Abstract
Apoptosis signal-regulating kinase 1 (ASK1), a member of the mitogen-activated protein kinase kinase kinase family, plays pivotal roles in reactive oxygen species (ROS)-induced cellular responses. In resting cells, endogenous ASK1 constitutively forms a homo-oligomerized but still inactive high-molecular-mass complex including thioredoxin (Trx), which we designated the ASK1 signalosome. Upon ROS stimulation, the ASK1 signalosome unbinds from Trx and forms a fully activated higher-molecular-mass complex, in part by recruitment of tumor necrosis factor receptor-associated factor 2 (TRAF2) and TRAF6. However, the precise mechanisms by which Trx inhibits and TRAF2 and TRAF6 activate ASK1 have not been elucidated fully. Here we demonstrate that the N-terminal homophilic interaction of ASK1 through the N-terminal coiled-coil domain is required for ROS-dependent activation of ASK1. Trx inhibited this interaction of ASK1, which was, however, enhanced by expression of TRAF2 or TRAF6 or by treatment of cells with H2O2. Furthermore, the H2O2-induced interaction was reduced by double knockdown of TRAF2 and TRAF6. These findings demonstrate that Trx, TRAF2, and TRAF6 regulate ASK1 activity by modulating N-terminal homophilic interaction of ASK1.
Apoptosis signal-regulating kinase 1 (ASK1), a serine/threonine protein kinase, is a member of the mitogen-activated protein kinase kinase kinase (MAPKKK) family that activates both the SEK1 (also termed MKK4)/MKK7-c-Jun NH2-terminal kinase (JNK) and MKK3/MKK6-p38 MAPK signaling cascades (6). ASK1 is activated in cells subjected to various types of stimuli, such as oxidative stress (17), tumor necrosis factor alpha (TNF-α) (14), lipopolysaccharide (11), endoplasmic reticulum (ER) stress (14), and calcium influx (19). Of the various stimuli tested, oxidative stress is one of the most potent activators of ASK1, and analyses of ASK1-deficient mice have revealed that ASK1 is required for the apoptosis induced by oxidative stress, ER stress, and TNF-α (17, 20). Studies have recently demonstrated that reactive oxygen species (ROS) play a critical role in lipopolysaccharide, β-amyloid-, and angiotensin II-induced activations of ASK1, which appear to be involved in inflammation, neurodegenerative disorders, and cardiac hypertrophy, respectively (4, 7).
We previously identified thioredoxin (Trx) as a negative regulator of the ASK1-JNK/p38 pathway through yeast two-hybrid screening for ASK1-binding proteins (2, 17). Trx, a reduction/oxidation (redox) regulatory protein, inhibits the kinase activity of ASK1 by directly binding to the N-terminal noncatalytic region of ASK1 (amino acids 1 to 655) (10, 17). Upon treatment of cells with ROS such as hydrogen peroxide (H2O2), the oxidized form of Trx (Trx-S2), which is induced by ROS through a disulfide bridge between Cys32 and Cys35 in the active center, is dissociated from ASK1, resulting in activation of ASK1 (17). In accordance with these findings, an ASK1 mutant lacking the N-terminal region (ASK1ΔN) has been found to behave as a constitutively active form and to induce various cellular activities, such as apoptosis and cell differentiation (5, 17, 18). A recent study showed that Cys250 in the N-terminal region of ASK1 is required for inhibition of ASK1 activity by Trx (22). The N-terminal region of ASK1 is thus important for Trx-mediated redox regulation of ASK1 kinase activity.
ASK1 forms a silent homo-oligomer in nonstressed cells by direct interaction through the C-terminal coiled-coil (CCC) domain (21). We recently showed that endogenous ASK1 constitutively forms a high-molecular-mass complex including Trx (approximately 1,500 to 2,000 kDa), which we designated the ASK1 signalosome (16). This signalosome is present as a homo-oligomerized but still inactive form, indicating that homo-oligomerization of ASK1 through the CCC domain is required but not sufficient for ASK1 activation. Following dissociation of Trx upon ROS stimulation, the ASK1 signalosome forms an active higher-molecular-mass complex, in part by the recruitment of TNF receptor-associated factor 2 (TRAF2) and TRAF6 (16). Importantly, upon H2O2 treatment, even ASK1ΔCCC, a deletion mutant of the CCC oligomerization domain, can still form homo-oligomers and undergo weak activation (21). ASK1 thus appears, upon ROS stimulation, to create a new but unidentified interface within a preexisting oligomer, which is important for trans-autophosphorylation of ASK1 and formation of an active ASK1 signalosome. However, the precise mechanisms by which ASK1 is activated after its release from Trx remain to be determined.
In this study, we found that amino acids 46 to 277 of the ASK1 N-terminal region are necessary and sufficient for the association with Trx. We also found that both TRAF2 and TRAF6 preferentially associate with amino acids 384 to 655 of ASK1. ASK1 possesses not only a CCC domain but also an N-terminal coiled-coil (NCC) domain. The NCC domain, between the Trx-binding domain and the TRAF-binding domain, was required for N-terminal homophilic interaction, which promotes ROS-dependent activation of ASK1. Trx interfered with this homophilic interaction of ASK1, which was, however, enhanced by overexpression of TRAF2 or TRAF6 or by treatment with H2O2. Furthermore, knockdown of TRAF2 and TRAF6 resulted in reduced homophilic interaction of ASK1 through the N-terminal region. These findings indicate that Trx inhibits ASK1 activity by disrupting N-terminal homophilic interaction, whereas TRAF2 and TRAF6 promote this homophilic interaction and, thus, activation of ASK1.
MATERIALS AND METHODS
Cell culture and transfection.
HEK293A cells were purchased from Invitrogen and cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 4.5 mg/ml glucose, and 100 units/ml penicillin G in a 5% CO2 atmosphere at 37°C. Transfection was performed using FuGENE6 (Roche Molecular Biochemicals) according to the manufacturer's instructions.
Antibodies.
Monoclonal antibodies to hemagglutinin (HA) (clones 3F10 and 12CA5) were purchased from Roche Molecular Biochemicals. A monoclonal antibody to Trx was purchased from Redox Bioscience Inc. Polyclonal antibodies to Trx and TRAF2 were purchased from Santa Cruz Biotechnology, Inc. Phospho-JNK (Thr-183/Tyr-185) antibody was purchased from Cell Signaling Technology. Anti-JNK and anti-ASK1 antibodies were purchased from Santa Cruz Biotechnology, Inc. An affinity-purified rabbit polyclonal antibody raised against phospho-ASK1 was described previously (21). An anti-Flag monoclonal antibody (M2) and anti-Flag M2-agarose affinity gel were purchased from Sigma. A polyclonal antibody to TRAF6 was purchased from MBL.
Plasmids.
pcDNA3-HA-ASK1WT, pcDNA3-HA-ASK1ΔN, pcDNA3-HA-ASK1ΔC, pcDNA3-HA-ASK1-NT, pcDNA3-HA-ASK1-CT, pcDNA3-Flag-ASK1WT, pcDNA3-Flag-Trx, pRK-Flag-TRAF2, and pCR3-Flag-TRAF6 have been described previously (12, 15, 17, 21). A Flag tag was inserted at the N terminus of Trx-CS in pcDNA3, which has been described previously (17). Flag-ASK1ΔN and Flag-ASK1ΔC were constructed in pcDNA3, using cDNAs from their respective HA-tagged vectors. cDNAs encoding ASK1-NT277, ASK1-NT384, ASK1-NT(277-655), ASK1-NT(384-655), ASK1Δ47-120, ASK1Δ244-277, ASK1Δ277, ASK1Δ384, and ASK1ΔNCC were generated by PCR, using mouse ASK1 cDNA as a template, and inserted into pcDNA3 with an HA or Flag tag. cDNAs encoding N-terminal fragments of ASK1 (1-277, 1-244, and 46-277) and Trx (Trx and Trx-CS) were subcloned into pEG202 and pJG4-5, respectively, for yeast two-hybrid analysis.
