Abstract
Despite its wide implications for many ecological issues, the global pattern of spatial turnover in the occurrence of species has been little studied, unlike the global pattern of species richness. Here, using a database on the breeding distributions of birds, we present the first global maps of variation in spatial turnover for an entire taxonomic class, a pattern that has to date remained largely a matter of conjecture, based on theoretical expectations and extrapolation of inconsistent patterns from different biogeographic realms. We use these maps to test four predictions from niche theory as to the form that this variation should take, namely that turnover should increase with species richness, towards lower latitudes, and with the steepness of environmental gradients and that variation in turnover is determined principally by rare (restricted) species. Contrary to prediction, we show that turnover is high both in areas of extremely low and high species richness, does not increase strongly towards the tropics, and is related both to average environmental conditions and spatial variation in those conditions. These results are closely associated with a further important and novel finding, namely that global patterns of spatial turnover are driven principally by widespread species rather than the restricted ones. This complements recent demonstrations that spatial patterns of species richness are also driven principally by widespread species, and thus provides an important contribution towards a unified model of how terrestrial biodiversity varies both within and between the Earth's major land masses.
Keywords: beta diversity, environmental gradients, global avifauna, niche theory, spatial turnover
1. Introduction
Spatial turnover in the composition of species assemblages (the gains and losses of species from place to place) is central to many important ecological questions, including the magnitude of and relationship between regional and global species richness, likely biotic responses to climate change and the design of protected area networks for conservation (Harrison 1993; Gaston 2000; Condit et al. 2002; Groves 2003; Wiersma & Urban 2005). Yet, in contrast to patterns of species richness per se, the global geographical patterns of spatial turnover remain poorly documented and their determinants little explored (Lawton 2000). While some regional analyses have been conducted, it is unclear how widely the results generalize (e.g. Willig & Sandlin 1991; Blackburn & Gaston 1996; Gregory et al. 1998; Williams et al. 1999; Lennon et al. 2001; Koleff et al. 2003a), particularly given recent demonstrations of marked variation in macroecological patterns between biogeographic realms (e.g. Orme et al. 2006).
Two principal theoretical frameworks exist for patterns in spatial turnover, rooted in niche limitation and dispersal limitation, respectively (Gaston et al. 2007). In the former, the distributions of individual species are limited by traits that influence the range of biotic and abiotic conditions under which they can survive and successfully reproduce, while in the latter, these distributions are limited simply by dispersal abilities. Although both influences doubtless play some role in structuring most assemblages, four simple predictions arise from niche limitation, with which most discussions of turnover at geographical scales have been concerned (Gaston et al. 2007). First, given that different species occur in different environments, and that on average environmental conditions are increasingly different with the distance separating sites (Williamson 1987), spatial turnover in species composition will tend to be positively correlated with species richness, with greater turnover enabling more species to persist in an area (Stevens 1989; Gaston & Williams 1996; Willig et al. 2003; Lomolino et al. 2006). Second, given that environmental conditions show particularly strong latitudinal gradients, spatial turnover will show a similar trend (Stevens 1989; Gaston & Williams 1996; Koleff et al. 2003a; Willig et al. 2003). Such a pattern might also be predicted to follow from a latitudinal gradient in species richness, if this gradient is a consequence of turnover. Third, the steeper the spatial gradient in environmental conditions, the fewer species will be shared between areas, the greater the numbers that will not be shared, and the greater will be the spatial turnover (Whittaker 1960, 1972). And finally, if comparatively rare (restricted) species tend to have narrower niches and more fragmented distributions (Gaston 1994), then we expect rarer species to drive turnover patterns more than common (widespread) species.
Here, we present the first global analyses of spatial turnover for a major taxon, and test the above predictions, using a database on the geographical distribution of the breeding ranges of extant bird species on an equal-area grid at a resolution comparable to 1° latitude×longitude, the finest considered practicable given in available data (Orme et al. 2005).
2. Material and methods
(a) Data
Analyses were based on a previously reported database of vector distribution maps for 9626 extant, recognized bird species (see Orme et al. 2005, 2006 for details). Briefly, the polygon ranges were converted into an equal area Behrmann grid at a resolution of 96.5×96.5 km (giving 17 924 grid cells for analysis). Species were scored as present in a grid cell if any of the available sources indicated that the breeding range fell within the cell boundaries.
Geographical patterns in spatial turnover are traditionally interpreted as reflecting gradients in environmental conditions and variation in species environmental adaptations, with greater turnover where these gradients are steeper (Whittaker 1960, 1972). However, average environmental conditions may also shape patterns of turnover through their influence both on the numbers of species occupying an area (Gaston 2000) and on the potential for individual species to become locally more widespread. The geographical variation of spatial turnover was modelled at a global scale using environmental factors selected a priori, based on previous empirical demonstrations of their possible importance as determinants of richness or turnover (Gaston 2000; Lennon et al. 2001). We used the mean and ‘roughness’ (the local gradient) of the availability of ambient energy (temperature) and productive energy (normalized difference vegetation index, NDVI), habitat diversity (number of land-cover types) and elevation.
