Abstract
The mechanism for maintaining complex food webs has been a central issue in ecology because theory often predicts that complexity (higher the species richness, more the interactions) destabilizes food webs. Although it has been proposed that prey anti-predator defence may affect the stability of prey–predator dynamics, such studies assumed a limited and relatively simpler variation in the food-web structure. Here, using mathematical models, I report that food-web flexibility arising from prey anti-predator defence enhances community-level stability (community persistence and robustness) in more complex systems and even changes the complexity–stability relationship. The model analysis shows that adaptive predator-specific defence enhances community-level stability under a wide range of food-web complexity levels and topologies, while generalized defence does not. Furthermore, while increasing food-web complexity has minor or negative effects on community-level stability in the absence of defence adaptation, or in the presence of generalized defence, in the presence of predator-specific defence, the connectance–stability relationship may become unimodal. Increasing species richness, in contrast, always lowers community-level stability. The emergence of a positive connectance–stability relationship however necessitates food-web compartmentalization, high defence efficiency and low defence cost, suggesting that it only occurs under a restricted condition.
Keywords: complexity–stability debate, anti-predator defence, food-web flexibility, adaptive food-web hypothesis
1. Introduction
In nature, a number of species, or trophic species, are interconnected by feeding interactions that form a complex network called food web (Belgrano et al. 2005; de Ruiter et al. 2005). However, theory often suggests that such complex food webs are unlikely to persist, as complexity tends to destabilize population dynamics (e.g. May 1972; Gilpin 1975; Chen & Cohen 2001). The apparent contradiction between theory and observation (Pimm 1991) has stimulated theoretical studies seeking a mechanism for the maintenance of complex food webs (e.g. DeAngelis 1975; Yodzis 1981; McCann et al. 1998; Neutel et al. 2002; Kondoh 2003a).
Ecological studies that relate food-web structure to population dynamics often assume a static food-web structure characterized by fixed topology or constant interaction strengths (May 1972; DeAngelis 1975; Gilpin 1975; Yodzis 1981; Pimm 1991; Chen & Cohen 2001; Neutel et al. 2002). However, trophic interaction can actually be more dynamic because of the adaptive behavioural or morphological shifts of prey and predator. A number of theoretical studies have shown that these adaptive phenotypic shifts alter the strengths of trophic interactions (Holling 1959; Abrams 1982) or shape of the food-web architecture (Matsuda & Namba 1991; Matsuda et al. 1994, 1996; Křivan & Schmitz 2003; Beckerman et al. 2006) and thus have a major impact on population dynamics (Abrams 1984, 1992; Bolker et al. 2003) and the complexity effect on population stability (Pelletier 2000; Kondoh 2003a,b, 2005).
The phenotypic shifts that modify trophic interactions and population dynamics include prey anti-predator defence (prey behaviour to reduce predation risks; Endler 1986; Lima & Dill 1990; for its effect on prey–predator dynamics, see Ives & Dobson 1987; Fryxell & Lundberg 1997; Křivan 1998; Abrams 2000; Bolker et al. 2003; Yoshida et al. 2003; Křivan & Sirot 2004; Vos et al. 2004). Theory suggests that the effect of prey defence on the coexistence of predators is influenced by the predator specificity of the defence. Consider a prey capable of anti-predator defence and its multiple predators. If the anti-predator defence is effective against a wide range of predator species (generalized defence; Sih et al. 1998), it brings about a negative trait-mediated indirect effect between the predator species and makes their coexistence difficult (Matsuda et al. 1996). In contrast, if the defence is predator specific and if there is a trade-off between these predator-specific defences (Soluk 1993; Sih et al. 1998; McCarthy & Fisher 2000; Magalhães et al. 2002), it may generate a minority-advantage mechanism and enhance the coexistence of the predator species (Lima 1992; Matsuda et al. 1993, 1994, 1996).
Despite intensive theoretical studies on the defence effect on prey–predator dynamics (Ives & Dobson 1987; Lima 1992; Matsuda et al. 1993, 1994, 1996; Abrams 2000; Vos et al. 2004), its role in more complex food web is still unclear. Furthermore, little is known about how the defence changes the relationship between food-web complexity (species richness and connectance) and stability. These are partly because previous studies tended to assume limited, and relatively simpler variation in food-web architecture and did not investigate how population dynamics change with simultaneous changes in the food-web structure and the level or type of defence adaptation.
