Abstract
Differential centrifugation of Triton X-100 or CHAPS lysates from control and cholesterol (CH) depleted MDCK II cells, segregated integral tight junction (TJ) proteins associated with detergent resistant membranes (DRMs) into two groups. Group A proteins (occludin, claudin-2 and -3) were detected in large, intermediate and small aggregates in both detergents, whereas group B proteins (claudin-1, -4 and -7) were observed in small aggregates in TX-100 and in intermediate and small aggregates in CHAPS. Depletion of CH altered the distribution of group A and B proteins among the three size categories in a detergent-specific manner. In lysates produced with octyl glucoside, a detergent that selectively extracts proteins from DRMs, group A proteins were undetectable in large aggregates and CH depletion did not alter the distribution of either group A or B proteins in intermediate or small aggregates. Neither occludin (group A) nor claudin-1 (group B) was in intimate enough contact with CH to be cross-linked to [3H]-photo-cholesterol. However, antibodies to either TJ protein co-immunoprecipitated caveolin-1, a CH-binding protein. Unlike claudins, occludin’s presence in TJs and DRMs did not require palmitoylation. Equilibrium density centrifugation on discontinuous OptiPrep gradients revealed detergent-related differences in the densities of TJ-bearing DRMs. There was little or no change in those densities after CH depletion. Removing CH from the plasma membrane increased tyrosine and threonine phosphorylation of occludin, and transepithelial electrical resistance (TER) within 30 min. After 2 h of CH efflux, phospho-occludin levels and TER fell below control values. We conclude that the association of integral TJ proteins with DRMS, pelleted at low speeds, is partially CH dependent. However, the buoyant density of TJ-associated DRMs is a function of the detergent used and is insensitive to decreases in CH.
Keywords: Tight junctions, occludin, claudins, cholesterol, detergent resistant microdomains
INTRODUCTION
Occludin [1] and the family of claudins [2, 3] are tetra-span integral membrane proteins that oligomerize to form the tight junction (TJ), a belt-like network of strands in the plane of the plasma membrane [4]. The TJ serves both as a fence between apical and basolateral membrane domains and as a regulated, selective permeability barrier in the paracellular space between adjacent epithelial and endothelial cells [3]. While a number of TJ integral membrane proteins have been identified, relatively little is known about how they are organized in the TJ. Using chemical cross-linking agents, detergent-free conditions and OptiPrep density gradients, it has recently been shown that proteins in the apical junctional complex are associated with a relatively small number of complexes [5]. The role of membrane lipids in segregating the various TJ proteins into these substructures, however, is not known. Since occludin and the claudins are oligomerized, tetra-span integral membrane proteins [6], it is reasonable to expect that lipids in the bilayer might play an important role in their assembly and organization into functional TJ stands [7].
One means used to test this notion is to determine the effect of modifying membrane lipids on TJ function. Increasing the fatty acid unsaturation index of clone-4 MDCK II glycerolipids with 18:3 (n-6), but not with 18:3 (n-3) or 18:1 (n-9), lengthened the time required to reseal TJs in a Ca2+ switch assay [8]. Altering the phospholipid polar head-groups of MDCK II cells, on the other hand, had no effect on TER [9]. That CH-rich lipid microdomains might play a role in TJ structure and function was suggested when it was shown that 72 h of incubation with Lovastatin, an inhibitor of CH synthesis, increased the rate of Ca2+ induced TJ assembly in MDCK II cells [10, 11]. When CH levels were reduced acutely, using methyl-β-cyclodextrin (MBCD) in monolayers with established TJs, there was a biphasic response. During the first 30-60 min TER increased by as much as 40%, and then decreased, approaching zero after 2 h of CH efflux [12]. If MBCD was removed at that time, CH content and TER returned to control levels within 12-24 h, indicating that CH depleted cells remained viable. Further evidence that membrane CH levels might be involved in TJ biology, came from studies which showed that occludin in TX-100 extracts of the T-84 intestinal epithelial cell line was recovered near the top of a sucrose gradient, where low-density CH/ glycosphingolipid, detergent resistant microdomains (DRM) are located; claudins were not examined [13]. In contrast, results from a recent study using the Caco-2 intestinal cell line, showed that occludin, claudin-1, -3 and -4 were detected in sucrose gradient fractions with densities greater than those containing flotillin, a known DRM associated protein. In the latter study TX-100 solubility of occludin, claudin-3 and -4, but not claudin-1, was increased by removal of CH [14]. Together these observations suggest that TJ stability is dependent, at least in part, on plasma membrane CH levels.