Sources and raw resolutions of the four selected environmental variables, which were then resampled to the 1° Behrmann grid, were as follows:
mean annual temperature data (°C) for the period 1961–1990 at 10 min resolution interpolated from station means (New et al. 2002);
mean annual remotely sensed NDVI for the period 1982–1996 at 0.25° resolution (Fourier-adjusted, sensor and solar zenith angle corrected, interpolated, reconstructed (FASIR) adjusted normalized difference vegetation index (NDVI): available at http://islscp2.sesda.com/ISLSCP2_1/html_pages/groups/veg/fasir_ndvi_monthly_xdeg.html);
number of land-cover types (habitat diversity) occurring in a grid cell, computed using remotely sensed data for the 12-month period between April 1992 and March 1993 at 30 arcsec resolution with types classified following the Global Ecosystems 100 category land-cover classification (Global Land Cover Characterization v. 2: available at http://edcdaac.usgs.gov/glcc/glcc.asp; Olson 1994a,b); and
elevation within each grid cell, from 30 arcsec resolution data (Global 30-arc-second Elevation Dataset (GTOPO30) developed by USGS EROS Data Centre: available at http://edcdaac.usgs.gov/gtopo30/gtopo30.asp).
Environmental analyses omitted grid cells falling within Oceania or Antarctica, since environmental data were not available for these realms. In order to standardize the definition of terrestrial land-area across raw environmental datasets, each was over-laid with a high-resolution terrestrial areas map (Digital Chart of the World, Environmental Systems Research Institute (ESRI), Inc., Redlands, CA, 1993) prior to re-sampling to the 1° Behrmann grid. Raw-data cells, or portions of cells, falling outside this definition of land-area were excluded from re-sampling calculations, and the latter were weighted by the land-area associated with each remaining raw-data cell.
NDVI and elevation were log-transformed to improve their fit to the assumptions of the analyses. Mean environmental conditions were those of the focal grid cell. With the exception of habitat diversity, roughness was computed as the mean of the absolute differences between the focal cell and each of its immediate neighbours for which environmental data were available. Roughness in habitat diversity was calculated by applying the equation for βsim (see below) to the habitats occurring in a given focal cell and its neighbours.
(b) Analyses
Measures of spatial turnover are typically derived from the matching/mismatching components a, b and c, where a (continuity) is the total number of species shared by two areas; b (gain) is the number of species present in the other area but not in the focal one; and c (loss) is the number of species present in the focal area but absent from the other one. We calculated the average values of each of these three components for each grid cell, from comparison with its n (maximum of eight) immediate neighbours (Lennon et al. 2001).
Numerous indices of spatial turnover in species composition have been derived from the matching components (Koleff et al. 2003b). We focus on three, which capture different facets of this phenomenon. The first, Whittaker's index (Whittaker 1960), is the most widely used and takes the form βw=(a+b+c)/(a+c), where the matching components for each grid cell are calculated relative to the species assemblage of the entire neighbourhood of n cells (Lennon et al. 2001). This is a ‘broad sense’ measure of turnover (Koleff et al. 2003b), in that it does not adjust for differences in composition attributable to local richness gradients. The second is a modified Simpson's index, which quantifies the relative magnitude of the species gains and losses and takes the form βsim=min(b, c)/(min(b, c)+a) (Lennon et al. 2001). This is a ‘narrow sense’ measure of turnover (Koleff et al. 2003b), that reflects the relative magnitude of species gains and losses rather than local richness gradients (Lennon et al. 2001). The third is the complement of Jaccard's index, again a ‘broad sense’ measure, which captures dissimilarity in composition in terms of the likelihood that any of the species occurring in two areas occurs in just one of them. It takes the form βj=(b+c)/(a+b+c) (Jaccard 1912). For the last two indices, mean values were calculated for each grid cell, from separate comparison with each of its n immediate neighbours.
These measures of turnover may be biased at high latitudes, because a geographical projection representing a sphere cannot faithfully preserve both the area of grid cells and the distances between them. We could have used a projection preserving distance, rather than area, but the problems posed by an unequal area grid dwarf those of an unequal distance grid. However, in using an equal area grid, both latitudinal and longitudinal distances between adjacent cells change with latitude. This effect is substantially mitigated by the averaging approach taken to calculating measures of turnover between focal and neighbouring cells. Specifically, while latitudinal distances between these cells increase with latitude, this is countered by a simultaneous decrease in longitudinal distances. Hence, the mean distances between cell centroids are approximately constant between c.50° S and c.50° N, and only increase markedly at very high latitudes (figure S1 in the electronic supplementary material). Such an effect cannot account for the key results documented in this paper, but needs nonetheless to be borne in mind when interpreting them.