Here, using mathematical models of more complex communities, I have studied the effect of adaptive defence on the two aspects of community-level stability concerning number of coexisting species, community persistence (the probability that all species persist; Kondoh 2003a), robustness (the expected number of coexisting species; Brose et al. 2003; Kondoh 2006) and their relationships with food-web complexity. The two indices of community-level stability are evaluated in an assemblage of randomly generated food-web models with different degrees of complexity (connectance and species richness) and different types (generalized or predator-specific), levels (fraction of adaptive prey, rate of adaptive shift) and efficiency or cost of anti-predator defence. Using this model, I report that (i) predator-specific defence enhances community-level stability under a variety of food-web topologies and complexity levels and thus provides a possible mechanism for maintaining natural food webs, (ii) community-level stability decreases with increasing species richness irrespective of the type of prey defence, as was also predicted in the classical theory, (iii) while the connectance–stability relationship is negative in the presence of generalized defence, predator-specific defence can give rise to a unimodal connectance–stability relationship, and (iv) community compartmentalization (May 1973), of sufficiently low defence cost and high defence efficiency are essential for the emergence of a positive connectance–stability relationship.
2. Model
(a) Potential food-web architecture and population dynamics
The topology of the food-web model is determined by species richness of basal and non-basal species, food-web topological constraints and connectance.
A community consists of N species, of which B are basal species (species 1 to B) and the rest, (N−B), are non-basal species (species (B+1) to N). While the basal species survive on their own and do not consume other species, the non-basal species need at least one resource species to survive. Three different topological constraints are used in connecting these species: the two-trophic model; the random model; and the cascade model (Cohen et al. 1990). In the two-trophic model, a food web consists of only two trophic levels: a basal level and a non-basal level. While a basal species never eats other species, a non-basal species eats a basal species with a given probability. The random and cascade models, in contrast, may consist of more than three trophic levels. In the random model, a non-basal species eats a randomly chosen species with a given probability; the cascade model assumes a trophic hierarchichy that constrains the trophic role as consumer or resource—for each pair of species i and j (i<j), species i never eats species j, while species j (greater than B) eats species i with a given probability. The assumption of trophic hierarchy was proposed to hold more for predator–prey interaction than for host–parasitoid interaction, as body size is considered as a possible mechanism for the constraint (Leaper & Huxham 2002). Neither cannibalism nor mutual predation is allowed in any of the three models.
A food web with given connectivity is generated as follows. Initially, a resource species is randomly selected for every non-basal species to confirm that all non-basal species have at least one resource species in a web. Then, each of the other potential pairs is connected by a link with probability, C. The expected number of links (L) is given as a function of N, B and C; i.e. {(N−B)+C(N−B)(B−1)} for the two-trophic model and {(N−B)+C(N−B)(N+B−3)/2} for the random and cascade models. Connectance, defined as (L/N2) (Martinez 1992), linearly increases with probability C (connectivity). Hereafter, C is used as an index of food-web connectance.
The population dynamics of species i is described by
| (2.1a) |
and
| (2.1b) |
for basal and non-basal species, respectively. Here, ri is the intrinsic growth rate; mi the mortality rate; si the self-regulation intensity; fij the foraging efficiency of species i consuming resource j; Pij (0–1) the effect of prey i defence against its potential predator j; ci (0–1) the effect of anti-predator defence on reproduction or consumption (i.e. defence cost); and eij the conversion rate of species i, when it consumes species j. For simplicity, eij is assigned a biologically feasible constant value, e (0.01–0.35). The linear functional response is used in the present study to facilitate comparison of the present model with former studies (Kondoh 2003a,b, 2005). Numerous simulations were carried out with different parameter values to test the robustness of the model results. In the present paper, mainly the results for (ri, mi, si, fij)=(1, [0.001, 0.1], 1, [0, 1]) have been shown.
(b) Adaptive dynamics
(i) Predator-specific defence
Consider that adaptive prey can reduce predation risk via defence behaviour and that defence is predator specific. Further, a defence behaviour is associated with decreased effort allocation to other predator-specific defences and reproduction. To introduce this trade-off among different predator-specific defences and reproduction, I assume that a species (x) has a fixed size of ‘effort budget’, of which bxy and bx0 are allocated to specific defence against predator y (0≤bxy≤1) and reproduction (intrinsic growth or resource consumption; 0≤bx0≤1), respectively ().
Predation rate of predator y on prey x decreases with increasing prey defence level, bxy; the effectiveness of the defence may vary among species. To represent this, Pxy (x, y=i, j or k) in equations (2.1a) and (2.1b) is set to (1−axy bxy), where axy (0≤axy≤1) is the defence efficiency of prey x against predator y. In addition, it is assumed that the reproductive ability, expressed as intrinsic growth rate or resource consumption rate, increases with increasing reproductive effort, bi0 by setting cx (x=i or k) in equations (2.1a) and (2.1b) to , where (0≤≤1) represents the magnitude of reduction in reproductive rate caused by a unit increase in defence effort. Thus, this means that reproductive ability decreases as the total defence effort increases (i.e. the reproduction–defence trade-off).
