Abstract
Eph receptors and ephrins have been implicated in a variety of cellular processes, including morphology and motility, because of their ability to modulate intricate signaling networks. Here we show that the phosphotyrosine binding (PTB) domain-containing proteins AIDA-1b and Odin are tightly associated with the EphA8 receptor in response to ligand stimulation. Both AIDA-1b and Odin belong to the ankyrin repeat and sterile alpha motif domain-containing (Anks) protein family. The PTB domain of Anks family proteins is crucial for their association with the juxtamembrane domain of EphA8, whereas EphA8 tyrosine kinase activity is not required for this protein-protein interaction. In addition, we found that Odin is a more physiologically relevant partner of EphA8 in mammalian cells. Interestingly, overexpression of the Odin PTB domain alone attenuated EphA8-mediated inhibition of cell migration in HEK293 cells, suggesting that it acts as a dominant-negative mutant of the endogenous Odin protein. More importantly, small interfering RNA-mediated Odin silencing significantly diminished ephrinA5-induced EphA8 signaling effects, which inhibit cell migration in HEK293 cells and retract growing neurites of Neuro2a cells. Taken together, our findings support a possible function for Anks family proteins as scaffolding proteins of the EphA8 signaling pathway.
Eph receptor tyrosine kinases regulate diverse cell behaviors by interacting with membrane-anchored ligands, ephrins, at sites of cell-cell contact (17, 31, 33). The signals of the receptor-ligand complex propagate bidirectionally into both the Eph receptor-expressing cells (forward signaling) and the ephrin-expressing cells (reverse signaling). Forward and reverse signaling by Eph receptors stimulates diverse signaling networks to regulate axon guidance, cell adhesion, and cell migration. The interplay between these signaling molecules and Eph-ephrin complexes is fairly complex, and it remains to be determined whether they play a pivotal role in axonal behavior and migration of physiologically relevant cells that express Eph receptors or ephrin ligands.
Eph receptors and their ligands have emerged as critical regulators of the dynamics of cell motility (4, 6, 9, 25, 43). It has been reported that the Rho family of small GTPases is a key converging point for the mechanisms regulating cell motility (36). Consistent with these observations, the relative activity of Rho versus Rac and Cdc42 activities is altered by Eph signaling. For example, ephrinA stimulation of EphA receptors is known to modulate the activity of ephexin, leading to RhoA activation and Cdc42 and Rac1 inhibition (35, 37). In contrast, activation of the EphB receptor induces recruitment of kalirin to the receptor clusters to activate Rac1 GTPase (32). Stimulation of the EphA2 receptor by ephrinA is also known to recruit the binding of Vav proteins or Tiam1 to the EphA2 receptor, leading to activation of Rac1 GTPase (13, 40). Likewise, EphB2 physically associates with intersectin and activates Cdc42 GTPase in cooperation with N-WASP (15). In addition, integrins (8, 14, 45), focal adhesion kinases (1, 23), Src family kinases (19, 21, 42), and signaling from Ras to the mitogen-activated protein kinase (MAPK) pathway (5, 24) are also affected by Eph signaling and have been implicated in modulating cell motility, although it is not clear whether these signaling pathways are really involved in regulating Rho activity. Similar mechanisms underlying Eph-regulated cell motility are likely to be involved in the migratory movements of axonal growth cones, a major function that has been attributed to the Eph receptor tyrosine kinase family (22, 26). Although a repulsive response involving the retraction of the growing axons was the first reported effect of Eph signaling, adhesive responses have also been reported (2, 18). At least two different mechanisms have been reported for the repulsive mechanism responsible for the retraction of cellular processes. One mechanism is the elimination of Eph-ephrin complexes through the metalloprotease-mediated cleavage of ephrins, which causes cell disengagement. ADAM10 (a disintegrin and metalloprotease 10) was reported to be involved in the cleavage of ephrinA2 upon interaction of ephrinA2 with EphA3 (11, 16). The other mechanism is that trans-endocytosis of Eph-ephrin complexes eliminates adhesive cell interactions, allowing the cells to detach from each other. For example, cell contact-induced EphB1/ephrinB1 or EphB4/ephrinB2 complexes are rapidly endocytosed during cell retraction and neuronal growth cone-induced cell-cell repulsion, and this process is mediated by a Rac-dependent mechanism (20, 44). Cell adhesion or attraction can also be mediated by Eph receptors and ephrin ligands. Several mechanisms have been reported for Eph's adhesive effects. These include inefficient activation of Eph-mediated repulsive signaling function due to either kinase-defective Eph receptors (12, 21) or phosphatases (30, 38). Cell adhesions are also mediated by cross talk between Eph receptors and integrins, by which cell adhesion to extracellular matrix protein is promoted, leading to membrane ruffling and axon extension (8, 10, 14, 39). Although it remains to be determined clearly how Eph receptors control repulsion versus attraction, the interplay between Eph signaling and various signaling molecules has been predicted to be a key factor in the control of its ultimate effects on cell behavior. In particular, understanding how Eph receptors alter the equilibrium between members of the Rho family of small GTPases will be critical for dissecting how the Eph receptor mediates attractive and repulsive effects.
In this study, we demonstrate that the EphA8 receptor interacts with AβPP intracellular domain-associated protein 1b (AIDA-1b) by using a yeast two-hybrid screen. Odin, the protein most closely related to AIDA-1b, is also shown to interact with the EphA8 receptor. Both AIDA-1b and Odin belong to the ankyrin repeat and sterile alpha motif (SAM) domain-containing (Anks) protein family. Importantly, members of the Anks protein family contain a phosphotyrosine binding (PTB) domain, which is crucial for interaction with the juxtamembrane (JM) region of EphA8. PTB domains are classified into three groups, namely, phosphotyrosine-dependent Shc-like, phosphotyrosine-dependent IRS-like, and phosphotyrosine-independent Dab-like PTB domains (41). The PTB domain in AIDA family proteins belongs to the phosphotyrosine-independent Dab-like PTB domain group. Consistent with this, association of Anks family proteins with the EphA8 receptor does not require EphA8 autokinase activity. Interestingly, it was previously shown that the EphA2 receptor interacts with the PTB and SH2 domains of Shc to stimulate the MAPK/extracellular signal-regulated kinase (ERK) signaling pathway. This was the first and only time a PTB domain-containing adaptor was found to be involved in Eph signaling (34). In fact, the majority of known PTB domain-containing proteins do not possess any catalytic domains, suggesting that their function is limited to that of adaptors/scaffolds of considerable diversity. Taken together with our novel findings, these studies suggest that PTB domain-containing proteins may function as crucial adaptors or scaffold proteins for the modulation of diverse signaling pathways downstream of Eph receptors. For example, AIDA-1b and Odin contain several motifs involved in protein-protein interactions, including six ankyrin repeats, two SAM domains, and a PTB domain. It was previously shown that AIDA-1b and Odin are involved in modulating AβPP processing and inhibiting platelet-derived growth factor-mediated cell proliferation, respectively (7, 28). In this paper, we show that relative to AIDA-1b, Odin is more abundant and more ubiquitously expressed in many different mammalian cell lines. More importantly, when Odin is downregulated, inhibition of cell migration or neurite retraction is significantly attenuated in response to ephrinA5 stimulation, suggesting that Odin is a key scaffolding protein for the modulation of EphA8-mediated cell behaviors.
MATERIALS AND METHODS
Construction of expression vectors.
