Abstract
Global analyses of gene expression in regulatory T (Treg) cells, whose development is critically dependent upon the transcription factor Foxp3, have provided many clues as to the molecular mechanisms these cells employ to control immune responses and establish immune tolerance. Through these studies, G protein-coupled receptor 83 (GPR83) was found to be expressed at high levels in Treg-cell populations. However, its function remained unclear. Recently, it has been suggested that GPR83 is involved in the induction of Foxp3 expression in the peripheral nonregulatory Foxp3− CD4 T cells. To examine a role for GPR83 in Treg-cell biology, we generated and characterized GPR83-deficient mice. We have shown that GPR83 abolition does not result in measurable pathology or changes in the numbers or function of Foxp3+ Treg cells. Furthermore, while in vitro analysis suggested a potential involvement of GPR83 in transforming growth factor β-dependent Foxp3 induction, there was no difference in the ability of nonregulatory GPR83-deficient and nondeficient Foxp3− T cells to acquire Foxp3 expression in vivo. Collectively, our results demonstrate that GPR83 is dispensable for Treg-cell development and function.
Over the past decade, regulatory T (Treg) cells have been proven to play a pivotal role in maintaining immunological tolerance (9, 22, 23). Recently, Foxp3, a member of the forkhead transcription factor family, has been identified as a key regulator of Treg-cell development and suppressor function and as a specific marker of Treg cells (see reference 8 for a review). The Foxp3+ Treg cells are able to actively suppress immune activation by dampening the intensity of immune responses to both self and non-self antigen through a variety of means (26). Despite the current progress in understanding the biology of Treg cells, precise molecular mechanisms of their differentiation and the suppression function that Treg cells employ in various inflammatory and autoimmune settings are still not well understood.
In the quest for further insights into, and better understanding of, the molecular and cellular mechanisms of Treg-cell development and function, several groups, including ours, have employed DNA microarray analyses to study genes that are differentially expressed in CD4+ CD25+ Foxp3+ Treg cells and activated and naïve non-Treg cells (7, 10, 11, 18, 24). In these studies, the gene for G protein-coupled receptor 83 (GPR83), an orphan G protein-coupled receptor, was identified among the genes selectively up-regulated in Treg cells (7, 24). The gene for GPR83, also known as glucocorticoid-induced receptor, was first identified as a glucocorticoid- and cyclic AMP-induced gene in a T-cell line, WEHI-7TG (13). Subsequently, high expression levels and distinct distribution patterns observed in the brain suggested that GPR83 may be involved in the control of feeding and emotional behavior, the regulation of stress, learning, and memory (20). Recently, Sugimoto and colleagues have shown GPR83 protein expression in Treg cells by flow cytometric analysis and immunohistochemistry (24). Despite the specific expression pattern of GPR83, however, the functional connection between GPR83 and Treg-cell development and function remains unresolved. Lately, a study by Hansen and colleagues showed that naïve Foxp3− T cells transduced with the GPR83 gene acquired Foxp3 expression more efficiently than nontransduced cells under inflammatory conditions in vivo. However, GPR83 overexpression alone failed to confer suppressor activity or up-regulate several Treg-cell-specific transcripts (12). This observation implied a direct functional link between GPR83 and Foxp3 expression. The anticipated interaction between GPR83 and its putative ligand, if confirmed, may provide a potential means to alter Treg-cell numbers or function by receptor agonists or antagonists. Nonetheless, the absence of a proper genetically modified animal model has precluded a stringent mechanistic analysis of the role of GPR83 in a physiological setting.
To examine a potential role of GPR83 in Treg-cell biology, we generated and characterized mice harboring a conditional Gpr83 allele. The elimination of the Gpr83 gene in the germ line had no detectable effect on the viability or fertility of mutant mice. The lack of the Gpr83 gene did not have a measurable effect on the development of Treg cells in the thymus, their suppressor function, or the induction of Foxp3 expression in the periphery in vivo. Consistent with these data, GPR83-deficient mice failed to develop any signs of autoimmunity. Hence, GPR83 is dispensable for the development and function of Treg cells.
MATERIALS AND METHODS
Generation of conditional GPR83-deficient mice.
The Gpr83-targeting vector was constructed using a 10-kb XmaI fragment of the Gpr83 gene containing exons 2 through 4. This fragment was subcloned from a 129-kb bacterial artificial chromosome clone containing the Gpr83 locus. A loxP element containing a 5′ XbaI restriction site was cloned into the XhoI site 347 bp upstream of exon 3, while the cassette comprising loxP and the FLP recombination target site-flanked neomycin resistance gene expressed from the phosphoglycerate kinase promoter (the FRT-PGK-NEO-FRT-loxP cassette) was cloned into the EcoRI site 703 bp downstream of exon 4. The targeting construct was electroporated into R1 embryonic stem (ES) cells, and neomycin-resistant clones were screened by PCR for evidence of homologous recombination. Positive clones were further verified for correct targeting by Southern blot analysis.
Two independently derived ES cell clones were injected into B6 blastocysts. Chimeric male offspring were mated first to FLP deleter mice to remove the neomycin resistance gene (6) and subsequently to Cre deleter mice to induce germ line Gpr83 gene deletion. Since in initial experiments we observed no phenotypic and functional differences between heterozygote Gpr83+/− and Gpr83+/+ littermates (data not shown), heterozygote Gpr83+/− mice were used as controls in the bulk of the experiments described here. All mice were bred and maintained under specific-pathogen-free conditions in the animal facility at the University of Washington in accordance with the institutional guidelines.