In view of the significant effect of CH depletion on TJ assembly and function, we proposed, in the present study to: 1. Determine whether occludin and claudins are in intimate contact with CH. 2. Examine whether depletion of membrane CH alters the solubility and buoyant density of these TJ proteins in two different classes of detergent. 3. Explore the possibility that, as in the case of the claudins [15], post-translational acylation of occludin is required for its incorporation into DRMs and TJs. 4. Test for the possibility that the early increase in TER, observed after addition of MBCD, might be associated with an increase of phosphorylated occludin, which has previously been shown to be present in epithelia with high, but not low TER [16].
MATERIALS AND METHODS
Reagents (as listed)
Dulbecco’s modified Eagle medium (DMEM)/Ham’s F-12, Earle’s balanced salt solution (EBSS), Dulbecco’s PBS (DPBS) (GIBCO, Grand Island, NY) and OptiPrep (Accurate Chemical Co. Westbury, NY). Newborn bovine calf serum (BCS) (Hyclone Laboratories, Logan, UT). Trypsin and deoxyribonuclease I (DNase I) (Worthington Diagnostic Systems, Freehold, NJ). Complete mini EDTA-free protease inhibitor tablets (Roche Diagnostics Corp. Indianapolis, IN). Trizma base, Celite, methyl-β-cyclodextrin (MBCD), sodium dodecylsulfate (SDS), Triton X-100 (TX-100), octyl β-D-glucopyranoside (octyl glucoside), sodium pyrophosphate and sodium orthovanadate (Sigma Chemical Co., St. Louis, MO). (3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate) (CHAPS) (Calbiochem Co. La Jolla CA). All organic solvents (Fisher Scientific, Pittsburgh, PA). Rabbit anti-occludin, -claudin-1, -claudin-2, -claudin-3, -claudin-7, -phosphothreonine, -phosphoserine polyclonal antibodies (pAb), mouse anti -claudin-4, anti-transferrin receptor and HRP-conjugated mouse anti-occludin monoclonal antibodies (mAb) (Zymed, South San Francisco, CA). HRP-conjugated mouse anti-phosphotyrosine (4G10) mAb (Upstate Biotechnology, Lake Placid, NY), rabbit anti-caveolin-1 pAb (Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-annexin II mAb (BD Biosciences, San Diego, CA), rabbit anti-actin, and the following HRP conjugated pAbs: goat anti-rabbit, rabbit anti-mouse and rabbit anti-rat (Sigma Chemical Co). Protein kinase inhibitors, genistein (Calbiochem, La Jolla, CA), staurosporine (Alexis Biochemicals, San Diego CA) and H7 (Sigma Chem. Co., St. Louis MO). Anti-ZO-1 mAb was generated from hybridoma R40.76 (gift form B.R. Stevenson). Photoactivatable CH and 10-azi-stearic acid were synthesized as described [7].
Cell Culture
MDCK II cells (CCL-34) (American Type Tissue Culture Collection, Rockville, MD) (passage levels 56-72) were maintained in antibiotic-free DMEM/Ham’s F-12, 10% BCS, sub-cultured weekly at a split ratio of 1:6 with a medium change at 2-3 d intervals. Experiments were conducted on monolayers plated on Falcon inserts (4.2 cm2, 0.4 μm pore size) (Becton Dickinson Labware, Franklin Lakes, NJ), Costar inserts (44 cm2, 0.4 μm pore size) (Corning, Corning, NY) or Millicell HA (0.6 cm2) (Millipore Corp. Bedford, MA) and cultured for 3-5 d in DMEM/Ham’s F-12, 1% BCS, 1% penicillin/ streptomycin (P/S).
CH depletion
Membrane CH was depleted by incubating monolayers in inserts with 10 mM MBCD in DMEM, 1% BCS applied to both apical and basolateral surfaces for either 0.5 h or 2 h at 37°C [12].
