Abstract
We previously reported that Otx2 is essential for photoreceptor cell fate determination; however, the functional role of Otx2 in postnatal retinal development is still unclear although it has been reported to be expressed in retinal bipolar cells and photoreceptors at postnatal stages. In this study, we first examined the roles of Otx2 in the terminal differentiation of photoreceptors by analyzing Otx2; Crx double-knockout mice. In Otx2+/−; Crx−/− retinas, photoreceptor degeneration and downregulation of photoreceptor-specific genes were much more prominent than in Crx−/− retinas, suggesting that Otx2 has a role in the terminal differentiation of the photoreceptors. Moreover, bipolar cells decreased in the Otx2+/−; Crx−/− retina, suggesting that Otx2 is also involved in retinal bipolar-cell development. To further investigate the role of Otx2 in bipolar-cell development, we generated a postnatal bipolar-cell-specific Otx2 conditional-knockout mouse line. Immunohistochemical analysis of this line showed that the expression of protein kinase C, a marker of mature bipolar cells, was significantly downregulated in the retina. Electroretinograms revealed that the electrophysiological function of retinal bipolar cells was impaired as a result of Otx2 ablation. These data suggest that Otx2 plays a functional role in the maturation of retinal photoreceptor and bipolar cells.
The vertebrate neural retina is comprised of six types of neurons and one type of glial cell, all derived from one population of multipotent progenitors (38, 39, 41). Transcription factors such as homeobox and basic helix-loop-helix factors have been known to play pivotal roles in the specification and development of retinal cell subtypes. Among the Otx-like homeobox genes, Otx2 and Crx play critical roles in retinal photoreceptor development. The expression of Otx2 covers most of the forebrain and midbrain neuroepithelium, including the eye domain, during development (32). Complete elimination of Otx2 functions in mice by gene targeting results in the absence of the forebrain and embryonic lethality (1, 3, 27). In a previous study, we have shown that Otx2 is essential and sufficient for the cell fate determination of retinal photoreceptors (29). Crx, on the other hand, is reported to be expressed abundantly in retinal photoreceptors and pinealocytes and also weakly in retinal bipolar cells (12, 19). It has also been reported that Crx regulates various photoreceptor-specific genes (12, 19, 20). Mutations of human CRX are associated with three types of photoreceptor diseases: cone-rod dystrophy 2, retinitis pigmentosa, and Leber's congenital amaurosis (15, 16, 33, 36). A gene-targeting study has revealed that Crx is essential for the terminal differentiation of photoreceptors and normal circadian entrainment (20). Thus, Otx2 and Crx have distinct roles in retinal photoreceptor development although their expression patterns in the retina overlap to some extent. However, they are structurally related transcription factors and can bind to a common DNA-binding sequence; therefore, it can be supposed that they have redundant roles in retinal development. It has been reported that Otx2 and Crx are expressed in the inner nuclear layer (INL) of the postnatal retina (4, 6), suggesting that they are involved in the development of retinal cells other than photoreceptors. In this study, we investigated the role of Otx2 in the developing postnatal retina. Histological analysis and examination of the expression of photoreceptor-specific genes in the Otx2+/−; Crx−/− retina suggested that Otx2 is also involved in the terminal differentiation of photoreceptors. Ablation of Otx2 in retinal progenitor cells with a Cre-expressing retrovirus led to a significant decrease in rod photoreceptors and bipolar cells, suggesting that Otx2 is involved also in retinal bipolar-cell development. Analysis of the Otx2 conditional-knockout (CKO) mouse line in which Otx2 is ablated in postnatal retinal bipolar cells revealed that immunohistochemical and electrophysiological maturation of the bipolar cells was impaired, suggesting that Otx2 is required for the terminal differentiation of bipolar cells.
MATERIALS AND METHODS
Generation of knockout mice.
To study the roles of Otx2 and Crx, we mated Otx2 knockout mice (27) with Crx knockout mice (20) to obtain four genotypes, i.e., the wild type (WT), Otx2+/−, Crx−/−, and Otx2+/−; Crx−/−. We established an Otx2 CKO mouse line by mating an Otx2/flox (37) mouse line with an L7-Cre knock-in (31) mouse line, which expresses cre recombinase under the control of the L7 promoter, in order to study the role of Otx2 in postnatal retinal bipolar cells. We analyzed Otx2flox/flox; L7-Cre-KI+/− mice as Otx2 CKO mice and used Otx2flox/flox; L7-Cre-KI+/+ mice as controls. We also generated an Otx2 CKO mouse line in the Crx−/− background by mating the Crx−/− mouse line with the Otx2 CKO mouse line. Phenotypic analysis of three or more independent litters was performed. All procedures conformed to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research, and the procedures were approved by the Institutional Safety Committee on Recombinant DNA Experiments and the Animal Research Committee of the Osaka Bioscience Institute.
Tissue sectioning.
Mouse eyeballs and pineal glands were fixed with 4% formaldehyde in phosphate-buffered saline (PBS) for immunostaining. To make plastic sections, we fixed the eyeballs with 4% formaldehyde and 1% glutaraldehyde in PBS, dehydrated them with ethyl alcohol, and embedded them in Historesin (Leica Microsystems, Wetzlar, Germany).
Antibodies and immunostaining.
We acquired mouse monoclonal antibodies against S-100β (Sigma, St. Louis, MO), protein kinase C (PKC) (Sigma), rhodopsin (RET-P1) (Sigma), and calbindin (Sigma); goat polyclonal antibodies against Brn3b (Santa Cruz Biotech, Santa Cruz, CA) and Otx2 (R&D Systems, Minneapolis, MN); and rabbit polyclonal antibodies against Pax6 (Zymed, South San Francisco, CA), S-opsin (Chemicon, Temecula, CA), phospho-histone H3 (Upstate, Lake Placid, NY), and active caspase 3 (Promega, Madison, WI). We raised polyclonal antibodies against M-opsin (Oriental Bioservice, Tokyo, Japan) and Chx10 (MBL, Nagoya, Japan) in rabbits. The antibody against Chx10 was raised as described previously (24), with some modification. We used either Alexa Fluor 488 (Invitrogen, Carlsbad, CA)- or Cy3 (Jackson ImmunoResearch Laboratories, West Grove, PA)-conjugated secondary antibodies. Cell nuclei were counterstained with TOTO-3 (Invitrogen). The specimens were observed under a laser confocal microscope (LSM510; Carl Zeiss, Oberkochen, Germany).
In situ hybridization.
In situ hybridization was performed as described previously (18, 19). An Otx2 probe was obtained as described previously (27).
Electron microscopy.
The animals were perfused with 4% glutaraldehyde in PBS, and the eyecups were dehydrated with ethyl alcohol. The ultrathin sections were stained with uranyl acetate and lead citrate and viewed on a transmission electron microscope (H-7650; Hitachi, Tokyo, Japan).
Lineage analysis.
In vivo infection of retinas was carried out by injection of retroviruses into postnatal day 0 (P0) mouse eyes. Infected retinas were dissected after 3 weeks, fixed, and stained for alkaline phosphatase. The procedures used for sectioning and counting of infected clones were previously described (19). More than 1,000 clones were counted for each virus.
RNA isolation and real-time PCR.
