Abstract
Some of the most spectacular exaggerated sexual ornaments are carotenoid dependent. It has been suggested that such ornaments have evolved because carotenoid pigments are limiting for both signal expression and in their role as antioxidants and immunostimulants. An implicit assumption of this hypothesis is that males which can afford to produce more elaborate carotenoid-dependent displays are signalling their enhanced ability to resist parasites, disease or oxidative stress and hence would be predicted to live longer. Therefore, in species with carotenoid-dependent ornaments where a parent's future longevity is crucial for determining offspring survival, there should be a mating preference for partners that present the lowest risk of mortality during the breeding attempt, as signalled by the ability to allocate carotenoids to sexual displays. In an experimental study using three-spined sticklebacks (Gasterosteus aculeatus), we show that when dietary carotenoid intake is limited, males attempt to maintain their sexual ornament at the expense of body carotenoids and hence suffer from reduced reproductive investment and a shorter lifespan. These males also suffer from an increased susceptibility to oxidative stress, suggesting that this may constitute the mechanism underlying the increased rate of ageing. Furthermore, in pairwise mate-choice trials, females preferred males that had a greater access to carotenoids and chance of surviving the breeding season, suggesting that females can make adaptive mate choice decisions based on a male's carotenoid status and potential future longevity.
Keywords: oxidative stress, reactive oxygen species, sexual selection, female preference
1. Introduction
It is widely accepted that there is a negative effect of current reproductive effort on subsequent survival (Dufresne et al. 1990; Lessels 1991; Roff 1992; Stearns 1992; Nilsson & Svensson 1996). This is usually assumed to reflect the existence of resource limitations, giving rise to a physiological trade-off between investment in reproduction and somatic maintenance (Kirkwood & Austad 2000; Mangel 2001). However, the underlying mechanisms are poorly understood, and in particular it is not clear which resources are the basis for this trade-off. Ecologists have tended to assume that energy is the primary limitation (e.g. by using it as the currency in most foraging models), but there is now increasing recognition that other nutrients may play a key role.
Carotenoid pigments have attracted considerable interest owing to their widespread role in sexual signalling and their importance as antioxidants and immunostimulants (Olson & Owens 1998). Since they cannot be synthesized de novo by vertebrates, and so must be obtained through the diet, carotenoid supply may be limiting, giving rise to trade-offs in their allocation within the body. Indeed, it has been suggested that carotenoid-based sexual ornaments may have evolved because carotenoids are limiting for both ornament expression and in their roles as immunomodulants and antioxidants (Lozano 1994; von Schantz et al. 1999). A key prediction of this hypothesis is that more elaborately ornamented individuals, which can afford to allocate more carotenoids to signalling, should have a greater capacity to resist parasites, diseases or oxidative stress (Lozano 1994), leading us to predict that such individuals should also live longer. Therefore, in species with carotenoid-dependent ornaments where a parent's future longevity is crucial for determining offspring survival, there should be a mating preference for partners with the greatest likelihood of surviving the breeding attempt, as signalled by the ability to allocate carotenoids to sexual displays. Recent experimental studies have confirmed that males with greater access to carotenoids have more colourful ornamental displays, better antioxidant defences and can mount larger immune responses (e.g. Blount et al. 2003; McGraw & Ardia 2003; Kolluru et al. 2006; Kurtz et al. 2006; but see Navara & Hill 2003). However, evidence of a link between carotenoid supply and adult survival has so far been correlational (Hill 1991; Hõrak et al. 2001) and no previous study has explicitly tested how carotenoid supply may influence resistance to senescence or longevity, and how these competing demands affect their ability to allocate carotenoids to sexual signals. This study is the first to address these questions.