Murine wild-type (WT) and kinase-inactive (KD) EphA8 cDNAs tagged with a nine-amino-acid hemagglutinin (HA) epitope (YPYDVPDYA) at their COOH termini have been described elsewhere (3). EphA8-enhanced green fluorescent protein (EphA8-EGFP) was generated as follows. First, the murine EphA8 cDNA (pSP38) was subcloned into the HindIII/SalI sites in pEGFP-N1 (Clontech) and used as a template for amplifying a 363-bp PCR product, using primers matching nucleotides (nt) 2731 to 2750 (5′-CCGGAATTCGCTCATGTCGTGAGCGTTCT-3′) and nt 3075 to 3090 (5′-ACCGTCGACTGGAGGTGCCGGCGGCGT-3′) of the insert. The resulting PCR product was digested with BssHII/SalI and subcloned into the corresponding region of full-length EphA8 cDNA in pEGFP-N1. The expression vector for human AIDA-1b (GenBank accession no. AY281131) has been described elsewhere (7), and mouse Odin (GenBank accession no. BC050847) was obtained from Invitrogen. To construct Odin-PTB (with a deletion of amino acids [aa] 63 to 864 of mouse Odin), a 308-bp PCR product was amplified using primers matching nt 1 to 20 and nt 289 to 308 of the Odin cDNA, and an 861-bp PCR product was amplified using primers matching nt 2715 to 2734 and nt 3558 to 3575 of the Odin cDNA. The resulting PCR products were digested with HindIII/EcoRI and EcoRI/XhoI, respectively, and then subcloned into pcDNA3 (Invitrogen). To construct Odin-ΔPTB (with a deletion of aa 865 to 1082 of mouse Odin), a 494-bp PCR product was amplified using primers matching nt 2221 to 2240 and nt 2695 to 2714 (5′-TTCTGGGCTTGTCTTAGCTGGGAAAACCTTG-3′) of the Odin cDNA, and a 207-bp PCR product was amplified using primers matching nt 3369 to 3385 (5′-CCCAGCTAAGACAAGCCCAGAAATCCAG-3′) and nt 3556 to 3575 of the Odin cDNA. The two partially complementary PCR fragments thus generated were annealed and used as the template in another PCR with primers matching nt 2221 to 2240 and nt 3556 to 3575 of the Odin cDNA. The resulting 687-bp PCR product was digested with BglI/XhoI and subcloned into the corresponding region of full-length Odin cDNA. Odin EGFP-PTB and Odin EGFP-ΔPTB constructs were generated by subcloning the Odin-PTB and Odin-ΔPTB cDNAs described above, respectively, into pEGFP-N1 (Clontech). The glutathione S-transferase (GST)-EphA8 JM construct has been described elsewhere (8). To generate the GST-AIDA PTB construct, a 1,035-bp XhoI/BamHI fragment was isolated from the partial AIDA cDNA (matching nt 739 to 1773 of the AIDA-1b transcript variant 2 [GenBank accession no. NM_181670]) identified through yeast library screening and subcloned into pGEX-5X-1. The His-AIDA PTB construct was also generated by subcloning a 780-bp XhoI/NheI fragment matching nt 739 to 1518 of the AIDA-1b transcript variant 2 into pET15b (Novagen). For the His-Odin PTB construct, an 861-bp PCR product was amplified using primers matching nt 2715 to 2734 and nt 3558 to 3575 of the Odin cDNA. The resulting PCR products were digested with EcoRI/XhoI and subcloned into pET28b (Novagen). The yeast expression construct for EphA8-JM1 was generated by amplifying a 216-bp PCR product, using primers matching nt 1765 to 1785 and nt 1960 to 1980 of the EphA8 cDNA, and subcloning it into the pBHA vector. The same procedures were used to generate the yeast bait constructs expressing EphA8-JM2, -JM3, -JM4, -JM5, and -JM6, EphA2-JM, EphA4-JM, and EphB2-JM, except that different primers were used: for EphA8-JM2, a 168-bp fragment was amplified using primers matching nt 1813 to 1833 and nt 1960 to 1980 of the EphA8 cDNA; for EphA8-JM3, a 142-bp fragment was amplified using primers matching nt 1839 to 1859 and nt 1960 to 1980 of the EphA8 cDNA; for EphA8-JM4, a 117-bp fragment was amplified using primers matching nt 1864 to 1884 and nt 1960 to 1980 of the EphA8 cDNA; for EphA8-JM5, 48-bp PCR products (nt 1765 to 1785 and nt 1798 to 1812 of the EphA8 cDNA) and 117-bp PCR products (nt 1864 to 1884 and nt 1960 to 1980 of the EphA8 cDNA) were combined for another PCR with primers matching nt 1765 to 1785 and nt 1960 to 1980 of the EphA8 cDNA; for EphA8-JM6, a 186-bp fragment was amplified using a 5′ primer matching both nt 1765 to 1782 and nt 1813 to 1837 and a 3′ primer matching nt 1960 to 1980 of the EphA8 cDNA; for EphA2-JM, a 165-bp fragment was amplified using primers matching nt 1791 to 1810 and nt 1936 to 1955 of the mouse EphA2 cDNA (GenBank accession no. NM_010139); for EphA4-JM, a 161-bp fragment was amplified using primers matching nt 1765 to 1784 and nt 1901 to 1920 of the mouse EphA4 cDNA (GenBank accession no. NM_007936); and for EphB2-JM, a 165-bp fragment was amplified using primers matching nt 1804 to 1823 and nt 1949 to 1968 of the mouse EphB2 cDNA (GenBank accession no. NM_010142). The yeast expression constructs for PSD-95 and NR-2A have been described elsewhere (27).
Yeast two-hybrid assay.
The mouse EphA8 JM domain (aa 563 to 634) cloned into the pBHA vector (pCK129) was used as bait to screen a human fetal brain cDNA library consisting of 3.0 × 106 independent clones (Clontech). Briefly, yeast strain L40 [MATa his3200 trp1-901 lue2-3,112 ade2 (LYS2::lexAop)4-HIS3 (URA3::lexAop)8-lacZ GAL4] was transformed with pCK129 and the fetal brain cDNA library. The resulting transformants were screened for histidine prototrophy by using 1 mM 3-aminotriazole (3-AT) on selective medium lacking His, Trp, and Leu. Histidine-positive (His+) clones were then assayed for blue coloring by colony lift with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal). Among several His+ LacZ+ clones, three identical clones encompassing the PTB domain of the AIDA-1b gene were identified.
Cell culture, cell transfection, RNA interference, and cell migration assay.
HEK293 and Neuro2a cells were cultured as described previously (10). For superior collicular neuron culture, the anterior half of the dorsal midbrain was isolated from embryonic day 14.5 (E14.5) mouse embryos. After incubation with trypsin in Hanks' balanced salt solution (Invitrogen) for 2 min at 37°C, tissues were dissociated into single cells and filtered using a nylon gauze to remove cell aggregates. Cells (1 × 105) were seeded onto a cover glass coated with poly-l-lysine (10 μg/ml; Sigma) and laminin (10 μg/ml; Sigma) and then cultured in Neurobasal medium (Invitrogen) with penicillin-streptomycin and N-2 supplement (Invitrogen). Transient transfection procedures were performed using Metafectin (Biontax) according to the manufacturer's instructions. For the silencing of Odin, a mouse- or human-specific SMARTpool was designed by Dharmacon. Human Odin-specific small interfering RNAs (siRNAs) (5′-CAACGUUGCUGACUCGAAAUU-3′ and 5′-UUUCGAGUCAGCAACGUUGUU-3′) were also purchased from Dharmacon. For every experiment performed, nontargeting, RNA-induced silencing complex-free siRNA was used as a nonsilencing control (Dharmacon). Three micrograms of double-stranded siRNA was introduced into 2 × 106 Neuro2a cells with Nucleofector II (Amaxa) set at program T-20, using a Cell Line Nucleofactor kit V (Amaxa) according to the manufacturer's instructions. Cell migration assays using 6.5-mm, 8-μm-pore-size Transwells (Costar) were performed essentially as described previously (25), except that cells were allowed to migrate through the filter for 2 h. EphrinA5-Fc preclustered with goat anti-human Fc at 5 μg/ml was added to the lower chamber. Cells were fixed with 4% paraformaldehyde and stained with 0.5% eosin.
Immunoprecipitation, Western blotting, and immunofluorescence staining.
Immunoprecipitation and Western blotting were performed essentially as described previously (8). For immunostaining of Neuro2a cells with anti-Odin or anti-EphA8 antibodies, cells (3 × 105) were seeded onto coverslips coated with 2 μg/ml of fibronectin (Sigma). After 24 h, cells were gently washed twice with phosphate-buffered saline, fixed with 4% paraformaldehyde-2% sucrose in phosphate-buffered saline for 20 min at room temperature, and rinsed three times for 5 min with TBST (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.1% Triton X-100). Cells were blocked with TBST containing 5% horse serum for 30 min at room temperature and then incubated with primary antibodies overnight at 4°C. Cells were washed twice with TBST and then blocked with TBST containing 0.1% bovine serum albumin for 30 min at room temperature. Cells were incubated with tetramethyl rhodamine isocyanate-conjugated anti-rabbit antibodies for 1 h at room temperature and washed three times with TBST. Cells were mounted with Vectashield (Vector Laboratory) and observed using a confocal microscope (model FV300; Olympus). For the colocalization study of Odin and EphAs in superior collicular neurons, differentiated cells were incubated with 2 μg/ml ephrinA5-Fc (preclustered with 10 μg/ml goat anti-human immunoglobulin G [IgG]) for 10 min, fixed with 4% paraformaldehyde-2% sucrose for 30 min, and then processed for immunofluorescence staining as described above. EphrinA5-Fc was detected by using fluorescein isothiocyanate (FITC)-conjugated goat anti-human IgG.
Neuronal differentiation and real-time microscopy.
For differentiation of Neuro2a cells, 1 × 104 cells were seeded onto a cover glass coated with poly-l-lysine (5 μg/ml; Sigma). After 12 h, cells were incubated with medium containing 0.5% serum and 0.1 mM dibutyryl cyclic AMP (cAMP; Sigma) for 3 days. Cells were placed in temperature/CO2 control chambers (Tokai) attached to an Olympus IX81-ZDC zero-drift microscope. After 1 h of acclimation, time-lapse images were collected 1 minute before adding EphrinA5-Fc or Fc control at 5 μg/ml. Images continued to be collected for 1 h at 1-min intervals, using a Cascade 512B camera (Roper Scientific) operated by the multidimensional acquisition package of MetaMorph v. 7.01 software (Molecular Devices).
Antibodies.
A polyclonal rabbit antibody specific for the JM domain of EphA8 was described previously (3). Anti-Odin antibodies were purchased from Calbiochem, antitubulin and antiactin antibodies were from Santa Cruz Biotechnology, anti-HA and anti-AIDA antibodies were from Zymed, and tetramethyl rhodamine isocyanate-conjugated anti-rabbit IgG and FITC-conjugated goat anti-human IgG antibodies were from Chemicon. Horseradish peroxidase-conjugated anti-rabbit IgG antibodies were acquired from Zymed.