Total RNAs from mouse retinas were isolated with TRIzol reagent (Invitrogen). Total RNA concentration was quantified spectrophotometrically. We synthesized cDNA from 1 μg total RNA in a 20-μl reaction mixture consisting of 10 U of Transcriptor reverse transcriptase according to the manufacturer's (Roche Diagnostics) instructions with 60 μM random primers (Roche Diagnostics), 1 μM each deoxynucleoside triphosphate (Invitrogen, San Diego, CA), and Transcriptor RT reaction buffer. We stored the cDNAs at −20°C prior to real-time PCR. Real-time PCRs were performed with the SYBR green reaction kit according to the manufacturer's instructions with the LightCycler (Roche Diagnostics). We diluted the cDNAs (2 μl each of a 1/10 dilution) to a volume of 20 μl with a PCR mixture (LightCycler DNA Master FastStart Plus SYBER Green Kit I) containing a final concentration of 0.5 μM primers. The cDNA contents of all samples were normalized for housekeeping gene expression (Gapdh). The relative levels of Rho, Rbp3, Pde6b, Sag, Bhlhb4, Cre, and Gapdh transcripts were determined with the following primer sets: Rho, 5′-TGCCACACTTGGAGGTGAAATC-3′ and 5′-ATGCGGGTGACTTCCTTCTCTG-3′; Rbp3, 5′-ATAGTGGTCCTGCGTGCTAAGG-3′ and 5′-GGTGCCTCGTCAAAGAAGTAAGAG-3′; Pde6b, 5′-ATCGTCTTCCCCCTGGACATTG-3′ and 5′-ATACCGTTCGCAGTTTAGATAGGC-3′; Sag, 5′-TTGTGAAGGGGAAGAAGGTGTATG-3′ and 5′-TGCTGAGGGAGACTGAGAGGTTC-3′; Bhlhb4, 5′-AGGTCCTTTGGAGAGGTCGTGG-3′ and 5′-TTCTGGGCTGTGGTCCGATTTGGGG-3′; Cre, 5′-GACGATGCAACGAGTGATGA-3′ and 5′-AGCATTGCTGTCACTTGGTC-3′; Gapdh, 5′-ACCACAGTCCATGCCATCAC-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′.
The relative expression levels of the genes were compared among the four genotypes and normalized to adjust the expression levels in the WT to 1.
Transfections and luciferase assay.
A reporter plasmid was generated by subcloning an 8.5-kb upstream fragment of the mouse Prkca gene into the pGL3 luciferase reporter vector (Promega). The Otx2 expression vector (pMIK-Otx2) was constructed previously (29). NIH 3T3 cells were used for the promoter activity assay. The procedures used for transfection were described previously (19).
Electroretinographic recordings.
Electroretinographic recordings were performed as described previously in detail (11). In brief, mice were dark adapted overnight and then anesthetized with an intramuscular injection of ketamine and xylazine. Electroretinograms (ERGs) were recorded with a gold wire loop electrode placed on the cornea. The mice were placed in a Ganzfeld bowl and stimulated with stroboscopic stimuli with a maximum intensity of 1.0 log candela-second (cd-s)/m2 (photopic units). Six levels of stimulus intensity ranging from −6.2 to 1.0 log cd-s/m2 were used for the scotopic ERG recordings, and four levels of stimuli ranging from −0.8 to 1.0 log cd-s/m2 were used for the photopic ERG recordings. The photopic ERGs were recorded on a rod-suppressing white background of 1.3 log cd/m2.
Statistical analysis.
All data were recorded as means ± standard deviations from three or more independent experiments. Statistical analysis was performed with Statview (SAS Institute, Cary, NC).
RESULTS
Retinal photoreceptor and bipolar-cell numbers decrease in the Otx2+/−; Crx−/− retina.
To examine the expression pattern of Otx2 in the postnatal retina, we performed in situ hybridization and immunostaining for Otx2. At P1, Otx2 was expressed diffusely in the outer layer of the retina (Fig. 1A and E). On the other hand, at the later stages, Otx2 was expressed strongly in the INL and weakly in the outer nuclear layer (ONL) (Fig. 1B to D and F to H) as previously reported (4). At the adult stage, subcellular localization of Otx2 in the photoreceptors changed from the nucleus to the cytoplasm (Fig. 1H), consistent with the previous report (4). Since Otx2 was expressed in postnatal photoreceptors, it raised the possibility that Otx2 plays a role in the terminal differentiation of the photoreceptors. To examine this issue, we mated Otx2 knockout mice with Crx knockout mice. Since homozygous mutation of Otx2 leads to embryonic lethality, we could obtain four genotypes, i.e., the WT, Otx2+/−, Crx−/−, and Otx2+/−; Crx−/−. These mice were all viable and fertile and showed no obvious abnormal behavior. Retinal sections showed that there was no obvious difference between WT and Otx2+/− mice at any stage observed (Fig. 2A, B, E, F, I, J, M, and N). In the Crx−/− retina, retinal organization was similar to that of the WT at P0 (Fig. 2C) and P7 (Fig. 2G); however, photoreceptors failed to develop inner and outer segments by P14 (Fig. 2K), followed by photoreceptor degeneration (Fig. 2O), which is consistent with our previous report (20). In the Otx2+/−; Crx−/− retina, there seemed to be no obvious abnormalities at P0 (Fig. 2D) but the development of the ONL, consisting of photoreceptor cell nuclei, appeared to be impaired at P7 (Fig. 2H). In addition, the decrease in the thickness of the ONL was much more prominent than in Crx−/− mice at P14 (Fig. 2L) and at 1 month of age (1M) (Fig. 2P). The pineal gland was hypoplastic in the Otx2+/−; Crx−/− mouse, while it developed normally in the other three genotypes (Fig. 2Q to T). Next, we performed immunostaining with various retinal cell type markers (Fig. 3). We counted the immunoreactive cells for each marker and the total cells in the microscopic field and calculated the percentages of immunoreactive cells for each marker. We also dissociated the retinas from the four genotypes and counted the total cells contained in the retina for each genotype. Then, we calculated the absolute number of immunoreactive cells per retina for each genotype and compared the totals among the four genotypes to avoid a bias due to the decrease in the total cell number in the Crx−/− and Otx2+/−; Crx−/− retinas. The numbers of immunoreactive cells for Brn3b (ganglion cell marker), Pax6 (ganglion and amacrine-cell marker), S-100β (Müller cell marker), and calbindin (horizontal-cell marker) were similar among the four genotypes (Fig. 3M). On the other hand, the numbers of immunoreactive cells for rhodopsin (rod photoreceptor marker) (Fig. 3A to D), M-opsin (M-cone photoreceptor marker), and S-opsin (S-cone photoreceptor marker), which decreased in the Crx−/− retina, much more obviously decreased in the Otx2+/−; Crx−/− retina (Fig. 3M). Interestingly, the numbers of immunoreactive cells for bipolar-cell markers, Chx10 (Fig. 3E to H) and PKC (Fig. 3I to L), decreased in the Otx2+/−; Crx−/− retina, while they did not significantly change in the Crx−/− retina (Fig. 3M). Especially, the numbers of Chx10-, PKC-, and rhodopsin-positive cells in the Otx2+/−; Crx−/− retinas were significantly smaller than those of the other three genotypes (Scheffe's test). The numbers of S-opsin- and M-opsin-positive cells in the Otx2+/−; Crx−/− retinas were significantly smaller than those in the WT and Otx2+/− retinas (Scheffe's test). We also immunostained the specimens with anti-phospho-histone H3, a mitosis marker (Fig. 4A to D), and anti-active caspase 3, an apoptosis marker (Fig. 4E to H) and counted the phospho-histone H3 (Fig. 4I)- and active caspase 3 (Fig. 4J)-positive cells on the sections. The numbers of phospho-histone H3-positive cells were similar among the four genotypes (Fig. 4A to D and I), while a larger number of active-caspase-3-positive cells were observed in the Otx2+/−; Crx−/− retinas than in the retinas of the other genotypes (Fig. 4E to H and J), suggesting that the decrease in photoreceptors and bipolar cells in the Otx2+/−; Crx−/− retina was due to apoptosis.
FIG. 1.
Expression of Otx2 in the postnatal mouse retina. In situ hybridization (A to D) and immunohistochemistry (E to H) of Otx2 in the WT retina at P1 (A and E), P6 (B and F), P9 (C and G), and the adult stage (D and H) are shown. Cell nuclei were counterstained in blue (E to H). Scale bars, 100 μm (A to D) and 50 μm (E to H).
FIG. 2.