SDS extraction
Monolayers were rinsed with ice-cold TBS (10 mM Tris-HCl, 150 mM NaCl, pH 8.0), scraped and lysed in 1% SDS in TBS with (in mM) 1 PMSF, 20 sodium fluoride, 10 sodium pyrophosphate, 1 sodium orthovanadate. Lysates were boiled with intermittent vortexing and sheared through a 27-gauge needle. Protein concentration was determined using a Bio-Rad DC Protein Assay kit (Bio-Rad, Hercules, CA). Equal amounts of protein were resolved using NuPage 4-12% Bis-Tris gels (InVitrogen, Carlsbad, CA) and identified by Western blotting on PVDF membranes (InVitrogen). MagicMark standards (InVitrogen) were used in each gel.
TX-100 extraction
Monolayers were rinsed with ice-cold TN buffer (in mM) (25 Tris, 150 NaCl, pH 7.4), scraped from the insert in 1% or 3.25% TX-100 in TN buffer, with protease and phosphatase inhibitors (PPI) and incubated for 30 min at 4°C. Lysates were homogenized using 25 strokes in a tight fitting Dounce homogenizer at 4°C. Protein content of homogenates was adjusted to equal concentrations. An equal volume from each sample was centrifuged at 1000 g for 30 min at 4°C. After adding 1% SDS in TBS with PPI to the low-speed pellets (LSP), the mixture was boiled with intermittent vortexing and sheared 6 times through a 27-gauge needle. An aliquot of low-speed supernatant (LSS) was harvested. The remaining LSS was centrifuged at 107,600 g in a Sorvall AH 650 rotor overnight at 4°C to yield high-speed supernatants (HSS) and high-speed pellets (HSP). Proteins in LSP, HSP and HSS were resolved using Nu-Page 4-12% Bis-Tris gels or NuPage 3-8% Tris-Acetate gels (for ZO-1), transferred electrophoretically to PVDF membranes, identified by Western blotting and quantified by densitometry.
CHAPS and octyl glucoside extractions
Monolayers were treated by the same procedure as that described for TX-100 extraction, except that 1.2% or 4% CHAPS or 1.75% (60 mM) octyl glucoside (OG) in TN buffer with PPI was used to lyse cells.
Western blotting
Membranes were pre-incubated for 1 h at RT in 5% nonfat dry milk, 0.05% Tween-20 in 10 mM Tris-HCl, 154 mM NaCl, pH 7.5, or 0.5% BSA, 0.1% Tween-20 instead of nonfat dry milk, when probing with anti-phospho-amino acid antibodies. Membranes were incubated for 1 h at RT with one of the following 1° rabbit pAbs that recognized occludin, claudin-1, -2, -3, -7, caveolin-1, actin, phospho-threonine, or phospho-serine. Annexin II, transferrin receptor and claudin-4 were detected using mouse mAbs. ZO-1 was identified using rat R40-76 hybridoma supernatant. All primary antibodies were followed by incubation with appropriate HRP-conjugated secondary antibodies. Alternatively, HRP-conjugated mouse anti-occludin mAb or HRP conjugated mouse anti-phosphotyrosine was used in a one step procedure. Detection was with Western Lightning Chemi-luminescence Reagent Plus (PerkinElmer, Boston, MA). Protein bands were quantified by densitometry using a Fluor-S Multi-Imager and Quantity One software, (Bio-Rad, Hercules, CA).
OptiPrep density gradient centrifugation
Monolayers were rinsed in TN buffer, scraped from the substrate in 1% TX-100/TN buffer or 1.2% CHAPS/TN buffer with PPI at 4° C, and incubated for 30 min. on ice. The lysates were homogenized in a Dounce homogenizer. Protein content of the lysates was adjusted to equal concentrations. Lysates were centrifuged at 1000 g for 30 min at 4°C. This step was necessary since its omission resulted in a broad smear of opaque, white material in fractions 7-10 that was difficult to separate into individual fractions. Duplicate step gradients (for protein identification and CH determination, respectively, in harvested fractions) were established by mixing cell lysates with OptiPrep at a final iodixinol concentration of 30, 20,10%. Preparations were loaded into Beckman Quick-Seal centrifuge tubes (13 × 51 mm) and centrifuged in a VTi 90 vertical rotor (Beckman, Fullerton CA) at 354,000 g for 3 h at 4°C. Ten 0.5 ml fractions were collected from each gradient, beginning at the top. The density of each fraction was determined by refractometry using an Abbe Mark II refractometer (Reichert-Jung, Jena, Germany). Protein in each fraction was precipitated with methanol and chloroform, as described [17]. After drying in a Speed-Vac centrifuge (Savant Instruments, Holbrook, NJ), pellets were dissolved in 1× NuPAGE lithium dodecylsulfate (LDS) sample buffer with NuPage reducing agent and boiled. Proteins were resolved electrophoretically on NuPage Bis-Tris or Tris-acetate gels and identified by Western blotting. Band density was quantified by densitometry.
