Abstract
The inflammation that occurs during atherosclerosis is characterized by the release of large amounts of group IIA secretory phospholipase A2 (sPLA2-IIA). This study was designed to define the function of the three peroxisome proliferator-activated receptors (PPARs) on sPLA2 expression in vascular smooth muscle cells (VSMCs). We found that PPAR ligands decreased sPLA2-IIA activity and inhibited mRNA accumulation under inflammatory conditions. Furthermore, interleukin-1β-induced sPLA2-IIA promoter activity was inhibited by the three PPAR ligands and in a similar way when cells were cotransfected with PPARα, PPARβ, or PPARγ, plus retinoid X receptor α (RXRα). Our study revealed that the regulation of sPLA2-IIA gene transcription by PPARα/RXR and PPARγ/RXR heterodimers requires an interaction with a PPAR response element (PPRE) of the sPLA2-IIA promoter. In contrast, PPARβ operates through a PPRE-independent mechanism. In addition, we demonstrated that VSMCs expressed the transcriptional repressor BCL-6. Overexpression of BCL-6 markedly reduced sPLA2-IIA promoter activity in VSMCs, while a dominant negative form of BCL-6 abrogated sPLA2 repression by PPARβ. The PPARβ agonist induced a BCL-6 binding to the sPLA2 promoter in VSMCs under inflammatory conditions. The knockdown of BCL-6 by short interfering RNA abolished the inhibitory effect of the PPARβ ligand on sPLA2 activity and prostaglandin E2 release. Thus, the inhibition of sPLA2-IIA activity by PPARβ agonists may provide a promising approach to impacting the initiation and progression of atherosclerosis.
The large family of phospholipase A2 (PLA2) enzymes hydrolyze ester bonds at the sn-2 position of glyceroacylphospholipids to produce lysophospholipids and nonesterified fatty acids such as arachidonic acid (C20:4 [n-6]), the precursor of various proinflammatory lipid mediators, such as eicosanoids (37). To date there are four major families of PLA2. Several observations argue for the implication of type IIA secretory PLA2 (sPLA2-IIA) in the pathogenesis of various inflammatory diseases, including cancer, septic shock, rheumatoid arthritis, and atherosclerosis (38). The plasma of patients with various inflammatory diseases, particularly atherosclerosis, contains high concentrations of sPLA2-IIA (18). The synthesis of sPLA2-IIA is stimulated by inflammatory cytokines, such as interleukin-1β (IL-1β), IL-6, and tumor necrosis factor alpha (TNF-α) (2, 20). Moreover, sPLA2-IIA is largely expressed in vascular smooth muscle cells (VSMCs) (18) in response to proinflammatory cytokines produced by infiltrated macrophages. Overproduction of human sPLA2-IIA in transgenic mice contributes to atherogenesis (19). This enzyme catalyzes the production of precursors of lipid mediators (mainly prostaglandin E2 [PGE2]), which in turn amplify the effect of cytokines on the dedifferentiation of VSMCs (11). The enzyme was reported to have other proatherogenic properties linked to its ability to hydrolyze phospholipid monolayers of high- and low-density lipoproteins (21). Previously, our laboratory demonstrated the interplay of various transcription factors (NF-κB, C/EBPβ, ETS, and liver X receptor) that bind on the proximal part of the sPLA2 promoter and, more recently, has provided evidence that sPLA2-IIA could stimulate its own production in VSMCs through positive feedback (2, 3, 22). Then a better understanding of the molecular mechanisms involved in the regulation of sPLA2-IIA gene expression, in an inflammatory context, may allow the development of new inhibitors.
The peroxisome proliferator-activated receptor (PPAR) family includes three members: PPARα, PPARβ, and PPARγ. These ligand-activated transcription factors, belonging to the nuclear receptor superfamily, form heterodimers with the retinoid X receptor (RXR) to regulate the expression of genes involved in lipid metabolism, glucose metabolism, and inflammation (13, 25, 34). The relevance of PPAR pathways to metabolic diseases is underscored by the use of fibrates (PPARα agonists) and thiazolidinediones (PPARγ agonists) to treat hyperlipidemia and hyperglycemia, respectively. Agonists of PPARα have positive effects on lipid metabolism both in animal models and in clinical practice. Indeed, agonists of PPARγ, the thiazolidinediones rosiglitazone and pioglitazone, improve insulin resistance in type 2 diabetes and pioglitazone improves the dyslipidemia associated with insulin resistance. In addition to these effects, both PPARα and PPARγ agonists have anti-inflammatory properties that can provide additional cardiovascular benefits (5). The role of PPARβ is less well understood. In keeping with its ubiquitous expression, PPARβ has been implicated in cellular proliferation and differentiation, lipid metabolism, and inflammation (4). Synthetic PPARβ agonists promote cholesterol uptake (29) and efflux (41) in macrophages. In muscle, the overexpression or activation of PPARβ by synthetic ligands induces lipid utilization (36, 42), while in the liver, PPARβ suppresses hepatic glucose output (25). For the heart, the protective role of PPARβ has been confirmed by in vitro studies showing that PPARβ agonists attenuate phenylephrine-induced cardiac hypertrophy (30). PPARβ has been suggested to control an inflammatory switch during atherogenesis (25). However, despite significant advancement in the search for the potential roles of PPARβ in atherogenic inflammation, responses remain obscure.
Because sPLA2-IIA is thought to exert crucial proinflammatory functions in VSMCs during atherosclerosis by hydrolyzing cell membrane phospholipids into free fatty acids, the major source of eicosanoids (mainly PGE2), we investigated the effects of different PPAR activators on the regulation of the sPLA2-IIA gene expression. We demonstrate here that PPARα, -γ, and -β significantly inhibited cytokine-stimulated sPLA2-IIA expression and secretion in VSMCs. While effects of PPARα and -γ are exerted through a PPAR response element (PPRE)-dependent mechanism, the anti-inflammatory effect of PPARβ required the proto-oncogene BCL-6 in a PPRE-independent manner.
MATERIALS AND METHODS
Isolation and culture of VSMCs from rat aortas.
VSMCs were isolated from the thoracic aortas of adult male Wistar rats as described previously (2). Cells were seeded on dishes coated with type I collagen from calf skin (Sigma) and cultured in Dulbecco's modified Eagle's medium supplemented with 10% (vol/vol) fetal calf serum (Gibco BRL, Cergy-Pontoise, France), 4 mM glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin. VSMCs were subcultured every 5 days, and experiments were performed on cells between passages 3 and 7. For the experiments, confluent cells were made quiescent by incubating them for 24 h in serum-free medium containing 0.2% fatty-acid-free bovine serum albumin before they were treated with the appropriate agents. The culture medium was then removed, and measurements of for sPLA2 activity were taken, and cells were lysed for total RNA, total proteins, or nuclear extracts preparation.
PLA2 activity.
sPLA2 activity was measured using the fluorescent substrate 1-hexadecanoyl-2-(1-pyrenyldecanoyl)-sn-glycero-3-phosphoglycerol (Interchim, France) as described previously (3). Total hydrolysis of the substrate obtained by 0.1 unit of PLA2 from bee venom (Sigma) was used as a reference to calculate the PLA2 activity in the samples. Spontaneous substrate hydrolysis was assayed in fresh culture medium and subtracted from each sample value.
RNA extraction and RT-PCR analysis.
Total RNA was extracted from rat VSMCs by using RNeasy kit columns (QIAGEN, Courtaboeuf, France) according to the supplier's instructions. RNA (1 μg) was reverse transcribed for 1 h at 37°C with 200 U of mouse mammary lentivirus-reverse transcriptase (RT) (Invitrogen), 100 μM random primers, and the buffer supplied by the manufacturer in a total volume of 20 μl. The reaction was terminated by heating to 95°C for 5 min. To ensure that subsequent amplification was not derived from contaminant genomic DNA, a control without mammary lentivirus-RT was included in parallel for each RNA sample. Reverse-transcribed mRNAs were amplified in a thermocycler (Hybaid Omnigene; Syngene, Ozyme, France) as described previously (3). The primer sequences used for the different tested genes are given in Table 1. PCR amplifications were performed, and PCR products were size separated by electrophoresis on 2% (wt/vol) agarose gel and visualized by ethidium bromide staining. PCR bands were quantified using the GeneGenius system (Syngene, Ozyme, France).
TABLE 1.
Primer sequences for the different tested genes
| Gene or probe | Primer sequence |
|---|---|
| Genes | |
| sPLA2-IIA | 5′GTG GCA GAG GAT CCC CCA AGG 3′ (forward) |
| sPLA2-IIA | 5′GCA ACT GGG CGT GTT CCC TCT GCA 3′ (reverse) |
| GAPDH | 5′CCA TGG AGA AGG CTG GGG 3′ (forward) |
| GAPDH | 5′CAA AGT TGT CAT GGA TGA CC 3′ (reverse) |
| EMSA probes | |
| AcoA-S-PPRE | 5′GGA AAC CAG GAC AAA GGT CAC GTT GCC 3′ (forward) |
| AcoA-S-PPRE | 5′GGC AAC GTG ACC TTT GTC CTG GTT TCC 3′ (reverse) |
| sPLA2-PPRE | 5′ACT AAG GTT GTC CTC TGA ACT CCA CAT 3′ (forward) |
| sPLA2-PPRE | 5′ATG TGG AGT TCA GAG GAC AAC CTT CTT AGT 3′ (reverse) |
| mPPRE | 5′ACT AAG GTC GTG TTC TGC GCT CCA CAT 3′ (forward) |
| mPPRE | 5′ATG TGG AGC GCA GAA CAC GAC CTT CTT AGT 3′ (reverse) |
| sPLA2-BCL-6 | 5′GTG CAT GCT CCT GGA AGT GGC TGC 3′ |
| sPLA2-BCL-6 | 5′GCA GCC ACT TCC AGG AGC ATG GAC 3′ |
| Consensus BCL-6 | 5′TTT AGT TTT TCT TCG AAG AGC TAG 3′ |
| Consensus BCL-6 | 5′CTA GCT CTT CGA AGA AAA ACT AAA 3′ |
Quantitative PCR.
