Abstract
Advanced societies owe their success to an efficient division of labour that, in some social insects, is based on specialized worker phenotypes. The system of caste determination in such species is therefore critical. Here, we examine in a leaf-cutting ant (Acromyrmex echinatior) how a recently discovered genetic influence on caste determination interacts with the social environment. By removing most of one phenotype (large workers; LW) from test colonies, we increased the stimulus for larvae to develop into this caste, while for control colonies we removed a representative sample of all workers so that the stimulus was unchanged. We established the relative tendencies of genotypes to develop into LW by genotyping workers before and after the manipulation. In the control colonies, genotypes were similarly represented in the large worker caste before and after worker removal. In the test colonies, however, this relationship was significantly weaker, demonstrating that the change in environmental stimuli had altered the caste propensity of at least some genotypes. The results indicate that the genetic influence on worker caste determination acts via genotypes differing in their response thresholds to environmental cues and can be conceptualized as a set of overlapping reaction norms. A plastic genetic influence on division of labour has thus evolved convergently in two distantly related polyandrous taxa, the leaf-cutting ants and the honeybees, suggesting that it may be a common, potentially adaptive, property of complex, genetically diverse societies.
Keywords: phenotype, reaction norm, Acromyrmex, caste, polyandry, genotype
1. Introduction
Division of labour is key to the success of many social animals, most notably, the social insects. In more derived cases, this division of labour involves individuals developing into distinct phenotypes (castes) that are morphologically adapted for their particular roles such as reproduction, foraging or defence. Such castes can perform work more efficiently than unspecialized individuals and the overall productivity of the group is thereby increased (Oster & Wilson 1978; Hölldobler & Wilson 1990). The group's output will be determined by the fit between the representation of, and requirement for, worker phenotypes, and so the system of caste determination is critical. This is more problematic than the equivalent behavioural task specialization that underlies division of labour in species with monomorphic workers because caste determination occurs while individuals are immature. The system of caste determination therefore has to predict the future needs of the colony.
Our understanding of the mechanism of caste determination is relatively limited, with only few species having been studied in any depth. From those that have, it is clear that environmental cues such as nutrition, temperature and adult pheromones can be important (Brian 1979; Nijhout & Wheeler 1982; Wheeler 1986; Hölldobler & Wilson 1990; Wheeler 1991; Passera et al. 1996). These most probably act in accordance with a stimulus–response threshold model. A certain threshold of a stimulus such as nutrition, results in differential gene expression and a switch from one developmental pathway to another (Evans & Wheeler 2001). Caste determination in social insects has therefore long appeared to represent a classic example of ‘nurture without nature’, i.e. pure phenotypic plasticity without any genetic variation.
Although an environmental influence on phenotype will be important in ensuring an optimal representation of castes, it is possible that having a diversity of reaction norms may allow a colony to respond more optimally to changing conditions (Crozier & Page 1985; Page et al. 1995; Fuchs & Moritz 1999; Crozier & Fjerdingstad 2001). Evidence for how this may work comes from the honeybee (Apis mellifera). Although honeybee workers are monomorphic, they exhibit task specialization and genetic polyethism: colonies contain multiple worker genotypes because queens are highly polyandrous (Palmer & Oldroyd 2000), and different genotypes have different response thresholds for particular tasks (Robinson 1992, 2002). A series of models indicate that genetic polyethism may result in a more optimal colony-level relationship between the available worker force and the tasks required when the demand for tasks changes (Bertram et al. 2003; Cox & Myerscough 2003; Myerscough & Oldroyd 2004; Graham et al. 2006). Empirical support for this has recently been obtained for thermoregulation (Jones et al. 2004).
It seems plausible that, in species with morphologically distinct castes, a genetic influence on caste determination (i.e. some form of genetic polymorphism for caste) could be beneficial for the same reason and may indeed be of even greater importance owing to the time delay intrinsic in morphological caste determination. However, this requires that the system of caste determination has both a significant genetic component and an appropriate level of phenotypic plasticity, i.e. that genotypes have variable and overlapping reaction norms to socially induced stimuli. A genetic influence on the determination of morphological worker castes has recently been demonstrated for a species of Acromyrmex leaf-cutting ant (Hughes et al. 2003). Workers of these ants are primarily divided into large workers (LW) that forage and small workers (SW) that work within the nest, caring for brood and the fungus garden (Weber 1972; Wetterer 1999; Hughes et al. 2003). As queens of free-living Acromyrmex species mate with multiple males (Sumner et al. 2004), their offspring belong to multiple patrilines that differ in their paternal genes while having everything else (maternal genes, environmental conditions during development) in common. Hughes et al. (2003) found that these patrilines were differentially represented in the two main worker castes. Evidence of a similar pattern has also been found in a harvester ant (Pogonomyrmex badius; Rheindt et al. 2005) while two other studies have found that genotype influences worker size in species without distinct size castes (Fraser et al. 2000; Schwander et al. 2005). In addition, several ant species have colony-specific caste ratios that are maintained under controlled environmental conditions (Johnston & Wilson 1985; Hölldobler & Wilson 1990; Billick 2002; Breed 2002), which is suggestive of a genetic component being involved.
