but induced memory CD8+ T-cell differentiation more rapidly in the vaccinated mice. Early inflammatory signals delivered by the vector to the immune system have been reported to enhance antigen-presenting activity and control the rate of memory CD8+ T-cell development (3, 4). It is likely that inflammatory signals also modulate the kinetics of differentiation of the secondary CD8+ T cells as well, since the differentiation of CD8+ T cells induced by the vectors was comparable in both the primary and secondary immune responses.
Differentiation of CD8+ T cells could also be modulated by the help provided by CD4+ T cells (10, 22). The rapid CD8+ T-cell differentiation seen in the DNA-primed mice that occurred despite the persistent expression of vaccine antigen might be explained by CD4+ T-cell help. CD4+ T cells have been reported to reverse activation-induced nonresponsiveness of antigen-specific CD8+ T cells through their secretion of IL-2 (13, 16). Indeed, IL-2 production by vaccine-elicited CD4+ T cells was seen in plasmid DNA-immunized mice, while no significant IL-2 production by CD4+ T cells was observed in mice primed with rAd. Nevertheless, the ultimate impact of these CD4+ T-cell responses on the quality of the vaccine-elicited secondary CD8+ T cells is unclear. CD4+ T-cell help has been shown to be important for the formation and maintenance of memory CD8+ T cells following priming (11, 22). CD4+ T cells may therefore contribute to the rapid generation of effector-memory CD8+ T cells elicited following boosting with rVac, since rVac is a potent inducer of CD4+ T-cell responses. However, the differentiation of effector-memory cells seen after DNA/rVac or rAd/rVac immunizations was similar to that seen after rVac/rVac immunization, despite the considerable difference in the levels of the CD4+ T-cell responses generated by these different immunization regimens. This finding suggests that CD4 help may not play a major role in the differentiation of secondary CD8+ T cells or that low-level production of IFN-γ and IL-2 by CD4+ T cells might be sufficient to provide help to secondary CD8+ T cells.
The robust expansion of secondary CD8+ T cells observed following heterologous boosting immunization with rVac is most likely explained by the absence of anti-vector immunity in these mice (19). This possibility is consistent with the higher expression of transgene seen in these mice than in the rVac/rVac-immunized mice. However, enhanced secondary CD8+ T-cell immune responses were not observed in mice receiving heterologous boost with rAd, and transgene expression was also reduced compared to the mice that received rAd/rAd immunization. It is possible that the low level of expression of transgene seen in rVac/rAd-immunized mice is due to the high level of effector-memory CD8+ T-cell responses generated in mice primed with rVac. Effector-memory CD8+ T cells might mediate a rapid clearance of cells expressing the antigen, resulting in reduced antigen-presenting activity and limited secondary responses (18, 24). This explanation, however, could not clarify the moderate secondary CD8+ T-cell responses observed in DNA/rAd-immunized mice, as the effector-memory population generated in DNA-primed mice was comparable to that seen in mice primed with rAd. Nevertheless, the prolonged expression of antigen following DNA immunization might result in large numbers of effector and effector-memory cells in the muscle (1). Expression of the secondary antigen following the subsequent intramuscular immunization with rAd might be reduced by the presence of these effector cells. Interestingly, it has been suggested that greater CD8+ T-cell responses can be achieved by priming and boosting the immune response using immunogens that employ alternate antigen presentation pathways (i.e., direct priming followed by cross-priming and vice versa) (17). It is possible that rVac, but not rAd, utilized a different antigen presentation pathway than that used by plasmid DNA. These findings suggest that the differentiation state of the primary CD8+ T cells may have a critical influence on the expression of antigen by the boosting immunogen.
The analysis of CD27 expression on subpopulations of p18-specific CD8+ T cells after a primary immunization revealed a distinct expression pattern associated with each vaccine vector. However, a general trend of decreasing CD27 expression over time on both the effector and effector-memory T cells was observed in mice receiving each of the vaccine modalities. The decrease in CD27 expression by each of these cell subpopulations is likely due to the maturation of the effector and effector-memory pools of CD27hi CD8+ T cells rather than simply their loss of CD27 expression. The loss of CD27 expression has been linked to terminal differentiation and death of CD8+ T cells (2, 7), yet we found that the percentage of p18-specific CD8+ T cells remained relatively constant over time in the vaccinated mice. The expression of CD27 on the secondary CD8+ T-cell subpopulations is consistent with recent studies showing that these cells predominantly differentiate into effector-memory cells (9). Interestingly, however, we observed that the effector CD8+ T cells generated by heterologous, but not by homologous, prime-boost immunization had a CD27 expression profile that was similar to that seen on this cell subset after priming. This suggests that heterologous prime-boost immunization confers on the secondary CD8+ T cells qualities of primary T cells. The present study also demonstrates an increase in CD27 expression on the central-memory CD8+ T-cell subsets following heterologous immunization, while CD27 expression on this cell subset was constant in mice receiving a homologous prime-boost immunization. In fact, this increase in CD27 expression was parallel to that seen on the effector-memory cells generated in the same mice. The reason for this phenomenon is not clear yet, but it supports the notion that heterologous and homologues prime-boost immunizations result in qualitatively different antigen-specific CD8+ T cells.