Lymphocyte isolation and flow cytometric analysis.
Single cell suspensions from thymus and spleen tissue were prepared from 6- to 8-week-old mice. In some experiments, mice of up to 12 to 14 months of age were used. Cells were labeled with fluorescein isothiocyanate-conjugated CD4 (L3T4), peridinin-chlorophyll-conjugated CD8 (53-6.7), allophycocyanin-conjugated Foxp3 (FJK-16S), and phycoerythrin-conjugated CD25 (PC-61), CD44 (IM7), CD62L (MEL-14), or CD69 (H1.2F3) antibodies. Flow cytometry analysis was performed using a FACSCanto flow cytometer (Becton Dickinson, CA), and results were analyzed using FlowJo software (Tree Star Inc., OR).
CD4+ T cells from GPR83-deficient and control mice were first purified by magnetic bead sorting using an AutoMACS (Miltenyi, Germany) followed by sorting on a FACSAria cell sorter after labeling with fluorescein isothiocyanate-conjugated anti-CD4, phycoerythrin-conjugated anti-CD25, and allophycocyanin-conjugated anti-CD62L. The purity of sorted populations of CD4+ CD25− naïve effector T cells expressing high levels of CD62L (CD62Lhi) and CD4+ CD25+ Treg cells was higher than 98%.
Proliferation and in vitro suppression assay.
To assess the proliferative capacities of non-Treg and Treg cells, purified CD4+ CD25− CD62Lhi or CD4+ CD25+ T cells (5 × 104) were cultured in the presence of titrated amounts of CD3-specific 2C11 antibody with T-cell-depleted splenocytes (15 × 104), irradiated at 2,000 rad, as antigen-presenting cells (APCs) in the wells of 96-well round-bottom plates for 72 h at 37°C. In vitro suppression assays were performed upon the culture of 5 × 104 CD4+ CD25− T cells with the indicated ratio of suppressor CD4+ CD25+ CD62Lhi T cells and T-cell-depleted splenocytes (15 × 104/well), irradiated at 2,000 rad, as APCs and 1 μg of anti-CD3 (2C11)/ml in 96-well round-bottom plates for 72 h at 37°C. T-cell proliferation was assessed by measuring [3H]thymidine ([3H]TdR) incorporation during the last 8 h of culture. The data are presented as mean counts per minute corresponding to the levels of [3H]TdR incorporation ± standard errors for triplicate cultures.
Cytokine secretion assays.
To access the secretion of interleukin-10 (IL-10) and transforming growth factor β (TGF-β), 105 purified T cells were stimulated in 96-well flat-bottom plates with plate-bound anti-CD3 (1 μg/ml) and anti-CD28 (10 μg/ml). Supernatants were collected after 24 and 48 h of culture, and cytokines were measured using IL-10 and TGF-β enzyme-linked immunosorbent assay kits according to the instructions of the manufacturers (eBioscience [San Diego, CA] and R&D Systems [Minneapolis, MN], respectively).
In vitro induction of Foxp3 expression and generation of Th17 cells.
For Foxp3 induction, 2 × 105 purified CD4+ CD25− CD62Lhi naïve T cells were cocultured with 2 × 106 irradiated T-cell-depleted splenocytes isolated from Ly5.1 C56BL/6 mice in the presence of CD3 (1-μg/ml) antibodies and 1-ng/ml human recombinant TGF-β (R&D Systems) in the wells of 24-well plates. For Th17 induction, mouse IL-6 was added to TGF-β-containing cultures at a 20-ng/ml final concentration. After 4 days of culture, cells were harvested and stained with CD4 and Ly5.1 antibodies and antibodies specific for Foxp3 and IL-17 by using established protocols. In some experiments, plate-bound CD3 (1-μg/ml) and CD28 (1-μg/ml) antibodies were used to stimulate T cells.
Cell transfer and tumor challenge.
Purified naïve CD4+ CD25− CD62Lhi T cells were injected intravenously into T-cell receptor βδ−/− (TCRβδ−/−) recipient mice. One day after cell transfer, mice were injected intradermally in the flank with 2 × 105 B16.F10 melanoma cells. Thirteen days after tumor challenge, mice were sacrificed, and cells from draining lymph nodes (LNs; axillary and inguinal LNs) and nondraining LNs (mesenteric LNs) were isolated and subjected to fluorescence-activated cell sorter analysis for the expression of CD4, TCRβ, and Foxp3.
RESULTS
Targeted deletion of GPR83.