An important outstanding question is how this genetic influence works in practice if the stimulus for a caste (or task) is increased. One possibility is that those genotypes already predisposed to develop into the required caste will become even more likely to develop into that caste. Alternatively, other genotypes whose response thresholds have now been met by the increased stimulus may respond and become more likely to develop into the required caste rather than other castes. A third possibility is that all genotypes may respond similarly (or not respond at all). Here, we shed light on this using the leaf-cutting ant Acromyrmex echinatior. By removing the majority of LW from colonies (‘LW-removal’ treatment), we increased the social stimulus for brood to develop into LW. To establish the impact of the increased stimulus on genetic polymorphism, we compared the representation of patrilines in the LW developing after this perturbation with those that developed under normal conditions. For comparison, we also investigated the same relationship in colonies from which a 1: 1 numerical ratio of LW and SW were removed (‘LWSW-removal’ treatment). It is important to note that LW and SW differ substantially in size and that removing a ratio based on numbers rather than mass is a conservative approach in that it might have biased the controls in the same direction as any treatment effect.
2. Material and methods
(a) Experimental procedure
The experiment involved 10 colonies of A. echinatior that were collected from Gamboa, Panama between 2000 and 2002. Colonies were set-up in plastic boxes with their fungus gardens within inverted, clear plastic beakers. Colony boxes also contained a plastic pot into which fresh bramble leaves (Rubus fruticosus) were added twice a week. The colonies were size matched into five pairs (table 1) and then one colony in each pair was randomly allocated to the LW-removal treatment and the other to the LWSW-removal treatment. Prior to the removal of workers, the numbers and size of workers in the foraging pots were recorded. We also estimated the volumes of the fungus gardens based on the proportion of beakers of known volume that the gardens occupied. Known amounts of fresh leaves were placed in each of the foraging pots, depending upon the size of the colony. Although leaves varied in area, they were allocated and cut by eye such as to add up to a known number of ‘standard leaf equivalents’. This allowed the amount of leaf material harvested between feeding intervals to be roughly estimated.
Table 1.
Numbers and proportions of workers removed. (LW, large workers; SW, small workers. As many LW as possible were removed from LW-removal colonies, while an equal number of LW and SW were removed from the LWSW-removal colonies such as to total an estimated 15% of the colony populations.)
| colony | removal treatment | size-matched pair | removal treatment | estimated population | individuals removed | estimated proportion removed | |||
|---|---|---|---|---|---|---|---|---|---|
| colony | LW | LW | SW | colony | LW | ||||
| 219 | LW | 1 | LW | 2000 | 450 | 256 | 0 | 0.13 | 0.64 |
| 112 | LW | 2 | LW | 1550 | 349 | 301 | 0 | 0.19 | 0.97 |
| 227 | LW | 3 | LW | 1450 | 326 | 243 | 0 | 0.17 | 0.84 |
| 223 | LW | 4 | LW | 1200 | 270 | 177 | 0 | 0.15 | 0.74 |
| 143 | LW | 5 | LW | 350 | 79 | 43 | 0 | 0.12 | 0.61 |
| 221 | LWSW | 1 | LWSW | 1750 | 394 | 131 | 131 | 0.15 | 0.38 |
| 220 | LWSW | 2 | LWSW | 1550 | 349 | 116 | 116 | 0.15 | 0.38 |
| 135b | LWSW | 3 | LWSW | 1550 | 349 | 116 | 116 | 0.15 | 0.38 |
| 226 | LWSW | 4 | LWSW | 1000 | 225 | 75 | 75 | 0.15 | 0.38 |
| 124 | LWSW | 5 | LWSW | 500 | 113 | 38 | 38 | 0.15 | 0.38 |
All LW were removed as far as possible from the LW-removal colonies. LW could be readily collected from outside the colony and from the surface of the fungus garden. However, many LW are normally located within the fungus garden and these could not be collected without destroying the structure of the garden and adding a considerable noise factor to the experiment. Fungus gardens were therefore observed for 30 min after the accessible LW had been removed and any further LW appearing on the surface of the garden were also removed. Given the sizes of the colonies and the relationship between garden volume and colony population (William O. H. Hughes 2007, unpublished data), it was estimated that approximately 15% of the colonies' total populations were removed from the LW-removal colonies (table 1). The populations of the LWSW-removal colonies were estimated from their garden volumes and 15% of their populations were also removed, with half being LW and half SW (table 1). The colonies were then left for eight weeks to allow new workers to be reared, eight weeks being the approximate time from egg to adult in Acromyrmex (Weber 1972). Four variables were recorded during this period to measure the impact of the worker removals on the colonies. The size of the fungus gardens and the amount of leaves harvested since the previous feeding were recorded twice a week. The number and size of workers in the foraging pots were recorded once a week.