CH determinations
CH content in individual OptiPrep fractions obtained from CHAPS extracted cells was determined using an Amplex Red CH assay kit (A-12216) (Molecular Probes, Eugene, OR), according to the manufacturer’s instructions. Because TX-100 interfered with the fluorescence signal of the Amplex Red reagent, CH content in OptiPrep fractions of TX-100 extracted cells was determined using an alternative fluorescent method [12, 18].
Immunoprecipitation
Cell monolayers were rinsed with DPBS supplemented with 0.5 mM MgCl2, 1 mM CaCl2 and lysed in RIPA buffer (1% Triton X-100, 0.5% sodium deoxycholate, 0.2% SDS, 150 mM NaCl, 10 mM HEPES, pH 7.3) with PPI. Lysates were incubated on ice for 30 min, sheared 10 times through a 22-gauge needle, and centrifuged at 10,000 g at 4°C for 10 min. Protein content of supernatants was brought to equal concentration before pre-clearing with Protein A-Sepharose beads (Zymed) overnight at 4°C. After centrifugation, supernatants were incubated with either rabbit anti-occludin, anti-claudin-1, or anti-caveolin-1 pAb and Protein A-Sepharose beads for 3 h at 4°C. Beads were washed with RIPA buffer and boiled in 2× NuPAGE sample buffer with reducing agent. Proteins were resolved by gel electrophoresis and analyzed by Western blotting. For co-immuno-precipitation experiments 1% CHAPS, 0.05% SDS were used as detergents.
Photoaffinity lipid labeling experiments
i. To complex [3H]-photo-CH with MBCD 200 μl of [3H]-photo-CH (2 mCi/ml) in ethanol, in a sterile glass vial, was dried down in a stream of N2 gas [7] and the residue vortexed with 7 mg MBCD dissolved in 400 μl H2O. MDCK II cells were plated at 90% confluence and cultured for 72 h in DMEM/F-12, 5% delipidized BCS (98% CH -free), 1% P/S supplemented with a sufficient amount of photo-CH-MBCD complex to provide 50 μCi/ml [3H]-photo-CH [7]. After rinsing in DPBS, 0.1 mM CaCl2, 1 mM MgCl2, 2% delipidized BCS, monolayers were irradiated in this solution for 1 h at RT, using a UV lamp placed 12 ins above the cells. Cells were harvested in RIPA buffer with PPI and prepared for sequential immuno-precipitation of occludin, claudin-1 and caveolin-1. Following PAGE and electrophoretic transfer, PVDF membranes were dried overnight at RT and exposed to Bio Max film using a BioMax TranScreen-LE Intensifying Screen (Kodak Inc., Rochester, NY) at -80°C for either one or six weeks. ii. [3H]-phosphatidylcholine: 10 azi-stearic acid (10-ASA) in serum was prepared as follows: 3 mg of 10-ASA in hexane was adsorbed to 100 mg of Celite, dried under N2 gas, and 4 ml of delipidized BCS added and stirred for 30 min at RT. The serum was sequentially centrifuged twice at 15,000 g for 10 min at 4°C and filter sterilized [19]. To label cells with [3H]-choline, HEPES buffered DMEM lacking choline chloride, phenol red and NaHCO3 (DMEM-) was prepared. Individual components, minus those listed above, were added to an Earle’s Balance Salts solution (Gibco). To label cells with [3H]-choline and 10-ASA, MDCK II cells were plated at 90% confluence and cultured overnight in DMEM- with 44 mM NaHCO3, 28.6 μM choline chloride, 6% BCS, 1% P/S (labeling medium). Monolayers were rinsed and incubated overnight in labeling medium supplemented with 66.7 μCi/ml [3H]-choline chloride and 33 μl/ml 10-ASA in delipidized BCS. Monolayers were rinsed, UV irradiated in labeling medium without NaHCO3 for 1 h at RT and harvested in RIPA buffer with PPI. Sequential immuno-precipitation was conducted. Following PAGE, and electrophoretic transfer, the dried membrane was exposed to Bio Max film, as described above.
