Abstract
For organisms with complex life cycles, variation among individuals in traits associated with survival in one life-history stage can strongly affect the performance in subsequent stages with important repercussions on population dynamics. To identify which individual attributes are the most influential in determining patterns of survival in a cohort of reef fish, we compared the characteristics of Pomacentrus amboinensis surviving early juvenile stages on the reef with those of the cohort from which they originated. Individuals were collected at hatching, the end of the planktonic phase, and two, three, four, six and eight weeks post-settlement. Information stored in the otoliths of individual fish revealed strong carry-over effects of larval condition at hatching on juvenile survival, weeks after settlement (i.e. smaller-is-better). Among the traits examined, planktonic growth history was, by far, the most influential and long-lasting trait associated with juvenile persistence in reef habitats. However, otolith increments suggested that larval growth rate may not be maintained during early juvenile life, when selective mortality swiftly reverses its direction. These changes in selective pressure may mediate growth-mortality trade-offs between predation and starvation risks during early juvenile life. Ontogenetic changes in the shape of selectivity may be a mechanism maintaining phenotypic variation in growth rate and size within a population.
Keywords: carry-over effects, compensatory growth, coral reef fishes, growth-mortality hypothesis, maternal effects, phenotypic selection
1. Introduction
The population dynamics of many animals including insects, amphibians, aquatic invertebrates and fishes involves multiple life stages, during which individuals change their habitat use or diet with ontogeny (i.e. ontogenetic niche shifts; Wilbur 1980). Niche-specific morphological, physiological and behavioural characteristics determine which individuals have better chances of avoiding predators and obtaining resources to grow and survive to the next life-history stage. As individuals shift between niches, their scope for growth and survival can vary in relation to the physical and biological environment they encounter (Altwegg & Reyer 2003). Understanding the mechanisms generating such phenotypic variation within and between ontogenetic stages is critical for animals with complex life cycles, where events occurring at one life stage can propagate through time (carry-over effects) and have profound demographic, life-history and genetic consequences on populations (e.g. Beckerman et al. 2002).
Historically, studies of animal populations have focused on growth parameters such as rates, durations, time of onset and offset as major factors influencing the survival of individuals between ontogenetic stages (Jones & German 2005). During the early life history of fishes, the role of growth is encapsulated by the growth–mortality hypothesis (Anderson 1988), which provides a theoretical framework to examine the carry-over effects of larval condition on post-metamorphic survival. The theory suggests that faster growing larvae may be able to gain survival advantages by shortening a developmental phase (the ‘stage-duration’ mechanism; Houde 1987) and potentially reducing predation and/or starvation risks by attaining a larger size at a given age (the ‘bigger-is-better’ mechanism; Miller et al. 1988). Advantages gained during larval phases are believed to extend to the juvenile phase, where they influence individual chances of survival (Sogard 1997; Vigliola & Meekan 2002).
Some aspects of the growth–mortality theory have been questioned, particularly the bigger-is-better mechanism (e.g. Fuiman 1989; Litvak & Leggett 1992). Substantial theoretical and empirical evidence has shown that survival of fish larvae is unlikely to be monotonically related to size (e.g. Pepin et al. 1992; Leggett & DeBlois 1994; Cowan et al. 1996). However, it is still widely accepted that larger individuals are more likely to survive than smaller conspecifics (e.g. McGurk 1986; Miller et al. 1988; Cowan & Houde 1992; Sogard 1997). While body size determines to a large extent the type and strength of ecological interactions experienced by an individual throughout its lifetime, and translates into size-specific patterns of survival at the population level, the generality of size-selective processes in natural populations remains controversial and may not be sufficient to explain the demography of individuals in the wild (Pfister & Wang 2005).
The back-calculation of growth and mortality patterns from otoliths has enabled recent studies to show that larval growth history strongly influences juvenile survivorship of marine fishes in benthic habitats (e.g. Searcy & Sponaugle 2001; Vigliola & Meekan 2002; Raventos & Macpherson 2005; Jenkins & King 2006). Events prior to hatching (via maternal effects) also have the potential to affect post-settlement survivorship (Vigliola & Meekan 2002; Raventos & Macpherson 2005), but these processes have not yet been quantitatively linked. Similarly, despite some evidence that events during post-settlement life have the capacity to breakdown patterns established at settlement (e.g. Webster 2002) and potentially obscure the effects of larval experience on juvenile success (Bertram et al. 1993; McCormick & Hoey 2004), few studies have linked larval and early post-settlement growth histories to persistence of juveniles in benthic habitats (but see Searcy & Sponaugle 2001; Vigliola & Meekan 2002; McCormick & Hoey 2004).
In the present study, we use growth histories recorded in the otoliths of a common coral reef fish, Pomacentrus amboinensis, to determine the extent to which variation in life-history characteristics, among individuals within a cohort, influences survival through ontogenetic niche shifts in the wild. Specifically, we aim to quantify the shape and magnitude of selective mortality acting on phenotypic variability from the end of the embryonic stage, through the planktonic larval phase, to weeks and months after settlement. By doing so, we identified the growth-related attributes that are the most influential in determining patterns of juvenile survival in benthic habitats.
2. Material and methods
(a) Study species
We studied the Ambon damselfish (P. amboinensis), a common and abundant coral reef fish on the Great Barrier Reef. The life cycle of this species begins around the time of the full moon in benthic nest sites, where females lay eggs that are tended by a male until the completion of embryonic development and hatching, a process that takes 4–5 days. Following hatching, P. amboinensis offspring undergo a 15- to 23-day planktonic phase (Kerrigan 1996) before returning to the reef at the time of the following new moon, when they rapidly (in less than 12 h) metamorphose into juveniles (McCormick et al. 2002) and settle directly into adult reef habitats. Once settled, P. amboinensis remains strongly site-attached throughout benthic life (Booth 2002).