RESULTS
Fetal brain cDNA library screening identifies AIDA-1b as an EphA8 JM domain-interacting protein.
Our previous studies indicated that a JM segment of the EphA8 receptor was critical for modulating cell adhesion and migration (8). To identify which proteins might interact with EphA8, we performed a yeast two-hybrid screen using the JM domain of EphA8 (72 aa) as a bait to screen a human fetal brain cDNA library (Fig. 1A). Three clones were identified as putative candidates, and all of them contained an identical PTB domain sequence of AIDA-1b (Fig. 1B). We confirmed the authenticity of the clones by transforming them back into yeast with the EphA8-JM protein (Fig. 1C). To further determine if JM domains of other Eph receptors interact with the PTB domain of AIDA-1b, we performed a yeast two-hybrid assay using the JM domains of EphA2, EphA4, and EphB2 as baits (Fig. 1D). Yeast transformants expressing various JM proteins, except for the EphA8-JM construct, did not grow on His− Trp− Leu− selective plates (data not shown) and failed to show X-Gal staining on Trp− Leu− selective plates (Fig. 1D). To identify the specific amino acids within the EphA8-JM construct that mediate the interaction with the AIDA-1b PTB domain, a panel of EphA8-JM deletion mutant constructs was generated and tested in the yeast two-hybrid system for interactions with the AIDA-1b PTB domain (Fig. 1E). As shown in Fig. 1F, deletion of the first seven amino acid residues of the EphA8 JM segment consistently abolished the ability of the EphA8 JM segment to bind to the AIDA-1b PTB domain. Taken together, these results demonstrate that the PTB domain of AIDA-1b is recruited to a unique docking site (KKRHCGY) in the EphA8 JM region and that this site is not present in the JM regions of EphA2, EphA4, and EphB2.
FIG. 1.
Yeast library screening identified AIDA-1 as an EphA8-interacting protein. (A) The JM amino acid sequences of the indicated Eph family members were aligned using the ClustalW program. Gaps are represented by dashes. The numbers above the two sets of sequences mark the locations of amino acids within the EphA8 JM region. The dotted underline indicates the specific amino acid residues of the EphA8 JM region that are critical for interaction with the AIDA-1b PTB domain. (B) Domain structures of EphA8, AIDA-1b, and the interacting clone of AIDA identified in the yeast two-hybrid screen. G, immunoglobulin-like domain; C, cysteine-rich motif; F, fibronectin type III repeat domain; T, transmembrane domain; JM, juxtamembrane domain; K, kinase domain; S, SAM domain; A, ankyrin repeat domain; P, PTB domain. (C) X-Gal staining analysis indicated that the PTB domain of AIDA-1 binds to the JM domain of EphA8. Yeast transformants were cultured on Trp− Leu− selective plates in the absence of 3-AT prior to X-Gal staining using a filter-lifting method. Yeast cells transformed with NR-2A and PSD-95 served as positive controls for these yeast two-hybrid systems. Yeast cells transformed with the tyrosine kinase domain (TKD) of EphA8 as bait served as a negative control. Yeast transformants showing negative X-Gal staining on Trp− Leu− selective plates did not grow on His− Trp− Leu− selective plates in the presence of 1 mM 3-AT (data not shown). (D) AIDA-1 PTB domain interacts only with the JM domain of EphA8, not with those of EphA2, EphA4, and EphB2, in yeast. The JM sequences of the Eph family members shown in panel A were expressed as bait proteins fused to the LexA DNA binding domain in yeast. Yeast transformants were cultured on Trp− Leu− selective plates, and X-Gal staining was performed essentially as described for panel C. (E) Schematic diagram showing the various EphA8-JM deletion constructs that were subcloned into the bait construct described for panels C and D. (F) The AIDA PTB domain was coexpressed in the yeast two-hybrid assay with various deletion mutants of EphA8-JM, and X-Gal staining was performed essentially as described for panel C.
The EphA8 receptor is associated with proteins of the Anks family in an EphA8 kinase activity-independent manner.
In order to demonstrate a direct interaction between the EphA8 JM domain and the AIDA-1b PTB domain, we utilized bacterially produced proteins in which EphA8-JM was fused to GST (Fig. 2A, bottom panel) and AIDA-1b PTB was fused to a His tag (Fig. 2A, middle panel). The purified proteins were mixed, and His tag pull-down experiments were performed (Fig. 2A, top panel). As expected, the GST-JM fusion protein, but not GST, was specifically coprecipitated with the PTB protein (lane 2). Conversely, the PTB protein was also coprecipitated with the GST-JM fusion protein in GST pull-down experiments (data not shown). The PTB domain was also fused to GST (Fig. 2B, middle panel) and mixed with a HEK293 cell lysate containing the EphA8 receptor for the GST pull-down experiment (Fig. 2B, bottom panel). It was evident that the PTB domain of AIDA-1b was sufficient for association with full-length EphA8 in vitro (Fig. 2B, lane 4). Likewise, the GST-JM fusion protein associated with the AIDA-1b protein in pull-down experiments (data not shown). AIDA-1b and Odin contain the highest degree of sequence identity in their PTB domains (over 82%), and they both belong to the Anks protein family. Since AIDA-1b and Odin are closely related, we investigated whether Odin-PTB binds to the EphA8 JM domain. As expected, the GST-JM fusion protein, but not the control GST protein, was readily detected in His tag pull-down experiments when EphA8-JM fused to GST was mixed with Odin-PTB fused to a His tag (Fig. 2C). Taken together, these results demonstrate that the EphA8 JM domain directly associates with the PTB domains of Anks family proteins.
FIG. 2.
Evidence that the EphA8 JM domain associates directly with the PTB domain of Anks family proteins. (A) Demonstration of AIDA-1 PTB domain interaction with the EphA8 JM domain, using purified proteins. Purified GST or GST-JM fusion proteins were mixed with purified His-tagged AIDA-1 PTB protein. Bound proteins were precipitated using His-bind resin, fractionated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and detected by Western blotting with anti-GST antibody (top panel). The same blot was stripped and reprobed with anti-AIDA antibody (middle panel). GST or GST-JM fusion proteins used for this experiment were directly detected by Coomassie staining (bottom panel). (B) Demonstration of AIDA-1 PTB domain interaction with the full-length EphA8 receptor. Purified proteins (GST or GST-PTB) were mixed with whole-cell lysates from HEK293 cells transfected with the control vector (lanes 1 and 2) or an EphA8 expression construct (lanes 3 and 4). Bound proteins were pulled down using glutathione beads, fractionated by 10% SDS-PAGE, and detected by Western blotting using anti-EphA8 antibody (top panel). The same blot was stripped and reprobed with anti-GST antibody (middle panel). A sample (2%) of each total cell lysate was analyzed directly by Western blotting using anti-EphA8 antibody (bottom panel). (C) Evidence that the Odin PTB domain interacts with the EphA8 JM domain, using purified proteins. Experiments were performed essentially as described for panel A, except that purified His-tagged Odin PTB protein and anti-Odin antibody were used. PD, pulldown; WB, Western blot.
Next, we assessed whether EphA8 and AIDA-1b interact in mammalian cells. Various isoforms of AIDA-1b proteins, derived from alternative splicing of the same transcript, have been described (7). The AIDA-1b isoform was primarily tested in these experiments. EphA8 and AIDA-1b constructs were transiently transfected into HEK293 cells. Total proteins were subjected to immunoprecipitation by anti-AIDA antibody, followed by immunoblotting with anti-EphA8 antibody (Fig. 3A, top panel). The results demonstrated that a significant level of EphA8 (approximately 5% of the total lysate) was coimmunoprecipitated with AIDA-1b from the cell lysate (Fig. 3A, lane 4), although some nonspecific immunoprecipitation was observed (0.5% of the cell lysate) (lane 3). Conversely, AIDA-1b (0.5% of the cell lysate) was coimmunoprecipitated with EphA8 from cell lysates by using anti-EphA8 antibody (Fig. 3B, top panel, lane 4), although the anti-EphA8 antibody also pulled down AIDA-1b nonspecifically (<0.1%) (lane 2). Interestingly, coimmunoprecipitation experiments using anti-EphA8 antibody revealed that AIDA-1b associates with a KD mutant of EphA8 (kdEphA8) to a similar extent to that with WT EphA8 (Fig. 3C). It was further determined that two additional AIDA-1 isoforms containing an intact PTB domain, namely, AIDA-1a and AIDA-DAnk, efficiently associated with EphA8 at similar levels (data not shown).
FIG. 3.