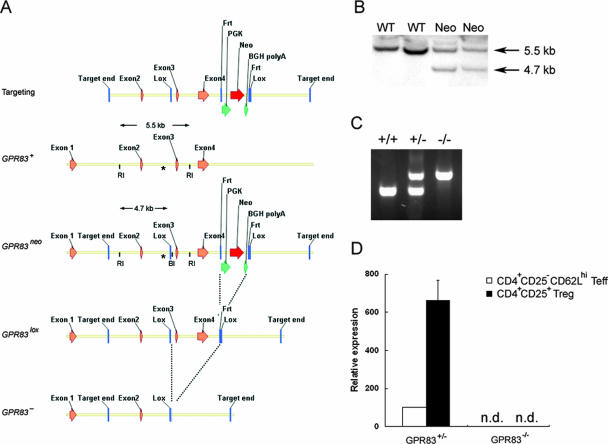
To understand an in vivo role for GPR83 in Treg-cell biology, we generated GPR83-deficient mice by using the strategy outlined in Fig. 1A. Southern blot analysis was used to screen for successful homologous recombination (Fig. 1B). Mice with the germ line abolition of the Gpr83 gene were generated upon the excision of the loxP “floxed” allele by crossing female Gpr83+/flox mice to Cre deleter mice expressing the ubiquitously expressed Cre transgene under the cytomegalovirus promoter (Gpr83−) (Fig. 1A). The transmission of the targeted allele was determined by genomic PCR analysis (Fig. 1C). In mutant mice, GPR83 message was undetectable by real-time PCR in both Treg and non-Treg cells, in contrast to readily detectable GPR83 mRNA in the corresponding cell subsets isolated from littermate control mice (Fig. 1D).
FIG. 1.
Generation of GPR83-deficient mice. (A) Schematic representation of the targeting construct used to generate mice harboring a GPR83 knockout allele. Two loxP sites were introduced, one prior to exon 3 and one after exon 4, as described in Materials and Methods. Chimeric males were mated first to FLP deleter mice to excise the neomycin resistance gene (neo) and next to Cre deleter mice to generate the GPR83 null allele. BGH, bovine growth hormone; RI, EcoRI; BI, XbaI. (B) Southern blot analysis was performed to screen positive ES clones for evidence of homologous recombination. Genomic DNA was digested with EcoRI and XbaI and hybridized with the probe depicted (labeled with an asterisk) in the panel. (C) PCR screening of Gpr83-targeted allele transmission. Breeding was set up by using a heterozygous male crossed with homozygous mutant females. (D) The expression of GPR83 mRNA in purified CD4+ CD25− CD62Lhi T cells (naïve effector T cells [Teff]) and CD4+ CD25+ CD62Lhi T cells (Treg cells) from GPR83−/− mice and control GPR83+/− littermates was measured by real-time RT-PCR (n.d., none detectable).
GPR83 is not required for the development and function of Treg cells.
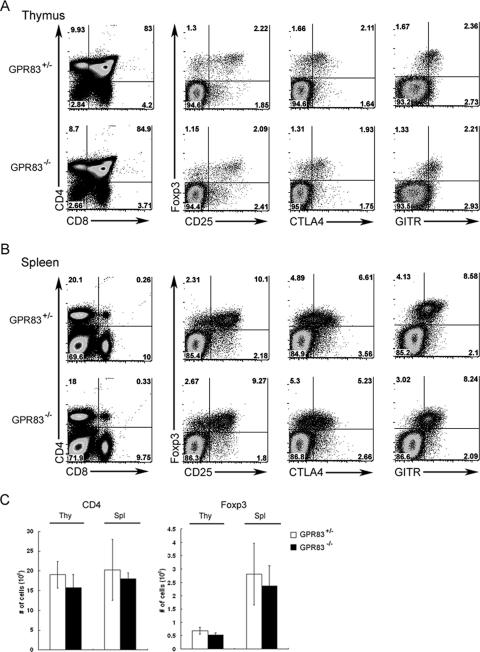
GPR83-deficient mice were viable and exhibited Mendelian inheritance of the targeted allele. Mice remained healthy, with no sign of autoimmunity or other pathology detectable at up to 12 to 14 months of age. The sizes of thymocyte, peripheral CD4 and CD8 T-cell, B-cell, and dendritic cell subsets and the phenotypes of the cell subsets in mutant mice were indistinguishable from those in wild-type littermates (Fig. 2 and data not shown). In addition, naïve T cells from GPR83-deficient and nondeficient littermates mounted comparable proliferative responses upon TCR engagement (Fig. 3A). A recent study showed that the overexpression of GPR83 upon retroviral transduction failed to induce Foxp3 expression or to confer suppression capacity on naïve T-cell populations (12). However, since GPR83 is up-regulated in Treg cells, we sought to assess a role for GPR83 in Treg-cell development. GPR83-deficient mice harbored normal numbers of Foxp3+ T cells with a surface phenotype indistinguishable from that of Foxp3+ Treg cells in wild-type littermates, as illustrated by comparable levels of CD25, cytotoxic-T-lymphocyte antigen 4, and glucocorticoid-induced tumor necrosis factor receptor expression (Fig. 2). Other characteristic features of Treg cells, such as elevated CD103 and reduced IL-7 receptor alpha protein expression, were unaffected by GPR83 deficiency (data not shown). Consistent with these results, we also observed a comparable increase in granzyme B and Helios and a similarly decreased level of phosphodiesterase 3B in mutant and wild-type Treg cells (data not shown).
FIG. 2.