Quantitative RT-PCR was performed with the Absolute QPCR SYBR green fluorescein mixture (ABgene, Epsom, United Kingdom). The total reaction mixture volume was 25 μl containing SYBR green PCR core reagents, 2.5 ng cDNA, and 300 nM of each specific primer. Amplification was performed on an iCycler (Bio-Rad Laboratories, Hercules, CA). Samples were denatured for 15 min at 95°C and amplified for 40 cycles as follows: denaturation for 15 s at 95°C, annealing for 30 s at the appropriate hybridization temperature, and elongation at 72°C for 30 s. Each real-time PCR run included cDNAs in triplicate in parallel with serial dilutions of a cDNA mix tested for each primer pair to generate a standard curve. The values were normalized to an internal control GAPDH (glyceraldehyde-3-phosphate dehydrogenase). This curve was then used to estimate the relative quantity of the relevant mRNA in each sample. The generation of specific PCR products was confirmed by melting curve analysis. The primers used to amplify rat sPLA2-IIA were 5′-GTGACTCATGACTGTTGTTAC-3′ (forward) and 5′-CAAAACATTCAGCGGCAGC-3′ (reverse) (hybridization temperature of 61°C). Those for rat cyclooxygenase 2 (COX-2) were 5′-TGTATGCTACCATCTGGCTTCGG-3′ (forward) and 5′-GTTTGGAACAGTCGCTCGTCATC-3′ (reverse). Finally, primers used to amplify rat GAPDH were 5′-CTCAATGACAACATTGTGAGC-3′(forward) and 5′-CTCTTGCTCTCAGTATCCTTGC-3′ (reverse).
Western blot analysis.
VSMCs were harvested and homogenized in a lysis buffer (50 mM Tris-HCl [pH 7.5], 1% Triton, 150 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol, 1 mM Na3VO4, 0.5 mM phenylmethylsulfonyl fluoride, 50 mM NaF, 5 mM PPi, 10% glycerol) supplemented with a cocktail of antiprotease (Complete; Roche). Homogenates were centrifuged at 13,000 × g for 10 min at 4°C. The resulting supernatants were stored at −20°C until used. Proteins (20 to 40 μg/lane) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10% gel) and electroblotted onto al 0.45-μm-pore-size polyvinylidene difluoride membrane (Immobilon-P; Millipore). After we determined the efficiency of protein transfer and well-to-well variability with Ponceau S (Sigma-Aldrich), the membrane was incubated overnight at 4°C with a PPARβ or BCL-6 rabbit polyclonal antibody (Santa Cruz Biotechnology, Inc.) at a dilution of 1:200 or 1:1,000, respectively, in 2% milk-Tris-buffered saline (TBS) with 0.1% Tween 20 (Sigma-Aldrich). The next day, the membrane was washed in TBS with 0.1% Tween before adding anti-rabbit immunoglobulin G (IgG)-horseradish peroxidase at a dilution of 1:1,000 in 5% milk-TBS with 0.1% Tween and then incubated for 1 h at room temperature. The detection of immune complex was performed using an enhanced chemiluminescence detection kit for Western blotting (Amersham) on BioMax MR Kodak film (Sigma-Aldrich).
Plasmid constructions and transfection.
The [−1153; +46]sPLA2-Luc construct was obtained by PCR amplification of −1153 to +46 bp of the sPLA2 promoter. The cloning of the rat sPLA2 promoter (−488 to +46 bp) into the pGL3-basic luciferase plasmid to create [−488; +46]sPLA2-Luc has been described previously (2). The mutant PPRE-sPLA2 construct (mPPRE-sPLA2) lacks the PPAR-binding element of the sPLA2 promoter, the mutant BCL-6-sPLA2 construct (mBCL-6-sPLA2) lacks the BCL-6-binding element, and the double-mutated BCL6-PPRE-sPLA2 construct (mBCL6-mPPRE-sPLA2) lacks both PPAR and BCL-6 binding sites. The sites were replaced with the BglII restriction site by using PCR-based, site-directed mutagenesis. VSMCs were seeded, 24 h before transfection, in 24-well plates at a concentration of 20,000 cells per plate, and at 70% confluence, cells were transfected using 1.5 ml of Lipofectamine Plus (Invitrogen), 0.4 μg of reporter DNA, and 0.1 μg of pCMV-β-galactosidase per well. For transactivation studies, 10 ng of either pcDNA3.1 PPARα, PPARβ, or PPARγ expression vectors and 10 ng of CMX-RXRα were added. The cells were refed with Dulbecco's modified Eagle's medium containing 0.2% bovine serum albumin 3 h after we added the DNA and incubated for 24 h. Then, indicated concentrations of PPARα agonist (WY14643), PPARβ agonist (L165041), or PPARγ agonist (GW1929) were added and incubation continued for a further 24 h. The plasmid construct containing PPRE-3-TK-LUC (luciferase gene under control of the herpes simplex virus thymidine kinase promoter and three PPRRs was used as a control of PPAR activation. Luciferase activity was measured using a luciferase reporter assay kit, with signal detection for 12 s by a luminometer (Berthold, Pforzheim, Germany), and normalized by dividing the relative light units by β-galactosidase activity. The degree of induction was calculated relative to the control.
EMSAs.
Electrophoretic mobility-shift assays (EMSAs) were performed as described previously (2). Sequences of the oligonucleotides used are shown in Table 1. Competition assays were performed using a 100-fold molar excess of an unlabeled oligonucleotide. Samples were electrophoresed on 0.5× Tris-borate-EDTA and a 6% polyacrylamide gel. After electrophoresis, the gel was dried at 80°C and autoradiographed overnight at room temperature.
ChIP.
Experiments were performed with a chromatin immunoprecipitation (ChIP) assay kit (Upstate), according to the manufacturer's procedures. Briefly, 5 × 106 cells were treated with 1% formaldehyde for 10 min at 37°C. Subsequent procedures were performed on ice in the presence of protease inhibitors. Cross-linked cells were harvested, washed with phosphate-buffered saline, and lysed in sodium dodecyl sulfate lysis buffer (1% sodium dodecyl sulfate, 10 mM EDTA, 50 mM Tris-HCl, pH 8.1) for 10 min at 4°C. Chromatin was sonicated with five 10-s pulses at 30% amplitude (Sonifier, Branson Ultrasonic Corp). After centrifugation (10 min, 4°C, 14,000 × g), the supernatant was diluted 10-fold with ChIP dilution buffer (0.01% sodium dodecyl sulfate, 1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris-HCl, pH 8.1, 167 mM NaCl). Diluted extracts were precleared in the presence of salmon sperm DNA-protein A-agarose beads (ChIP assay kit; Upstate). One-tenth of the diluted extract was kept for a direct quantitative PCR (input). The remaining extracts were incubated for 16 h at 4°C in the presence of 1 μg of specific antibodies per milliliter, followed by 1 h of incubation with salmon sperm DNA-protein A-agarose beads. Anti-BCL6 antibodies (N-3) were purchased from Santa Cruz. Following extensive washing, bound DNA fragments were eluted with a 30-min incubation in elution buffer (1% sodium dodecyl sulfate, 0.1 M NaHCO3). The DNA was recovered for 4 h at 65°C in elution buffer containing 200 mM NaCl and then incubated in the presence of proteinase K (20 μg/ml) for 1 h at 45°C. DNA was extracted in the presence of phenol-chloroform and chloroform-isoamyl alcohol and ethanol precipitated before being subjected to PCR.
siRNA transfection.
Small interfering RNA (siRNA) duplexes designed against rat BCL-6 were siRNA BCL-6_1 (5′-AUGCAACCUUAAUCUU-3′) and siRNA BCL-6_3 (5′-ACCAUACAAAUGUGACCGCUU-3′). Scrambled negative control 1 siRNA (15) was used as the control siRNA. siRNAs were transfected into cells by electroporation in an Amaxa electroporation device. One million cells were resuspended in 100 μl of Amaxa electroporation transfection solution, and 2.5 μl of siRNA (20 μM) was added. The D-33 program was used. Transfected cells were plated in two wells of a six-well plate, each containing 1 ml of complete cell culture medium. Twenty-four hours after siRNA treatment, the cells were harvested.
Statistical analysis.
Analysis of variance and paired or unpaired t test were performed for statistical analysis as appropriate. Probability values less than 0.05 were considered to be statistically significant. Results are expressed as means ± standard error of the means (SEM).
RESULTS
PPAR-selective synthetic ligands inhibit IL-1β-induced sPLA2-IIA activity and mRNA accumulation in rat VSMCs.
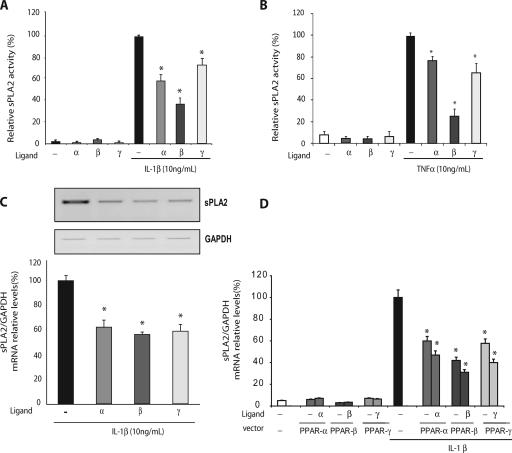
Experiments were conducted on primary cultures of VSMCs isolated from rat aortas. These cells are especially appropriate for studies of inflammatory processes, since they undergo a phenotypic change in response to proinflammatory cytokines, with an increase in the expression of adhesion proteins (VCAM-1 and MCP-1), extracellular metalloproteases, and acute-phase enzymes, such as secreted sPLA2-IIA and COX-2 (26). In the case of vascular inflammation, particularly during atherogenesis, VSMCs are activated by several proinflammatory cytokines, including IL-1β (23). Given their antiatherogenic and anti-inflammatory properties, PPAR agonists have been proposed as promising for a therapeutic approach to treat cardiovascular diseases (7). To explore the role of PPAR activation on IL-1β-induced sPLA2, cultured VSMCs were pretreated with PPAR agonists for 6 h prior to IL-1β treatment (24 h). We first tested the effects of WY146043, L165041, and GW1929, agonists of PPARα, PPARβ, and PPARγ, respectively, and then measured the enzyme activity of sPLA2 in the cell supernatant. The three PPAR agonists caused significant decreases in IL-1β-induced sPLA2 activity in the medium of VSMCs (Fig. 1A). In order to determine whether this effect was IL-1β specific, we conducted sPLA2 assays on TNF-α-induced VSMCs. In the same way, we pretreated the cells with the PPAR agonists for 6 h prior to TNF-α treatment (24 h) and then measured the enzyme activity of sPLA2 in the cell supernatant (Fig. 1B). As expected, the three PPAR agonists caused significant decreases in sPLA2 activity in the supernatant.