Yeast two-hybrid system.
The reporter plasmid pJK103, encoding β-galactosidase, and a prey plasmid encoding Trx or Trx-CS fused to the transcriptional activation domain were cotransformed into Saccharomyces cerevisiae EGY188 with bait plasmids encoding the N-terminal (NT) fragments corresponding to amino acids (aa) 1 to 277, 1 to 244, and 46 to 277 of ASK1 fused to the DNA-binding domain. Each transformant was patched onto a 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal; Calbiochem)-containing galactose-positive and His− Trp− Ura− plate (SG-HWU/X-Gal).
Immunoblotting analysis.
Cells were lysed in lysis buffer A, containing 150 mM NaCl, 20 mM Tris-HCl, pH 7.5, 10 mM EDTA, 1% Triton X-100, 1% deoxycholate, 1 mM phenylmethylsulfonyl fluoride, and 1.5% aprotinin, or lysis buffer B, containing 150 mM NaCl, 50 mM Tris-HCl, pH 8.0, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 1 mM phenylmethylsulfonyl fluoride, and 1.5% aprotinin. Cell extracts were clarified by centrifugation, and the supernatants were resolved by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membranes. After being blocked with 5% skim milk in TBS-T (150 mM NaCl, 50 mM Tris-HCl, pH 8.0, and 0.05% Tween 20) for 3 h, the membranes were probed with antibodies. The antibody-antigen complexes were detected using an enhanced chemiluminescence system (Amersham Biosciences).
Coimmunoprecipitation assay.
Cells were lysed in lysis buffer A. Cell extracts were clarified by centrifugation, and the supernatants were immunoprecipitated with anti-Flag M2 affinity gel or anti-HA, anti-Trx, or anti-immunoglobulin G1 (anti-IgG1) antibody, using protein G-Sepharose (Amersham Biosciences). The beads were washed twice with a washing buffer containing 1% Triton X-100, 500 mM NaCl, 20 mM Tris-HCl, pH 7.5, 5 mM EGTA, and 2 mM dithiothreitol, followed by two washes with a washing buffer containing 150 mM NaCl, 20 mM Tris-HCl, pH 7.5, and 5 mM EGTA. The beads were subjected to SDS-PAGE followed by immunoblotting analysis.
Measurement of caspase-3 activity.
HEK293A cells were transiently transfected with vector alone, Flag-ASK1WT, Flag-ASK1Δ277, or Flag-ASK1Δ384. After 48 h, cells were washed with phosphate-buffered saline and lysed in lysis buffer B. Caspase-3-like activity was measured with a CPP32/caspase-3 fluorometric protease assay kit (MBL) following the manufacturer's instructions, in which a fluorogenic synthetic peptide, DEVD-7-amino-4-trifluoromethyl coumarine (AFC), was used as a substrate. The fluorescence of the released AFC was measured at an excitation wavelength of 380 nm and an emission wavelength of 510 nm. To confirm the appropriate expression of transfected plasmids, the same lysates in duplicate wells were combined and verified by immunoblot analysis.
RNA interference.
Small interfering RNAs (siRNAs) for human TRAF2 and TRAF6 were purchased from Invitrogen (Stealth Select RNAi RNAs HSS110961 and HSS110969, respectively). A Stealth RNA interference negative control (Invitrogen) was used as a control. HEK293A cells were transfected with siRNAs by using Lipofectamine 2000 (Invitrogen). After 24 h, cells were transfected with the indicated combinations of Flag-ASK1ΔC and HA-ASK1ΔC. After another 24 h, cells were treated with 5.0 mM H2O2 for 20 min. Cell lysates were immunoprecipitated with anti-Flag antibody. Immunoprecipitates and aliquots of each lysate were subjected to SDS-PAGE followed by immunoblotting with the antibodies indicated in Fig. 6C.
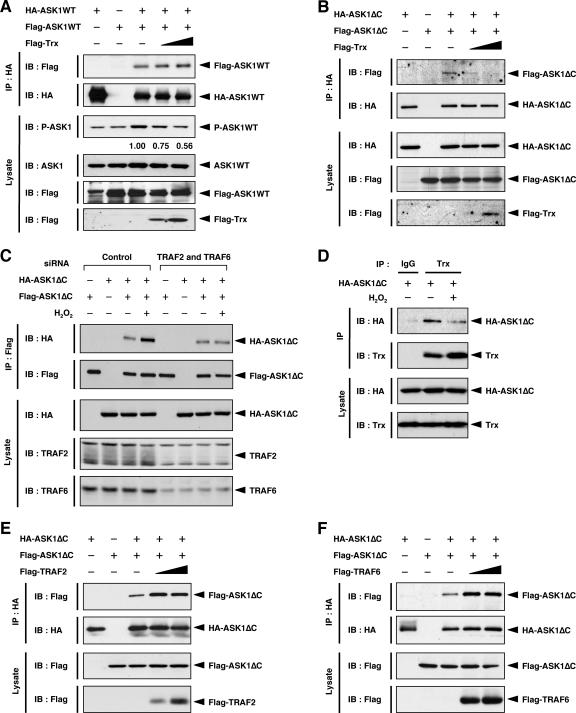
FIG. 6.
Trx inhibits homophilic interaction of ASK1 through the N-terminal region. (A) Expression of Trx has no effect on homophilic interaction of ASK1WT. HEK293A cells were transfected with the indicated combinations of Flag-ASK1WT, HA-ASK1WT, and Flag-Trx. Cell lysates were immunoprecipitated with anti-HA antibody. Immunoprecipitates and aliquots of each lysate were subjected to SDS-PAGE followed by immunoblotting with the indicated antibodies. The amounts of activated ASK1 (P-ASK1) relative to total protein (ASK1) were calculated and are shown as x-fold increases by considering the value for cells expressing HA-ASK1WT and Flag-ASK1WT but not Flag-Trx (lane 3) as 1.00. (B) Expression of Trx inhibits homophilic interaction of ASK1ΔC. HEK293A cells were transfected with the indicated combinations of Flag-ASK1ΔC, HA-ASK1ΔC, and Flag-Trx. Cell lysates were immunoprecipitated with anti-HA antibody. Immunoprecipitates and aliquots of each lysate were subjected to SDS-PAGE followed by immunoblotting with the indicated antibodies. (C) TRAF2 and TRAF6 are required for H2O2-induced homophilic interaction of ASK1ΔC. HEK293A cells were transfected with the indicated siRNAs. After 24 h, cells were transfected with the indicated combinations of Flag-ASK1ΔC and HA-ASK1ΔC. After another 24 h, cells were treated with 5.0 mM H2O2 for 20 min. Cell lysates were immunoprecipitated with anti-Flag antibody. Immunoprecipitates and aliquots of each lysate were subjected to SDS-PAGE followed by immunoblotting with the indicated antibodies. (D) Dissociation of Trx from ASK1ΔC in response to H2O2. HEK293A cells were transfected with HA-ASK1ΔC. After 24 h, cells were treated with 1.0 mM H2O2 for 20 min. Cell lysates were immunoprecipitated with anti-Trx or anti-IgG1 antibody. Immunoprecipitates and aliquots of each lysate were subjected to SDS-PAGE followed by immunoblotting with anti-HA and anti-Trx antibodies. (E) Expression of TRAF2 promotes homophilic interaction of ASK1ΔC. HEK293A cells were transfected with the indicated combinations of Flag-ASK1ΔC, HA-ASK1ΔC, and Flag-TRAF2. Samples were analyzed as described for panel B. (F) Expression of TRAF6 promotes homophilic interaction of ASK1ΔC. HEK293A cells were transfected with the indicated combinations of Flag-ASK1ΔC, HA-ASK1ΔC, and Flag-TRAF6. Samples were analyzed as described for panel B. IP, immunoprecipitation; IB, immunoblotting.
