Abstract
Phosphoinositide 3-kinase (PI3K) activation and synthesis of phosphatidylinositol-3,4-bisphosphate (PI-3,4-P2) and phosphatidylinositol-3,4,5-trisphosphate (PI-3,4,5-P3) lipids mediate growth factor signaling that leads to cell proliferation, migration, and survival. PI3K-dependent activation of Akt is critical for myoblast differentiation induced by serum withdrawal, suggesting that in these cells PI3K signaling is activated in an unconventional manner. Here we investigate the mechanisms by which PI3K signaling and Akt are regulated during myogenesis. We report that PI-3,4-P2 and PI-3,4,5-P3 accumulated in the plasma membranes of serum-starved 3T3-L6 myoblasts due to de novo synthesis and increased lipid stability. Surprisingly, only newly synthesized lipids were capable of activating Akt. Knockdown of the lipid phosphatase PTEN moderately increased PI3K lipids but significantly increased Akt phosphorylation and promoted myoblast differentiation. Knockdown of the lipid phosphatase Ship2, on the other hand, dramatically increased the steady-state levels of PI-3,4,5-P3 but did not affect Akt phosphorylation and increased apoptotic cell death. Together, these results reveal the existence of two distinct pools of PI3K lipids in differentiating 3T3-L6 myoblasts: a pool of nascent lipids that is mainly dephosphorylated by PTEN and is capable of activating Akt and promoting myoblast differentiation and a stable pool that is dephosphorylated by Ship2 and is unable to activate Akt.
Phosphoinositides generated by phosphoinositide 3-kinase (PI3K) play critical roles in cell metabolism, motility, proliferation, and survival (3). PI3K recruitment to cell membranes in response to extracellular signals leads to the synthesis of phosphatidylinositol-3,4,5-trisphosphate (PI-3,4,5-P3) and phosphatidylinositol-3,4-bisphosphate (PI-3,4-P2). These lipids can trigger the activation of a plethora of intracellular signaling molecules, such as Akt, Rac, and protein kinase C (24). Akt, a serine/threonine kinase also known as protein kinase B, is the most well-studied downstream effector of PI3K. Akt has been shown to mediate several of the physiological responses to growth factor-activated PI3K, including proliferation and survival, as well as responses to insulin, such as GLUT-4-mediated glucose uptake in muscle and adipocytes (14, 31).
Growth factor activation of Akt is initiated by the binding of its plekstrin homology (PH) domain to PI-3,4-P2 or PI-3,4,5-P3. This interaction is thought to bring Akt in proximity to the enzyme PDK1 (phosphoinositide-dependent kinase 1), which also contains a PH domain capable of binding to PI-3,4-P2 or PI-3,4,5-P3. PDK1 phosphorylates Akt at Thr308, and this phosphorylation is thought to prime Akt for phosphorylation at Ser473 by the kinase previously referred to as PDK2 (1). PDK2 has now been shown to be mTORC2, a protein kinase complex which contains mTOR (Target of Rapamycin), rictor, mLST8, and mSIN (10, 25). Phosphorylation at both sites, T308 and S473, is necessary for full activation of Akt and its ability to induce the phosphorylation (directly or indirectly) of most of its downstream targets (23). Activation of mTOR leads to S6 kinase activation, which in turn can phosphorylate insulin receptor substrate 1 (IRS1) and prevent PI3K recruitment to growth factor receptors, triggering a negative-feedback loop in which IRS1-dependent PI3K activation is suppressed (20).
Lipid phosphatases that regulate PI3K lipid products play critical roles in Akt activation. PTEN is a 3′ phosphatase, which can dephosphorylate phosphatidylinositol-3-phosphate (PI-3-P), PI-3,4-P2, and PI-3,4,5-P3 (18). Ship2 is a phosphoinositide phosphatase that removes the 5′ phosphate of the inositol ring. In vitro, Ship2 can dephosphorylate several substrates, including inositol phosphates, but it seems to preferentially use PI-3,4,5-P3 as a substrate to generate PI-3,4-P2 (22). SKIP is also a phosphatase for PI-3,4,5-P3 that preferentially removes the phosphate from the 5′ position of the inositol ring to generate PI-3,4-P2 (11). The roles of these phosphatases in Akt regulation have been tested by overexpression of the wild type or dominant-negative mutants and by gene knockout or protein knockdown in various cell lines (4, 6). Although it is clear from numerous reports that PTEN can regulate Akt activation, the role of Ship2 has not been so clear (6, 26, 30). Since Akt can be activated either by the substrate or by the product of Ship2, it is possible that Ship2's effect on Akt phosphorylation is determined by the activity level of PTEN in a particular cell line or in response to a specific stimulus.
Although PI3K signaling and Akt signaling have been mostly associated with cell proliferation, they have also been shown to play critical roles in several steps of the myoblast differentiation process. Myoblasts obtained from adult skeletal muscle can be cultured and induced to differentiate into myocytes. After a proliferation stage and as the culture reaches confluence or is starved from growth factors present in serum, myoblasts exit the cell cycle, become resistant to apoptosis, and start to differentiate. First, they undergo a series of cell-cell contact events, including cell alignment and elongation, which culminate in cell fusion and the formation of multinucleated myotubes. In myoblasts, serum withdrawal promotes Akt phosphorylation and activation in a PI3K-dependent manner (8, 9). Expression of a constitutively active form of PI3K enhances myoblast differentiation, while expression of dominant-negative forms of PI3K or treatment with PI3K inhibitors impairs the process (13, 15, 16). Akt activation is thought to mediate the PI3K effects on myogenesis. Constitutively active Akt enhances myotube formation and overcomes the effect of PI3K inhibition, while dominant-negative forms of Akt inhibit the process (12, 32). Despite all the evidence suggesting a role for PI3K in myogenesis, the mechanism by which PI3K signaling is regulated in differentiating myoblasts is not well understood and is of special interest, given that the process is triggered by serum withdrawal and cell cycle arrest.
Here we investigate how phosphoinositide metabolism and Akt activation are regulated during differentiation of 3T3-L6 myoblasts. Our results show that PI-3,4-P2 and PI-3,4,5-P3 levels were induced during 3T3-L6 myoblast differentiation, reaching maximum levels when the cultures were near confluence and the cells were starting to elongate. Serum withdrawal further and dramatically increased the levels of these lipids due to increased lipid stability. Despite the high levels of PI-3,4-P2 and PI-3,4,5-P3 that accumulate in serum-starved myoblast cultures, Akt phosphorylation was dependent on de novo synthesis of PI3K lipids. Ship2 knockdown in these cells dramatically increased PI-3,4,5-P3 and moderately increased PI-3,4-P2, while Akt phosphorylation was not affected or even decreased. PTEN knockdown, on the other hand, significantly increased Akt phosphorylation but only moderately increased PI3K lipids. Green fluorescent protein (GFP)-PH domain studies showed that PI-3,4,5-P3 was mainly located at the plasma membrane of myoblasts and was concentrated in areas of membrane ruffles but was excluded from areas of cell-cell contact. Immunocytochemistry studies showed that Akt was recruited to membrane ruffles located on the edge of cell extensions, where PTEN and PI-3,4,5-P3 colocalize. Increased Akt phosphorylation in PTEN knockdown cells correlated with enhanced myoblast differentiation, while increased steady-state levels of PI-3,4,5-P3 in Ship2 knockdown cells correlated with increased apoptotic cell death.
MATERIALS AND METHODS
Cell lines.
3T3-L6 cells (rat myoblasts), HeLa cells, U87 cells (human glioblastoma cells), and 293 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS). 3T3-L6 cells were subcultured before they reached 50% confluence.
DNA constructs.
pSuper.retro.puro (OligoEngine) and pReSI-hyg (7) vectors were used to express the short hairpin RNAs (shRNAs) for Ship2 and PTEN. The 19-nucleotide target sequences for Ship2 and Ship2 II shRNAs were 5′-GGTGTTTGACCAGCAGAGC-3′ and 5′-GGCCTACATTGAGTTTGAG-3′, respectively, and the 19-nucleotide target sequence for PTEN was 5′-GATCTTGACCAATGGCTAA-3′. The vectors were ligated with the PTEN and Ship2 target sequences according to the manufacturer's protocol. These vectors are referred to as pS-Ship2, pS-Ship2 II, pS-PTEN, or pReSI-Ship2 and pReSI-PTEN. Control vectors (pS-C1 or pReSI-C1) were constructed using a 19-nucleotide sequence with no significant homology to any mammalian gene sequence and therefore served as nonsilencing controls. For double-knockdown experiments, puromycin-resistant pSuper-infected cells were reinfected with pReSI vectors and selected with hygromycin.
pBabe-Hygromycin mammalian expression vector was used to express RNA interference (RNAi)-insensitive PTEN and Ship2 proteins. To this end, RNAi-resistant mutant constructs containing at least three silent mutations in the 19-nucleotide target sequence were generated using a site-directed mutagenesis kit (BD Biosciences). Vectors containing RNAi-resistant wild-type mouse Ship2, catalytic-dead Ship2 (D608A), wild-type human PTEN, catalytic-dead PTEN (C124S), and lipid phosphatase-dead PTEN (G129E) were constructed.
Retrovirus infection.