FIG. 1.
IL-1β-induced sPLA2 activity was repressed in response to PPAR ligands. VSMCs were preincubated for 6 h with a specific agonist of PPARα (WY14643; 100 μM), PPARβ (L165041; 50 μM), or PPARγ (GW1929; 1 μM) and then treated or not treated with either IL-1β (A) or TNF-α. (Β) Culture mediums were collected 24 h after IL-1β treatment, and the sPLA2 activity was measured spectrofluorimetrically. (C) Semiquantitative RT-PCR was performed on 1 μg of total RNA from each sample. Amplification was performed with sPLA2 primers and with GAPDH used as a standard gene, as shown in a representative blot. mRNA levels were quantified by the Gene Tools system. (D) VSMCs were transiently transfected with 50 ng of PPARα, PPARβ, or PPARγ expression vector and RXR vector in equimolar quantities. Twenty-four hours after transfection, cells were pretreated for 6 h with a specific agonist of PPARα (WY14643; 100 μM), PPARβ (L165041; 50 μM), or PPARγ (GW1929; 1 μM) and then treated or not treated with IL-1β (10 ng/ml). sPLA2-IIA transcription was measured by reverse transcription/real-time PCR using the expression of the GAPDH gene as an internal standard. Results are representative of three independent experiments. *, P < 0.05 (PPAR agonist-treated versus IL-1β-treated cells). Error bars indicate SEM.
We then performed semiquantitative RT-PCR analysis of total RNA from rat VSMCs to determine whether the inhibitory effect of the PPAR-selective agonists on cytokine-induced sPLA2 activity correlated with decreased sPLA2 mRNA levels. Treatment of VSMCs with WY14643, L165041, or GW1929 led to a significant decrease in sPLA2 mRNA content (Fig. 1C). These results confirmed that PPAR ligands are potent anti-inflammatory agents that suppress sPLA2-IIA production in cultured rat VSMCs. We obtained similar results with IL-1β induction prior to PPAR ligand treatments (data not shown). We transiently transfected VSMCs with pcDNA3.1 expression vectors encoding PPARα, PPARβ, or PPARγ, together with an RXR expression vector, as PPAR forms a heterodimer with RXR to activate the target genes. We then performed quantitative RT-PCR analysis of total RNA from rat VSMCs. As shown in Fig. 1D, the overexpression of PPARα, -β, or -γ led to a strong inhibition of the endogenous IL-1β-induced sPLA2 gene activity with or without the presence of specific ligands.
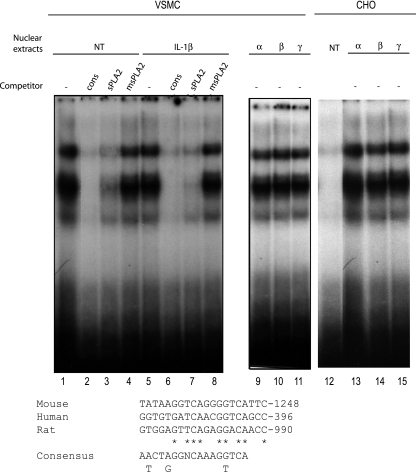
Activators of PPARα (WY14643, LY171883, and clofibrate) have been shown to enhance the transcription of the sPLA2-IIA gene in rat mesangial cells via the PPRE-1 site (bp −909 to −888) in the rat sPLA2-IIA promoter (32). We also identified a putative PPRE binding site in the human and mouse sPLA2-IIA gene (Fig. 2). The sequence alignment of the putative sPLA2-PPRE was shown to match the PPAR-binding sequence of the known PPAR target gene for acyl-CoA oxidase. So, we performed EMSA on nuclear extracts from VSMCS to examine the binding activity of the PPARs to the rat sPLA2-PPRE. When nuclear extracts from VSMCs or CHO cells were incubated with radiolabeled PPRE as a probe, gel retardation bands were observed (Fig. 2, lanes 1 and 5). The major retarded band was obviously removed by a nonlabeled competitor with the same sequence (sPLA2-PPRE) or the consensus acyl-CoA oxidase PPRE sequence. In contrast, mutated cold probes substituted from AGGTTGTCCTCTGAACTCCACA to AGGTTGTGATCTGCGCTCCACA (the two-base mismatches are underlined) (mPPRE) did not abolish the formation of PPAR-PPRE complexes, strongly suggesting a specific binding of PPAR to the sPLA2-PPRE (Fig. 2, lanes 4 and 8). In addition, nuclear extracts from cells transfected with each of the three PPAR isotypes, along with RXR, showed the same migration pattern (lanes 9 to 11). Altogether, these results demonstrate that the putative PPRE from the rat sPLA2-IIA gene binds PPARs in VSMCs.
FIG. 2.
PPAR/RXR binds to the sPLA2 promoter in VSMCs. EMSA was performed on nuclear extracts of VSMCs incubated for 24 h with IL-1β (10 ng/ml). Radiolabeled DNA probe corresponding to nucleotides −928 to −909 of the rat sPLA2 gene promoter (sPLA2-PPRE) was incubated with VSMC nuclear extracts with or without (−) excess of unlabeled probe corresponding to sPLA2, the consensus PPRE (cons), or mPPRE (lanes 1 to 4). A radiolabeled probe corresponding to the sPLA2-PPRE incubated with nuclear extracts of VSMCs transfected with vector expressing PPARα (lane 9), β (lane 10), and γ (lane 11), or with nuclear extracts of CHO transfected with vector expressing PPARα (lane 13), -β (lane 14), and -γ (lane 15) were used as a positive control for PPAR/RXR binding. Asterisks indicate nucleotides matching the consensus sequence.
Functional characterization of the PPRE present in the sPLA2-type IIA promoter.
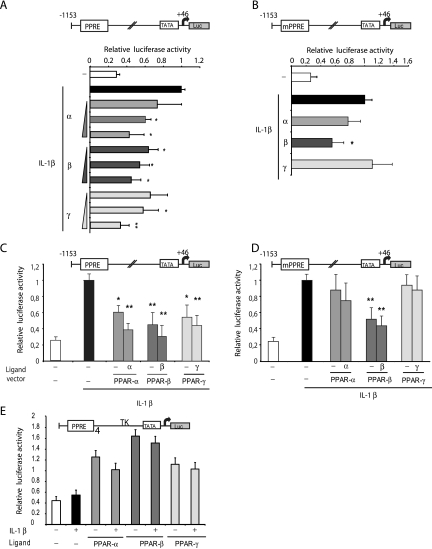
The ability of PPAR ligands to modulate sPLA2 expression suggested that the sPLA2 gene was a target of the PPAR-signaling pathway in VSMCs. We examined the ability of PPARs to regulate sPLA2 promoter activity as a way of assessing the regulation of sPLA2 by PPAR pathways. A pGL3-basic luciferase reporter construct containing the PPRE of the sPLA2 promoter ([−1153; +46]sPLA2-Luc) was transiently transfected into VSMCs. The transfected cells were preincubated for 6 h with specific agonists, and then IL-1β (10 ng/ml) was added for 24 h. The activity of the sPLA2 promoter in VSMCs was 3.5-fold induced by IL-1β alone (Fig. 3A). When cultured VSMCs were pretreated with PPAR-selective ligands prior to IL-1β treatment, we observed that the activity of the reporter was significantly decreased in a dose-dependent manner with the three ligands (Fig. 3A). To assess the role of the PPRE in the inhibition of the sPLA2 promoter activity by the ligands of PPAR, we tested the transcriptional activity of the [−1153; +46 bp]sPLA2 promoter containing mPPRE-sPLA2. VSMCs were also transfected with this mutated version of the promoter and then stimulated with IL-1β in the absence or presence of WY14643 (100 μM), L165041 (50 μM), or GW1929 (1 μM) (Fig. 3B). As a first result, the mutation of the PPRE did not significantly influence the stimulation level observed when cells were treated with IL-1β. However, as shown in Fig. 3B, the inhibition of the luciferase activity by PPARα agonist WY14643 or PPARγ agonist GW1929 was limited with the PPRE mutant. In contrast, inhibition by PPARβ agonist L165041 was maintained with the mutated construct. To get rid of possible PPAR-independent effects induced by the three PPAR ligands, we transiently transfected VSMCs with pcDNA3.1 expression vectors encoding PPARα, PPARβ, or PPARγ, together with an RXR expression vector, as PPAR forms a heterodimer with RXR to activate the target genes. As shown in Fig. 3C, the overexpression of PPARα, -β, or -γ led to a strong inhibition of the wild-type sPLA2 promoter activity with or without the presence of specific ligands. While the overexpression of PPARα and -γ did not significantly affect the activity of the mutated sPLA2 promoter, the inhibition was kept up when PPARβ expression vector was added (Fig. 3D). These results confirm the PPRE-independent PPARβ inhibition of the sPLA2 gene promoter in a proinflammatory context induced by IL-1β. Moreover, this phenomenon was clearly dependent on the sPLA2 promoter construct, since we used a construct containing four copies of the PPAR-binding site promoter (PPRE-TK-Luc) as a positive control for these experiments (Fig. 3E). We observed a stimulation of this synthetic promoter by PPAR-selective ligands; this stimulation was maintained with IL-1β treatment. Thus, the PPRE-dependent inhibitory effects of PPARα and -γ ligands on the IL-1β-induced sPLA2 promoter activity observed in transient transfection assays are consistent with the ability of their respective ligands to down-regulate sPLA2 gene expression in VSMCs. In contrast, the unexpected PPRE-independent inhibition of the sPLA2 gene promoter by PPARβ in IL-1β-stimulated VSMCs prompted us to further investigate new indirect molecular mechanisms.
FIG. 3.
