Abstract
The extracellular signal-regulated kinase (ERK) cascade is activated in response to a multitude of extracellular signals and converts these signals into a variety of specific biological responses, including cell differentiation, cell movement, cell division, and apoptosis. The specificity of the biological response is likely to be controlled in large measure by the localization of signaling, thus enabling ERK activity to be directed towards specific targets. Here we show that the RACK1 scaffold protein functions specifically in integrin-mediated activation of the mitogen-activated protein kinase/ERK cascade and targets active ERK to focal adhesions. We found that RACK1 associated with the core kinases of the ERK pathway, Raf, MEK, and ERK, and that attenuation of RACK1 expression resulted in a decrease in ERK activity in response to adhesion but not in response to growth factors. RACK1 silencing also caused a reduction of active ERK in focal adhesions, an increase in focal adhesion length, a decreased rate of focal adhesion disassembly, and decreased motility. Our data further suggest that focal adhesion kinase is an upstream activator of the RACK1/ERK pathway. We suggest that RACK1 tethers the ERK pathway core kinases and channels signals from upstream activation by integrins to downstream targets at focal adhesions.
The extracellular signal-regulated kinase (ERK) signaling cascade, comprised of the protein kinases Raf, MEK, and ERK, is an integral part of evolutionarily conserved signaling cascades that enable eukaryotic cells to sense and read a multitude of extracellular signals. ERK signaling converts the extracellular signals into a variety of specific intracellular biological responses, such as differentiation, cell movement, cell division, apoptosis, and oncogenic transformation. Extracellular stimuli, including growth factors, hormones, and adhesion to extracellular matrix proteins, activate Raf, which phosphorylates and activates MEK, which in turn phosphorylates and activates ERK (3, 27, 29). Although a great deal is known about the biochemical steps involved in the transduction of signals through this pathway, considerably less is known about how these signals are implemented into specific biological responses. Following activation, active ERK localizes to different subcellular compartments, including the nucleus, the plasma membrane, endosomes, the Golgi apparatus, and focal adhesions (11, 20, 23). At these various locations, ERK can phosphorylate a specific constellation of diverse substrates, leading to changes in gene expression, intracellular trafficking, and cell movement appropriate for the cell and stimulus.
Activated ERK localizes to focal adhesions, structures that link extracellular matrix proteins to the actin cytoskeleton and anchor the cell to the substratum. Focal adhesion formation is initiated by ligand binding to integrins and subsequent clustering of the receptors, which is followed by recruitment of both structural and signaling proteins into the adhesion complex (7, 26, 41). Integrin engagement is also sufficient to activate ERK, and in cells spreading on fibronectin active ERK localizes to newly formed focal adhesions (5, 11). However, the functional significance of active ERK in focal adhesions has not been well understood.
One mechanism by which ERK signaling can be directed to specific locations and functions is by scaffolds or anchor proteins. These nonenzymatic components associate with and enhance functional interaction of the components of the ERK pathway and can regulate the amplitude, timing, and location of signals (20, 24, 32). Several mitogen-activated protein kinase (MAPK)/ERK pathway scaffolds have been reported to control ERK pathway activity at specific subcellular compartments. For example, KSR regulates ERK activation at the plasma membrane, β-arrestins at early endosomes, and the MP1/p14 complex at late endosomes (22, 25, 36).
In our laboratory we identified MP1 as a 14-kDa scaffold protein bridging MEK and ERK (31). Because of its small size, we predicted that MP1 would function as a scaffold by engaging in interactions with multiple other scaffolds, thus enabling a combinatorial diversity in ERK signaling (32, 34, 39). Indeed, in addition to its role in endosomes, MP1 has been shown to regulate ERK pathway activation by integrating ERK and parallel Rac/Cdc42-PAK pathway signaling. MP1, by virtue of its ability to interact with MEK1 and PAK, regulates phosphorylation of serine residue 298 (S298) of MEK1 and primes MEK for activation by Raf (28). To identify additional MP1 interactors, we performed a yeast two-hybrid screen with MP1 as bait and identified a novel protein that we named MORG1 (MAPK organizing protein 1) that interacts with MP1 and regulates the ERK pathway activation in response to a subset of mitogenic stimuli.
We also identified the WD40-domain scaffold RACK1 as an MP1-interacting protein in our yeast two-hybrid screen. Here we demonstrate that RACK1 is required for efficient ERK pathway activation in response to adhesion but is not necessary for stimulation by epidermal growth factor (EGF). We also show that RACK1 targets active ERK to a specific intracellular location, focal adhesions, and that at this location ERK promotes focal adhesion disassembly and consequently cell motility. Thus, RACK1 is shown to be a novel scaffold protein for the ERK pathway that couples it specifically to an upstream activator and to localized downstream effectors.
MATERIALS AND METHODS
Plasmid constructs.
MP1, Raf-1, epitope-tagged MEK, and ERK expression constructs have been described previously (39). For expression of tagged RACK1, cDNA containing the mouse RACK1 coding sequence was either hemagglutinin (HA) or FLAG tagged at the amino terminus by PCR and subcloned to pcDNA3 vector (Invitrogen). Chicken paxillin fused to green fluorescent protein (GFP) (40) was subcloned to pcDNA5-FRT vector (Invitrogen) to generate an NIH 3T3 cell line stably expressing GFP-paxillin. Cells were derived according to the manufacturer's instructions (Invitrogen).
Cell lines and transfections.
NIH 3T3 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) (Gibco BRL) supplemented with 10% calf serum (Gibco BRL). CCL39, RAT2, and REF52 cells were maintained in DMEM supplemented with 10% fetal bovine serum (FBS) (Gibco BRL). CCL39 cells were transfected using Lipofectamine (Gibco BRL) according to the manufacturer's instructions. A FlipIn system (Invitrogen) was used for generation of an NIH 3T3 cell line stably expressing GFP-paxillin. Briefly, NIH 3T3 cells were transfected with pFRT/LacZeo vector, and a zeomycin-resistant clone (NIH 3T3-LacZeo) was isolated. NIH 3T3-LacZeo cells were then cotransfected with pOG44 and GFP-paxillin-pcDNA5-FRT constructs, and a hygromycin-resistant cell population (NIH 3T3-GFP-paxillin) was established. NIH 3T3-GFP-paxillin cells have been shown to be GFP-paxillin positive by Western blotting and total internal reflection fluorescence (TIRF) microscopy (data not shown).
