Abstract
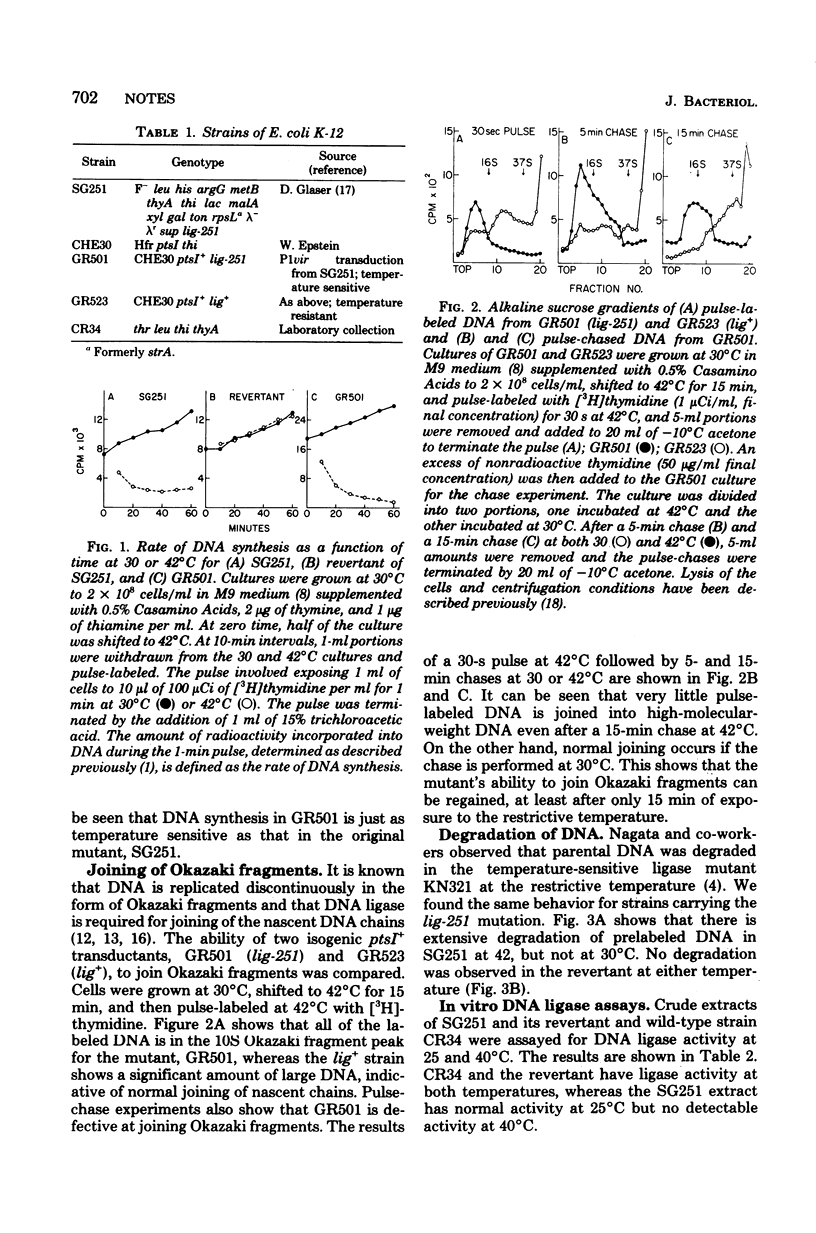
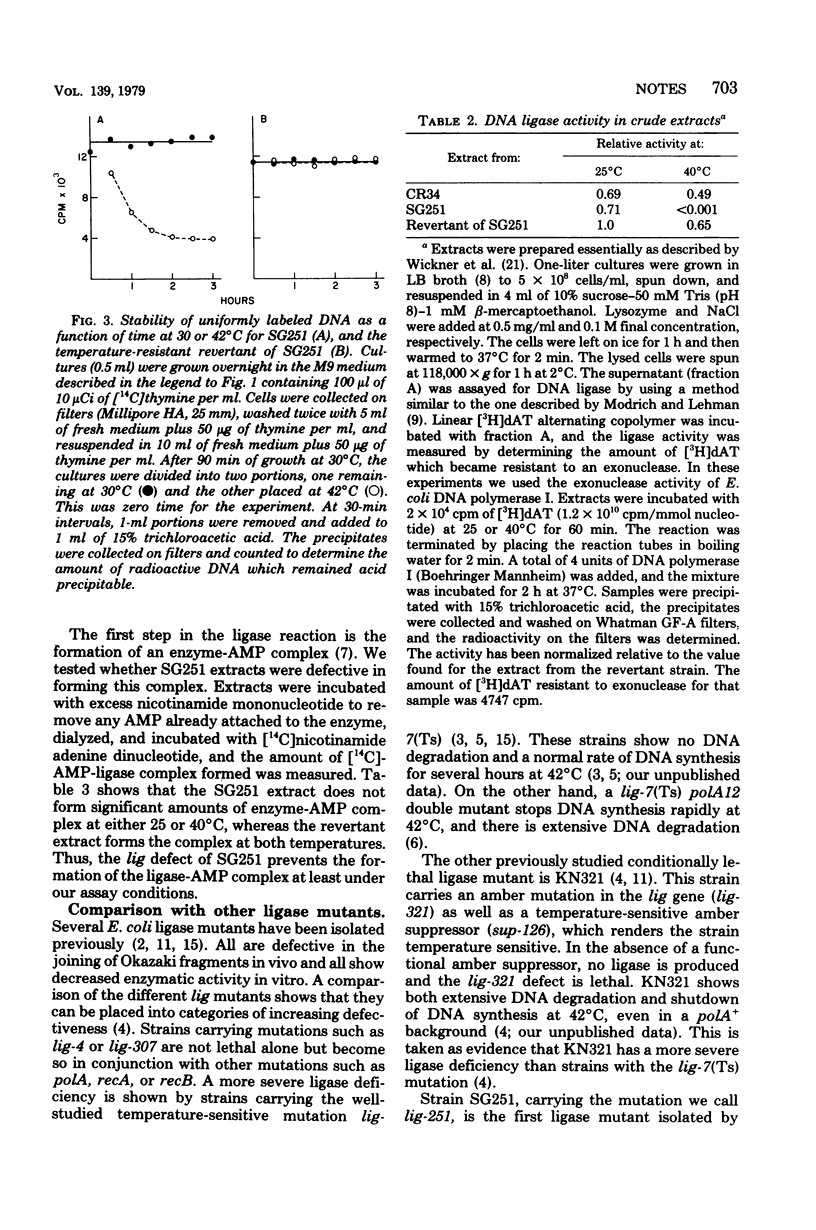
A new Escherichia coli deoxyribonucleic acid (DNA) ligase mutant has been identified among a collection of temperature-sensitive DNA replication mutants isolated recently (Sevastopoulos, Wehr, and Glaser, Proc. Natl. Acad. Sci. U.S.A. 74:3485-3489, 1977). At the nonpermissive temperature DNA synthesis in the mutant stops rapidly, the DNA is degraded to acid-soluble material, and cell death ensures. This suggests that the mutant may be among the most ligase-deficient strains yet characterized.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dermody J. J., Bourguignon G. J., Foglesong P. D., Sternglanz R. Nalidixic acid-sensitive and resistant modes of DNA replication in Escherichia coli. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1340–1347. doi: 10.1016/s0006-291x(74)80431-6. [DOI] [PubMed] [Google Scholar]
- Gellert M., Bullock M. L. DNA ligase mutants of Escherichia coli. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1580–1587. doi: 10.1073/pnas.67.3.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M. M., Hicks M. L., Gellert M. Genetics and function of DNA ligase in Escherichia coli. J Mol Biol. 1973 Jul 15;77(4):531–547. doi: 10.1016/0022-2836(73)90221-0. [DOI] [PubMed] [Google Scholar]
- Horiuchi T., Sato T., Nagata T. DNA degradation in an amber mutant of Escherichia coli K12 affecting DNA ligase and viability. J Mol Biol. 1975 Jun 25;95(2):271–287. doi: 10.1016/0022-2836(75)90395-2. [DOI] [PubMed] [Google Scholar]
- Konrad E. B., Modrich P., Lehman I. R. DNA synthesis in strains of Escherichia coli K12 with temperature-sensitive DNA ligase and DNA polymerase I. J Mol Biol. 1974 Nov 25;90(1):115–126. doi: 10.1016/0022-2836(74)90260-5. [DOI] [PubMed] [Google Scholar]