Phenotype of Otx2+/−; Crx−/− mice. Plastic sections of WT (A, E, I, M, and Q), Otx2+/− (B, F, J, N, and R), Crx−/− (C, G, K, O, and S), and Otx2+/−; Crx−/− (D, H, L, P, and T) mice are shown. Retinas at P0 (A to D), P7 (E to H), P14 (I to L), and 1M (M to P) and pineal glands (arrows) at P14 (Q to T) were stained with toluidine blue. Scale bars, 100 μm (A to H and Q to T) and 50 μm (I to P). GCL, ganglion cell layer; NBL, neuroblastic layer; RPE, retinal pigment epithelium; OS, outer segment; IS, inner segment.
FIG. 3.
Photoreceptor and bipolar-cell numbers decrease in Otx2+/−; Crx−/− retinas. Immunostaining of WT (A, E, and I), Otx2+/− (B, F, and J), Crx−/− (C, G, and K), and Otx2+/−; Crx−/− (D, H, and L) retinas at P14 is shown. The antibodies applied were antirhodopsin (A to D), anti-Chx10 (E to H), and anti-PKC (I to L). Scale bar, 50 μm. GCL, ganglion cell layer. (M) Total cell numbers of each retinal cell type in WT, Otx2+/−, Crx−/−, and Otx2+/−; Crx−/− retinas at P14. Error bars represent the standard deviations of the means. Statistical analysis was performed by ANOVA.
FIG. 4.
Apoptosis is promoted in Otx2+/−; Crx−/− retinas. (A to H) Immunostaining of WT (A and E), Otx2+/− (B and F), Crx−/− (C and G), and Otx2+/−; Crx−/− (D and H) retinas at P0 (A to D) or P7 (E to H) is shown. The antibodies applied were anti-phospho-histone H3 (A to D) and anti-active caspase 3 (E to H). Scale bar, 50 μm. GCL, ganglion cell layer; NBL, neuroblastic layer. (I and J) Numbers of phospho-histone H3 (I)- and active caspase 3 (J)-positive cells per section. Standard deviations are represented by error bars. Statistical analysis was performed by ANOVA and Scheffe's test. N. S., not significant.
Otx2 regulates photoreceptor-specific genes cooperatively with Crx.
To examine whether Otx2 regulates photoreceptor-specific genes, we compared the expression levels of photoreceptor-specific genes in the retinas among the four genotypes by real-time PCR. We previously reported that the expression of various photoreceptor-specific genes, including that for rhodopsin (rho), was reduced in Crx−/− mice (20). The expression of rho, noticeably downregulated in the Crx−/− retina, was almost completely absent in the Otx2+/−; Crx−/− retina (Fig. 5A). The expression of the β subunit of phosphodiesterase (Pde6b) and S-antigen (Sag), moderately downregulated in the Crx−/− retina, was strongly downregulated in the Otx2+/−; Crx−/− retina (Fig. 5C and D). These results suggest that Otx2 is involved in the transactivation of these photoreceptor-specific genes along with Crx. The results also suggest that one copy of Otx2 is not sufficient to upregulate rho, while Pde6b and Sag can be upregulated to some extent by only one copy of Otx2. Alternatively, it might be possible that Pde6b and Sag could be upregulated to a small extent by a factor or factors other than Otx2 and Crx. In contrast, the expression levels of interphotoreceptor retinoid-binding protein (Rbp3) among the four genotypes were not significantly changed by analysis of variance (ANOVA) (Fig. 5B), suggesting that one copy of Otx2 is sufficient for upregulating Rbp3 or that there is another factor(s) that regulates Rbp3 transcription.
FIG. 5.
Otx2 regulates photoreceptor-specific genes. (A to D) Quantitative RT-PCR of Rho (A), Rbp3 (B), Pde6b (C), and Sag (D) in WT, Otx2+/−, Crx−/−, and Otx2+/−; Crx−/− retinas at P10 is shown. Standard deviations are represented by error bars. Statistical analysis was performed by ANOVA and Scheffe's test.
Otx2 is required for retinal bipolar-cell development.
Since the numbers of immunoreactive cells for bipolar-cell markers in the Otx2+/−; Crx−/− retina decreased (Fig. 3E to M), we examined whether or not Otx2 is involved in retinal bipolar-cell development. We infected the retinal progenitor cells of Otx2flox/flox mice (37) with LIA-Cre virus at P0 to ablate both alleles of the Otx2 gene by cre-mediated recombination (Fig. 6A). As a control, retinal progenitors were also infected with LIA virus. At P21, when retinal development was complete, the infected retinas were stained for alkaline phosphatase activity and clonal analysis was performed by reconstructing serially sectioned retinas. Cell type was determined by the characteristic morphologies and locations of terminally differentiated cells. The cellular composition of clones infected with LIA-Cre virus was clearly altered relative to clones infected with LIA virus (Table 1). The LIA-Cre virus-infected clones exhibited a statistically significant increase in the percentage of clones containing amacrine cells (Fig. 6D). The LIA-Cre virus-infected clones also showed a clear decrease in the percentages of clones containing rod photoreceptors only (Fig. 6B), at least one rod photoreceptor (Fig. 6C), and bipolar cells (Fig. 6E), suggesting that Otx2 is required for retinal bipolar-cell development, as well as for retinal photoreceptor development. The percentages of clones containing Müller glia were not significantly changed (Fig. 6F). The clone sizes, indicating the number of cells per clone, significantly decreased upon infection with the LIA-Cre virus, suggesting a slight promotion of cell death (Fig. 6G).
FIG. 6.
Otx2 is required for retinal photoreceptor and bipolar-cell development. (A) Viral constructs used to express Cre. pLIA was derived from Moloney murine leukemia virus and was designed to express a marker gene, AP (alkaline phosphatase), through an internal ribosome entry site (IRES) sequence and another gene under the control of the long terminal repeat (LTR) promoter. (B to F) Percentages of clones containing rods only (B) and at least one rod (C), amacrine cell (D), bipolar cell (E), and Müller glia (F) out of the total number of clones infected by LIA or LIA-Cre virus. (G) Average number of cells contained in each clone infected by LIA or LIA-Cre virus. Error bars represent the standard deviations of the means. *, P < 0.0001; **, P < 0.01 (Student's t test).
TABLE 1.
Clonal composition following infection of Otx2flox/flox retinas with retroviruses encoding cre recombinase
| Litter no. or parameter, virus | No. of clones containing at least one of the following/total (%):
|
No. of rod-only clones/total (%) | |||
|---|---|---|---|---|---|
| Rod | Bipolar | Amacrine | Müller | ||
| 1, LIA | 126/135 (93.3) | 34/135 (25.2) | 14/135 (10.4) | 7/135 (5.2) | 90/135 (66.7) |
| 2, LIA | 121/128 (94.5) | 30/128 (23.4) | 7/128 (5.5) | 11/128 (8.6) | 83/128 (64.8) |
| 3, LIA | 225/241 (93.4) | 53/241 (22.0) | 21/241 (8.7) | 11/241 (4.6) | 159/241 (66.0) |
| 4, LIA | 229/243 (94.2) | 46/243 (18.9) | 21/243 (8.6) | 9/243 (3.7) | 169/243 (69.5) |
| 5, LIA | 350/380 (92.1) | 78/380 (20.5) | 37/380 (9.7) | 19/380 (5.0) | 256/380 (67.4) |
| 6, LIA-Cre | 55/91 (60.4) | 3/91 (3.3) | 48/91 (52.7) | 9/91 (9.9) | 33/91 (36.3) |
| 7, LIA-Cre | 138/235 (58.7) | 4/235 (1.7) | 125/235 (53.2) | 18/235 (7.7) | 93/235 (39.6) |
| 8, LIA-Cre | 177/336 (52.7) | 9/336 (2.7) | 181/336 (53.9) | 17/336 (5.1) | 141/336 (42.0) |
| 9, LIA-Cre | 271/479 (56.6) | 2/479 (0.4) | 235/479 (49.1) | 17/479 (3.5) | 235/479 (49.1) |
| Mean ± SD (%) | |||||
| LIA | 93.5 ± 0.9 | 22.0 ± 2.5 | 8.6 ± 1.9 | 5.4 ± 1.9 | 66.9 ± 1.8 |
| LIA-Cre | 57.1 ± 3.3 | 2.0 ± 1.3 | 52.0 ± 1.9 | 6.6 ± 2.8 | 41.8 ± 5.4 |
Phenotypic analysis of Otx2 CKO mice in postnatal retinal bipolar cells.