Immunoprecipitations and immunoblotting.
Cells were lysed 24 h posttransfection in FLAG lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, 10% glycerol, 0.5 mM EDTA, 0.5 mM EGTA, pH 7.3, supplemented with 50 mM NaF, 5 mM Na4P2O7, 0.2 mM Na3VO4, and protease inhibitors). Clarified extracts were incubated for 2 h with M2 anti-FLAG affinity resin (Sigma) at 4°C. For Raf-1 immunoprecipitations, clarified extracts were incubated with Raf-1 antibody absorbed to protein A resin. Immune complexes were washed four times with lysis buffer, resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to a nitrocellulose membrane. Immunoblotting was carried out with the following antibodies: anti-FLAG M2 (Sigma), anti-HA 12CA5 (Babco), anti-Raf-1 C20 (Santa Cruz Biotechnologies), and anti-MP1 (28). Secondary goat anti-rabbit and anti-mouse antibodies coupled to horseradish peroxidase and enhanced chemiluminescence reagents (Amersham Biosciences) were used for protein detection.
Cell stimulation.
To determine ERK activation status in response to EGF, REF52 cells were cultured in 10% FBS and either left untreated or serum deprived for 4 h and stimulated with EGF in DMEM for 10 min. To determine ERK activation status in response to adhesion, REF52 cells were cultured in 10% FBS and detached by trypsin; trypsin detachment was stopped by addition of trypsin inhibitor. Cells were pelleted, resuspended in DMEM, kept in suspension for 90 min, and plated on dishes precoated with fibronectin (10 μg/ml) for 15 min. Cells were lysed in radioimmunoprecipitation assay lysis buffer and processed as described above with the following antibodies: anti-ERK2 B3B9, anti-pS298, and anti-focal adhesion kinase (FAK) (Upstate), anti-pS218/222 and anti Raf-1 C20 (Santa Cruz Biotechnologies), anti-phospho-c-Raf (Ser338) (Cell Signaling), anti-ppERK (42), anti-RACK1 and antipaxillin (Signal Transduction Laboratories), and anti-FAKpY397 and anti-paxillin pY118 (Biosource International). Western blot membranes were developed using enhanced chemiluminescence (Amersham).
siRNA gene silencing assay.
A double-stranded small interfering RNA (siRNA) targeting the 21-nucleotide sequence AAGGTGTGGAATCTGGCTAAC conserved between the mouse and rat RACK1 genes was synthesized by Qiagen, and the nonspecific control Duplexes-XIII was obtained from Dharmacon Research. The siRNA was transfected into REF52 and RAT2 cells by use of a calcium phosphate protocol, and 48 to 72 h posttransfection cell lysates were probed as indicated. Mock control cells received transfection reagent only. NIH 3T3 cells were transfected with Lipofectamine 2000 (Invitrogen). A second RACK1 siRNA (GCTAAAGACCAACCACATTTT) was utilized as well and generated biological results identical to those shown in this paper. The FAK siRNA oligonucleotide has been described previously (37).
Immunocytochemistry.
Cells were washed twice with PBS, fixed in 1% paraformaldehyde in PHEM buffer {60 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)], 25 mM HEPES, 10 mM EGTA, 4 mM MgSO4, pH 6.9} supplemented with 0.2 mM vanadate and 50 mM β-glycerophosphate for 20 min, and extracted with 1% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate} in PHEM buffer for 5 min. Active ERK was visualized by following a modified avidin-biotin amplification protocol described previously (11). Cells were blocked in 20% normal goat serum (NGS) (Invitrogen) in MBST (50 mM MOPS [morpholinepropanesulfonic acid], 150 mM NaCl, 0.05% Tween 20, pH 7.4) for 1 h and incubated with rabbit polyclonal anti-ppERK antibody diluted in 5% NGS in MBST for 1 h. Cells were washed with MBST three times and subsequently treated with secondary goat anti-rabbit antibody conjugated to biotin (Vector Laboratories) diluted in 5% NGS in MBST for 1 h, followed by incubation with Texas Red-avidin (Vector Laboratories). Paxillin was detected with mouse monoclonal antipaxillin antibody and visualized with goat anti-mouse antibody conjugated to either fluorescein isothiocyanate (Jackson Laboratories) or Cy5 (Molecular Probes). The following primary antibodies were used: rabbit polyclonal anti-ppERK (diluted 1:50 to 300; Cell Signaling) and mouse monoclonal antipaxillin clone 5H11 (1:1,000; Upstate). Fluorescent images were acquired using a Nikon Eclipse E600 upright fluorescence microscope equipped with a Hamamatsu Orca charge-coupled-device camera. Images were captured and analyzed with OpenLab (Improvision) software.
OpenLab (Improvision) software was used to measure the lengths of the focal adhesions. For each experimental condition, the lengths (in pixels) of approximately 200 focal adhesions from 10 cells were measured. The statistical analyses (Mann-Whitney nonpaired [not assuming Gaussian distribution] t test) were done using Prism software (GraphPad Software, Inc.).
TIRF microscopy and focal adhesion disassembly quantification.
To record dynamics of focal adhesions, an NIH 3T3-GFP-paxillin stable cell line was grown on Bioptechs T-dishes (Fisher Scientific) to confluence. To induce cell migration, a wound was made and 5 h later cell images were acquired every 3 min. Cells were visualized using a Nikon Eclipse TE2000-E inverted microscope equipped with a TIRF illuminator, a 488 argon laser, and a 60× differential interference contract TIRF objective (numerical aperture, 1.45) equipped with a Bioptechs objective heater. Images were acquired with a Retiga 1300i charge-coupled-device camera and QCapture Pro software (Q Imaging). To quantify the rates of focal adhesion assembly and disassembly, OpenLab (Improvision) software was used to measure the fluorescence intensity of individual focal adhesions. Changes in fluorescence intensity of focal adhesion were plotted in semilogarithmic plots as a function of time, and the constant rate of focal adhesion disassembly was calculated from the slope as described before (40). Plotting of individual focal adhesion disassembly rate and statistical analysis (Mann-Whitney nonpaired t test) were done using Prism software (GraphPad Software).
Cell migration assay.
The bottom sides of 24-well-plate cell culture inserts (Biocoat, 8-μm pores; Becton Dickinson) were precoated with fibronectin (1.0 μg/ml) overnight at 4°C. To determine cell migration, 1 × 105 REF52 cells were plated on the top of the insert in serum-free media and allowed to migrate for 6 h. Nonmigrating cells were wiped from the top by cotton swab. Cells were washed with PBS, fixed in 3% paraformaldehyde, and stained with crystal violet (0.1% in 20% ethanol). Cell migration was determined by counting the cells at the bottom from five fields.