The three-spined stickleback (Gasterosteus aculeatus) is an ideal species in which to investigate carotenoid trade-offs between sexual signalling and somatic maintenance: red male nuptial coloration is based on carotenoids that can be limiting in the diet (Wedekind et al. 1998; Barber et al. 2000) and females most often prefer redder males (reviewed by Rowland 1994). Males build a nest in which a female will deposit all her eggs, and all subsequent parental care (including intensive fanning of the eggs and guarding of the nest and fry) is by the male. Both sexes might participate in several breeding attempts within a single breeding season, but reproduction is costly, and in annual populations most fish senesce and die before the end of the season (Chellappa et al. 1989; Poizat et al. 1999). Reproductive effort in each breeding attempt is influenced by this short-life expectancy, with individuals of both sexes investing more in reproduction as future reproductive opportunities decline (Candolin 1998; Poizat et al. 1999). For males in particular, reproductive activities are energetically expensive (Chellappa et al. 1989; Fitzgerald et al. 1989) and males in poor condition may die during the parental phase and thus fail to rear the offspring to independence or they may eat the eggs in their nest in order to improve their physical condition (Whoriskey & FitzGerald 1994). Given this breeding system and the rapid rate of senescence, there should be selection pressure on females to choose males who are capable of remaining alive and healthy for the duration of parental care. In this experimental study, we tested the hypotheses that (i) carotenoid availability limits reproductive rate and lifespan and (ii) a carotenoid-based ornament reveals potential lifespan, by feeding sticklebacks nutritionally identical food to which we added either high or low (but biologically realistic) levels of carotenoids.
2. Material and methods
(a) Study animals
Juvenile three-spined sticklebacks were captured with dip nets from the River Endrick, Scotland (56°04′ N, 4°23′ W) during November 2004. This is an annual population, the overwhelming majority of fish dying after a single spawning season (N. B. Metcalfe 2004, personal observation). After capture, individual fish were allocated randomly to 1 of 12 holding aquaria, each containing only a water filter and several artificial plants (to provide refuges and reduce stress), and held until the start of the breeding season. Throughout this time, fish were fed to satiation daily on a customized diet containing either high (200 μg carotenoids g−1 food) or low (10 μg g−1) levels of carotenoids (astaxanthin, lutein and zeaxanthin; see electronic supplementary material for full details of dietary manipulation). 2-Chloro-4,6-bis-(ethylamino)-s-triazine (Algae Destroyer, Aquarium Pharmaceuticals) was added to the water to control algal growth and the salinity adjusted (with seawater) to approximately 0.4 ppt to prevent the risk of whitespot (Ichthyophthirius multifiliis) infection. Throughout the experiment, the temperature and photoperiod were adjusted weekly to match those at the source river. None of the fish exhibited external signs of parasitic infection.
(b) Experimental procedures
Holding tanks were checked daily and when males began to develop blue eye coloration (an indicator of sexual maturation), they were transferred to individual experimental aquaria (33×18×19 cm), where they were maintained on the same experimental diets. The experimental aquaria contained only a filter and an artificial plant and had the same photoperiod, temperature and water conditions as the holding aquaria. Individual aquaria were separated by opaque partitions, so males were not in visual or olfactory contact with each other.
When a sufficient number of males had reached sexual maturation, a nesting dish filled with 1 cm sand and around one hundred 5 cm long strands of polyester thread as nesting material were added to each aquarium. In order to stimulate nest building, each male was shown a gravid female enclosed in a Plexiglas container for 5 min twice a day for 10 days, after which time all males had completed nest building, developed red nuptial coloration and entered the courtship phase. They were then shown a gravid female for 10 min, immediately netted, anaesthetized (with benzocaine) and their standard length (±0.01 mm) and wet mass (±0.001 g) were determined. A random sample of these males (high-carotenoid males, n=17 and low-carotenoid males, n=14) were then sacrificed with an overdose of anaesthetic, photographed using a standardized technique (Frischknecht 1993; Candolin 1999) to determine a ‘redness’ score (measured as the proportion of colour attributable to the red region of the spectrum; see electronic supplementary material) and the region of skin containing the nuptial coloration immediately removed (Wedekind et al. 1998) and weighed (±0.001 g). These males, along with a further random sample of high- (n=12) and low-carotenoid diet (n=8) females, were then dissected to remove the stomach, intestine and, for females, any eggs, and each carcass weighed (±0.001 g) and homogenized in phosphate-buffered saline (containing 1.15% w/v potassium chloride) at 5% w/v. All samples were then snap-frozen in liquid nitrogen and stored separately, under nitrogen gas, at −80°C in the dark for up to six months until analysis. Methods for the high-performance liquid chromatography (HPLC) quantification of carotenoids and the determination of susceptibility to oxidative stress (using malondialdehyde (MDA), a secondary product of lipid peroxidation) are given in the electronic supplementary material.
