Abstract
Humans are quite unusual compared to other great apes in that reproduction typically takes place within long-term, iteroparous pairings—social arrangements that have been culturally reified as the institution of marriage. With respect to male behaviour, explanations of marriage fall into two major schools of thought. One holds that marriage facilitates a sexual division of labour and paternal investment, both important to the rearing of offspring that are born helpless and remain dependent for remarkably long periods (provisioning model). And the other suggests that the main benefits which men receive from entering into marriage derive from monopolizing access to women's fertility (mating effort model). In this paper, we explore extramarital sexual relationships and the conditions under which they occur as a means of testing predictions derived from these two models. Using data on men's extramarital sexual relationships among Tsimane forager–horticulturists in lowland Bolivia, we tested whether infidelity was more common when men had less of an opportunity to invest in their children or when they risked losing less fertility. We found that Tsimane men appear to be biasing the timing of their affairs to when they are younger and have fewer children, supporting the provisioning model.
Keywords: extramarital, affairs, Tsimane, paternal investment, mating effort
1. Introduction
Marriage is common to all cultures (Murdock 1949), suggesting that it plays a central role in our species' reproductive strategy. Explanations for why men enter into marriages fall into two major schools of thought. One argument, referred to as the provisioning model here, emphasizes the fact that marriage facilitates a sexual division of labour and the providing of biparental care, both important to the rearing of offspring that are born helpless and remain dependent for remarkably long periods (Lancaster & Lancaster 1980, 1983; Lovejoy 1981; Fisher 1989). Another view, stemming largely from the contention that men in foraging populations do not evidence true paternal concern (Hawkes 1991, 1993; Bleige Bird et al. 2001), holds that men form long-term relationships with women in order to obtain fertility benefits and monopolize women's reproductive careers (Hawkes et al. 1995; Blurton Jones et al. 2000). This is referred to here as the mating effort model.
Although marriage is common throughout the world, so too is infidelity in the form of extramarital affairs. In this paper, we argue that extramarital sexual relationships and the conditions under which they occur can be used to test predictions derived from the two models of marriage described above. Extramarital affairs may provide increased fertility for men, but such infidelity also represents a disinvestment in their families and can result in divorce or mate desertion (and hence a loss of access to his wife's fertility)—essentially the opposites of the two benefits proposed by the competing models. Here, we test predictions derived from the two models of marriage with data on men's extramarital sexual relationships among Tsimane forager–horticulturists in lowland Bolivia.
(a) Previous studies on infidelity
Previous studies have revealed unsurprisingly that extramarital relationships are viewed negatively by victimized partners the world over (Jankowiak et al. 2002). Among the Tsimane, the study population of the research presented in this paper, women believe that a husband's philandering can directly lead to his children's sickness and death. Suspected infidelity is a common subject of dispute between spouses and even among the competing women (Rucas et al. 2006). Betzig (1989) reported that infidelity was the most commonly cited factor as a potential cause of divorce for both men and women in the standard cross-cultural sample.
Despite the numerous consequences, many studies have shown that sexual infidelity is quite common (Collumbien et al. 2001; Lawoyin & Larsen 2002; Allen et al. 2005) and have revealed a number of factors that are associated with an increased risk of engaging in such behaviour. Perhaps the most frequently cited is gender—men are typically found to be more likely to commit adultery than women (e.g. Wiederman 1997; Atkins et al. 2001). Other factors found to be associated with extramarital activity include young age (Edwards & Booth 1976; Wiederman 1997; Kimuna & Kjamba 2005), personality traits like narcissism, psychoticism and low conscientiousness (Buss & Shackelford 1997; Atkins et al. 2001), and contextual factors such as marital satisfaction (Thompson 1983), length of marriage (Fair 1978; Liu 2000) and opportunity (Greeley 1994; Atkins et al. 2001).
Unfortunately, most studies simply report correlations and lack a cohesive, explanatory theoretical framework (although see Walster et al. 1978; Drigotas et al. 1999; Liu 2000). A few evolutionary studies on infidelity have focused on factors expected to alter the benefits of pursuing extramarital relationships such as attractiveness (Gangestad & Thornhill 1997) and ovulatory stage (Bellis & Baker 1990; Gangestad et al. 2002). These studies, however, do not consider familial characteristics that mediate the costs of investing in extramarital affairs, such as the dependency load of the family and the amount of future fertility the man risks losing through divorce and desertion by his marital partner. Those costs are considered in §1b to differentiate predictions derived from the two theories of marriage.