It has been reported that Otx2 is expressed in the nuclei of retinal bipolar cells at postnatal stages (4), suggesting that Otx2 plays a role in the late development of bipolar cells. We confirmed that Otx2 was colocalized with Chx10, a bipolar-cell marker (see Fig. S1A to C in the supplemental material), but not with Pax6, a marker of amacrine and ganglion cells (see Fig. S1D to F in the supplemental material), and calbindin, a horizontal-cell marker (see Fig. S1G to I in the supplemental material) at 1M. To investigate the roles of Otx2 in retinal bipolar-cell development at the postnatal stages, we generated bipolar-cell-specific Otx2 CKO mice. The L7/Pcp-2 protein is a protein of unknown function expressed in retinal rod bipolar cells and cerebellar Purkinje cells at postnatal stages (5, 21). We used L7-Cre knock-in (L7-Cre-KI) mice in which cre recombinase is specifically expressed under the control of the L7 promoter (31) to generate CKO mice. Quantitative reverse transcription (RTPCR) revealed that there was significant expression of Cre mRNA in the L7-Cre-KI−/− retina compared to the WT retina, even at P0, although the expression level was lower than that at P6 (see Fig. S2A in the supplemental material). We mated this L7-Cre-KI mouse line with the Otx2/flox mouse line. In the Otx2/flox allele, the second exon, where the initiating codon is located, is flanked by two loxP sites (37). Therefore, the recombinant allele is considered to act as a null allele. We analyzed Otx2flox/flox; L7-Cre-KI+/− mice as Otx2 CKO mice and used Otx2flox/flox; L7-Cre-KI+/+ mice as controls. We examined the expression of Otx2 protein in the CKO retina by immunostaining. The numbers of Otx2 immunoreactive cells in the INL were similar between the control and CKO retinas at P7 (see Fig. S2B and C in the supplemental material); however, Otx2-positive cells decreased at P9 and P14 in the CKO retina (see Fig. S2D to H in the supplemental material). The discrepancy between the stages of Cre expression and the decrease in Otx2-positive cells suggests that in this CKO mouse line, the expression level of cre recombinase is sufficient to ablate both alleles of Otx2 after P9.
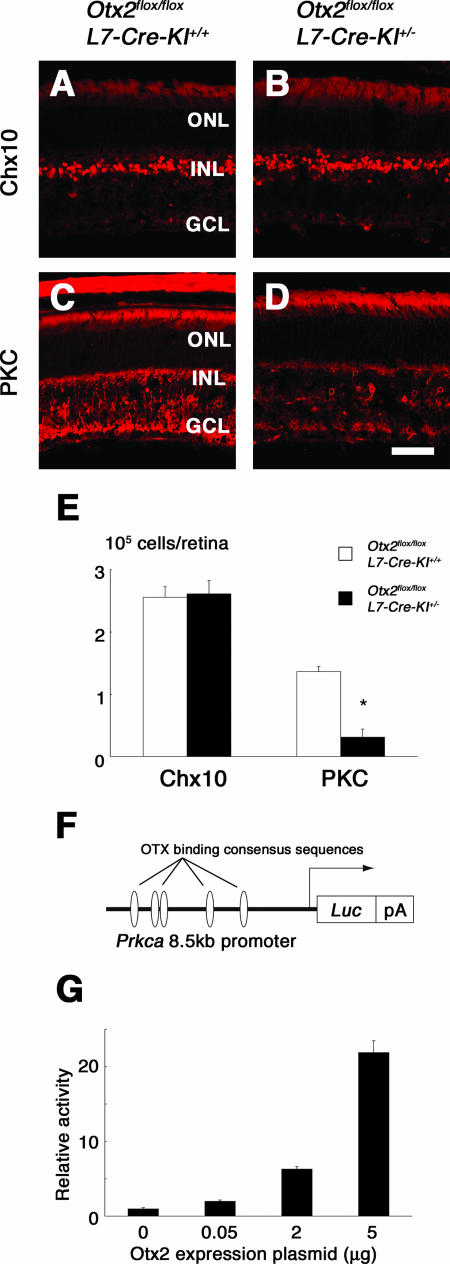
We performed immunostaining with retinal cell type markers as described above. The expression of Brn3b, HPC-1, Pax6, S-100β, calbindin, rhodopsin, M-opsin, and S-opsin (data not shown) was similar between the CKO and control retinas (data not shown). The expression of Chx10, a transcription factor which is essential for retinal bipolar-cell development (10) and is expressed in both bipolar progenitor cells and mature bipolar cells (24), was also similar between the two genotypes (Fig. 7A, B, and E). On the other hand, the number of cells immunoreactive for PKC, a marker of mature bipolar cells (28, 42), was notably decreased in the CKO retinas (Fig. 7C, D, and E), suggesting that the terminal differentiation of bipolar cells was impaired in the CKO retinas. Electron microscopy revealed that in the CKO retina, there were no obvious abnormalities and the synaptic termini appeared to be morphologically normal both in the outer plexiform layer (see Fig. S3A and B in the supplemental material) and in the INL (see Fig. S3C and D in the supplemental material). Next, we examined whether Otx2 can transactivate the Prkca promoter because there are at least five OTX binding consensus sequences in the 8.5-kb Prkca upstream region (Fig. 7F). The reporter activity was enhanced by Otx2 in a dose-dependent manner (Fig. 7G), suggesting that Otx2 can regulate Prkca transcription directly. The decrease in Chx10-positive cells in the Otx2+/−; Crx−/− retinas (Fig. 3H) suggests that Otx2 has a role in bipolar-cell survival in cooperation with Crx. It can be speculated that in the CKO retinas, Crx expression may prevent apoptosis in the bipolar cells. To verify this speculation, we analyzed the expression of Chx10 in the Otx2 CKO retinas with a Crx−/− background. The expression of Chx10 in the CKO retina with a Crx−/− background was notably reduced compared to that in the Crx−/− retina at 1M (Fig. 8C, D, and E) but not at P14 (Fig. 8A, B, and E), suggesting that determination of the fate of Chx10-positive cells occurred normally in the CKO retina with a Crx−/− background but that their survival was impaired by a lack of both Otx2 and Crx. Furthermore, in order to evaluate retinal function in vivo, ERGs were recorded from the Otx2 CKO and control mice when they were 6 weeks old. The intensity response series of the scotopic ERGs recorded from both types of mice are shown in Fig. 9A. In normal control mice, only a positive b-wave, originating from rod bipolar cells, was seen at low stimulus intensities (−5.0 to −2.6 log cd-s/m2) (30). At higher stimulus intensities (−1.4 to 1.0 log cd-s/m2), a negative a-wave, originating from the rod photoreceptors, appeared (9). In the Otx2 CKO mouse, the amplitude of the b-wave was reduced to less than one-half of that of the control but the amplitude of the a-wave remained completely normal. The intensity response series of the photopic ERGs for both types of mice are shown in Fig. 9B. As in the scotopic ERGs, the amplitude of the b-wave was attenuated while the amplitude of the a-wave remained normal. These results clearly indicate that the function of retinal bipolar cells is impaired in the Otx2 CKO mouse, whereas the function of photoreceptor cells remains unchanged.
FIG. 7.