RESULTS
Identification of the Trx-binding region of ASK1.
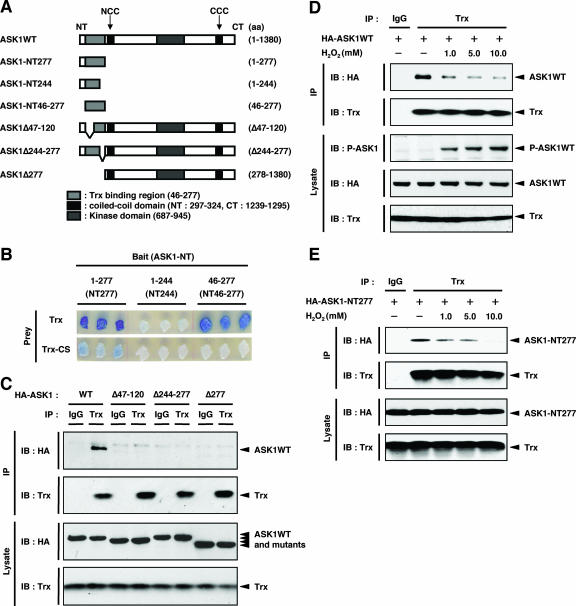
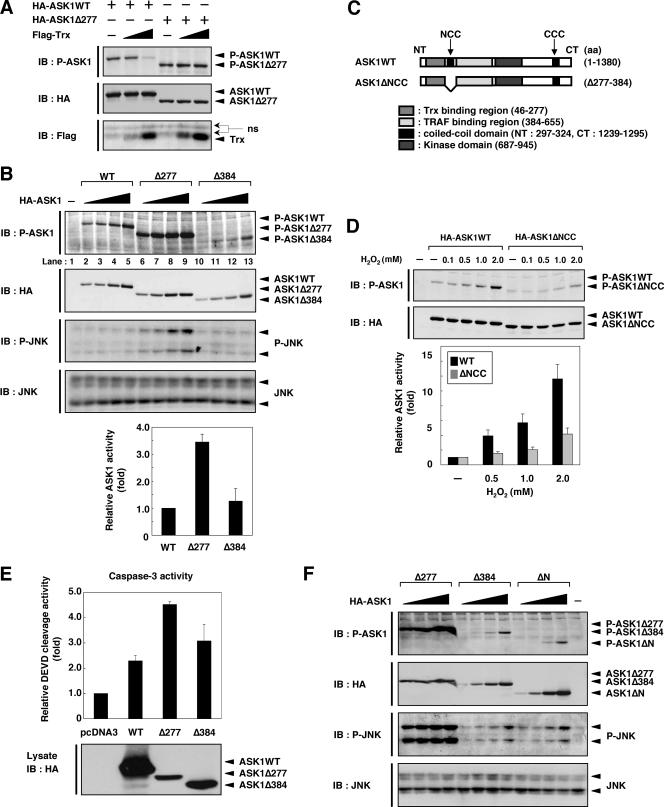
To elucidate the mechanism of inhibition of ASK1 by Trx, we first specified the Trx-binding region within the N terminus of ASK1 by using a yeast two-hybrid system. A peptide fragment corresponding to aa 1 to 277 but not one corresponding to aa 1 to 244 of mouse ASK1 was associated with Trx, indicating that the region of aa 244 to 277 is required for Trx binding (Fig. 1A and B). A fragment containing aa 46 to 277 also associated with Trx, suggesting that the most N-terminal 45-aa region is not required for Trx binding. Consistent with our previous finding that a redox-inactive mutant, Trx-CS, does not bind to ASK1 (17), Trx-CS associated with neither the fragment containing aa 1 to 277 nor that containing aa 46 to 277. We next examined Trx-ASK1 binding by coimmunoprecipitation analysis using mammalian cells. Cell lysates from HEK293A cells transiently transfected with HA-tagged ASK1 (HA-ASK1) were subjected to immunoprecipitation with anti-Trx polyclonal antibody, and coimmunoprecipitated ASK1 was detected by immunoblotting with anti-HA antibody (Fig. 1C). Consistent with the binding region determined for yeast (aa 46 to 277), ASK1Δ277, which lacked the N-terminal 277 aa, did not associate with Trx. ASK1Δ47-120 and ASK1Δ244-277, which lacked the N- and C-terminal portions of the Trx-binding region of ASK1 (aa 46 to 277 in ASK1), respectively, also failed to associate with Trx. We also examined the interaction between the HA-tagged aa 46-to-277 region of ASK1 and endogenous Trx in HEK293A cells. However, we failed to detect the interaction because of the nonspecific background (see Fig. S1A in the supplemental material). We then transfected the Flag-tagged aa 46-to-277 region of ASK1 (Flag-ASK1-NT46-277) into HEK293A cells. Cell lysates were subjected to immunoprecipitation with anti-Flag antibody, and coimmunoprecipitated endogenous Trx was detected by immunoblotting with anti-Trx antibody (see Fig. S1B in the supplemental material). This observation clearly demonstrates that endogenous Trx interacts with ASK1-NT46-277. Taken together, these results suggest that aa 46 to 277 of ASK1 are necessary and sufficient for association with Trx.
FIG. 1.
Identification of Trx-binding region of ASK1. (A) Schematic representation of mouse ASK1WT and its deletion mutants, with amino acid numbers indicated. The NCC and CCC domains are indicated. (B) Interaction of ASK1 with Trx in yeast. A reporter plasmid encoding β-galactosidase and a prey plasmid encoding Trx or Trx-CS fused to the transcriptional activation domain were cotransformed into yeast strain EGY188 with bait plasmids encoding N-terminal fragments corresponding to aa 1 to 277, 1 to 244, and 46 to 277 of ASK1 fused to the DNA-binding domain. Each transformant was patched onto an indicator plate. Galactose-dependent blue spots indicate positive interactions. (C) Interaction of ASK1 with endogenous Trx in HEK293A cells. HEK293A cells were transfected with HA-ASK1WT, HA-ASK1Δ47-120, HA-ASK1Δ244-277, and HA-ASK1Δ277. Cell lysates were immunoprecipitated with anti-Trx or anti-IgG1 antibody. Immunoprecipitates and aliquots of each lysate were subjected to SDS-PAGE followed by immunoblotting with anti-HA and anti-Trx antibodies. (D) Dissociation of Trx from ASK1 in response to H2O2. HEK293A cells were transfected with HA-ASK1WT. After 24 h, cells were treated with the indicated concentrations of H2O2 for 20 min. Cell lysates were immunoprecipitated with anti-Trx or anti-IgG1 antibody. Immunoprecipitates and aliquots of each lysate were subjected to SDS-PAGE followed by immunoblotting with the indicated antibodies. P-ASK1 represents the activated form of ASK1. (E) Dissociation of Trx from ASK1-NT277 in response to H2O2. HEK293A cells were transfected with HA-ASK1-NT277. After 24 h, cells were treated with the indicated concentrations of H2O2 for 20 min. Cell lysates were immunoprecipitated with anti-Trx or anti-IgG1 antibody. Immunoprecipitates and aliquots of each lysate were subjected to SDS-PAGE followed by immunoblotting with anti-HA and anti-Trx antibodies. IP, immunoprecipitation; IB, immunoblotting.