(b) Predictions derived from the two models of marriage and infidelity
The successful rearing of human offspring entails substantial costs for a number of reasons. First, human infants are born relatively helpless, and their cognitive development depends on energetically expensive post-natal brain growth (Martin 1983; Flinn et al. 2005). Additionally, the period of dependence for human offspring is remarkably extended, usually until the late teens, and multiple children are reared simultaneously (Kaplan 1994; Kaplan & Lancaster 2003). Thus, in pre-demographic transition populations, human families are typically characterized by multiple offspring of varying levels of dependence, usually including helpless infants, providing a great challenge to parents who must provide care, protection and food. Proponents of the provisioning model suggest that marriage provides a solution to this problem (Lovejoy 1981; Lancaster & Lancaster 1983). The augmented paternity confidence that the sexual exclusivity of marriage confers allows men to effectively invest in their known children. Furthermore, by linking their reproductive interests, marriage allows men and women to cooperate much more effectively. Labour is typically divided, with women focusing on the direct care of children and the economic tasks that are compatible with such care, and men focusing on provisioning and other riskier, more labour-intensive tasks (Murdock 1949; Brown 1970; Lancaster & Lancaster 1980; Kaplan & Lancaster 2003).
This model implies that the ultimate goal of male familial involvement is the enhancement of offspring quality. Because time and resources invested in the pursuit of extramarital affairs are unavailable for familial investment, men face a trade-off between investments in children within marriage and the fertility benefits of seeking additional mates outside of marriage. The returns to familial investment are largely dependent on the level of need of a man's progeny, which can be approximated by the number of dependents co-residing with the man (Hames 1992). Therefore, this model predicts that men's pursuit of extramarital relationships will be negatively correlated with their number of dependent children in their marital family. Two previous studies have explored such an effect in western populations and failed to find a significant relationship (Edwards & Booth 1976; Liu 2000). These studies, however, failed to capture the time-sensitive nature of the proposed effect of dependency load by using an ever-had-affair variable (Edwards & Booth 1976) or including adult children (Liu 2000).
In a series of papers, Hawkes and colleagues (Hawkes 1991, 1993; Hawkes & Bleige Bird 2002) have criticized the provisioning model, arguing that the widespread sharing of meat that is typical in most hunting and gathering populations precludes men from efficiently provisioning their families through hunting, especially that of large game. According to this view, men could more efficiently provision their families by gathering or pursuing smaller game species that are less shared. The fact that men continue to focus on large game indicates that the goal of men's work is not to provide food for their wives and children, but rather to gain social and mating benefits through displays of their hunting prowess and the distribution of their kill (Hawkes 1993; Hawkes & Bleige Bird 2002). It follows that men must enter pair bonds in pursuit of goals other than parental investment. There are several variants of this view. One holds that pair bonds provided a solution to costly male–male contests (Hawkes et al. 1995, 2001), while others contend that women offer men reproductive exclusivity in exchange for their protective services (Mesnick 1997; Wrangham et al. 1999; Blurton Jones et al. 2000). The commonality among all of these is that the primary benefits that men receive from entering into long-term reproductive relationships derive from access to women's fertility (the mating effort model).
Men's philandering not only results in opportunity costs associated with an inability to invest in one's family, but also the very real risk of spousal abandonment and the loss of the wife's fertility. The fitness costs imposed by such a loss of fertility are directly related to the wife's reproductive value, which varies inversely with her age. A man who is deserted by a post-menopausal wife suffers no loss of fertility. Additionally, a woman with many dependents and low reproductive value may be more reluctant to desert a philandering husband owing to lower prospects of remarriage. Husbands of older wives may therefore not only experience reduced costs of divorce but also experience reduced risk. Although this logic follows that of the mating effort model, Marlowe (2003) actually argued that the very fact that women are angered by their husbands' pursuits of other women (combined with the typical female preference for good providers) indicates that women are most probably benefiting from men's provisioning.