Otx2 is required for the terminal differentiation of bipolar cells. (A to D) Immunostaining of retinal sections from Otx2flox/flox; L7-Cre-KI+/+ (control) (A and C) and Otx2flox/flox; L7-Cre-KI+/− (Otx2 CKO) (B and D) mice at 1M with antibodies against Chx10 (A and B) and PKC (C and D). Scale bar, 50 μm. GCL, ganglion cell layer. (E) Numbers of Chx10- and PKC-positive cells in 1M retinas from Otx2flox/flox; L7-Cre-KI+/+ (control) and Otx2flox/flox; L7-Cre-KI+/− (Otx2 CKO) mice. Error bars represent the standard deviations of the means. *, P < 0.0005 by Student's t test. (F) Schematic of the Prkca promoter-reporter constructs for a transcription assay. The five OTX binding consensus sequences located in the Prkca 8.5-kb promoter region are indicated. (G) Luciferase reporter plasmids and 0, 0.05, 2, or 5 μg of Otx2 expression plasmids were transferred into NIH 3T3 cells with internal control vector pβSV. Three cell culture replicates per Otx2 expression plasmid level are shown. Error bars indicate the means ± the standard deviations of three replicated cell cultures.
FIG. 8.
Otx2 and Crx are cooperatively required for bipolar-cell survival. (A to D) The retinas of a control mouse with a Crx−/− background (A and C) and an Otx2 CKO mouse with a Crx−/− background (B and D) at P14 (A and B) and 1M (C and D) were immunostained with an antibody against Chx10. (E) Numbers of Chx10-positive cells in the P14 and 1M retinas of Otx2flox/flox; L7-Cre-KI+/+; Crx−/−, and Otx2flox/flox; L7-Cre-KI+/−; Crx−/− mice. Error bars represent the standard deviations of the means. *, P < 0.005 by Student's t test.
FIG. 9.
ERGs recorded from 6-week-old Otx2 CKO and control mice. (A) Dark-adapted ERGs elicited with six different stimulus intensities. (B) Light-adapted ERGs elicited with four different stimulus intensities. Vertical dotted lines show the onset of the stimulus. The ERGs of an Otx2 CKO mouse showed a selective reduction of the b-wave amplitude which originates from bipolar cells in both rod and cone pathways.
DISCUSSION
Otx2 could have roles in terminal differentiation of retinal photoreceptors along with Crx.
In previous studies, we have reported that Otx2 and Crx have distinct roles in retinal photoreceptor development. Conditional ablation of Otx2 in retinal photoreceptors and pinealocytes under the control of a Crx 12-kb promoter (17) leads to a complete loss of photoreceptors caused by a cell fate shift from photoreceptors to amacrine cells (29). In contrast, in Crx−/− mice, early development of the photoreceptors appears to occur normally but the photoreceptors fail to form the outer segments and subsequently degenerate (20). These reports suggest that Otx2 plays a role mainly in the early development of photoreceptors while Crx has a role mainly in terminal differentiation and survival. Furthermore, the temporal and spatial expression patterns of Otx2 and Crx, such as the downregulation of Otx2 expression in photoreceptors after birth while retaining Crx expression (29), support this idea. However, since Otx2 and Crx can bind to the same DNA sequence (7, 19), we speculate that Otx2 may play a role in the terminal differentiation of retinal photoreceptors. In Otx2+/−; Crx−/− mice, the decrease in the thickness of the ONL was much more prominent than in Crx−/− mice at P14 (Fig. 2L) and 1M (Fig. 2P). The possibility that the photoreceptor differentiation might be delayed in the Otx2+/−; Crx−/− retina cannot be excluded. However, we observed that the outer segment of the photoreceptors was not formed at 1M (Fig. 2P) or at stages later than 1M (data not shown) and that the thickness of the ONL was not recovered at stages later than 1M (data not shown). The persistence of the neuroblastic layer in Fig. 2H and the subsequent loss of photoreceptors in Fig. 2L and P raise the possibilities of failure of photoreceptor development and subsequent cell death or a decrease in photoreceptor generation from progenitors. In the normal retina, Otx2 was expressed in the neuroblastic layer at P1 (Fig. 1A and E), suggesting that Otx2 is expressed in either proliferating common progenitor cells or postmitotic retinal precursor cells. According to a previous study, Baas et al. reported that Otx2 is expressed in newly postmitotic retinal cells but not in proliferating progenitor cells (4). If Otx2 is expressed in retinal progenitors and a smaller number of postmitotic cells are generated in the Otx2+/−; Crx−/− retina, it is inconsistent with the finding that the actual numbers of amacrine and Müller cells, which have progenitors in common with photoreceptors and bipolar cells, did not significantly change in the Otx2+/−; Crx−/− retina compared to those in the other genotypes (Fig. 3M). Therefore, this suggests that Otx2 may not play a critical role in cell proliferation. Cell fate change from photoreceptors to other cell types is another possible explanation for the decrease in photoreceptor generation from progenitors. However, if a cell fate change occurred in the Otx2+/−; Crx−/− retina, the actual numbers of cells other than photoreceptors should have increased. Therefore, the selective decrease in the numbers of photoreceptor and bipolar cells and the unchanged numbers of Müller and amacrine cells in the Otx2+/−; Crx−/− retina (Fig. 3M) suggest that selective cell death in photoreceptors and bipolar cells, rather than cell fate change or impairment of cell proliferation, caused the change in retinal cell proportion. Immunohistochemically, we could not find phospho-histone H3-positive cells in the Otx2+/−; Crx−/− retina at P14 (data not shown). On the other hand, active-caspase 3- positive cell numbers increased in the Otx2+/−; Crx−/− retina at P7 (Fig. 4H and J). Taken together, this information suggests that it is likely that the decrease in the numbers of photoreceptor nuclei in Fig. 2L and P was due to photoreceptor degeneration, but the possibility of delayed differentiation still persists. In addition, the expression of photoreceptor-specific genes, such as Rho, Pde6b, and Sag, was much more downregulated than in Crx−/− mice (Fig. 5). It is unlikely that the reduction of the expression levels of these genes in the Otx2+/−; Crx−/− retinas in Fig. 5 is only a result of photoreceptor degeneration. First, the expression of Rbp3 was not downregulated in the Otx2+/−; Crx−/− retinas (Fig. 5B). Since Rbp3 is predominantly expressed in retinal photoreceptors (and retinal pigment epithelial cells, which were not present in the specimens), Rbp3 expression in the Otx2+/−; Crx−/− retinas should be significantly downregulated if most of the photoreceptors had degenerated. Second, we dissociated the retinal cells (75% of which are occupied by photoreceptors) and determined their total number at P10. We could not find a significant difference in the numbers of retinal cells among the four genotypes (data not shown). These results suggest that Otx2 is also involved in the late development of photoreceptors by promoting terminal differentiation and upregulating photoreceptor-specific genes.
Otx2 and Crx are required for bipolar-cell and pinealocyte development.
It has been reported that Otx2 and Crx are also expressed in the INL cells (4, 6), suggesting roles for Otx2 and Crx in the development of INL cells. Previous studies have reported that rod photoreceptors and bipolar cells are developmentally related. The addition of ciliary neurotrophic factor to postnatal rat retinal explants results in a reduction in the number of rod photoreceptors and an increase in the number of bipolar cells (14). Lipofection of retinal precursors in a Xenopus laevis embryo with XOtx2 increases bipolar-cell numbers and suppresses opsin expression (40). In our present study, both photoreceptor and bipolar-cell numbers decreased in the Otx2+/−; Crx−/− retina (Fig. 3D, H, and L). The fact that a decrease in the number of bipolar cells was not observed in the other genotypes suggests that Otx2 and Crx are cooperatively required for bipolar-cell development. Similarly, these two genes also appear to be cooperatively required for pinealocyte development because the pineal gland was hypoplastic only in Otx2+/−; Crx−/− mice (Fig. 2T). It has been reported that Otx2 and Otx1, another member of the Otx family, are cooperatively required for the development of the brain and the retina (25, 34, 35). A similar mechanism may apply to Otx2 and Crx in retinal bipolar-cell and pinealocyte development.