(b) Field sampling
In late October 2004, we monitored the daily spawning activity of P. amboinensis at six sites (approx. 1.5–2 km apart) on the fringing reef around Lizard Island (14°40′ S, 145°28′ E) on the northern Great Barrier Reef, Australia. These sites were distributed across all habitats and depths, where P. amboinensis occurs at the study location so as to obtain as representative a sample as possible of traits that may influence survival in the population reproductive output for that pulse. We collected a total of 42 egg clutches spawned on plastic half-pipes that had been adopted by males as nest sites (Gagliano & McCormick 2007a). Prior to dusk on the night of hatching, clutches were brought into the laboratory and placed in well-aerated aquaria of flowing seawater at 28°C (ambient). All wild clutches successfully hatched in the laboratory and all individuals from each clutch hatched within a 20–30 min period. Subsamples of approximately 100 newly hatched larvae were collected from each clutch using a small hand net and a fine brush, and immediately preserved in 30% ethanol freshwater solution stored at −19°C. This allowed accurate measurement of morphology and otolith dimensions of newly hatched larvae with a negligible error due to shrinkage (Gagliano et al. 2006).
During the new moon in November, over 7000 newly metamorphosed P. amboinensis were captured using light traps (see fig. 1 in Meekan et al. 2001 for trap design) as they approached the reefs surrounding Lizard Island. Traps were moored over sand approximately 100 m apart and 30–50 m from the reef edge. They were suspended from a buoy 1 m below the surface prior to dusk and then cleared of fish just after dawn the following morning for 12 days centred around the time of the new moon. This period was likely to encompass the majority of the settlement pulse for the month (Kerrigan 1996). Fish collected by light traps were sacrificed immediately following capture and preserved in 70% ethanol. Individuals from this same lunar cohort (i.e. those who originally settled on the reef during the new moon in November) were sampled by divers using SCUBA two, three, four, six and eight weeks after settlement from reef habitats using hand nets and an anaesthetic (ethanol : clove oil mixture, 5 : 1). A total of 635 juveniles were collected from the reef. Effort was spread over different habitats and locations on large sections (500–800 m) of the fringing reef around Lizard Island to ensure that sampling would not bias subsequent collections and account for potential spatial variability of the examined traits (Vigliola & Meekan 2002). After each collection, juvenile fish were immediately preserved in 70% ethanol.
(c) Data collection
Twenty newly hatched larvae were randomly selected from each clutch and photographed individually against a scale bar under a dissecting microscope. Larval body dimensions, including standard length (SL), yolk-sac area (YK) and oil globule area (OG), were measured on these images using image analysis software (Optimas v. 6.5). Sagittal otoliths or ear bones of individual hatchlings were located under a compound microscope at 40× magnification using a polarized light source and otolith size (maximum diameter in micrometres) was recorded as a measure of size-at-hatching.
Over 7000 P. amboinensis settlers were caught by light traps in November. Preserved individuals were measured to the nearest 0.1 mm SL and then size-corrected to account for shrinkage effects (estimated at 11% for SL during the first month of preservation, after which shrinkage of this attribute becomes negligible). A total of 410 individuals were then randomly subsampled in proportion to the numbers occurring in 1 mm size classes of SL within the size range of the entire light trap collection.
To compare the early life history of P. amboinensis surviving on the reef with that of the cohort at earlier times, we examined the information stored in the otoliths of fish from each of the seven collections (newly hatched through to eight weeks post-settlement). After removal from the fish, thin cross-sections of otoliths were obtained for analysis by mounting sagitta in thermoplastic cement (Crystal Bond) on a glass microscope slide. These were then ground and polished using 12–0.3 μm lapping films, as described by Wilson & McCormick (1997). Sections were viewed using a compound microscope at 400× magnification and analysed along the longest axis of the cross-section by a video image-analysis system linked to the microscope.
As in many other tropical and temperate fishes (Wellington & Victor 1989), P. amboinensis forms an increment closest to the spherical nucleus of the otolith on the day of hatching. Furthermore, settlement coincides with deposition of a single dark increment followed by a sharp decline in increment width in the otolith (Wilson & McCormick 1997). Formation of daily increments after settlement has been validated by Pitcher (1988). To ensure that our analyses included only fishes that were part of the same November cohort, we counted the number of increments from the settlement mark to the edge of the otolith of all benthic juveniles in our collections and excluded fishes that were older or younger (less than 15%) than expected at the date of capture from the reef.
(d) Data analyses
We tested for the presence of size- and growth-selective mortality in the cohort of newly settled P. amboinensis by comparing the distribution of otolith characteristics at a given age among successive collections from the time of hatching to eight weeks after settlement. For example, we compared distributions of size-at-settlement of all benthic juveniles (i.e. post-settlement survivors) with those of all newly metamorphosed settlers caught by light traps (i.e. our samples of the cohort immediately prior to settlement from which these survivors originated) to test for size-selective mortality acting on this trait at the time of settlement. A shift (i.e. change in skewness) in the distribution of traits of survivors to the left and/or decline in mean size-at-settlement would suggest that smaller individuals have higher rates of survival than larger conspecifics at settlement (i.e. negative directional selection). Similarly, a shift to the right and/or an increase in mean size would indicate that selection favours larger individuals. Size and growth rate distributions (survivors versus original cohort) for each pair of samples (i.e. ages) were compared using the non-parametric Kolmogorov–Smirnov two-sample test owing to its sensitivity to changes in location, dispersion and skewness of the distributions, while making no assumptions on the distribution of data (Sokal & Rohlf 2001). The significance of all comparisons was based on an α-level of 0.05. We examined the frequency distributions of pelagic larval duration (PLD), size-at-hatching, size-at-settlement, size-at-age (2, 3 and 4 weeks post-settlement) and growth (pelagic larval phase, 2–4 weeks post-settlement). In our comparison of selected traits at hatching of newly hatched larvae with those of the cohort at older ages, we assumed that hatchlings collected from benthic nests around Lizard Island were a representative sample of the cohort of fish that settled on the fringing reefs around the island. Although this is an untestable assumption, we believe it is justifiable, given that recent evidence has suggested a high degree of self-recruitment at this location (e.g. Jones et al. 1999; James et al. 2002) and the extensive range of habitats and environmental conditions sampled around Lizard Island during the present study.