The two models of marriage generate opposing predictions about the conditions under which men will engage in extramarital relationships. The provisioning model predicts that men's pursuit of extramarital affairs will vary inversely with the number of dependent children in their marital family, which will increase with marital duration, at least during the first decade or two of marriage. In stark contrast, the logic of the mating effort model predicts that wife's age, holding all else constant, will be positively associated with a man's investment in extramarital relationships, which will increase with marital duration. This paper presents the results of testing those predictions among Tsimane men.
2. Material and methods
(a) The Tsimane population
The Tsimane are forager–horticulturists living in the lowland rainforests and savannas just east of the Andes in the Beni region of Bolivia. Approximately, 8000 Tsimane reside in around 80 villages along the banks of the Maniqui River and its many tributaries (VAIPO 1998). The majority of their food comes from swidden agriculture, hunting and fishing, with varying amounts coming from market goods. The two villages in which the data were collected are located along the Maniqui River with a total population of around 280 individuals. The Tsimane do not commemorate weddings with formal ceremonies, but consider a pair to be married when they sleep together in the same house. Polygyny is widely accepted, but relatively uncommon (around 5–10% of men). Marriages among the Tsimane are very stable with roughly 20% of marriages ending in divorce (15 of 76 marriages begun over 20 years ago), which is most common in the first year.
(b) The interview
Retrospective longitudinal interviews were conducted by Winking to assess the frequency of extramarital encounters throughout the course of individuals' marriages. Men who had been divorced or widowed were asked questions concerning their current or latest marriage, and men who were married polygynously were excluded to avoid ambiguity concerning the pursuit of additional wives versus extramarital affairs. It is important to note that the Tsimane do not have strong taboos concerning conversations about sexual behaviour, and freely make humorous remarks concerning sexual matters in large groups and even around children. Winking informed all men within the two communities, on an individual-by-individual basis, that he would be conducting such interviews and that their participation should be completely voluntary. No translators were used during the interviews to increase the participants' comfort levels and the likelihood that they would provide accurate responses. Each participant was assured that the details of his particular interview would not be discussed with others. Additionally, the names of women with whom the participants had had relationships were never discussed. The interview primarily aimed to determine the years of marriage in which the individual had an affair, the number of women with whom he had affairs and the length of each affair. This was done by first asking with how many women the individual had had affairs, and then elucidating the timing and duration of each relationship. Since the Tsimane are often unaware of their age or particular dates, this was determined by asking which of their children had been born when the affair occurred (for a description of demography methods, see Gurven et al. 2007). At the end of the interview, interviewees were given four fishhooks and 10 m of fishing line.
(c) Data analysis
From the interviews, we extrapolated a dataset consisting of data points for each year of each individual's marriage. The number of dependent children was calculated for each risk year, with dependent children being defined as biological children under the age of 10 years (following Hames 1992). We used the generalized estimating equations (GEE) method in the Genmod procedure of SAS to test for effects of the hypothesized variables (Liang & Zegler 1986). For all GEE analyses presented in this paper, the model assumes an autoregressive correlation structure since affairs can last longer than a year and may therefore be clumped in time. Personal identification numbers were used as repeated subjects, and all parameter estimates presented are logit estimates. To assess the goodness of fit of the different models, we used a statistic proposed by Horton et al. (1999), which actually tests for a lack of fit and has been found to be robust with continuous independent variables (Evans & Li 2005).
3. Results
(a) Descriptives
The sample consisted of 34 men who provided 500 risk years of marriage, with an average of 14.7 years. Age at first marriage ranged from 16 to 26 years with a mean age of 19.8 years. Five of the sampled men gave responses relating to second marriages, which occurred at ages ranging from 24 to 38 years and a mean age of 32.4 years. Three of the cases resulted from the first wife's death and the other two from the desertion of the first wife.
In order to test the reliability of informants’ responses, two logistic regressions were performed. There was no effect on the length of time since the first year of marriage and the probability of reporting an affair in the first year (Wald Χ2=0.677, n=34, p=0.411), nor was any such effect found when including all years of marriages (Wald Χ2=0.244, n=500, p=0.621).