Otx2 is involved in survival and terminal differentiation of retinal bipolar cells.
It has been reported that Otx2 is expressed in the nuclei of retinal bipolar cells and the cytoplasm of rod photoreceptors at postnatal stages (4). To examine the role of Otx2 in postnatal retinal bipolar-cell development, we generated L7 promoter-mediated Otx2 CKO mice in which Otx2 is ablated in postnatal bipolar cells. In this CKO retina, there was a discrepancy between the expression of two bipolar-cell markers, Chx10 and PKC (Fig. 7B, D, and E), suggesting that the terminal differentiation of bipolar cells was impaired. The impairment of bipolar-cell maturation in the CKO retina was also verified by an electrophysiological study (Fig. 9). It has also been reported that L7 is expressed specifically in retinal rod bipolar cells and cerebellar Purkinje cells (5, 21). The reduction of the amplitude of the b wave in the photopic ERGs of the CKO mice (Fig. 9B) suggests the possibility that cre recombinase is also expressed in a subset of cone bipolar cells in the CKO mice. However, it is also possible that in the CKO mice cre recombinase is expressed exclusively in rod bipolar cells and the dysfunction of rod bipolar cells affects the photopic b-wave, as well as the scotopic b-wave. Alternatively, immature rod bipolar cells may affect the cone bipolar cells and prevent their functional maturation. The evidence that the amplitude of the photopic b-wave was reduced in Bhlhb4-deficient mice in which maturation of rod bipolar cells is impaired (8) supports the idea that immature rod bipolar cells can affect not only the scotopic b-wave but also the photopic b-wave either directly or indirectly.
There is a discrepancy between the results of LIA-Cre virus infection and the CKO retinas. When the LIA-Cre virus infected the Otx2flox/flox retinas, the number of retinal bipolar cells decreased (Fig. 6E). In the CKO retina, however, when Otx2 was ablated under the control of the L7 promoter, the number of Chx10-positive bipolar cells was unchanged (Fig. 7A, B, and E). One possible explanation is that a subpopulation of Chx10-positive cells might not show bipolar-cell morphology. In the lineage analysis, we judged the cell type on the basis of the morphology and cell position in the retina; therefore, it may be possible that there is no difference in Chx10-positive cells between the clones infected with the LIA and LIA-Cre viruses. However, this possibility is rather unlikely because we could find few morphologically unclassifiable cells in the Cre virus-infected clones. Alternatively, the difference in the stages of Cre expression between the two experiments may affect the results. In the lineage analysis study, the retinas were infected with the LIA-Cre virus at P0. At this stage, the expression of Crx has not reached its maximum level (19). It was reported that the expression of Crx is regulated by both Otx2 and Crx itself (17, 29). If Otx2 is downregulated at P0, it is possible that Crx cannot be upregulated by either Otx2 or Crx itself. On the other hand, in the CKO retina, Cre expression was observed at P0 but the expression level at this stage is lower than that at later stages (see Fig. S2A in the supplemental material). The downregulation of Otx2 in the bipolar-cell layer was observed after P9 in the CKO retina (see Fig. S2D to H in the supplemental material). At this stage, the expression of Crx does reach its peak level (19) and this level of Crx is supposed to be able to upregulate itself by autoregulation (17). We speculate that Crx is also involved in survival and/or maturation of the bipolar cells in the postnatal retina in cooperation with Otx2. To verify this hypothesis, we generated and analyzed the phenotype of Otx2 CKO mice with a Crx−/− background. The resulting decrease in Chx10-positive cells in the CKO retina with a Crx−/− background (Fig. 8C, D, and E) but not in the CKO retina with a normal background (Fig. 7A, B, and E) supports this idea. Moreover, this hypothesis is consistent with the finding of a decrease in Chx10-positive cells in the Otx2+/−; Crx−/− retina (Fig. 3H and M).
In addition to Chx10, several other transcription factors have been reported to be involved in bipolar-cell development. Bhlhb4 is essential for the maturation of rod bipolar cells (8). The expression of Bhlhb4 in the retina peaks at P8 and then becomes very low after P10 (8). On the other hand, Otx2-positive cells in the CKO retina decreased after P9 (see Fig. S2D to H in the supplemental material). Therefore, it is unlikely that Otx2 directly regulates Bhlhb4. Indeed, the expression levels of Bhlhb4 were not significantly changed between the CKO and control retinas at P8 (see Fig. S4 in the supplemental material). However, the phenotype of Bhlhb4−/− mice, impairment of bipolar-cell maturation and reduction of the amplitudes of scotopic and photopic b-waves in ERGs (8), was very similar to that of the Otx2 CKO mice (Fig. 9). This ERG pattern, the reduced b-wave amplitudes and normal a-wave amplitudes under both dark-adapted and light-adapted conditions, was previously reported in mutant mice deficient in mGluR6 (26) and Gao (13), in both of which the functional maturation of bipolar cells is impaired. It is possible that Otx2 and Bhlhb4 have downstream factors in common which are involved in retinal bipolar-cell maturation.
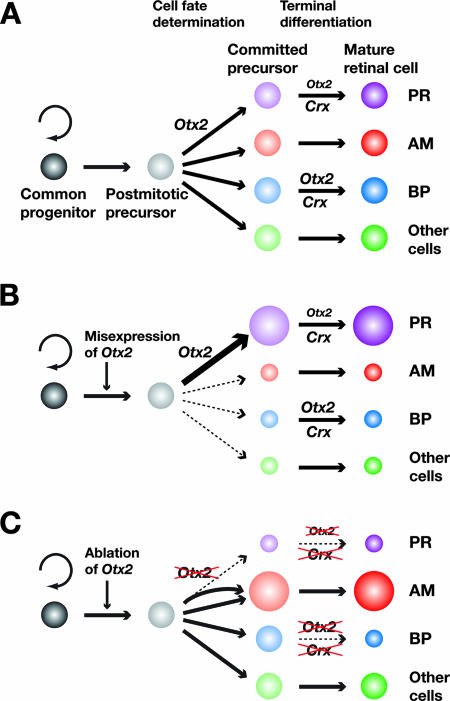
The results of the lineage analysis, the decrease in the clones containing rod photoreceptors (Fig. 6B and C) and the increase in the clones containing amacrine cells after infection with the LIA-Cre virus, are consistent with our previous results indicating that the photoreceptors were totally depleted and the amacrine cells were significantly increased by cell fate change in the Otx2 CKO mice mediated by the Crx promoter (29). The marked increase in amacrine-cell-containing clones (Fig. 6D) strongly suggests that the decrease in the numbers of photoreceptors in this experiment is mainly due to cell fate change, although it is possible that the increase in cell death modified the result to some extent. The number of clones containing bipolar cells also decreased after LIA-Cre virus infection (Fig. 6E), suggesting the possibility that the fate of the bipolar cells was affected or that the bipolar cells were directed to cell death by the ablation of Otx2. However, the possibility of bipolar-cell fate change is rather unlikely, considering our previous report that bipolar-cell-containing clones decreased when Otx2 was misexpressed in the retinal progenitor cells (29). To explain this paradox, we hypothesize that Otx2 is involved in retinal photoreceptor cell fate but not in bipolar-cell fate in early development while it is involved in the terminal differentiation of both photoreceptors and bipolar cells in late development in cooperation with Crx (Fig. 10A). According to this hypothesis, misexpression of Otx2 directs retinal cell fate to the photoreceptor lineage, resulting in an increase in rod photoreceptors and a decrease in the other cell types, including bipolar cells (Fig. 10B). In contrast, ablation of Otx2 from the retinal progenitor cells induces a retinal-cell fate shift from the photoreceptor lineage to the amacrine-cell lineage, as we previously reported (29). The fate of bipolar and Müller cells is not affected, but maturation and/or survival of bipolar cells is impaired by the lack of Otx2 and, possibly, its downstream factor Crx. As a result, amacrine-cell numbers increase, rod photoreceptor and bipolar-cell numbers decrease, and the number of Müller cells is not affected (Fig. 10C). This hypothesis is consistent with the results of both our present and previous studies (29). In conclusion, the evidence suggests that Otx2 is involved in survival and/or terminal differentiation of retinal photoreceptors and bipolar cells in cooperation with Crx.