For the remaining males, experimental aquaria were then rearranged into pairs (n=17 pairs) so that each male with a nest was next to a size-matched male of the opposite diet treatment. Neither body weight (paired t-test: t16=1.20, p=0.25) nor length (t16=0.93, p=0.36) differed between pairs, and left- and right-hand sides were randomized. Female preference between these paired males was tested once in the initial breeding round using an established protocol (Milinski & Bakker 1990) in a dichotomous choice design. A 33×18×19 cm aquarium tank, divided into two equally sized ‘choice zones’ by a 9 cm opaque partition protruding from the front wall, containing a single female was placed immediately in front of each pair of males. After 1 min of acclimatization, it was observed for 5 min and the time spent in front of each male (in the ‘choice zone’) was recorded and used to calculate female preference (expressed as the time spent with the high-carotenoid male as a proportion of the time spent with both males). Trait preferences recorded using this method have been shown to correspond to those shown by females that are actually able to spawn with their preferred male (Cubillos & Guderley 2000), although females were not allowed to spawn in this experiment. After the mate-choice trial, nesting material was removed. After 5 days, it was replaced for 10 days and the cycle was repeated continuously, using novel stimulatory females, until all males stopped building nests. The males were then monitored (while remaining on experimental diets) until they died. True birth dates were unknown because the fish were wild-caught as underyearlings and lifespans are therefore expressed as days from the start of the experiment (12 November 2004). All experiments were performed under license from the UK Home Office.
(c) Statistical analyses
All continuous data met the assumptions of normality and hence were analysed using either ANOVA, t-tests or, in the case of lifespan data, Cox regression. Non-continuous data were analysed using suitable non-parametric statistics. Proportional data were arcsine-square root transformed prior to analysis. Post hoc pairwise comparisons were performed using sequentially Bonferroni-adjusted (α=0.10) independent sample t-tests. All statistical tests were two-tailed. n denotes the sample size and mean values are presented ±s.e.
3. Results
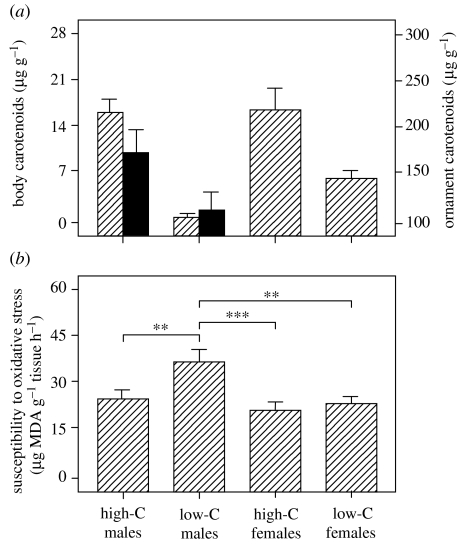
Fish raised on the low-carotenoid diet had significantly lower concentrations of carotenoids in their body than high-carotenoid diet fish (effect of diet: F1,47=40.07, p<0.001; sex: F1,47=2.32, p=0.14; diet×sex interaction: F1,47=1.60, p=0.21; figure 1a). Furthermore, when we looked at the effect of diet on the expression of nuptial coloration, we found that males on the low-carotenoid diet had significantly reduced standardized coloration scores, measured from photographs (high-carotenoid males: 0.75±0.01; low-carotenoid males: 0.71±0.01; t29=2.97, p=0.006), and tended to deposit less carotenoids in their ornament than high-carotenoid males, although the difference between the two groups was not statistically significant (t29=1.81, p=0.081; figure 1a).
Figure 1.
(a) Mean+s.e. total concentration of carotenoids in the body of males and females (hashed bars) and the nuptial coloration of males (black bars) and (b) mean+s.e. susceptibility to oxidative stress (measured in terms of MDA, a by-product of lipid peroxidation) in males and females fed on either high- (high-C) or low- (low-C) carotenoid diets. **p<0.01, ***p<0.001.
The concentration of carotenoids contained in the area of nuptial coloration outweighed body carotenoid concentrations by a factor of 15.8, on average (figure 1a), suggesting that the maintenance of nuptial coloration may impose considerable costs. We therefore explored whether low-carotenoid males were paying a higher price in an attempt to maintain their sexual coloration. Consistent with this, males on the low-carotenoid diet invested a significantly greater proportion of their total pool of carotenoids (the sum of carotenoids in the sexual signal and body) in nuptial coloration (80.7±5.5%) when compared with high-carotenoid males (20.9±2.6%; t29=10.45, p<0.001).