Relationships between matching components, spatial turnover indices and species richness, spatial turnover indices and environmental variables (with and without also fitting, and hence accounting for, species richness), and with spatial turnover indices for different range size quartiles, were assessed using either normal error or Poisson error mixed modelling methods (SAS; Littell et al. 1996) that accounted for spatial autocorrelation in the residuals by fitting exponential spatial covariance structures. The longitudes and latitudes of cell centroids were used as spatial coordinates. Models were implemented using Proc Mixed (for normal errors) and Proc Glimmix v. 1.0 add-in (for Poisson errors) in SAS v. 9.1.3 (Littell et al. 1996). The fit of quadratic as well as linear terms for predictors was tested in order to allow for nonlinear relationships. For all spatial models (both normal and Poisson error), differences among major biogeographical realms in spatial autocorrelation were accounted for by using equivalent independent error models to estimate the maximum geographical distance (the range parameter ρ), measured in degrees, over which spatial autocorrelation in model residuals was observed to occur. This involved estimating ρ from the semi-variogram of residuals of non-spatial models that included the relevant combination of predictors, separately for each realm. All the eight estimates (six for environmental models) of ρ were then entered as spatial covariance parameters in global models, with spatial autocorrelation taken into account within the same realm. Spatial Poisson error models used the pseudo-likelihood (PL) procedure (Wolfinger & O'Connell 1993) that obtains a maximum-likelihood-like estimate of the scale parameter (φ; Littell et al. 1996). PL does not compute a true log-likelihood, precluding use of model selection procedures based on Akaike's information criterion. Hence, relative importance of predictors was determined by inspection of F values. Since variance explained cannot be derived from spatial models, we used R2 taken from equivalent normal error OLS (non-spatial) models as a rough estimate. Similarly, in Poisson error cases, we used percentage of total deviance explained from equivalent non-spatial models as an indication.
3. Results and discussion
(a) Matching components
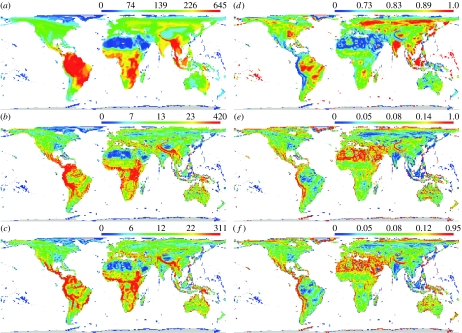
For birds, the matching components exhibit marked large-scale spatial heterogeneity (figure 1). Species continuity a shows rather smooth gradients of variation with strong tropical peaks, being highest across much of the Indo-Malayan realm, sub-Saharan Africa, and much of the Neotropics, including Amazon and the Atlantic coastal forests (figure 1a). In large part, this reflects patterns of global species richness, but expressing continuity as a proportion of overall richness in each cell continues to result in a number of tropical peaks, along with others in Holarctic regions (figure 1d). The gain and loss components, b and c, have a hump-shaped quadratic relationship (both variables log transformed, b as the dependent and c as the independent variable, F1,8968=145.44 (linear term) and F1,8968=174.02 (quadratic term); p<0.001 in both cases and R2=0.360). They also show tropical peaks (figure 1b,c), but the relative variation in these components in tropical areas is much greater than for shared species (the patterns are patchier), and is particularly marked in some mountain ranges, whose topographic complexity is commonly associated with high levels of species richness and endemism (Orme et al. 2005), and thus of spatial gains and losses in species identities. This is not, however, the sole explanation of high gains and losses, and these occur more widely, picking out such features as the Amazon and the Atlantic Forest in the Neotropics, and the woody savannas of the Afrotropics. Expressing gains and losses as a proportion of overall species richness retains peaks in the Andes and Himalayas, but additionally highlights some desert areas, most notably the Sahara (figure 1e,f).
Figure 1.
Global distributions of species gain and loss between neighbouring grid cells. The mean number of species (a,d) shared (matching component a), (b,e) gained (matching component b) and (c,f) lost (matching component c) in comparisons between each focal grid cell and its adjacent neighbours, expressed as raw numbers (a–c) and as a proportion of the total number of species in the focal cell. The colour scales are histogram equalized and the quartile values are indicated.