The combination of vectors selected to prime and boost the immune response also influenced the function of the secondary T-cell responses. As shown previously, higher levels of IFN-γ and IL-2 were produced by vaccine-elicited CD4+ T cells following heterologous rather than homologous prime-boost immunizations (15, 20). However, the combination of prime-boost vectors largely regulates the level of this cytokine production. Production of IFN-γ and IL-2 by the secondary CD8+ T cells varied less than the CD4+ T-cell responses, and the only combination of vectors that produced considerably higher levels of these cytokines was rVac/rAd. This finding suggests that, in contrast to the observation that the phenotypic profile of the secondary CD8+ T cells is shaped for the most part by the boosting vector, the functional capabilities of the cells are modulated by both the priming and boosting immunogens. Finally, the data presented in this study (summarized in Table 1) suggest that secondary CD8+ T cells have distinct differentiation and functional profiles that are determined by the vectors used in the specific immunization regimen.
TABLE 1.
Summary of vector-associated differences in the secondary T-cell responses
| Immunization (prime/boost) | Antigen expression (RLU)a | CD8+ T-cell response (%)
|
CD4+ T-cell response (%)
|
||||
|---|---|---|---|---|---|---|---|
| Tetramerb | E/EMc | IFN-γ | IL-2 | IFN-γ | IL-2 | ||
| rVAC | 8 × 107 | 8 | 30/60 | 50 | 30 | 0.02 | 0.04 |
| rAd | 5 × 105 | 10 | 70/30 | 35 | 20 | 0.012 | 0.008 |
| DNA | 108 | 1 | 40/40 | 45 | 25 | 0.02 | 0.012 |
| DNA/DNA | 7 × 106 | 8 | 50/40 | 30 | 15 | 0.04 | 0.08 |
| rAd/rAd | 5 × 104 | 30 | 60/30 | 30 | 15 | 0.01 | 0.03 |
| rVac/rVac | 6 × 105 | 23 | 40/45 | 35 | 10 | 0.03 | 0.05 |
| DNA/rAd | 2 × 105 | 15 | 60/30 | 25 | 15 | 0.06 | 0.04 |
| DNA/rVac | 8 × 107 | 32 | 45/45 | 30 | 8 | 0.15 | 0.18 |
| rAd/rVac | 2 × 107 | 60 | 45/45 | 35 | 8 | 0.1 | 0.1 |
| rVac/rAd | 2 × 104 | 20 | 55/30 | 55 | 30 | 0.1 | 0.11 |
As determined in vivo by IVIS. RLU, relative light units.
Results are the percentages of the highest tetramer-positive response following immunization.
Percentages of effector (E) and effector-memory (EM) subsets at ∼10 weeks postimmunization.
Acknowledgments
This work was supported by the National Institute of Allergy and Infectious Diseases Center for HIV/AIDS Vaccine Immunology grant AI067854.
We are grateful to Brianne Barker, Yue Sun, and Itai Roni Eyal for technical assistance and scientific discussions. The HIV-1 IIIB gp120 overlapping peptides were provided by the EU Program EVA/MRC Centralized Facility for AIDS Reagents, National Institute for Biological Standards and Control, United Kingdom.
Footnotes
Published ahead of print on 19 September 2007.