We then examined the interaction of Trx with ASK1 and the activation of ASK1 in response to H2O2 in HEK293A cells (Fig. 1D). As we demonstrated previously (17), treatment of cells with increasing concentrations of H2O2 dose-dependently decreased the amount of wild-type ASK1 (ASK1WT) coimmunoprecipitated with Trx. Furthermore, dose-dependent activation of ASK1 by H2O2 was observed in parallel (Fig. 1D, third panel). We next examined whether the interaction of Trx with the Trx-binding domain of ASK1 was sensitive to the ROS-dependent dissociation of Trx from ASK1. HEK293A cells were transiently transfected with an HA-tagged N-terminal fragment corresponding to aa 1 to 277 (HA-ASK1-NT277), and the Trx-ASK1 complexes were immunoprecipitated with anti-Trx antibody and detected with anti-HA antibody (Fig. 1E). Treatment of cells with increasing concentrations of H2O2 dose-dependently decreased the amount of ASK1-NT277 coimmunoprecipitated with Trx. These findings suggested that the most N-terminal 277 aa of ASK1 are essential for the redox-dependent association/dissociation of the Trx-ASK1 complex.
Identification of the TRAF-binding region of ASK1.
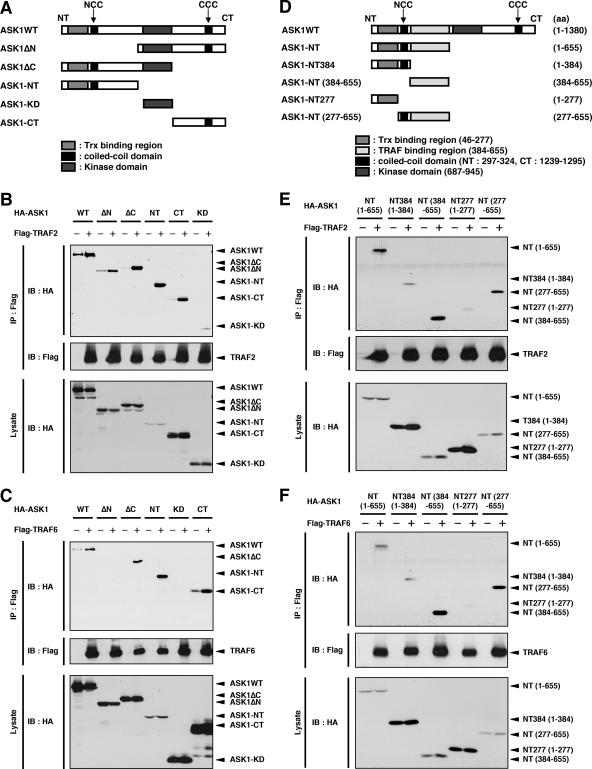
TRAF2 and TRAF6 have been found to be required for ROS-induced activation of ASK1 (16). To elucidate the mechanism of activation of ASK1 by TRAF2 and TRAF6, we next determined the TRAF2- and TRAF6-binding regions within ASK1. When various HA-tagged deletion mutants of ASK1 were cotransfected with Flag-TRAF2 or Flag-TRAF6 into HEK293A cells, both the N-terminal (ASK1ΔC and ASK1-NT) and C-terminal (ASK1ΔN and ASK1-CT) domains, but not a catalytic domain (ASK1-KD), of ASK1 were found to be associated with TRAF2 (Fig. 2A and B). These findings are consistent with those of a previous study, which showed that TRAF2 interacted with both the ASK1 N-terminal (aa 1 to 460) and C-terminal (aa 937 to 1375) noncatalytic regions (9). High levels of ASK1-NT were associated with TRAF2, even though ASK1-NT was expressed only at low levels (Fig. 2B). This implies a much stronger interaction between TRAF2 and ASK1-NT than that with the other truncated ASK1 mutants, suggesting that TRAF2 preferentially associates with ASK1 at the N-terminal region. Similar to TRAF2, TRAF6 interacted with both the N- and C-terminal regions of ASK1 (Fig. 2C). Furthermore, TRAF6 also interacted strongly with ASK1 at the N-terminal region. Note that in the upper panels of Fig. 2B and C, HA-ASK1 in some lanes without TRAF2 or TRAF6 expression represents nonspecifically coprecipitated HA-ASK1 obtained with the beads. Nevertheless, TRAF-dependent interactions with ASK1 at the N-terminal region were clearly detected, suggesting that both TRAF2 and TRAF6 preferentially interact with ASK1 at the N-terminal region.
FIG. 2.
Identification of TRAF2- and TRAF6-binding region of ASK1. (A) Schematic representation of ASK1WT and its deletion mutants. (B) Interaction of ASK1 with TRAF2 in HEK293A cells. HEK293A cells were cotransfected with Flag-TRAF2 and either ASK1WT or its deletion mutants, as indicated. Cell lysates were immunoprecipitated with anti-Flag antibody. Immunoprecipitates and aliquots of each lysate were subjected to SDS-PAGE followed by immunoblotting with anti-HA and anti-Flag antibodies. (C) Interaction of ASK1 with TRAF6 in HEK293A cells. HEK293A cells were cotransfected with Flag-TRAF6 and either ASK1WT or its deletion mutants, as indicated. Samples were analyzed as described for panel B. (D) Schematic representation of mouse ASK1WT, ASK1-NT, and deletion mutants, with amino acid numbers indicated. (E) TRAF2 preferentially interacts with aa 384 to 655 of ASK1. HEK293A cells were cotransfected with Flag-TRAF2 and either ASK1-NT or its deletion mutants, as indicated. Samples were analyzed as described for panel B. (F) TRAF6 preferentially interacts with aa 384 to 655 of ASK1. HEK293A cells were cotransfected with Flag-TRAF6 and either ASK1-NT or its deletion mutants, as indicated. Samples were analyzed as described for panel B. IP, immunoprecipitation; IB, immunoblotting.
We next examined the TRAF-binding region within the N terminus of ASK1. Peptide fragments corresponding to aa 384 to 655 and aa 277 to 655 but not to aa 1 to 384 and aa 1 to 277 of mouse ASK1 associated with TRAF2, indicating that aa 384 to 655 are important for TRAF2 binding (Fig. 2D and E). Similar results were obtained with TRAF6 (Fig. 2F). These findings demonstrate that both TRAF2 and TRAF6 strongly interact with the aa 384-to-655 region of ASK1.
Trx-binding-region truncated mutants of ASK1 strongly interact with TRAF2 and TRAF6.