In order to determine the representation of patrilines in the LW and SW populations under normal conditions, samples of 50 LW and 50 SW were collected from each colony prior to the manipulations and stored in alcohol. Ants were collected from the surface of the fungus gardens and were chosen to be of similar cuticular coloration in order to minimize the age variation between them. Samples of 50 LW and 50 SW of light cuticular coloration (indicating recent eclosion) were also collected from each colony at the end of the eight week experimental period in order to determine the representation of patrilines in the LW and SW populations following the experimental manipulations. The sampling protocol targeting light-coloured individuals ensured that the workers sampled at the end of the eight weeks were individuals that had developed during the experiment.
(b) Molecular analysis
DNA was extracted from the legs of sampled ants using Chelex beads (BioRad) and amplified at four microsatellite loci that are highly polymorphic in A. echinatior: Ech1390, Ech3385, Ech4126 and Ech4225 (4, 6, 8 and 10 alleles, respectively; Ortius-Lechner et al. 2000; Hughes & Boomsma 2006). PCR products were run on 5% polyacrylamide gels and analysed with an ABI377 automatic sequencer (Applied BioSystems). Allele sizes were scored by comparison with internal size markers. The multi-locus offspring genotypes were used to infer the genotypes of colony queens and their multiple mates. The sampled workers were then assigned to patrilines according to their paternal alleles. Patrilines were distinguished from the other patrilines within the colony by having a unique paternal allele at one or more loci. When individuals were heterozygous and had the same alleles as a heterozygous mother queen, the paternal allele could not be identified. Where this occurred at the diagnostic loci for patriline identification, the individual could then not be assigned accurately to a patriline. In order to be conservative in our identifications, such individuals (approximately 3% of those sampled) were excluded from the analysis.
(c) Statistical analysis
The variables measured over the course of the experiment (fungus garden volume, leaf material harvested, number and size of foragers) were log or Box–Cox transformed, and the transformed variables confirmed to be normally distributed with homogenous variances using Shapiro–Wilk's and Leven's tests. They were then analysed with repeated measures analyses of variance, using the Greenhouse–Geisser correction to control for deviations from the assumption of sphericity (Field 2000). To determine the relationship between the representation of patrilines in the LW and SW samples normally (pre-removal) and eight weeks after the experimental manipulation (post-removal), we calculated the ratios of LW to SW for each patriline in both samples. We then analysed the empirical logits of these ratios using a general linear model with the LW/SW ratio post-removal as the dependent variable, the ratio pre-removal as a covariate, and with treatment (LW- or LWSW-removal) and colony nested within treatment as main effects.