(b) Turnover, richness and latitude
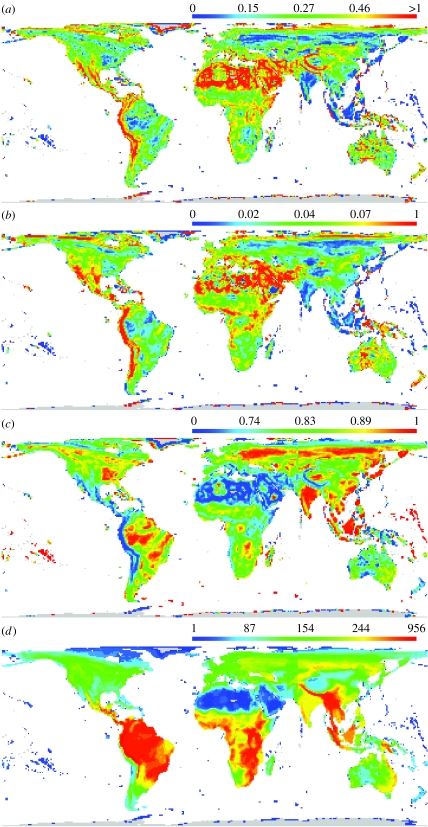
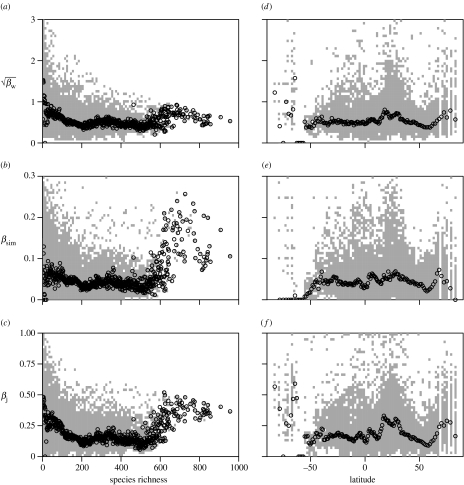
All three indices of spatial turnover show broadly similar patterns of spatial variation (figure 2). βw has peaks principally along the Himalayas, along the Andes and north into Mexico, and in the Sahara and the Middle East (figure 2a), βsim also has peaks in the Andes, the Sahara and the Middle East but not in the Himalayas (figure 2b) and βj has highest values in these regions (figure 2c). In part, and contrary to the first prediction derived from niche limitation theory, these patterns reflect high proportional turnovers in regions of relatively low species richness (figure 2d), the high turnover being generated by gains and losses of very small numbers, but high proportions, of species (figure 1). Thus, with the turnover measures as response variables, βw has an initially decelerating negative relationship with species richness (figure 3a; table 1; square-root transformed), as does βsim (figure 3b; table 1) and βj (figure 3c; table 1). This trend is conspicuously weak for βsim, suggesting that the broad-sense turnover-richness relationship is driven largely by local richness gradients (for which βsim controls, but the other two measures do not). In all cases, however, and in agreement with prediction, plots of turnover against species richness indicate particularly high turnover in those areas with the highest richness, suggesting that the world's avian biodiversity hotspots are a patchwork of spatially diverse assemblages (figure 3a–c). In areas of high richness, high turnover is generated by gains and losses of large numbers and proportions of species (figure 1). The strongly nonlinear global relationships between spatial turnover and species richness mean that regional studies are likely to find different such relationships, depending on what range of richness variation they capture. Although doubtless there are other causes as well, this may in part explain why published turnover–richness relationships have been so variable (Koleff et al. 2003a).
Figure 2.
Global distributions of spatial turnover indices and species richness. (a) βw, (b) βsim, (c) βj and (d) species richness. The colour scales are histogram equalized and the quartile values are indicated.
Figure 3.
Relationships of three indices of spatial turnover with species richness and latitude. Associations are shown for species richness (a–c), and latitude (d–f), for (a,d) square-root transformed βw, (b,e) βsim and (c,f) βj. The range of values at each latitude and species richness, respectively, is shown in grey and the latitudinal or species richness medians are plotted as open circles. In order to show these relationships more clearly, the graph for βw is truncated at 3 and that of βsim at 0.3.The omitted values constitute 1.8% (βw) and 0.7% (βsim) of the dataset: all have low species richness (max=186, median=4) but cover a wide range of latitudes. Southern latitudes are indicated as negative, northern ones as positive.
Table 1.