Metabolic labeling with [3H]-palmitic acid
MDCK II cells were plated at 50% confluence. Three h later cells were rinsed with and changed to serum-free DMEM and incubated for 2 h. The medium was then changed to DMEM, 5% dialyzed serum, 1% penicillin/streptomycin and 0.2 mCi/ ml of [3H]-palmitic acid (Amersham Biosciences) and incubated for 18 h. Cells in RIPA buffer were sheared through a 22-gauge needle. The lysate was centrifuged for 10 min at 10,000 g. Occludin, claudin-1 and claudin-2 were sequentially immunoprecipitated using the respective antibodies. Immunoprecipitates were electrophoresed under non-reducing conditions and transferred electrophoretically to PVDF membranes for Western blotting and autoradiography. A Bio-Max Transcreen was used with Bio-Max MS film and the film was exposed for either one or six weeks at -80°C.
Electron microscopy
LSP and HSP were generated from MDCK cell monolayers that had been homogenized with 1 or 3.25% TX-100 or with 1.2 or 4% CHAPS. The pellets were fixed in 3% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4 for 40 min followed by 2% glutaraldehyde in 0.1 M phosphate buffer for 40 min on ice. Pellets were dehydrated and embedded in Epon. Thin sections were stained with lead citrate and uranyl acetate and examined in a Philips 300 electron microscope.
RESULTS AND DISCUSSION
Cholesterol is highly enriched in the plasma membrane of eukaryotic cells, where a fraction of it has been shown to co-aggregate with the saturated acyl chains of sphingolipids and glycerophospholipids to form DRMs [20-22]. A number of biochemical and structural features of integral membrane proteins promote their inclusion in DRMs. These include: a. interactions between lipids and hydrophobic amino acid sequences in the trans-membrane domain [23], b. single and double acylation of specific amino acid side chains [21], c. addition of glycosylphosphatidylinositol anchors [24] and d. a close physical association with CH [7, 25]. Many of the proteins associated with DRMs are involved in signaling pathways [21]. Interestingly, TJ proteins share at least some of the attributes common to DRM associated proteins [6].
Studies summarized in a recent review [26] suggest that if the solubility profiles of a group of proteins are similar in two or more detergents, it is likely that their lipid microenvironments are similar. With this in mind, using MDCK II cells, we compared six integral TJ proteins with each other, and with a set of non-TJ-related proteins, with respect to their solubility in TX-100 and CHAPS before and after MBCD induced CH efflux. Although both of these detergents yield DRM fractions that are enriched in CH [27-29], they differ in that TX-100, is a non-ionic detergent that tends to denature proteins and preserve protein-protein interactions, while CHAPS is a zwitterionic detergent that preserves native structure, but more effectively disrupts weak, nonspecific protein-protein interactions [30]. TX-100 and CHAPS lysates can be harvested for analysis by subjecting them to either differential centrifugation or to density gradient centrifugation. However, whether a particular protein is identified as a component of a DRM is a function of the detergent used, its concentration and the particular cell type under investigation [29, 32-34]. The concentrations of 1% TX-100 [29] and 1.2% CHAPS [34], selected for use in these experiments, are widely used to extract DRMs. To confirm that these concentrations are sufficient to completely solubilize TJ-bearing membranes, the experiments were repeated using three-fold higher concentrations of each detergent; OG was used at a single concentration of 1.75%. The calculated protein to detergent ratio of each of the three detergents is indicated in Table 1. Using differential centrifugation, we first compared the ability of these three detergents to extract TJ proteins.
Table 1.