Phenotypic analysis of CD4 and CD8 T-cell subsets and surface phenotype of Treg cells in GPR83-deficient mice. (A and B) Flow cytometric analysis of CD4 and CD8 cell subsets in the thymus (A) and spleen (B). Foxp3, CD25, cytotoxic-T-lymphocyte antigen 4 (CTLA4), and glucocorticoid-induced tumor necrosis factor receptor (GITR) expression by thymic CD4 single-positive thymocytes (A) or splenic CD4 T cells (B) was analyzed by flow cytometry. Representative data are shown. More than five animals in each group were analyzed in three independent experiments. (C) Numbers of CD4 single-positive cells in thymus tissue (thy) and of CD4+ T cells in spleen tissue (spl) as well as numbers of CD4+ Foxp3+ Treg cells in both thymus and spleen tissues are indicated. The data are presented as means ± standard deviations (SD).
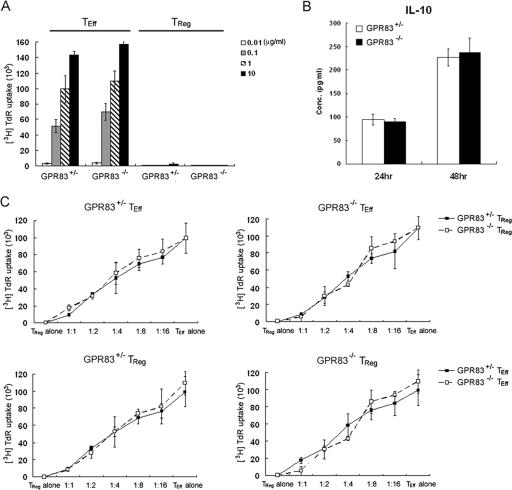
FIG. 3.
Functional analysis of GPR83-deficient Treg cells. (A) Proliferative responses of naïve CD4 T cells and Treg cells isolated from Gpr83−/− or control Gpr83+/− mice to the indicated concentrations of CD3 antibodies in the presence of irradiated T-cell-depleted splenocytes. The data are presented as the mean [3H]TdR incorporation for triplicate 72-h cultures ± SD. TEff, effector T cells. (B) IL-10 production by Gpr83−/− or Gpr83+/− Treg cells in response to plate-bound anti-CD3 and anti-CD28 antibody was measured by enzyme-linked immunosorbent assay kits after 24 and 48 h of culture. The data are shown as the mean IL-10 concentration for triplicate cultures ± SD. (C) In vitro analysis of suppressor capacity of GPR83-deficient Treg cells. Responder CD4 T cells and different numbers of Treg cells isolated from Gpr83−/− or control Gpr83+/− mice were cocultured for 72 h in the presence of anti-CD3 and irradiated T-cell-depleted splenocytes. The data shown as mean [3H]TdR incorporation for the triplicate cultures represent one of three identical experiments.
Next, we tested whether Treg cells could exhibit normal suppressor activity in the absence of GPR83 expression. As shown in Fig. 3A, CD62Lhi CD25hi Treg cells isolated from GPR83-deficient mice remained anergic in response to anti-CD3 in vitro, like those isolated from wild-type littermate control mice. Recently, Buer and colleagues suggested that GPR83 overexpression is associated with increased IL-10 expression (12). However, we have found no significant difference in levels of IL-10 secretion by Treg cells upon activation in vitro in the absence or presence of GPR83 (Fig. 3B). Finally, we examined the suppressor activity of GPR83-deficient Treg cells. As shown in Fig. 3C, GPR83-deficient and nondeficient Treg cells were similarly efficient at the suppression of wild-type T-cell-proliferative responses. Furthermore, the proliferative responses of naïve T cells from GPR83-deficient mice were suppressed by GPR83 mutant and wild-type Treg cells in similar fashions (Fig. 3C). Together, these data demonstrated that GPR83 is dispensable for the development and function of Foxp3+ Treg cells.
GPR83 deficiency results in a modest reduction in the efficiency of Foxp3 up-regulation upon Foxp3− CD4+-T-cell activation in the presence of TGF-β.
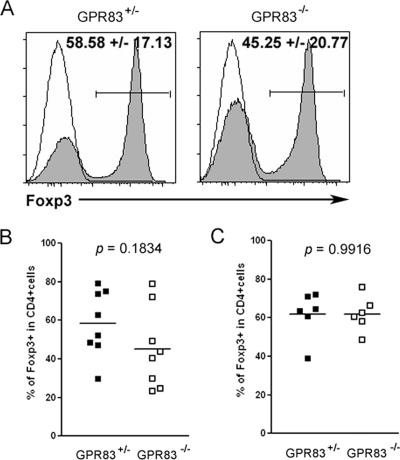
Although the analysis of TCR repertoires of thymic and peripheral Treg cells suggested that a majority of peripheral Treg cells likely acquire Foxp3 expression in the thymus, peripheral nonregulatory T cells are also able to up-regulate Foxp3 expression upon TCR stimulation in vitro in the presence of TGF-β (2, 14). In addition, chronic antigenic exposure of T cells in vivo or T-cell proliferation under lymphopenic conditions can facilitate the induction of Foxp3 expression in antigen-specific T cells (4, 15). A recent study utilizing retroviral transduction of naïve CD4+ T cells with GPR83 suggested that GPR83 augments the induction of Foxp3 expression in non-Treg cells (12). However, GPR83 mRNA is expressed, albeit at a low level, in Foxp3− T cells. Thus, it is not clear whether GPR83 is absolutely required for the Foxp3 induction in peripheral T cells. To address this issue, we examined an effect of GPR83 deficiency on the induction of Foxp3 expression in T cells subjected to TCR stimulation in vitro in the presence of TGF-β. In vitro Treg-cell conversion was performed as described previously (16). In these experiments, CD4+ CD25− CD62Lhi naïve T cells isolated from GPR83-deficient or littermate control mice were cocultured with T-cell-depleted splenocytes as APCs with or without recombinant TGF-β. Similar to the results of previous studies, ∼60% of T cells isolated from wild-type mice up-regulated Foxp3 while a modest decrease in the induction of Foxp3 expression was observed in the absence of GPR83 (∼45% of T cells expressed Foxp3) (Fig. 4A and B). This trend, albeit statistically insignificant, was observed in multiple experiments. Interestingly, this trend was not observed upon activation by plate-bound anti-CD3 and anti-CD28 in the absence of APCs (Fig. 4C). Collectively, in agreement with the findings of the previous study utilizing forced expression of GPR83, our results suggested a possible auxiliary role of GPR83 signaling in TGF-β-dependent Foxp3 induction and provided an indirect argument in favor of GPR83 interaction with a putative ligand supplied by APCs.