Effects of specific PPAR activation on the sPLA2 promoter activity in VSMCs. (A) VSMCs were transfected with the sPLA2-Luc reporter plasmid: [−1153; +46]sPLA2Luc. Twenty-four hours after transfection, cells were pretreated for 6 h with increasing amounts of specific agonist of PPARα (WY14643; 1, 100, and 500 μM), PPARβ (L165041; 1, 10, and 50 μM), or PPARγ (GW1929; 10, 100, 1,000 nM) and stimulated with IL-1β (10 ng/ml) for 24 h. Cells were lysed, and luciferase activity was determined and normalized to β-galactosidase activity. (B) VSMCs were transfected with mPPRE-sPLA2-Luc. Cells were pretreated for 6 h with WY14643 (500 μM), L165041 (50 μM), or GW1929 (1,000 nM) and stimulated with IL-1β (10 ng/ml) for 24 h. (C and D) VSMCs were transiently transfected with the [−1153; +46]sPLA2Luc construct (C) or with mPPRE-sPLA2-Luc (D), together with 50 ng of PPARα, PPARβ, or PPARγ expression vector and RXR vector in equimolar quantities. Twenty-four hours after transfection, cells were pretreated for 6 h with a specific agonist of PPARα (WY14643; 100 μM), PPARβ (L165041; 50 μM), or PPARγ (GW1929; 1 μM) and then treated or not treated with IL-1β (10 ng/ml). (E) VSMCs were transiently cotransfected by a plasmid construct containing the PPREγ-TK-LUC. Luciferase activity was determined and normalized to β-galactosidase activity. Results are means ± SEM (error bars) and are representative of three independent experiments. *, P < 0.05 compared with IL-1β-treated VSMCs; **, P < 0.01; −, absence of; +, presence of.
BCL-6 is a repressor of sPLA2 expression in VSMCs.
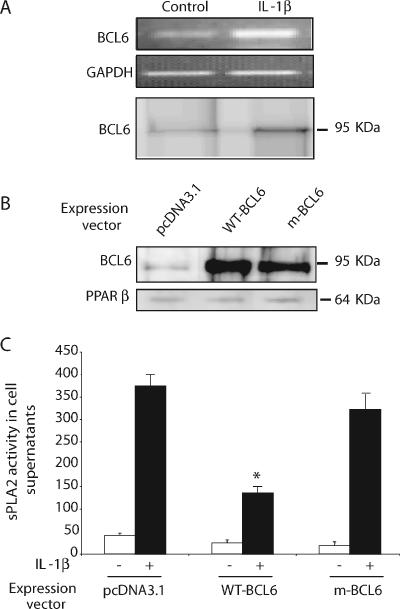
Several lines of strong evidence indicate that in addition to these effects on lipid metabolism, PPARβ plays a role in inflammation in atherosclerosis (25) by sequestering the transcriptional repressor BCL-6 in macrophages. Because L165041 decreased IL-1β-induced sPLA2 expression, we examined whether VSMCs express BCL-6. We first determined the expression of BCL-6 in rat VSMCs by RT-PCR and Western blot analysis (Fig. 4A). We observed that this expression was somewhat augmented in IL-1β-treated cells. The effects of BCL-6 on sPLA2 expression were assessed in the cultured VSMCs transfected with wild-type BCL-6 or a mutated BCL-6 H114N/K21I (mBCL-6) for 24 h and then treated with IL-1β (10 ng/ml) for 24 h. Western blot analysis showed efficient overexpression of wild-type BCL-6 and mBCL-6, while PPARβ protein expression was not affected (Fig. 4B). We then sought to determine whether the overexpression of BCL-6 influences the expression of sPLA2 in cultured rat VSMCs. Cells transfected with BCL-6 exhibited a decrease of sPLA2 activity of about 65% (P < 0.01) in the supernatant of cells treated with IL-1β (Fig. 4C). For the mBCL6 construct, the neutralization of the charged pocket has been shown to suppress the repression activity of BCL-6 (1). Consistent with that result, the overexpression of the mBCL-6 construct did not inhibit IL-1β-induced sPLA2 secretion. These results indicate that BCL-6 exerts a repressive effect on IL-1β-stimulated expression of the sPLA2-IIA endogenous gene in VSMCs.
FIG. 4.
BCL-6 inhibits sPLA2 activity in rat VSMCs. VSMCs were cultured in the absence or presence of Il-1β (10 ng/ml) for 24 h. (A) The expression of BCL-6 mRNAs and protein was assessed by RT-PCR and Western blotting analysis (Table 1). (B) VSMCs were transfected with a vector expressing a wild-type (WT) BCL-6 or mBCL-6, and BCL-6 protein expression was assessed by Western blot analysis. (C) VSMCs overexpressing BCL-6 or mBCL6 were treated or not treated with IL-1β (10 μg/ml) for 24 h. Culture mediums were collected, and the PLA2 activity was measured spectrofluorimetrically. Luciferase activity was determined and normalized to β-galactosidase activity. Results are means ± SEM (error bars) and are representative of three independent experiments. *, P < 0.05 (compared with IL-1β-treated VSMCs).
BCL-6 represses IL-1β-induced activation of the sPLA2 promoter.
In an effort to examine whether BCL-6 repressed transcription from the promoter of the sPLA2-IIA gene, VSMCs were transiently transfected with the luciferase reporter plasmid containing the sPLA2 promoter ([−1153; +46]sPLA2-Luc). To examine the repressor activity of Bcl6, the reporter gene and BCL-6 expression vectors were cotransfected into VSMCs, and the transfected cells were stimulated with IL-1β (10 ng/ml) for 24 h after a 6-h pretreatment with PPARβ agonist. We observed that up to 20% of the promoter activity was dose dependently suppressed without adding pcDNA3.1-BCL6 (Fig. 5A). Moreover, the inhibition observed after transfection by the BCL-6 vector alone was strengthened after treatment by PPARβ agonist. These results suggest that BCL-6 functions as a potent repressor for the sPLA2-IIA gene in VSMCs. As induction of the sPLA2-IIA gene expression was shown to be lowered by the PPARβ ligand, VSMCs were transiently cotransfected with the reporter gene and mBCL-6 and then incubated with PPARβ ligand for 6 h before IL-1β treatment. As demonstrated previously by Ahmad et al. (1), the neutralization of the charged pocket in this mutant resulted in a loss of repression by BCL-6. As shown in Fig. 5B, the PPARβ ligand failed to significantly inhibit the sPLA2 gene promoter in cells transfected with the inactive mBCL-6, despite expression of the recombinant protein in immunoblots (Fig. 4A). Altogether, these results demonstrate that the transcriptional repressor complex containing BCL-6 can repress sPLA2-IIA transcriptional repression.
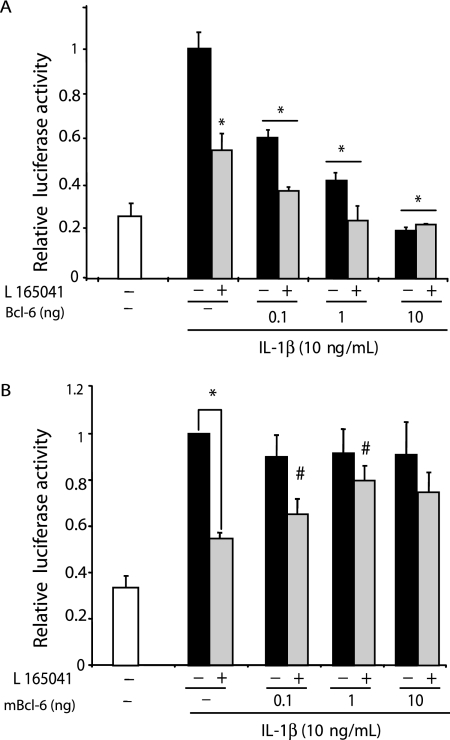
FIG. 5.
BCL-6 can repress IL-1β-induced sPLA2-IIA gene transcription in VSMCs. (A) VSMCs were cotransfected with increasing amounts (0.1 to 10 ng) of pcDNA3.1 vectors expressing BCL-6. (B) VSMCs were cotransfected with luciferase reporter construct containing [−1153; +46]sPLA2Luc and increasing amounts (0.1 to 10 ng) of pcDNA3.1 vectors expressing the mBCL6. As a control, cells were transfected with pcDNA3.1 vector alone. Twenty-four hours after transfection, cells were pretreated with vehicle (dimethyl sulfoxide) or 50 μM of PPARβ agonist L165041 and then incubated with IL-1β for 24 h. The relative inductions of luciferase activity were calculated after normalization to β-galactosidase activity. Results are means ± SEM (error bars) and are representative of three independent experiments. *, P < 0.01 (compared with IL-β-treated cells); #, P < 0.05 (compared with cells transfected with empty vector and L165041 pretreated cells); ##, P < 0.01 (compared with cells transfected with empty vector and L165041-pretreated cells).
PPARβ activation induces BCL-6 binding to the sPLA2 promoter.
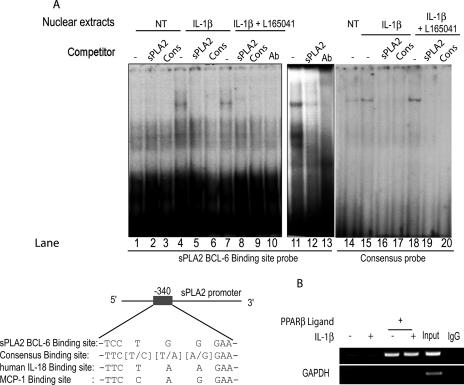
The Bcl6 binding sequence was found 340 bp upstream of exon 1. This sPLA2 sequence was compared with the BCL-6 binding sequences of known Bcl6 target genes (IL-18, mcp1, and consensus) (Fig. 6C). DNA gel shift analysis was conducted next, focusing on a 20-bp region of the sPLA2 promoter at −340 bp as a probe (Table 1). Nuclear extracts from VSMCs bound to this promoter region, and a specific high-molecular-mass complex was identified by competitions with an unlabeled oligonucleotide (Fig. 6A, lanes 2, 3, 5, 6, 8, and 9) corresponding to consensus or sPLA2 BCL-6 binding site. We observed an increase in the intensity of this complex with extracts from IL-1β-treated cells (Fig. 6A, lane 4). The major protein/DNA complex was highly induced in extracts from VSMCs overexpressing BCL-6 (Fig. 6, lane 11). Furthermore, the addition of a BCL-6 antibody removed the specific complex (Fig. 6A, lanes 10 and 13). These results indicate that BCL-6 is a component of a high-molecular-mass complex bound to a region of the sPLA2 promoter.