3. Results
(a) Changes over experiment
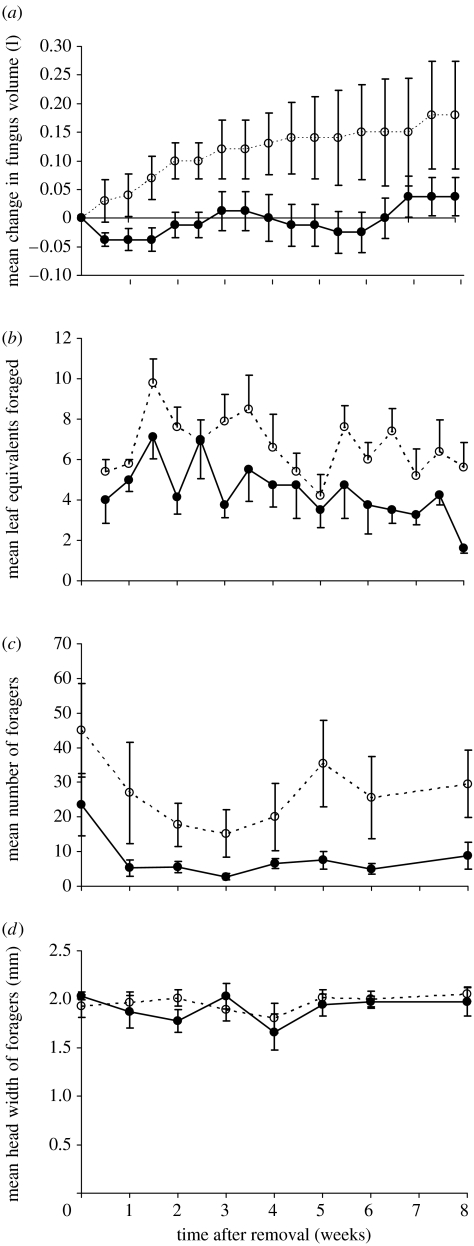
Before the experimental manipulations (time 0 in figure 1), the LW- and LWSW-removal colonies were similar in the size of their fungus gardens (F1,8=0.07, p=0.8), the number of foragers (F1,8=0.604, p=0.459) and the size of foragers (F1,8=0.66, p=0.444). Following the removal of workers, the volume of fungus gardens increased gradually in the LWSW-removal colonies (figure 1a). It initially decreased in the LW-removal colonies before returning to around its initial level (figure 1a). Although neither time (F1.5,12.1=2.68, p=0.118) nor the treatment×time interaction was significant (F1.5,12.1=1.96, p=0.187), there was a significant overall difference in fungus garden volume between the treatments (F1,8=6.64, p=0.033). The treatments did not differ in the way the quantities of leaves harvested changed over the eight week period (F4.8,38=1.04, p=0.406), with the quantity harvested decreasing significantly in both cases (F4.8,38=4.44, p=0.003; figure 1b). LW-removal colonies harvested less leaf material than did LWSW-removal colonies, although the difference was marginally non-significant (F1,8=4.83, p=0.059; figure 1b).
Figure 1.
Changes over the course of the eight week experiment period in (a) the mean (±s.e.) volume of fungus garden, (b) the amount of leaf material harvested since the previous record, (c) the number of foragers in the food pot, and (d) the average size (head width) of the foragers for colonies from which LW were removed (filled circles and lines) and colonies where both LW and SW were removed (open circles and dashed lines). In (b), error bars have been removed from below the latter colonies and above the former for clarity.
In both treatments, the numbers of foragers initially decreased significantly after worker removal and then increased gradually for the remainder of the eight weeks (time: F6,48=3.94, p=0.003; treatment×time interaction: F6,48=1.15, p=0.35; figure 1c). The decrease was greater and the increase slower in the LW-removal colonies, so overall there were significantly fewer foragers after the experimental manipulation in the LW- than in the LWSW-removal colonies (F1,8=6.47, p=0.035; figure 1c). The mean sizes of foraging ants did not change over time (F7,49=1.2, p=0.32) and did not differ between the two treatments (treatment×time interaction: F7,49=0.0.907, p=0.509; treatment: F1,7=0.1, p=0.76; figure 1d).
(b) Caste genetics
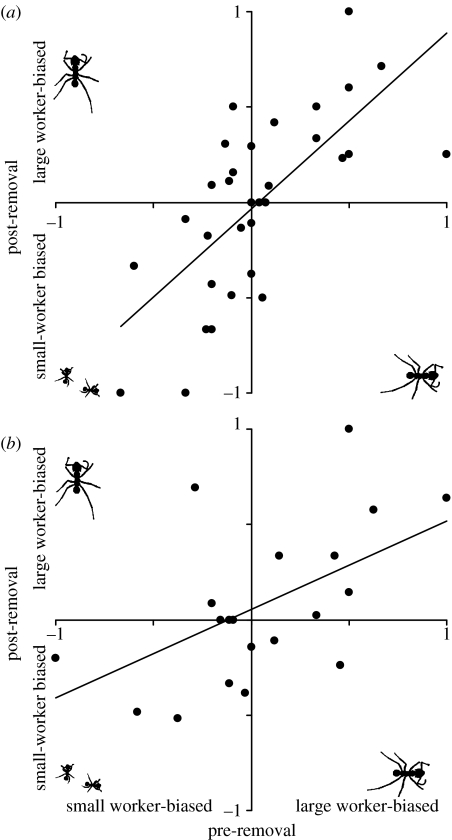
As expected, given that the same overall proportions of LW and SW were sampled for each colony, the treatments did not differ overall in the LW/SW ratios of their patrilines (F1,42=0.631, p=0.432) and there were also no differences between the colonies within treatments (F7,42=0.51, p=0.822). In both the LW- and LWSW-removal colonies, there was a positive, highly significant relationship between the LW/SW ratios of patrilines before and after treatment (F1,42=36.2, p<0.0001; figure 2). However, this relationship was approximately twice as steep for the LWSW-removal colonies as for the LW-removal colonies (interaction term: F1,42=4.17, p=0.047; figure 2). In both treatments, therefore, patrilines whose larvae tended to develop into one or other caste prior to the experimental manipulation tended to maintain that bias following the removal treatment, but this relationship was significantly weaker for the colonies from which most of the LW had been removed, both in terms of slope and variance explained (figure 2).