Significant relationships between spatial turnover species richness and selected environmental variables (both linear and squared terms). (There were 8968 degrees of freedom for all species richness terms; for environmental terms, there were 7891 degrees of freedom in the case of linear models and 7890 in the case of quadratic models. For βsim and βj estimated proportion of variance explained is reported as R2 values taken from equivalent OLS regression models. In the case of βw, the overall proportion of total deviance explained (Pp. expl. D) for equivalent non-spatial Poisson error models is used as an estimate of variance explained. For significant linear and quadratic terms, + and − indicate positive and negative slopes, respectively, with level of significance coded as: +++/−−−, p<0.001; ++/−−, 0.001≤p<0.01; +/−, 0.01≤p<0.05.)
| Effect | βw | βsim | βj | |||
|---|---|---|---|---|---|---|
| F | Pp. expl. D | F | R2 | F | R2 | |
| species richness | 765.12−−− | 0.273 | 84.57−−− | 0.086 | 538.67−−− | 0.268 |
| species richness2 | 22.48+++ | 44.58+++ | 139.83+++ | |||
| environmental mean | ||||||
| elevation | 16.22−−− | 0.435 | 32.44+++ | 0.018 | — | — |
| elevation2 | — | — | — | |||
| habitat diversity | 74.26−−− | 0.547 | 27.1−−− | 0.047 | 58.82−−− | 0.161 |
| habitat diversity2 | 12.69+++ | 17.98+++ | 23.04+++ | |||
| temperature | 80.37−−− | 0.458 | — | — | 61.69−−− | 0.045 |
| temperature2 | 95.37+++ | — | 74.50+++ | |||
| NDVI | 55.99−−− | 0.588 | 46.91−−− | 0.074 | 155.07−−− | 0.271 |
| NDVI2 | 5.29+ | — | 45.55+++ | |||
| environmental roughness | ||||||
| elevation | 85.05+++ | 0.431 | 41.90+++ | 0.034 | 50.16+++ | 0.044 |
| elevation2 | 103.17−−− | — | ||||
| habitat diversity | — | — | 13.5+++ | 0.0008 | 6.00+ | 0.029 |
| habitat diversity2 | — | — | ||||
| temperature | 100.72−−− | 0.429 | 38.37+++ | 0.032 | 62.58+++ | 0.039 |
| temperature2 | — | 4.73− | 38.60−−− | |||
| NDVI | 50.42−−− | 0.434 | 13.93+++ | 0.003 | 11.38+++ | 0.027 |
| NDVI2 | — | — | ||||
There is a marked latitudinal gradient in global avian species richness (Orme et al. 2005). However, contrary to the second prediction derived from niche limitation theory and some regional studies (Stevens 1989; Gaston & Williams 1996; Koleff et al. 2003a; Willig et al. 2003), there is no simple global relationship between spatial turnover and latitude (figure 3d–f). The weak correlations between turnover indices and absolute cell latitudes are all highly significant (Pearson correlations: βwr=−0.03, βsimr=−0.04, βjr=−0.02; n=17 921 and p<0.001 in all cases), but this is confounded by longitudinal spatial autocorrelation. When this autocorrelation is removed by averaging across longitudes, neither βsim nor βj show a strong or statistically significant correlation with absolute latitude (Pearson correlations: βsimr=0.12; βjr=0.14; n=152 and p>0.05 in both cases). By contrast, βw does show a significant correlation (r=−0.25, n=152, p=0.002), but this is driven by extreme values, as shown by repeating the analysis omitting the 0.9% of cells with βw>5 (r=0.09, n=152, p=0.28). It has been argued that relatively simple latitudinal gradients in species richness hide much of the rich spatial variation in species numbers, which may be important to understanding what determines the richness occurring in different areas (Hawkins & Diniz-Filho 2004). Our results reveal that for spatial turnover almost the converse argument may apply. There is little evidence for simple latitudinal gradients in turnover, in large part because turnover peaks in both low and high richness areas (figure 3).
(c) Turnover and environment
Our global model revealed that, after controlling for spatial autocorrelation, spatial turnover decreased with mean habitat diversity and mean NDVI, and showed inconsistent patterns with mean elevation and mean temperature (table 1). Although increasing with elevation, spatial turnover generally showed inconsistent patterns with roughness in the environmental variables (table 1). Most relationships were stronger for mean than roughness values, suggesting that at this spatial resolution patterns of spatial turnover are not solely driven by patterns in environmental turnover. For βw and βj, some of these relationships changed direction when species richness was controlled for, while for βsim the patterns were generally weaker than for the other turnover measures and were maintained (table S1 in the electronic supplementary material). Nevertheless, the principal finding—that relationships were not consistently stronger with roughness than mean environmental values—held irrespective of whether or not species richness was included in the models, and was contrary to the third prediction derived from niche limitation theory.
The potential importance of average environmental conditions for global patterns of spatial turnover for birds fits with recent demonstrations that overall species population sizes increase, and average species population sizes increase or remain constant, with resource availability (Kaspari et al. 2000; Hurlbert 2004; Evans et al. 2006; Mönkkönen et al. 2006). As the population size and range size of species are frequently positively correlated (Brown 1984; Gaston et al. 2000), the larger populations in resource-rich areas are likely to occupy more of that region, thus elevating mean species occupancy (Bonn et al. 2004). This increase in occupancy may increase the number of species shared between two areas within a region while decreasing species loss or gain, and thus decreasing our three measures of spatial turnover. Such a negative effect is seen both for mean temperature and NDVI, two commonly used indices of resource availability (table 1). Unfortunately, the combinations of mean and roughness of environmental variables that typically result from using multivariate models incorporating the two kinds of variables are virtually impossible to interpret mechanistically and provide limited additional insight into their relative importance.