All comparisons of relative size and growth within the targeted cohort were based on and refer to otolith measurements only, unless specified otherwise. The advantage of using otolith traits for studies of phenotypic selection is that these measurements are permanent individual records unmodified by subsequent age and growth, thus providing a convenient means for quantifying phenotypic selection (cf. Swain 1992). We estimated the intensity of linear (Si) and nonlinear selection (Ci) as
where before and after are the mean; and Varbefore and Varafter are the variance of size (or growth) z before- and after-selection, respectively (Brodie et al. 1995).
Since phenotypic selection can have both linear and nonlinear forms, we also described selection acting on size and growth rate of larval and juvenile P. amboinensis, using the non-parametric approach pioneered by Schluter (1988) and modified by Anderson (1995) and Sinclair et al. (2002) for cross-sectional data. This spline-based regression method describes relative survival as a smoothly changing function of size or growth, making no assumptions about the underlying fitness function, and allows calculation of 95% confidence bands about the curve. Briefly, we first estimated the conditional probability h(z) that a fish of size (or growth) z at a given age was caught in the sample of survivors, given that it was caught in one of the two samples. To do this, fish caught prior to the occurrence of selection were coded as 0 and those caught after selection as 1, and h(z) was estimated using a generalized additive model assuming a binomial error distribution and a logit link as per Sinclair et al. (2002) and given by
where u is a cubic B-spline smooth function of z, with a smoothing parameter λ chosen by generalized cross validation. The relative survival function f(z) was then given by
where nbefore and nafter are the numbers in the before- and after-selection group, respectively.
Finally, the probability (π) of a newly hatched fish surviving the pelagic phase and reaching benthic habitats as a function of its larval characteristics (SL, standard length; YK, yolk-sac area; OG, oil globule area) was estimated using logistic regression analysis. These larval characteristics are important because they are directly influenced by maternal provisioning to offspring (McCormick 2006; Gagliano & McCormick 2007a) and thus enable potential carry-over effects of maternally induced variation on offspring survival to be quantified. Although individual survivorship was not directly recorded, we used these larval characteristics associated with change in the distributions of otolith size-at-hatching as a proxy for survival. To do this, we first examined both the size–frequency distributions at settlement (i.e. comparing newly hatched larvae with settled fish) and the form of phenotypic selection acting on otolith size-at-hatching during the planktonic phase and identified the range of otolith sizes representing individuals unlikely to survive to settlement, where a low probability of survival was defined as those individuals representing less than 1% of the surviving size–frequency distribution (see figure 1 in the electronic supplementary material). We then dummy-coded all hatchlings likely to survive as ‘1’ and all those unlikely to survive the pelagic phase as ‘0’ based on their otolith size-at-hatching. To identify the minimum number of variables that predicted survival to settlement, we used the best subset model. This involves a likelihood score criterion and the Wald test (Z) to evaluate the statistical significance of each of the regression coefficients. All traits included in the analysis met the assumption of no collinearity.
3. Results
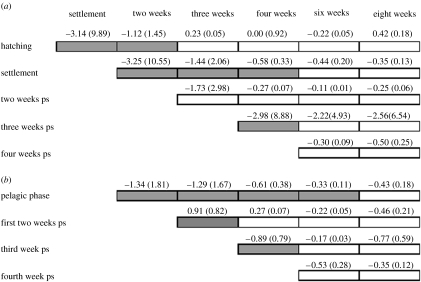
Individuals surviving the intense size-selective mortality, which occurred during the pelagic phase, had significantly smaller otoliths at hatching (before=0.029 mm, after=0.023 mm; Kolmogorov–Smirnov test, p<0.001; figure 1a and electronic supplementary material, figure 1a). Selective mortality of individuals with larger otoliths at hatching was also pronounced during the first two weeks following settlement on reef habitats (before=0.023 mm, after=0.022 mm; Kolmogorov–Smirnov test, p<0.001), indicating that selection for this trait operated between the life-history stages (figure 1a). Logistic regression analysis based on dummy-coded individuals showed that the probability (π) of a newly hatched fish surviving the planktonic phase and reaching reef habitats depended on both body size, SL (Wald test Z=22.40, p<0.001) and amount of yolk-sac reserves, YK (Wald test Z=8.81, p<0.003) at hatching (logit (π)=5.60−2.14SL +8.25YK). Specifically, smaller body size and larger yolk-sac reserves at the time of hatching were associated with smaller otolith size and higher survival probability.
Figure 1.
Quantitative estimates of selective mortality based on (a) size and (b) growth of P. amboinensis from hatching to eight weeks post-settlement (ps). Size and growth attributes are listed on the left-hand side and the length of time, a given trait was examined for, is represented by each horizontal bar. The extent to which a given trait influences survival over subsequent life periods is indicated by the grey (p<0.05) and white (p>0.05) boxes based on Kolmogorov–Smirnov two-sample tests. For example, size-at-hatching has a significant influence on survival at settlement and over the first two weeks ps, but it does not affect survival over the succeeding life periods. Values on each box describe change in units of phenotypic standard deviations and represent the intensities of linear and nonlinear (in brackets) selection acting on individual traits at a given time.