FIG. 4.
Moderate diminution in the efficiency of TGF-β-facilitated Foxp3 induction in the absence of GPR83. (A) Flow cytometric analysis of Foxp3 expression induced in sorted naïve CD25− CD62Lhi CD4+ T cells upon stimulation with anti-CD3 and irradiated splenic APCs in the presence (shaded histogram) or absence (open histogram) of recombinant TGF-β1. Percentages of Foxp3+ cells within the Ly5.1− CD4+-T-cell population are indicated. (B and C) Percentages of Foxp3+ cells induced in the presence of TGF-β1 upon the stimulation of naïve T cells with soluble CD3 antibody in the presence of APCs (B) or plate-bound anti-CD3 and anti-CD28 antibody in the absence of APCs (C). Each symbol represents one independent experiment with two to three mice per group.
GPR83 is not involved in the generation of the Th17 cell population.
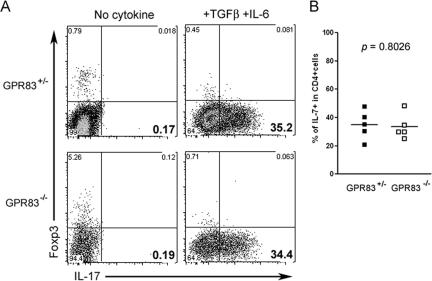
While TGF-β has been shown to be essential for Foxp3 induction in peripheral T cells, recent studies have also demonstrated an indispensable role of TGF-β in the generation of Th17 cells with the combination of IL-6 signaling (1, 17, 25). In contrast to the Foxp3+ Treg-cell population, with its dedicated suppressor function, Th17 cells have been shown to be involved in immunity to extracellular bacterial and yeast infection. In addition, Th17 cells have been implicated in many autoimmune disorders in mice and humans, such as experimental autoimmune encephalitis and collagen-induced arthritis (28). Hence, we sought to examine the possibility that GPR83 signaling may contribute to the development of Th17 cells. In agreement with the results in a number of published studies (1, 17, 25), the activation of naïve CD4 T cells in the presence of both TGF-β and IL-6 induced the differentiation of Th17 cells while Foxp3 up-regulation was abolished. However, unlike the mild reduction in Foxp3 induction in GPR83-deficient T cells, no detectable difference in the frequency of IL-17-producing T cells in the presence or absence GPR83 could be found (Fig. 5).
FIG. 5.
Th17 differentiation in vitro is unaffected by GPR83 deficiency. (A) Purified naïve CD25− CD62Lhi CD4+ T cells were stimulated with soluble CD3 antibodies and Ly5.1-marked T-cell-depleted splenocytes in the presence or absence of recombinant TGF-β1 and IL-6 for 4 days. During the last 6 h, cells were cultured with phorbol myristate acetate-ionomycin and IL-17 secretion by Ly5.1− CD4+ T cells was measured using intracellular flow cytometric analysis. (B) Percentages of IL-17-expressing T cells among G protein-coupled receptor-deficient and nondeficient CD4 T cells activated under Th17 polarizing conditions. Each square represents an average number of IL-17+ cells in an individual experiment with two to three mice per group.
GPR83 plays a dispensable role in Foxp3 induction in Foxp3− T cells in vivo.