- Konrad E. B., Modrich P., Lehman I. R. Genetic and enzymatic characterization of a conditional lethal mutant of Escherichia coli K12 with a temperature-sensitive DNA ligase. J Mol Biol. 1973 Jul 15;77(4):519–529. doi: 10.1016/0022-2836(73)90220-9. [DOI] [PubMed] [Google Scholar]
- Little J. W., Zimmerman S. B., Oshinsky C. K., Gellert M. Enzymatic joining of DNA strands, II. An enzyme-adenylate intermediate in the dpn-dependent DNA ligase reaction. Proc Natl Acad Sci U S A. 1967 Nov;58(5):2004–2011. doi: 10.1073/pnas.58.5.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrich P., Lehman I. R. Enzymatic characterization of a mutant of Escherichia coli with an altered DNA ligase. Proc Natl Acad Sci U S A. 1971 May;68(5):1002–1005. doi: 10.1073/pnas.68.5.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrich P., Lehman I. R. Enzymatic joining of polynucleotides. IX. A simple and rapid assay of polynucleotide joining (ligase) activity by measurement of circle formation from linear deoxyadenylate-deoxythymidylate copolymer. J Biol Chem. 1970 Jul 25;245(14):3626–3631. [PubMed] [Google Scholar]
- Nagata T., Horiuchi T. An amber dna mutant of Escherichia coli K12 affecting DNA ligase. J Mol Biol. 1974 Aug 5;87(2):369–373. doi: 10.1016/0022-2836(74)90156-9. [DOI] [PubMed] [Google Scholar]
- Newman J., Hanawalt P. Intermediates in T4 DNA replication in a T4 ligase deficient strain. Cold Spring Harb Symp Quant Biol. 1968;33:145–150. doi: 10.1101/sqb.1968.033.01.018. [DOI] [PubMed] [Google Scholar]