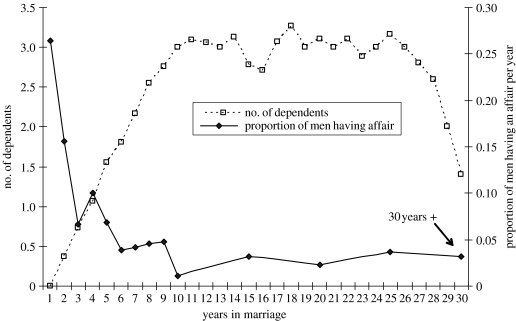
Figure 1 demonstrates how the number of dependents and frequency of affairs vary through the course of a marriage. As a marriage progresses, the number of dependents grows until there are as many children reaching 10 years of age as there are new children being born. This equilibrium is typically reached in the second decade of marriage at around three dependents, lasting until the wife ceases reproducing, usually around 25 years into the marriage. Of the 29 men who had been married for more than 5 years, 9 of them, or 31%, reported at least one affair in those first 5 years of marriage. Affairs are concentrated in the first 5 years of marriages, after which men maintain a rate of less than 0.05 affairs per person-year.
Figure 1.
Number of dependents and proportion of men having an affair per year versus years in marriage. For 10 years and beyond, five-year averages of the proportion of men having an affair per year were used due to declining sample sizes.
(b) Effects of predictor variables on extramarital behaviour
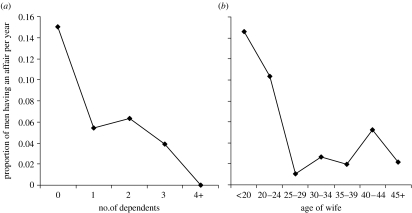
Table 1 presents the results of a GEE analysis including the two predictor variables (log of wife's age was used as it proved a better fit), and as demonstrated in figure 2a,b, both the number of dependents and log of wife's age are negatively associated with the frequency of extramarital affairs. With both variables in the model together, however, only the number of dependents reaches significance, supporting the provisioning model prediction. Even after controlling for the number of dependents, the effect of wife's age approaches significance in the opposite direction than that predicted by the mating effort model. Although there is some collinearity, the correlation is not great enough to impair interpretation (r=0.291, n=500, p<0.001).
Table 1.
Results of GEE analysis including two predictor variables. (Lack of fit Χ2=6.434, p=0.696.)
| variable | parameter estimate | s.e. | Z | p |
|---|---|---|---|---|
| no. of dependents | −0.394 | 0.191 | −2.06 | 0.039 |
| log of wife's age | −1.505 | 0.868 | −1.73 | 0.083 |
Figure 2.
Proportion of men having an affair per year versus (a) number of dependents (children under 10 years of age) and (b) age of wife.
Since previous research suggests that a man's age and perhaps even the number of years within a marriage may have an effect on the probability of having an affair, these variables were added as controls. Within each marriage, however, the husband's age, the wife's age and the number of years of marriage covary perfectly. Any true effect of any one of these variables will result in all of them being significant in separate univariate analyses; in multivariate analyses, such collinearity would lead to unclear and questionable results. Therefore, in the full model, we included the number of dependents, the log of wife's age, the difference in age between husband and wife (husband−wife) and the wife's age at marriage. In this manner, after controlling for the log of wife's age, the age difference serves as a measure of the man's age and the wife's age of marriage serves as a measure of the number of years within marriage. In this model, the number of dependents remains a marginally significant predictor, while the age difference is also significant and the log of wife's age approaches significance (table 2). The fact that the latter two at least approach significance and are in the same direction implies that these two variables are capturing the effect of man's age, with younger men having more affairs. If we include only log of man's age and the number of dependents in the model, we see that the log of man's age is much more significant than the log of wife's age as shown in table 1, and the number of dependents remains marginally significant (parameter estimate=−2.292, Z=−2.59, n=500, p=0.010; parameter estimate=−0.338, Z=−1.95, n=500, p=0.052, respectively).
Table 2.
Results of GEE analysis of full model. (Lack of fit Χ2=8.999, p=0.437.)
| variable | parameter estimate | s.e. | Z | p |
|---|---|---|---|---|
| no. of dependents | −0.385 | 0.198 | −1.95 | 0.051 |
| log of wife's age | −1.796 | 0.979 | −1.83 | 0.067 |
| age difference (husband−wife) | −0.152 | 0.070 | −2.17 | 0.030 |
| wife's age at marriage | −0.019 | 0.081 | −0.23 | 0.816 |
4. Discussion
The simplified model, including only the hypothesized predictors, shows that the frequency of affairs varies inversely with the number of dependents within the family, supporting the provisioning model. The parameter estimate of the log of wife's age is actually negative, in the opposite direction of that predicted by the mating effort model. After including variables to account for the man's age and number of years in marriage, it appears that the number of dependents and the man's age are the most predictive variables of men's extramarital behaviour, both in the negative direction. This points to an apparent life-history trend in which younger men invest more heavily in mating effort, apparently switching to a long-term parental strategy as they grow older and become fathers.