RESULTS
Identification of RACK1 as a MAPK/ERK pathway scaffold.
To determine whether RACK1 associates with components of the MAPK/ERK pathway in living cells, we coexpressed RACK1 together with individual components of the ERK pathway and examined their association. RACK1 associated with MP1 as it did in yeast (Fig. 1A), as well as with Raf-1 (Fig. 1B), B-Raf (data not shown), ERKs (Fig. 1C), and MEKs (Fig. 1D), supporting the concept that RACK1 can serve a scaffolding function for the ERK pathway.
FIG. 1.
RACK1 associates with components of the ERK pathway. (A) Coimmunoprecipitation of MP1 with RACK1 from CCL39 cells transiently transfected with either control vector or FLAG-RACK1 and MP1 constructs. RACK1 was precipitated and detected with FLAG M2 antibody. Coprecipitated MP1 was detected with MP1 antibody, and cell lysates were probed with the FLAG and MP1 antibodies to verify comparable expression levels of RACK1 and MP1 protein. (B) Coimmunoprecipitation of Raf-1 with RACK1. CCL39 cells were transfected with either control vector or FLAG-RACK1 and untagged Raf-1. RACK1 precipitation and immunodetection were performed as described for panel A, with the exception that Raf-1 antibody was used to detect Raf-1. (C) Coimmunoprecipitation of RACK1 with ERK1 and ERK2. CCL39 cells were transfected with control vector or FLAG-ERK1 or FLAG-ERK2 constructs and HA-RACK1. ERK precipitation and immunodetection were performed as described for panel A, and RACK1 was detected with HA antibody. (D) Coimmunoprecipitation of RACK1 with MEK1 and MEK2. CCL39 cells were transfected with control vector or FLAG-MEK1 or FLAG-MEK2 constructs and HA-RACK1. MEK precipitation and immunodetection were performed as described for panel C. The upper band in the MEK blot represents the FLAG antibody heavy chain. IP, immunoprecipitate.
RACK1 regulates adhesion-induced ERK activation.
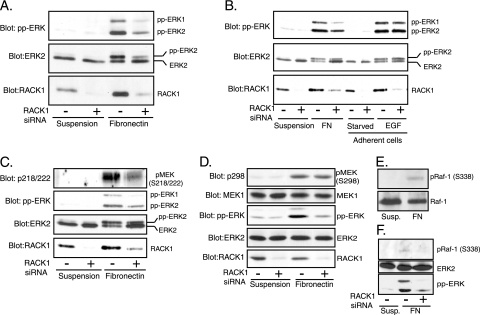
RACK1 has been reported to interact with integrin β subunits and with the protein kinase Src (2, 21). Integrins, Src, and MP1 all play important roles in ERK activation during cell adhesion (5, 28, 33). We therefore hypothesized that RACK1 may also be important for ERK activation during adhesion. Silencing of RACK1 expression by siRNA in REF52 fibroblasts suppressed ERK activation in cells plated on fibronectin (Fig. 2A). RACK1 function was specific to adhesion-induced ERK activation, as RACK1 silencing did not inhibit ERK activation in response to EGF (Fig. 2B).
FIG. 2.
Suppression of RACK1 interferes with ERK, MEK, and Raf-1 activation in response to adhesion. (A) Downregulation of endogenous RACK1 protein inhibits ERK activation in response to adhesion. REF52 cells were transfected with siRNA for 48 h, suspended in serum-free medium for 90 min, and replated on fibronectin-coated dishes for 15 min. Cell lysates were probed with antibody recognizing the doubly phosphorylated active form of ERK and with antibody directed against ERK2 and RACK1 to confirm equal loading of proteins and knockdown efficiency, respectively. Depletion of RACK1 protein by siRNA targeting different regions of RACK1 mRNA also inhibited ERK activation in response to adhesion to fibronectin, confirming the siRNA specificity (data not shown). (B) Downregulation of RACK1 protein inhibits ERK phosphorylation in response to adhesion but not in response to EGF. REF52 cells were treated as described for panel A, with the exception that cells were stimulated in side-by-side comparisons by replating of cells on fibronectin (FN) for 15 min or by stimulation of adherent cells with EGF (2 ng/ml) for 15 min. Cell lysates were probed as described for panel A. (C) Downregulation of RACK1 protein inhibits MEK phosphorylation on serines 218/222. REF52 cells were treated as described for panel A, and cell lysates were probed with antibody recognizing MEK phosphorylated on serines 218/222 (p218/222) required for the MEK activation. (D) Downregulation of RACK1 protein does not inhibit MEK phosphorylation on serine 298. REF52 cells were treated as described for panel A, and cell lysates were probed with antibody recognizing MEK phosphorylated on serine 298 (p298). (E) Cell adhesion to fibronectin induces Raf-1 phosphorylation on serine 338. REF52 cells were treated as described for panel A, and Raf-1 was precipitated as described in Materials and Methods. Immune complexes were probed with antibody recognizing Raf-1 phosphorylated on S338 and with antibody directed against Raf-1 to confirm equal amounts of proteins. (F) Downregulation of RACK1 protein inhibits Raf-1 phosphorylation on serine 338. REF52 cells were treated as described for panel A, and cell lysates were probed with antibody recognizing Raf-1 phosphorylated on S338.
RACK1 depletion also reduced adhesion-induced MEK activation, as determined by the phosphorylation status of serines 218/222 (Fig. 2C). RACK1 depletion did not affect PAK phosphorylation of MEK on S298 (Fig. 2D), which has been reported to be regulated by MP1 (28). Since serines 218/222 are phosphorylated by Raf, we examined whether RACK1 knockdown affects Raf activation. Phosphorylation of Raf-1 on serine residue 338 has been shown to be critical for Raf-1 activation (8, 17, 38). Consistent with a previous report (4), we observed that adhesion of REF52 cells to fibronectin induced phosphorylation of Raf-1 serine 338 (Fig. 2E). Importantly, RACK1 depletion inhibited phosphorylation of Raf-1 on serine 338 in response to adhesion (Fig. 2F).