A prey species is ‘adaptive’ with probability F (0≤F≤1). While a non-adaptive prey species (i) allocates its effort equally to all behaviours (bij=constant for all j), an adaptive prey (i) can adjust its effort allocation, bij, in a way that increases the per capita growth rate (Wi=(dXi/dt)/Xi). For simplicity, it is assumed that an adaptive prey allocates more effort to the behaviour (predator-specific defence or reproduction) if its pay-off per unit effort, dWi/dbij, is higher than the average (). There are two properties for the adaptive dynamics to satisfy in order to confirm its biological plausibility: (i) the dynamics of bij is bounded within the range 0–1 (i.e. the limits of anti-predator defence) and (ii) the total effort of prey i is kept constant (the size of effort budget conserved over time, at any t). The simplest form in which to represent the above-mentioned adaptive dynamics of the defence effort (bij) is
| (2.2) |
where Gi is the adaptation rate of species i and Wi is the per capita growth rate, (dXi/dt)/Xi (see Kondoh 2003a). Note that if , there is no cost of anti-predator defence and effort is allocated only among predator-specific defences (bi0=0). The initial defence effort, bij(0), and initial reproductive effort, bi0(0), are set to 1/(1+(number of potential predator species of species i)) for >0, or to 1/(number of potential predator species of species i) and 0, respectively, for .
(ii) Generalized defence
Next, consider that predation risk by any predator species is reduced via the same defence behaviour (generalized defence) and that defence behaviour is associated with decreased effort allocation to reproduction. To represent this reproduction–defence trade-off, I assume that species x allocates effort bx (0≤bx≤1) and the rest, (1−bx), to generalized defence and reproduction, respectively. In equations (2.1a) and (2.1b), Pxy (x, y=i, j or k) is set to (1−axy bx) to represent the defence effect on the trophic interaction, where axy (≤1) is the defence efficiency of prey x against predator y. A decrease in the reproductive effort, (1−bx) (where x=i or k), lowers the reproductive rate, as is represented by setting cx to (0≤cx≤1), where (0≤≤1) is the cost per unit defence.
An adaptive prey changes its effort allocation in a way that increases the per capita growth [Wi=(dXi/dt)/Xi]. In addition, bi should be a non-negative value smaller than 1. The simplest form of the adaptive dynamics to satisfy those constraints is
| (2.3) |
where Gi is the adaptation rate of species i. The term [bi(1−bi)] is to keep the dynamics of bi bounded between 0 and 1. The initial defence level, bi(0), is set to 0.5, where the changing rate, [Gbi(1−bi)], is maximized.
(c) Community-level stability
Community persistence (Kondoh 2003a) and community robustness (Brose et al. 2003; Kondoh 2006) were used as stability indices at community level. For a given set of adaptation rates (G), probabilities that a prey species is adaptive (F), defence efficiencies (aij), defence costs (), species richnesses (N) and connection probabilities (C), the number of species with population level (Xi) higher than the extinction threshold (10−13; other threshold population levels were also used to check the robustness of the result) was counted at t=105, in an ensemble of 10 000 stochastically generated food-web models. Community persistence is defined as a proportion of simulation runs where all species survive. Community robustness is defined as an expected proportion of persisting species, i.e. , where k is the proportion of surviving species and pk is the probability that proportion k of species survive, and calculated from an ensemble of 1000 stochastically generated food-web models. As community robustness shows a pattern similar to community persistence, hereafter I mainly present results for community persistence. The initial density, Xi(0), is set to a random value between the extinction threshold and 0.1.
3. Results
First, consider that the defence is predator specific, the defence efficiency is highest (aij=1) and the defence cost is zero (c=0). In the presence of adaptive defence, the number of actual trophic links, i.e. links with Pij>10−4, is smaller than the potential link number (see figure A1 in the electronic supplementary material), as a prey–predator interaction does not occur when all prey defence effort is allocated to the predator. Furthermore, the population densities and defence levels often show short-term fluctuations (figure A2 in the electronic supplementary material), implying the temporal variability in food-web structure.
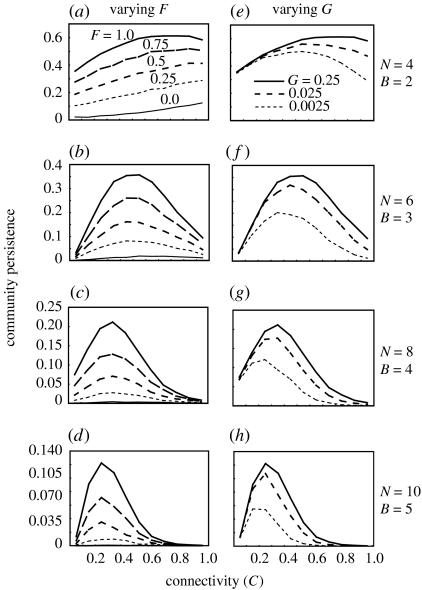
Community persistence increases with increasing fraction of adaptive prey (F) or increasing adaptive rate (G) under any topological constraints (figures 1 and 2) as long as species richness is sufficiently high (N≥4). Community robustness shows a similar pattern (figure A3 in the electronic supplementary material). The qualitative pattern is not altered by changing the conversion rate (e=0.01, 0.15 and 0.35), the loss rate of non-basal species (mi=0.01–0.001 or 0.1–0.01), the self-regulation rate (si=1, 0.001, 0.01 or 0.1) or the number of basal species ((N, B)=(4, 2), (8, 4), (12, 6), (6, 2), (9, 3) or (12, 4)). Moreover, a change in the extinction threshold does not qualitatively change the resultant pattern, if the threshold is sufficiently low.