Protein to Detergent Mass Ratios
| Detergent | Concentration (%) | Protein (mg/ml) | Protein to detergent mass ratio |
|---|---|---|---|
| TX-100 | 1 | 1.74 ± 0.09 | 1:6.1 ± 0.3 |
| 3.25 | 1.50 ± 0.07 | 1:23.0 ± 1.04 | |
| CHAPS | 1.2 | 1.69 ± 0.14 | 1:7.1 ± 0.62 |
| 4 | 1.65 ± 0.09 | 1:24.3 ±1.24 | |
| Octyl glucoside | 1.75 | 1.68 ± 0.11 | 1:10.4 ± 0.75 |
MDCK II cell monolayers were lysed either in TX100, CHAPS or OG. Protein to detergent mass ratios were calculated from a minimum of four lysates for each of the indicated concentrations of detergents. Calculated values are the mean ± SD
Selected integral TJ proteins in TX-100 and CHAPS lysates are associated, in a partially CH dependent manner, with DRMs that are separable into three fractions by differential centrifugation
In our initial studies, we observed that the amount of occludin recovered in the low-speed, post-nuclear supernatant of TX-100 extracts from MBCD treated cells was increased relative to untreated control cells. This raised the possibility that in a relatively weak detergent, such as TX-100, a fraction of occludin is linked via CH dependent interactions to readily sedimented sub-cellular structures such as the cytoskeleton. To examine this in more detail and to extend our observations to other integral TJ proteins, TX-100 and CHAPS lysates of control MDCK II cells and those incubated with 10 mM MBCD for 0.5 or 2 h were subjected to differential centrifugation. The LSP, HSP and HSS fractions, recovered after differential centrifugation, were harvested as described in Materials and Methods. They were subjected to Western blot analysis using antibody probes for occludin, claudin-1, -2, -3, -4, -7 as well as several non-integral TJ proteins (Fig. 1). The latter included: a. ZO-1, a peripheral TJ protein [35], b. caveolin-1, a membrane protein that binds CH on the inner lipid leaflet of the plasma membrane [7, 36], c. annexin II, a protein that binds to acidic phospholipids in a calcium dependent manner and whose association with the plasma membrane is, in part, CH dependent [37, 38], d. actin, a cytoskeletal protein linked to the TJ via cytoplasmic adaptors [39] and e. transferrin receptor a membrane protein that is reported not to be associated with DRMs [40]. The data shown are representative of those obtained from experiments that were repeated a minimum of two times, using 1 and 3.25% TX-100 or 1.2 and 4% CHAPS. Similar differential centrifugation experiments were conducted with MDCK cell monolayers incubated for 30 min with MBCD and then lysed and homogenized with 1.75% OG.
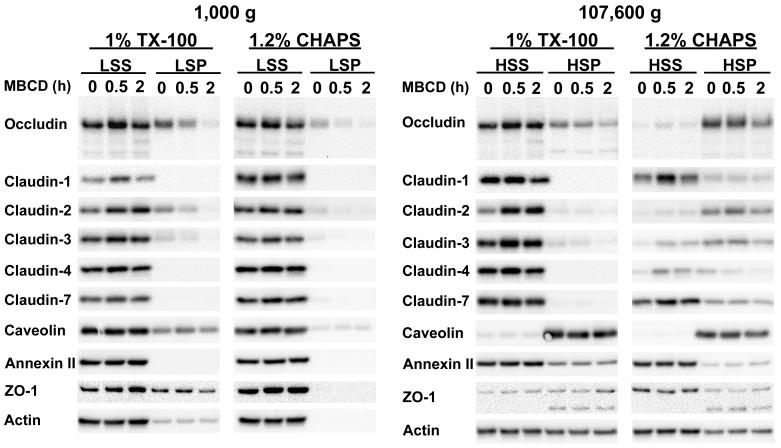
Figure 1.
Western blot analysis of MDCK II cell monolayers incubated with 10 mM MBCD for 0, 0.5 or 2 h. Monolayers were lysed either in TX-100 or CHAPS at 4°C and centrifuged at 1000 g for 30 min. Low speed pellets (LSP) and aliquots of the low speed supernatants (LSS) were retrieved. The remaining low speed supernatants were centrifuged at 4°C for 18 h at 107,600 g. High-speed supernatants (HSS) and high-speed pellets (HSP) were harvested. All supernatant and pellet fractions were processed for Western blotting and probed for occludin, claudin-1, -2, -3, -4, -7, caveolin-1, annexin-2, ZO-1 and actin. The data shown are representative of those obtained from a minimum of two independent experiments using TX-100 and CHAPS.