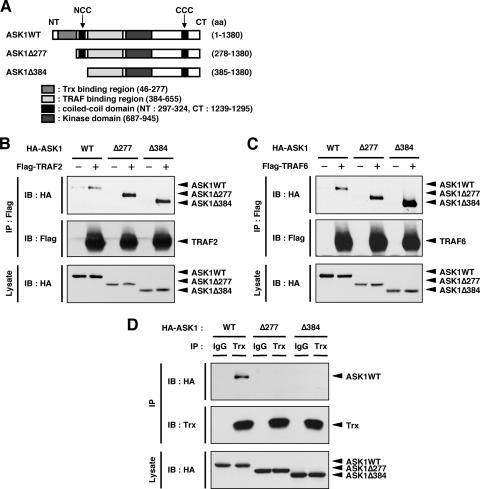
In resting cells, Trx directly binds to and inhibits the kinase activity of ASK1. In previous studies, we found that ROS-dependent dissociation of Trx and reciprocal recruitment of TRAF2 and TRAF6 to ASK1 are required for the activation of ASK1 (16). In addition, it has been shown that overexpression of Trx inhibits ASK1-TRAF2 interaction and that overexpression of TRAF2 inhibits Trx-ASK1 interaction (9). These previous findings suggest the possibility that the regions of Trx and TRAFs that bind ASK1 overlap in the primary structure of ASK1. However, coimmunoprecipitation analysis (Fig. 1 and 2) revealed that the regions that bind ASK1 are close but not overlapping in the primary structure of ASK1, suggesting that Trx and TRAFs bind competitively to ASK1 when ASK1 forms its tertiary structure. We then employed Trx-binding-region truncated mutants of ASK1, namely, ASK1Δ277 (aa 278 to 1380) and ASK1Δ384 (aa 385 to 1380), to examine the interaction between ASK1 and TRAFs in the absence of effects of Trx (Fig. 3A). When HA-tagged ASK1 and its mutants were cotransfected with Flag-TRAF2 or Flag-TRAF6 in HEK293A cells, coimmunoprecipitation analysis showed that both ASK1Δ277 and ASK1Δ384 associated with TRAF2 and TRAF6 much more strongly than did the WT (Fig. 3B and C). ASK1Δ277 and ASK1Δ384 were confirmed not to interact with endogenous Trx in HEK293A cells (Fig. 3D). These results further support the previous finding that the binding of Trx to ASK1 interferes with access of TRAF2 to ASK1 (9).
FIG. 3.
Trx-binding-region truncated mutants of ASK1 strongly interact with TRAF2 and TRAF6. (A) Schematic representation of mouse ASK1WT and its N-terminal deletion mutants, with amino acid numbers indicated. (B) ASK1Δ277 and ASK1Δ384 strongly interact with TRAF2. HEK293A cells were cotransfected with Flag-TRAF2 and either ASK1WT, ASK1Δ277, or ASK1Δ384. Cell lysates were immunoprecipitated with anti-Flag antibody. Immunoprecipitates and aliquots of each lysate were subjected to SDS-PAGE followed by immunoblotting with anti-HA and anti-Flag antibodies. (C) ASK1Δ277 and ASK1Δ384 strongly interact with TRAF6. HEK293A cells were cotransfected with Flag-TRAF6 and either ASK1WT, ASK1Δ277, or ASK1Δ384. Samples were analyzed as described for panel B. (D) Interaction of ASK1 mutants with endogenous Trx in HEK293A cells. HEK293A cells were transfected with HA-ASK1WT, HA-ASK1Δ277, and HA-ASK1Δ384. Cell lysates were immunoprecipitated with anti-Trx or anti-IgG1 antibody. Immunoprecipitates and aliquots of each lysate were subjected to SDS-PAGE followed by immunoblotting with anti-HA and anti-Trx antibodies. IP, immunoprecipitation; IB, immunoblotting.
The NCC domain is required for ROS-induced activation of ASK1.
The finding that ASK1Δ277 and ASK1Δ384 did not interact with Trx but strongly interacted with TRAF2 and TRAF6 prompted us to examine their kinase activities. We first examined the effect of Trx binding on basal activity of ASK1 by immunoblotting analysis using phospho-ASK1 antibody. The basal activity of WT ASK1 (ASK1WT) but not that of ASK1Δ277 was strongly inhibited by coexpression of Trx (Fig. 4A), confirming that Trx suppresses ASK1 activity by direct binding to the N terminus of ASK1. We next compared the kinase activities of ASK1WT, ASK1Δ277, and ASK1Δ384 (Fig. 4B). ASK1Δ277, which contains the NCC domain but not the Trx-binding domain, exhibited higher basal activity than did ASK1WT and induced the activation of endogenous JNK (Fig. 4B). In contrast, the kinase activity of ASK1Δ384, which lacks not only the Trx-binding region but also the NCC domain, was lower than that of ASK1Δ277, whereas both of the mutants interacted strongly with TRAF2 and TRAF6 (Fig. 4B). These findings suggested that binding of TRAF2 or TRAF6 to ASK1 is not sufficient for activation and that the NCC domain of ASK1 is also required for full activation of ASK1. In order to determine whether the NCC domain is required for ASK1 activity, we generated an ASK1 mutant that lacks the NCC domain, namely, ASK1ΔNCC (Fig. 4C). When HEK293A cells transfected with HA-ASK1WT or HA-ASK1ΔNCC were stimulated with H2O2, ASK1ΔNCC exhibited much lower activity than did ASK1WT (Fig. 4D). These findings suggested that not only TRAF2 or TRAF6 binding to ASK1 but also certain function of the NCC domain of ASK1 is required for ROS-induced activation of ASK1.
FIG. 4.
The NCC domain is required for ROS-induced activation of ASK1. (A) Expression of Trx inhibits ASK1 activity but does not inhibit ASK1Δ277 activity. HEK293A cells were transfected with the indicated combinations of HA-ASK1WT, HA-ASK1Δ277, and Flag-Trx. Cell lysates were subjected to SDS-PAGE followed by immunoblotting with the indicated antibodies. P-ASK1 represents the activated form of ASK1. (B) ASK1Δ277 exhibits high basal activity. HEK293A cells were transfected with various amounts of HA-ASK1WT, HA-ASK1Δ277, and HA-ASK1Δ384. Cell lysates were subjected to SDS-PAGE followed by immunoblotting with the indicated antibodies. P-ASK1 and P-JNK represent the activated forms of ASK1 and JNK, respectively. Relative activities for ASK1WT, ASK1Δ277, and ASK1Δ384 in lanes 4, 8, and 13, respectively, are shown in the graph. Values are the means ± standard errors (SE) for three independent experiments. (C) Schematic representation of mouse ASK1WT and its NCC domain-deleted mutant, with amino acid numbers indicated. (D) H2O2-induced activation of ASK1WT and ASK1ΔNCC. HEK293A cells were transfected with HA-ASK1WT or HA-ASK1ΔNCC. Cell lysates were subjected to SDS-PAGE followed by immunoblotting with the indicated antibodies. P-ASK1 represents the activated form of ASK1. Amounts of activated ASK1 (P-ASK1WT or P-ASK1ΔNCC) relative to total protein (HA-ASK1WT or HA-ASK1ΔNCC) were calculated and are shown as x-fold increases by considering the value for the cells treated without H2O2 as 1.00. Values are the means ± SE for three independent experiments. (E) ASK1 induces caspase-3-like activity in HEK293A cells. HEK293A cells were transfected with HA-ASK1WT, HA-ASK1Δ277, HA-ASK1Δ384, or pcDNA3. After 48 h, cells were lysed and caspase-3-like activity in lysates was measured, using DEVD-AFC as a cleaved substrate. Activity is shown as the x-fold increase relative to the value for the extract from pcDNA3-transfected cells. Values are the means ± SE for three independent experiments (upper panel). Cell lysates were subjected to SDS-PAGE followed by immunoblotting with anti-HA antibody (lower panel). (F) ASK1Δ277 exhibits the highest activity among the N-terminal deletion mutants. HEK293A cells were transfected with various amounts of HA-ASK1Δ277, HA-ASK1Δ384, and HA-ASK1ΔN. Cell lysates were subjected to SDS-PAGE followed by immunoblotting with the indicated antibodies. IB, immunoblotting; ns, nonspecific.