Figure 1.
Relationships between food-web complexity and community persistence with varying fractions of adaptive prey (a–d; F=0.0, 0.25, 0.5, 0.75, 1.0) and adaptive rate (e–h; G=0.25, 0.025, 0.0025) in the two-trophic model. The other parameters are B=N/2, F=1 (e–h), G=0.25 (a–d), si=1, e=0.15, m=0.001–0.01, a=1.
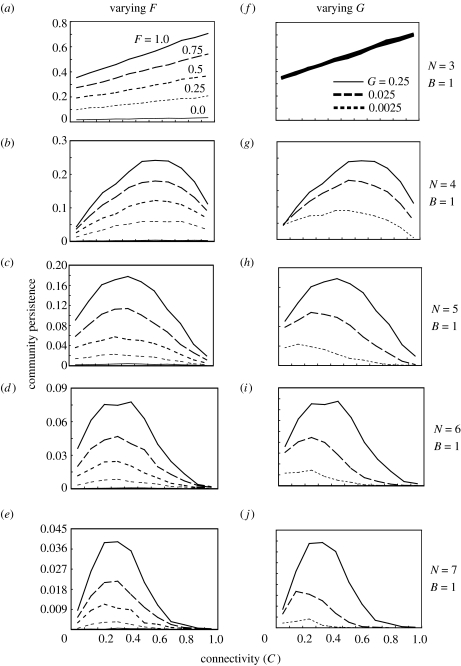
Figure 2.
Relationships between food-web complexity and community persistence with varying fractions of adaptive prey (a–e; F=0.0, 0.25, 0.5, 0.75, 1.0) and adaptive rate (f–j; G=0.25, 0.025, 0.0025) in the cascade model. The other parameters are B=1, F=1 (f–j), G=0.25 (a–e), si=1, e=0.15, m=0.001–0.01, a=1. The patterns are qualitatively the same for the random model.
While increasing species richness always lowers community persistence, the relationship between connectance and community persistence is qualitatively altered by changing the fraction of adaptive prey (F) or the adaptation rate (G) (figures 1 and 2). There are three notable patterns. First, in random and cascade models, with lower species richness, community persistence increases steadily with increasing C (figure 2a,f). The positive relationship is clearer for higher F, while changing G has only a slight effect. Second, when species richness is higher (figure 2b–e, g–j) or a two-trophic model is used (figure 1), the connectance–persistence relationship is unimodal, and the shoulder becomes steeper with increasing G or F. In the absence of adaptation (F=0, G=0), most model food webs are not persistent (the persistent models are less than 1% of all the models examined). Third, the C value that maximizes community persistence increases with G, while changing F has less influence on the maximum position. Again, community robustness shows a similar pattern (figure A3 in the electronic supplementary material). These patterns are not altered qualitatively by changing the metabolic rate, the mortality rate or the fraction of basal species.
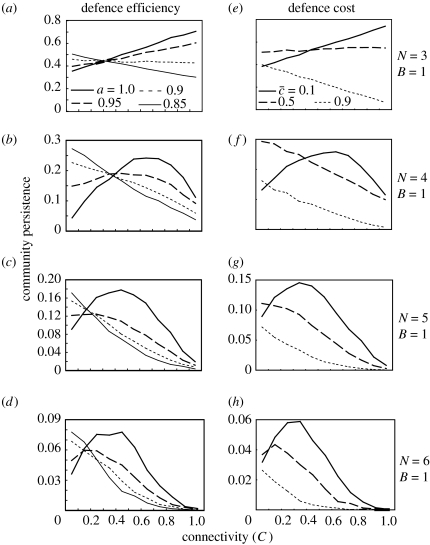
The defence efficiency (aij=a) and defence cost () qualitatively alters the connectance–persistence relationship (figure 3). The unimodal relationship for N≥4 is observed only when the defence efficiency is sufficiently high (a≥0.95) and the defence cost is sufficiently low. When the efficiency is lower or the defence cost is higher, in contrast, community persistence always decreases with increasing C. The C value that maximizes persistence increases with increasing defence efficiency (a) or decreasing defence cost ().