Susceptibility to oxidative stress depended on dietary carotenoid intake, but this effect differed between the sexes (effect of diet: F1,47=7.51, p=0.009; sex: F1,47=10.48, p=0.002; diets×sex interaction: F1,47=4.10, p=0.049; figure 1b). In accordance with the hypothesis that low-carotenoid availability can increase an individual's susceptibility to oxidative stress and that there is a trade-off between signalling and antioxidant protection, post hoc tests revealed that males on the low-carotenoid diet showed a significantly greater susceptibility to oxidative stress than either high-carotenoid males or females, or low-carotenoid females. None of the other groups differed in their susceptibility (figure 1b).
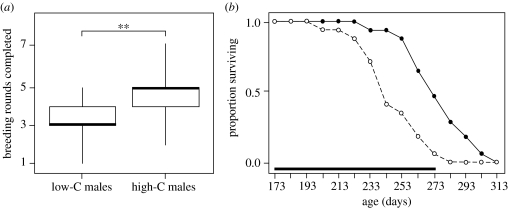
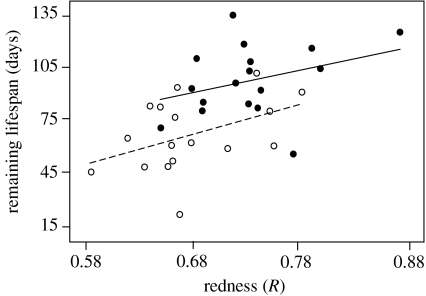
Low-carotenoid males completed significantly fewer breeding rounds than high-carotenoid males (Mann–Whitney test: w17=217.5, p=0.005; figure 2a). In addition, while there was no effect of initial body weight on longevity (Cox regression: Χ12=0.05, p=0.82), males on the low-carotenoid diet had significantly shorter lifespans than those on the high-carotenoid diet (Χ12=9.56, p=0.002; figure 2b) with the great majority (93%) of low-carotenoid diet males dying during the breeding season when compared with only about half (54%) of the high-carotenoid diet males (figure 2b). Furthermore, a male's lifespan was significantly predicted by its standardized coloration score (ANCOVA, diet treatment group: F1,31=8.59, p=0.006; coloration score: F1,31=6.75, p=0.014; figure 3), with redder males living longer than less red males.
Figure 2.
(a) Distribution of breeding rounds completed by males on the high- (high-C) and low- (low-C) carotenoid diets (the median is indicated by the thick line), and (b) survival curves for males in the high- (filled circles and solid line) and low- (open circles and dashed line) carotenoid diet groups. Age is expressed as time in days since the start of the dietary manipulation. The duration of the breeding season (from date of first to date of last nest built by any male) is indicated by the horizontal bar. **p<0.01.
Figure 3.
The relationship between signal redness and remaining lifespan for males on the high- (filled circles and solid line) and low- (open circles and dashed line) carotenoid diets. Lines of least squares are shown for each diet treatment group (high carotenoids: r2=0.14; low carotenoids: r2=0.18; see text for details).
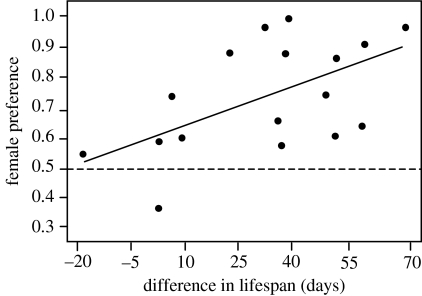
In pairwise choice tests, females exhibited a significant preference for males on the high-carotenoid diet (mean±s.e. proportion of time spent with the high-carotenoid male: 0.74±0.05; one-sample t-test against a test mean of 0.5 (no preference): t16=5.25, p<0.001; figure 4). Moreover, there was a significant positive correlation between the proportion of time that a female spent with the high-carotenoid male and the difference in subsequent longevity between two males that it saw (F1,15=5.30, p=0.036; figure 4), suggesting that females were preferring to associate with males which ultimately lived longest.
Figure 4.
The relationship between female preference (the proportion of time spent with the high-carotenoid male) and the difference in subsequent longevity between the high- and low-carotenoid males it saw. The dashed line represents no preference. The line of least squares is shown (see text for details).