Anks family proteins associate with EphA8 in HEK293 cells. (A) Coimmunoprecipitation of AIDA-1b and EphA8 in HEK293 cells. Cells were transfected with AIDA-1b and EphA8 as indicated in each lane, and then total cell lysates (100 μg) were immunoprecipitated (IP) with anti-AIDA antibody, followed by immunoblotting (WB) with anti-EphA8 antibody (top panel). The same blot was stripped and reprobed with anti-AIDA antibody (middle panel). A sample (1%) of each total cell lysate (WCL) was analyzed directly by Western blotting using anti-EphA8 antibody (bottom panel). (B) Reciprocal coimmunoprecipitation of AIDA-1b and EphA8 in HEK293 cells. Experiments were performed essentially as described for panel A, except that reciprocal antibodies were used, as indicated in each panel. (C) Coimmunoprecipitation of AIDA-1b and KD EphA8 in HEK293 cells. Experiments were performed essentially as described for panel B. (D) Coimmunoprecipitation of Odin and EphA8 in HEK293 cells. Experiments were performed essentially as described for panel A, except that AIDA-1b was replaced by Odin. (E) Reciprocal coimmunoprecipitation of Odin and EphA8 in HEK293 cells. Experiments were performed as described for panel D, except that reciprocal antibodies were used, as indicated in each panel. (F) Coimmunoprecipitation of Odin and KD EphA8 in HEK293 cells as described for panel E. (G) Binding of Odin-PTB to EphA8-JM is inhibited by a specific peptide (KKRHCGY), which contains the docking site in EphA8-JM for the PTB domain. Purified proteins (GST or GST-JM) were mixed with whole-cell lysates from HEK293 cells transfected with control vector (lanes 1 and 3) or an Odin-PTB expression construct (lanes 2, 4, and 5). In the peptide competition experiment, 50 μM of specific peptides was preincubated with cell lysates for 30 min on ice (lane 5). GST pull-down experiments using glutathione beads were performed essentially as described in the legend to Fig. 2B, and bound proteins were detected by Western blotting using anti-Odin antibodies (top panel). The same blot was stripped and reprobed with anti-GST antibodies (middle panel). A sample (2%) of each total cell lysate was analyzed directly by Western blotting using anti-Odin antibodies (bottom panel). (H and I) Evidence that both Odin-PTB and Odin-ΔPTB interact with EphA8. Experiments were performed essentially as described for panel D, except that the indicated constructs and antibodies were used for transfection and immunoprecipitation, respectively.
Since Odin is most closely related to AIDA-1b and its PTB domain directly binds to the EphA8 JM domain (Fig. 2C), we next sought to demonstrate a specific interaction between Odin and EphA8 in transiently transfected HEK293 cells. As shown in Fig. 3D, immunoprecipitation of Odin from HEK293 cells expressing both Odin and EphA8 resulted in the specific recovery of EphA8 (lane 4). Conversely, Odin was also coimmunoprecipitated with EphA8 from the same cell lysates by using anti-EphA8 antibody (Fig. 3E, top panel, lane 4). Like the AIDA-1b protein shown in Fig. 3C, Odin was readily detected in anti-EphA8 immunoprecipitates from cells transfected with both Odin and a KD mutant of EphA8 (kdEphA8) (Fig. 3F). We further investigated whether the Odin PTB domain overexpressed in HEK293 cells interacts with the bacterially expressed EphA8 JM domain and whether this interaction is specifically inhibited by a specific peptide (KKRHCGY) which acts as a docking site in EphA8-JM for the PTB domain. As shown in Fig. 3G, GST-JM, but not the control GST protein, could bind to the Odin-PTB protein expressed in HEK293 cells (lane 4), and this binding was significantly reduced by the specific peptide (lane 5). Taken together, these results demonstrate that EphA8 forms a protein complex with AIDA-1b or Odin through a specific interaction between the JM domain and the PTB domain in mammalian cells and that EphA8 tyrosine kinase activity does not play a role in this association.
We next sought to determine whether or not the PTB domain-deleted Odin protein would fail to bind to the full-length EphA8 receptor. Interestingly, in HEK293 cells cotransfected with EphA8 and Odin lacking the PTB domain (Odin-ΔPTB), it appeared that the EphA8 receptor was coprecipitated with Odin-ΔPTB (Fig. 3H, lane 3). EphA8 was also readily detected in anti-Odin immunoprecipitates from cells transfected with Odin-PTB as a positive control (Fig. 3H, lane 4). However, this finding could result from the presence of endogenous Odin in HEK293 cells. To further confirm the possibility that EphA8 can associate with Odin-ΔPTB, we generated an EGFP-Odin fusion protein lacking the PTB domain (EGFP-ΔPTB) and performed coimmunoprecipitation experiments using anti-EGFP antibody. As shown in Fig. 3I, EphA8 was able to bind to the EGFP-ΔPTB fusion protein as well as to the EGFP-PTB fusion protein in HEK293 cells, suggesting the potential importance of other motifs in Odin for interactions with EphA8.
Treatment with ephrin ligand enhances the specific association between EphA8 and Anks family proteins.
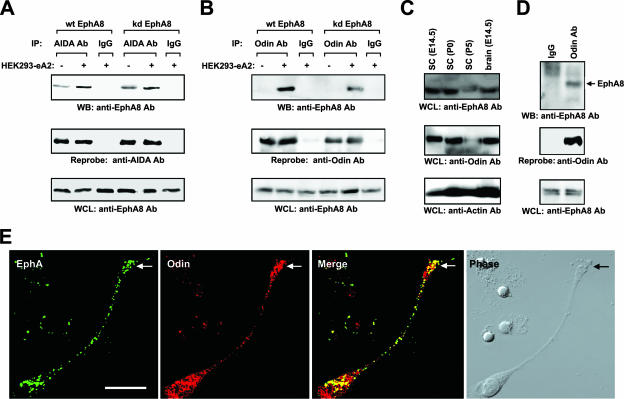
To further investigate whether ephrin ligand stimulation results in an increased association between EphA8 and AIDA-1b, HEK293 cells stably transfected with ephrinA2 were added directly to cells transiently transfected with both AIDA1-b and EphA8 constructs. After 15 min of stimulation, the cells were washed once and then subjected to an immunoprecipitation assay using anti-AIDA1 antibody. As shown in Fig. 4A, upon stimulation using ephrinA2-expressing cells, the level of the WT EphA8 protein coimmunoprecipitated with AIDA-1b was at least threefold higher than the basal level in cells treated with control HEK293 cells (top panel, lanes 1 to 3). In addition, the level of the kinase-inactive EphA8 receptor coimmunoprecipitated with AIDA-1b was also threefold higher following treatment of ephrinA2-expressing cells (Fig. 4A, top panel, lanes 4 to 6). We also assessed whether ephrinA2 stimulation results in increased association between EphA8 and Odin in HEK293 cells. As shown in Fig. 4B, it was evident that the level of EphA8 protein coimmunoprecipitated with Odin was strongly elevated in response to ephrinA2 stimulation and that this occurred irrespective of EphA8 autokinase activity. Taken together, these results indicate that the recruitment of Anks family proteins to the EphA8 receptor is potentiated by ephrin ligand stimulation and that EphA8 tyrosine kinase activity does not play any significant role in this association. In contrast to a previous report that Odin is tyrosine phosphorylated in epidermal growth factor (EGF)-stimulated cells (28), we observed that neither AIDA-1b nor Odin was tyrosine phosphorylated in cotransfected HEK293 cells, regardless of ephrin ligand stimulation (data not shown).
FIG. 4.
EphrinA5 stimulation enhances specific complex formation of EphA8 with AIDA-1 family proteins. (A) HEK293 cells were transiently transfected with AIDA-1b and WT or KD EphA8. Forty-eight hours after transfection, cells were stimulated with ephrinA2-expressing HEK293 cells for 15 min. Total cell lysates (100 μg) were immunoprecipitated with anti-AIDA antibody or control IgG and then immunoblotted with anti-EphA8 antibody (top panel). The same blot was stripped and reprobed with anti-AIDA antibody (middle panel). A sample (1%) of each total cell lysate was analyzed directly by Western blotting using anti-EphA8 antibody (bottom panel). (B) Experiments were performed essentially as described for panel A, except that AIDA-1b was replaced by Odin. Note that the coprecipitated EphA8 protein was barely detectable in the absence of ephrinA2 stimulation because of the very short exposure time (top panel, lanes 1 and 4). (C) Mouse brains at the indicated developmental stages were homogenized in lysis buffer, and the lysates were analyzed directly by Western blotting with the indicated antibodies. SC, superior colliculus; E, embryonic day; P, postnatal day. (D) Association of endogenous Odin and EphA8 in the mouse superior colliculus. Lysates from E14.5 mouse superior colliculi were immunoprecipitated with anti-Odin antibodies or with control IgG and then immunoblotted with anti-EphA8 antibodies (top panel). The same blot was stripped and reprobed with anti-Odin antibodies (middle panel). A sample (10%) of each lysate was analyzed directly by Western blotting using anti-EphA8 antibodies (bottom panel). (E) Odin colocalizes with EphA in superior collicular neurons. E14.5 mouse superior collicular neurons were treated with aggregated ephrinA5-Fc for 10 min, fixed, and then stained for Odin (red) and EphA (green). Bar = 20 μm. WB, Western blot; WCL, whole-cell lysate.