Figure 3.
The complexity–persistence relationship of a cascade food web consisting of adaptive prey with different defence efficiencies (a–d; a=1.0, 0.95, 0.9, 0.85) and defence costs (e–h; =0.1, 0.5, 0.9). The other parameters are G=0.25, F=1.0, si=1, e=0.15, m=0.001–0.01, N=3–6, B=1, a=1, =0. The result is qualitatively the same for the random model.
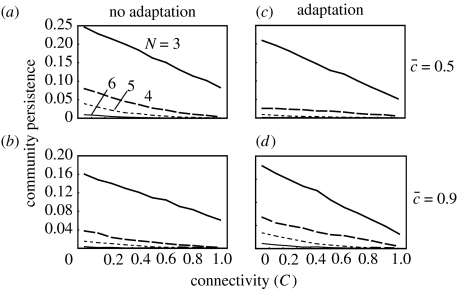
The pattern is completely different for webs with generalized defence (figure 4). The presence of adaptive defence tends to lower community persistence for smaller (less than 0.5), while enhancing community persistence for large (greater than 0.7) in all ranges of food-web complexity (C=0–1, N=3–6, B=1; figure 4). Decreasing defence cost strongly lowers community persistence in the presence of adaptation (F=1, G=0.25). Indeed, when the defence cost is very low (≤0.3), most model food webs are not persistent, and the persistence is lower than 0.001 for N≥4. Generalized defence does not qualitatively alter the connectance–persistence relationship (figure 4). Community persistence steadily decreases with increasing C in all cases irrespective of the presence of defence adaptation and other parameters. A similar pattern was observed for community robustness (figure A3 in the electronic supplementary material).
Figure 4.
The effect of generalized defence on the relationships between food-web complexity (C) and community persistence for varying . The left column and right column are for non-adaptive webs (F=0.0; a, b) and adaptive webs (G=0.25, F=1; c, d), respectively. Community persistence in the presence of adaptation is <0.001 for N≥5 when is smaller (≤0.5). The parameters are B=1, si=1, e=0.15, m=0.001–0.01, a=1. The cascade model is used. The choice of topological constraint does not qualitatively change the result.
4. Discussion
To my knowledge, this constitutes the first study examining systematically the interacting effect of adaptive defence and food-web complexity on community-level stability. The model analysis shows that predator-specific defence enhances community persistence and robustness under a wide range of food-web topologies or complexities, suggesting that adaptive prey behaviour potentially contributes to persistence of communities. This is in agreement with the previous theoretical prediction derived from the analysis of simpler systems (Matsuda et al. 1993, 1994, 1996). This result, taken together with previous studies on the stabilizing effect of predation behaviour (McCann et al. 1998; Pelletier 2000; Kondoh 2003a,b, 2005), suggests that adaptation, a distinguishing characteristic of organisms, may contribute to food-web maintenance.
While generalized defence has only a minor effect on the complexity–stability relationships, predator-specific defence qualitatively changes the relationship between connectance and stability. More specifically, the connectance–stability relationship becomes unimodal with its shoulders becoming steeper and region of the positive relationship being wider with increasing fraction of adaptive prey or increasing adaptive rate. The prediction is not altered even if connectance is measured by ‘realized connectance’, the connectance taking account only of actual prey–predator interactions occurring at a specific period (Kondoh 2003a), as realized connectance always increases with increasing potential connectance (see figure A1 in the electronic supplementary material). These results imply a possible emergence of positive connectance–stability relationship in the presence of adaptive prey. However, the model also predicts that this occurs only if species richness is sufficiently low. Furthermore, community-level stability is predicted to decrease with increasing species richness. These predictions suggest that the stabilizing effect of connectance necessitates the system size being sufficiently small. A positive relationship may only occur in small subgroups of food web, connected by few and weak interactions, as proposed in the classic hypothesis of ‘ecosystem compartmentalization’ (May 1973; Krause et al. 2003).
The mechanism through which predator-specific defence generates the unimodal relationship is intuitively explained as follows. In an extremely simple food web, a predator relies on a few prey species that have no other predators. If a prey species is capable of predator-specific defence, such a subsystem is unlikely to persist, as the prey allocates its all defence effort to the predator. The addition of another predator to the system, however, can rescue the focal predator, because the adaptive prey switches its defence effort to the more abundant predator, encouraging the persistence of less abundant predator species (Matsuda et al. 1993, 1994, 1996). This rescue effect generates the positive part of the unimodal relationship. However, addition of more predators increases resource competition between the predators, as is implied by the realized links increasing linearly with increasing potential links (figure 4), and lowers the persistence of the subsystem, giving rise to the negative part of the relationship. These mechanisms together result in the unimodal relationship. In the absence of an adaptive defence switch, in contrast, the addition of predators only increases resource competition between the predators and thus leads to the negative relationship.