REFERENCES
- 1.Bachmann, M. F., P. Wolint, K. Schwarz, P. Jager, and A. Oxenius. 2005. Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor alpha and CD62L. J. Immunol. 175:4686-4696. [DOI] [PubMed] [Google Scholar]
- 2.Hamann, D., P. A. Baars, M. H. Rep, B. Hooibrink, S. R. Kerkhof-Garde, M. R. Klein, and R. A. van Lier. 1997. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 186:1407-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haring, J. S., V. P. Badovinac, and J. T. Harty. 2006. Inflaming the CD8+ T cell response. Immunity 25:19-29. [DOI] [PubMed] [Google Scholar]
- 4.Heath, W. R., and F. R. Carbone. 2001. Cross-presentation in viral immunity and self-tolerance. Nat. Rev. Immunol. 1:126-134. [DOI] [PubMed] [Google Scholar]
- 5.Hendriks, J., L. A. Gravestein, K. Tesselaar, R. A. van Lier, T. N. Schumacher, and J. Borst. 2000. CD27 is required for generation and long-term maintenance of T cell immunity. Nat. Immunol. 1:433-440. [DOI] [PubMed] [Google Scholar]
- 6.Hendriks, J., Y. Xiao, and J. Borst. 2003. CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. J. Exp. Med. 198:1369-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hintzen, R. Q., R. de Jong, S. M. Lens, and R. A. van Lier. 1994. CD27: marker and mediator of T-cell activation? Immunol. Today 15:307-311. [DOI] [PubMed] [Google Scholar]
- 8.Huster, K. M., V. Busch, M. Schiemann, K. Linkemann, K. M. Kerksiek, H. Wagner, and D. H. Busch. 2004. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc. Natl. Acad. Sci. USA 101:5610-5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jabbari, A., and J. T. Harty. 2006. Secondary memory CD8+ T cells are more protective but slower to acquire a central-memory phenotype. J. Exp. Med. 203:919-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janssen, E. M., N. M. Droin, E. E. Lemmens, M. J. Pinkoski, S. J. Bensinger, B. D. Ehst, T. S. Griffith, D. R. Green, and S. P. Schoenberger. 2005. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature 434:88-93. [DOI] [PubMed] [Google Scholar]
- 11.Janssen, E. M., E. E. Lemmens, T. Wolfe, U. Christen, M. G. von Herrath, and S. P. Schoenberger. 2003. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421:852-856. [DOI] [PubMed] [Google Scholar]
- 12.Kaech, S. M., J. T. Tan, E. J. Wherry, B. T. Konieczny, C. D. Surh, and R. Ahmed. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4:1191-1198. [DOI] [PubMed] [Google Scholar]
- 13.Khanolkar, A., M. J. Fuller, and A. J. Zajac. 2004. CD4 T cell-dependent CD8 T cell maturation. J. Immunol. 172:2834-2844. [DOI] [PubMed] [Google Scholar]
- 14.Masopust, D., S. J. Ha, V. Vezys, and R. Ahmed. 2006. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J. Immunol. 177:831-839. [DOI] [PubMed] [Google Scholar]
- 15.McShane, H., and A. Hill. 2005. Prime-boost immunisation strategies for tuberculosis. Microbes Infect. 7:962-967. [DOI] [PubMed] [Google Scholar]
- 16.Mescher, M. F., J. M. Curtsinger, P. Agarwal, K. A. Casey, M. Gerner, C. D. Hammerbeck, F. Popescu, and Z. Xiao. 2006. Signals required for programming effector and memory development by CD8+ T cells. Immunol. Rev. 211:81-92. [DOI] [PubMed] [Google Scholar]
- 17.Radcliffe, J. N., J. S. Roddick, P. S. Friedmann, F. K. Stevenson, and S. M. Thirdborough. 2006. Prime-boost with alternating DNA vaccines designed to engage different antigen presentation pathways generates high frequencies of peptide-specific CD8+ T cells. J. Immunol. 177:6626-6633. [DOI] [PubMed] [Google Scholar]
- 18.Sallusto, F., D. Lenig, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708-712. [DOI] [PubMed] [Google Scholar]
- 19.Sharpe, S., N. Polyanskaya, M. Dennis, G. Sutter, T. Hanke, V. Erfle, V. Hirsch, and M. Cranage. 2001. Induction of simian immunodeficiency virus (SIV)-specific CTL in rhesus macaques by vaccination with modified vaccinia virus Ankara expressing SIV transgenes: influence of pre-existing anti-vector immunity. J. Gen. Virol. 82:2215-2223. [DOI] [PubMed] [Google Scholar]
- 20.Skeiky, Y. A., and J. C. Sadoff. 2006. Advances in tuberculosis vaccine strategies. Nat. Rev. Microbiol. 4:469-476. [DOI] [PubMed] [Google Scholar]
- 21.Staats, H. F., C. P. Bradney, W. M. Gwinn, S. S. Jackson, G. D. Sempowski, H. X. Liao, N. L. Letvin, and B. F. Haynes. 2001. Cytokine requirements for induction of systemic and mucosal CTL after nasal immunization. J. Immunol. 167:5386-5394. [DOI] [PubMed] [Google Scholar]
- 22.Sun, J. C., M. A. Williams, and M. J. Bevan. 2004. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat. Immunol. 5:927-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahashi, H., Y. Nakagawa, C. D. Pendleton, R. A. Houghten, K. Yokomuro, R. N. Germain, and J. A. Berzofsky. 1992. Induction of broadly cross-reactive cytotoxic T cells recognizing an HIV-1 envelope determinant. Science 255:333-336. [DOI] [PubMed] [Google Scholar]
- 24.Wherry, E. J., and R. Ahmed. 2004. Memory CD8 T-cell differentiation during viral infection. J. Virol. 78:5535-5545. [DOI] [PMC free article] [PubMed] [Google Scholar]