Consistent with previous results, our expression pattern analyses revealed that AIDA1b and its isoforms are specifically expressed in many regions of the mouse adult brain but not in mouse embryonic brain tissues where the EphA8 receptor is expressed (data not shown). Additionally, it was found that AIDA-1b proteins are not expressed in any of the mammalian cells we have tested so far (data not shown). In contrast, Odin, the only AIDA-1b-related protein, is abundantly expressed in mouse embryonic brain tissues (Fig. 4C) as well as in most mammalian cells (see Fig. 5C and 7A). Because Odin and EphA8 appear to be coexpressed in the superior colliculus during the development of mesencephalon (Fig. 4C), we next asked whether endogenous Odin and EphA8 associate with one another in the brain. Odin was immunoprecipitated from cell extracts derived from E14.5 superior colliculus, and the immunoprecipitates were probed with anti-EphA8 antibodies. We found that EphA8 coprecipitated with Odin but not with the control IgG, indicating that Odin is a physiologically relevant partner of the EphA8 receptor (Fig. 4D). We further examined whether Odin and EphA receptors colocalize in E14.5 mouse superior collicular neurons by using preclustered ephrinA5-Fc and anti-Odin antibodies. As shown in Fig. 4E, EphA receptors form clusters along neurites in response to ephrinA5 stimulation and these EphA clusters colocalize with Odin clusters. We were unable to directly demonstrate the colocalization of EphA8 and Odin due to the lack of reliable mouse antibodies for immunostaining of EphA8 or Odin. Nevertheless, our results strongly suggest that Odin is a physiologically relevant partner of the EphA8 receptor.
FIG. 5.
(A) EphrinA5 inhibits cell migration in an EphA8 kinase activity-dependent manner. The lower chambers of Transwell inserts were coated with fibronectin, and then 1 × 105 cells were added to the top chamber and allowed to migrate toward the lower chamber, filled with medium containing 5 μg/ml ephrinA5-Fc or Fc, for 2 h. Cells were fixed and stained with eosin prior to being counted under an inverted microscope. WT-6, -7, and -9 and KD-3, -7, and -8 represent different clones of stably transfected cells. Values represent means plus standard deviations. Asterisks mark cell migration whose inhibition was significantly different (P < 0.01) from that of the vector-transfected cells. (B and C) A sample (10%) of each total cell lysate was analyzed directly by Western blotting using anti-EphA8 antibody (B) or anti-Odin antibody (C). (D) Overexpression of Odin-PTB reverses the inhibitory effect of cell migration by the ephrinA5-stimulated EphA8 receptor. A Boyden chamber migration assay was performed as described for panel A. The EphA8-expressing cells used for this experiment correspond to the WT-7 clone shown in panel A. Values represent means plus standard deviations. Asterisks mark cell migration whose inhibition was significantly different (P < 0.01) from that of the vector-transfected cells. (E to G) A sample (10%) of each total cell lysate was analyzed directly by Western blotting using anti-Odin antibody (E and F) or anti-EphA8 antibody (G). (H) Evidence that Odin-PTB competes with endogenous Odin for binding to EphA8. Cells were stimulated with preclustered ephrinA5-Fc (+) or Fc (−) for 15 min, and equal amounts of protein (500 μg) from each cell lysate were immunoprecipitated with anti-EphA8 antibodies and analyzed by immunoblotting with anti-Odin antibodies. The WT-7, EphA8/PTB-2, and EphA8/ΔPTB-2 clones shown in panels A and D were used for this experiment. (I) The same blot shown in panel H was stripped and reprobed with anti-EphA8 antibodies. (J to L) A sample (5%) of each total cell lysate was analyzed directly by Western blotting using anti-EphA8 (J) or anti-Odin (K and L) antibodies. WB, Western blot.
FIG. 7.
(A) Coexpression of EphA8 and Odin in Neuro2a cells. One hundred micrograms of whole-cell extract (WCL) from PC12 or Neuro2a cells was resolved by SDS-PAGE and then subjected to Western blot analysis using the indicated antibodies. Note that Odin was detected as a smaller protein in PC12 cells than in Neuro2a cells. This may be because PC12 cells predominantly express a 1,125-amino-acid rat Odin protein, which is 25 amino acids shorter than mouse Odin in Neuro2a cells. (B) Coimmunoprecipitation of Odin and EphA8 in Neuro2a cells. Neuro2a cells stably expressing the WT EphA8 receptor were generated as described previously. Total cell lysates (500 μg) were immunoprecipitated with anti-Odin antibody and then immunoblotted with the anti-EphA8 antibody (top panel). The same blot was stripped and reprobed with anti-Odin antibody (middle panel). A sample (0.5%) of each total cell lysate was analyzed directly by Western blotting using anti-EphA8 antibody (bottom panel). (C) Odin colocalizes with EphA8 in the neurite tips of Neuro2a cells. Neuro2a cells stably expressing the EphA8 receptor were stained with either anti-EphA8 or anti-Odin antibody followed by Alex Fluor 594-anti-rabbit secondary antibody (top panels). Neuro2a cells transiently transfected with EphA8-EGFP were stained with anti-Odin antibody followed by Alex Fluor 594-anti-rabbit secondary antibody (bottom panels). WB, Western blot.
The EphA8-ephrinA5 interaction mediates inhibition of cell migration, and this signaling function requires both EphA8 tyrosine kinase activity and Odin.
Our previous studies showed that HEK293 cells expressing EphA8 could be induced to migrate by adding fibronectin to the lower chamber of a modified Boyden chamber cell migration system (9). We also found that the addition of preclustered ephrinA5-Fc to the lower chamber potently suppressed cell migration in this system. As shown in Fig. 5A, HEK293 cells stably expressing WT EphA8 and the KD EphA8 receptor were used to investigate the role of both EphA8 tyrosine kinase activity and its interaction with Odin in the regulation of cell migration. HEK293 cells provide an ideal model system because they express undetectable or very low levels of Eph-related kinases. The migration of WT EphA8-expressing cells was significantly inhibited by preclustered ephrinA5-Fc compared with Fc (Fig. 5A, bars 3 to 8), whereas that of KD EphA8-expressing cells was not significantly changed, just like vector-transfected cells (bars 9 to 14). Taken together, these results show that the EphA8-mediated inhibition of cell migration requires both ephrinA5 stimulation and EphA8 tyrosine kinase activity.
We next investigated whether overexpression of the Odin PTB domain alone influences cell migration by inhibiting the specific interaction between Odin and EphA8 in HEK293 cells. Western blot analysis of HEK293 cell lysates revealed a significant level of endogenous Odin protein expression (Fig. 5C and E). As shown in Fig. 5F and G, both EphA8 and the Odin PTB domain were stably expressed in HEK293 cells, and two different stable cell lines were chosen for the cell migration experiments. Consistent with the results presented in Fig. 5A, the migration of WT EphA8-expressing cells was significantly inhibited by ephrinA5-Fc compared with Fc (Fig. 5D, bars 7 and 8). No significant change in migration was observed in vector- or PTB domain-transfected cells (Fig. 5D, bars 1 to 6). Surprisingly, the migration of cells expressing both EphA8 and Odin-PTB was not significantly altered by ephrinA5-Fc compared with Fc (Fig. 5D, bars 9 to 12), strongly suggesting that Odin-PTB acts as a dominant-negative mutant of the endogenous Odin protein in HEK293 cells. In contrast, the migration of cells expressing both EphA8 and Odin lacking the PTB domain (Odin-ΔPTB) was inhibited as much as that in WT EphA8-expressing cells by ephrinA5-Fc (Fig. 5D, bars 13 to 16). We next sought to determine if Odin-PTB but not Odin-ΔPTB could compete for endogenous Odin binding to EphA8 (Fig. 5H). As expected, in the cells expressing both EphA8 and Odin-PTB, the level of the endogenous Odin protein that coimmunoprecipitated with EphA8 was not significantly altered by ephrinA5 stimulation (Fig. 5H, lanes 3 and 4). In contrast, in cells expressing EphA8 alone (Fig. 5H, lanes 1 and 2) or both EphA8 and Odin-ΔPTB (lanes 5 and 6), the level of the endogenous Odin protein that coimmunoprecipitated with EphA8 was significantly elevated in response to ephrinA5 stimulation. Given that Odin-PTB competes for endogenous Odin binding to EphA8 and that this competition inhibits the EphA8 signaling function, this result suggests that the binding between the Odin PTB domain and the JM domain of EphA8 is essential for the signaling function, although these two proteins may have other binding sites between them.
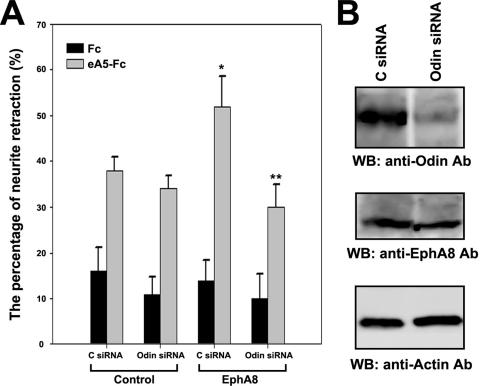
To further test the hypothesis that the Odin protein in HEK293 cells is critically involved in EphA8-mediated inhibition of cell migration, Odin was knocked down by transfecting siRNA into cells stably expressing EphA8. Either a SMARTpool or a specific siRNA was very effective in downregulating endogenous Odin expression, whereas control siRNA was not (Fig. 6B, top panel). The level of the EphA8 receptor or the β-actin protein was not significantly altered by siRNA transfection (Fig. 6B, middle and bottom panels). Consistent with siRNA-mediated downregulation of Odin expression, the EphA8-mediated inhibition of cell migration in response to ephrinA5 treatment was significantly attenuated in siRNA-transfected cells (Fig. 6A, bars 4 and 6). To exclude the possibility that the effects seen with Odin siRNA were nonspecific, we reintroduced an Odin rescue plasmid into siRNA-transfected cells to reverse the effect of EphA8 after Odin knockdown. Using this analysis, we found that the level of Odin was increased by the Odin rescue plasmid (Fig. 6D, lane 3), whereas the decrease in Odin expression was still detected in cells transfected with the control plasmid (lane 2). Accordingly, increasing the Odin level in HEK293 cells resulted in the inhibition of cell migration in response to ephrinA5 stimulation (Fig. 6C, bars 5 and 6), demonstrating the specificity of the Odin siRNA used in this study. Taken together, these data strongly support the observation that a specific interaction between Odin and EphA8 is necessary for mediating inhibition of cell migration in response to ephrinA5 stimulation.