It should be noted that a complex food web becomes stable only under restricted conditions; that is, the defence is predator specific and sufficiently efficient, defence cost is sufficiently low and the system is compartmentalized. This may imply that the present mechanism alone is too weak for a complex food web to be stable in nature. However, when working together with adaptive foraging (Kondoh 2003a,b, 2005), the adaptive predator-specific defence can still play an important role in the maintenance of complex food webs. On one hand, adaptive foragers tend to use only a few prey species and thus decrease food-web connectance (Matsuda & Namba 1991; Kondoh 2003a) and compartmentalize a large system (McCann et al. 2005); on the other hand, adaptive defence with low efficiency or high cost is still strongly stabilizing at low complexity levels (figure 3). Together, these observations suggest that a predator's adaptive food choice may not only create the positive relationship on its own, but also sets the stage for defence adaptation to stabilize the food web. The interacting effect of adaptive defence and adaptive diet choice should be tested explicitly in future studies.
The adaptation-oriented view of food-web ecology is gaining increasing attention (e.g. Drossel et al. 2001; Kondoh 2003a; Takimoto 2003; Yoshida et al. 2003; Cattin et al. 2004; Loeuille & Loreau 2005; Beckerman et al. 2006). Yet, there is still a large gap between theory and empirical studies, especially as to how temporally variable interaction strength influences long-term population dynamics in food webs. Although recent studies (e.g. Neutel et al. 2002; Emmerson & Raffaelli 2004), which analysed the dynamic property of food-web models parameterized by empirical data, have shown how prey–predator interaction strength strongly influences long-term population dynamics of food webs, these are based on the fundamental assumption that the interaction strength is independent of other non-focal species in the community. An essential next step is to capture the variable nature of interaction strength by further empirical measurement and evaluate its dependence on densities of species in the web, which will allow extension of the modelling approach to flexible food webs. For example, it would be interesting to examine in nature if interaction strength changes in a way that favours less abundant species, as predicted by theoretical studies—more predation pressure to more abundant preys and more anti-predator defence to more abundant predators.
Acknowledgments
I thank H. Jones, S. Diehl, D. L. DeAngelis and H. Matsuda for valuable comments on the earlier manuscript. This study is supported by JSPS Research Fellowship for Young Scientists (no. 17770022), the MEXT Grant-in-Aid for the 21st Century COE Program (A2 to Kyoto University) and Science and Technology Fund of Ryukoku University.
Supplementary Material
Relationship between potential link number and realised link number for different fraction of adaptive prey species
An example of temporal fluctuation in population and adaptive dynamics
Relationships between food-web complexity and community robustness
References
- Abrams P.A. Functional responses of optimal foragers. Am. Nat. 1982;120:382–390. doi:10.1086/283996 [Google Scholar]
- Abrams P.A. Foraging time optimization and interactions in food webs. Am. Nat. 1984;124:80–96. doi:10.1086/284253 [Google Scholar]
- Abrams P.A. Predators that benefit prey and prey that harm predators: unusual effects of interacting foraging adaptations. Am. Nat. 1992;140:573–600. doi:10.1086/285429 [Google Scholar]
- Abrams P.A. The evolution of predator–prey interactions: theory and evidence. Annu. Rev. Ecol. Syst. 2000;31:79–105. doi:10.1146/annurev.ecolsys.31.1.79 [Google Scholar]
- Beckerman A.P, Petchey O.L, Warren P.H. Foraging adaptation predicts food web complexity. Proc. Natl Acad. Sci. USA. 2006;103:13 745–13 749. doi: 10.1073/pnas.0603039103. doi:10.1073/pnas.0603039103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgrano A, Scharler U.M, Dunne J, Ulanowicz R.E. Oxford University Press; Oxford, UK: 2005. Aquatic food webs: an ecosystem approach. [Google Scholar]
- Bolker B, Holyoak M, Křivan V, Rowe L, Schmitz O. Connecting theoretical and empirical studies of trait-mediated interactions. Ecology. 2003;84:1101–1114. [Google Scholar]