Figure 2.
Caste bias towards LW or SW for (a) 32 patrilines from five colonies from which both large workers and small workers were removed in equal numbers (LWSW-removal treatment), and (b) for 21 patrilines from five colonies from which only large workers were removed (LW-removal treatment). Data presented are for the same patrilines prior to (pre-removal; x-axes) and eight weeks after (post-removal; y-axes) the removal of workers. Each dot represents the bias of a single patriline with 0 indicating a 1 : 1 split between large workers and small workers, 1 indicating that a patriline was entirely large-worker-biased, and −1 indicating that a patriline was entirely small-worker-biased. Lines of best fit are: (a) y=0.95x−0.045, r2=0.51 and (b) y=0.473x+0.097, r2=0.33.
4. Discussion
The aim of the experiment was to increase the stimulus for large worker production in the LW-removal colonies as compared to the LWSW-removal colonies. The protocol achieved this goal with an estimated three quarters of LW being removed and there being clear evidence that this had a negative effect on the colonies. Compared with the LWSW-removal colonies, LW-removal colonies had fewer foragers, harvested less leaf material and built less fungus garden. This was in spite of the conservative approach of removing an equal numerical ratio of LW to SW from the LWSW-removal colonies rather than a ratio based on mass. Interestingly, the size of foragers did not differ between the treatments. There was thus no evidence of SW or intermediate-sized ants switching task to replace the LW removed from the LW-removal colonies. This was also found to be the case in a similar experiment with the leaf-cutting ant Atta cephalotes (Wilson 1983). In that study, colonies of A. cephalotes appeared to cope with the immediate impact of a loss of foragers by the remaining foragers increasing their activity (Wilson 1983). Acromyrmex echinatior may compensate for the loss of foragers in a similar manner in the short term. In addition, foragers spend the first weeks of their life within the fungus garden and it may be that these young foragers sped up their behavioural development to become active foragers, much as occurs in the honeybee (Huang & Robinson 1996; Chapman et al. 2007).
For the LWSW-removal colonies, the caste-skew of patrilines normally (i.e. at the start of the experiment) and at the end of the experimental period was very similar with the slope of the relationship being very close to 1. As the workers removed from these colonies were both LW and SW, the stimulus for brood to develop into LW should have been unchanged. The same patrilines would therefore be expected to be over- or under-represented in the LW before and after the removal, as was found. That the genotypic pattern was consistent over time in these colonies provides further confirmation that it is due to a genuine genetic influence.
The patrilines in the LW-removal colonies also tended to exhibit similar caste-skew before and after the experiment. Here, the majority of the LW had been removed so that the stimulus for brood to develop into LW was increased. As hypothesized earlier, there are essentially three possible responses to this increased stimulus: (i) patrilines normally biased towards LW may become even more biased, (ii) patrilines that are not normally biased to become LW, or which normally tend to become SW, may now become LW-biased, or (iii) all patrilines may respond similarly (or not at all). The slopes of the caste-skew relationships across patrilines allow us to distinguish between these possibilities. The first possibility would result in the LW-removal colonies having a steeper slope than the LWSW-removal colonies; the second possibility, in the LW-removal colonies having a shallower slope or even no relationship, while under the third possibility the relationships would be the same. The actual slope for the LW-removal colonies was about half that of the LWSW-removal colonies, demonstrating that the likelihood of some patrilines to become LW had been altered by the increase in stimulus. However, the fact that the relationship was still significantly positive indicates that the disposition of at least some patrilines to become LW under the increased stimulus was not completely divorced from that under normal conditions. Just as in honeybees (Fewell & Page 1993, 2000; Fewell & Bertram 1999; Hunt et al. 2003; Jones et al. 2004; Chapman et al. 2007), the genetic influence on division of labour is plastic and has thus evolved convergently in two distantly related taxa with complex societies and polyandrous queens.