FIG. 6.
Inhibition of cell migration by ephrinA5-stimulated EphA8 requires Odin function in HEK293 cells. The EphA8-expressing cells used for this experiment correspond to the WT-7 clone shown in Fig. 5A. (A) HEK293 cells were transfected with control siRNA or human Odin siRNA, and 72 h later, cells were subjected to cell migration assay in the presence of Fc or ephrinA5-Fc as described in the legend to Fig. 5A. An asterisk marks cell migration whose inhibition was significantly different (P < 0.01) from that of Odin siRNA-transfected cells. (B) Seventy-two hours after siRNA transfection, cell lysates (10%) were probed by immunoblotting with anti-Odin antibodies (top panel), anti-EphA8 antibodies (middle panel), or anti-actin antibodies (bottom panel). (C) Experiments were performed essentially as described for panel A, except that the control vector (bars 1 to 4) or Odin expression construct was retransfected after 72 h of transfection with siRNA. An asterisk marks cell migration whose inhibition was significantly different (P < 0.01) from that of cells transfected with Odin siRNA and the control vector (bar 4). (D) Experiments were performed essentially as described for panel B. WB, Western blot.
EphA8 and Odin form complexes in Neuro2a cells and are colocalized to the neurite tips.
Western blot analysis of various cell lysates using anti-Odin antibody revealed that Odin was highly expressed in many different cell lines, including PC12 and Neuro2a (Fig. 7A, middle panel). The EphA8 receptor was also endogenously expressed in Neuro2a cells (Fig. 7A, top panel, lane 2), although it was barely coprecipitated with Odin in an immunoprecipitation assay due to its very low expression level (data not shown). To verify that the EphA8-Odin interaction also occurs in Neuro2a cells, we generated Neuro2a cells stably expressing the WT EphA8 receptor. As shown in Fig. 7B, immunoprecipitation of cell extracts from this cell line with an anti-Odin antibody, but not with a control antibody (lane 2), specifically recovered EphA8 (top panel, lane 1). To compare the localization of EphA8 and Odin in EphA8-expressing Neuro2a cells, dibutyryl cAMP was used to induce neurite outgrowth and then differentiated cells were labeled with EphA8 antibody or Odin antibody (Fig. 7C, top panels). The immunostaining data suggested that Odin might be colocalized with EphA8 at neurite tips in Neuro2a cells (Fig. 7C, compare panels 1 and 3), which is consistent with what we observed in superior collicular neurons (Fig. 4E). To further confirm the colocalization of these two proteins, we analyzed the localization of an EphA8-EGFP fusion protein in differentiated Neuro2a cells. The results demonstrated that Odin protein was very well colocalized with EphA8 at neurite tips. Taken together, these data support the potential importance of the Odin protein in modulating Eph-dependent neurite tip extension or retraction associated with axon guidance.
Odin is required for ephrinA5-induced neurite retraction in EphA8-expressing Neuro2a cells.
Next, we investigated whether the neurite tips of EphA8-expressing Neuro2a cells are influenced by preclustered ephrinA5-Fc treatment. To monitor dynamic neurite changes, we used time-lapse microscopy of living Neuro2a cells that had been treated with dibutyryl cAMP to induce neurite outgrowth. The behavior of neurite tips was recorded every 1 min, starting 1 min before and lasting 60 min after the addition of ephrinA5 to the cultures, and neurite tips displaying significant length reductions (as much as or more than two cell body lengths) and a collapsed growth cone morphology were counted as retracted neurites. As shown in Fig. 8A and B, the vector-transfected or KD EphA8-expressing cells showed approximately 35% ephrinA5-induced neurite retraction, which appeared to be the basal level resulting from ligand treatment under our experimental conditions. In contrast, the WT EphA8-expressing cells showed at least 50% ephrinA5-induced neurite retraction, consistent with the role of the Eph receptor in repulsive axon guidance and collapse (see Fig. S1 to S3 in the supplemental material).
FIG. 8.
Neurite retraction is induced by ephrinA5-stimulated EphA8 in Neuro2a cells. Neuro2a cells stably transfected with the indicated constructs were cultured on a poly-l-lysine-coated cover glass for 2 to 3 days in the presence of dibutyryl cAMP to induce neurite outgrowth. (A) Neurites were imaged by time-lapse microscopy at 1 frame per min. The 0-min images show neuronal tips just prior to treatment with preclustered ephrinA5-Fc. (B) Neurite retraction was scored for Fc-treated and ephrinA5-Fc-treated cells by using time-lapse microscopy. The data represent three or more separate experiments in which at least 20 neurites were scored for each cell. Asterisks mark neurite retraction that was significantly different (*, P < 0.01; **, P < 0.02) from that of the vector transfectants. (C) A sample (10%) of each total cell lysate was analyzed directly by Western blotting (WB) using anti-EphA8 antibody.
To test whether Odin is essential for ephrinA5-induced neurite retraction in WT EphA8-expressing cells, Odin was knocked down by transfecting siRNA into Neuro2a cells stably expressing EphA8. Immunoblot analysis revealed that SMARTpool siRNA was effective in downregulating endogenous Odin expression, whereas control siRNA was not (Fig. 9B). For the neurite retraction assay, FITC-labeled control siRNA was included in each transfection, and the transfected cells with fluorescence were localized and monitored for neurite changes for 60 min by time-lapse microscopy (differential interference contrast imaging system). In the vector-transfected cells, SMARTpool Odin siRNA transfection did not result in a significant change in ephrinA5-induced neurite retraction compared with control siRNA transfection (Fig. 9A, bars 2 and 4). However, in EphA8-expressing cells, the ephrinA5-induced neurite retraction in SMARTpool siRNA-transfected cells was significantly decreased compared with that in control siRNA-transfected cells (Fig. 9A, bars 6 and 8). Taken together, these data strongly indicate that a specific interaction between Odin and EphA8 is necessary for mediating neurite retraction in response to ephrinA5 stimulation.
FIG. 9.
Odin is required for neurite retraction induced by ephrinA5-stimulated EphA8 in Neuro2a cells. Experiments were performed essentially as described in the legend to Fig. 8A and B, except that FITC-labeled control siRNA was included in each transfection to visualize the siRNA-transfected cells. Once the transfectants were localized by fluorescence, neurite retraction was observed by time-lapse microscopy, using a differential interference contrast imaging system. The data represent three or more separate experiments in which at least 20 neurites were scored for each cell. *, P < 0.05 compared with cells transfected with the control vector; **, P < 0.02 compared with cells transfected with control siRNA. (B) Neuro2a cells were transfected with control siRNA or Odin siRNA, and 72 h later, cell lysates (10%) were probed by immunoblotting with anti-Odin antibodies (top panel), anti-EphA8 antibodies (middle panel), or antiactin antibodies (bottom panel). WB, Western blot.
DISCUSSION
In this study, we show that when ephrinA5 binds to EphA8, the PTB domain-containing Anks family proteins AIDA-1b and Odin are recruited to the JM region of EphA8. These Anks family proteins are not phosphorylated on tyrosine residues, despite the finding that the tyrosine kinase activity of EphA8 is crucial for its inhibitory effect on cell migration. We observed that Odin is the physiologically relevant partner of EphA8 in mammalian cells and is critically involved in EphA8-mediated inhibition of cell migration. In addition, Odin is enriched and colocalized with the EphA8 receptor to the neurite tips of differentiated Neuro2a cells. Accordingly, Neuro2a cells defective in the Odin-dependent EphA8 signaling pathway fail to collapse and retract their neurite tips in response to ephrinA5 stimulation. Taken together, these results suggest that Odin is a scaffolding protein that regulates the EphA8 signaling pathway leading to cell migration and axonal targeting. These findings raise several important questions, including the following. (i) What are the specific motifs in EphA8 that are critical for its interaction with the PTB domain, and is the PTB domain important for other Eph receptors? (ii) What is the molecular mechanism underlying the Odin-dependent EphA8 signaling pathway? (iii) What is the physiological role of the Odin-dependent EphA8 signaling function in the mouse embryonic brain?