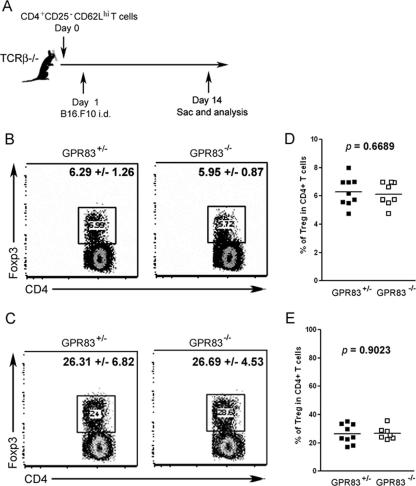
The results thus far suggested that GPR83 can modestly enhance the induction of Foxp3 expression in peripheral Foxp3− CD4+ T cells in vitro. Next, we sought to examine whether GPR83 is also required for Foxp3 up-regulation in Foxp3− CD4+ T cells in vivo. The adoptive transfer of naïve Foxp3− CD4 T cells into T-cell-deficient recipient mice results in the acquisition of Foxp3 expression by some transferred T cells upon their “homeostatic” proliferation (4). In addition, accumulating experimental evidence strongly supports the possibility that naïve T cells acquire Foxp3 expression in the presence of tumors (27, 29). These tumor-induced Treg cells were shown to accumulate within the tumor as well as in the tumor-draining LNs and were implicated in the attenuation of antitumor immune responses (3, 19). Therefore, we decided to examine whether GPR83 has a role in Foxp3 induction in non-Treg cells in tumor-bearing mice. For this purpose, naïve CD4+ CD25− CD62Lhi T cells from GPR83-deficient or control mice, including Foxp3+ T cells at a frequency of ∼1% or less, were transferred into TCRβδ−/− mice (data not shown), and the TCRβδ−/− mice were injected intradermally with B16.F10 melanoma cells 1 day after T-cell transfer. Thirteen days after tumor inoculation, mice were sacrificed and donor T cells were examined for Foxp3 expression by using flow cytometry (Fig. 6A). Since TGF-β is abundant in the normal gastrointestinal tract and has been implicated in peripheral Foxp3 induction (5), we first examined the presence of Foxp3+ T cells in mesenteric LNs draining the gastrointestinal tract. Homeostatic proliferation resulted in a level of induction of Foxp3 comparable to that in normal mice independent of GPR83 expression, as illustrated by the observation of ∼6% of donor GPR83-deficient or nondeficient T cells in mesenteric LNs 2 weeks after transfer (Fig. 6B and C). Furthermore, as shown in Fig. 6D and E, an analysis of Foxp3+ cells within GPR83-deficient and wild-type CD4+ TCRβ+ cell populations in the tumor-draining axillary and inguinal LNs showed comparable ∼4-fold increases in their frequencies in draining versus nondraining LNs. Taken together, these results indicate that GPR83 is not required for Foxp3 induction in nonregulatory T cells in vivo.
FIG. 6.
GPR83 is dispensable for Foxp3 up-regulation in CD25− CD62Lhi CD4+ T cells facilitated by homeostatic proliferation or tumor growth. (A) Schematic representation of the experimental design. Foxp3 expression was examined in tumor-draining and distal LNs 14 days after the adoptive transfer of Gpr83−/− or Gpr83+/− CD25− CD62Lhi CD4+ T cells into TCRβδ−/− mice followed by the inoculation of B16.F10 melanoma cells. i.d., intradermal; sac, sacrifice. (B to E) Flow cytometric analysis of Foxp3+ cells within the CD4 T-cell population present in non-tumor-draining mesenteric LNs (B and C) and tumor-draining axillary and inguinal LNs (D and E). (B and D) CD4 and Foxp3 expression in LN cells gated for TCRβ+ cells is shown. (C and E) Each symbol represents the proportion of Foxp3+ T cells in a single animal. Results are from two independent experiments each with four to five animals per group.
DISCUSSION
GPR83, an orphan G-protein coupled receptor, is expressed at high levels in Foxp3+ Treg cells, and forced Foxp3 expression in non-Treg cells results in GPR83 up-regulation, suggesting a close link between Foxp3 and GPR83 expression (7, 12, 24). However, the functional significance of GPR83 expression in Treg cells has remained unclear. Our study showed that GPR83-deficient mice harbor normal numbers of thymic and peripheral Foxp3+ cells with no significant changes in surface phenotypes or functions. GPR83-deficient Treg cells were anergic, as revealed by in vitro TCR stimulation, and were able to suppress target T-cell populations from either GPR83-deficient or wild-type control mice. Moreover, in contrast to a previously published report suggesting a role for GPR83 in the regulation of IL-10 secretion (12), we found unaltered IL-10 production by Treg cells in the absence of GPR83. In addition to the normal and fully functional Treg cell subset, the non-Treg CD4 T-cell population in GPR83-deficient mice was numerically and functionally intact. These cells were able to proliferate and secrete effector cytokines like gamma interferon in response to TCR stimulation at a level comparable to that in wild-type mice (data not shown). Furthermore, the elimination of the GPR83 conditional allele specifically in Treg cells induced by a Cre recombinase gene knocked into the 3′ untranslated region of the Foxp3 locus did not lead to an impairment in suppressor Treg-cell function, and the resulting mice remained healthy (M. A. Gavin, J. P. Rasmussen, and A. Y. Rudensky, unpublished observations). Altogether, we found no detectable adverse effects of GPR83 deficiency on Treg-cell development and function.
A recent study suggested that the overexpression of GPR83 in Foxp3− T cells facilitates the acquisition of Foxp3 expression in vivo in an inflammatory setting (12). We were able to detect at best a slightly reduced efficiency of Foxp3 induction in GPR83-deficient naïve peripheral T cells upon their activation in vitro in the presence of TGF-β. This mild difference cannot be explained by attenuated TGF-β signaling in GPR83-deficient T cells, since we found comparable levels of Smad2/3 phosphorylation and nuclear translocation in GPR83-deficient and nondeficient T cells (data not shown). Another, albeit indirect, argument against a general effect of GPR83 deficiency on TGF-β signaling is our observation that, while Foxp3 induction was diminished in the absence of GPR83, the generation of Th17 cells, another TGF-β-dependent T-cell lineage, was not affected. Finally, it is noteworthy that a subtle difference in Foxp3 induction in GPR83-deficient and wild-type T cells was not observed in the absence of APCs, suggesting that APCs may serve as a source of putative GPR83 ligand and that GPR83-ligand interactions can facilitate the acquisition of Foxp3 by peripheral T cells.