Three alternative explanations of the observed patterns are that (i) during the early years of marriage, men are less committed while they are still evaluating the potential of the relationship, (ii) during these years, men have more success in their pursuits of extramarital relationships, and (iii) the number of dependents, controlling for wife's age and/or time in marriage, may reflect a measure of fertility rate, and men with less fertile wives may be more apt to pursue extramarital relationships. The first few months or even years of a marriage may function as a period of evaluation for both partners, and thus represent the time of greatest risk of divorce (Blurton Jones et al. 2000). During these first few years, spouses judge not only the personality and behaviour of their partners but also their fecundity. Despite these assertions, these men are having elevated rates of affairs until the fifth year of marriage, when the average household has 1.5 dependent children. By this time, it is hard to imagine that these men are still unsure of their intentions with their wives.
Although it is impossible to rule out that younger men might enjoy higher returns to their extramarital pursuits, a number of factors imply that this may not be the case. Young men spend more time in waged labour, which typically takes them away from their communities, providing greater opportunity for extramarital relationships. In this sample, however, time spent in waged labour was not significantly associated with the probability of having an affair in the past year among individuals younger than 35 years (controlling for age, logistic, n=21 men, B=4.23, Wald Χ2=0.704, p=0.402). Although women place greater importance on attractiveness for short-term relationships (Buss & Schmitt 1993), there is little evidence that women find youth particularly attractive (Buss 1989; Jones 1995). It is possible, however, that women are less willing to enter into relationships with men who have many dependents. Younger men could also enjoy a competitive advantage in the intrasexual or male–male aspect of short-term mating competition (as opposed to female choice). These advantages, however, could not be conferred through greater physical ability, as Tsimane men's strength profiles do not peak until the late twenties (Gurven et al. 2006), during which the frequency of affairs is dramatically decreasing. Finally, it is difficult to believe that any of the effects described above could cause men's competitiveness to drop so precipitously in the first few years of marriage as implied by the declining frequency of affairs.
Finally, men may be responding to the ability of their wives to produce children, in which men who are married to less fertile women invest more in extramarital affairs either due to a lower concern for the dissolution of the marriage or because they are more likely to pursue alternative marital partners. This would lead to a negative effect of the number of dependents on the frequency of affairs after controlling for wife's age and/or time in marriage. This reasoning, however, cannot account for the sharp decline in the number of affairs throughout the first decade of marriage.
The unpredictability of early marriages, differing return rates to extramarital pursuits and men's responses to less fertile wives appear to be poor explanations for the observed pattern of affair behaviour. Contrary to the mating effort model, men's concern with the amount of fertility they risk losing also fails to explain the pattern. Among this Tsimane sample, the pattern seems to indicate that men are essentially oblivious to the potential fertility that they are risking by pursuing extramarital relations. Men are engaging in a behaviour that unambiguously angers their wives at a time when the wives have the most to offer the men and the least to lose from leaving them. Although divorce is relatively uncommon among the Tsimane, of those couples that do divorce, roughly two-thirds do so within the first 5 years (21 of 32 divorces recorded in demography of four villages). The brazenness of men's exploits during this period and the seeming carelessness with which they risk their marriage is underscored by the fact that most men during this time are living with their wives' families.
Supporting the provisioning model, the number of dependents was significantly negatively associated with frequency of affairs in the simplified model and marginally so in the full model. Additionally, younger men appeared to be having significantly more affairs than their older counterparts. This is perhaps indicative of a general life-history pattern characterized by a strategic shift from a mating effort-intensive strategy towards one of greater pair bond and parental involvement as the ability to win a wife and the opportunity to invest in children increase. We see the lowest frequency of extramarital affairs during the thirties, while the number of dependents under the age of 10 years is holding at a stable maximum between three and four in a typical marriage.