These data indicate that RACK1 is required specifically for integrin-induced ERK activation upstream or at the level of Raf-1 and by a mechanism clearly distinct from MP1. The RACK1-MP1 interaction suggests that functionally complete scaffolds could be formed from individual subunits which regulate the convergence of separate signaling pathways, ensuring the efficiency and specificity of ERK pathway signaling.
RACK1 is required for active ERK localization to focal adhesions.
Next, we sought to explore the consequences of ERK and RACK1 signaling in response to adhesion. Since RACK1 depletion inhibited ERK activation mediated by integrin engagement to the extracellular matrix, we investigated whether RACK1 controlled the localization of active ERK to focal adhesions. Control or RACK1-depleted REF52 cells were plated on fibronectin-coated coverslips for 15 min in serum-free media, fixed and permeabilized under conditions that retain focal adhesions, and immunostained with antibody recognizing diphosphorylated, active ERK and antibody recognizing the focal adhesion marker paxillin. Consistent with previous reports (11, 35), we found that control cells spreading on fibronectin formed peripheral focal adhesions and that a fraction of the cellular pool of active ERK localized to these focal adhesions (Fig. 3A). RACK1 silencing did not inhibit cell spreading on fibronectin or assembly of focal adhesions. In fact, RACK1-depleted cells formed visibly more focal adhesions than control cells (discussed below). Importantly, RACK1 silencing resulted in the absence of active ERK in focal adhesions (Fig. 3A).
FIG. 3.
RACK1 regulates the localization of activated ERK to focal adhesions. (A) RACK1 silencing inhibits active ERK localization to focal adhesions in response to adhesion. REF52 cells were transfected with siRNA for 48 h, suspended, and replated on fibronectin for 15 min. Cells were costained with antibodies recognizing the active form of ERK (red) and paxillin (green). The inset shows a higher magnification of the boxed area. (B) RACK1 silencing inhibits active ERK localization to focal adhesions in adherent cells. REF52 cells were transfected with siRNA for 48 h in the presence of 10% serum. Adherent cells were costained with antibodies recognizing the active form of ERK (red) and paxillin (green). Cell lysates were probed with antibody directed against RACK1 and active ERK2 to confirm knockdown efficiency and equal loading of proteins, respectively (right).
In addition to cells acutely spreading on fibronectin after plating, active ERK has been shown to localize to focal adhesions in adherent cells with a polarized migratory phenotype grown in the presence of serum (11). Figure 3B shows that RACK1 silencing reduced the level of active ERK in focal adhesions under these conditions as well. Biochemical analysis of cell lysates showed equal levels of ERK activation in these asynchronously growing cells, regardless of whether RACK1 had been silenced (Fig. 3B, right). This finding suggests that RACK1 is essential for localization of active ERK to focal adhesions and that the existing cellular pool of active ERK cannot be stably targeted to focal adhesions in the absence of RACK1. RACK1 silencing also reduced the level of active ERK in focal adhesions of adherent cells migrating into a wound (Fig. 4A), further supporting the idea that active ERK is targeted to newly formed focal adhesions in a RACK1-dependent process.
FIG. 4.
RACK1 regulates focal adhesion disassembly and cell motility. (A) RACK1 regulates the localization of active ERK to focal adhesions in wounded cell monolayers. NIH 3T3 cells were transfected with siRNA, and 48 h after the transfection a wound was made in a confluent monolayer of cells in the presence of 10% serum. Five hours after the wound, cells were costained with antibodies recognizing the active form of ERK (red) and paxillin (green). The inset shows a higher magnification of the boxed area. (B) RACK1 regulates focal adhesion turnover. Series of images from time-lapse TIRF microscopy recording the dynamics of GFP-paxillin adhesions in control and RACK1 knockdown cells. Arrows indicate examples of dynamic focal adhesion at a protrusive region of the cell where both assembly and disassembly were seen. A longer time frame of GFP-paxillin dynamic is shown in RACK1-silenced cells to demonstrate the slower rate of focal adhesion disassembly. NIH 3T3 cells stably expressing GFP-paxillin were transfected and wounded as described for panel A. GFP-paxillin-containing focal adhesions were imaged 5 h after wounding by time-lapse TIRF microscopy by collecting images every 3 min. (C) Rates of focal adhesion disassembly. Rate constants of focal adhesion disassembly were determined from values obtained from 37 focal adhesions in four cells (control cells) or 30 focal adhesions in five cells (RACK1 siRNA-treated cells). Each point represents one focal adhesion analyzed. Only focal adhesions that displayed an initial increase in fluorescence intensity (i.e., focal adhesion assembly) were included in the analysis. (D) Rates of focal adhesion assembly. Rate constants of focal adhesion assembly were determined from values obtained from 16 focal adhesions in four cells (control cells) or 15 focal adhesions in five cells (RACK1 siRNA-treated cells). (E) RACK1 depletion inhibits cell migration toward fibronectin. REF52 cells were transfected with siRNA for 48 h and suspended, and cell migration toward the fibronectin was measured in triplicate in a Boyden chamber assay. The data represent averages ± standard deviations. Cell lysates were probed with antibodies directed against RACK1 and ERK2 to confirm knockdown efficiency and equal loading of proteins, respectively (bottom). Depletion of RACK1 protein by siRNA targeting different regions of RACK1 mRNA also inhibited cell migration toward fibronectin (data not shown).
RACK1 promotes focal adhesion disassembly and cell motility.