293 packaging cells (GPG or Phoenix) were plated on 100-mm culture dishes and transfected on the following day with various DNA constructs using Lipofectamine Plus transfection reagent (Invitrogen). Viral supernatants were harvested 2 days after transfection, filtered, and added to the cells in the presence of 4 μg/μl Polybrene. After 24 h of incubation, the medium was removed and replaced with fresh culture medium, and cells were selected with the appropriate antibiotics. Infected cells were used for a maximum of 3 weeks. Expression of exogenous proteins or knockdown of endogenous proteins was checked by Western blotting along with each experiment.
Western blot analysis.
Protein lysates were prepared in lysis buffer containing 50 mM Tris-HCl (pH 7.5), 500 mM NaCl, 1 mM EDTA, 1% Triton X-100, and 10% glycerol as well as protease and phosphatase inhibitors. The cells were scraped off the culture dishes, and the lysates were centrifuged at 14,000 × g to remove the insoluble fraction. The lysates were normalized against total protein content measured by the Bio-Rad protein assay based on the method of Bradford (2a). Lysates were mixed with sodium dodecyl sulfate (SDS) loading buffer, boiled, and separated by 10% SDS-polyacrylamide gel electrophoresis. The proteins were transferred to nitrocellulose membranes, which were blocked with 5% milk dissolved in Tris-buffered saline (TBS) plus 1 mM Na orthovanadate. The membranes were probed overnight with the following primary antibodies: anti-phospho-T308 Akt and anti-phospho-S473 Akt (Cell Signaling Technology), anti-Ship2 (Santa Cruz), anti-PTEN (Cell Signaling Technology), anti-total Akt (Cell Signaling Technology), antitubulin (BD Biosciences), anti-phospho-Erk (antibody against phosphorylated extracellular signal-regulated kinase) (Cell Signaling Technology), anti-total Erk (Santa Cruz), anti-phospho-S9 glycogen synthase kinase 3β (Cell Signaling Technology), and anti-phospho-S256 FKHR (Cell Signaling Technology). After the membranes were washed, they were incubated for 60 min with the appropriate secondary antibodies conjugated to IR680 (Rockland and Molecular Probes) or IR800 (Rockland). The membranes were washed in TBS-Tween, and bound antibodies were detected and quantified using the Odyssey infrared imaging system (Li-Cor).
Phosphoinositide analysis.
Cells were metabolically labeled with 200 μCi/ml inorganic 32P for 1.5 to 4 h in phosphate-free DMEM or with 10 μCi/ml [3H]inositol for 24 or 48 h in inositol-free DMEM supplemented with dialyzed FCS (Gibco) and 200 mM l-glutamine. After the cells were labeled, they were treated as indicated and lysed in 1 N HCl. Lipids were extracted in chloroform-methanol (1:1, vol/vol) and deacylated as described previously (28). Phosphoinositides were separated by anion-exchange high-performance liquid chromatography (HPLC) (Beckman), detected by a flow scintillation analyzer (Perkin-Elmer), and quantified using ProFSA software (Perkin-Elmer). The 3H-labeled PI-3-P, PI-3,4-P2, and PI-3,4,5-P3 peaks were identified using 32P-labeled, in vitro-synthesized internal lipid standards, prepared with baculovirus-expressed PI3K, while the 32P-labeled PI-3,4-P2 and PI-3,4,5-P3 peaks were identified using chemically synthesized 3H internal standards (a gift from C.-S. Chen, University of Kentucky). For the 32P labeling, the counts in each peak were normalized against total phosphoinositide phosphate counts or against PI-4,5-P2 counts. For the [3H]inositol labeling, the counts in each peak were normalized against the counts found in the phosphatidylinositol peak. The same results were obtained whether the data were normalized against phosphatidylinositol or PI-4,5-P2.
PDK2 activity assay.
3T3-L6 cells were transiently transfected with vector containing the sequence for HA-myr-Akt (hemagglutinin-labeled myristoylated Akt) (a gift from A. Toker) using Lipofectamine Plus reagent (Invitrogen). Total protein lysates were prepared 48 h after transfection in lysis buffer containing 50 mM Tris-HCl (pH 7.5), 100 mM NaCl, 1 mM EDTA, 1% Triton X-100, and 10% glycerol as well as protease and phosphatase inhibitors and immunoprecipitated using anti-HA antibody and protein G-containing beads. The cells were treated with 20 nM rapamycin (Cell Signaling Technology) or not treated for 24 h or treated with 100 nM wortmannin (Sigma) for 30 min prior to lysis. Immunoprecipitates were washed three times with phosphate-buffered saline (PBS) containing 1% Triton X-100, mixed with SDS loading buffer, boiled, and separated by 10% SDS-polyacrylamide gel electrophoresis. The proteins were transferred to nitrocellulose membranes, which were blocked with 5% milk dissolved in TBS plus 1 mM Na orthovanadate. Membranes were probed overnight with anti-phospho-S473 and anti-HA, washed, and incubated for 60 min with the appropriate secondary antibodies conjugated to IR680 or IR800. The membranes were washed in TBS-Tween, and bound antibodies were detected and quantified using the Odyssey infrared imaging system (Li-Cor).
GFP-PH domain localization and immunocytochemistry.
3T3-L6 cells were plated on coverslips and transiently transfected with vector containing the sequence for GFP-PH domain from Btk (a gift from S. Field) or GFP-PH domain from Akt (a gift from S. Grinstein) using Lipofectamine Plus reagent (Invitrogen). Cells were fixed 3 days after transfection with 4% paraformaldehyde in PBS for 10 min, permeabilized, and blocked with 0.3% Triton X-100 in PBS containing 5% donkey serum. For the immunocytochemistry studies, fixed cells (transfected or not) were incubated with primary antibodies overnight, washed, and incubated with the appropriate secondary antibodies conjugated to Cy2 or Cy3 (Jackson Laboratory). Coverslips were mounted in Fluorsave (Calbiochem), and cells were analyzed by confocal microscopy using a Nikon microscope attached to a Bio-Rad confocal microscope.
Apoptosis assay and myoblast differentiation assay.
The rate of apoptosis was measured by Western blotting using antibody directed against cleaved caspase 3 (Cell Signaling Technology) as described above, except that semiconfluent cell cultures were scraped off the culture dishes without removing the medium and centrifuged before lysis.
The rate of myoblast differentiation was measured by Western blotting using anti-MHC (anti-myosin heavy chain) antibody (MF20 or F59) as described above or by determining the percentage of fusing colonies in the population. Approximately 500 cells were seeded into a 100-mm tissue culture dish and kept in DMEM supplemented with 10% FCS for 6 days and then in DMEM supplemented with 2% FCS for 2 days. The cells were fixed in 4% paraformaldehyde, and the nuclei were stained with 4′,6′-diamidino-2-phenylindole (DAPI) (Calbiochem). The colonies with myotubes containing 20 or more nuclei per cell were counted using a fluorescence microscope.
RESULTS
PI-3,4-P2 and PI-3,4,5-P3 levels are induced during 3T3-L6 cell differentiation due to de novo synthesis and increased lipid stability.
Myoblasts can be differentiated into myocytes by serum starvation and/or by high cell density. In 3T3-L6 cells, the expression of some myoblast determination markers (such as Myf-5 and MyoD) decreases as the cells start to differentiate. Other differentiation markers (such as myogenin and m-cadherin) are transiently induced just prior to the beginning of the process, while myocyte protein markers, such as MHC, are massively expressed as myotubes start to form. In order to study phosphoinositide metabolism during myogenesis, we metabolically labeled 3T3-L6 cells with [3H]inositol in regular medium (10% FCS) or in low-serum medium (1% FCS) on different days throughout the process of myoblast differentiation and measured the levels of 3H-labeled PI3K lipids. In order to monitor the differentiation process, we also collected protein lysates from cultures maintained under similar conditions and analyzed them for the expression of myoblast differentiation markers (Fig. 1A). Figure 1B shows that PI-3,4-P2 and PI-3,4,5-P3 levels significantly increased as 3T3-L6 myoblasts reached confluence (day 4) and were starting to elongate (Fig. 1C to H). The PI-3,4-P2 and PI-3,4,5-P3 peaks followed the myogenin peak (not shown) and the m-cadherin peak and preceded the MHC peak (Fig. 1A). These high levels of PI-3,4-P2 and PI-3,4,5-P3 were further and dramatically increased (approximately 10-fold) in cells cultured for 24 h in 1% FCS. PI-3-P levels, on the other hand, did not increase with serum withdrawal and decreased only slightly from day 2 to day 3 of the process. Compared to other well-studied cell lines, we found that the maximum levels of PI-3,4-P2 and PI-3,4,5-P3 together in 3T3-L6 cells (day 4) were more than 10-fold higher than the levels of these lipids in serum-starved U87 cells (a glioblastoma cell line where PTEN is not expressed) or growth factor-stimulated HeLa cells, labeled under the same conditions (data not shown).
FIG. 1.