The significant selective loss of individuals with faster larval growth during the planktonic phase continued to occur weeks to months after settlement, influencing survivorship of subsequent life stages on benthic habitats (figure 1b and electronic supplementary material, figure 1b). Growth during the planktonic phase was a stronger determinant of survivorship than growth at older ages (figure 1b). Importantly, we found that selective mortality based on larval growth was generally nonlinear and changed the form across ontogenetic stages (figures 1b and 2).
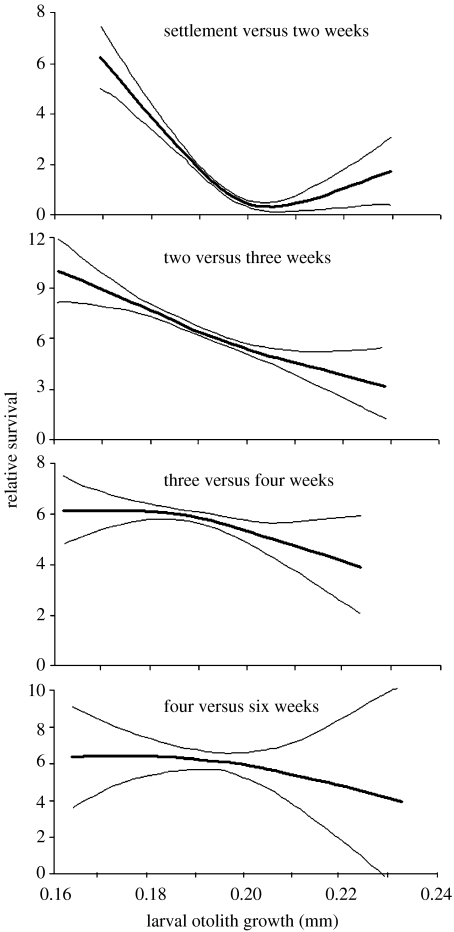
Figure 2.
Changes in intensity and shape of selective pressure acting on the pelagic growth of P. amboinensis from settlement to six weeks later. Thin lines are 95% confidence bands.
We detected no selective mortality based on PLD (Kolmogorov–Smirnov test, p>0.10). Juveniles collected from the reef settled at similar ages to recruits collected at settlement by light traps (mean PLD: 17.80 days and 17.66, respectively), and variation in PLD among individuals was relatively low (recruits PLD range: 15–22 days; juvenile PLD range: 15–22 days; CV<9%).
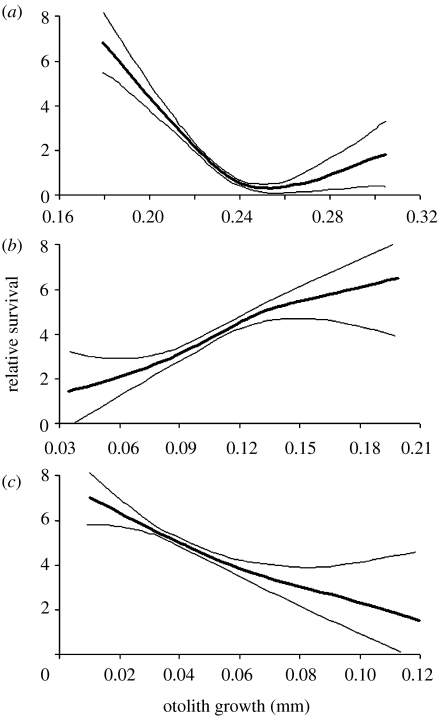
Although selective mortality of this cohort after settlement was strongly associated with larval characteristics, we also detected significant selection based on juvenile traits, including otolith size-at-settlement and growth over the first two to three weeks following settlement on the reef (figure 1 and electronic supplementary material, figure 1). We found that smaller initial size conferred higher survival probability to newly settled P. amboinensis. However, despite of being slower-growing as larvae and the smaller members of the cohort at settlement, survivors of the early juvenile period were those individuals who grew faster during the first two weeks on the reef (figures 1b and 3a,b). We found that the growth rates in the larval and early juvenile period (first two weeks) were inversely related (r=−0.46, p<0.001). Interestingly, those individuals who continued to grow at a faster rate throughout the third week post-settlement were preferentially lost from the cohort (figure 1), as shown by a significant switch in the direction of the selection curve (figure 3b,c).
Figure 3.
Growth-dependent relative survival of P. amboinensis (a) from hatching to settlement, (b) during the first two weeks and (c) the third week post-settlement. Thin lines are 95% confidence bands.
4. Discussion
By exploring the links between life-history stages of a coral reef fish, we have demonstrated how juvenile survivorship can be significantly influenced by processes taking place in the pelagic environment or even before hatching of larvae via parental effects. Pomacentrus amboinensis individuals that survived intense size-selective mortality, which occurred during the pelagic phase, had significantly smaller otoliths at hatching. We found that otolith size-at-hatching closely reflected a combination of early larval characteristics (i.e. larval body size and amount of yolk-sac reserves); contrary to expectations of the bigger-is-better hypothesis (Miller et al. 1988), there was no apparent disadvantage of small size at hatching, when body size was coupled with large energy reserves. Previous studies have directly linked larval and yolk-sac size to maternal condition in this species (McCormick 2003, 2006; Gagliano & McCormick 2007a) and suggested that females can constrain size of offspring in favour of quality (i.e. yolk-sac size). For example, McCormick (2006) showed that maternal stress from competition increases cortisol in P. amboinensis ovaries and decreases larval size but not yolk-sac reserve. In the light of the present study, we suggest that ‘smaller-is-better’ may be a maternal strategy for increasing early survivorship of larvae rather than decreasing it. Clearly, the relationship between the condition of parental stock, larval characteristics and otolith size deserves further investigation. Furthermore, selective mortality of individuals with larger otoliths at hatching was also pronounced during the first two weeks following settlement on reef habitats, indicating that there was a carry-over effect on selection for this trait that operated across life-history stages.