(d) Turnover and range size
Spatial patterns of species richness tend to be driven foremost by the distribution of the more widespread rather than the more restricted species, with the former having the stronger relationships with mean environmental conditions (Jetz & Rahbek 2002; Lennon et al. 2004). Contrary to the prediction based on the assumption that restricted species have narrower niches and more fragmented distributions, the more widespread species tend also to have a disproportionate influence on observed patterns of spatial turnover. Dividing species into the quartiles of the species-range size distribution, and determining the relationships between overall patterns of turnover and the patterns of turnover for each quartile separately, shows the clear pattern that more widespread species better predict species turnover (table S2 in the electronic supplementary material). Owing to this, it is likely that the relationships we identify here between turnover, richness and environment will reflect the geographical distributions of widespread rather than restricted-range species. The relative importance of widespread species in determining patterns of species richness has been explained in terms of the stronger responses that these species show to patterns of mean environmental variation (Jetz & Rahbek 2002), and this would fit with the evident importance of such variation in determining patterns of spatial turnover (table 1).
4. In conclusion
While it would be valuable to repeat them at yet finer spatial resolutions (including using site based data) and to address issues of scale dependence, the global analyses reported here have been conducted at the finest practicable given available data, and at a resolution that is now commonly adopted for the study of species richness patterns across geographical scales (Jetz & Rahbek 2002; Orme et al. 2005). The results thus have important implications for present understanding of global patterns of biodiversity. Foremost, despite exhibiting marked spatial patterns, spatial turnover is not simply correlated with species richness, latitude or turnover in the environment, contrary to the predictions derived from niche limitation theory. Rather, turnover is high when species richness is low, where the loss or gain of a very few species exerts a strong influence, and when species richness is very high, where the same factors that promote high richness produce gradients of rapid change in species composition.
Acknowledgments
We thank T. Allnutt, B. Beehler, T. Brooks, B. Coates, J. Cromie, H. Fry, P. Higgins, D. McNicol, D. Mehlman, C. Perrins, R. Porter, H. Pratt, N. Redman, R.S. Ridgely, C. Robertson, A. Silcocks, A.J. Stattersfield, M. Strange, M. Unwin, M. Weston, M. Whitby, P. Williams, D. Wynn, B. Young, J. Zook, A. & C. Black, Academic Press, BirdGuides Ltd, Birds Australia, Christopher Helm, Conservation International, NatureServe, Oxford University Press, Ornithological Society of New Zealand, and Princeton University Press for access to data; L. Birch, R. Prys-Jones, B. Sheldon, the Alexander Library (Oxford University), and the Natural History Museum (Tring) for access to libraries; O. Schabenberger for analytical advice; O. Barbosa, F. Bokma, J. Booth, J.H. Brown, K.L. Evans, R.A. Fuller, I.S. Fishburn, B. Goettsch, S.F. Jackson, O.L. Petchey and an anonymous reviewer for their comments and discussion. This work was supported by The Natural Environment Research Council (grant nos NER/O/S/2001/01258, NER/O/S/2001/01257, NER/O/S/2001/01230, and NER/O/S/2001/01259, NE/B503492/1). K.J.G. holds a Royal Society-Wolfson Research Merit Award.