One caveat to this conclusion is the lack of a demonstrable difference in the efficiency of Foxp3 acquisition by naïve T cells in vivo in the absence or presence of GPR83, whereas it was suggested by others that GPR83 may facilitate the acquisition of Foxp3 expression (12). Although we cannot exclude the possibility that under certain conditions GPR83 signaling may be absolutely necessary, our results suggest that in vivo endogenous GPR83 plays a redundant role in the induction of Foxp3 expression in peripheral T cells, perhaps due to signals provided by other receptors, such as neuropeptide Y receptor 2, able to compensate for GPR83 deficiency (21). Moreover, it is possible that the discrepancy between our results and those of the study by Buer and coauthors is due to the reliance of the latter on the overexpression of GPR83 in naïve Foxp3− T cells, which normally express GPR83 at low levels in both resting and activated states.
Further analysis of GPR83 mutant mice may be able to address the potential redundancy in GPR83 signaling in peripheral Treg-cell differentiation in vivo. Furthermore, GPR83-deficient mice will be instrumental in the generation of GPR83-specific antibodies and in the evaluation of the utility of such a reagent for the specific detection of Treg cells in genetically unmodified mice and in humans. Finally, these mice will serve as a valuable resource for studying a recently proposed role for GPR83 in the control of feeding and emotional behavior (20). In fact, we observed a trend of GPR83-deficient mice to become obese with age (L.-F. Lu and A. Y.Rudensky, unpublished observation).
In conclusion, through the analysis of GPR83-deficient mice, we examined a role for GPR83 in Treg-cell development and function. Our studies demonstrated that GPR83 is dispensable for thymic development and the maintenance of Treg cells and for their suppression. Although our in vitro studies suggested a subtle, but detectable, role for GPR83 in potentiating TGF-β-dependent Foxp3 induction in non-Treg cells, in vivo experiments indicate that the role of GPR83 in this process is redundant. Overall, the findings of our study suggest that under physiologic settings GPR83 does not play a nonredundant role in Treg-cell biology.
Acknowledgments
We thank T. Chu and L. Karpik for great care of mice. We also thank members of the Rudensky lab for discussions.
This study was supported by the Howard Hughes Medical Institute (A.Y.R.), the Leukemia and Lymphoma Society (L.-F.L.), and the U.S. National Institutes of Health (A.Y.R.).
Footnotes
Published ahead of print on 24 September 2007.
REFERENCES
- 1.Bettelli, E., Y. Carrier, W. Gao, T. Korn, T. B. Strom, M. Oukka, H. L. Weiner, and V. K. Kuchroo. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441:235-238. [DOI] [PubMed] [Google Scholar]
- 2.Chen, W., W. Jin, N. Hardegen, K. J. Lei, L. Li, N. Marinos, G. McGrady, and S. M. Wahl. 2003. Conversion of peripheral CD4+ CD25− naive T cells to CD4+ CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 198:1875-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curiel, T. J., G. Coukos, L. Zou, X. Alvarez, P. Cheng, P. Mottram, M. Evdemon-Hogan, J. R. Conejo-Garcia, L. Zhang, M. Burow, Y. Zhu, S. Wei, I. Kryczek, B. Daniel, A. Gordon, L. Myers, A. Lackner, M. L. Disis, K. L. Knutson, L. Chen, and W. Zou. 2004. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 10:942-949. [DOI] [PubMed] [Google Scholar]
- 4.Curotto de Lafaille, M. A., A. C. Lino, N. Kutchukhidze, and J. J. Lafaille. 2004. CD25− T cells generate CD25+ Foxp3+ regulatory T cells by peripheral expansion. J. Immunol. 173:7259-7268. [DOI] [PubMed] [Google Scholar]
- 5.Faria, A. M., and H. L. Weiner. 2005. Oral tolerance. Immunol. Rev. 206:232-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farley, F. W., P. Soriano, L. S. Steffen, and S. M. Dymecki. 2000. Widespread recombinase expression using FLPeR (flipper) mice. Genesis 28:106-110. [PubMed] [Google Scholar]
- 7.Fontenot, J. D., J. P. Rasmussen, M. A. Gavin, and A. Y. Rudensky. 2005. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 6:1142-1151. [DOI] [PubMed] [Google Scholar]
- 8.Fontenot, J. D., and A. Y. Rudensky. 2005. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat. Immunol. 6:331-337. [DOI] [PubMed] [Google Scholar]
- 9.Gavin, M., and A. Rudensky. 2003. Control of immune homeostasis by naturally arising regulatory CD4+ T cells. Curr. Opin. Immunol. 15:690-696. [DOI] [PubMed] [Google Scholar]