FIG. 6.
BCL-6 binds to the sPLA2 promoter in VSMCs in vitro and in vivo. (A) EMSA was performed on nuclear extracts of VSMCs pretreated with vehicle (NT) or 50 μM of the PPARβ agonist L165041 and then incubated with IL-1β for 24 h. Radiolabeled DNA probe corresponding to nucleotides −402 to −411 of the rat sPLA2 gene promoter (BCL-6 BS) was incubated with VSMC nuclear extracts with or without (−) excess of unlabeled probe corresponding either to the sPLA2 promoter BCL-6 binding site (sPLA2) (lanes 2, 5, and 8) or to the consensus BCL-6 binding site (Cons) (lanes 3, 6, and 9). The band was removed by the addition of anti-Bcl6 antibody (Ab) (lane 10). A radiolabeled probe corresponding to the sPLA2 promoter BCL-6 binding site incubated with VSMC nuclear extracts transfected with vector expressing BCL-6 was used as a positive control for BCL-6 binding (lanes 11 to 13). Radiolabeled DNA probe corresponding to the consensus BCL-6 binding site (lanes 14 to 20) was incubated with VSMC nuclear extracts with or without excess of unlabeled probe corresponding either to sPLA2 (lanes 16 and 19) or to the consensus (lanes 17 and 20). (B) VSMCs were pretreated with vehicle (NT) or 50 μM of the PPARβ agonist L165041 and then incubated with IL-1β for 24 h. DNA-protein cross-linking was induced with formaldehyde. After fragmentation, the chromatin was precipitated with rabbit antibody against BCL-6, followed by anti-rabbit IgG. To control nonspecific precipitation, the chromatin was incubated with anti-goat IgG alone. Immunoprecipitated chromatin was amplified by PCR using primers (−488/+46) designed to amplify the rat sPLA2 promoter containing the BCL-6 binding site (−340 bp). As a positive control, lysate chromatin (Input) was also amplified. −, absence of; +, presence of.
Our previous observations predicted that BCL-6 complexes associate with the sPLA2 promoter. To confirm that BCL-6 binds to the endogenous sPLA2 promoter and that PPARβ ligands may induce this binding in vivo, we next performed ChIP experiments. For PCR amplification, we used primer pairs that cover a region from −488 to +46 in the sPLA2 promoter containing the putative BCL-6 binding site (Fig. 6B). Our results demonstrated that BCL-6 is bound to the sPLA2 promoter region under PPARβ agonist-treated conditions. ChIP analysis with primers against an unrelated sPLA2 promoter region revealed no BCL-6 binding (data not shown). In summary, this ChIP assay shows that BCL-6 binds in vivo to a specific region within the PLA2G-IIA locus.
BCL-6 targets a PPARβ ligand-responsive region of the sPLA2 promoter.
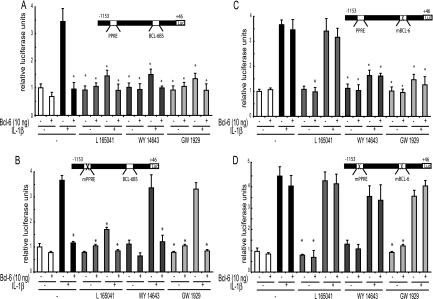
Next, we tested the role played by the putative BCL-6-binding site in the repression of IL-1β-induced sPLA2 activation by the PPARβ ligand. VSMCs were transiently transfected with the luciferase reporter plasmid containing either the [−1153; +46]sPLA2 promoter (Fig. 7A), mPPRE[−1153; +46]sPLA2 promoter (Fig. 7B), the mBCL-6[−1153; +46]sPLA2 promoter (Fig. 7C), or mPPRE-mBCL-6[−1153; +46] (Fig. 7D). As a first result, the PPRE mutation did not affect the basal promoter activity and preserved the IL-1β induction. Moreover, inhibition by BCL-6 overexpression was still observed with the PPRE-deleted construct in the presence or absence of PPARβ agonist (Fig. 7A and B). While PPARβ repression was maintained with this construct, sPLA2 repression by PPARα and PPARγ ligands was inhibited (Fig. 7B). This result is consistent with the one presented in Fig. 3 and confirms the diverse effects of PPARα and -γ versus PPARβ. Noticeably, the double mutation of the PPAR and BCL-6 binding sites slightly and positively influenced the [−1153; +46]sPLA2 promoter induction by IL-1β (Fig. 7D). In addition, the BCL-6 binding site mutation counteracted the inhibitory effect of BCL-6 in the presence or absence of PPARβ agonist (Fig. 7C and D) as well as the repression by the PPARβ ligand. In contrast, this BL-6 binding site doesn't appear as a key element of the IL-1β-induced-sPLA2 repression by PPARα and PPARγ ligands. Altogether, these results suggest that the ability of PPARβ to inhibit the sPLA2-IIA promoter is dependent on the BCL-6 binding site located between positions −342 and −351 relative to the transcription initiation site.
FIG. 7.
Analysis of PPRE-deleted and BCL-6-mutated constructs of the sPLA2 promoter. VSMCs were transfected with the wild-type sPLA2 promoter ([−1153; +46]sPLA2-Luc) (A), its PPRE-mutated version ([−1153; +46]sPLA2-IIA) (B), its BCL-6-mutated version (mBCL-6[−1153; +46]) (C), or its double-mutated version (mPPRE-mBCL6[−1153; +46]) (D), and cotransfected with pcDNA3.1 vectors expressing (+) or not expressing (−) BCL-6. SMCs were then treated with IL-1β and/or PPARβ ligand (10 mM) (L165041), PPARα ligand (100 μM) (WY14643), or PPARγ ligand (1 μM) (GW1929) for 24 h. The relative inductions of luciferase activity were calculated after normalization to β-galactosidase activity. Results are means ± SEM and are representative of three independent experiments. *, P < 0.05 (compared with IL-1β-treated VSMCs).
BCL-6-specific siRNA reverses PPAR-mediated inhibition of sPLA2 expression in VSMCs.
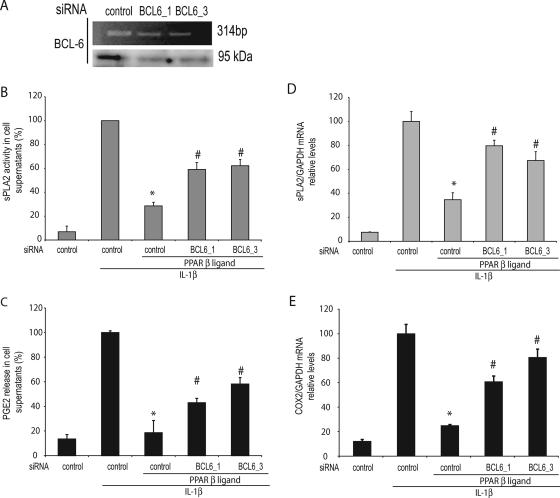
We next used BCL-6-specific siRNA to evaluate whether inhibition of BCL-6 expression results in a reversal of the repression of sPLA2 activity by PPAR ligands. To minimize potential off-target effects, we used two independent BCL-6 siRNAs and both effectively reduced endogenous BCL-6 expression (Fig. 8A). The inhibition of BCL-6 expression by using two BCL-6-specific siRNAs resulted in the attenuation of PPARβ ligand-mediated inhibition of sPLA2 activity compared with control siRNA-transfected cells (40.7% and 37% inhibition for BCL-6_1/BCL-6_3 versus 70.9% inhibition for control siRNA, respectively) (Fig. 8B). As expected, the attenuation of PPARβ ligand-mediated inhibition of sPLA2 activity was associated with a decrease in sPLA2-IIA gene expression (Fig. 8D). Whereas PPARβ alone inhibited 65.5% in sPLA2-IIA gene expression compared with that inhibited by IL-1β-treated cells, BCL-6_1 and BCL-6_3 siRNAs induced a 20.7% and 32.6% decrease, respectively, in sPLA2-IIA gene expression compared with that induced by IL-1β-treated cells (Fig. 8D). Taken together, these data indicate that the inhibition of sPLA2 gene in VSMC transcription is mediated, at least in part, through a BCL-6-dependent mechanism. As a control, we verified that BCL-6 knockdown affects neither PPARα nor PPARγ repression of IL-1β-induced sPLA2 activity (data not shown).
FIG. 8.