In REF52 cells adhering to fibronectin, RACK1 silencing resulted in a noticeable change in focal adhesion architecture, particularly with respect to focal adhesion distribution. The cells with silenced RACK1 contained more focal adhesions, and these focal adhesions became significantly longer in adherent cells (Fig. 3). These observations suggested that regulation of adhesion lifetime could be affected by attenuation of RACK1. Previously published observations suggested that ERK activity is necessary for proper focal adhesion turnover, particularly focal adhesion disassembly (40). Since ERK presumably needs to act at these focal adhesions, we hypothesized that RACK1 silencing would specifically alter disassembly of focal adhesions. To determine whether RACK1 affects the dynamic behavior of focal adhesions in real time, we established an NIH 3T3 cell line stably expressing GFP-paxillin and measured the dynamics of focal adhesions in a wound-healing assay. Focal adhesions containing GFP-paxillin were recorded using time-lapse TIRF microscopy because TIRF microscopy selectively excites fluorophores close to the solid surface (within ∼100 nm), thus minimizing the background fluorescence of cytoplasmic GFP-paxillin. The wound-healing assay was chosen because the cells migrating into the wound display a uniform direction of migration and the focal adhesions are very dynamic, rapidly appearing and disappearing at the front of the migrating cell (see Movie S1 in the supplemental material). In a wounded NIH 3T3 monolayer, active ERK localized to dynamic focal adhesions at the leading edge of the cells and RACK1 silencing almost completely abolished the appearance of active ERK at the focal adhesions in the cells migrating into the wound (Fig. 4A). Both control cells and cells with silenced RACK1 migrating into the wound were capable of forming new focal adhesions at the front of the cell. However, in RACK1-depleted cells focal adhesions at the front of the cell persisted for a longer time than focal adhesions in control cells (see Movies S1 and S2 in the supplemental material). The kinetics of focal adhesion disassembly showed that in the control cells focal adhesions were disassembled in approximately 10 min (Fig. 4B, top). By contrast, the disassembly of focal adhesions in cells with silenced RACK1 was delayed greatly and this defect in focal adhesion disassembly resulted in enhanced focal adhesion growth and elongation (Fig. 4B, bottom). Analysis of the assembly and disassembly rates of dynamic focal adhesions at the front of the cell showed that RACK1 silencing reduced the disassembly rate by more than 50% (Fig. 4C) but had no effect on the rate of focal adhesion assembly (Fig. 4D). These data suggest that RACK1 is a functional component of the ERK signaling pathway that regulates focal adhesion disassembly. Since the disassembly of focal adhesions is an important step in the regulation of cell motility (40), we determined whether RACK1 regulates cell motility. In REF52 cells, RACK1 was depleted by siRNA and cell migration toward fibronectin was measured in a Boyden chamber assay. Consistent with the reduced focal adhesion disassembly rate, we found that RACK1 silencing resulted in a significantly reduced migration toward fibronectin (Fig. 4E).
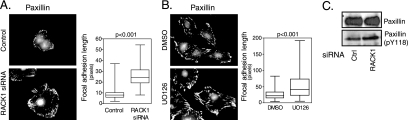
A reduced rate of focal adhesion disassembly was also reflected in an increase of focal adhesion length in cells migrating into a wound (Fig. 4B). The quantification of the focal adhesion length showed that in cells plated on fibronectin in the presence of serum, RACK1 knockdown resulted in approximately a twofold increase in focal adhesion length concomitant with the absence of active ERK from these adhesions (Fig. 5A).
FIG. 5.
RACK1 and ERK regulate focal adhesion length. (A) RACK1 silencing increases focal adhesion length. RAT2 cells were transfected with siRNA, and 48 h after the transfection cells were plated on fibronectin (1 μg/ml)-coated coverslips in the presence of 10% serum. Four hours after the plating, cells were stained with antibody recognizing paxillin. The right panel shows the quantification of focal adhesion length in control and RACK1 siRNA-transfected cells (determined from values obtained from ∼200 focal adhesions in 10 cells). Only peripheral focal adhesions were included in the analysis. Depletion of RACK1 protein by siRNA targeting different regions of RACK1 mRNA also increased the length of the focal adhesions (data not shown). (B) MEK inhibition induces an increase in focal adhesion length. RAT2 cells were plated on fibronectin (1 μg/ml)-coated coverslips and treated with MEK inhibitor UO126 (10 μM) for 48 h. Cell staining and quantification of focal adhesion length were done as described for panel A. (C) RACK1 silencing does not inhibit paxillin Y118 phosphorylation. RAT2 cells were treated as described for panel A, and cell lysates were probed with antibodies directed against paxillin and paxillin pY118. DMSO, dimethyl sulfoxide; Ctrl, control.
FAK, RACK1, and ERK are components of a signaling pathway that regulates active ERK localization to focal adhesions and focal adhesion dynamics.
RACK1 has been shown previously to associate with Src and regulate its activity (2). In addition, Src phosphorylates several focal adhesion proteins and regulates focal adhesion disassembly. This raises the question whether the defects in focal adhesion dynamics seen in RACK1 knockdown cells are a consequence of impaired Src signaling. We thus determined whether pharmacological inhibition of ERK by the MEK inhibitor UO126 affects focal adhesion length. Similarly to RACK1 knockdown in RAT2 cells, ERK inhibition resulted in a significant increase of focal adhesion length (Fig. 5B). In addition, we observed an increase in focal adhesion length in cells with silenced ERKs (data not shown). These data are consistent with the previous finding that ERK pharmacological inhibition reduces the rate of focal adhesion disassembly (40). In addition, biochemical analysis of cell lysates from control and RACK1 siRNA-transfected cells showed that RACK1 did not inhibit but rather slightly increased phosphorylation of paxillin on tyrosine residue 118 (Fig. 5C). Since tyrosine residue 118 is a known Src phosphorylation site that is necessary for efficient focal adhesion disassembly (40), we conclude that RACK1 regulates focal adhesion disassembly primarily by linking the ERK pathway to Src but not by affecting the phosphorylation of Src substrates.
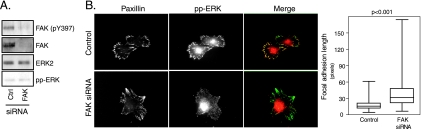
Our data suggested that integrin engagement is necessary for active ERK localization to focal adhesions. The activation of FAK is a central step in integrin signaling, and it has been proposed that both FAK and ERK signaling are linked to focal adhesion disassembly (40). These findings indicated that FAK and RACK1 may be components of a signaling pathway that regulates focal adhesion dynamics through ERK. If FAK regulates focal adhesion disassembly through ERK, it should also regulate active ERK localization to focal adhesions. To test this, we depleted the FAK protein level by siRNA and determined the localization of active ERK. FAK silencing efficiently decreased both the total level of FAK protein and FAK activation as measured by phosphorylation of tyrosine residue 397 (Fig. 6A). Importantly, in adherent cells grown in the presence of serum, FAK silencing completely abrogated active ERK appearance in focal adhesions (Fig. 6B). In addition, similarly to RACK1 knockdown we observed that FAK depletion by siRNA resulted in focal adhesion elongation at the front edge of cells migrating into the wound (data not shown) and in cells spreading on fibronectin for 4 h in the presence of serum (Fig. 6B).
FIG. 6.