Supplementary Material
(i)Significant relationships between spatial turnover and selected environmental variables, controlling for species richness. There were 7891 degrees of freedom (d.f.) in the case of linear models and 7890 in the case of quadratic models. Methods and abbreviations for estimates of variance explained are as for table 1. For significant linear and quadratic terms, + and − indicate positive and negative slopes, respectively, with level of significance coded as: +++/−−−, p<0.001; ++/−−, 0.001≤p<0.01; +/−, 0.01≤p<0.05. (ii)F-values and estimates of variance explained for relationships between overall patterns of turnover and the patterns of turnover for each quartile of the species-range size distribution separately. Methods and abbreviations for estimates of variance explained are as for table 1. In each case there are 8969 degrees of freedom, slopes are positive, and p<0.0001. (iii)Latitudinal variation in the mean distance between the centroid of a focal cell and that of its immediate neighbours on the equal area Behrmann grid employed in this study
References
- Blackburn T.M, Gaston K.J. The distribution of bird species in the New World: patterns in species turnover. Oikos. 1996;77:146–152. doi:10.2307/3545594 [Google Scholar]
- Bonn A, Storch D, Gaston K.J. Structure of the species–energy relationship. Proc. R. Soc. B. 2004;271:1685–1691. doi: 10.1098/rspb.2004.2745. doi:10.1098/rspb.2004.2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.H. On the relationship between abundance and distribution of species. Am. Nat. 1984;124:255–279. doi:10.1086/284267 [Google Scholar]
- Condit R, et al. Beta-diversity in tropical forest trees. Science. 2002;295:666–669. doi: 10.1126/science.1066854. doi:10.1126/science.1066854 [DOI] [PubMed] [Google Scholar]
- Evans K.L, James N.A, Gaston K.J. Abundance, species richness and energy availability in the North American avifauna. Glob. Ecol. Biogeogr. 2006;15:372–385. doi:10.1111/j.1466-822X.2006.00228.x [Google Scholar]
- Gaston K.J. Chapman and Hall; London, UK: 1994. Rarity. [Google Scholar]
- Gaston K.J. Global patterns in biodiversity. Nature. 2000;405:220–227. doi: 10.1038/35012228. doi:10.1038/35012228 [DOI] [PubMed] [Google Scholar]
- Gaston K.J, Williams P.H. Spatial patterns in taxonomic diversity. In: Gaston K.J, editor. Biodiversity: a biology of numbers and difference. Blackwell Science; Oxford, UK: 1996. pp. 202–229. [Google Scholar]
- Gaston K.J, Blackburn T.M, Greenwood J.J.D, Gregory R.D, Quinn R.M, Lawton J.H. Abundance–occupancy relationships. J. Appl. Ecol. 2000;37(Suppl. 1):39–59. doi:10.1046/j.1365-2664.2000.00485.x [Google Scholar]
- Gaston, K. J., Evans, K. L. & Lennon, J. J. 2007 The scaling of spatial turnover: pruning the thicket. In Scaling biodiversity (eds D. Storch, P. M. Marquet & J. H. Brown). Cambridge, UK: Cambridge University Press.
- Gregory R.D, Greenwood J.J.D, Hagermeijer E.J.M. The EBCC atlas of European breeding birds: a contribution to science and conservation. Biol. Conserv. Fauna. 1998;102:38–49. [Google Scholar]
- Groves C.R. Island Press; Washington, DC: 2003. Drafting a conservation blueprint: a practitioner's guide to planning for biodiversity. [Google Scholar]
- Harrison S. Species diversity, spatial scale, and global change. In: Kareiva P.M, Kingsolver J.G, Huey R.B, editors. Biotic interactions and global change. Sinauer; Sunderland, MA: 1993. pp. 388–401. [Google Scholar]
- Hawkins B.A, Diniz-Filho J.A.F. ‘Latitude’ and geographic patterns in species richness. Ecography. 2004;27:268–272. doi:10.1111/j.0906-7590.2004.03883.x [Google Scholar]
- Hurlbert A.H. Species–energy relationships and habitat complexity in bird communities. Ecol. Lett. 2004;7:714–720. doi:10.1111/j.1461-0248.2004.00630.x [Google Scholar]
- Jaccard P. The distribution of the flora in the alpine zone. New Phytol. 1912;11:37–50. doi:10.1111/j.1469-8137.1912.tb05611.x [Google Scholar]
- Jetz W, Rahbek C. Geographic range size and determinants of species richness in African birds. Science. 2002;297:1548–1551. doi: 10.1126/science.1072779. doi:10.1126/science.1072779 [DOI] [PubMed] [Google Scholar]
- Kaspari M, O'Donnell S, Alonso L. Three energy variables predict ant abundance at a geographic scale. Proc. R. Soc. B. 2000;267:485–490. doi: 10.1098/rspb.2000.1026. doi:10.1098/rspb.2000.1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koleff P, Lennon J.J, Gaston K.J. Are there latitudinal gradients in species turnover? Glob. Ecol. Biogeogr. 2003a;12:483–498. doi:10.1046/j.1466-822X.2003.00056.x [Google Scholar]
- Koleff P, Gaston K.J, Lennon J.J. Measuring beta diversity for presence–absence data. J. Anim. Ecol. 2003b;72:367–382. doi:10.1046/j.1365-2656.2003.00710.x [Google Scholar]