- Olivera B. M., Lehman I. R. Linkage of polynucleotides through phosphodiester bonds by an enzyme from Escherichia coli. Proc Natl Acad Sci U S A. 1967 May;57(5):1426–1433. doi: 10.1073/pnas.57.5.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauling C., Hamm L. Properties of a temperature-sensitive, radiation-sensitive mutant of Escherichia coli. II. DNA replication. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1195–1202. doi: 10.1073/pnas.64.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C. C., Masamune Y., Live T. R., Jacquemin-Sablon A., Weiss B., Fareed G. C. Studies on the joining of DNA by polynucleotide ligase of phage T4. Cold Spring Harb Symp Quant Biol. 1968;33:151–164. doi: 10.1101/sqb.1968.033.01.019. [DOI] [PubMed] [Google Scholar]
- Sevastopoulos C. G., Wehr C. T., Glaser D. A. Large-scale automated isolation of Escherichia coli mutants with thermosensitive DNA replication. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3485–3489. doi: 10.1073/pnas.74.8.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternglanz R., Wang H. F., Donegan J. J. Evidence that both growing DNA chains at a replication fork are synthesized discontinuously. Biochemistry. 1976 May 4;15(9):1838–1843. doi: 10.1021/bi00654a008. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Genetic analysis of non-essential bacteriophage T7 genes. J Mol Biol. 1973 Sep 15;79(2):227–236. doi: 10.1016/0022-2836(73)90002-8. [DOI] [PubMed] [Google Scholar]
- Wechsler J. A., Gross J. D. Escherichia coli mutants temperature-sensitive for DNA synthesis. Mol Gen Genet. 1971;113(3):273–284. doi: 10.1007/BF00339547. [DOI] [PubMed] [Google Scholar]
- Wickner W., Brutlag D., Schekman R., Kornberg A. RNA synthesis initiates in vitro conversion of M13 DNA to its replicative form. Proc Natl Acad Sci U S A. 1972 Apr;69(4):965–969. doi: 10.1073/pnas.69.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]