FAK regulates active ERK localization to focal adhesion. (A) Efficiency of FAK knockdown. RAT2 cells were transfected with FAK siRNA for 48 h, suspended in medium with 10% serum, and replated on fibronectin-coated dishes for 4 h. Cell lysates were probed with antibodies recognizing FAK, active FAK [FAK (pY397)], ERK, and active ERK. (B) FAK silencing inhibits active ERK localization to focal adhesions. RAT2 cells were transfected with FAK siRNA for 48 h, suspended in medium with 10% serum, and replated on fibronectin-coated coverslips. Four hours after the plating, cells were costained with antibodies recognizing the active form of ERK (red) and paxillin (green). Ctrl, control.
DISCUSSION
Taken together, the data presented here show that RACK1 acts as a scaffold for the ERK pathway core kinases, presents these enzymes to specific upstream activators in the integrin/FAK adhesion signaling pathway, and controls the delivery of active ERK to focal adhesions, where it can act on substrates essential for focal adhesion disassembly and motility. Although RACK1 selectively facilitates ERK activation in response to cellular adhesion, it is dispensable for EGF-mediated ERK activation.
RACK1 associates with multiple components of the ERK pathway, including ERK1/2, MEK1/2, Raf-1, and B-Raf. In addition, RACK1 has been reported to associate with components of the adhesion signaling pathway, including integrin β-subunits and the Src protein kinase (2, 21), and we show here that it functionally cooperates with FAK. Integrin engagement to extracellular matrix proteins potently activates Src (30), and genetic evidence suggests that Src is an upstream component in integrin-mediated ERK activation. In Src-deficient cells, ERK activation is inhibited in response to adhesion but the Src family protein kinases are dispensable for ERK activation by the soluble growth factor platelet-derived growth factor (19, 33). Similarly to Src, depletion of RACK1 inhibits ERK activation in response to integrin engagement but not in response to EGF. Thus, the functional and binding characteristics of RACK1 are consistent with its functioning as a scaffold in integrin-mediated activation of the ERK pathway and suggest that RACK1 regulates ERK activation probably by its ability to assemble adhesion-specific signaling complexes containing the RACK1 partners integrin and Src and components of the ERK pathway. Therefore, RACK1 displays a functional resemblance to the prototypical yeast scaffold protein STE5. Similarly to RACK1, STE5 tethers components of the yeast mating MAPK pathway and links the pathway to membrane-associated G proteins (10). This scaffold-based organization links cell surface receptors with specific, localized effectors, ensuring efficient and specific biological response.
Integrin engagement during adhesion not only is sufficient to induce ERK activation but also is required to target active ERK to focal adhesions. This is shown by the fact that integrin-independent cell adhesion to polylysine neither activates ERK nor targets active ERK to focal adhesions. Moreover, in cells spreading on fibronectin or in cells with a polarized migratory phenotype, active ERK associates intensively with the presumed sites of acute integrin engagement to extracellular matrix and de novo focal adhesion formation (5, 11). Taken together, these data suggest that in migrating cells integrin attachment to the substratum coordinately induces both new focal adhesion assembly and ERK activation that is spatially linked to these newly formed focal adhesions. We observed that RACK1 regulates ERK activation specifically in response to integrin engagement to extracellular matrix and that depletion of RACK1 results in the absence of active ERK in focal adhesions. Importantly, the bulk cellular pool of active ERK is not able to compensate for the absence of active ERK in focal adhesions when RACK1 is depleted. RACK1 has been reported to localize to focal adhesions (6) and to regulate focal adhesion abundance (14). Our data show that RACK1 regulates focal adhesion disassembly, and this observation was confirmed by a report that came out during the review of the manuscript (9). Thus, RACK1 may promote ERK pathway activation at the sites of integrin engagement and at the sites of de novo focal adhesion formation. At focal adhesions, ERK signaling facilitates focal adhesion disassembly, consistent with the previous finding that ERK signaling regulates this process (40).
Integrin engagement leads to FAK activation and active FAK localization to focal adhesions. FAK is a central transducer of integrin signaling, as it activates numerous pathways, including ERK. Previous reports suggested that FAK plays an important role in regulation of focal adhesion dynamics. It has been shown that FAK null cells display an increase in focal adhesion size and number, probably as a consequence of reduced focal adhesion disassembly (15, 40). FAK regulates focal adhesion turnover at least partly through the ERK pathway because the reduced focal adhesion disassembly in FAK null cells can be rescued by expression of constitutively active MEK. These data suggest that the ERK pathway acts downstream of FAK to regulate focal adhesion disassembly. Since we show that active ERK localization to focal adhesions is necessary for proper focal adhesion disassembly, these data suggest that FAK may regulate activation of an ERK pool at focal adhesions. Indeed, we observed that FAK silencing resulted in the absence of active ERK in focal adhesions. In addition, similarly to RACK1 knockdown and ERK pharmacological inhibition, focal adhesions in FAK-silenced cells were significantly longer, suggesting that FAK silencing inhibited focal adhesion disassembly. Collectively, these data suggest that the tools for focal adhesion disassembly are generated coordinately in space and time with the assembly of focal adhesions through the ability of RACK1 to bind integrins and the MAPK enzymes and to locate these signaling components proximal to the upstream activator FAK.
FAK activation induces Src binding to FAK, Src activation, and Src-mediated phosphorylation of several focal adhesion components (26). Src phosphorylation of focal adhesion proteins, e.g., phosphorylation of paxillin tyrosine residue 118, has been implicated in the regulation of focal adhesion disassembly. It has been shown that the mutation of paxillin tyrosine residue 118 to alanine reduces focal adhesion disassembly (40). Since RACK1 also associates with Src, it has been suggested that RACK1 modulates focal adhesion disassembly through Src (9) independently of the ERK pathway. However, we found that RACK1 knockdown does not inhibit but rather slightly increases paxillin Y118 phosphorylation, suggesting that RACK1 knockdown does not impair the phosphorylation of Src substrates. To further support this, we found that phosphorylation of MEK1 serine residue 298, which is Src dependent (35), is not affected by RACK1 depletion. In addition, several lines of evidence implicate ERK as a key effector in regulating focal adhesion disassembly. Expression of constitutively active MEK rescues focal adhesion disassembly in FAK null cells, and, conversely, MEK inhibition decreases the adhesion disassembly rate (40). In addition, we observed that both MEK inhibition and RACK1 knockdown result in similar phenotypes, i.e., increase in focal adhesion length. These data suggest that RACK1 regulates focal adhesion dynamics through the ERK pathway, specifically coupling the ERK pathway to the upstream activator Src, consistent with a RACK1 scaffolding function.