- Lawton J.H. Concluding remarks: a review of some open questions. In: Hutchings M.J, John E.A, Stewart A.J.A, editors. The ecological consequences of environmental heterogeneity. Blackwell Science; Oxford, UK: 2000. pp. 401–424. [Google Scholar]
- Lennon J.J, Koleff P, Greenwood J.J.D, Gaston K.J. The geographical structure of British bird distributions: diversity, spatial turnover and scale. J. Anim. Ecol. 2001;70:966–979. doi:10.1046/j.0021-8790.2001.00563.x [Google Scholar]
- Lennon J.J, Koleff P, Greenwood J.J.D, Gaston K.J. Contribution of rarity and commonness to patterns of species richness. Ecol. Lett. 2004;7:81–87. doi:10.1046/j.1461-0248.2004.00548.x [Google Scholar]
- Littell R.C, Milliken G.A, Stroup W.W, Wolfinger R.D. SAS Institute; Cary, NC: 1996. SAS system for mixed models. [Google Scholar]
- Lomolino M.V, Riddle B.R, Brown J.H. 3rd edn. Sinauer Associates; Sunderland, MA: 2006. Biogeography. [Google Scholar]
- Mönkkönen M, Forsmann J.T, Bokma F. Energy availability, abundance, energy-use and species richness in forest bird communities: a test of the species–energy theory. Glob. Ecol. Biogeogr. 2006;15:290–302. [Google Scholar]
- New M, Lister D, Hulme M, Makin I. A high-resolution data set of surface climate over global land areas. Climate Res. 2002;21:1–25. (available from Climate Research Unit of University of East Anglia at http://www.cru.uea.ac.uk/cru/data/tmc.htm) [Google Scholar]
- Olson J.S. USGS EROS Data Center; Sioux Falls, SD: 1994a. Global ecosystem framework—translation strategy. [Google Scholar]
- Olson J.S. USGS EROS Data Center; Sioux Falls, SD: 1994b. Global ecosystem framework—definitions. [Google Scholar]
- Orme C.D.L, et al. Global hotspots of species richness are not congruent with endemism or threat. Nature. 2005;436:1016–1019. doi: 10.1038/nature03850. doi:10.1038/nature03850 [DOI] [PubMed] [Google Scholar]
- Orme C.D.L, et al. Global patterns of geographic range size in birds. PLoS Biol. 2006;4:1276–1283. doi: 10.1371/journal.pbio.0040208. doi:10.1371/journal.pbio.0040208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens G.C. The latitudinal gradient in geographical range: how so many species coexist in the tropics. Am. Nat. 1989;133:240–256. doi:10.1086/284913 [Google Scholar]
- Whittaker R.H. Vegetation of the Siskiyou mountains, Oregon and California. Ecol. Monogr. 1960;30:279–338. doi:10.2307/1943563 [Google Scholar]
- Whittaker R.H. Evolution and measurement of species diversity. Taxon. 1972;21:213–251. doi:10.2307/1218190 [Google Scholar]
- Wiersma Y.F, Urban D.L. Beta-diversity and nature reserve system design: a case study from the Yukon. Conserv. Biol. 2005;19:1262–1272. doi:10.1111/j.1523-1739.2005.00099.x [Google Scholar]
- Williams P.H, de Klerk H.M, Crowe T.M. Interpreting biogeographical boundaries among Afrotropical birds: spatial patterns in richness gradients and species replacement. J. Biogeogr. 1999;26:459–474. doi:10.1046/j.1365-2699.1999.00294.x [Google Scholar]
- Williamson M. Are communities ever stable? In: Gray A.J, Crawley M.J, Edwards P.J, editors. Colonisation, succession and stability. Blackwell Scientific; Oxford, UK: 1987. pp. 353–371. [Google Scholar]
- Willig M.R, Sandlin E.A. Gradients of species density and species turnover in New World bats: a comparison of quadrat and band methodologies. In: Mares M.A, Schmidly D.J, editors. Latin American mammalogy: history, biodiversity and conservation. University of Oklahoma Press; Norman, OK: 1991. pp. 81–96. [Google Scholar]
- Willig M.R, Kaufman D.M, Stevens R.D. Latitudinal gradients of biodiversity: pattern, process, scale, and synthesis. Annu. Rev. Ecol. Evol. Syst. 2003;34:273–309. doi:10.1146/annurev.ecolsys.34.012103.144032 [Google Scholar]
- Wolfinger R, O'Connell M. Generalized linear mixed models: a pseudolikelihood approach. J. Stat. Comput. Sim. 1993;48:233–243. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(i)Significant relationships between spatial turnover and selected environmental variables, controlling for species richness. There were 7891 degrees of freedom (d.f.) in the case of linear models and 7890 in the case of quadratic models. Methods and abbreviations for estimates of variance explained are as for table 1. For significant linear and quadratic terms, + and − indicate positive and negative slopes, respectively, with level of significance coded as: +++/−−−, p<0.001; ++/−−, 0.001≤p<0.01; +/−, 0.01≤p<0.05. (ii)F-values and estimates of variance explained for relationships between overall patterns of turnover and the patterns of turnover for each quartile of the species-range size distribution separately. Methods and abbreviations for estimates of variance explained are as for table 1. In each case there are 8969 degrees of freedom, slopes are positive, and p<0.0001. (iii)Latitudinal variation in the mean distance between the centroid of a focal cell and that of its immediate neighbours on the equal area Behrmann grid employed in this study