Throughout the world, men appear to go through this transition. Young and sexually loose Maasai warriors mature into elders and are then allowed to marry. Ache men become less likely to be named as a secondary father, a distinction of possible but not probable paternity, and more likely to be named as primary fathers of children as they age (Hill & Hurtado 1996). Sexually motivated college students grow into minivan-driving fathers. Similarly, Tsimane men appear to curtail their sexual exploits as their progeny and the ability to invest in their family grows. The scale of this transition may be attenuated in populations in which men are less involved in family provisioning, divorce rates are high, and/or a greater pool of extramarital partners and potential long-term alternatives is available. Among the Tsimane, fathers account for a large portion of a family's food and divorce rates are extremely low, resulting in relatively few unattached women. And while an aversion to losing one's wife must be curbing Tsimane men's extramarital behaviour to some degree, it appears that the desire to invest in one's family provides an even greater deterrent.
Acknowledgments
This research was supported by LAII Field Research grants, funded by the Tinker Foundation, and by NSF grant BCS-0136274. We would also like to thank the communities in which we worked for their wonderful hospitality and cooperative spirit.
References
- Allen E.S, Atkins D.C, Baucom D.H, Snyder D.K, Gordon K.C, Glass S.P. Intrapersonal, interpersonal, and contextual factors in engaging in and responding to extramarital involvement. Clin. Psychol. Sci. Pract. 2005;12:101–130. doi:10.1093/clipsy/bpi014 [Google Scholar]
- Atkins D.C, Jacobson N.S, Baucom D.H. Understanding infidelity: correlates in a national random sample. J. Fam. Psychol. 2001;15:735–749. doi: 10.1037//0893-3200.15.4.735. doi:10.1037/0893-3200.15.4.735 [DOI] [PubMed] [Google Scholar]
- Bellis M.A, Baker R.R. Do females promote sperm competition: data for humans. Anim. Behav. 1990;40:997–999. doi:10.1016/S0003-3472(05)81008-5 [Google Scholar]
- Betzig L. Causes of conjugal dissolution: a cross-cultural study. Curr. Anthropol. 1989;30:654–675. doi:10.1086/203798 [Google Scholar]
- Bleige Bird R, Smith E.A, Bird D.W. The hunting handicap: costly signaling in human foraging strategies. Behav. Ecol. Sociobiol. 2001;50:9–19. doi:10.1007/s002650100338 [Google Scholar]
- Blurton Jones N.G, Marlowe F, Hawkes K, O'connel J.F. Paternal investment and hunter–gatherer divorce rates. In: Cronk L, Chagnon N, Irons W, editors. Adaptation and human behavior: an anthropological perspective. Aldine de Gruyter; New York, NY: 2000. pp. 69–90. [Google Scholar]
- Brown J.K. A note on the division of labor by sex. Am. Anthropol. 1970;72:1073–1078. doi:10.1525/aa.1970.72.5.02a00070 [Google Scholar]
- Buss D.M. Sex differences in human mate preferences: evolutionary hypotheses tested in 37 cultures. Behav. Brain Sci. 1989;12:1–14. [Google Scholar]
- Buss D.M, Schmitt D.P. Sexual strategies theory: an evolutionary perspective on human mating. Psychol. Rev. 1993;100:204–232. doi: 10.1037/0033-295x.100.2.204. doi:10.1037/0033-295X.100.2.204 [DOI] [PubMed] [Google Scholar]
- Buss D.M, Shackelford T.K. Susceptibility to infidelity in the first year of marriage. J. Res. Pers. 1997;31:193–221. doi:10.1006/jrpe.1997.2175 [Google Scholar]
- Collumbien M, Das B, Campbell O.M.R. Why are condoms used, and how many are needed? Estimates from Orissa, India. Int. Fam. Plan. Perspect. 2001;27:171–178. doi:10.2307/2673852 [Google Scholar]