The molecular mechanism(s) by which ERK and RACK1 regulate focal adhesion disassembly remains to be elucidated. Myosin light-chain kinase is a potential downstream target, as it has been shown to be phosphorylated by ERK and to regulate focal adhesion disassembly (18, 40). ERK may also activate proteases such as calpain to induce calpain-mediated proteolysis of focal adhesion components and thus regulate focal adhesion disassembly (1, 12, 13). In addition, ERK phosphorylates paxillin on serine residue 83, which may modulate the association of specific adhesion molecules (16).
In summary, we propose that RACK1 serves as a scaffold protein that organizes integrin- and FAK-induced ERK activation and localization to focal adhesions. In this way, a pool of active ERK is created that is spatially and temporally restricted to the site of integrin engagement and focal adhesion formation and is separated from bulk cellular ERK signaling, providing a specific channel for activation and regulation of the MAPK pathway. This localized ERK activation, which is generated coordinately with integrin-induced focal adhesion assembly, represents a negative regulatory loop which promotes focal adhesion disassembly. By coordinating the formation of focal adhesions with a mechanism to disassemble focal adhesions, RACK1 fosters the dynamic cycle of assembly and disassembly of focal adhesions that is necessary for cell migration.
Supplementary Material
Acknowledgments
We thank Karen Martin, Rob Tilghman, and Jill Slack-Davis for help with immunocytochemistry and all members of the Parsons-Weber-Parsons laboratories for helpful discussions.
This work was supported by grants CA104106, CA105402 (to M.J.W.), CA40042, and CA29243 (to J.T.P.) from the National Institutes of Health and Academy of Sciences of the Czech Republic Institutional Research Concept AV02 50200510 and grant IAA500200716 (to T.V.).
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
Published ahead of print on 1 October 2007.
REFERENCES
- 1.Carragher, N. O., M. A. Westhoff, V. J. Fincham, M. D. Schaller, and M. C. Frame. 2003. A novel role for FAK as a protease-targeting adaptor protein: regulation by p42 ERK and Src. Curr. Biol. 13:1442-1450. [DOI] [PubMed] [Google Scholar]
- 2.Chang, B. Y., K. B. Conroy, E. M. Machleder, and C. A. Cartwright. 1998. RACK1, a receptor for activated C kinase and a homolog of the beta subunit of G proteins, inhibits activity of Src tyrosine kinases and growth of NIH 3T3 cells. Mol. Cell. Biol. 18:3245-3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang, L., and M. Karin. 2001. Mammalian MAP kinase signalling cascades. Nature 410:37-40. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhary, A., W. G. King, M. D. Mattaliano, J. A. Frost, B. Diaz, D. K. Morrison, M. H. Cobb, M. S. Marshall, and J. S. Brugge. 2000. Phosphatidylinositol 3-kinase regulates Raf1 through Pak phosphorylation of serine 338. Curr. Biol. 10:551-554. [DOI] [PubMed] [Google Scholar]
- 5.Chen, Q., M. S. Kinch, T. H. Lin, K. Burridge, and R. L. Juliano. 1994. Integrin-mediated cell adhesion activates mitogen-activated protein kinases. J. Biol. Chem. 269:26602-26605. [PubMed] [Google Scholar]
- 6.Cox, E. A., D. Bennin, A. T. Doan, T. O'Toole, and A. Huttenlocher. 2003. RACK1 regulates integrin-mediated adhesion, protrusion, and chemotactic cell migration via its Src-binding site. Mol. Biol. Cell 14:658-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeMali, K. A., K. Wennerberg, and K. Burridge. 2003. Integrin signaling to the actin cytoskeleton. Curr. Opin. Cell Biol. 15:572-582. [DOI] [PubMed] [Google Scholar]
- 8.Diaz, B., D. Barnard, A. Filson, S. MacDonald, A. King, and M. Marshall. 1997. Phosphorylation of Raf-1 serine 338-serine 339 is an essential regulatory event for Ras-dependent activation and biological signaling. Mol. Cell. Biol. 17:4509-4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doan, A. T., and A. Huttenlocher. 2007. RACK1 regulates Src activity and modulates paxillin dynamics during cell migration. Exp. Cell Res. 313:2667-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elion, E. A. 2001. The Ste5p scaffold. J. Cell Sci. 114:3967-3978. [DOI] [PubMed] [Google Scholar]
- 11.Fincham, V. J., M. James, M. C. Frame, and S. J. Winder. 2000. Active ERK/MAP kinase is targeted to newly forming cell-matrix adhesions by integrin engagement and v-Src. EMBO J. 19:2911-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franco, S. J., M. A. Rodgers, B. J. Perrin, J. Han, D. A. Bennin, D. R. Critchley, and A. Huttenlocher. 2004. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat. Cell Biol. 6:977-983. [DOI] [PubMed] [Google Scholar]
- 13.Glading, A., R. J. Bodnar, I. J. Reynolds, H. Shiraha, L. Satish, D. A. Potter, H. C. Blair, and A. Wells. 2004. Epidermal growth factor activates m-calpain (calpain II), at least in part, by extracellular signal-regulated kinase-mediated phosphorylation. Mol. Cell. Biol. 24:2499-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hermanto, U., C. S. Zong, W. Q. Li, and L. W. Wang. 2002. RACK1, an insulin-like growth factor I (IGF-I) receptor-interacting protein, modulates IGF-I-dependent integrin signaling and promotes cell spreading and contact extracellular matrix. Mol. Cell. Biol. 22:2345-2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ilic, D., Y. Furuta, S. Kanazawa, N. Takeda, K. Sobue, N. Nakatsuji, S. Nomura, J. Fujimoto, M. Okada, and T. Yamamoto. 1995. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 377:539-544. [DOI] [PubMed] [Google Scholar]
- 16.Ishibe, S., D. Joly, Z. X. Liu, and L. G. Cantley. 2004. Paxillin serves as an ERK-regulated scaffold for coordinating FAK and Rac activation in epithelial morphogenesis. Mol. Cell 16:257-267. [DOI] [PubMed] [Google Scholar]