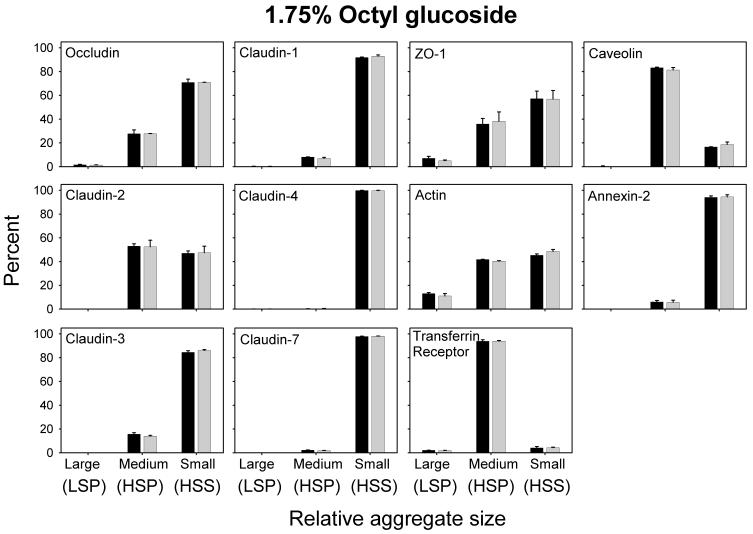
To simplify the discussion, we will refer to materials in the LSP, HSP, and HSS as large, medium and small sized aggregates, respectively. It should be noted that the HSS is likely a mixture of detergent-stabilized individual membrane proteins and protein aggregates, with or without accompanying lipids that are too small to be sedimented at 107,600 g. Our results (Figs. 1 and 2 A - D) show that TJ proteins from control cells (not incubated with MBCD) may be broadly separated into two groups, based on their distribution among large, medium and small aggregates following differential centrifugation of TX-100 or CHAPS lysates. Members of group A, (occludin, claudin-2 and -3), were found in all three size-classes in both TX-100 and CHAPS lysates (Fig 2A). In TX-100 extracts these proteins were located predominantly in the largest and smallest of the complexes, whereas in CHAPS lysates they were primarily in complexes of intermediate size. The fraction of occludin, claudin-2 and -3 associated with the large complexes in TX-100 lysates, decreased by 30-40% when the experiment was repeated in 1M NaCl, indicating that polar interactions play a role in maintaining the association of TJ proteins with the large and medium sized complexes. However, there was little or no effect of 1M NaCl on the distribution of the TJ proteins among the three fractions in CHAPS extracts (data not shown), an observation that is consistent with the zwitterionic nature of the latter detergent. Reducing cell CH content by 75% during 2 h of incubation with 10 mM MBCD [12], reduced the fraction of these three TJ proteins in the largest complexes by approximately 70%, 85% and 85%, respectively, while that in the HSS, containing the smallest complexes, increased. In CHAPS extracts, by contrast, 60-80% of the group A proteins in control cells were in the intermediate size complexes. Although only 15-20% of the remainder were associated with the largest complexes (LSP), that fraction declined by more than 50% after MBCD treatment with most of these proteins being recovered in either the intermediate size (HSP) or smallest complexes (HSS). That the displacement of group A proteins from the LSP was associated with a decrease in CH content was confirmed by analysis of the LSP for CH. Regardless of whether the fractions were derived from TX-100 or CHAPS lysates, the CH content in all cases was reduced by 70-95% (data not shown). These findings are consistent with there being a CH-dependent association between at least a fraction of TJ proteins in group A with other cell components, including members of the cytoplasmic scaffold proteins and/or the actin cytoskeleton [6, 41] to form complexes that sediment at low speeds. The data suggest further that the group A TJ proteins may reside in CH-rich microdomains that resist solubilization in TX-200 and CHAPS [29, 34]. Since DRMs are not solubilized by octylglucoside (OG), it has been suggested that proteins insoluble in TX-100 and CHAPS, but soluble in OG are bound to DRMS [31, 40]. We therefore, lysed control and MBCD-treated cells with 1.75% OG and subjected them to differential centrifugation. Nearly all of the TJ proteins in group A were displaced from the largest aggregates (LSP) into either the intermediate or smallest, most soluble fractions. Since OG solubilized the TJ proteins in the largest aggregates, we were unable to detect any change in the distribution of the group A proteins among the three fractions after MBCD-induced CH depletion.