Among the traits considered in this study, pelagic larval growth was, by far, the most influential and long-lasting trait associated with juvenile persistence on the reef. Selective mortality based on larval growth was generally nonlinear and changed form across ontogenetic stages. Such changes in the shape and magnitude of selective mortality over time may help maintain phenotypic variation in larval growth and ultimately preserve (genetic) variation in fish populations (Swain 1992; Hare & Cowen 1997). This could also explain why we do not see a progressive evolution towards faster larval growth rates, as might be predicted if faster-growing individuals within a cohort enjoy higher probability of survival (the growth-rate mechanism; Anderson 1988).
Unlike pelagic larval growth, we detected no patterns of selective mortality based on PLD. The low variation in this trait among individuals suggests that selective mortality with respect to this trait had limited potential to occur within this cohort (cf. Sogard 1997). There appears to be low intra-cohort variability in larval duration in this family of reef fishes (Robertson et al. 1990) and our results combined with previous findings on other pomacentrid species (e.g. Macpherson & Raventos 2005; Bay et al. 2006) suggest that theoretical predictions of the stage-duration mechanism (Houde 1987) are unlikely to be applicable to this group of fishes.
Although our results showed that larval traits strongly influenced patterns of selective mortality within this cohort weeks after settlement, juvenile characteristics also significantly shaped early survivorship of individuals on reef habitats. Specifically, smaller rather than larger initial size conferred higher survival probability to newly settled P. amboinensis. This result contrasts with predictions of the growth-mortality hypothesis (Anderson 1988), which proposes that faster growth at this time enhances survival through the covariation of size with behavioural, physiological and other morphological attributes, which reduce potential predation and/or starvation risks. However, the present finding supports recent evidence indicating that the extent of size-selective mortality of newly settled reef fish can differ among locations separated by only hundreds of metres (Holmes & McCormick 2006). This suggests that the characteristics of the predator assemblage and prevailing environmental conditions can lessen or even negate any advantage to being large at settlement. Ultimately, the lack or even the possibility of negative covariance between size and survival could be indicative of a trade-off between growth and size against behavioural, physiological and other morphological attributes rather than a growth-mortality hypothesis.
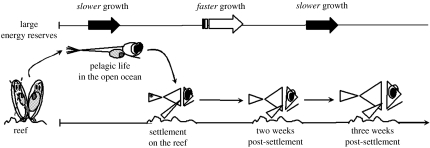
Interestingly, survivors of the early juvenile period were those individuals who were slower-growing as larvae and smaller at settlement but grew faster during the first two weeks on the reef (figure 4). Our finding of an inverse relationship between the growth rates in the larval and early juvenile periods (first two weeks) is consistent with earlier laboratory studies (Bertram et al. 1993) and recent field experiments (McCormick & Hoey 2004), in that it demonstrates that growth rates throughout the planktonic life are not necessarily maintained during the early post-settlement period. This also suggests that changes in the direction of phenotypic selection can promote the occurrence of compensatory responses during early juvenile life (see review by Ali et al. 2003).
Figure 4.
Growth trajectory of a young fish from embryonic conditions in benthic nests, through pelagic life in the open ocean, to settlement and subsequent survival back on reef habitats. The arrows at the top of the diagram indicate the optimal growth rate at a given stage (e.g. at settlement on the reef) to ensure survival into a subsequent stage (e.g. at two weeks post-settlement). The black arrows indicate slower growth and the white arrow illustrates faster growth.
Faster growth during the first few days on the reef is expected to be advantageous by enabling initially smaller settlers to quickly outgrow high vulnerability to gape-limited predators (bigger-is-better hypothesis; Anderson 1988). However, we found that individuals who maintained a faster growth trajectory throughout the third week post-settlement were preferentially lost from the cohort (figure 4). It may be that young fish, faced with intense selective pressure to grow at a faster rate during the earlier periods of benthic life, had high foraging motivation (Nicieza & Metcalfe 1999) and may be willing to take a potentially greater predation risk for possible gains in food resources (Biro et al. 2004, 2005). If this is the case, significant changes in behaviourally mediated mortality could be expected to occur over narrow time frames.