Changes in PI-3,4-P2 and PI-3,4,5-P3 levels during 3T3-L6 myoblast differentiation. Cells were seeded at a low density (10% confluence) and maintained in medium supplemented with 10% FCS. Twenty-four hours prior to harvesting the lipids or proteins, the medium was changed to DMEM containing 10% FCS (dashed lines) or 1% FCS (solid black lines), supplemented with [3H]inositol (B, I, and J). (A) The expression of the myogenic markers m-cadherin and MHC during the course of 3T3-L6 differentiation was determined by Western blot analysis. (B) The levels of PI-3,4-P2 (squares) and PI-3,4,5-P3 (triangles) in differentiating 3T3-L6 myoblasts were measured by HPLC analysis after metabolic labeling of the cells for 24 h. Also shown are the levels of PI-3-P in cells labeled in medium containing 10% FCS or 1% FCS. (C to H) Phase-contrast microscopy photos of the 3T3-L6 cultures in medium containing 1% FCS (C to E) or 10% FCS (F to H), before cells were collected at day 2 (C and F), day 4 (D and G), or day 5 (E and H). (I and J) HPLC profile of the deacylated phosphoinositides in 3T3-L6 cells labeled for 24 h with [3H]inositol in medium containing 10% FCS (I) or 1% FCS (J) on day 4 of differentiation. The data shown are representative of more than three independent measurements.
Using 32P labeling for short periods, we examined whether PI-3,4-P2 and PI-3,4,5-P3 are de novo synthesized after serum withdrawal. For this purpose, 3T3-L6 cells, maintained in regular medium or in low-serum medium for 24 h, were labeled with inorganic 32P for 1 to 4 h, and the levels of 32P-labeled phosphoinositides were determined. Table 1 shows that 32P-labeled PI-3,4,5-P3 was present in confluent 3T3-L6 cells kept in either 1% FCS or in 10% FCS (equivalent to day 4 [Fig. 1D and G]), demonstrating that this lipid is being synthesized even after 24 h of serum starvation. In contrast, no 32P-labeled PI-3,4,5-P3 peak was detected in sparse 3T3-L6 cultures in either 10% or 1% FCS (equivalent to day 2 [Fig. 1C and F]) even after 4 h of labeling (data not shown), indicating that PI3K activity increases as 3T3-L6 cells become confluent. After 1.5 h of labeling, the levels of [32P]PI-3,4,5-P3 in cells growing in 10% FCS were twice as high as in serum-starved cells, while after 3 h of labeling, the levels of [32P]PI-3,4,5-P3 in serum-starved cells surpassed the levels in cells growing in regular medium. [32P]PI-3,4-P2 was detected only after 3 h of labeling (Table 1), suggesting that it may be a subproduct of PI-3,4,5-P3. Table 1 also shows that the levels of [32P]PI-3-P in serum-starved cells were approximately 10-fold higher than the levels of [32P]PI-3,4,5-P3 and [32P]PI-3,4-P2, which implies that PI-3-P is synthesized at a much faster rate. In contrast, when cells were labeled for 24 to 48 h with [3H]inositol (Table 1), the combined levels of PI-3,4-P2 and PI-3,4,5-P3 were similar to or higher than the levels of PI-3-P (see also Fig. 1B, days 3, 4, and 5). Together, these results show that PI-3,4-P2 and PI-3,4,5-P3 are synthesized in serum-starved 3T3-L6 cells at a lower rate than in cells growing in regular medium but reach a higher steady-state level, most likely due to increased lipid stability.
TABLE 1.
Phosphoinositide levels in 3T3-L6 myoblasts labeled with 32P or [3H]inositol
| Serum concn in cell medium | Lipid | Phosphoinositide levela in cells labeled with 32P or [3H]inositol for the following time:
|
|||
|---|---|---|---|---|---|
|
32P
|
[3H]inositol, 48 hb | ||||
| 1.5 h | 3 h | 4 h | |||
| 0.1-1% FCS | PI-3-P | 1.004 | 2.254 | 1.322 | 3.203 ± 0.504 |
| PI-3,4-P2 | 0.000 | 0.091 | 0.288 | 2.798 ± 0.656 | |
| PI-3,4,5-P3 | 0.067 | 0.093 | 0.166 | 1.725 ± 0.405 | |
| 10% FCS | PI-3-P | 1.099 | 1.684 | 1.266 | 3.178 ± 0.148 |
| PI-3,4-P2 | 0.000 | 0.019 | 0.000 | 0.647 ± 0.241 | |
| PI-3,4,5-P3 | 0.116 | 0.083 | 0.099 | 0.336 ± 0.098 | |
3T3-L6 myoblasts were labeled with 32P or [3H]inositol for the time indicated in medium supplemented with a low or high concentration of serum, as indicated. Data represent the counts detected in each peak as a percentage of the counts detected in the PI-4,5-P2 peak.
Data are shown as means ± standard errors of the means.
To test this possibility, we measured the degradation rates for PI-3-P, PI-3,4-P2, and PI-3,4,5-P3 in serum-starved 3T3-L6 cells treated with the PI3K inhibitor wortmannin. First, 3T3-L6 cells (serum starved for 24 h or not) were pretreated with wortmannin or dimethyl sulfoxide (DMSO) for 25 min and then labeled with 32P for 1.5 h. In wortmannin-treated cells, PI-3,4,5-P3 was not detected (data not shown), while in DMSO-treated cells, a 32P-labeled PI-3,4,5-P3 peak was present, demonstrating that the majority of the PI-3,4,5-P3 synthesized in serum-starved 3T3-L6 cells is sensitive to wortmannin. Next, we measured the degradation rate of newly synthesized PI-3,4,5-P3 after wortmannin treatment of cells prelabeled with 32P for 2 h. Figure 2A shows that PI-3-P levels in serum-starved and non-serum-starved cells decreased at the same rate. In contrast, PI-3,4,5-P3 levels decreased at a higher rate in non-serum-starved cells than in serum-starved cells (75% and 55% decrease, respectively, after 15 min of wortmannin treatment). After 30 min of wortmannin treatment, 60% of the PI-3,4,5-P3 in serum-starved cells was wortmannin resistant. These results show that in serum-starved cells, there is a pool of stable PI-3,4,5-P3 which is 2.5 to 3 times larger than the pool in non-serum-starved cells. To measure the degradation rate of the stable pool of PI-3,4,5-P3, we labeled cells with [3H]inositol for 24 h prior to wortmannin treatment. Figure 2B shows that, after PI3K inhibition, the steady-state levels of PI-3-P decreased rapidly (70% decrease after 15 min). In contrast, the steady-state levels of PI-3,4-P2 and PI-3,4,5-P3 decreased slowly but steadily. We conclude that serum withdrawal increases the stability of PI-3,4-P2 and PI-3,4,5-P3 in differentiating 3T3-L6 cells.
FIG. 2.
Effects of serum withdrawal and insulin stimulation on the stability of phosphoinositides (PI) and on phospho-Akt levels. (A) 3T3-L6 cells were kept in medium containing 10% FCS (gray symbols) or serum starved in medium containing 1% FCS (black symbols). Twenty-four hours after serum withdrawal, the cells were labeled with 32P for 2 h and then treated with wortmannin (100 nM) for 15 or 30 min or not treated, as indicated. The levels of deacylated PI-3-P (circles) and PI-3,4,5-P3 (triangles) were measured after HPLC separation and normalized against total phosphoinositide phosphate levels. After 2 h of 32P labeling, the levels of [32P]PI-3,4,5-P3 present in serum-starved cells (time zero) were similar to the levels present in non-serum-starved cells. (B) 3T3-L6 cells were labeled with [3H]inositol for 24 h in medium containing 1% FCS and treated with 100 nM wortmannin or not treated for the indicated times, and the levels of deacylated PI-3-P (circles), PI-3,4-P2 (squares), and PI-3,4,5-P3 (triangles) were measured after HPLC separation. Phospho-Akt levels (diamonds) and phospho-Erk1 (stars) in serum-starved cells (1% FCS) treated with 100 nM wortmannin or not treated were measured by Western blotting using anti-pS473 and anti-pErk antibodies. The data shown are representative of more than three independent measurements. (C) 3T3-L6 cells were labeled with [3H]inositol in medium containing 10%, 1%, or 0.1% FCS, and the levels of deacylated PI-3-P, PI-3,4-P2, and PI-3,4,5-P3 were measured after HPLC separation. Phospho-Akt (pAkt) levels were measured by Western blotting, using protein lysates prepared from cells kept under conditions similar to those used for lipid labeling and anti-pS473 antibody. The relative data, normalized against the cells kept in 10% FCS, were plotted in a direct (y axis) and inverse (x axis) logarithmic scale and show the averages ± standard errors (error bars) for three independent experiments. (D) Cells were labeled as described above for panel B, treated with insulin (10 nM) or not treated for 1 min (PI measurements) or 5 min (phospho-Akt measurements), before treatment with wortmannin (white symbols) or DMSO (black symbols) for the time indicated. Time zero indicates the time when wortmannin was added. Time −5 indicates the basal levels of phospho-Akt, and time −1 indicates the basal levels of PI3K lipids. Data were plotted relative to the levels of PIs and phospho-Akt at time zero.
The bulk of PI-3,4-P2 and PI-3,4,5-P3 in 3T3-L6 cells cannot stimulate Akt phosphorylation.