FIG. 10.
Involvement of Otx2 in photoreceptor and bipolar-cell development. (A) In normal development, Otx2 is required for the cell fate determination of photoreceptors. Otx2 is also involved in the terminal differentiation of photoreceptors, but the contribution is relatively small compared to that of Crx. In contrast, in bipolar cells, Otx2 is required not for cell fate determination but instead for terminal differentiation cooperatively with Crx. (B) When Otx2 is misexpressed, a larger number of postmitotic precursors is directed to choose the photoreceptor lineage. As a result, the number of photoreceptors increases and the numbers of other retinal cells, including amacrine and bipolar cells, decrease. (C) When Otx2 is ablated, postmitotic precursors originally fated to become photoreceptors are forced to change their cell fate to the amacrine lineage. Crx, which is induced by Otx2, is also downregulated. As a result, the number of photoreceptors decreases and the number of amacrine cells increases. In the bipolar-cell lineage, cell fate determination is not affected but terminal differentiation is impaired. Therefore, the number of bipolar cells also decreases. PR, photoreceptor; AM, amacrine cell; BP, bipolar cells.
There still remain unresolved issues in Otx2 and retinal development. Not only Otx2 and Crx but also several transcription factors such as the basic helix-loop-helix genes (2, 22, 23) or OTX family genes (25) are cooperatively involved in retinal development. Revealing the specific and common roles of these transcription factors will give insights into the multiple-requirement mechanisms of transcription factors in development.
Supplementary Material
Acknowledgments
We thank K. Myata, T. Koyasu, A. Tani, Y. Kambara, M. Murai, Y. Hirao, T. Terada, and H. Yoshii for technical assistance.
This work was supported by Molecular Brain Science, a Grant-in Aid for Scientific Research on Priority Areas and Grant-in-Aid for Scientific Research (B and C), a Grant-in-Aid for Exploratory Research, the Takeda Science Foundation, the Senri Life Science Foundation, The Uehara Memorial Foundation, and the Mochida Memorial Foundation for Medical and Pharmaceutical Research.
Footnotes
Published ahead of print on 1 October 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Acampora, D., S. Mazan, Y. Lallemand, V. Avantaggiato, M. Maury, A. Simeone, and P. Brulet. 1995. Forebrain and midbrain regions are deleted in Otx2−/− mutants due to a defective anterior neuroectoderm specification during gastrulation. Development 121:3279-3290. [DOI] [PubMed] [Google Scholar]
- 2.Akagi, T., T. Inoue, G. Miyoshi, Y. Bessho, M. Takahashi, J. E. Lee, F. Guillemot, and R. Kageyama. 2004. Requirement of multiple basic helix-loop-helix genes for retinal neuronal subtype specification. J. Biol. Chem. 279:28492-28498. [DOI] [PubMed] [Google Scholar]
- 3.Ang, S. L., O. Jin, M. Rhinn, N. Daigle, L. Stevenson, and J. Rossant. 1996. A targeted mouse Otx2 mutation leads to severe defects in gastrulation and formation of axial mesoderm and to deletion of rostral brain. Development 122:243-252. [DOI] [PubMed] [Google Scholar]
- 4.Baas, D., K. M. Bumsted, J. A. Martinez, F. M. Vaccarino, K. C. Wikler, and C. J. Barnstable. 2000. The subcellular localization of Otx2 is cell type specific and developmentally regulated in the mouse retina. Brain Res. Mol. Brain Res. 78:26-37. [DOI] [PubMed] [Google Scholar]
- 5.Berrebi, A. S., J. Oberdick, L. Sangameswaran, S. Christakos, J. I. Morgan, and E. Mugnaini. 1991. Cerebellar Purkinje cell markers are expressed in retinal bipolar neurons. J. Comp. Neurol 308:630-649. [DOI] [PubMed] [Google Scholar]
- 6.Bibb, L. C., J. K. Holt, E. E. Tarttelin, M. D. Hodges, K. Gregory-Evans, A. Rutherford, R. J. Lucas, J. C. Sowden, and C. Y. Gregory-Evans. 2001. Temporal and spatial expression patterns of the CRX transcription factor and its downstream targets. Critical differences during human and mouse eye development. Hum. Mol. Genet. 10:1571-1579. [DOI] [PubMed] [Google Scholar]
- 7.Bobola, N., P. Briata, C. Ilengo, N. Rosatto, C. Craft, G. Corte, and R. Ravazzolo. 1999. OTX2 homeodomain protein binds a DNA element necessary for interphotoreceptor retinoid binding protein gene expression. Mech. Dev. 82:165-169. [DOI] [PubMed] [Google Scholar]
- 8.Bramblett, D. E., M. E. Pennesi, S. M. Wu, and M. J. Tsai. 2004. The transcription factor Bhlhb4 is required for rod bipolar cell maturation. Neuron 43:779-793. [DOI] [PubMed] [Google Scholar]
- 9.Breton, M. E., A. W. Schueller, T. D. Lamb, and E. N. Pugh, Jr. 1994. Analysis of ERG a-wave amplification and kinetics in terms of the G-protein cascade of phototransduction. Investig. Ophthalmol. Vis. Sci. 35:295-309. [PubMed] [Google Scholar]
- 10.Burmeister, M., J. Novak, M. Y. Liang, S. Basu, L. Ploder, N. L. Hawes, D. Vidgen, F. Hoover, D. Goldman, V. I. Kalnins, T. H. Roderick, B. A. Taylor, M. H. Hankin, and R. R. McInnes. 1996. Ocular retardation mouse caused by Chx10 homeobox null allele: impaired retinal progenitor proliferation and bipolar cell differentiation. Nat. Genet. 12:376-384. [DOI] [PubMed] [Google Scholar]
- 11.Chen, S., K. Kadomatsu, M. Kondo, Y. Toyama, K. Toshimori, S. Ueno, Y. Miyake, and T. Muramatsu. 2004. Effects of flanking genes on the phenotypes of mice deficient in basigin/CD147. Biochem. Biophys. Res. Commun. 324:147-153. [DOI] [PubMed] [Google Scholar]
- 12.Chen, S., Q. L. Wang, Z. Nie, H. Sun, G. Lennon, N. G. Copeland, D. J. Gilbert, N. A. Jenkins, and D. J. Zack. 1997. Crx, a novel Otx-like paired-homeodomain protein, binds to and transactivates photoreceptor cell-specific genes. Neuron 19:1017-1030. [DOI] [PubMed] [Google Scholar]
- 13.Dhingra, A., A. Lyubarsky, M. Jiang, E. N. Pugh, Jr., L. Birnbaumer, P. Sterling, and N. Vardi. 2000. The light response of ON bipolar neurons requires Gao. J. Neurosci. 20:9053-9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ezzeddine, Z. D., X. Yang, T. DeChiara, G. Yancopoulos, and C. L. Cepko. 1997. Postmitotic cells fated to become rod photoreceptors can be respecified by CNTF treatment of the retina. Development 124:1055-1067. [DOI] [PubMed] [Google Scholar]
- 15.Freund, C. L., C. Y. Gregory-Evans, T. Furukawa, M. Papaioannou, J. Looser, L. Ploder, J. Bellingham, D. Ng, J. A. Herbrick, A. Duncan, S. W. Scherer, L. C. Tsui, A. Loutradis-Anagnostou, S. G. Jacobson, C. L. Cepko, S. S. Bhattacharya, and R. R. McInnes. 1997. Cone-rod dystrophy due to mutations in a novel photoreceptor-specific homeobox gene (CRX) essential for maintenance of the photoreceptor. Cell 91:543-553. [DOI] [PubMed] [Google Scholar]