A possible explanation for why the removal of LW did not alter the genotype–caste relationship to a greater extent may be that leaf-cutting ant colonies are buffered against the loss of foragers by virtue of having a store of their fungal food. A recent experiment examining genetic caste plasticity in the honeybee Apis mellifera produced a similar finding (Chapman et al. 2007). Here too, patrilines over- or under-represented in the foragers under normal conditions were also over- or under-represented after foragers were removed, and here too colonies may be buffered against this loss by their food stores of honey and pollen. In contrast, increases in stimuli for nursing, thermoregulation, pollen collecting and nest defence all result in more or different honeybee patrilines engaging in the tasks (Fewell & Page 1993, 2000; Fewell & Bertram 1999; Hunt et al. 2003; Jones et al. 2004; Chapman et al. 2007). It seems probable that the plasticity of the genetic influence on caste or task determination is therefore linked to the relative importance of the caste or task, with more genotypes responding to increases in stimuli for castes or tasks that are more immediately critical.
Hughes et al. (2003) found that the combination of genetic polymorphism and polyandry does not significantly affect the overall degree of worker polymorphism expressed by a colony. Rather, it was suggested that genetic polymorphism may increase the flexibility of the colony's response to changing conditions (Hughes et al. 2003), just as genetic polyethism can improve the division of labour in honeybee colonies (Jones et al. 2004). While the current study did not test whether genetic polymorphism had such an adaptive benefit, it did probe how it acts in practice. The fact, that the representation of patrilines changed when the demand for LW was increased, confirms that the genetic influence on morphological caste is phenotypically plastic and interacts with stimuli from the social environment. Rather than all patrilines being equally likely to become LW at a particular level of demand, they differ so that colony-level responses to changing demand become smoother and possibly more optimal (Crozier & Page 1985; Page et al. 1995; Fuchs & Moritz 1999; Bertram et al. 2003; Cox & Myerscough 2003; Hughes et al. 2003; Myerscough & Oldroyd 2004; Graham et al. 2006). Colonies are thus made up of genotypes with overlapping reaction norms in much the same way as genetic variation for phenotypic plasticity is expressed for many life-history traits (Stearns 1992). The convergent evolution of phenotypically plastic genetic influences on division of labour in leaf-cutting ants and honeybees suggests that they may be an intrinsic property of complex, genetically diverse societies. Further experiments are needed to establish whether genetic polymorphism for caste is indeed adaptive in bestowing a more flexible colony-level phenotype.
Acknowledgments
We thank the Smithsonian Tropical Research Institute for providing facilities in Gamboa for the collection of ant colonies, the Autoridad Nacional del Ambiente y el Mar (ANAM) for permission to export the ants from Panama to Denmark, Sylvia Mathiasen for technical assistance and two anonymous reviewers whose constructive comments helped improve the manuscript. We are grateful to the Carlsberg Foundation and the Danish Natural Sciences Research Council for funding.