PI-3,4-P2 and PI-3,4,5-P3 were shown to activate Akt by directly binding to its PH domain and to the PH domain of PDK1, bringing these two molecules together to promote the phosphorylation and activation of Akt (3). In order to determine whether the stable pool of PI3K lipids can stimulate Akt phosphorylation, we measured the effects of serum withdrawal on the levels of phospho-Akt and PI3K lipid. Figure 2C shows that, in spite of the dramatic increases in PI-3,4,5-P3 and PI-3,4-P2 levels, phospho-Akt levels decreased by at least 50% after serum withdrawal. Erk-1 and Erk-2 were highly phosphorylated in 3T3-L6 cells, and serum withdrawal did not significantly change phospho-Erk levels (not shown). Figure 2D shows that insulin stimulation of serum-starved 3T3-L6 cells leads to small increases in the high basal levels of PI-3,4-P2 and PI-3,4,5-P3 (approximately 1.2-fold) and to significant increases in phospho-Akt (9-fold for phosphorylated Ser473 [pS473] and phosphorylated Thr308 [pT308]) and phospho-Erk1/2 (approximately 2-fold) (see also Fig. 3). While wortmannin treatment of 3T3-L6 cells for 15 min decreased serum-starved and insulin-induced PI-3,4-P2 and PI-3,4,5-P3 levels by only 20% (Fig. 2B and D), it completely inhibited phospho-Akt levels in serum-starved cells (Fig. 2B), exponentially growing cells (see Fig. 9B), and insulin-treated cells (Fig. 2D). These results demonstrate that Akt phosphorylation is stimulated by newly synthesized PI3K lipids, but not by the bulk of the PI3K lipids that accumulate in 3T3-L6 cells. Interestingly, wortmannin inhibition of Erk1 and Erk2 phosphorylation correlated with inhibition of the stable pool of PI3K lipids (Fig. 2B, phospho-Erk1).
FIG. 3.
RNAi knockdown of Ship2 and PTEN in 3T3-L6 cells. The expression of PTEN and Ship2 in 3T3-L6 cells infected with the pSuper-derived retrovirus pS-C1, pS-Ship2, or pS-PTEN were measured by Western blotting using PTEN- and Ship2-specific antibodies, as indicated. Akt and Erk phosphorylation were also determined by Western blotting using phospho-specific antibodies. Protein lysates were prepared from serum-starved 3T3-L6 cells, treated with insulin (10 nM) for 10 min (+) or not treated with insulin (−). Antitubulin or anti-Erk antibodies were used as loading controls. Blots shown are representative of more than 10 separate experiments and separate infections. Akt-pT308, Akt with phosphorylated Thr308; pErk, phosphorylated Erk.
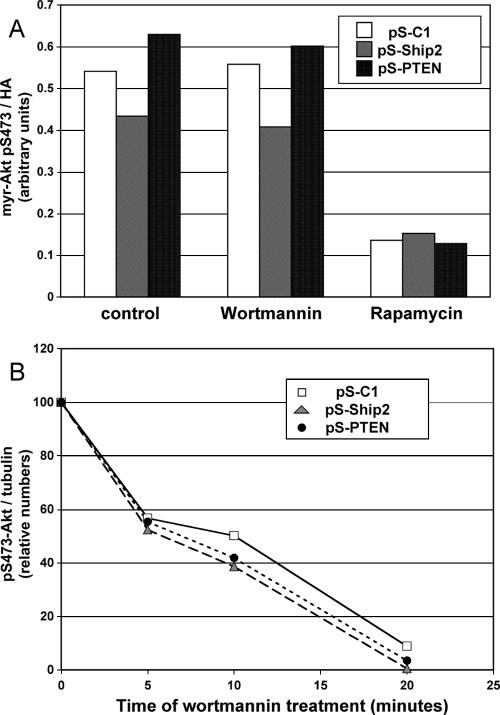
FIG. 9.
Effect of Ship2 or PTEN knockdown on PDK2 activity and on Akt dephosphorylation rate. (A) Phosphorylation of myr-Akt at S473 in pS-C1-, pS-Ship2-, and pS-PTEN-infected 3T3-L6 cells was measured by Western blotting of anti-HA immunoprecipitates with phospho-S473 antibody. Cells were transiently transfected with HA-myr-Akt and treated with rapamycin for 24 h or with wortmannin for 30 min or left untreated (control). The pS473 band was quantified and normalized against total HA. The data shown are representative of three experiments. (B) Dephosphorylation of Akt at S473 was measured by Western blotting of total lysates from 3T3-L6 cells infected with pS-C1, pS-Ship2, or pS-PTEN, maintained in 10% FCS, and treated with wortmannin for 5, 10 and 20 min or not treated, as indicated. The data shown represent the quantification of the pS473 bands, normalized against tubulin data and plotted relative to the values for untreated cells. Data are representative of two experiments.
PTEN dephosphorylates newly synthesized PI3K lipids, while Ship2 dephosphorylates the bulk of PI-3,4,5-P3 in 3T3-L6 cells.
In order to study the roles of Ship2 and PTEN in regulating the levels of PI3K lipids in 3T3-L6 cells, we suppressed the expression of these proteins using RNAi. Retroviruses derived from pSuper vectors containing the shRNA target sequence for Ship2 (pS-Ship2) or PTEN (pS-PTEN) or a control, unrelated sequence (pS-C1) were used to infect 3T3-L6 myoblasts. Figure 3 shows that in the pS-PTEN-infected cells, 95% of the endogenous PTEN protein was suppressed, while in pS-Ship2-infected cells, 70% of the endogenous Ship2 was suppressed compared to the control cells infected with pS-C1. In pS-Ship2-infected cells, approximately 20% of Ship1 expression was also suppressed (Rat Ship1 and Ship2 sequences share homology in 16 out of the 19 nucleotides present in the target sequence). A second Ship2 target sequence (pS-Ship2 II), which does not affect Ship1 expression, also knocked down 70% of Ship2 expression (not shown). pS-Ship2 infection did not affect the expression of PTEN, and pS-PTEN infection did not affect the expression of Ship2. None of the constructs affected the expression of tubulin, total Erk (Fig. 3), or total Akt (not shown). Figure 3 also shows that 3T3-L6 myoblasts have low basal levels of phospho-Akt (as shown in Fig. 2) and high basal levels of phospho-Erk. PTEN knockdown significantly increased the basal and insulin-stimulated levels of phospho-Akt, but not phospho-Erk. On the other hand, Ship2 knockdown with either pS-Ship2 or pS-Ship2 II did not affect basal phospho-Akt levels and slightly decreased insulin-stimulated phospho-Akt (discussed below).
We then metabolically labeled the pSuper-infected 3T3-L6 cells before (Fig. 4A) or after (Fig. 4B) differentiation with [3H]inositol to measure the effects of Ship2 and PTEN knockdown on the steady-state levels of PI3K lipids. We also labeled these cells with inorganic 32P to measure newly synthesized lipids before and after insulin treatment (Fig. 5A and B). Ship2 knockdown with pS-Ship2 caused a remarkable increase in the level of PI-3,4,5-P3 and a small increase in the level of PI-3,4-P2 (Fig. 4 and Fig. 5A and B; see also Fig. 8 [discussed below]) but did not significantly affect the level of PI-3-P, PI-4-P, or PI-4,5-P2 (not shown). Ship2 knockdown with pS-Ship2 II also significantly increased PI-3,4,5-P3 levels, but to a lesser extent than with pS-Ship2 (not shown), suggesting that Ship1 may partially compensate for Ship2 knockdown. Increases in PI-3,4,5-P3 levels caused by Ship2 knockdown were observed in more than 20 independent sets of experiments, and the increases ranged from 2- to 20-fold. Ship2 knockdown increased PI-3,4,5-P3 levels regardless of whether the cells were undifferentiated (Fig. 4A and 5) or fully differentiated (Fig. 4B) and regardless of whether the cells were labeled in medium with 10% FCS (not shown) or 0.1 to 2% FCS. After Ship2 knockdown, the levels of PI-3,4-P2 and PI-3,4,5-P3 were often three- to fourfold higher than the levels of PI-3-P. In contrast to Ship2 knockdown, PTEN knockdown caused only a small increase (twofold) to no change in the level of PI-3,4,5-P3 and a modest (two- to threefold) increase in the level of PI-3,4-P2 (Fig. 4 and Fig. 5A and B; see also Fig. 8 [discussed below]). In some labeling experiments, but not all, we also noticed that PTEN knockdown caused small increases (less than twofold) in PI-3-P levels. As with Ship2 knockdown, PTEN knockdown did not significantly affect the level of PI-4-P or PI-4,5-P2.
FIG. 4.
Effects of Ship2 or PTEN knockdown on the steady-state level of phosphoinositide in 3T3-L6 myoblasts and myocytes. Phosphoinositide levels in 3T3-L6 cells infected with pS-C1, pS-Ship2, or pS-PTEN retrovirus were measured in myoblasts (A) or myocytes (B) labeled with [3H]inositol for 48 h in 0.1% or 2% FCS, respectively. The data in panel A represent the averages ± standard errors (error bars) of 5 (control), 10 (Ship2), and 4 (PTEN) independent measurements. The data in panel B represent the average (bars) and range (error bars) for the values from two separate experiments.
FIG. 5.