Overall, our analyses revealed that strong size- and growth-selective mortality generally removed the larger and faster growing members of the cohort (i.e. smaller-is-better). Larval growth during planktonic life was by far the most enduring of all the traits examined, influencing survivorship of young fish settled on reef habitats. The selective loss of individuals with faster larval growth observed in the present study is counter to the prediction of the growth-rate mechanism (Anderson 1988). While the theory is supported by a large number of both field and laboratory studies, there are a growing number of examples of studies that have found that faster larval growth does not always confer greater survival benefits (e.g. Cowan et al. 1996; Fuiman et al. 2005) or detected no selective mortality based on larval growth (e.g. Searcy & Sponaugle 2001). Empirical evidence from other animal systems has also demonstrated that in some environments, individuals growing slower experience a greater advantage, in terms of survival, than do faster-growing conspecifics (e.g. amphibians, Werner 1991; mammals, Negus et al. 1992; insects, Gotthard et al. 1994; reptiles, Olsson & Shine 2002). When rapid growth entails physiological changes that lead to a reduced capacity to respond to environmental stress (Arendt 1997), the costs of growing too fast may increase under harsh conditions. So, why do some individuals still grow faster when rapid growth compromises their early survival? One possibility is that individuals follow a growth pathway defined early in their development (e.g. prior to or at hatching) and they are unable of modifying its trajectory until later in life (e.g. after settlement; see Gagliano & McCormick 2007b). In fish, where early pelagic larvae have limited or no control of the spatially and temporally variable environment to which they are exposed, the variation per se in growth trajectories among individuals of the same cohort may be adaptive. Ultimately, if this is the case, the lack of consistency in trends of selective mortality based on larval growth may be the result of masked ontogenetic changes in the form and intensity of selectivity. While this is clearly a complicating factor to our understanding of selective processes influencing early survival of young fish, unveiling changes in selective curves over different portions of the life history may ultimately enable us to better appreciate the dynamics governing the complex life cycles of many species.
Acknowledgments
We thank S. Kowalewsky, B. Higgins, A. Putz, M. Hasshoff, M. Depczynski and J. Moore who spent many hours helping us with the fish collections and dissections, and otolith preparation. We also thank C. Anderson, W. Blanckenhorn, R. Rowe, D. Schluter, A. Sinclair and particularly D. Swain for their precious advices on selection analyses. Two anonymous reviewers greatly improved an earlier version of the manuscript with their thoughtful suggestions. Financial support was provided by a JCU Doctoral Scholarship and a Nancy Vernon Rankine award to M.G. and an Australian Research Council Discovery grant to M.I.M. and M.G.M. This study was conducted under appropriate permits from the Great Barrier Reef Marine Park Authority and the JCU Animal Ethics Committee.
Supplementary Material
(a) Percent frequency of occurrence for P. amboinensis otolith size-at-age from hatching to 8 wks post-settlement (ps). Initial (white bars) and surviving portions of the targeted cohort (grey bars) are compared and relative sample sizes for each age are indicated next to the boxes (initial and surviving sample). (b) Percent frequency of occurrence for P. amboinensis otolith growth from hatching to 8 wks post-settlement (ps). Initial (white bars) and surviving portions of the targeted cohort (grey bars) are compared and relative sample sizes for each age are indicated next to the boxes (initial and surviving sample).
References
- Ali M, Nicieza A, Wootton R.J. Compensatory growth in fishes: a response to growth depression. Fish Fish. 2003;4:147–190. [Google Scholar]
- Altwegg R, Reyer H.U. Patterns of natural selection on size at metamorphosis in water frogs. Evolution. 2003;57:872–882. doi: 10.1111/j.0014-3820.2003.tb00298.x. doi:10.1554/0014-3820(2003)057[0872:PONSOS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Anderson J.T. A review of size dependent survival during pre-recruit stages of fishes in relation to recruitment. J. Northwest Atl. Fish. Soc. 1988;8:55–66. [Google Scholar]
- Anderson C. S. 1995 Calculating size-dependent relative survival from samples taken before and after selection. In Recent developments in fish otolith research, vol. 19 (eds D. H. Secor, J. M. Dean & S. E. Campana), pp. 455–466. Belle W. Baruch library in marine sciences, Columbia, SC: University of South Carolina Press.
- Arendt J.D. Adaptive intrinsic growth rates: an integration across taxa. Q. Rev. Biol. 1997;72:149–177. doi:10.1086/419764 [Google Scholar]
- Bay L.K, Buechler K, Gagliano M, Caley M.J. Intraspecific variation in the pelagic larval duration of tropical reef fishes. J. Fish Biol. 2006;68:1206–1214. doi:10.1111/j.0022-1112.2006.01016.x [Google Scholar]
- Beckerman A, Benton T.G, Ranta E, Kaitala V, Lundberg P. Population dynamic consequences of delayed life-history effects. Trends Ecol. Evol. 2002;17:263–269. doi:10.1016/S0169-5347(02)02469-2 [Google Scholar]