- 10.Gavin, M. A., S. R. Clarke, E. Negrou, A. Gallegos, and A. Rudensky. 2002. Homeostasis and anergy of CD4+ CD25+ suppressor T cells in vivo. Nat. Immunol. 3:33-41. [DOI] [PubMed] [Google Scholar]
- 11.Gavin, M. A., J. P. Rasmussen, J. D. Fontenot, V. Vasta, V. C. Manganiello, J. A. Beavo, and A. Y. Rudensky. 2007. Foxp3-dependent programme of regulatory T-cell differentiation. Nature 445:771-775. [DOI] [PubMed] [Google Scholar]
- 12.Hansen, W., K. Loser, A. M. Westendorf, D. Bruder, S. Pfoertner, C. Siewert, J. Huehn, S. Beissert, and J. Buer. 2006. G protein-coupled receptor 83 overexpression in naive CD4+ CD25− T cells leads to the induction of Foxp3+ regulatory T cells in vivo. J. Immunol. 177:209-215. [DOI] [PubMed] [Google Scholar]
- 13.Harrigan, M. T., G. Baughman, N. F. Campbell, and S. Bourgeois. 1989. Isolation and characterization of glucocorticoid- and cyclic AMP-induced genes in T lymphocytes. Mol. Cell. Biol. 9:3438-3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsieh, C. S., Y. Liang, A. J. Tyznik, S. G. Self, D. Liggitt, and A. Y. Rudensky. 2006. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat. Immunol. 7:401-410. [DOI] [PubMed] [Google Scholar]
- 15.Jaeckel, E., K. Kretschmer, I. Apostolou, and H. von Boehmer. 2006. Instruction of Treg commitment in peripheral T cells is suited to reverse autoimmunity. Semin. Immunol. 18:89-92. [DOI] [PubMed] [Google Scholar]
- 16.Kim, J. M., and A. Rudensky. 2006. The role of the transcription factor Foxp3 in the development of regulatory T cells. Immunol. Rev. 212:86-98. [DOI] [PubMed] [Google Scholar]
- 17.Mangan, P. R., L. E. Harrington, D. B. O'Quinn, W. S. Helms, D. C. Bullard, C. O. Elson, R. D. Hatton, S. M. Wahl, T. R. Schoeb, and C. T. Weaver. 2006. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 441:231-234. [DOI] [PubMed] [Google Scholar]
- 18.McHugh, R. S., M. J. Whitters, C. A. Piccirillo, D. A. Young, E. M. Shevach, M. Collins, and M. C. Byrne. 2002. CD4+ CD25+ immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity 16:311-323. [DOI] [PubMed] [Google Scholar]
- 19.Quezada, S. A., K. S. Peggs, M. A. Curran, and J. P. Allison. 2006. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J. Clin. Investig. 116:1935-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sah, R., L. M. Pritchard, N. M. Richtand, R. Ahlbrand, K. Eaton, F. R. Sallee, and J. P. Herman. 2005. Expression of the glucocorticoid-induced receptor mRNA in rat brain. Neuroscience 133:281-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sah, R., S. L. Parker, S. Sheriff, K. Eaton, A. Balasubramaniam, and F. R. Sallee. 2007. Interaction of NPY compounds with the rat glucocorticoid-induced receptor (GIR) reveals similarity to the NPY-Y2 receptor. Peptides 28:302-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakaguchi, S., N. Sakaguchi, J. Shimizu, S. Yamazaki, T. Sakihama, M. Itoh, Y. Kuniyasu, T. Nomura, M. Toda, and T. Takahashi. 2001. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol. Rev. 182:18-32. [DOI] [PubMed] [Google Scholar]
- 23.Shevach, E. M. 2002. CD4+ CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2:389-400. [DOI] [PubMed] [Google Scholar]
- 24.Sugimoto, N., T. Oida, K. Hirota, K. Nakamura, T. Nomura, T. Uchiyama, and S. Sakaguchi. 2006. Foxp3-dependent and -independent molecules specific for CD25+ CD4+ natural regulatory T cells revealed by DNA microarray analysis. Int. Immunol. 18:1197-1209. [DOI] [PubMed] [Google Scholar]
- 25.Veldhoen, M., R. J. Hocking, R. A. Flavell, and B. Stockinger. 2006. Signals mediated by transforming growth factor-beta initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat. Immunol. 7:1151-1156. [DOI] [PubMed] [Google Scholar]
- 26.von Boehmer, H. 2005. Mechanisms of suppression by suppressor T cells. Nat. Immunol. 6:338-344. [DOI] [PubMed] [Google Scholar]
- 27.Wang, H. Y., D. A. Lee, G. Peng, Z. Guo, Y. Li, Y. Kiniwa, E. M. Shevach, and R. F. Wang. 2004. Tumor-specific human CD4+ regulatory T cells and their ligands: implications for immunotherapy. Immunity 20:107-118. [DOI] [PubMed] [Google Scholar]
- 28.Weaver, C. T., L. E. Harrington, P. R. Mangan, M. Gavrieli, and K. M. Murphy. 2006. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity 24:677-688. [DOI] [PubMed] [Google Scholar]
- 29.Zhou, G., and H. I. Levitsky. 2007. Natural regulatory T cells and de novo-induced regulatory T cells contribute independently to tumor-specific tolerance. J. Immunol. 178:2155-2162. [DOI] [PubMed] [Google Scholar]