Effects of Ship2 or PTEN knockdown on basal and insulin-stimulated PI3K lipids and phospho-Akt. (A and B) The levels of PI-3-P, PI-3,4-P2, and PI-3,4,5-P3 in 3T3-L6 cells infected with pS-C1, pS-Ship2, or pS-PTEN were measured in cells serum starved for 24 h, labeled with 32P for 4 h, and stimulated with 10 nM insulin for 10 min (B) or not stimulated with insulin (A). PIPs, phosphoinositide phosphates. (C and D) Phospho-S473 (C) and phospho-T308 (D) levels in 3T3-L6 cells infected with pS-C1, pS-Ship2, or pS-PTEN, serum starved for 24 h, and stimulated with insulin (10 nM) for 10 min or not stimulated with insulin were measured by Western blotting using phospho-specific antibodies. Data shown are the averages ± standard errors (error bars) for six experiments (for Akt with phosphorylated Ser473 [pS473-Akt]) and four experiments (for Akt with phosphorylated Thr308 [pT308-Akt]).
FIG. 8.
Phosphoinositide levels and Akt phosphorylation in Ship2 and PTEN double-knockdown cells. (A) Phospho-Akt, Ship2, and PTEN levels in 3T3-L6 cells infected with pSuper and pReSI vectors to generate single and double-knockdown cells were measured by Western blotting using anti-pS473, anti-Ship2, and anti-PTEN antibodies, as indicated. (B) Phospho-Akt levels from three independent experiments were quantified, normalized against tubulin data, and plotted relative to the PTEN knockdown values. pS473-Akt, Akt with phosphorylated Ser473. (C to F) The levels of PI-3,4-P2 (C and E) and PI-3,4,5-P3 (D and F) in [3H]inositol-labeled 3T3-L6 cells were measured after HPLC separation of the deacylated lipids and normalized against phosphatidylinositol (PI). The cells in panels C and D were labeled for 48 h in medium containing 0.1% FCS. Results shown represent the averages plus standard errors (error bars) of three separate experiments. (E and F) pS-C1/pReSI-C1-infected cells (C1 C1) or pS-PTEN/pReSI-Ship2 cells (PTEN Ship2) were labeled for 24 h in medium containing either 10% FCS or 1% FCS.
Together, these results show that Ship2 is an active phosphatase that dephosphorylates PI-3,4,5-P3 in 3T3-L6 cells. In contrast, PTEN seems to have little role in regulating the bulk of PI-3,4,5-P3 in these cells. Unexpectedly, Ship2 knockdown with pS-Ship2, but not with pS-Ship2 II, also consistently increased the levels of PI-3,4-P2 to similar or higher levels than the levels obtained after PTEN knockdown. Given that Ship2 is a 5′-specific PI-3,4,5-P3 phosphatase, we expected that knocking down Ship2 would cause a decrease, rather than an increase, in the levels of PI-3,4-P2. However, it is possible that the excessively high levels of PI-3,4,5-P3 in pS-Ship2-infected cells caused an increase in the activity of Skip, another PI-3,4,5-P3 phosphatase, that like Ship2 is able to generate PI-3,4-P2. Consistent with this explanation, we found that increased levels of PI-3,4-P2 in Ship2 knockdown cells were detected only after 24 to 48 h [3H]inositol labeling experiments, but not after short 4-h 32P labeling experiments (compare Fig. 4 with Fig. 5A and B). Alternatively, Ship2 may collaborate with a PI-3,4-P2 phosphatase to coordinate the degradation of both lipids. Therefore, it is likely that the increases in PI-3,4-P2 observed in Ship2 knockdown cells are not a direct consequence of the loss of Ship2 activity.
The effect of Ship2 or PTEN knockdown on basal or insulin-induced phospho-Akt levels was examined (Fig. 3 and Fig. 5C and D). Like many other cells, Akt phosphorylation in serum-starved 3T3-L6 cells is low and is rapidly induced after insulin stimulation (10-fold induction). In Ship2 knockdown cells, despite the robust increase in PI-3,4,5-P3 levels, basal phospho-Akt levels were low and similar to the levels in control cells. After insulin stimulation, phospho-Akt levels increased in Ship2 knockdown cells but to levels slightly (but consistently) lower than the levels in insulin-stimulated control cells. PTEN knockdown, on the other hand, increased basal (3- to 10-fold) and insulin-stimulated (1.5- to 2.25-fold) phospho-Akt levels (see also Fig. 7 and 8 [discussed below]). Similar results were obtained regardless of whether anti-pS473 (Fig. 5C) or anti-pT308 (Fig. 5D) antibodies were used.
FIG. 7.
Reexpression of wild-type and lipid phosphatase-dead PTEN in PTEN knockdown cells. Expression of PTEN (A) or Akt phosphorylation (B and C) was measured by Western blotting using anti-PTEN antibody or anti-pS473 and lysates from cells infected with pS-C1 or pS-PTEN reinfected with the pBabe empty vector or pBabe expressing wild-type, RNAi-insensitive PTEN (pBabe-PTEN) or lipid phosphatase-dead PTEN (pBabe-PTEN-LD). The cells in panel B were serum starved for 24 h in medium supplemented with 0.1% FCS, and the cells in panel C were kept in medium supplemented with 10% FCS. Data were normalized against the data for tubulin and are averages ± standard errors (error bars) for three experiments.
The specificity of the effect of Ship2 knockdown on PI-3,4,5-P3 levels was assessed by reexpression of Ship2 protein into Ship2 knockdown cells, as described in Materials and Methods. Exogenous Ship2 expression was twofold higher than endogenous Ship2, as seen from Fig. 6A (compare the two leftmost lanes). Figure 6B and C show that reexpression of wild-type Ship2 in Ship2 knockdown cells decreased PI-3,4,5-P3 levels by 60% and increased PI-3,4-P2 levels by 20%, which is consistent with Ship2 being a 5′ PI-3,4,5-P3 phosphatase. Wild-type Ship2 expression had no effect on PI-3,4,5-P3 levels in control cells expressing endogenous Ship2. Expression of catalytic-dead Ship2, on the other hand, increased PI-3,4,5-P3 levels in control cells, but not in knockdown cells, consistent with the finding that this mutant can function as a dominant-negative mutant by competing with endogenous Ship2 (33). Expression of wild-type Ship2 did not affect the levels of phospho-Akt in 1% or 10% FCS (not shown). Together, these results confirm that Ship2 is an active PI-3,4,5-P3-specific phosphatase in 3T3-L6 cells.
FIG. 6.
Reexpression of wild-type and catalytic-dead Ship2 in Ship2 knockdown cells. (A) Expression of Ship2 was measured by Western blotting using anti-Ship2 antibody after 3T3-L6 cells infected with pS-C1 or pS-Ship2 were reinfected with empty pBabe virus (−) or pBabe virus carrying the sequence for RNAi-insensitive wild-type (WT) or catalytic-dead (CD) Ship2, as indicated. (B and C) PI-3,4,5-P3 (B) and PI-3,4-P2 (C) levels in 3T3-L6 cells expressing RNAi-insensitive wild-type Ship2 (pBabe-Ship2), catalytic-dead Ship2 (pBabe-Ship2-CD), or empty vector (pBabe) were measured by labeling the cells for 48 h with [3H]inositol in medium containing 0.1% FCS. Phosphoinositide levels were plotted relative to the levels in the control cells (cells infected with empty pBabe virus). The results shown are the averages ± standard errors (error bars) obtained from two independent experiments.
The specificity of the effect of PTEN knockdown on phospho-Akt levels was assessed by reexpression of wild-type PTEN or a lipid phosphatase-dead mutant of PTEN, which retains the ability to dephosphorylate peptide substrates (21). The levels of exogenous PTEN expression in 3T3-L6 cells were low and never reached the levels of endogenous PTEN, despite several attempts (Fig. 7A). Nevertheless, reexpression of wild-type PTEN, but not catalytic-dead PTEN (not shown) or lipid-dead PTEN, decreased the basal levels of phosphorylated Akt in PTEN knockdown cells growing in medium containing 10% FCS (Fig. 7C) or 0.1% FCS (Fig. 7B). These results confirmed that lipid phosphatase activity is important for PTEN′s ability to regulate Akt phosphorylation in 3T3-L6 cells, despite the fact that it did not significantly affect the bulk of PI3K lipids in these cells.
Together, these results clearly show that in 3T3-L6 cells the bulk of PI-3,4-P2 and/or PI-3,4,5-P3 cannot stimulate Akt phosphorylation, confirming the results shown in Fig. 2. Most importantly, the data show that PTEN knockdown, but not Ship2 knockdown, induces Akt phosphorylation. Since Akt phosphorylation was shown to be regulated by newly synthesized PI3K lipids, we conclude that PTEN dephosphorylates PI-3,4-P2 and PI-3,4,5-P3 at the sites where they are being synthesized, while Ship2 dephosphorylates the bulk of PI-3,4,5-P3 that turns over slowly and is not involved in Akt activation.