- Drigotas S.M, Safstrom C.A, Gentilia T. An investment model prediction of dating infidelity. J. Pers. Soc. Psychol. 1999;77:509–524. doi:10.1037/0022-3514.77.3.509 [Google Scholar]
- Edwards J.N, Booth A. Sexual behavior in and out of marriage: an assessment of correlates. J. Marriage Fam. 1976;38:73–81. doi:10.2307/350551 [Google Scholar]
- Evans S, Li L. A comparison of goodness of fit tests for the logistic GEE model. Stat. Med. 2005;24:1245–1261. doi: 10.1002/sim.2023. doi:10.1002/sim.2023 [DOI] [PubMed] [Google Scholar]
- Fair R.C. Theory of extramarital affairs. J. Polit. Econ. 1978;86:45–61. doi:10.1086/260646 [Google Scholar]
- Fisher H.E. Evolution of human serial pairbonding. Am. J. Phys. Anthropol. 1989;78:331–354. doi: 10.1002/ajpa.1330780303. doi:10.1002/ajpa.1330780303 [DOI] [PubMed] [Google Scholar]
- Flinn M.V, Geary D.C, Ward C.V. Ecological dominance, social competition, and coalitionary arms races: why humans evolved extraordinary intelligence. Evol. Hum. Behav. 2005;26:10–46. doi:10.1016/j.evolhumbehav.2004.08.005 [Google Scholar]
- Gangestad S.W, Thornhill R. The evolutionary psychology of extrapair sex: the role of fluctuating asymmetry. Evol. Hum. Behav. 1997;18:69–88. doi:10.1016/S1090-5138(97)00003-2 [Google Scholar]
- Gangestad S.W, Thornhill R, Garver C.E. Changes in women's sexual interests and their partners' mate-retention tactics across the menstrual cycle: evidence for shifting conflicts of interest. Proc. R. Soc. B. 2002;269:975–982. doi: 10.1098/rspb.2001.1952. doi:10.1098/rspb.2001.1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greeley A. Marital infidelity. Society. 1994;31:9–13. [Google Scholar]
- Gurven M, Kaplan H, Gutierrez M. How long does it take to become a proficient hunter? Implications on the evolution of delayed growth. J. Hum. Evol. 2006;51:454–470. doi: 10.1016/j.jhevol.2006.05.003. doi:10.1016/j.jhevol.2006.05.003 [DOI] [PubMed] [Google Scholar]
- Gurven M, Kaplan H, Zelada Supa A. Mortality experience of Tsimane Amerindians: regional variation and temporal trends. J. Hum. Biol. 2007;19:376–398. doi: 10.1002/ajhb.20600. doi:10.1002/ajhb.20600 [DOI] [PubMed] [Google Scholar]
- Hames R. Variation in paternal care among the Yanomamo. In: Hewlett B.S, editor. Father–child relations: cultural and biosocial contexts. Aldine de Gruyter; New York, NY: 1992. pp. 85–110. [Google Scholar]
- Hawkes K. Showing off: tests of an hypothesis about men's foraging goals. Ethol. Sociobiol. 1991;12:29–54. doi:10.1016/0162-3095(91)90011-E [Google Scholar]
- Hawkes K. Why hunter–gatherers work. Curr. Anthropol. 1993;34:341–361. doi:10.1086/204182 [Google Scholar]
- Hawkes K, Bleige Bird R. Showing off, handicap signaling, and the evolution of men's work. Evol. Anthropol. 2002;11:58–67. doi:10.1002/evan.20005 [Google Scholar]
- Hawkes K, Rogers A.R, Charnov E.L. The male's dilemma: increased offspring production is more paternity to steal. Evol. Ecol. 1995;9:662–677. doi:10.1007/BF01237661 [Google Scholar]
- Hawkes K, O'Connel J.F, Blurton Jones N.G. Hunting and nuclear families: some lessons from the Hadza about men's work. Curr. Anthropol. 2001;42:681–709. doi:10.1086/322559 [Google Scholar]
- Hill K, Hurtado A.M. Aldine de Gruyter; New York, NY: 1996. Ache life history: the ecology and demography of a foraging people. [Google Scholar]
- Horton N.J, Bebchuk J.D, Jones C.L, Lipsitz S.R, Catalano P.J, Zahner G.E.P, Fitzmaurice G.M. Goodness of fit for GEE: an example with mental health service utilization. Stat. Med. 1999;18:213–222. doi: 10.1002/(sici)1097-0258(19990130)18:2<213::aid-sim999>3.0.co;2-e. doi:10.1002/(SICI)1097-0258(19990130)18:2<213::AID-SIM999>3.0.CO;2-E [DOI] [PubMed] [Google Scholar]