Knockdown of BCL6 abolishes PPARβ ligand repression of sPLA2 activity in VSMCs. VSMCs were transfected with control siRNA or with BCL-6-specific siRNAs (BCL-6_1/BCL-6_3). RT-PCR and Western blot analysis were performed in order to verify the knockdown effects. Following transfection, cells were pretreated for 6 h with vehicle (dimethyl sulfoxide) or PPARβ ligand (10 μM) before stimulation with IL-1β (10 ng/ml) for 24 h. (B) sPLA2 activity was measured in the supernatant. (C) PGE2 release into the supernatant was quantified by enzyme-linked immunosorbent assay. sPLA2-IIA (D) and COX-2 (E) transcriptions were measured by reverse transcription/real-time PCR using GAPDH gene expression as the internal standard. The results are expressed as induction compared with the expression in cells treated with phosphate-buffered saline alone. Results are means ± SEM (error bars) and are representative of three independent experiments. *, P < 0.01 (compared with IL-1β-treated VSMCs); #, P < 0.05 (compared with cells transfected with control siRNA and L165041-pretreated cells).
sPLA2 activity is essential for the production of the proinflammatory cytokine PGE2 (17). As PPARβ ligand was shown to inhibit sPLA2-IIA enzymatic activity in the supernatant of IL-1β-treated VSMCs, we assumed that PGE2 release could also be repressed by PPARβ ligand under similar experimental conditions. We measured PGE2 in the culture supernatant of VSMCs pretreated with the PPARβ agonists for 6 h prior to IL-1β treatment (24 h) (Fig. 8C). L165041 completely abolished the IL-1β-induced PGE2 release by the VSMCs. Given that PPARβ regulates sPLA2 activity in IL-1β-induced cells through BCL-6, we examined whether BCL-6 has a role in PGE2 release. To test this possibility, we depleted endogenous BCL-6 with siRNAs and then monitored the PGE2 release of VSMCs. The knockdown of BCL-6 revealed a partial reversion of PPARβ inhibition on PGE2 release from VSMCs: 80.2% to 64.7% and 80.2 to 59.2% for siRNA_BCL6_1 and siRNA_BCL6_3, respectively (Fig. 8C). PGE2 accumulation was shown to be associated with the induction of sPLA2-IIA. However, other enzymes are responsible for the synthesis of PGE2 from arachidonic acid, including COX. Therefore, we asked whether the expression of the inducible COX-2 gene was affected in response to PPARβ in VSMCs treated with IL-1β. IL-1β alone increased COX-2 transcription (Fig. 8E). As expected, pretreatment of IL-1β-treated cells with PPARβ decreased COX-2 gene expression, while BCL-6 knockdown revealed a reversion of PPARβ inhibition on COX-2 expression (80.2 to 37.5% and 80.2 to 21.2% for siRNA_BCL6_1 and siRNA_BCL6_3, respectively) (Fig. 8E). Taken together, these results suggest that PPARβ regulation decreased sPLA2 activity and, consequently, the PGE2 released from VSMCs through a BCL-6-dependent pathway.
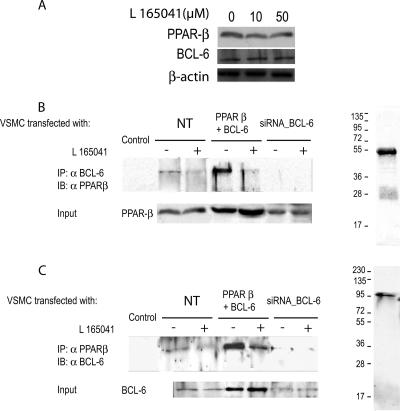
PPARβ activation reduces its interaction with BCL-6.
The augmentation in the DNA binding activity of BCL-6 after L165041 treatment may result from different molecular mechanisms. First, these changes may be caused by an augmentation in the expression of BCL-6. However, this possibility is unlikely because no significant changes were observed in the protein expression of either PPARβ or BCL-6 after L165041 stimulation (Fig. 9A). In addition, BCL-6 may interact physically with PPARs. This association has been described for PPARβ and prevents this nuclear receptor from binding to its response element and thereby inhibits its ability to repress gene transcription (25). Whether a similar mechanism affects PPARβ in VSMCs is not yet known. To evaluate this possibility, we performed coimmunoprecipitation studies with isolated nuclear extracts from cotransfected VSMCs expressing PPARβ and BCL-6. Immunoprecipitation was conducted in VSMCs either with antibody to BCL-6 (Fig. 9B) or with antibody to PPARβ (Fig. 9C). Data shown in Fig. 9B and C demonstrate that L165041 stimulation lowered the physical interaction between BCL-6 and PPARβ in transfected cells, suggesting that a decreased association between these two proteins is the mechanism through which BCL-6-repressing activity is increased after L165041 stimulation. As a control, we used siRNA to knock down BCL-6 expression. The lowered BCL-6 expression resulted in an elimination of the interaction between PPARβ and BCL-6.
FIG. 9.
Protein-protein interaction between BCL-6 and PPARβ in primary aortic SMCs. L165041 stimulation does not affect the protein level of either BCL-6 or PPARβ. Nuclear protein extracts from VSMCs stimulated with L165041 for 6 h were assayed by Western blot analysis with PPARβ or BCL-6 antibodies (A). Coimmunoprecipitation was performed in VSMCs transfected or not transfected (NT) with PPARβ and BCL-6 or siRNA against BCL-6 (BCL-6_3) in the absence (−) or presence (+) of PPARβ ligand. Nuclear extracts from VSMCs stimulated or not stimulated with L165041 (50 μM) for 4 h were subjected to immunoprecipitation using anti-BCL-6 antibody (B) or anti-PPARβ antibody (C) coupled to protein A-agarose beads. Immunoprecipitates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted with an anti-PPARβ antibody (B) or an anti-BCL-6 antibody (C). IP, immunoprecipitation; IB, immunoblotting; Input, 10% of cell lysate used for IP, showing an expression level of PPARβ and BCL-6.
DISCUSSION
In the present study, we investigated the anti-inflammatory properties of the three PPARs (PPARα, -β, and -γ) commonly expressed in VSMCs (27) by using both pharmacological and molecular approaches. Our findings indicate that IL-1β-induced sPLA2 is significantly inhibited by the three ligands and by overexpression of PPARα, PPARβ, and PPARγ. Furthermore, our results revealed that PPARβ inhibits IL-1β-induced sPLA2 production through the proto-oncogene BCL-6 independently of the PPRE.
Inflammation is one of the major pathological responses in the cardiovascular system, and sPLA2 is known to be one of the key regulators. In VSMCs, the synthesis of this enzyme is stimulated by proinflammatory cytokines and the enzyme is responsible for the production of proinflammatory mediator promoting atherogenesis (i.e., PGE2). sPLA2-IIA is thus the culprit of an inflammation amplification mechanism (18). PPARs are currently best understood as regulators of lipid metabolism. However, PPARs are expressed in the major cell types that make up atherosclerotic lesions, including macrophages, SMCs, lymphocytes, and endothelial cells, suggesting that ligands for these receptors may act both systemically and locally to influence lesion development. In fact, the anti-inflammatory activities of PPARs have been documented extensively in vitro and in vivo (8, 23, 31). PPARα has been shown to inhibit vascular inflammation, oxidative stress, and cell growth and migration through blocking NF-κB, TGF-β/Smad, and mitogen-activated protein kinase pathways (14). Evidence has also emerged suggesting that direct vascular actions of PPARγ may also play an antiatherogenic role. PPARγ has been detected in both endothelium and VSMCs. It has been long known that PPARγ has anti-inflammatory effects on monocytes. PPARγ activation can reduce cytokine (TNF-α, IL-1, and IL-6) production (23), probably by inhibiting the activities of proinflammatory transcription factors, such as NF-κB, AP-1, and STAT. In addition, PPARγ agonists may indirectly suppress systemic production of a proinflammatory milieu, mainly by inhibiting TNF-α, plasminogen activator inhibitor-1, and IL-6 expression in adipose tissue (34). The lesser known isoform PPARβ has emerged as a powerful metabolic regulator in various tissues, including fat, skeletal muscle, and heart (29). An understanding of PPARβ function has been augmented through a series of preclinical studies in which PPARβ activation diminishes metabolic perturbations and obesity, apparently by increasing lipid uptake and oxidation in skeletal muscle (5, 9). Recent studies reveal that PPARβ activation in the liver suppresses hepatic glucose output, contributing to improved glucose homeostasis (25). Additionally, PPARβ may suppress inflammation through mechanisms involving the release of anti-inflammatory factors or the stabilization of repressive complexes at inflammatory gene promoters (25).
In the present study, we found that the amounts of IL-1β-induced sPLA2 mRNA and protein in VSMCs are inhibited by the PPAR agonists WY14643, L165041, and GW1929 (Fig. 1 and 2). Besides, transient transfection assays demonstrate that the sPLA2 promoter is a valid target for PPARα/RXR, PPARβ/RXR, and PPARγ/RXR heterodimer transcription factors. By EMSA, we demonstrated that those heterodimers bind to a PPRE in positions −909 to −888 of the rat sPLA2 promoter (Fig. 2). These results indicate the ability of the PPAR heterodimers to modulate sPLA2 expression at a transcriptional level. IL-1β-induced sPLA2 inhibition by PPARα and PPARγ indicates that those isoforms share common features. Unexpectedly, PPARα and PPARγ isoforms need the presence of the PPRE located in the sPLA2 promoter (between −909 and −888) to be able to exert their inhibitory effects in VSMCs (Fig. 3). In accordance with this anti-inflammatory effect, PPARα agonists have been shown to suppress the NF-κB-dependent induction of COX-2 and IL-6 in VSMCs (34). In contrast, PPARα appears to potentiate sPLA2-IIA expression in rat mesangial cells (32). The major experiments were repeated with CHO cells, showing that PPAR ligands repressed the transcription of the endogenous sPLA2-IIA and the use of expression vector expressing PPARα and PPARγ freed us of subsidiary effects possibly caused by PPAR agonists (Fig. 5). Besides, PPARγ-dependent inhibitory mechanism has also been reported through competition on a composite PPRE/AP1 site of the latter promoter (16). Altogether, these results emphasize the multiplicity and complexity of the molecular mechanisms responsible of the gene expression modulation. After activation of the PPAR/RXRα heterodimer at the PPRE, the PPAR/RXRα complex can recruit a great variety of nuclear receptor cofactors that modulate transcriptional activity of PPAR and RXRα receptor heterodimer. Therefore, multiple mechanisms are involved in controlling the transcription of PPAR target genes in a given cell or tissue, depending on its molecular environment.
Here, we demonstrated that PPARβ ligands (e.g., L165041) inhibited sPLA2 production in cultured VSMCs (Fig. 1 and 8). Transient transfection confirmed the effects observed with PPARβ ligand on the endogenous sPLA2 (Fig. 3). Moreover, the use of PPARβ expression vector abolished IL-1β transcriptional activation of sPLA2 in a similar manner. In this regard, unknown endogenous PPARβ ligands may be sufficient to activate the more abundant PPARβ to exert their inhibition on sPLA2 expression. Unlike PPARα and -γ isoforms, mutations in the PPRE of the sPLA2-IIA did not significantly abolish the action of PPARβ on the promoter activity. This observation prompted us to investigate the role of BCL-6 for the PPARβ ligand-mediated inhibition of the sPLA2-IIA gene transcription. In cultured macrophages, overexpression of PPARβ exhibits a proinflammatory effect by sequestering BCL-6, whereas the deletion of PPARβ increased the availability of this inflammatory suppressor (25). A recent report indicated that a PPARβ-selective ligand, L165041, inhibits phenylephrine-induced expression of an NF-κB target gene, MCP-1, in cultured rat neonatal cardiomyocytes (9). However, the effects of PPARβ on inflammatory responses, such as IL-1β-induced sPLA2 production in VSMCs, remained obscure.