Next, we investigated whether Ship2 can cooperate with PTEN to regulate phosphoinositides and Akt phosphorylation. Double knockdowns of Ship2 and PTEN were generated as described in Materials and Methods. Figure 8A shows the expression of Ship2 and PTEN in these cells. Figure 8A and B show that Ship2 knockdown can further increase Akt phosphorylation (more than twofold) in serum-starved 3T3-L6 cells lacking PTEN expression, but not in cells where PTEN is expressed. Figure 8C and D show the levels of [3H]inositol-labeled PI-3,4-P2 and PI-3,4,5-P3 in serum-starved 3T3-L6. Ship2 and PTEN double-knockdown cells had higher levels of both PI-3,4-P2 and PI-3,4,5-P3 than single-knockdown cells did. In fact, knockdown of Ship2 and PTEN synergized to increase PI-3,4,5-P3 and PI-3,4-P2 and also to increase phospho-Akt levels. Increases in phospho-Akt levels were accompanied by comparable increases in the phosphorylation of the Akt substrates FOXO1 (FKHR), FOXO4 (AFX), and glycogen synthase kinase 3β (data not shown), confirming that measurements of Akt phosphorylation reflected the state of Akt activation in these cells. The finding that Ship2 knockdown regulates Akt when PTEN is absent suggests that Ship2 also dephosphorylates newly synthesized PI-3,4,5-P3 in 3T3-L6 cells. However, PTEN seems to compensate for the loss of Ship2 at the sites of Akt activation.
In non-serum-starved cells, the increases in PI-3,4-P2 and PI-3,4,5-P3 levels caused by Ship2 and PTEN double knockdown were comparable to the increases caused by serum withdrawal of control cells (Fig. 8E and F). These results indicate that in 10% FCS, PI-3,4-P2 and PI-3,4,5-P3 levels are kept low due to the action of these phosphatases. However, serum withdrawal further increased the levels of PI3K lipids in double-knockdown cells, showing that inactivation of PTEN and Ship2 can only partially explain the increase in lipid stability caused by serum withdrawal.
Steady-state levels of PI-3,4,5-P3 and PI-3,4-P2 cannot stimulate Akt phosphorylation: possible models.
The observations that serum-starved 3T3-L6 cells have low phospho-Akt levels, despite the high levels of PI-3,4-P2 and PI-3,4,5-P3, and that Ship2 knockdown dramatically increased PI-3,4,5-P3 levels without affecting phospho-Akt, led us to seek potential explanations for this discrepancy. First, we investigated whether PDK2 activity is limiting Akt phosphorylation in serum-starved 3T3-L6 cells, when PI-3,4,5-P3 and PI-3,4-P2 levels are high. Although phosphorylation at S473 is a good indicator of PDK2 activity, it is unclear how phospho-T308 can affect phospho-S473 and vice versa. Thus, we used a constitutively active form of Akt (myr-Akt) to measure phosphorylation at S473, independently of T308. We found that phosphorylation of myr-Akt at S473 was constitutively elevated, as previously observed for other cell lines (36), and was wortmannin insensitive, validating the use of this reagent as a tool for measuring PDK2 activity, independently of T308 phosphorylation. Rapamycin treatment decreased myr-Akt phosphorylation at S473 by 80%, indicating that TORC2 activity accounts for most of the PDK2 activity in these cells. Figure 9A also shows that the levels of PDK2 activity in 3T3-L6 cells with Ship2 and PTEN knockdown were similar to those in control 3T3-L6 cells. The phosphorylation of myr-Akt was high, regardless of whether the cells were cultured in 10% FCS or 1% FCS or treated with insulin (not shown). This result strongly suggests that PDK2 activity is not limiting Akt phosphorylation in 3T3-L6 cells.
In order to investigate whether the low levels of phospho-Akt in Ship2 knockdown cells could be caused by increased dephosphorylation of Akt, we followed the rate of phospho-Akt dephosphorylation after wortmannin treatment. Figure 9B shows that the rate of Akt dephosphorylation was the same, regardless of whether PTEN or Ship2 had been knocked down.
Next, we investigated the possibility that in 3T3-L6 cells the bulk of PI-3,4-P2 and PI-3,4,5-P3 and Akt localize into distinct subcellular compartments. For this purpose, we expressed exogenous GFP-PH domains from Akt (Fig. 10b) or from Btk (Fig. 10a and c to g) in 3T3-L6 cells (Fig. 10a, b, and h to l), in Ship2 knockdown cells (Fig. 10c, d, e, and f) or in Ship2 and PTEN double-knockdown cells (Fig. 10g) and immunostained them for total endogenous Akt, phospho-Akt, PTEN, or m-cadherin. In serum-starved 3T3-L6 cells, the PH domains from Btk and Akt were mostly cytoplasmic, but subfractions clearly localized to specific areas of the plasma membrane (Fig. 10a and b). In serum-starved Ship2 knockdown cells, where steady-state levels of PI-3,4,5-P3 are elevated, the GFP-Btk PH domain and endogenous Akt colocalized into spread areas of the plasma membrane or into multiple membrane speckles (Fig. 10c, d, e, and f). We were able to distinguish three distinct patterns of plasma membrane staining by PH domains: (i) the tip of cell extensions, which are rich in actin and found in elongating cells (Fig. 10a, b, d, e, and f); (ii) multiple small membrane speckles, which are common in Ship2 knockdown cells (Fig. 10c and g); (iii) membrane ruffles, which are present in serum-starved cells (Fig. 10d, e, and f) and are abundant in insulin-treated cells (not shown). Treatment with wortmannin for short periods (which has been shown in Fig. 2 to inhibit de novo synthesis of PI3K lipids and Akt phosphorylation, but not the steady-state levels of these lipids) decreased the size and abundance of the membrane ruffles. Nevertheless, in wortmannin-treated cells, the PH domains and endogenous Akt clearly colocalized at the cell membrane (Fig. 10f and g). Interestingly, when cells were costained for m-cadherin, we found that the GFP-PH domain of Btk was excluded from regions of cell-cell contact (Fig. 10d).
FIG. 10.
Subcellular localization of GFP-PH domain, Akt, and PTEN. (a to g) Confocal microscopy of 3T3-L6 cells transiently expressing the GFP-PH domain of Akt (b) or Btk (a and c to g). After transfection, cells were serum starved for 24 h and left untreated (a to e) or treated with wortmannin for 30 min (f to g). The cells in panels d were also stained with antibody against m-cadherin, and the cells in panels e to g were stained with antibody against total Akt. (h to l) Confocal microscopy showing endogenous Akt and PTEN localization in 3T3-L6 cells, serum starved and left untreated (h and j) or treated with insulin for 10 min (i and k) or wortmannin for 30 min (l) and stained with antibodies against total Akt and m-cadherin (h) or with phospho-Akt and fluorescent phalloidin (i), total Akt and PTEN (j and l), or phospho-Akt and PTEN (k). In panels d to l, the leftmost panels (labeled with the number 1) show the red channel, the middle panels (number 2) show the green channel, and the rightmost panels (number 3) show both channels.
Immunostaining of serum-starved 3T3-L6 cells with antibodies against Akt showed that this protein was mostly cytoplasmic but that a subfraction also concentrated at the edge of cell extensions and membrane ruffles and that it was excluded from areas of cell-cell contact (Fig. 10h). Anti-phospho-Akt antibody stained the tip of membrane extensions and membrane ruffles in insulin-stimulated cells or in PTEN knockdown cells only (Fig. 10i and k and not shown). Anti-PTEN antibody also stained the tip of cell extensions (Fig. 10j to l), where Akt and phospho-Akt were also found. In PTEN knockdown cells, PTEN staining at the cell extensions and cytoplasm was significantly decreased (not shown). Together, these results show that in 3T3-L6 cells, PI-3,4-P2 and PI-3,4,5-P3 localized to the plasma membrane, where Akt was recruited. In addition, the data also suggest that Akt phosphorylation occurs at specific sites of the membrane, such as membrane extensions, to which PTEN can be recruited.
Effects of PTEN and Ship2 knockdown on myoblast proliferation, survival, and differentiation.
PI3K signaling has been implicated in several steps of the myogenic process. In order to investigate how the two pools of PI3K lipids affect some of these steps, we measured the effect of Ship2 or PTEN knockdown on proliferation, survival, MHC expression, and fusion. Figure 11A shows a proliferation curve for 3T3-L6 cells infected with pS-C1, pS-Ship2, or pS-PTEN. PTEN knockdown cells can reach a higher saturation density than control cells (twofold increase), but they have similar proliferation rates. In 3T3-L6 cells where Ship2 expression has been suppressed, proliferation rate and saturation density were not significantly affected.
FIG. 11.
Effects of Ship2 and PTEN knockdowns on cells. (A) Proliferation rates of 3T3-L6 myoblasts infected with pS-C1-, pS-Ship2-, or pS-PTEN-derived retrovirus were measured in 10% FCS by counting the number of viable cells for a period of 8 days. (B) Percentages of apoptosis in 3T3-L6 myoblasts infected with pS-C1, pS-Ship2, or pS-PTEN retrovirus and kept for 48 h in 10% FCS or 0.1% FCS were measured by Western blotting using antibody against cleaved caspase 3. The results shown are the average ± standard errors (error bars) for three experiments. (C) Early differentiation rate was determined by measuring the MHC expression in myoblasts infected with pS-C1, pS-Ship2, or pS-PTEN retroviruses after 24 h in 0.1% FCS by Western blotting. The results shown are the averages of two experiments. (D) Percent differentiation of 3T3-L6 myoblasts infected with pS-C1, pS-Ship2, or pS-PTEN retroviruses was measured by counting the number of colonies that present more than 20 nuclei per myotube after 4 days in medium containing 2% FCS. One hundred colonies were counted for each cell line.