- Bertram D.F, Chambers R.C, Leggett W.C. Negative correlations between larval and juvenile growth rates in winter flounder: implications of compensatory growth for variation in size-at-age. Mar. Ecol. Prog. Ser. 1993;96:209–215. [Google Scholar]
- Biro P.A, Abrahams M.V, Post J.R, Parkinson E.A. Predators select against high growth rates and risk-taking behaviour in domestic trout populations. Proc. R. Soc. B. 2004;271:2233–2237. doi: 10.1098/rspb.2004.2861. doi:10.1098/rspb.2004.2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro P.A, Post J.R, Abrahams M.V. Ontogeny of energy allocation reveals selective pressure promoting risk-taking behaviour in young fish cohorts. Proc. R. Soc. B. 2005;272:1443–1448. doi: 10.1098/rspb.2005.3096. doi:10.1098/rspb.2005.3096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth D.J. Distribution changes after settlement in six species of damselfish (Pomacentridae) in One Tree Island lagoon, Great Barrier Reef. Mar. Ecol. Prog. Ser. 2002;226:157–164. [Google Scholar]
- Brodie E.D, Moore A.J, Janzen F.J. Visualizing and quantifying natural selection. Trends Ecol. Evol. 1995;10:313–318. doi: 10.1016/s0169-5347(00)89117-x. doi:10.1016/S0169-5347(00)89117-X [DOI] [PubMed] [Google Scholar]
- Cowan J.H, Houde E.D. Size-dependent predation on marine fish larvae by ctenophores, scyphomedusae, and planktivorous fish. Fish. Oceanogr. 1992;1:113–126. [Google Scholar]
- Cowan J.H, Houde E.D, Rose K.A. Size-dependent vulnerability of marine fish larvae to predation: an individual-based numerical experiment. ICES J. Mar. Sci. 1996;53:23–37. doi:10.1006/jmsc.1996.0003 [Google Scholar]
- Fuiman L.A. Vulnerability of Atlantic herring larvae to predation by yearling herring. Mar. Ecol. Prog. Ser. 1989;51:291–299. [Google Scholar]
- Fuiman L.A, Cowan J.H, Smith M.E, O'Neal J.P. Behavior and recruitment success in fish larvae: variation with growth rate and the batch effect. Can. J. Fish. Aquat. Sci. 2005;62:1337–1349. doi:10.1139/f05-053 [Google Scholar]
- Gagliano M, McCormick M.I. Maternal condition influences phenotypic selection on offspring. J. Anim. Ecol. 2007a;76:174–182. doi: 10.1111/j.1365-2656.2006.01187.x. doi:10.1111/j.1365-2656.2006.01187.x [DOI] [PubMed] [Google Scholar]
- Gagliano M, McCormick M.I. Compensating in the wild: is flexible growth the key to early juvenile survival? Oikos. 2007b;116:111–120. doi:10.1111/j.2006.0030-1299.15418.x [Google Scholar]
- Gagliano M, Kowalewsky S, McCormick M.I. An alternative method for the preservation of tropical fish larvae. J. Fish Biol. 2006;68:634–639. doi:10.1111/j.0022-1112.2006.00911.x [Google Scholar]
- Gotthard K, Nylin S, Wiklund C. Adaptive variation in growth rates: life history costs and consequences in the speckled wood butterfly, Pararge aegeria. Oecologia. 1994;99:281–289. doi: 10.1007/BF00627740. doi:10.1007/BF00627740 [DOI] [PubMed] [Google Scholar]
- Hare J.A, Cowen R.K. Size, growth, development, and survival of the planktonic larvae of Pomatomus saltatrix (Pisces: Pomatomidae) Ecology. 1997;78:2451–2431. doi:10.2307/2265903 [Google Scholar]
- Holmes T.H, McCormick M.I. Location influences size-selective predation on newly-settled reef fish. Mar. Ecol. Prog. Ser. 2006;317:203–209. [Google Scholar]
- Houde E.D. Fish early dynamics and recruitment variability. Am. Fish. Soc. Symp. 1987;2:17–29. [Google Scholar]
- James M.K, Armsworth P.R, Mason L.B, Bode L. The structure of reef fish metapopulations: modelling larval dispersal and retention patterns. Proc. R. Soc. B. 2002;269:2079–2086. doi: 10.1098/rspb.2002.2128. doi:10.1098/rspb.2002.2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins G.P, King D. Variation in larval growth can predict the recruitment of a temperate, seagrass-associated fish. Oecologia. 2006;147:641–649. doi: 10.1007/s00442-005-0336-5. doi:10.1007/s00442-005-0336-5 [DOI] [PubMed] [Google Scholar]
- Jones D.C, German R.Z. Variation in ontogeny. In: Hallgrímsson B, Hall B.K, editors. Variation: a central concept in biology. Academic Press; San Diego, CA: 2005. pp. 71–85. [Google Scholar]
- Jones G.P, Milicich M.J, Emslie M.J, Lunow C. Self-recruitment in a coral reef fish population. Nature. 1999;402:802–804. doi:10.1038/45538 [Google Scholar]
- Kerrigan B.A. Temporal patterns in size and condition at settlement in two tropical reef fishes (Pomacentridae: Pomacentrus amboinensis and P. nagasakiensis) Mar. Ecol. Prog. Ser. 1996;135:27–41. [Google Scholar]
- Leggett W.C, DeBlois E.M. Recruitment in marine fishes: is it regulated by starvation and predation in the egg and larval stages? Neth. J. Sea Res. 1994;32:119–134. doi:10.1016/0077-7579(94)90036-1 [Google Scholar]
- Litvak M.K, Leggett W.C. Age and size-selective predation on larval fishes: the bigger is better paradigm revisited. Mar. Ecol. Prog. Ser. 1992;81:13–24. [Google Scholar]
- Macpherson E, Raventos N. Settlement patterns and post-settlement survival in two Mediterranean littoral fishes: influences of early-life traits and environmental variables. Mar. Biol. 2005;148:167–177. doi:10.1007/s00227-005-0059-5 [Google Scholar]
- McCormick M.I. Consumption of coral propagules after mass spawning enhances larval quality of damselfish through maternal effects. Oecologia. 2003;136:37–45. doi: 10.1007/s00442-003-1247-y. doi:10.1007/s00442-003-1247-y [DOI] [PubMed] [Google Scholar]