4. Discussion
The results of this study on three-spined sticklebacks support our hypothesis that limited access to dietary carotenoids can constrain reproductive output and lifespan, and that females exhibit a mating preference for males with the greatest future longevity. In species where adults provide an extended period of parental care, during which there is a high risk of mortality, there should be strong selection pressure to choose a mate who is capable of remaining alive and healthy long enough to successfully complete the rearing of their offspring. Consistent with this, we have shown that in this population females preferred males with access to greater amounts of carotenoids and these males completed more nest-building rounds and ultimately lived longer. These results would be predicted based on earlier correlational evidence of a relationship between carotenoid coloration and survival/lifespan in birds (Hill 1991; Hõrak et al. 2001), and suggest that females are able to maximize their chances of mating with a male who will survive the parental phase and so complete rearing their offspring to independence.
Males on the high-carotenoid diet had redder carotenoid-based sexual ornaments than low-carotenoid diet males, which is consistent with the hypothesis that future survival prospects may be signalled through the intensity of nuptial coloration. This coloration may have been the basis for female choice. However, we cannot rule out the possibility that other cues may also have been used, such as the vigour of a male's courtship display (Wootton 1985). Conceivably, exercise tolerance could be affected by carotenoid supply through effects on susceptibility to fatigue or parasites and diseases (Blount & Matheson 2006). Whatever trait, or suite of traits, were the focus of female choice, our experimental data clearly show that high-carotenoid diet males were preferred by females and lived longer.
Consistent with previous theories concerning the evolution of carotenoid-based sexual signals (Lozano 1994; von Schantz et al. 1999), in this study low-carotenoid diet males traded off future reproductive opportunities for a large investment in current reproduction, diverting carotenoids away from somatic maintenance in order to increase their sexual coloration in an attempt to maintain sexual attractiveness. In fact, the negative effects of low-carotenoid availability were exacerbated by the trade-off between using carotenoids for somatic maintenance and allocating them to reproduction, since low-carotenoid diet females (which do not have the costs of producing and maintaining carotenoid-rich nuptial coloration and did not spawn in this experiment) had more body carotenoids than males on the same diet, and unlike the males showed no significant increase in their susceptibility to oxidative stress when compared with fish fed high-carotenoid diets. It thus appears that low-carotenoid diet males were attempting to cheat by allocating more carotenoids to their nuptial coloration, at the expense of their health. However, the deception was inadequate since they failed to achieve levels of redness as great as that of high-carotenoid males, and so the signal remained honest. The rapidly increasing mortality rate of low-carotenoid diet males means that they would not be able to maintain their investment in sexual ornamentation through the breeding season; previous research has indeed shown that the male's red throat becomes a more honest signal of his gross nutritional reserves as the season progresses (Candolin 2000), as predicted by theory (Proulx et al. 2002). Given the high rate of extrinsic mortality in many stickleback populations (Wootton 1985), it is likely that males with poor access to carotenoids would achieve higher fitness by investing in sexual signalling at the expense of survival than by investing in somatic maintenance and forfeiting the current breeding round. However, in populations with low extrinsic mortality, where the probability of survival to subsequent breeding rounds is higher, the latter strategy is likely to predominate.
The mechanism causing the accelerated rate of ageing in low-carotenoid diet males may have been an increased susceptibility to oxidative stress, as revealed by their elevated body MDA levels (see also Nakano et al. 1999; Alonso-Alvarez et al. 2004; Bertrand et al. 2006). Antioxidants, including carotenoids (Burton 1989; Kiokias & Gordon 2004), are used to inactivate reactive oxygen species (ROS) and so protect DNA, proteins and lipids from oxidation. Inadequate access to such antioxidants allows the accumulation of damage to biomolecules caused by ROS (i.e. conditions of ‘oxidative stress’), and it is this damage that is thought to underlie the ageing process through its effect on cellular senescence (Harman 1991; Sohal et al. 1994; Beckman & Ames 1998; Finkel & Holbrook 2000). It would be impossible to prove a direct relationship between an individual stickleback's susceptibility to oxidative stress and lifespan, because such intervention would seriously impair reproductive behaviour, physiology and lifespan itself although this link is strongly suggested by our data. It is possible, however, that a lack of carotenoids impaired the immune system of low-carotenoid diet males to a degree that they succumbed to disease or parasites (Milinski & Bakker 1990; Lozano 1994).