- 16.Freund, C. L., Q. L. Wang, S. Chen, B. L. Muskat, C. D. Wiles, V. C. Sheffield, S. G. Jacobson, R. R. McInnes, D. J. Zack, and E. M. Stone. 1998. De novo mutations in the CRX homeobox gene associated with Leber congenital amaurosis. Nat. Genet. 18:311-312. [DOI] [PubMed] [Google Scholar]
- 17.Furukawa, A., C. Koike, P. Lippincott, C. L. Cepko, and T. Furukawa. 2002. The mouse Crx 5′-upstream transgene sequence directs cell-specific and developmentally regulated expression in retinal photoreceptor cells. J. Neurosci. 22:1640-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furukawa, T., C. A. Kozak, and C. L. Cepko. 1997. rax, a novel paired-type homeobox gene, shows expression in the anterior neural fold and developing retina. Proc. Natl. Acad. Sci. USA 94:3088-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furukawa, T., E. M. Morrow, and C. L. Cepko. 1997. Crx, a novel otx-like homeobox gene, shows photoreceptor-specific expression and regulates photoreceptor differentiation. Cell 91:531-541. [DOI] [PubMed] [Google Scholar]
- 20.Furukawa, T., E. M. Morrow, T. Li, F. C. Davis, and C. L. Cepko. 1999. Retinopathy and attenuated circadian entrainment in Crx-deficient mice. Nat. Genet. 23:466-470. [DOI] [PubMed] [Google Scholar]
- 21.Grünert, U., and P. R. Martin. 1991. Rod bipolar cells in the macaque monkey retina: immunoreactivity and connectivity. J. Neurosci. 11:2742-2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatakeyama, J., K. Tomita, T. Inoue, and R. Kageyama. 2001. Roles of homeobox and bHLH genes in specification of a retinal cell type. Development 128:1313-1322. [DOI] [PubMed] [Google Scholar]
- 23.Inoue, T., M. Hojo, Y. Bessho, Y. Tano, J. E. Lee, and R. Kageyama. 2002. Math3 and NeuroD regulate amacrine cell fate specification in the retina. Development 129:831-842. [DOI] [PubMed] [Google Scholar]
- 24.Liu, I. S., J. D. Chen, L. Ploder, D. Vidgen, D. van der Kooy, V. I. Kalnins, and R. R. McInnes. 1994. Developmental expression of a novel murine homeobox gene (Chx10): evidence for roles in determination of the neuroretina and inner nuclear layer. Neuron 13:377-393. [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Morales, J. R., M. Signore, D. Acampora, A. Simeone, and P. Bovolenta. 2001. Otx genes are required for tissue specification in the developing eye. Development 128:2019-2030. [DOI] [PubMed] [Google Scholar]
- 26.Masu, M., H. Iwakabe, Y. Tagawa, T. Miyoshi, M. Yamashita, Y. Fukuda, H. Sasaki, K. Hiroi, Y. Nakamura, R. Shigemoto, et al. 1995. Specific deficit of the ON response in visual transmission by targeted disruption of the mGluR6 gene. Cell 80:757-765. [DOI] [PubMed] [Google Scholar]
- 27.Matsuo, I., S. Kuratani, C. Kimura, N. Takeda, and S. Aizawa. 1995. Mouse Otx2 functions in the formation and patterning of rostral head. Genes Dev. 9:2646-2658. [DOI] [PubMed] [Google Scholar]
- 28.Negishi, K., S. Kato, and T. Teranishi. 1988. Dopamine cells and rod bipolar cells contain protein kinase C-like immunoreactivity in some vertebrate retinas. Neurosci. Lett. 94:247-252. [DOI] [PubMed] [Google Scholar]
- 29.Nishida, A., A. Furukawa, C. Koike, Y. Tano, S. Aizawa, I. Matsuo, and T. Furukawa. 2003. Otx2 homeobox gene controls retinal photoreceptor cell fate and pineal gland development. Nat. Neurosci. 6:1255-1263. [DOI] [PubMed] [Google Scholar]
- 30.Robson, J. G., and L. J. Frishman. 1995. Response linearity and kinetics of the cat retina: the bipolar cell component of the dark-adapted electroretinogram. Vis. Neurosci. 12:837-850. [DOI] [PubMed] [Google Scholar]
- 31.Saito, H., H. Tsumura, S. Otake, A. Nishida, T. Furukawa, and N. Suzuki. 2005. L7/Pcp-2-specific expression of Cre recombinase using knock-in approach. Biochem. Biophys. Res. Commun. 331:1216-1221. [DOI] [PubMed] [Google Scholar]
- 32.Simeone, A., D. Acampora, A. Mallamaci, A. Stornaiuolo, M. R. D'Apice, V. Nigro, and E. Boncinelli. 1993. A vertebrate gene related to orthodenticle contains a homeodomain of the bicoid class and demarcates anterior neuroectoderm in the gastrulating mouse embryo. EMBO J. 12:2735-2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sohocki, M. M., L. S. Sullivan, H. A. Mintz-Hittner, D. Birch, J. R. Heckenlively, C. L. Freund, R. R. McInnes, and S. P. Daiger. 1998. A range of clinical phenotypes associated with mutations in CRX, a photoreceptor transcription-factor gene. Am. J. Hum. Genet. 63:1307-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suda, Y., I. Matsuo, and S. Aizawa. 1997. Cooperation between Otx1 and Otx2 genes in developmental patterning of rostral brain. Mech. Dev. 69:125-141. [DOI] [PubMed] [Google Scholar]
- 35.Suda, Y., I. Matsuo, S. Kuratani, and S. Aizawa. 1996. Otx1 function overlaps with Otx2 in development of mouse forebrain and midbrain. Genes Cells 1:1031-1044. [DOI] [PubMed] [Google Scholar]
- 36.Swain, P. K., S. Chen, Q. L. Wang, L. M. Affatigato, C. L. Coats, K. D. Brady, G. A. Fishman, S. G. Jacobson, A. Swaroop, E. Stone, P. A. Sieving, and D. J. Zack. 1997. Mutations in the cone-rod homeobox gene are associated with the cone-rod dystrophy photoreceptor degeneration. Neuron 19:1329-1336. [DOI] [PubMed] [Google Scholar]
- 37.Tian, E., C. Kimura, N. Takeda, S. Aizawa, and I. Matsuo. 2002. Otx2 is required to respond to signals from anterior neural ridge for forebrain specification. Dev. Biol. 242:204-223. [DOI] [PubMed] [Google Scholar]
- 38.Turner, D. L., and C. L. Cepko. 1987. A common progenitor for neurons and glia persists in rat retina late in development. Nature 328:131-136. [DOI] [PubMed] [Google Scholar]
- 39.Turner, D. L., E. Y. Snyder, and C. L. Cepko. 1990. Lineage-independent determination of cell type in the embryonic mouse retina. Neuron 4:833-845. [DOI] [PubMed] [Google Scholar]
- 40.Viczian, A. S., R. Vignali, M. E. Zuber, G. Barsacchi, and W. A. Harris. 2003. XOtx5b and XOtx2 regulate photoreceptor and bipolar fates in the Xenopus retina. Development 130:1281-1294. [DOI] [PubMed] [Google Scholar]
- 41.Wetts, R., and S. E. Fraser. 1988. Multipotent precursors can give rise to all major cell types of the frog retina. Science 239:1142-1145. [DOI] [PubMed] [Google Scholar]
- 42.Zhang, D. R., and H. H. Yeh. 1991. Protein kinase C-like immunoreactivity in rod bipolar cells of the rat retina: a developmental study. Vis. Neurosci. 6:429-437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.