During the process of myogenesis, a large fraction of the myoblasts undergoes apoptosis (34). Confluent Ship2 knockdown cells seem to have increased rates of cell death compared to control cells, as judged by phase-contrast microscopy, especially after serum withdrawal. In the experiment shown in Fig. 11B, we used an antibody against cleaved caspase 3 to measure apoptosis in 3T3-L6 control cells and in Ship2 or PTEN knockdown cells. Confirming our initial observations, we found that Ship2 knockdown with pS-Ship2 caused a dramatic increase in cleaved caspase 3, indicating a correlation between dramatically elevated levels of PI-3,4,5-P3 and apoptosis.
The effect of Ship2 or PTEN knockdown on myoblast differentiation was determined by measuring MHC levels when the myocytes begin to fuse. We also measured the rate of differentiation by counting the number of colonies that show high levels of fusion (more than 20 nuclei per myotube). Figure 11C shows that PTEN knockdown increased MHC expression in the early stages of fusion, while Ship2 knockdown had no effect. At late stages of myogenesis, there were no significant differences in MHC expression between control 3T3-L6, Ship2 knockdown, or PTEN knockdown (not shown) cells. Figure 11D shows that PTEN knockdown doubled the percentage of colonies in the culture that differentiated into multinucleated myotubes. These results show that PTEN knockdown, but not Ship2 knockdown, can enhance the differentiation process, indicating that Akt activation promotes myoblast differentiation, as suggested by other reports (12). These results also show that the increases in the steady-state levels of PI-3,4-P2 and PI-3,4,5-P3 by Ship2 knockdown impaired differentiation and enhanced apoptosis.
DISCUSSION
The data presented here describe, for the first time, a cell line in which PI-3,4-P2 and PI-3,4,5-P3 accumulate in response to serum withdrawal, as a result of de novo synthesis and increased stability of these lipids. Matrix-dependent and cell-cell contact-dependent generation of PI3K lipids has been previously implicated in the accumulation of PI-3,4,5-P3 in serum-starved Cos cells and epithelial cells (17, 35). Therefore, it is possible that, in confluent myoblasts, synthesis of PI-3,4,5-P3 after serum withdrawal is due to activation of class I PI3Ks by extracellular matrix or m-cadherin. Alternatively, PI3K activation in serum-starved cells may result from increased secretion of endogenous growth factors (such as insulin-like growth factor 1, for example) and autocrine stimulation. Our data indicate that in 3T3-L6 cells, PI-3,4-P2 is generated by dephosphorylation of PI-3,4,5-P3 by Ship2. Although most of the PI-3,4-P2 and PI-3,4,5-P3 present in these cells was wortmannin sensitive, we cannot rule out the possibility that class II PI3K also contributes to PI-3,4-P2 generation during differentiation.
PI3K activation alone (Table 1) cannot account for the 10-fold increase in PI-3,4-P2 and PI-3,4,5-P3 after serum withdrawal (Fig. 1B). The difference in lipid stability in serum-starved cells versus non-serum-starved cells (Fig. 2A) is the most likely cause of the higher steady-state levels of PI-3,4-P2 and PI-3,4,5-P3 in differentiating 3T3-L6 cells. The mechanism for this increased stability is not completely understood, but we suspect that inactivation of lipid phosphatases may play a role. In 3T3-L6 cells, PTEN knockdown caused moderate or no increase in PI3K lipids, indicating that its activity may be restricted to certain subcellular compartments. PTEN activity was previously shown to be regulated by membrane recruitment (through binding to PDZ-containing proteins) or by phosphorylation and/or oxidation (19). We speculate that recruitment of PTEN to specific locations of the plasma membrane where PI-3,4,5-P3 is being synthesized can lead to local activation of this enzyme as a result of a reducing microenvironment.
Unlike PTEN, Ship2 seems to be very active in 3T3-L6 cells. PI-3,4-P2 is the most abundant PI3K lipid in these cells, and Ship2 knockdown dramatically increased the level of PI-3,4,5-P3. Surprisingly, Ship2 knockdown alone did not increase Akt phosphorylation, but PTEN and Ship2 double knockdown cooperated to increase PI-3,4,5-P3 and to induce Akt phosphorylation. The effect of Ship2 knockdown or knockout on phospho-Akt has been tested in various cell lines. Ship2 was shown to affect growth factor-stimulated Akt phosphorylation in mouse embryonic fibroblasts, but not in 3T3-L1 cells (2, 30). Our results are consistent with the results from Sharrad and Maitland (29), where Ship2 knockdown was shown to increase Akt phosphorylation only in cells where PTEN is absent.
Wortmannin treatment of 3T3-L6 cells together with phosphatase knockdown experiments allowed us to distinguish two separate pools of PI3K lipids in these cells: a pool of newly synthesized lipids that is mainly dephosphorylated by PTEN and a pool of stable lipids that is dephosphorylated by Ship2. While newly synthesized PI3K lipids were clearly necessary for Akt phosphorylation, the bulk of PI-3,4-P2 and PI-3,4,5-P3 was unable to stimulate Akt phosphorylation. These results are in partial agreement with the results of Scheid and collaborators, who showed that PI-3,4,5-P3 was not sufficient for Akt phosphorylation at S473 in mast cells and PI-3,4-P2 was required for full activation of Akt (27). However, in 3T3-L6 cells, we find that PI-3,4-P2 was not sufficient either, since Ship2 knockdown increased the steady-state levels of PI-3,4-P2 to the same extent as PTEN knockdown, without increasing phospho-Akt. On the basis of our data, we propose a model where newly synthesized PI3K lipids can diffuse to areas of the membranes where PTEN is absent or inactive to generate the stable pool of lipids. It is possible that the nascent pool of lipids can effectively promote the encounter of PDK1 and Akt, while the stable, but diffuse pool, cannot. Another possibility is that serum withdrawal increases the expression of a PI-3,4-P2 and/or PI-3,4,5-P3-binding protein that protects these lipids from being dephosphorylated and prevents them from activating Akt. These findings may help us design novel strategies for regulating Akt activation by a specific stimulus by bringing lipid phosphatases closer to the site of lipid synthesis.
Increased phosphorylation of Akt in PTEN knockdown correlated with enhanced myoblast differentiation, suggesting that the pool of newly synthesized PI3K lipids play an important role in myoblast differentiation through Akt phosphorylation. Although we have been unable to detect elevated levels of PI-3,4,5-P3 in differentiating C2 myoblasts, we and others have observed that, as for 3T3-L6 myoblasts, Akt phosphorylation increases dramatically in C2 cells as they exit the cell cycle and start to differentiate (9; D. Sarkes et al., unpublished data). Therefore, it is likely that serum withdrawal-induced synthesis of PI3K lipids is a trait of differentiating myoblasts, but only in 3T3-L6 myoblasts, due to the stability of these lipids, was this phenomenon detectable.
The role of the stable pool of PI-3,4-P2 and PI-3,4,5-P3 in signaling and myoblast differentiation remains to be discovered. We observed that in serum-starved 3T3-L6 cells, phospho-Erk levels were high and were slowly inhibited by wortmannin treatment, as were the bulk levels of the PI3K lipids. Integrin-stimulated Raf-MAPK (mitogen-activated protein kinase)-Erk pathway activation was shown to be PI3K dependent (17). Therefore, it is possible that the stable pool of PI3K lipids can regulate the Raf-MAPK pathway, which has been previously shown to inhibit myoblast differentiation (5, 9) and may be involved in the maintenance of a nondifferentiated population of myoblasts in adult muscle. This possibility is currently being investigated in our lab.
Acknowledgments
We thank Sergio Grinstein, Alex Toker, Seth Field, and Scott Frank for DNA constructs; Jennifer Chen and Janice Dominov for antibodies; Sheila Thomas for retrovirus reagents; and Lew Cantley and Steen Hansen for insightful discussions.
This work was supported by grant NIDDK 63219 from the National Institute of Health.
Footnotes
Published ahead of print on 24 September 2007.