The finding that males with a lower dietary intake of carotenoids produced fewer red ornaments, despite diverting carotenoids from essential somatic maintenance, demonstrates that the stickleback's carotenoid-dependent sexual ornament acts as an honest signal of health and potential lifespan (and hence ability to provide parental care). Our data thus provide strong support for the hypothesis that trade-offs limit the expression of carotenoid-based signals (Lozano 1994) and provide the first evidence that such trade-offs might impact lifespan. Given the widespread use of carotenoid coloration in nature (Olson & Owens 1998), this finding could be relevant across a range of taxa. In particular, in any short-lived species where there is a fitness cost to rearing offspring in the absence of the father (as in many monogamous birds; Bart & Tornes 1989) or where there is obligate paternal care (the case in many teleosts), there would be selection pressure on females to accurately evaluate a male's future longevity. For the opposite reasons, selection for the ability to judge the lifespan of potential mates is likely to be relaxed in longer lived species. Thus species-specific life expectancies may both limit the taxonomic distribution of carotenoid-based sexual signals and provide the founding impetus for their evolution.
Acknowledgments
We thank Stirling University's Institute of Aquaculture for providing the antioxidant-free food, J. Laurie and G. Adam for animal husbandry, W. Mullen and A. Adams for their help with the chemical analyses and P. Monaghan, J. A. Endler, A. J. Moore and two anonymous referees for their helpful comments on the manuscript. The work was funded by a grant from the Natural Environment Research Council (to N.B.M, J.D.B and J.L.). J.D.B. was supported by a Royal Society University Research Fellowship. This research adhered to the Association for the Study of Animal Behaviour Guidelines for the Use of Animals in Research and was performed under licence from the UK Home Office.
Supplementary Material
This file contains further details on the preparation of the experimental diets, photographic analysis of ornament coloration, high-performance liquid chromatography (HPLC) of carotenoids and determination of susceptibility to oxidative stress
References
- Alonso-Alvarez C, Bertrand S, Devevey G, Gaillard M, Prost J, Faivre B, Sorci G. An experimental test of the dose-dependent effect of carotenoids and immune activation on sexual signals and antioxidant activity. Am. Nat. 2004;164:651–659. doi: 10.1086/424971. doi:10.1086/424971 [DOI] [PubMed] [Google Scholar]
- Barber I, Arnott S.A, Braithwaite V.A, Andrew J, Mullen W, Huntingford F.A. Carotenoid-based sexual coloration and body condition in nesting male sticklebacks. J. Fish Biol. 2000;57:777–790. doi:10.1111/j.1095-8649.2000.tb00274.x [Google Scholar]
- Bart J, Tornes A. Importance of monogamous male birds in determining reproductive success—evidence for house wrens and a review of male-removal studies. Behav. Ecol. Sociobiol. 1989;24:109–116. doi:10.1007/BF00299642 [Google Scholar]
- Beckman K.B, Ames B.N. The free radical theory of aging matures. Phys. Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- Bertrand S, Alonso-Alvarez C, Devevey G, Faivre B, Prost J, Sorci G. Carotenoids modulate the trade-off between egg production and resistance to oxidative stress in zebra finches. Oecologia. 2006;147:576–584. doi: 10.1007/s00442-005-0317-8. doi:10.1007/s00442-005-0317-8 [DOI] [PubMed] [Google Scholar]
- Blount J.D, Matheson S. Effects of carotenoid supply on escape-flight responses in zebra finches Taeniopygia guttata. Anim. Behav. 2006;72:595–601. doi:10.1016/j.anbehav.2005.11.014 [Google Scholar]
- Blount J.D, Metcalfe N.B, Birkhead T.R, Surai P.F. Carotenoid modulation of immune function and sexual attractiveness in zebra finches. Science. 2003;300:125–127. doi: 10.1126/science.1082142. doi:10.1126/science.1082142 [DOI] [PubMed] [Google Scholar]