ASK1 has been shown to execute apoptosis mainly through mitochondrion-dependent caspase-9 and -3 activation (5). To examine the biological activities of these mutants, we measured caspase-3-like activity in HEK293A cells expressing ASK1WT and each mutant of ASK1 (Fig. 4E). Although the level of expression of ASK1Δ277 was much lower than that of ASK1WT, ASK1Δ277 induced caspase-3-like activity more strongly than did ASK1WT. On the other hand, ASK1Δ384 induced caspase-3-like activity to a moderate extent, in good correlation with its relative kinase activity, as shown in Fig. 4B. These findings suggest that the NCC domain is required for full activation of ASK1, which contributes to effective transmission of proapoptotic signals.
We have previously shown that ASK1ΔN (aa 656 to 1380), which lacks the entire N-terminal region, acts as a constitutively active mutant (17). We therefore compared the kinase activity of ASK1ΔN with those of ASK1Δ277 and ASK1Δ384. Unexpectedly, ASK1ΔN exhibited almost the same activity as ASK1Δ384, although ASK1ΔN does not possess a TRAF-binding region (aa 384 to 655) (Fig. 4F). ASK1ΔN and ASK1Δ384 may mimic an intermediately active form that is liberated from inhibition by Trx but not fully activated due to lack of the NCC domain.
Homophilic interaction of ASK1 through the NCC domain.
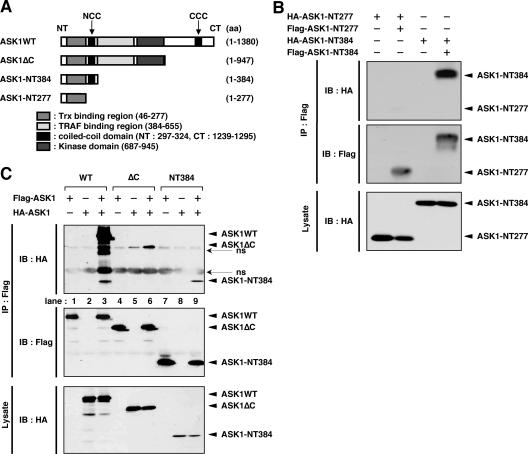
We previously demonstrated that the CCC domain is required for homo-oligomerization of ASK1, which prompted us to examine whether the NCC domain is also required for homophilic interaction of ASK1. We generated the ASK1 fragments ASK1-NT384 and ASK1-NT277 (Fig. 5A). When Flag-tagged ASK1-NT384 (Flag-ASK1-NT384), which contains the NCC domain in addition to the Trx-binding domain, was cotransfected with HA-ASK1-NT384 into HEK293A cells, the interaction of Flag-ASK1-NT384 with HA-ASK1-NT384 was much stronger than that of Flag-ASK1-NT277 with HA-ASK1-NT277 (Fig. 5B). These findings suggested that the NCC domain contributes to homophilic interaction of ASK1. The existence of such a contribution was supported by the finding that homophilic interaction of ASK1ΔC, which lacks the C-terminal region, including the CCC domain (Fig. 5A), was also detected (Fig. 5C, lane 6). Homophilic interaction of ASK1WT was much stronger than that of ASK1ΔC or ASK1-NT384 (Fig. 5C), indicating that ASK1 proteins associate with each other mainly through the C-terminal region, as previously reported (21). Importantly, however, these findings also suggest that the NCC domain serves as an additional interface for the CCC domain-mediated preformed ASK1 complex.
FIG. 5.
Homophilic interaction of ASK1 through the NCC domain. (A) Schematic representation of mouse ASK1WT and its C-terminal deletion mutants, with amino acid numbers indicated. (B) The NCC domain contributes to homophilic interaction of ASK1. HEK293A cells were transfected with the indicated combinations of Flag- or HA-tagged ASK1-NT277 and ASK1-NT384. Cell lysates were immunoprecipitated with anti-Flag antibody. Immunoprecipitates and aliquots of each lysate were subjected to SDS-PAGE followed by immunoblotting with anti-HA and anti-Flag antibodies. (C) Homophilic interaction of ASK1 through the N-terminal region is much weaker than that through the C-terminal region. HEK293A cells were transfected with the indicated combinations of Flag- or HA-tagged ASK1WT, ASK1ΔC, and ASK1-NT384. Cell lysates were immunoprecipitated with anti-Flag antibody. Immunoprecipitates and aliquots of each lysate were subjected to SDS-PAGE followed by immunoblotting with anti-HA and anti-Flag antibodies. IP, immunoprecipitation; IB, immunoblotting; ns, nonspecific.
Trx inhibits homophilic interaction of ASK1 through the N-terminal region.
Since the NCC domain is adjacent to the Trx-binding region, we tested whether Trx affects the homophilic interaction of ASK1 through the NCC domain. We first designed an experiment using full-length ASK1 (ASK1WT). HEK293A cells were transfected with Flag- and HA-ASK1WT together, with or without Flag-Trx, and cell lysates were subjected to coimmunoprecipitation analysis. Immunoblotting using a phospho-ASK1 antibody that detects the activated state of ASK1 revealed that the basal activity of exogenously expressed ASK1 (Flag- and HA-ASK1) was inhibited by coexpression of Flag-Trx (Fig. 6A, third panel). However, the association of Flag-ASK1 with HA-ASK1 was unaffected by coexpression of Flag-Trx (Fig. 6A, top panel). These findings suggested that the inhibitory effect of Trx on ASK1 activity does not require disruption of basal homo-oligomerization of ASK1, a result consistent with our recent finding that the homo-oligomer-based, steady-state ASK1 complex includes Trx (16).
To evaluate the effect of Trx on the ROS-dependent weak interaction through the NCC domain, we next designed a similar experiment, as shown in Fig. 6A, but using ASK1ΔC instead of full-length ASK1. We found that the association of Flag-ASK1ΔC with HA-ASK1ΔC was readily inhibited by coexpression of Flag-Trx (Fig. 6B, top panel), suggesting that Trx inhibits NCC domain- but not CCC domain-dependent homophilic interaction of ASK1. Endogenous Trx may interrupt the homophilic interaction of exogenously expressed ASK1ΔC to some extent. If this is the case, inactivation of endogenous Trx should enhance the homophilic interaction of ASK1ΔC. To test this hypothesis, we examined the effect of H2O2 on the homophilic interaction of ASK1ΔC. Cell lysates from H2O2-treated and untreated HEK293A cells coexpressing Flag- and HA-ASK1ΔC were subjected to coimmunoprecipitation analysis. The association of Flag-ASK1ΔC with HA-ASK1ΔC was augmented by treatment with H2O2 (Fig. 6C, control lanes), although this augmentation was undetectable in the case of ASK1WT (21). Furthermore, endogenous Trx dissociated from ASK1ΔC upon treatment with H2O2 (Fig. 6D). These findings suggested that the NCC domain-mediated homophilic interaction of ASK1, which is suppressed by association with Trx under nonstress conditions, is induced by ROS-dependent dissociation of Trx from ASK1.