- 17.King, A. J., H. Sun, B. Diaz, D. Barnard, W. Miao, S. Bagrodia, and M. S. Marshall. 1998. The protein kinase Pak3 positively regulates Raf-1 activity through phosphorylation of serine 338. Nature 396:180-183. [DOI] [PubMed] [Google Scholar]
- 18.Klemke, R. L., S. Cai, A. L. Giannini, P. J. Gallagher, P. deLanerolle, and D. A. Cheresh. 1997. Regulation of cell motility by mitogen-activated protein kinase. J. Cell Biol. 137:481-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klinghoffer, R. A., C. Sachsenmaier, J. A. Cooper, and P. Soriano. 1999. Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J. 18:2459-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolch, W. 2005. Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat. Rev. Mol. Cell Biol. 6:827-837. [DOI] [PubMed] [Google Scholar]
- 21.Liliental, J., and D. D. Chang. 1998. Rack1, a receptor for activated protein kinase C, interacts with integrin beta subunit. J. Biol. Chem. 273:2379-2383. [DOI] [PubMed] [Google Scholar]
- 22.Luttrell, L. M., F. L. Roudabush, E. W. Choy, W. E. Miller, M. E. Field, K. L. Pierce, and R. J. Lefkowitz. 2001. Activation and targeting of extracellular signal-regulated kinases by beta-arrestin scaffolds. Proc. Natl. Acad. Sci. USA 98:2449-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mor, A., and M. R. Philips. 2006. Compartmentalized Ras/MAPK signaling. Annu. Rev. Immunol. 24:771-800. [DOI] [PubMed] [Google Scholar]
- 24.Morrison, D. K., and R. J. Davis. 2003. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu. Rev. Cell Dev. Biol. 19:91-118. [DOI] [PubMed] [Google Scholar]
- 25.Muller, J., S. Ory, T. Copeland, H. Piwnica-Worms, and D. K. Morrison. 2001. C-TAK1 regulates Ras signaling by phosphorylating the MAPK scaffold, KSR1. Mol. Cell 8:983-993. [DOI] [PubMed] [Google Scholar]
- 26.Parsons, J. T. 2003. Focal adhesion kinase: the first ten years. J. Cell Sci. 116:1409-1416. [DOI] [PubMed] [Google Scholar]
- 27.Pearson, G., F. Robinson, G. T. Beers, B. E. Xu, M. Karandikar, K. Berman, and M. H. Cobb. 2001. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 22:153-183. [DOI] [PubMed] [Google Scholar]
- 28.Pullikuth, A., E. McKinnon, H. J. Schaeffer, and A. D. Catling. 2005. The MEK1 scaffolding protein MP1 regulates cell spreading by integrating PAK1 and Rho signals. Mol. Cell. Biol. 25:5119-5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubinfeld, H., and R. Seger. 2005. The ERK cascade: a prototype of MAPK signaling. Mol. Biotechnol. 31:151-174. [DOI] [PubMed] [Google Scholar]
- 30.Sandilands, E., C. Cans, V. J. Fincham, V. G. Brunton, H. Mellor, G. C. Prendergast, J. C. Norman, G. Superti-Furga, and M. C. Frame. 2004. RhoB and actin polymerization coordinate Src activation with endosome-mediated delivery to the membrane. Dev. Cell 7:855-869. [DOI] [PubMed] [Google Scholar]
- 31.Schaeffer, H. J., A. D. Catling, S. T. Eblen, L. S. Collier, A. Krauss, and M. J. Weber. 1998. MP1: a MEK binding partner that enhances enzymatic activation of the MAP kinase cascade. Science 281:1668-1671. [DOI] [PubMed] [Google Scholar]
- 32.Schaeffer, H. J., and M. J. Weber. 1999. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol. Cell. Biol. 19:2435-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schlaepfer, D. D., M. A. Broome, and T. Hunter. 1997. Fibronectin-stimulated signaling from a focal adhesion kinase-c-Src complex: involvement of the Grb2, p130cas, and Nck adaptor proteins. Mol. Cell. Biol. 17:1702-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma, C., T. Vomastek, A. Tarcsafalvi, A. D. Catling, H. J. Schaeffer, S. T. Eblen, and M. J. Weber. 2005. MEK partner 1 (MP1): regulation of oligomerization in MAP kinase signaling. J. Cell. Biochem. 94:708-719. [DOI] [PubMed] [Google Scholar]
- 35.Slack-Davis, J. K., S. T. Eblen, M. Zecevic, S. A. Boerner, A. Tarcsafalvi, H. B. Diaz, M. S. Marshall, M. J. Weber, J. T. Parsons, and A. D. Catling. 2003. PAK1 phosphorylation of MEK1 regulates fibronectin-stimulated MAPK activation. J. Cell Biol. 162:281-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teis, D., W. Wunderlich, and L. A. Huber. 2002. Localization of the MP1-MAPK scaffold complex to endosomes is mediated by p14 and required for signal transduction. Dev. Cell 3:803-814. [DOI] [PubMed] [Google Scholar]
- 37.Tilghman, R. W., J. K. Slack-Davis, N. Sergina, K. H. Martin, M. Iwanicki, E. D. Hershey, H. E. Beggs, L. F. Reichardt, and J. T. Parsons. 2005. Focal adhesion kinase is required for the spatial organization of the leading edge in migrating cells. J. Cell Sci. 118:2613-2623. [DOI] [PubMed] [Google Scholar]
- 38.Tran, N. H., and J. A. Frost. 2003. Phosphorylation of Raf-1 by p21-activated kinase 1 and Src regulates Raf-1 autoinhibition. J. Biol. Chem. 278:11221-11226. [DOI] [PubMed] [Google Scholar]
- 39.Vomastek, T., H. J. Schaeffer, A. Tarcsafalvi, M. E. Smolkin, E. A. Bissonette, and M. J. Weber. 2004. Modular construction of a signaling scaffold: MORG1 interacts with components of the ERK cascade and links ERK signaling to specific agonists. Proc. Natl. Acad. Sci. USA 101:6981-6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Webb, D. J., K. Donais, L. A. Whitmore, S. M. Thomas, C. E. Turner, J. T. Parsons, and A. F. Horwitz. 2004. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat. Cell Biol. 6:154-161. [DOI] [PubMed] [Google Scholar]
- 41.Zamir, E., and B. Geiger. 2001. Components of cell-matrix adhesions. J. Cell Sci. 114:3577-3579. [DOI] [PubMed] [Google Scholar]
- 42.Zecevic, M., A. D. Catling, S. T. Eblen, L. Renzi, J. C. Hittle, T. J. Yen, G. J. Gorbsky, and M. J. Weber. 1998. Active MAP kinase in mitosis: localization at kinetochores and association with the motor protein CENP-E. J. Cell Biol. 142:1547-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.