- McCormick M.I. Mothers matter: crowding leads to stressed mothers and smaller offspring in marine fish. Ecology. 2006;87:1104–1109. doi: 10.1890/0012-9658(2006)87[1104:mmclts]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- McCormick M.I, Hoey A.S. Larval growth history determines juvenile growth and survival in a tropical marine fish. Oikos. 2004;106:225–242. doi:10.1111/j.0030-1299.2004.13131.x [Google Scholar]
- McCormick M.I, Makey L, Dufour V. Comparative study of metamorphosis in tropical reef fishes. Mar. Biol. 2002;141:841–853. doi:10.1007/s00227-002-0883-9 [Google Scholar]
- McGurk M.D. Natural mortality of marine fish eggs and larvae: role of spatial patchiness. Mar. Ecol. Prog. Ser. 1986;34:227–242. [Google Scholar]
- Meekan M.G, Wilson S.G, Halford A, Retzel A. A comparison of catches of fishes and invertebrates by two light trap designs, in tropical NW Australia. Mar. Biol. 2001;139:373–381. doi:10.1007/s002270100577 [Google Scholar]
- Miller T.J, Crowder L.B, Rice J.A, Marschall E.A. Larval size and recruitment mechanisms in fishes: towards a conceptual framework. Can. J. Fish. Aquat. Sci. 1988;45:1657–1670. [Google Scholar]
- Negus N.C, Berger P.J, Pinter A.J. Phenotypic plasticity of the montane vole (Microtus montanus) in unpredictable environments. Can. J. Zool. 1992;70:2121–2124. [Google Scholar]
- Nicieza A.G, Metcalfe N.B. Costs of rapid growth: the risk of aggression is higher for fast-growing salmon. Funct. Ecol. 1999;13:793–800. doi:10.1046/j.1365-2435.1999.00371.x [Google Scholar]
- Olsson M, Shine R. Growth to death in lizards. Evolution. 2002;56:1867–1870. doi: 10.1111/j.0014-3820.2002.tb00202.x. doi:10.1554/0014-3820(2002)056[1867:GTDIL]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pepin P, Shears T.H, Delafontaine Y. Significance of body size to the interaction between a larval fish (Mallotus villosus) and a vertebrate predator (Gasterosteus aculeatus) Mar. Ecol. Prog. Ser. 1992;81:1–12. [Google Scholar]
- Pfister C.A, Wang M. Beyond size: matrix projection models for populations where size is an incomplete descriptor. Ecology. 2005;86:2673–2683. [Google Scholar]
- Pitcher C.R. Validation of a technique for reconstructing daily patterns in the recruitment of coral reef damselfish. Coral Reefs. 1988;7:105–111. doi:10.1007/BF00300969 [Google Scholar]
- Raventos N, Macpherson E. Effect of pelagic larval growth and size-at-hatching on post-settlement survivorship in two temperate labrid fish of the genus Symphodus. Mar. Ecol. Prog. Ser. 2005;285:205–211. [Google Scholar]
- Robertson D.R, Petersen C.W, Brawn J.D. Lunar reproductive-cycles of benthic-brooding reef fishes: reflections of larval biology or adult biology. Ecol. Monogr. 1990;60:311–329. doi:10.2307/1943060 [Google Scholar]
- Schluter D. Estimating the form of natural selection on a quantitative trait. Evolution. 1988;42:849–861. doi: 10.1111/j.1558-5646.1988.tb02507.x. doi:10.2307/2408904 [DOI] [PubMed] [Google Scholar]
- Searcy S.P, Sponaugle S. Selective mortality during the larval–juvenile transition in two coral reef fishes. Ecology. 2001;82:2452–2470. doi:10.2307/2679928 [Google Scholar]
- Sinclair A.F, Swain D.P, Hanson J.M. Measuring changes in the direction and magnitude of size-selective mortality in a commercial fish population. Can. J. Fish. Aquat. Sci. 2002;59:361–371. doi:10.1139/f02-015 [Google Scholar]
- Sogard S.M. Size-selective mortality in the juvenile stage of teleost fishes: a review. Bull. Mar. Sci. 1997;60:1129–1157. [Google Scholar]
- Sokal R.R, Rohlf F.J. 3rd edn. W. H. Freeman; New York, NY: 2001. Biometry. [Google Scholar]
- Swain D.P. The functional basis of natural selection for vertebral traits of larvae in the stickleback Gasterosteus aculeatus. Evolution. 1992;46:987–997. doi: 10.1111/j.1558-5646.1992.tb00614.x. doi:10.2307/2409751 [DOI] [PubMed] [Google Scholar]
- Vigliola L, Meekan M.G. Size at hatching and planktonic growth determine post-settlement survivorship of a coral reef fish. Oecologia. 2002;131:89–93. doi: 10.1007/s00442-001-0866-4. doi:10.1007/s00442-001-0866-4 [DOI] [PubMed] [Google Scholar]
- Webster M.S. Role of predators in the early post-settlement demography of coral-reef fishes. Oecologia. 2002;131:52–60. doi: 10.1007/s00442-001-0860-x. doi:10.1007/s00442-001-0860-x [DOI] [PubMed] [Google Scholar]
- Wellington G.M, Victor V.B. Planktonic larval duration of one hundred species of Pacific and Atlantic damselfishes (Pomacentridae) Mar. Biol. 1989;101:557–567. doi:10.1007/BF00541659 [Google Scholar]
- Werner E.E. Nonlethal effects of a predator on competitive interactions between two anuran larvae. Ecology. 1991;72:1709–1720. doi:10.2307/1940970 [Google Scholar]
- Wilbur H.M. Complex life-cycles. Annu. Rev. Ecol. Syst. 1980;11:67–93. doi:10.1146/annurev.es.11.110180.000435 [Google Scholar]
- Wilson D.T, McCormick M.I. Spatial and temporal validation of settlement-marks in the otoliths of tropical reef fishes. Mar. Ecol. Prog. Ser. 1997;153:259–271. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a) Percent frequency of occurrence for P. amboinensis otolith size-at-age from hatching to 8 wks post-settlement (ps). Initial (white bars) and surviving portions of the targeted cohort (grey bars) are compared and relative sample sizes for each age are indicated next to the boxes (initial and surviving sample). (b) Percent frequency of occurrence for P. amboinensis otolith growth from hatching to 8 wks post-settlement (ps). Initial (white bars) and surviving portions of the targeted cohort (grey bars) are compared and relative sample sizes for each age are indicated next to the boxes (initial and surviving sample).