- Burton G.W. Antioxidant actions of carotenoids. J. Nutr. 1989;119:109–111. doi: 10.1093/jn/119.1.109. [DOI] [PubMed] [Google Scholar]
- Candolin U. Reproduction under predation risk and the trade-off between current and future reproduction in the threespine stickleback. Proc. R. Soc. B. 1998;265:1171–1175. doi:10.1098/rspb.1998.0415 [Google Scholar]
- Candolin U. The relationship between signal quality and physical condition: is sexual signalling honest in the three-spined stickleback? Anim. Behav. 1999;58:1261–1267. doi: 10.1006/anbe.1999.1259. doi:10.1006/anbe.1999.1259 [DOI] [PubMed] [Google Scholar]
- Candolin U. Changes in expression and honesty of sexual signalling over the reproductive lifetime of sticklebacks. Proc. R. Soc. B. 2000;267:2425–2430. doi: 10.1098/rspb.2000.1301. doi:10.1098/rspb.2000.1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellappa S, Huntingford F.A, Strang R.H.C, Thomson R.Y. Annual variation in energy reserves in male 3-spined stickleback, Gasterosteus aculeatus L. (Pisces, Gasterosteidae) J. Fish Biol. 1989;35:275–286. doi:10.1111/j.1095-8649.1989.tb02976.x [Google Scholar]
- Cubillos E.R, Guderley H.E. Analysis of the factors related with mate choice and reproductive success in male three-spined sticklebacks. J. Fish Biol. 2000;56:1201–1216. doi:10.1111/j.1095-8649.2000.tb02134.x [Google Scholar]
- Dufresne F, FitzGerald G.J, Lachance S. Age and size-related differences in reproductive success and reproductive cost in threespine stickleback. Behav. Ecol. 1990;1:140–147. doi:10.1093/beheco/1.2.140 [Google Scholar]
- Finkel T, Holbrook N.J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. doi:10.1038/35041687 [DOI] [PubMed] [Google Scholar]
- Fitzgerald G.J, Guderley H, Picard P. Hidden reproductive costs in threespined stickleback (Gasterosteus aculeatus L.) J. Exp. Biol. 1989;48:295–300. [PubMed] [Google Scholar]
- Frischknecht M. The breeding coloration of male threespined sticklebacks (Gasterosteus aculeatus) as an indicator of energy investment in vigour. Evol. Ecol. 1993;7:439–450. doi:10.1007/BF01237640 [Google Scholar]
- Harman D. Aging—a theory based on free-radical and radiation-chemistry. J. Gerontol. 1991;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Hill G.E. Plumage coloration is a sexually selected indicator of male quality. Nature. 1991;350:337–339. doi:10.1038/350337a0 [Google Scholar]
- Hõrak P, Ots I, Vellau H, Spottiswoode C, Møller A.P. Carotenoid-based plumage coloration reflects hemoparasite infection and local survival in breeding great tits. Oecologia. 2001;126:166–173. doi: 10.1007/s004420000513. doi:10.1007/s004420000513 [DOI] [PubMed] [Google Scholar]
- Kiokias S, Gordon M.H. Antioxidant properties of carotenoids in vitro and in vivo. Food Rev. Int. 2004;20:99–121. doi:10.1081/FRI-120037155 [Google Scholar]
- Kirkwood T.B.L, Austad S.N. Why do we age? Nature. 2000;408:233–238. doi: 10.1038/35041682. doi:10.1038/35041682 [DOI] [PubMed] [Google Scholar]
- Kolluru G.R, Grether G.F, South S.H, Dunlop E, Cardinali A, Liu L, Carapiet A. The effects of carotenoid and food availability on resistance to a naturally occurring parasite (Gyrodactylus turnbulli) in guppies (Poecilia reticulata) Biol. J. Linn. Soc. 2006;89:301–309. doi:10.1111/j.1095-8312.2006.00675.x [Google Scholar]
- Kurtz J, Wegner K.M, Kalbe M, Reusch T.B.H, Schaschl H, Hasselquist D, Milinski M. MHC genes and oxidative stress in sticklebacks: an immuno-ecological approach. Proc. R. Soc. B. 2006;273:1407–1414. doi: 10.1098/rspb.2005.3450. doi:10.1098/rspb.2005.3450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessels C.M. The evolution of life histories. In: Krebs J.R, Davies N.B, editors. Behavioural ecology. Blackwell; Oxford, UK: 1991. pp. 32–68. [Google Scholar]
- Lozano G.A. Carotenoids, parasites, and sexual selection. Oikos. 1994;70:309–311. doi:10.2307/3545643 [Google Scholar]
