Abstract
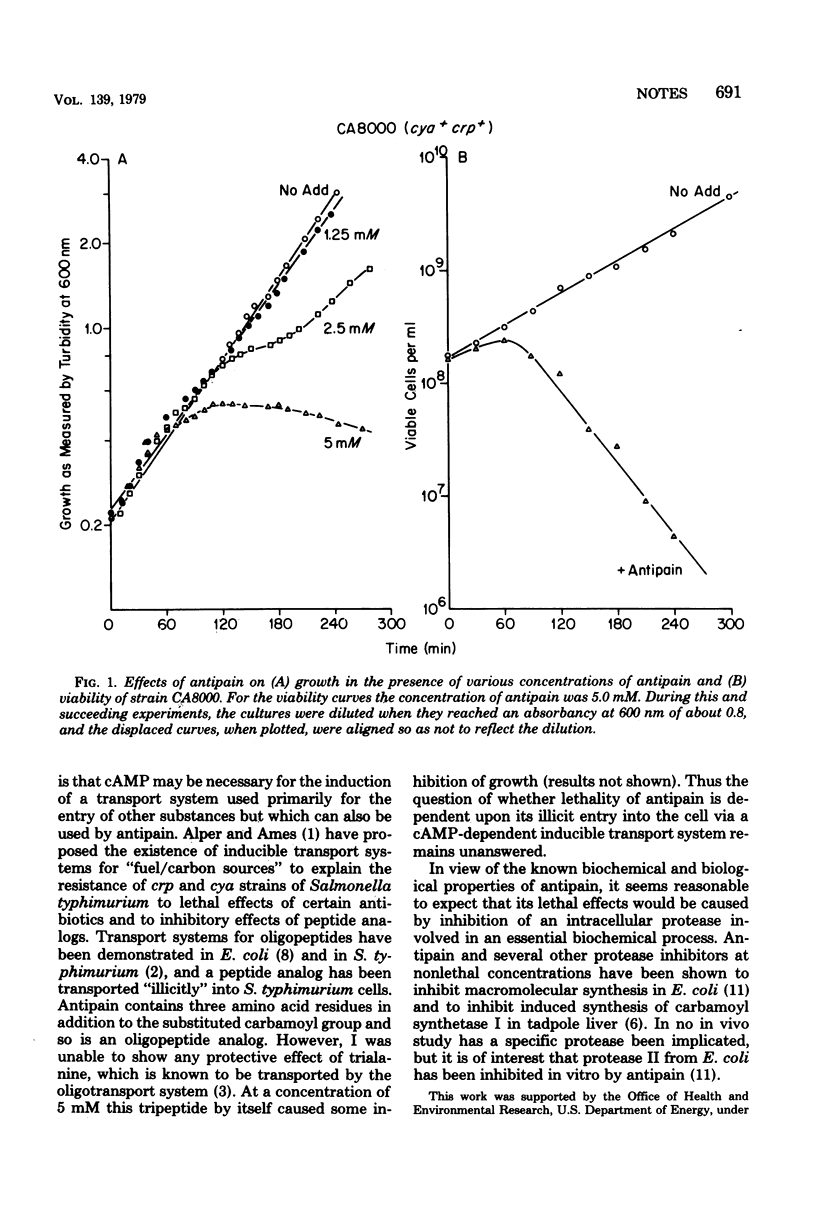
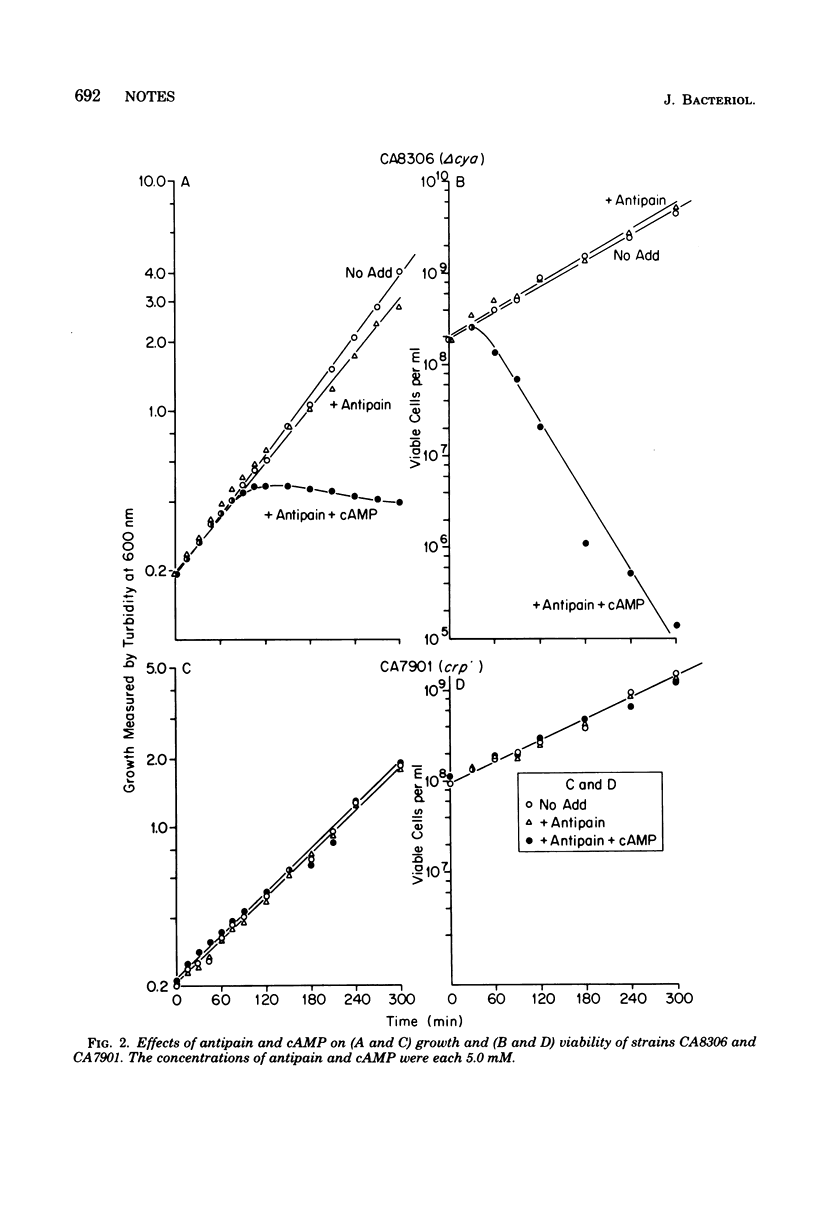
Antipain kills Escherichia coli K-12 cells in an exponential manner beginning 1 h after its addition. Mutant strains, delta cya and crp, which are unable to synthesize cyclic adenosine 3',5'-monophosphate (cAMP) and the cAMP receptor protein, respectively, are not affected. Addition of cAMP (5 mM) to antipain-treated mutant strains causes killing of delta cya cells, but not crp cells. Thus the lethal effect of antipain is dependent upon cAMP and its receptor protein.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper M. D., Ames B. N. Transport of antibiotics and metabolite analogs by systems under cyclic AMP control: positive selection of Salmonella typhimurium cya and crp mutants. J Bacteriol. 1978 Jan;133(1):149–157. doi: 10.1128/jb.133.1.149-157.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames B. N., Ames G. F., Young J. D., Tsuchiya D., Lecocq J. Illicit transport: the oligopeptide permease. Proc Natl Acad Sci U S A. 1973 Feb;70(2):456–458. doi: 10.1073/pnas.70.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diddens H., Zähner H., Kraas E., Göhring W., Jung G. On the transport of tripeptide antibiotics in bacteria. Eur J Biochem. 1976 Jun 15;66(1):11–23. doi: 10.1111/j.1432-1033.1976.tb10420.x. [DOI] [PubMed] [Google Scholar]
- Hammer-Jespersen K., Nygaard P. Multiple regulation of nucleoside catabolizing enzymes in Escherichia coli: effects of 3:5' cyclic AMP and CRP protein. Mol Gen Genet. 1976 Oct 18;148(1):49–55. doi: 10.1007/BF00268545. [DOI] [PubMed] [Google Scholar]
- Meyn M. S., Rossman T., Troll W. A protease inhibitor blocks SOS functions in Escherichia coli: antipain prevents lambda repressor inactivation, ultraviolet mutagenesis, and filamentous growth. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1152–1156. doi: 10.1073/pnas.74.3.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M., Cohen P. P. Antipain inhibits thyroxine-induced synthesis of carbamyl phosphate synthetase I in tadpole liver. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5339–5343. doi: 10.1073/pnas.75.11.