Since the TRAF-binding region is adjacent to the NCC domain as well as the Trx-binding region, we next examined whether TRAF2 and TRAF6 affect the homophilic interaction of ASK1ΔC. In contrast to Trx, we found that association of Flag-ASK1ΔC with HA-ASK1ΔC was promoted by coexpression of Flag-TRAF2 or Flag-TRAF6 (Fig. 6E and F, top panels), suggesting that TRAF2 and TRAF6 activate ASK1 by promoting N-terminal homophilic interaction of ASK1. Furthermore, the H2O2-induced homophilic interaction of ASK1ΔC was inhibited by double knockdown of TRAF2 and TRAF6 (Fig. 6C, right half), suggesting that TRAF2 and TRAF6 are required for ROS-induced homophilic interaction of ASK1 through the NCC domain.
DISCUSSION
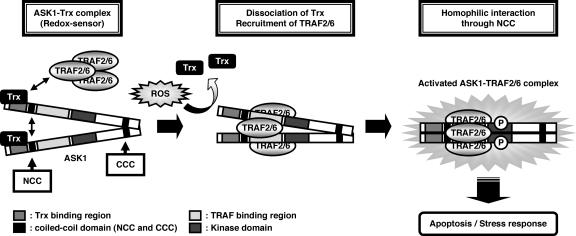
In the present study, we extended our previous findings on the redox-sensitive ASK1 signalosome, focusing in particular upon the ASK1-Trx and ASK1-TRAF systems by examining in detail the molecular mechanisms by which Trx inhibits and TRAF2 and TRAF6 promote ASK1 activity. The principal findings of this study can be summarized as follows: (i) the N-terminal region (aa 46 to 277) of ASK1 is the Trx-binding region and is necessary and sufficient for association with Trx; (ii) both TRAF2 and TRAF6 preferentially interact with the N-terminal region (aa 384 to 655) of ASK1; (iii) ASK1 mutants lacking the Trx-binding region, i.e., ASK1Δ277 and ASK1Δ384, strongly interact with TRAF2 and TRAF6; (iv) the ASK1Δ277 mutant, which lacks the Trx-binding region, is a constitutively active form and strongly induces the activation of JNK and caspase-3, while ASK1Δ384, which lacks not only the Trx-binding region but also the NCC domain (aa 297 to 324), has reduced activity compared with ASK1Δ277; (v) homophilic interaction of the N terminus of ASK1 is mediated by the NCC domain of ASK1, adjacent to the Trx-binding region and the TRAF-binding region; (vi) the NCC domain is required for ROS-induced activation of ASK1; and (vii) Trx inhibits NCC-dependent homophilic interaction of ASK1, which is promoted by overexpression of TRAFs or oxidative stress. Based on these observations, we present a schematic model of the mechanisms of regulation of ASK1 activation by Trx and TRAFs (Fig. 7). In nonstressed cells, the reduced form of Trx binds to the Trx-binding region of ASK1 and inhibits NCC domain- but not CCC domain-mediated homophilic interaction, possibly through mechanisms involving steric hindrance. Upon ROS stimulation, Trx is readily oxidized and ASK1 thus unbinds from oxidized Trx, enabling ASK1 to interact with TRAF2 and TRAF6. ASK1 is thus activated by trans-autophosphorylation, which is dependent on TRAF-mediated homophilic interaction through the N-terminal region of ASK1.
FIG. 7.
Predicted model of mechanisms of regulation of Trx-mediated ASK1 activation. In resting cells, Trx binds to the Trx-binding domain of ASK1, inhibiting N-terminal homophilic interaction. Upon ROS stimulation, oxidized Trx dissociates from ASK1, with reciprocal recruitment of TRAF2 and TRAF6 to ASK1, promoting N-terminal homophilic interaction. This interaction contributes to effective autophosphorylation and activation of ASK1.
We found that the ASK1Δ277 mutant, which lacks the Trx-binding region, is a constitutively and fully activated form that strongly induces cell death. We have previously shown that the N-terminal region of ASK1 is important for Trx-mediated regulation of ASK1 kinase activity and that ASK1ΔN (aa 656 to 1380), which lacks the entire N-terminal region, acts as an active mutant (17). In fact, the kinase activity of ASK1ΔN is stronger than that of ASK1WT (data not shown) but weaker than that of ASK1Δ277 (Fig. 4F). ASK1ΔN lacks not only the Trx-binding region but also the NCC domain and the TRAF-binding region. It may thus be a partially active form that is liberated from inhibition by Trx but not fully activated due to a lack of recruitment of TRAF2 and TRAF6, whereas ASK1Δ277 mimics a fully activated form of ASK1. Unexpectedly, however, ASK1Δ277 exhibited slight activation in response to ROS, although the increases of activated ASK1Δ277 were lower than those of activated ASK1WT (see Fig. S2 in the supplemental material). It is conceivable that endogenous ASK1 that formed a complex with ASK1Δ277 was activated by ROS and thereby also activated ASK1Δ277. On the other hand, these results may also suggest that there are unidentified ROS-dependent modifications of ASK1, TRAF2, or TRAF6.
We found that the Trx-binding region is adjacent to the NCC domain, which is required for homophilic interaction and ROS-induced activation of ASK1. The presence of an inhibitory domain adjacent to the activation domain may favor rapid and precise regulation. It has been reported that many other negative regulators of ASK1 directly bind to or modify the N-terminal region of ASK1. Raf-1 interacts with the N-terminal region of ASK1 and inhibits ASK1 activity in a fashion independent of Raf-1 kinase activity (1). Akt directly phosphorylates Ser83 in the human ASK1 N-terminal region and down-regulates ASK1 activity (8). The type 1 insulin-like growth factor receptor suppresses ASK1 activity by phosphorylation of multiple Tyr residues in the N-terminal region of ASK1 (3). The N-terminal region of ASK1 is a common target site for these negative regulators of ASK1 as well as for Trx. These molecules might thus inhibit ASK1 activity through interference with NCC domain homophilic interaction.
We have recently shown that endogenous ASK1 constitutively forms the so-called ASK1 signalosome as a homo- oligomerized but still inactive high-molecular-mass complex including Trx (16), indicating that homo-oligomerization of ASK1 through the CCC domain is required but not sufficient for ASK1 activation. Consistent with this, the present study clearly showed that not only CCC domain-mediated homo-oligomerization but also ROS-induced NCC domain interaction is required for full activation of ASK1. Furthermore, upon ROS stimulation, the ASK1 signalosome unbinds from oxidized Trx and forms a fully activated higher-molecular-mass complex, in part by recruitment of TRAF2 and TRAF6 (16). However, the precise mechanisms by which TRAF2 and TRAF6 function as activators of ASK1 have remained poorly understood (9, 15). In this study, we also identified the TRAF-binding region adjacent to the NCC domain, which may effectively promote the N-terminal homophilic interaction of ASK1, resulting in full activation of ASK1. Given our previous observation that a TRAF2 mutant lacking a ring finger domain can no longer activate ASK1 (15), it is conceivable that the ring finger domains of TRAF2 and TRAF6 are required to accelerate N-terminal homophilic interaction of ASK1 and are thus necessary for activation of ASK1. Interestingly, the ring finger domain is required for the E3 ubiquitin ligase activity of TRAF2 and TRAF6. ROS-dependent ubiquitination of TRAF2 and TRAF6 themselves or of ASK1 might thus be involved in ROS-induced mechanisms of activation of ASK1.
Exposure of aerobic organisms to oxidative stress is continuous and unavoidable. ROS generation and redox imbalance are closely linked to a wide range of diseases and conditions, such as inflammation, ischemia-reperfusion injury, cancer, neurodegenerative disorders, and aging. It has been reported that ROS-mediated ASK1 activation is involved in a variety of disorders, such as inflammation, neurodegeneration, and cardiac hypertrophy and remodeling (13). Further studies on the mechanisms of regulation of ASK1 and the development of ASK1-targeting drugs may contribute to the treatment of various diseases caused by oxidative stress.