- Jankowiak W, Nell M.D, Buckmaster A. Managing infidelity: a cross-cultural perspective. Ethnology. 2002;41:85–101. [Google Scholar]
- Jones D. Sexual selection, physical attractiveness, and facial neoteny: cross-cultural evidence and implications. Curr. Anthropol. 1995;36:723–748. doi:10.1086/204427 [Google Scholar]
- Kaplan H. Evolutionary and wealth flows theories of fertility: empirical tests and new models. Popul. Dev. Rev. 1994;20:753–791. doi:10.2307/2137661 [Google Scholar]
- Kaplan H.S, Lancaster J.B. An evolutionary and ecological analysis of human fertility, mating patterns, and parental investment. In: Wachter K.W, Bulatao R.A, editors. Offspring: human fertility behavior in biodemographic perspective. National Academies Press; Washington, DC: 2003. pp. 170–223. [PubMed] [Google Scholar]
- Kimuna S.R, Kjamba Y.K. Wealth and extramarital sex among men in Zambia. Int. Fam. Plan. Perspect. 2005;31:83–89. doi: 10.1363/3108305. doi:10.1363/3108305 [DOI] [PubMed] [Google Scholar]
- Lancaster J.B, Lancaster C.S. The division of labor and the evolution of human-sexuality. Behav. Brain Sci. 1980;3:193. [Google Scholar]
- Lancaster J.B, Lancaster C.S. Parental investment: the hominid adaptation. In: Ortner D.J, editor. How humans adapt: a biocultural odyssey. Smithsonian Institution Press; Washington, DC: 1983. pp. 33–69. [Google Scholar]
- Lawoyin T, Larsen U. Male sexual behaviour during wife's pregnancy and postpartum abstinence period in Oyo State, Nigeria. J. Biosoc. Sci. 2002;34:51–63. [PubMed] [Google Scholar]
- Liang K.Y, Zegler S.L. Longitudinal data analysis using general linear models. Biometrika. 1986;73:13–22. doi:10.1093/biomet/73.1.13 [Google Scholar]
- Liu C. A theory of marital sexual life. J. Marriage Fam. 2000;62:363–374. doi:10.1111/j.1741-3737.2000.00363.x [Google Scholar]
- Lovejoy O. The origin of man. Science. 1981;211:341–350. doi: 10.1126/science.211.4480.341. doi:10.1126/science.211.4480.341 [DOI] [PubMed] [Google Scholar]
- Marlowe F. A critical period for provisioning by Hadza men: implications for pair bonding. Evol. Hum. Behav. 2003;24:217–229. doi:10.1016/S1090-5138(03)00014-X [Google Scholar]
- Martin, R. D. 1983 Human brain evolution in an ecological context. In Fifty-second James Arthur Lecture New York, NY: American Museum of Natural History.
- Mesnick S.L. Sexual alliances: evidence and evolutionary implications. In: Gowaty P.A, editor. Feminism and evolutionary biology. Chapman and Hall; New York, NY: 1997. pp. 207–260. [Google Scholar]
- Murdock G.P. Macmillan; New York, NY: 1949. Social structure. [Google Scholar]
- Rucas S, Kaplan H, Winking J, Gurven M, Gangestad S, Crespo M. Female intrasexual competition and reputational effects on attractiveness among the Tsimane of Bolivia. Evol. Hum. Behav. 2006;21:40–52. doi:10.1016/j.evolhumbehav.2005.07.001 [Google Scholar]
- Thompson A.P. Extramarital sex: a review of the research literature. J. Sex Res. 1983;19:1–22. [Google Scholar]
- VAIPO. Vice-Ministerio de Asuntos Indigenas y Pueblos Originarios; Trinidad, Bolivia: 1998. Pueblos indigenas y originarios de Bolivia. [Google Scholar]
- Walster E, Traupmann J, Walster G.W. Equity and extramarital sexuality. Arch. Sex. Behav. 1978;7:127–142. doi: 10.1007/BF01542062. doi:10.1007/BF01542062 [DOI] [PubMed] [Google Scholar]
- Wiederman M.W. Extramarital sex: prevalence and correlates in a national survey. J. Sex Res. 1997;34:167–174. [Google Scholar]
- Wrangham R.W, Jones J.H, Laden G, Pilbeam D, Conklin-Brittain N. The raw and the stolen: cooking and the ecology of human origins. Curr. Anthropol. 1999;40:567–594. doi:10.1086/300083 [PubMed] [Google Scholar]