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Adhya S. Cyclic adenosine 5'-monophosphate in Escherichia coli. Bacteriol Rev. 1976 Sep;40(3):527–551. doi: 10.1128/br.40.3.527-551.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W. Proteolytic cleavage of bacteriophage lambda repressor in induction. Proc Natl Acad Sci U S A. 1975 Jan;72(1):147–151. doi: 10.1073/pnas.72.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman T., Norris C., Troll W. Inhibition of macromolecular synthesis in Escherichia coli by protease inhibitors. Specific reversal by glutathione of the effects of chloromethyl ketones. J Biol Chem. 1974 Jun 10;249(11):3412–3417. [PubMed] [Google Scholar]
- Swenson P. A., Joshi J. G., Schenley R. L. Regulation of cessation of respiration and killing by cyclic 3',5'-adenosine monophosphate and its receptor protein after far-ultraviolet irradiation of Escherichia coli. Mol Gen Genet. 1978 Feb 16;159(2):125–130. doi: 10.1007/BF00270885. [DOI] [PubMed] [Google Scholar]
- Swenson P. A., Schenley R. L. Effects of antipain (a protease inhibitor) on respiration, viability, and excision of pyrimidine dimers in UV-irradiated Escherichia coli cells. J Bacteriol. 1978 Sep;135(3):1167–1170. doi: 10.1128/jb.135.3.1167-1170.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson P. A., Schenley R. L., Joshi J. G. Role of cyclic adenosine 3',5'-monophosphate on cessation of respiration in ultraviolet-irradiated Escherichia coli. J Bacteriol. 1977 Aug;131(2):707–709. doi: 10.1128/jb.131.2.707-709.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa S., Tatsuta K., Fujimoto K., Tsuchiya T., Umezawa H. Structure of antipain, a new Sakaguchi-positive product of streptomyces. J Antibiot (Tokyo) 1972 Apr;25(4):267–270. doi: 10.7164/antibiotics.25.267. [DOI] [PubMed] [Google Scholar]
- Witkin E. M., George D. L. Ultraviolet mutagenesis in polA and UvrA polA derivatives of Escherichia coli B-R: evidence for an inducible error-prone repair system. Genetics. 1973 Apr;73(Suppl):91–10. [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