Supplementary Material
Acknowledgments
We thank T. Hatai and N. Osaka for assistance with yeast two-hybrid analyses. We also thank all members of the Cell Signaling Laboratory for helpful discussions.
This work was supported by grants-in-aid for scientific research from the Ministry of Education, Sciences, and Culture of Japan, by CREST, the Japan Science and Technology Corporation, and by the Center of Excellence (COE) program.
Footnotes
Published ahead of print on 27 August 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Chen, J., K. Fujii, L. Zhang, T. Roberts, and H. Fu. 2001. Raf-1 promotes cell survival by antagonizing apoptosis signal-regulating kinase 1 through a MEK-ERK independent mechanism. Proc. Natl. Acad. Sci. USA 98:7783-7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujino, G., T. Noguchi, K. Takeda, and H. Ichijo. 2006. Thioredoxin and protein kinases in redox signaling. Semin. Cancer Biol. 16:427-435. [DOI] [PubMed] [Google Scholar]
- 3.Galvan, V., A. Logvinova, S. Sperandio, H. Ichijo, and D. E. Bredesen. 2003. Type 1 insulin-like growth factor receptor (IGF-IR) signaling inhibits apoptosis signal-regulating kinase 1 (ASK1). J. Biol. Chem. 278:13325-13332. [DOI] [PubMed] [Google Scholar]
- 4.Hashimoto, Y., T. Niikura, T. Chiba, E. Tsukamoto, H. Kadowaki, H. Nishitoh, Y. Yamagishi, M. Ishizaka, M. Yamada, M. Nawa, K. Terashita, S. Aiso, H. Ichijo, and I. Nishimoto. 2003. The cytoplasmic domain of Alzheimer's amyloid-beta protein precursor causes sustained apoptosis signal-regulating kinase 1/c-Jun NH2-terminal kinase-mediated neurotoxic signal via dimerization. J. Pharmacol. Exp. Ther. 306:889-902. [DOI] [PubMed] [Google Scholar]
- 5.Hatai, T., A. Matsuzawa, S. Inoshita, Y. Mochida, T. Kuroda, K. Sakamaki, K. Kuida, S. Yonehara, H. Ichijo, and K. Takeda. 2000. Execution of apoptosis signal-regulating kinase 1 (ASK1)-induced apoptosis by the mitochondria-dependent caspase activation. J. Biol. Chem. 275:26576-26581. [DOI] [PubMed] [Google Scholar]
- 6.Ichijo, H., E. Nishida, K. Irie, P. ten Dijke, M. Saitoh, T. Moriguchi, M. Takagi, K. Matsumoto, K. Miyazono, and Y. Gotoh. 1997. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 275:90-94. [DOI] [PubMed] [Google Scholar]
- 7.Kadowaki, H., H. Nishitoh, F. Urano, C. Sadamitsu, A. Matsuzawa, K. Takeda, H. Masutani, J. Yodoi, Y. Urano, T. Nagano, and H. Ichijo. 2005. Amyloid beta induces neuronal cell death through ROS-mediated ASK1 activation. Cell Death Differ. 12:19-24. [DOI] [PubMed] [Google Scholar]
- 8.Kim, A. H., G. Khursigara, X. Sun, T. F. Franke, and M. V. Chao. 2001. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol. Cell. Biol. 21:893-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu, H., H. Nishitoh, H. Ichijo, and J. M. Kyriakis. 2000. Activation of apoptosis signal-regulating kinase 1 (ASK1) by tumor necrosis factor receptor-associated factor 2 requires prior dissociation of the ASK1 inhibitor thioredoxin. Mol. Cell. Biol. 20:2198-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu, Y., and W. Min. 2002. Thioredoxin promotes ASK1 ubiquitination and degradation to inhibit ASK1-mediated apoptosis in a redox activity-independent manner. Circ. Res. 90:1259-1266. [DOI] [PubMed] [Google Scholar]
- 11.Matsuzawa, A., K. Saegusa, T. Noguchi, C. Sadamitsu, H. Nishitoh, S. Nagai, S. Koyasu, K. Matsumoto, K. Takeda, and H. Ichijo. 2005. ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nat. Immunol. 6:587-592. [DOI] [PubMed] [Google Scholar]
- 12.Morita, K., M. Saitoh, K. Tobiume, H. Matsuura, S. Enomoto, H. Nishitoh, and H. Ichijo. 2001. Negative feedback regulation of ASK1 by protein phosphatase 5 (PP5) in response to oxidative stress. EMBO J. 20:6028-6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagai, H., T. Noguchi, K. Takeda, and H. Ichijo. 2007. Pathophysiological roles of ASK1-MAP kinase signaling pathways. J. Biochem. Mol. Biol. 40:1-6. [DOI] [PubMed] [Google Scholar]
- 14.Nishitoh, H., A. Matsuzawa, K. Tobiume, K. Saegusa, K. Takeda, K. Inoue, S. Hori, A. Kakizuka, and H. Ichijo. 2002. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 16:1345-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishitoh, H., M. Saitoh, Y. Mochida, K. Takeda, H. Nakano, M. Rothe, K. Miyazono, and H. Ichijo. 1998. ASK1 is essential for JNK/SAPK activation by TRAF2. Mol. Cell 2:389-395. [DOI] [PubMed] [Google Scholar]
- 16.Noguchi, T., K. Takeda, A. Matsuzawa, K. Saegusa, H. Nakano, J. Gohda, J. Inoue, and H. Ichijo. 2005. Recruitment of tumor necrosis factor receptor-associated factor family proteins to apoptosis signal-regulating kinase 1 signalosome is essential for oxidative stress-induced cell death. J. Biol. Chem. 280:37033-37040. [DOI] [PubMed] [Google Scholar]
- 17.Saitoh, M., H. Nishitoh, M. Fujii, K. Takeda, K. Tobiume, Y. Sawada, M. Kawabata, K. Miyazono, and H. Ichijo. 1998. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 17:2596-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takeda, K., T. Hatai, T. S. Hamazaki, H. Nishitoh, M. Saitoh, and H. Ichijo. 2000. Apoptosis signal-regulating kinase 1 (ASK1) induces neuronal differentiation and survival of PC12 cells. J. Biol. Chem. 275:9805-9813. [DOI] [PubMed] [Google Scholar]
- 19.Takeda, K., A. Matsuzawa, H. Nishitoh, K. Tobiume, S. Kishida, J. Ninomiya-Tsuji, K. Matsumoto, and H. Ichijo. 2004. Involvement of ASK1 in Ca2+-induced p38 MAP kinase activation. EMBO Rep. 5:161-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tobiume, K., A. Matsuzawa, T. Takahashi, H. Nishitoh, K. Morita, K. Takeda, O. Minowa, K. Miyazono, T. Noda, and H. Ichijo. 2001. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2:222-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tobiume, K., M. Saitoh, and H. Ichijo. 2002. Activation of apoptosis signal-regulating kinase 1 by the stress-induced activating phosphorylation of preformed oligomer. J. Cell Physiol. 191:95-104. [DOI] [PubMed] [Google Scholar]
- 22.Zhang, R., R. Al-Lamki, L. Bai, J. W. Streb, J. M. Miano, J. Bradley, and W. Min. 2004. Thioredoxin-2 inhibits mitochondria-located ASK1-mediated apoptosis in a JNK-independent manner. Circ. Res. 94:1483-1491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.