References
- Bertram S.M, Gorelick R, Fewell J.H. Colony response to graded resource changes: an analytical model of the influence of genotype, environment, and dominance. Theor. Popul. Biol. 2003;64:151–162. doi: 10.1016/s0040-5809(03)00064-9. doi:10.1016/S0040-5809(03)00064-9 [DOI] [PubMed] [Google Scholar]
- Billick I. The relationship between the distribution of worker sizes and new worker production in the ant Formica neorufibarbis. Oecologia. 2002;132:244–249. doi: 10.1007/s00442-002-0976-7. doi:10.1007/s00442-002-0976-7 [DOI] [PubMed] [Google Scholar]
- Breed M.D. Allometry in the giant tropical ant, Paraponera clavata. Insectes Soc. 2002;49:125–128. doi:10.1007/s00040-002-8290-2 [Google Scholar]
- Brian M.V. Caste differentiation and division of labour. In: Hermann H.R, editor. Social insects. vol. 1. Academic Press; New York, NY: 1979. pp. 121–222. [Google Scholar]
- Chapman, N. C., Oldroyd, B. P. & Hughes, W. O. H. 2007 Differential responses of honey bee (Apis mellifera) patrilines to changes in stimuli for the generalist tasks of nursing and foraging. Behav. Ecol. Sociobiol (doi:10.1007/s00265-006-0348-0)
- Cox M.D, Myerscough M.R. A flexible model of foraging by a honey bee colony: the effects of individual behaviour on foraging success. J. Theor. Biol. 2003;223:179–197. doi: 10.1016/s0022-5193(03)00085-7. doi:10.1016/S0022-5193(03)00085-7 [DOI] [PubMed] [Google Scholar]
- Crozier R.H, Fjerdingstad E.J. Polyandry in social Hymenoptera—disunity in diversity? Ann. Zool. Fenn. 2001;38:267–285. [Google Scholar]
- Crozier R.H, Page R.E. On being the right size—male contributions and multiple mating in social Hymenoptera. Behav. Ecol. Sociobiol. 1985;18:105–115. doi:10.1007/BF00299039 [Google Scholar]
- Evans J.D, Wheeler D.E. Gene expression and the evolution of insect polyphenisms. Bioessays. 2001;23:62–68. doi: 10.1002/1521-1878(200101)23:1<62::AID-BIES1008>3.0.CO;2-7. doi:10.1002/1521-1878(200101)23:1<62::AID-BIES1008>3.0.CO;2-7 [DOI] [PubMed] [Google Scholar]
- Fewell J.H, Bertram S.M. Division of labor in a dynamic environment: response by honeybees (Apis mellifera) to graded changes in colony pollen stores. Behav. Ecol. Sociobiol. 1999;46:171–179. doi:10.1007/s002650050607 [Google Scholar]
- Fewell J.H, Page R.E. Genotypic variation in foraging responses to environmental stimuli by honey bees, Apis mellifera. Experientia. 1993;49:1106–1112. doi:10.1007/BF01929923 [Google Scholar]
- Fewell J.H, Page R.E. Colony-level selection effects on individual and colony foraging task performance in honeybees, Apis mellifera L. Behav. Ecol. Sociobiol. 2000;48:173–181. doi:10.1007/s002650000183 [Google Scholar]
- Field A. SAGE Publications; London, UK: 2000. Discovering statistics using SPSS for windows. [Google Scholar]
- Fraser V.S, Kaufmann B, Oldroyd B.P, Crozier R.H. Genetic influence on caste in the ant Camponotus consobrinus. Behav. Ecol. Sociobiol. 2000;47:188–194. doi:10.1007/s002650050010 [Google Scholar]
- Fuchs S, Moritz R.F.A. Evolution of extreme polyandry in the honeybee Apis mellifera L. Behav. Ecol. Sociobiol. 1999;45:269–275. doi:10.1007/s002650050561 [Google Scholar]
- Graham S, Myerscough M, Jones J, Oldroyd B. Modelling the role of intracolonial genetic diversity on regulation of brood temperature in honey bee (Apis mellifera L.) colonies. Insectes Soc. 2006;53:226–232. doi:10.1007/s00040-005-0862-5 [Google Scholar]
- Hölldobler B, Wilson E.O. Belknap Press; Harvard, MA: 1990. The ants. [Google Scholar]
- Huang Z.Y, Robinson G.E. Regulation of honey bee division of labor by colony age demography. Behav. Ecol. Sociobiol. 1996;39:147–158. doi:10.1007/s002650050276 [Google Scholar]
- Hughes W.O.H, Boomsma J.J. Does genetic diversity hinder parasite evolution in social insect colonies? J. Evol. Biol. 2006;19:132–143. doi: 10.1111/j.1420-9101.2005.00979.x. doi:10.1111/j.1420-9101.2005.00979.x [DOI] [PubMed] [Google Scholar]