The first issue addresses the peptide motifs that are critical for EphA8 interaction with the PTB domains of Anks family proteins. The PTB domains of Anks family proteins belong to the Dab-like PTB domain subgroup, which is not dependent upon tyrosine phosphorylation of the peptide ligand (41). Consistent with this, the association of EphA8 with Anks family proteins does not require its tyrosine kinase activity. Using various JM deletion mutants, it was determined that the first seven amino acid residues (KKRHCGY) of the EphA8 JM segment act as a docking site for the AIDA-1b PTB domain (Fig. 1). This peptide motif is not present in other Eph receptors, including EphA2, EphA4, and EphB2. This is very consistent with our result that the AIDA-1b PTB domain does not bind to the JM domains of these Eph receptors in a yeast two-hybrid assay. In contrast to these data, we found that the AIDA-1b PTB domain is sufficient for association with full-length EphA4 expressed in HEK293 cells (unpublished result). This finding suggests that a certain peptide motif in EphA4, but not in its JM region, is involved in EphA4 association with the AIDA-1b PTB domain. Interestingly, we observed that the EphA8 receptor lacking the PTB binding motif in its JM region is also able to form a complex with Odin, suggesting the potential presence of additional motifs in EphA8 for interacting with Odin (unpublished result). These as yet unidentified motifs in EphA8 might be highly conserved throughout all Eph receptors. In addition, we found that an Odin protein lacking the PTB domain was able to associate with EphA8 in intact cells, suggesting the potential importance of other motifs in Odin for interacting with EphA8 (Fig. 3H and I). Taken together, the results show that as yet unidentified motifs are likely to be required for stable complex formation between these two proteins. The characterization of these interacting modules is currently under investigation in our laboratory.
The next question concerns the mechanism by which the activated EphA8 receptor induces inhibition of cell migration through the Odin-dependent signaling function. Odin was originally identified as a novel signaling molecule in the EGF receptor signaling pathway (28), whereas AIDA-1b was identified as a protein that interacts with the intracellular domain of AβPP (7). It was previously shown that Odin undergoes tyrosine phosphorylation upon addition of EGF or PDGF and that it plays a negative role in growth factor receptor signaling pathways (28). AIDA-1b was shown to be important for modulating AβPP processing by inhibiting γ-secretase (7). However, the signaling mechanisms of these proteins have not yet been elucidated. In our study, it was shown that the EphA8 receptor does not induce tyrosine phosphorylation of Odin but requires its autokinase activity for modulating cell migration. An important question is how Odin mediates the EphA8 signaling function in an EphA8 tyrosine kinase activity-dependent manner. Odin does not have any catalytic domains but contains several motifs involved in protein-protein interactions, including six ankyrin repeats, two SAM domains, and a PTB domain. Although we did not determine the function of the ankyrin repeats in this study, they could possibly couple EphA8-Odin signaling complexes with cytoskeletal components. Other motifs, including the SAM domains in Odin, also recruit various signaling proteins to the EphA8 receptor at the plasma membrane. Whereas the PTB domain alone effectively blocked the Odin-dependent EphA8 signaling function, AIDA-1 isoforms, such as AIDA-1a and AIDA-DAnk proteins, did not work as dominant-negative mutants (unpublished result). In fact, the PTB domain and the two SAM domains are very well conserved among all AIDA-1 isoforms (7), suggesting that the SAM domain might also be critically involved in the Odin-dependent EphA8 signaling pathway. In this respect, we would like to propose that Odin is the physiologically relevant scaffolding protein for the regulation of the EphA8 signaling pathway. Although the recruitment of Odin to the EphA8 receptor is not dependent on EphA8 autokinase activity, unidentified signaling proteins associated with Odin are likely to be tyrosine phosphorylated by the EphA8 receptor for the inhibition of cell migration. Which molecules are likely to be involved in these phenotypic changes downstream of the EphA8-Odin signaling complexes? Recent studies have begun to expand our understanding of the Eph receptor signaling pathway implicated in cell adhesion and migration. Phosphorylation of R-Ras, suppression of MAPKs, and activation of small GTP binding proteins and their exchange factors are all potential targets of EphA8-Odin signaling complexes.
The final issue concerns the biological and physiological relevance of the Odin-dependent EphA8 signaling function in the embryonic brain. AIDA-1b exists in many different alternative spliced forms, and its shorter isoforms appear to be expressed in the adult brain but not in the embryonic brain (7). In contrast to AIDA-1b, Odin is more abundant in the embryonic brain and ubiquitously expressed in many different cell lines. Unlike AIDA-1b, Odin does not show alternative splice forms of different molecular weights in brain tissues and tissue culture lines. These results suggest that AIDA-1b and Odin may exert different functions during animal development. Although AIDA-1b and Odin are localized to the cytoplasm, they could be recruited to the plasma membrane via interaction with the EphA8 receptor. The selective recruitment of AIDA-1b or Odin to the plasma membrane may induce a signaling complex critical for the regulation of cell migration or axonal targeting. As shown in Fig. 4D, we found that Odin is bound to the EphA8 receptor in embryonic tissues at E14.5. Although the data are not shown in this report, we also observed that Odin, but not AIDA-1b, is bound to the EphA8 receptor in neonatal tissues at P0, which corresponds to the time when neuronal wiring is actively being established. Using ephA8 knockout mice, it was previously shown that the EphA8 receptor plays an important role in the axonal pathfinding of a subset of superior collicular commissural axons (29). In this study, Odin was shown to be critical for EphA8-mediated axonal retraction in response to ephrinA5. In this respect, Odin may serve as a scaffold for the assembly of an EphA8 signaling complex at the plasma membrane, thereby triggering a signal for proper axon guidance. In adult brain tissues, AIDA-1b may associate with other members of the Eph subfamily of receptor tyrosine kinases. We also found that other Eph receptors are expressed in adult brain tissues, such as the cerebral cortex and hippocampus (unpublished result). Although it remains to be determined whether other Eph receptors interact with AIDA-1b in adult brain tissues, AIDA-1b may regulate the Eph-mediated signaling pathway in a similar way to AβPP processing. The findings of this study open an exciting opportunity to elucidate the molecular mechanism underlying Eph-mediated axonal targeting during the development of the mammalian nervous system.
Supplementary Material
Acknowledgments
We are indebted to Eunjoon Kim for yeast expression constructs and to Luciano D'Adamio for various AIDA-1 constructs. We also thank Sungbo Shim and Jieun Kim for excellent technical assistance.
This research was supported by a grant (M103KV010007 06K220100710) from the Brain Research Center of the 21st Century Frontier Research Program, funded by the Ministry of Science and Technology (MOST); a grant (R11-2005-017-01002-0) from the SRC program, funded by MOST/KOSEF (Research Center for Women's Diseases); a Korea Research Foundation grant (KRF-2006-005-J02201); and a KRF MOEHRD Basic Research Promotion Fund grant (KRF-2006-312-C00338).
Footnotes
Published ahead of print on 17 September 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Carter, N., T. Nakamoto, H. Hirai, and T. Hunter. 2002. EphrinA1-induced cytoskeletal re-organization requires FAK and p130(cas). Nat. Cell Biol. 4:565-573. [DOI] [PubMed] [Google Scholar]
- 2.Chen, Z. Y., C. Sun, K. Reuhl, A. Bergemann, M. Henkemeyer, and R. Zhou. 2004. Abnormal hippocampal axon bundling in EphB receptor mutant mice. J. Neurosci. 24:2366-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi, S., and S. Park. 1999. Phosphorylation at Tyr-838 in the kinase domain of EphA8 modulates Fyn binding to the Tyr-615 site by enhancing tyrosine kinase activity. Oncogene 18:5413-5422. [DOI] [PubMed] [Google Scholar]
- 4.Dail, M., M. Richter, P. Godement, and E. B. Pasquale. 2006. Eph receptors inactivate R-Ras through different mechanisms to achieve cell repulsion. J. Cell Sci. 119:1244-1254. [DOI] [PubMed] [Google Scholar]
- 5.Elowe, S., S. J. Holland, S. Kulkarni, and T. Pawson. 2001. Downregulation of the Ras-mitogen-activated protein kinase pathway by the EphB2 receptor tyrosine kinase is required for ephrin-induced neurite retraction. Mol. Cell. Biol. 21:7429-7441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foo, S. S., C. J. Turner, S. Adams, A. Compagni, D. Aubyn, N. Kogata, P. Lindblom, M. Shani, D. Zicha, and R. H. Adams. 2006. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell 124:161-173. [DOI] [PubMed] [Google Scholar]