REFERENCES
- 1.Alessi, D. R., S. R. James, C. P. Downes, A. B. Holmes, P. R. Gaffney, C. B. Reese, and P. Cohen. 1997. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr. Biol. 7:261-269. [DOI] [PubMed] [Google Scholar]
- 2.Blero, D., J. Zhang, X. Pesesse, B. Payrastre, J. E. Dumont, S. Shurmans, and C. Erneux. 2005. Phosphatidylinositol 3,4,5-trisphosphate modulation in Ship2 deficient mouse embryonic fibroblast. FEBS J. 272:2512-2522. [DOI] [PubMed] [Google Scholar]
- 2a.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 3.Cantley, L. C. 2002. The phosphoinositide 3-kinase pathway. Science 296:1655-1657. [DOI] [PubMed] [Google Scholar]
- 4.Clement, S., U. Krause, F. Desmedt, J. F. Tanti, J. Behrends, X. Pesesse, T. Sasaki, J. Penninger, M. Doherty, W. Malaisse, J. E. Dumont, Y. Le Marchand-Brustel, C. Erneux, L. Hue, and S. Schurmans. 2001. The lipid phosphatase SHIP2 controls insulin sensitivity. Nature 409:92-97. [DOI] [PubMed] [Google Scholar]
- 5.Coolican, S. A., D. S. Samuel, D. Z. Ewton, F. J. McWade, and J. R. Florini. 1997. The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J. Biol. Chem. 272:6653-6662. [DOI] [PubMed] [Google Scholar]
- 6.Downes, C. P., N. R. Leslie, I. H. Batty, and J. van der Kaay. 2007. Metabolic switching of PI3K-dependent lipid signals. Biochem. Soc. Trans. 35:188-192. [DOI] [PubMed] [Google Scholar]
- 7.Frank, S. R., M. R. Adelstein, and S. H. Hansen. 2006. GIT2 represses Crk- and Rac1-regulated cell spreading and Cdc42-mediated focal adhesion turnover. EMBO J. 25:1848-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujio, Y., K. Guo, T. Mano, Y. Mitsuuchi, J. R. Testa, and K. Walsh. 1999. Cell cycle withdrawal promotes myogenic induction of Akt, a positive modulator of myocyte survival. Mol. Cell. Biol. 19:5073-5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez, I., G. Tripathi, E. J. Carter, L. J. Cobb, D. A. Salih, F. A. Lovett, C. Holding, and J. M. Pell. 2004. Akt2, a novel functional link between p38 mitogen-activated protein kinase and phosphatidylinositol 3-kinase pathways in myogenesis. Mol. Cell. Biol. 24:3607-3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hresko, R. C., and M. Mueckler. 2005. mTOR.RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J. Biol. Chem. 280:40406-40416. [DOI] [PubMed] [Google Scholar]
- 11.Ijuin, T., and T. Takenawa. 2003. SKIP negatively regulates insulin-induced GLUT4 translocation and membrane ruffle formation. Mol. Cell. Biol. 23:1209-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang, B. H., M. Aoki, J. Z. Zheng, J. Li, and P. K. Vogt. 1999. Myogenic signaling of phosphatidylinositol 3-kinase requires the serine-threonine kinase Akt/protein kinase B. Proc. Natl. Acad. Sci. USA 96:2077-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang, B. H., J. Z. Zheng, and P. K. Vogt. 1998. An essential role of phosphatidylinositol 3-kinase in myogenic differentiation. Proc. Natl. Acad. Sci. USA 95:14179-14183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang, Z. Y., Q. L. Zhou, K. A. Coleman, M. Chouinard, Q. Boese, and M. P. Czech. 2003. Insulin signaling through Akt/protein kinase B analyzed by small interfering RNA-mediated gene silencing. Proc. Natl. Acad. Sci. USA. 100:7569-7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaliman, P., J. Canicio, P. R. Shepherd, C. A. Beeton, X. Testar, M. Palacin, and A. Zorzano. 1998. Insulin-like growth factors require phosphatidylinositol 3-kinase to signal myogenesis: dominant negative p85 expression blocks differentiation of L6E9 muscle cells. Mol. Endocrinol. 12:66-77. [DOI] [PubMed] [Google Scholar]
- 16.Kaliman, P., F. Vinals, X. Testar, M. Palacin, and A. Zorzano. 1996. Phosphatidylinositol 3-kinase inhibitors block differentiation of skeletal muscle cells. J. Biol. Chem. 271:19146-19151. [DOI] [PubMed] [Google Scholar]
- 17.King, W. G., M. D. Mattaliano, T. O. Chan, P. N. Tsichlis, and J. S. Brugge. 1997. Phosphatidylinositol 3-kinase is required for integrin-stimulated AKT and Raf-1/mitogen-activated protein kinase pathway activation. Mol. Cell. Biol. 17:4406-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leslie, N. R., and C. P. Downes. 2002. PTEN: the down side of PI 3-kinase signalling. Cell. Signal. 14:285-295. [DOI] [PubMed] [Google Scholar]
- 19.Leslie, N. R., and C. P. Downes. 2004. PTEN function: how normal cells control it and tumour cells lose it. Biochem. J. 382:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin, D. E., and M. N. Hall. 2005. The expanding TOR signaling network. Curr. Opin. Cell Biol. 17:158-166. [DOI] [PubMed] [Google Scholar]
- 21.Myers, M. P., I. Pass, I. H. Batty, J. Van der Kaay, J. P. Stolarov, B. A. Hemmings, M. H. Wigler, C. P. Downes, and N. K. Tonks. 1998. The lipid phosphatase activity of PTEN is critical for its tumor suppressor function. Proc. Natl. Acad. Sci. USA 95:13513-13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pesesse, X., C. Moreau, A. L. Drayer, R. Woscholski, P. Parker, and C. Erneux. 1998. The SH2 domain containing inositol 5-phosphatase SHIP2 displays phosphatidylinositol 3,4,5-trisphosphate and inositol 1,3,4,5-tetrakisphosphate 5-phosphatase activity. FEBS Lett. 437:301-303. [DOI] [PubMed] [Google Scholar]
- 23.Polak, P., and M. N. Hall. 2006. mTORC2 caught in a SINful Akt. Dev. Cell 11:433-434. [DOI] [PubMed] [Google Scholar]
- 24.Rameh, L. E., and L. C. Cantley. 1999. The role of phosphoinositide 3-kinase lipid products in cell function. J. Biol. Chem. 274:8347-8350. [DOI] [PubMed] [Google Scholar]
- 25.Sarbassov, D. D., D. A. Guertin, S. M. Ali, and D. M. Sabatini. 2005. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307:1098-1101. [DOI] [PubMed] [Google Scholar]
- 26.Sasaoka, T., T. Wada, K. Fukui, S. Murakami, H. Ishihara, R. Suzuki, K. Tobe, T. Kadowaki, and M. Kobayashi. 2004. SH2-containing inositol phosphatase 2 predominantly regulates Akt2, and not Akt1, phosphorylation at the plasma membrane in response to insulin in 3T3-L1 adipocytes. J. Biol. Chem. 279:14835-14843. [DOI] [PubMed] [Google Scholar]
- 27.Scheid, M. P., M. Huber, J. E. Damen, M. Hughes, V. Kang, P. Neilsen, G. D. Prestwich, G. Krystal, and V. Duronio. 2002. Phosphatidylinositol (3,4,5)P3 is essential but not sufficient for protein kinase B (PKB) activation: phosphatidylinositol (3,4)P2 is required for PKB phosphorylation at Ser-473: studies using cells from SH2-containing inositol-5-phosphatase knockout mice. J. Biol. Chem. 277:9027-9035. [DOI] [PubMed] [Google Scholar]
- 28.Serunian, L. A., K. R. Auger, and L. C. Cantley. 1991. Identification and quantification of polyphosphoinositides produced in response to platelet-derived growth factor stimulation. Methods Enzymol. 198:78-87. [DOI] [PubMed] [Google Scholar]
- 29.Sharrard, R. M., and N. J. Maitland. 2007. Regulation of protein kinase B activity by PTEN and SHIP2 in human prostate-derived cell lines. Cell. Signal. 19:129-138. [DOI] [PubMed] [Google Scholar]
- 30.Tang, X., A. M. Powelka, N. A. Soriano, M. P. Czech, and A. Guilherme. 2005. PTEN, but not SHIP2, suppresses insulin signaling through the phosphatidylinositol 3-kinase/Akt pathway in 3T3-L1 adipocytes. J. Biol. Chem. 280:22523-22529. [DOI] [PubMed] [Google Scholar]
- 31.Toker, A. 2000. Protein kinases as mediators of phosphoinositide 3-kinase signaling. Mol. Pharmacol. 57:652-658. [PubMed] [Google Scholar]
- 32.Tureckova, J., E. M. Wilson, J. L. Cappalonga, and P. Rotwein. 2001. Insulin-like growth factor-mediated muscle differentiation: collaboration between phosphatidylinositol 3-kinase-Akt-signaling pathways and myogenin. J. Biol. Chem. 276:39264-39270. [DOI] [PubMed] [Google Scholar]
- 33.Wada, T., T. Sasaoka, M. Funaki, H. Hori, S. Murakami, M. Ishiki, T. Haruta, T. Asano, W. Ogawa, H. Ishihara, and M. Kobayashi. 2001. Overexpression of SH2-containing inositol phosphatase 2 results in negative regulation of insulin-induced metabolic actions in 3T3-L1 adipocytes via its 5′-phosphatase catalytic activity. Mol. Cell. Biol. 21:1633-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang, J., and K. Walsh. 1996. Resistance to apoptosis conferred by Cdk inhibitors during myocyte differentiation. Science 273:359-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watton, S. J., and J. Downward. 1999. Akt/PKB localisation and 3′ phosphoinositide generation at sites of epithelial cell-matrix and cell-cell interaction. Curr. Biol. 9:433-436. [DOI] [PubMed] [Google Scholar]
- 36.Yoeli-Lerner, M., G. K. Yiu, I. Rabinovitz, P. Erhardt, S. Jauliac, and A. Toker. 2005. Akt blocks breast cancer cell motility and invasion through the transcription factor NFAT. Mol. Cell 20:539-550. [DOI] [PubMed] [Google Scholar]