- Brose U, Williams R.J, Martinez N.D. Comments on “foraging adaptation and the relationship between food-web complexity and stability”. Science. 2003;301:918. doi: 10.1126/science.1085902. doi:10.1126/science.1085902 [DOI] [PubMed] [Google Scholar]
- Cattin M.-F, Bersier L.-F, Banasek-Richter C, Baltensperger R, Gabriel J.-P. Phylogenetic constraints and adaptation explain food-web structure. Nature. 2004;427:835–839. doi: 10.1038/nature02327. doi:10.1038/nature02327 [DOI] [PubMed] [Google Scholar]
- Chen X, Cohen J.E. Global stability, local stability and permanence in model food webs. J. Theor. Biol. 2001;212:223–235. doi: 10.1006/jtbi.2001.2370. doi:10.1006/jtbi.2001.2370 [DOI] [PubMed] [Google Scholar]
- Cohen J.E, Briand F, Newman C.M. Springer; Berlin, Germany: 1990. Community food webs: data and theory. [Google Scholar]
- de Ruiter P.C, Wolters V, Moore J.C. Academic Press; San Diego, CA: 2005. Dynamic food webs: multispecies assemblages, ecosystem development, and environmental change. [Google Scholar]
- DeAngelis D.L. Stability and connectance in food web models. Ecology. 1975;56:238–243. doi:10.2307/1935318 [Google Scholar]
- Drossel B, Higgs P.G, McKane A.J. The influence of predator–prey population dynamics on the long-term evolution of food web structure. J. Theor. Biol. 2001;208:91–107. doi: 10.1006/jtbi.2000.2203. doi:10.1006/jtbi.2000.2203 [DOI] [PubMed] [Google Scholar]
- Emmerson M.C, Raffaelli D.G. Predator–prey body size, interaction strength and the stability of a real food web. J. Anim. Ecol. 2004;73:399–409. doi:10.1111/j.0021-8790.2004.00818.x [Google Scholar]
- Endler J.A. Defence against predators. In: Feder M.E, Lauder G.V, editors. Predator–prey relationships. University of Chicago Press; Chicago, IL: 1986. pp. 109–134. [Google Scholar]
- Fryxell L.M, Lundberg P. Chapman & Hall; New York, NY: 1997. Individual behavior and community dynamics. [Google Scholar]
- Gilpin M.E. Stability of feasible predator–prey systems. Nature. 1975;254:137–139. doi:10.1038/254137a0 [Google Scholar]
- Holling C.S. Some characteristics of simple types of predation and parasitism. Can. Entomol. 1959;91:385–398. [Google Scholar]
- Ives A.R, Dobson A.P. Antipredator behavior and the population dynamics of simple predator–prey systems. Am. Nat. 1987;130:431–437. doi:10.1086/284719 [Google Scholar]
- Křivan V. Effects of optimal antipredator behavior of prey on predator–prey dynamics: the role of refuges. Theor. Popul. Biol. 1998;53:131–142. doi: 10.1006/tpbi.1998.1351. doi:10.1006/tpbi.1998.1351 [DOI] [PubMed] [Google Scholar]
- Křivan V, Schmitz O.J. Adaptive foraging and flexible food web topology. Evol. Ecol. Res. 2003;5:623–652. [Google Scholar]
- Křivan V, Sirot E. Do short-term behavioral responses of consumers in tri-trophic food chains persist at the population time scale? Evol. Ecol. Res. 2004;6:1063–1081. [Google Scholar]
- Kondoh M. Foraging adaptation and the relationship between food-web complexity and stability. Science. 2003a;299:1388–1391. doi: 10.1126/science.1079154. doi:10.1126/science.1079154 [DOI] [PubMed] [Google Scholar]
- Kondoh M. Response to comment on “foraging adaptation and the relationship between food-web complexity and stability”. Science. 2003b;301:918. doi: 10.1126/science.1085902. doi:10.1126/science.1087539 [DOI] [PubMed] [Google Scholar]
- Kondoh M. Is biodiversity maintained by food-web complexity?—The adaptive food-web hypothesis. In: Belgrano A, Scharler U.M, Dunne J, Ulanowicz R.E, editors. Aquatic food webs: an ecosystem approach. Oxford University Press; Oxford, UK: 2005. pp. 130–142. [Google Scholar]
- Kondoh M. Does foraging adaptation create the positive complexity–stability relationship in realistic food-web structure? J. Theor. Biol. 2006;238:646–651. doi: 10.1016/j.jtbi.2005.06.028. doi:10.1016/j.jtbi.2005.06.028 [DOI] [PubMed] [Google Scholar]
- Krause A.E, Frank K.A, Mason D.M, Ulanowicz R.E, Taylor W.W. Compartments revealed in food web structure. Nature. 2003;426:282–285. doi: 10.1038/nature02115. doi:10.1038/nature02115 [DOI] [PubMed] [Google Scholar]
- Leaper R, Huxham M. Size constraints in a real food web: predator, parasite and prey body-size relationships. Oikos. 2002;99:443–456. doi:10.1034/j.1600-0706.2002.10888.x [Google Scholar]