Because PPARβ activation strongly decreases sPLA2 induction by cytokines, we examined whether VSMCs express BCL-6 mRNA and protein. VSMCs transfected with a BCL-6 expression plasmid showed a great reduction of IL-1β sPLA2 activation. Those results indicate, for the first time, the presence of the proto-oncogene BCL-6 in rat VSMCs and moreover, its ability to inhibit a proinflammatory enzyme, sPLA-IIA. Our study adds sPLA2-IIA to the list of IL-5, IL-18, prdm1, and BCL-6 itself that are direct targets of BCL-6 (35, 40, 43). Much work has been directed toward elucidating the role of BCL-6 in physiology. Along with many other immunological defects, BCL-6−/− mice develop a profound inflammatory disease characterized by tissue infiltration of activated eosinophils, macrophages, and T-helper type 2 (Th2) cells. Noticeably, this “Th2-type” inflammatory disease, primarily affecting the heart, results from a specific heart defect (43). Thus, the identification of direct targets is important in distinguishing the precise function of this transcription factor independently of the “downstream” changes induced in the cell.
The use of a wild-type and a mutated BCL-6 expression vector showed that the PPARβ effects on IL-1β-induced inflammation are BCL-6 dependent. This mechanism appears to require the POZ/BTB domain of BCL-6 for its inhibitory effects (1). In light of the findings that the overexpression of a wild type but not a corepressor binding mutant of BCL-6 suppressed sPLA2 promoter activation in cultured VSMCs, a plausible mechanism could be that PPARβ activation in VSMCs recruits a corepressor complex through the proto-oncogene BCL-6. Coimmunoprecipitation experiments demonstrate that PPARβ interacts with BCL-6 in the nucleus of VSMCs (Fig. 9). In the absence of PPARβ ligand, this association prevents BCL-6 from binding to its response element (Fig. 6B) and thereby inhibits its ability to repress sPLA2 transcription. These findings are in concordance with the results reported by Lee et al. (25) on another proinflammatory gene, MCP-1. Moreover, it was recently demonstrated that the lack of BCL-6 expression in pancreatic beta cells prevented PPARβ-mediated repression of inflammatory responses (24). BCL-6 can act directly or indirectly to repress chemokine expression (12). BCL-6 can also negatively regulate NF-κB (10) by repressing its transcription and inhibiting its nuclear binding activity. Independently of BCL-6, PPARs may also exert anti-inflammatory effects via cross talk with NF-κB. Recently, it was shown that PPARβ itself may physically interact with the P65 subunit of NF-κB (30). Conversely, synthetic ligand-mediated PPARβ activation can inhibit NF-κB activation (6). These regulatory mechanisms vary between tissues, as illustrated by the differences in PPARβ-mediated repression of MCP-1 expression in liver and aorta (39). These findings implicate that PPARβ might act through different mechanisms to modulate inflammation in different cell types. Although the role of PPARβ in SMC proliferation has not been examined extensively, it has been reported that PPARβ activation might promote VSMC proliferation (44), while other results with the selective PPARβ agonist GW501516 (33) showed no effect on SMC proliferation. Indisputably, more studies will be needed to understand the tissue-specific effects of this near-ubiquitous receptor and its full potential to impact the action of metabolic syndrome and its associated disorders. The physiological relevance of the PPARβ ligands in VSMCs was illuminated by measuring the impact of the ligands on the production of proinflammatory mediators. Prostanoids, including PGE2, are lipid mediators produced by sequential catalysis of COX and the respective synthase. They play a major role in the induction and/or progression of the inflammatory reaction in various models of inflammatory diseases (28). In particular, PGE2 is enhanced in atherosclerotic lesions (10). Hence, the precise regulation of sPLA2-IIA and PGE2 release strongly suggests that the synthesis of prostanoids is strongly regulated during atherogenesis. We have demonstrated here that PPARβ inhibits the expression of sPLA2-IIA in VSMCs sensitized with IL-1β, resulting in a reduction in the release of PGE2 by VSMCs. Taken together, the results of our study suggest that BCL-6-dependent PPARβ regulation activity might act as a potent repressor of inflammatory events, since it appears to regulate gradual accumulation of two major inflamatory factors, sPLA2-IIA and PGE2.
Nevertheless, each active compound must be evaluated carefully in clinical studies to determine definitively whether PPARβ ligand may have beneficial effects for the treatment of inflammatory vascular diseases.
Acknowledgments
This work was supported by the Centre National de la Recherche Scientifique and the Université Pierre et Marie Curie. L.R. was supported by doctoral fellowships from the French Ministère de l'Education Nationale, de la Recherche et de la Technologie.
We gratefully thank Ari Melnick (Department of Developmental and Molecular Biology of Albert Einstein College of Medecine) for providing the BCL-6 plasmids. We thank Walter Wahli (Center for Integrative Genomics, University of Lausanne) for providing us the PPRE-TK-Luc construct and PPAR expression vectors. The English text was edited by Paul Lazarow.
Footnotes
Published ahead of print on 1 October 2007.
REFERENCES
- 1.Ahmad, K. F., A. Melnick, S. Lax, D. Bouchard, J. Liu, C. L. Kiang, S. Mayer, S. Takahashi, J. D. Licht, and G. G. Prive. 2003. Mechanism of SMRT corepressor recruitment by the BCL6 BTB domain. Mol. Cell 12:1551-1564. [DOI] [PubMed] [Google Scholar]
- 2.Antonio, V., A. Brouillet, B. Janvier, C. Monne, G. Bereziat, M. Andreani, and M. Raymondjean. 2002. Transcriptional regulation of the rat type IIA phospholipase A2 gene by cAMP and interleukin-1beta in vascular smooth muscle cells: interplay of the CCAAT/enhancer binding protein (C/EBP), nuclear factor-kappaB and Ets transcription factors. Biochem. J. 368:415-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonio, V., B. Janvier, A. Brouillet, M. Andreani, and M. Raymondjean. 2003. Oxysterol and 9-cis-retinoic acid stimulate the group IIA secretory phospholipase A2 gene in rat smooth-muscle cells. Biochem. J. 376:351-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barak, Y., D. Liao, W. He, E. S. Ong, M. C. Nelson, J. M. Olefsky, R. Boland, and R. M. Evans. 2002. Effects of peroxisome proliferator-activated receptor delta on placentation, adiposity, and colorectal cancer. Proc. Natl. Acad. Sci. USA 99:303-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barish, G. D., V. A. Narkar, and R. M. Evans. 2006. PPAR delta: a dagger in the heart of the metabolic syndrome. J. Clin. Investig. 116:590-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berry, E. B., J. A. Keelan, R. J. Helliwell, R. S. Gilmour, and M. D. Mitchell. 2005. Nanomolar and micromolar effects of 15-deoxy-delta 12,14-prostaglandin J2 on amnion-derived WISH epithelial cells: differential roles of peroxisome proliferator-activated receptors gamma and delta and nuclear factor kappa B. Mol. Pharmacol. 68:169-178. [DOI] [PubMed] [Google Scholar]
- 7.Brown, J. D., and J. Plutzky. 2007. Peroxisome proliferator-activated receptors as transcriptional nodal points and therapeutic targets. Circulation 115:518-533. [DOI] [PubMed] [Google Scholar]
- 8.Castrillo, A., and P. Tontonoz. 2004. PPARs in atherosclerosis: the clot thickens. J. Clin. Investig. 114:1538-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng, L., G. Ding, Q. Qin, Y. Huang, W. Lewis, N. He, R. M. Evans, M. D. Schneider, F. A. Brako, Y. Xiao, Y. E. Chen, and Q. Yang. 2004. Cardiomyocyte-restricted peroxisome proliferator-activated receptor-delta deletion perturbs myocardial fatty acid oxidation and leads to cardiomyopathy. Nat. Med. 10:1245-1250. [DOI] [PubMed] [Google Scholar]
- 10.Cipollone, F., C. Prontera, B. Pini, M. Marini, M. Fazia, D. De Cesare, A. Iezzi, S. Ucchino, G. Boccoli, V. Saba, F. Chiarelli, F. Cuccurullo, and A. Mezzetti. 2001. Overexpression of functionally coupled cyclooxygenase-2 and prostaglandin E synthase in symptomatic atherosclerotic plaques as a basis of prostaglandin E(2)-dependent plaque instability. Circulation 104:921-927. [DOI] [PubMed] [Google Scholar]
- 11.Clément, N., M. Glorian, M. Raymondjean, M. Andreani, and I. Limon. 2006. PGE2 amplifies the effects of IL-1beta on vascular smooth muscle cell de-differentiation: a consequence of the versatility of PGE2 receptors 3 due to the emerging expression of adenylyl cyclase 8. J. Cell. Physiol. 208:495-505. [DOI] [PubMed] [Google Scholar]
- 12.Dent, A. L., F. H. Vasanwala, and L. M. Toney. 2002. Regulation of gene expression by the proto-oncogene BCL-6. Crit. Rev. Oncol. Hematol. 41:1-9. [DOI] [PubMed] [Google Scholar]