- Hughes W.O.H, Sumner S, Van Borm S, Boomsma J.J. Worker caste polymorphism has a genetic basis in Acromyrmex leaf-cutting ants. Proc. Natl Acad. Sci. USA. 2003;100:9394–9397. doi: 10.1073/pnas.1633701100. doi:10.1073/pnas.1633701100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt G.J, Guzman-Novoa E, Uribe-Rubio J.L, Prieto-Merlos D. Genotype–environment interactions in honeybee guarding behaviour. Anim. Behav. 2003;66:459–467. doi:10.1006/anbe.2003.2253 [Google Scholar]
- Johnston A.B, Wilson E.O. Correlates of variation in the major minor ratio of the ant, Pheidole dentata (Hymenoptera, Formicidae) Ann. Entomol. Soc. Am. 1985;78:8–11. [Google Scholar]
- Jones J.C, Myerscough M.R, Graham S, Oldroyd B.P. Honey bee nest thermoregulation: diversity promotes stability. Science. 2004;305:402–404. doi: 10.1126/science.1096340. doi:10.1126/science.1096340 [DOI] [PubMed] [Google Scholar]
- Myerscough M.R, Oldroyd B.P. Simulation models of the role of genetic variability in social insect task allocation. Insectes Soc. 2004;51:146–152. doi:10.1007/s00040-003-0713-1 [Google Scholar]
- Nijhout H.F, Wheeler D.E. Juvenile hormone and the physiological basis of insect polymorphisms. Q. Rev. Biol. 1982;57:109–133. doi:10.1086/412671 [Google Scholar]
- Ortius-Lechner D, Gertsch P.J, Boomsma J.J. Variable microsatellite loci for the leaf cutter ant Acromyrmex echinatior and their applicability to related species. Mol. Ecol. 2000;9:114–116. doi: 10.1046/j.1365-294x.2000.00764-5.x. doi:10.1046/j.1365-294x.2000.00764-5.x [DOI] [PubMed] [Google Scholar]
- Oster G.F, Wilson E.O. Princeton University Press; Princeton, NJ: 1978. Caste and ecology in the social insects. [PubMed] [Google Scholar]
- Page R.E, Robinson G.E, Fondrk M.K, Nasr M.E. Effects of worker genotypic diversity on honey bee colony development and behavior (Apis mellifera L) Behav. Ecol. Sociobiol. 1995;36:387–396. doi:10.1007/s002650050161 [Google Scholar]
- Palmer K.A, Oldroyd B.P. Evolution of multiple mating in the genus Apis. Apidologie. 2000;31:235–248. doi:10.1051/apido:2000119 [Google Scholar]
- Passera L, Roncin E, Kaufmann B, Keller L. Increased soldier production in ant colonies exposed to intraspecific competition. Nature. 1996;379:630–631. doi:10.1038/379630a0 [Google Scholar]
- Rheindt F.E, Strehl C.P, Gadau J. A genetic component in the determination of worker polymorphism in the Florida harvester ant Pogonomyrmex badius. Insectes Soc. 2005;52:163–168. doi:10.1007/s00040-004-0787-4 [Google Scholar]
- Robinson G.E. Regulation of division of labor in insect societies. Annu. Rev. Entomol. 1992;37:637–665. doi: 10.1146/annurev.en.37.010192.003225. doi:10.1146/annurev.en.37.010192.003225 [DOI] [PubMed] [Google Scholar]
- Robinson G.E. Genomics and integrative analyses of division of labor in honeybee colonies. Am. Nat. 2002;160:S160–S172. doi: 10.1086/342901. doi:10.1086/342901 [DOI] [PubMed] [Google Scholar]
- Schwander T, Rosset H, Chapuisat M. Division of labour and worker size polymorphism in ant colonies: the impact of social and genetic factors. Behav. Ecol. Sociobiol. 2005;59:215–221. doi:10.1007/s00265-005-0027-6 [Google Scholar]
- Stearns S.C. Oxford University Press; Oxford, UK: 1992. The evolution of life histories. [Google Scholar]
- Sumner S, Hughes W.O.H, Pedersen J.S, Boomsma J.J. Ant parasite queens revert to mating singly. Nature. 2004;428:35–36. doi: 10.1038/428035a. doi:10.1038/428035a [DOI] [PubMed] [Google Scholar]
- Weber N.A. Gardening ants: the attines. Mem. Am. Phil. Soc. 1972;92:1–146. [Google Scholar]
- Wetterer J.K. The ecology and evolution of worker size-distribution in leaf-cutting ants (Hymenoptera: Formicidae) Sociobiology. 1999;34:119–144. [Google Scholar]
- Wheeler D.E. Developmental and physiological determinants of caste in social Hymenoptera—evolutionary implications. Am. Nat. 1986;128:13–34. doi:10.1086/284536 [Google Scholar]
- Wheeler D.E. The developmental basis of worker caste polymorphism in ants. Am. Nat. 1991;138:1218–1238. doi:10.1086/285279 [Google Scholar]
- Wilson E.O. Caste and division of labor in leaf-cutter ants (Hymenoptera, Formicidae, Atta). 3. Ergonomic resiliency in foraging by Atta cephalotes. Behav. Ecol. Sociobiol. 1983;14:47–54. doi:10.1007/BF00366655 [Google Scholar]