- Lima S.L. Life in a multi-predator environment: some considerations for antipredatory vigilance. Annales Zoologici Fennici. 1992;29:217–226. [Google Scholar]
- Lima S.L, Dill L.M. Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 1990;68:619–640. [Google Scholar]
- Loeuille N, Loreau M. Evolutionary emergence of size-structured food webs. Proc. Natl Acad. Sci. USA. 2005;102:5761–5766. doi: 10.1073/pnas.0408424102. doi:10.1073/pnas.0408424102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhães S, Janssen A, Hanna R, Sabelis M.W. Flexible antipredator behaviour in herbivorous mites through vertical migration in a plant. Oecologia. 2002;132:143–149. doi: 10.1007/s00442-002-0950-4. doi:10.1007/s00442-002-0950-4 [DOI] [PubMed] [Google Scholar]
- Martinez N.D. Constant connectance in community food webs. Am. Nat. 1992;139:1208–1218. doi:10.1086/285382 [Google Scholar]
- Matsuda H, Namba T. Food web graph of a coevolutionarily stable community. Ecology. 1991;72:267–276. doi:10.2307/1938920 [Google Scholar]
- Matsuda H, Abrams P.A, Hori M. The effect of adaptive antipredator behavior on exploitative competition and mutualism between predators. Oikos. 1993;68:549–559. doi:10.2307/3544924 [Google Scholar]
- Matsuda H, Hori M, Abrams P.A. Effects of predator-specific defence on community complexity. Evol. Ecol. 1994;8:628–638. doi:10.1007/BF01237846 [Google Scholar]
- Matsuda H, Hori M, Abrams P.A. Effects of predator-specific defence on biodiversity and community complexity in two-trophic-level communities. Evol. Ecol. 1996;10:13–28. doi:10.1007/BF01239343 [Google Scholar]
- May R.M. Will a large complex system be stable? Nature. 1972;238:413–414. doi: 10.1038/238413a0. doi:10.1038/238413a0 [DOI] [PubMed] [Google Scholar]
- May, R. M. 1973 Stability and complexity in model ecosystems Princeton, NJ: Princeton University Press.
- McCann K.S, Hastings A, Huxel G.R. Weak trophic interactions and the balance of nature. Nature. 1998;395:794–798. doi:10.1038/27427 [Google Scholar]
- McCann K.S, Rasmussen J.B, Umbanhowar J. The dynamics of spatially coupled food webs. Ecol. Lett. 2005;8:513–523. doi: 10.1111/j.1461-0248.2005.00742.x. doi:10.1111/j.1461-0248.2005.00742.x [DOI] [PubMed] [Google Scholar]
- McCarthy T.M, Fisher W.A. Multiple predator-avoidance behaviours of the freshwater snail Physella heterostropha pomila: responses vary with risk. Ecol. Lett. 2000;44:387–397. doi:10.1046/j.1365-2427.2000.00576.x [Google Scholar]
- Neutel A, Heesterbeek J.A.P, de Ruiter P.C. Stability in real food webs: weak links in long loops. Science. 2002;296:1120–1123. doi: 10.1126/science.1068326. doi:10.1126/science.1068326 [DOI] [PubMed] [Google Scholar]
- Pelletier J.D. Are large complex ecosystems more unstable? A theoretical reassessment with predator switching. Math. Biosci. 2000;163:91–96. doi: 10.1016/s0025-5564(99)00054-1. doi:10.1016/S0025-5564(99)00054-1 [DOI] [PubMed] [Google Scholar]
- Pimm S.L. University of Chicago Press; Chicago, IL: 1991. The balance of nature? [Google Scholar]
- Sih A, Englund G, Wooster D. Emergent impacts of multiple predators on prey. Trends Ecol. Evol. 1998;13:350–355. doi: 10.1016/s0169-5347(98)01437-2. doi:10.1016/S0169-5347(98)01437-2 [DOI] [PubMed] [Google Scholar]
- Soluk D.A. Multiple predator effects: predicting combined functional response of stream fish and invertebrate predators. Ecology. 1993;74:219–225. doi:10.2307/1939516 [Google Scholar]
- Takimoto G. Adaptive plasticity in ontogenetic niche shifts stabilizes consumer–resource dynamics. Am. Nat. 2003;162:93–109. doi: 10.1086/375540. doi:10.1086/375540 [DOI] [PubMed] [Google Scholar]
- Vos M, Kooi B.W, DeAngelis D.L, Mooij W.M. Inducible defences and the paradox of enrichment. Oikos. 2004;105:471–480. doi:10.1111/j.0030-1299.2004.12930.x [Google Scholar]
- Yodzis P. The stability of real ecosystems. Nature. 1981;289:674–676. doi:10.1038/289674a0 [Google Scholar]
- Yoshida T, Jones L.E, Ellner S.P, Fussmann G.F, Hairston N.G., Jr Rapid evolution drives ecological dynamics in a predator–prey system. Nature. 2003;424:303–306. doi: 10.1038/nature01767. doi:10.1038/nature01767 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Relationship between potential link number and realised link number for different fraction of adaptive prey species
An example of temporal fluctuation in population and adaptive dynamics
Relationships between food-web complexity and community robustness