- 7.Ghersi, E., C. Noviello, and L. D'Adamio. 2004. Amyloid-beta protein precursor (AbetaPP) intracellular domain-associated protein-1 proteins bind to AbetaPP and modulate its processing in an isoform-specific manner. J. Biol. Chem. 279:49105-49112. [DOI] [PubMed] [Google Scholar]
- 8.Gu, C., and S. Park. 2001. The EphA8 receptor regulates integrin activity through p110gamma phosphatidylinositol-3 kinase in a tyrosine kinase activity-independent manner. Mol. Cell. Biol. 21:4579-4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu, C., and S. Park. 2003. The p110 gamma PI-3 kinase is required for EphA8-stimulated cell migration. FEBS Lett. 540:65-70. [DOI] [PubMed] [Google Scholar]
- 10.Gu, C., S. Shim, J. Shin, J. Kim, J. Park, K. Han, and S. Park. 2005. The EphA8 receptor induces sustained MAP kinase activation to promote neurite outgrowth in neuronal cells. Oncogene 24:4243-4256. [DOI] [PubMed] [Google Scholar]
- 11.Hattori, M., M. Osterfield, and J. G. Flanagan. 2000. Regulated cleavage of a contact-mediated axon repellent. Science 289:1360-1365. [DOI] [PubMed] [Google Scholar]
- 12.Holmberg, J., D. L. Clarke, and J. Frisen. 2000. Regulation of repulsion versus adhesion by different splice forms of an Eph receptor. Nature 408:203-206. [DOI] [PubMed] [Google Scholar]
- 13.Hunter, S. G., G. Zhuang, D. Brantley-Sieders, W. Swat, C. W. Cowan, and J. Chen. 2006. Essential role of Vav family guanine nucleotide exchange factors in EphA receptor-mediated angiogenesis. Mol. Cell. Biol. 26:4830-4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huynh-Do, U., E. Stein, A. A. Lane, H. Liu, D. P. Cerretti, and T. O. Daniel. 1999. Surface densities of ephrin-B1 determine EphB1-coupled activation of cell attachment through alphavbeta3 and alpha5beta1 integrins. EMBO J. 18:2165-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irie, F., and Y. Yamaguchi. 2002. EphB receptors regulate dendritic spine development via intersectin, Cdc42 and N-WASP. Nat. Neurosci. 5:1117-1128. [DOI] [PubMed] [Google Scholar]
- 16.Janes, P. W., N. Saha, W. A. Barton, M. W. Kolev, S. H. Wimmer-Kleikamp, E. Nievergall, C. P. Blobel, J. P. Himanen, M. Lackmann, and D. B. Nikolov. 2005. Adam meets Eph: an ADAM substrate recognition module acts as a molecular switch for ephrin cleavage in trans. Cell 123:291-304. [DOI] [PubMed] [Google Scholar]
- 17.Klein, R. 2004. Eph/ephrin signaling in morphogenesis, neural development and plasticity. Curr. Opin. Cell Biol. 16:580-589. [DOI] [PubMed] [Google Scholar]
- 18.Knoll, B., K. Zarbalis, W. Wurst, and U. Drescher. 2001. A role for the EphA family in the topographic targeting of vomeronasal axons. Development 128:895-906. [DOI] [PubMed] [Google Scholar]
- 19.Knoll, B., and U. Drescher. 2004. Src family kinases are involved in EphA receptor-mediated retinal axon guidance. J. Neurosci. 24:6248-6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marston, D. J., S. Dickinson, and C. D. Nobes. 2003. Rac-dependent trans-endocytosis of ephrinBs regulates Eph-ephrin contact repulsion. Nat. Cell Biol. 5:879-888. [DOI] [PubMed] [Google Scholar]
- 21.Matsuoka, H., H. Obama, M. L. Kelly, T. Matsui, and M. Nakamoto. 2005. Biphasic functions of the kinase-defective Ephb6 receptor in cell adhesion and migration. J. Biol. Chem. 280:29355-29363. [DOI] [PubMed] [Google Scholar]
- 22.McLaughlin, T., and D. O'Leary. 2005. Molecular gradients and development of retinotopic maps. Annu. Rev. Neurosci. 28:327-355. [DOI] [PubMed] [Google Scholar]
- 23.Miao, H., E. Burnett, M. Kinch, E. Simon, and B. Wang. 2000. Activation of EphA2 kinase suppresses integrin function and causes focal-adhesion-kinase dephosphorylation. Nat. Cell Biol. 2:62-69. [DOI] [PubMed] [Google Scholar]
- 24.Miao, H., B. Wei, D. M. Peehl, Q. Li, T. Alexandrou, J. Schelling, J. Rhim, J. Sedor, E. Burnett, and B. Wang. 2001. Activation of EphA receptor tyrosine kinase inhibits the Ras/MAPK pathway. Nat. Cell Biol. 3:527-530. [DOI] [PubMed] [Google Scholar]
- 25.Miao, H., K. Strebhardt, E. B. Pasquale, T. Shen, J. Guan, and B. Wang. 2005. Inhibition of integrin-mediated cell adhesion but not directional cell migration requires catalytic activity of EphB3 receptor tyrosine kinase: role of Rho family small GTPases. J. Biol. Chem. 280:923-932. [DOI] [PubMed] [Google Scholar]
- 26.Murai, K., and E. B. Pasquale. 2005. New exchanges in Eph-dependent growth cone dynamics. Neuron 46:161-163. [DOI] [PubMed] [Google Scholar]
- 27.Niethammer, M., E. Kim, and M. Sheng. 1996. Interaction between the C terminus of NMDA receptor subunits and multiple members of the PSD-95 family of membrane-associated guanylate kinases. J. Neurosci. 16:2157-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pandey, A., B. Blagoev, I. Kratchmarova, M. Fernandez, M. Nielsen, T. Z. Kristiansen, O. Ohara, A. V. Podtelejnikov, S. Roche, H. F. Lodish, and M. Mann. 2002. Cloning of a novel phosphotyrosine binding domain containing molecule, Odin, involved in signaling by receptor tyrosine kinases. Oncogene 21:8029-8036. [DOI] [PubMed] [Google Scholar]
- 29.Park, S., J. Frisen, and M. Barbacid. 1997. Aberrant axonal projections in mice lacking EphA8 (Eek) tyrosine protein kinase receptors. EMBO J. 16:3106-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parri, M., F. Buricchi, M. L. Taddei, E. Giannoni, G. Raugei, G. Ramponi, and P. Chiarugi. 2005. EphrinA1 repulsive response is regulated by an EphA2 tyrosine phosphatase. J. Biol. Chem. 280:34008-34018. [DOI] [PubMed] [Google Scholar]
- 31.Pasquale, E. B. 2005. Eph receptor signalling casts a wide net on cell behaviour. Nat. Rev. Mol. Cell. Biol. 6:462-475. [DOI] [PubMed] [Google Scholar]
- 32.Penzes, P., A. Beeser, J. Chernoff, M. R. Schiller, B. A. Eipper, R. E. Mains, and R. L. Huganir. 2003. Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB-EphB receptor activation of the Rho-GEF kalirin. Neuron 37:263-274. [DOI] [PubMed] [Google Scholar]
- 33.Poliakov, A., M. Cotrina, and D. G. Wilkinson. 2004. Diverse roles of Eph receptors and ephrins in the regulation of cell migration and tissue assembly. Dev. Cell 7:465-480. [DOI] [PubMed] [Google Scholar]
- 34.Pratt, R. L., and M. S. Kinch. 2002. Activation of the EphA2 tyrosine kinase stimulates the MAP/ERK kinase signaling cascade. Oncogene 21:7690-7699. [DOI] [PubMed] [Google Scholar]
- 35.Sahin, M., P. L. Greer, M. Z. Lin, H. Poucher, J. Eberhart, S. Schmidt, T. M. Wright, S. M. Shamah, S. O'Connell, C. W. Cowan, L. Hu, J. L. Goldberg, A. Debant, G. Corfas, C. E. Krull, and M. E. Greenberg. 2005. Eph-dependent tyrosine phosphorylation of ephexin1 modulates growth cone collapse. Neuron 46:191-204. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt, A., and A. Hall. 2007. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 16:1587-1609. [DOI] [PubMed] [Google Scholar]
- 37.Shamah, S. M., M. Z. Lin, J. L. Goldberg, S. Estrach, M. Sahin, L. Hu, M. Bazalakova, R. L. Neve, G. Corfas, A. Debant, and M. E. Greenberg. 2001. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell 105:233-244. [DOI] [PubMed] [Google Scholar]
- 38.Shintani, T., M. Ihara, H. Sakuta, H. Takahashi, I. Watakabe, and M. Noda. 2006. Eph receptors are negatively controlled by protein tyrosine phosphatase receptor type O. Nat. Neurosci. 9:761-769. [DOI] [PubMed] [Google Scholar]
- 39.Stein, E., A. A. Lane, D. P. Cerretti, H. O. Schoecklmann, A. D. Schroff, R. L. Van Etten, and T. O. Daniel. 1998. Eph receptors discriminate specific ligand oligomers to determine alternative signaling complexes, attachment, and assembly responses. Genes Dev. 12:667-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanaka, M., R. Ohashi, R. Nakamura, K. Shinmura, T. Kamo, R. Sakai, and H. Sugimura. 2004. Tiam1 mediates neurite outgrowth induced by ephrin-B1 and EphA2. EMBO J. 23:1075-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uhlik, M. T., B. Temple, S. Bencharit, A. J. Kimple, D. P. Siderovski, and G. L. Johnson. 2005. Structural and evolutionary division of phosphotyrosine binding (PTB) domains. J. Mol. Biol. 345:1-20. [DOI] [PubMed] [Google Scholar]
- 42.Vindis, C., D. P. Cerretti, T. O. Daniel, and U. Huynh-Do. 2003. EphB1 recruits c-Src and p52Shc to activate MAPK/ERK and promote chemotaxis. J. Cell Biol. 162:661-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang, H. U., and D. J. Anderson. 1997. Eph family transmembrane ligands can mediate repulsive guidance of trunk neural crest migration and motor axon outgrowth. Neuron 18:383-396. [DOI] [PubMed] [Google Scholar]
- 44.Zimmer, M., A. Palmer, J. Kohler, and R. Klein. 2003. EphB-ephrinB bi-directional endocytosis terminates adhesion allowing contact mediated repulsion. Nat. Cell Biol. 5:869-878. [DOI] [PubMed] [Google Scholar]
- 45.Zou, J. X., B. Wang, M. S. Kalo, A. H. Zisch, E. Pasquale, and E. Ruoslahti. 1999. An Eph receptor regulates integrin activity through R-Ras. Proc. Natl. Acad. Sci. USA 96:13813-13818. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.