- Mangel M. Complex adaptive systems, aging and longevity. J. Theor. Biol. 2001;213:559–571. doi: 10.1006/jtbi.2001.2431. doi:10.1006/jtbi.2001.2431 [DOI] [PubMed] [Google Scholar]
- McGraw K.J, Ardia D.R. Carotenoids, immunocompetence, and the information content of sexual colors: an experimental test. Am. Nat. 2003;162:704–712. doi: 10.1086/378904. doi:10.1086/378904 [DOI] [PubMed] [Google Scholar]
- Milinski M, Bakker T.C.M. Female sticklebacks use male coloration in mate choice and hence avoid parasitized males. Nature. 1990;344:330–333. doi:10.1038/344330a0 [Google Scholar]
- Nakano T, Kanmuri T, Sato M, Takeuchi M. Effect of astaxanthin rich red yeast (Phaffia rhodozyma) on oxidative stress in rainbow trout. Biochem. Biophys. Acta. 1999;1426:119–125. doi: 10.1016/s0304-4165(98)00145-7. doi:10.1016/S0304-4165(98)00145-7 [DOI] [PubMed] [Google Scholar]
- Navara K.J, Hill G.E. Dietary carotenoid pigments and immune function in a songbird with extensive carotenoid-based plumage coloration. Behav. Ecol. 2003;14:909–916. doi:10.1093/beheco/arg085 [Google Scholar]
- Nilsson J.-Å, Svensson E. The cost of reproduction: a new link between current reproductive effort and future reproductive success. Proc. R. Soc. B. 1996;263:711–714. doi:10.1098/rspb.1996.0106 [Google Scholar]
- Olson V.A, Owens I.P.F. Costly sexual signals: are carotenoids rare, risky or required? Trends Ecol. Evol. 1998;13:510–514. doi: 10.1016/s0169-5347(98)01484-0. doi:10.1016/S0169-5347(98)01484-0 [DOI] [PubMed] [Google Scholar]
- Poizat G, Rosecchi E, Crivelli A.J. Empirical evidence of a trade-off between reproductive effort and expectation of future reproduction in female three-spined sticklebacks. Proc. R. Soc. B. 1999;266:1543–1548. doi:10.1098/rspb.1999.0813 [Google Scholar]
- Proulx S.R, Day T, Rowe L. Older males signal more reliably. Proc. R. Soc. B. 2002;269:2291–2299. doi: 10.1098/rspb.2002.2129. doi:10.1098/rspb.2002.2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roff D.A. Chapman and Hall; New York, NY: 1992. The evolution of life histories. [Google Scholar]
- Rowland W.J. Proximate determinants of stickleback behaviour: an evolutionary perspective. In: Bell M.A, Foster S.A, editors. The evolutionary biology of the threespine stickleback. Oxford University Press; Oxford, UK: 1994. pp. 297–344. [Google Scholar]
- Sohal R.S, Ku H.H, Agarwal S, Forster M.J, Lal H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech. Ageing Dev. 1994;74:121–133. doi: 10.1016/0047-6374(94)90104-x. doi:10.1016/0047-6374(94)90104-X [DOI] [PubMed] [Google Scholar]
- Stearns S.C. Oxford University Press; Oxford, UK: 1992. The evolution of life histories. [Google Scholar]
- von Schantz T, Bensch S, Grahn M, Hasselquist D, Wittzell H. Good genes, oxidative stress and condition-dependent sexual signals. Proc. R. Soc. B. 1999;266:1–12. doi: 10.1098/rspb.1999.0597. doi:10.1098/rspb.1999.0597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedekind C, Meyer P, Frischknecht M, Niggli U.A, Pfander H. Different carotenoids and potential information content of red coloration of male three-spined stickleback. J. Chem. Ecol. 1998;24:787–801. doi:10.1023/A:1022365315836 [Google Scholar]
- Whoriskey F.G, FitzGerald G.J. Ecology of the threespine stickleback on the breeding grounds. In: Bell M.A, Foster S.A, editors. The evolutionary biology of the threespine stickleback. Oxford University Press; Oxford, UK: 1994. pp. 189–206. [Google Scholar]
- Wootton R.J. Energetics of reproduction. In: Tytler P, Calow P, editors. Fish energetics: new perspectives. Croom Helm; London, UK: 1985. pp. 231–254. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file contains further details on the preparation of the experimental diets, photographic analysis of ornament coloration, high-performance liquid chromatography (HPLC) of carotenoids and determination of susceptibility to oxidative stress