- 13.Devchand, P. R., H. Keller, J. M. Peters, M. Vazquez, F. J. Gonzalez, and W. Wahli. 1996. The PPARalpha-leukotriene B4 pathway to inflammation control. Nature 384:39-43. [DOI] [PubMed] [Google Scholar]
- 14.Diep, Q. N., R. M. Touyz, and E. L. Schiffrin. 2000. Docosahexaenoic acid, a peroxisome proliferator-activated receptor-alpha ligand, induces apoptosis in vascular smooth muscle cells by stimulation of p38 mitogen-activated protein kinase. Hypertension 36:851-855. [DOI] [PubMed] [Google Scholar]
- 15.Elbashir, S. M., J. Martinez, A. Patkaniowska, W. Lendeckel, and T. Tuschl. 2001. Functional anatomy of siRNAs for mediating efficient RNAi in Drosophila melanogaster embryo lysate. EMBO J. 20:6877-6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.François, M., P. Richette, L. Tsagris, M. Raymondjean, M. C. Fulchignoni-Lataud, C. Forest, J. F. Savouret, and M. T. Corvol. 2004. Peroxisome proliferator-activated receptor-gamma down-regulates chondrocyte matrix metalloproteinase-1 via a novel composite element. J. Biol. Chem. 279:28411-28418. [DOI] [PubMed] [Google Scholar]
- 17.Hajjar, D. P., and K. B. Pomerantz. 1992. Signal transduction in atherosclerosis: integration of cytokines and the eicosanoid network. FASEB J. 6:2933-2941. [DOI] [PubMed] [Google Scholar]
- 18.Hurt-Camejo, E., S. Andersen, R. Standal, B. Rosengren, P. Sartipy, E. Stadberg, and B. Johansen. 1997. Localization of nonpancreatic secretory phospholipase A2 in normal and atherosclerotic arteries. Activity of the isolated enzyme on low-density lipoproteins. Arterioscler. Thromb. Vasc. Biol. 17:300-309. [DOI] [PubMed] [Google Scholar]
- 19.Ivandic, B., L. W. Castellani, X. P. Wang, J. H. Qiao, M. Mehrabian, M. Navab, A. M. Fogelman, D. S. Grass, M. E. Swanson, M. C. de Beer, F. de Beer, and A. J. Lusis. 1999. Role of group II secretory phospholipase A2 in atherosclerosis. 1. Increased atherogenesis and altered lipoproteins in transgenic mice expressing group IIa phospholipase A2. Arterioscler. Thromb. Vasc. Biol. 19:1284-1290. [DOI] [PubMed] [Google Scholar]
- 20.Jacques, C., G. Bereziat, L. Humbert, J. L. Olivier, M. T. Corvol, J. Masliah, and F. Berenbaum. 1997. Posttranscriptional effect of insulin-like growth factor-I on interleukin-1beta-induced type II-secreted phospholipase A2 gene expression in rabbit articular chondrocytes. J. Clin. Investig. 99:1864-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaross, W., R. Eckey, and M. Menschikowski. 2002. Biological effects of secretory phospholipase A(2) group IIA on lipoproteins and in atherogenesis. Eur. J. Clin. Investig. 32:383-393. [DOI] [PubMed] [Google Scholar]
- 22.Jaulmes, A., B. Janvier, M. Andreani, and M. Raymondjean. 2005. Autocrine and paracrine transcriptional regulation of type IIA secretory phospholipase A2 gene in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 25:1161-1167. [DOI] [PubMed] [Google Scholar]
- 23.Jiang, C., A. T. Ting, and B. Seed. 1998. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature 391:82-86. [DOI] [PubMed] [Google Scholar]
- 24.Kharroubi, I., C. H. Lee, P. Hekerman, M. I. Darville, R. M. Evans, D. L. Eizirik, and M. Cnop. 2006. BCL-6: a possible missing link for anti-inflammatory PPAR-delta signalling in pancreatic beta cells. Diabetologia 49:2350-2358. [DOI] [PubMed] [Google Scholar]
- 25.Lee, C. H., A. Chawla, N. Urbiztondo, D. Liao, W. A. Boisvert, R. M. Evans, and L. K. Curtiss. 2003. Transcriptional repression of atherogenic inflammation: modulation by PPARdelta. Science 302:453-457. [DOI] [PubMed] [Google Scholar]
- 26.Lusis, A. J. 2000. Atherosclerosis. Nature 407:233-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marx, N., H. Duez, J. C. Fruchart, and B. Staels. 2004. Peroxisome proliferator-activated receptors and atherogenesis: regulators of gene expression in vascular cells. Circ. Res. 94:1168-1178. [DOI] [PubMed] [Google Scholar]
- 28.Murakami, M., H. Naraba, T. Tanioka, N. Semmyo, Y. Nakatani, F. Kojima, T. Ikeda, M. Fueki, A. Ueno, S. Oh, and I. Kudo. 2000. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J. Biol. Chem. 275:32783-32792. [DOI] [PubMed] [Google Scholar]
- 29.Oliver, W. R., Jr., J. L. Shenk, M. R. Snaith, C. S. Russell, K. D. Plunket, N. L. Bodkin, M. C. Lewis, D. A. Winegar, M. L. Sznaidman, M. H. Lambert, H. E. Xu, D. D. Sternbach, S. A. Kliewer, B. C. Hansen, and T. M. Willson. 2001. A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport. Proc. Natl. Acad. Sci. USA 98:5306-5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Planavila, A., R. Rodriguez-Calvo, M. Jove, L. Michalik, W. Wahli, J. C. Laguna, and M. Vazquez-Carrera. 2005. Peroxisome proliferator-activated receptor beta/delta activation inhibits hypertrophy in neonatal rat cardiomyocytes. Cardiovasc. Res. 65:832-841. [DOI] [PubMed] [Google Scholar]
- 31.Ricote, M., J. Huang, L. Fajas, A. Li, J. Welch, J. Najib, J. L. Witztum, J. Auwerx, W. Palinski, and C. K. Glass. 1998. Expression of the peroxisome proliferator-activated receptor gamma (PPARgamma) in human atherosclerosis and regulation in macrophages by colony stimulating factors and oxidized low density lipoprotein. Proc. Natl. Acad. Sci. USA 95:7614-7619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scholz-Pedretti, K., A. Gans, K. F. Beck, J. Pfeilschifter, and M. Kaszkin. 2002. Potentiation of TNF-alpha-stimulated group IIA phospholipase A(2) expression by peroxisome proliferator-activated receptor alpha activators in rat mesangial cells. J. Am. Soc. Nephrol. 13:611-620. [DOI] [PubMed] [Google Scholar]
- 33.Seimandi, M., G. Lemaire, A. Pillon, A. Perrin, I. Carlavan, J. J. Voegel, F. Vignon, J. C. Nicolas, and P. Balaguer. 2005. Differential responses of PPARalpha, PPARdelta, and PPARgamma reporter cell lines to selective PPAR synthetic ligands. Anal. Biochem. 344:8-15. [DOI] [PubMed] [Google Scholar]
- 34.Staels, B., W. Koenig, A. Habib, R. Merval, M. Lebret, I. P. Torra, P. Delerive, A. Fadel, G. Chinetti, J. C. Fruchart, J. Najib, J. Maclouf, and A. Tedgui. 1998. Activation of human aortic smooth-muscle cells is inhibited by PPARalpha but not by PPARgamma activators. Nature 393:790-793. [DOI] [PubMed] [Google Scholar]
- 35.Takeda, N., M. Arima, N. Tsuruoka, S. Okada, M. Hatano, A. Sakamoto, Y. Kohno, and T. Tokuhisa. 2003. Bcl6 is a transcriptional repressor for the IL-18 gene. J. Immunol. 171:426-431. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka, T., J. Yamamoto, S. Iwasaki, H. Asaba, H. Hamura, Y. Ikeda, M. Watanabe, K. Magoori, R. X. Ioka, K. Tachibana, Y. Watanabe, Y. Uchiyama, K. Sumi, H. Iguchi, S. Ito, T. Doi, T. Hamakubo, M. Naito, J. Auwerx, M. Yanagisawa, T. Kodama, and J. Sakai. 2003. Activation of peroxisome proliferator-activated receptor delta induces fatty acid beta-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc. Natl. Acad. Sci. USA 100:15924-15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tischfield, J. A. 1997. A reassessment of the low molecular weight phospholipase A2 gene family in mammals. J. Biol. Chem. 272:17247-17250. [DOI] [PubMed] [Google Scholar]
- 38.Touqui, L., and M. Alaoui-El-Azher. 2001. Mammalian secreted phospholipases A2 and their pathophysiological significance in inflammatory diseases. Curr. Mol. Med. 1:739-754. [DOI] [PubMed] [Google Scholar]
- 39.Tous, M., N. Ferre, A. Rull, J. Marsillach, B. Coll, C. Alonso-Villaverde, J. Camps, and J. Joven. 2006. Dietary cholesterol and differential monocyte chemoattractant protein-1 gene expression in aorta and liver of apo E-deficient mice. Biochem. Biophys. Res. Commun. 340:1078-1084. [DOI] [PubMed] [Google Scholar]
- 40.Tunyaplin, C., A. L. Shaffer, C. D. Angelin-Duclos, X. Yu, L. M. Staudt, and K. L. Calame. 2004. Direct repression of prdm1 by Bcl-6 inhibits plasmacytic differentiation. J. Immunol. 173:1158-1165. [DOI] [PubMed] [Google Scholar]
- 41.Vosper, H., L. Patel, T. L. Graham, G. A. Khoudoli, A. Hill, C. H. Macphee, I. Pinto, S. A. Smith, K. E. Suckling, C. R. Wolf, and C. N. Palmer. 2001. The peroxisome proliferator-activated receptor delta promotes lipid accumulation in human macrophages. J. Biol. Chem. 276:44258-44265. [DOI] [PubMed] [Google Scholar]
- 42.Wang, Y. X., C. H. Lee, S. Tiep, R. T. Yu, J. Ham, H. Kang, and R. M. Evans. 2003. Peroxisome-proliferator-activated receptor delta activates fat metabolism to prevent obesity. Cell 113:159-170. [DOI] [PubMed] [Google Scholar]
- 43.Yoshida, T., T. Fukuda, M. Hatano, H. Koseki, S. Okabe, K. Ishibashi, S. Kojima, M. Arima, I. Komuro, G. Ishii, T. Miki, S. Hirosawa, N. Miyasaka, M. Taniguchi, T. Ochiai, K. Isono, and T. Tokuhisa. 1999. The role of Bcl6 in mature cardiac myocytes. Cardiovasc. Res. 42:670-679. [DOI] [PubMed] [Google Scholar]
- 44.Zhang, J., M. Fu, X. Zhu, Y. Xiao, Y. Mou, H. Zheng, M. A. Akinbami, Q. Wang, and Y. E. Chen. 2002. Peroxisome proliferator-activated receptor delta is up-regulated during vascular lesion formation and promotes post-confluent cell proliferation in vascular smooth muscle cells. J. Biol. Chem. 277:11505-11512. [DOI] [PubMed] [Google Scholar]