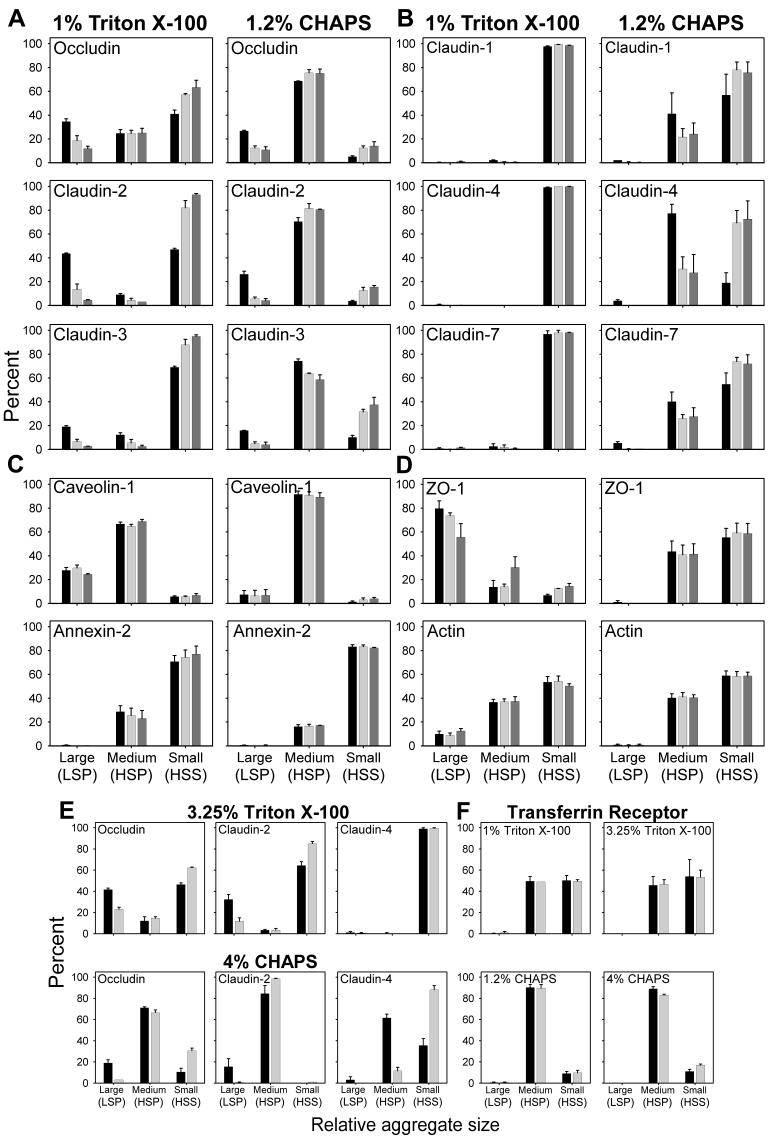
Figure 2.
Densitometric data derived from control MDCK II cells (black bar), treated for 30 min (light gray bars) or 2 h (dark gray bars) with 10 mM MBCD and lysed in either TX-100 or CHAPS at 4°C. The large aggregates are derived from low-speed pellets (LSP), the medium size aggregates are from high-speed pellets (HSP) and the small aggregates are obtained from high-speed supernatants (HSS). Densitometric measurements are derived from experiments described in the Fig. 1 legend. Values on the ordinate represent the percent of the protein in each fraction. Densitometric data from the two groups of integral TJ proteins (A and B), the lipid binding proteins (C) and TJ associated cytoplasmic proteins (D). Densitometric data from cell monolayers incubated with MBCD for 30 min and extracted with either 3.25% TX-100 or 4% CHAPS (E). Densitometric data of transferrin receptor from cells treated as in (E) and extracted with either of two concentrations of TX-100 or CHAPS (F). In (D) and (E) only data from control cultures and those incubated with MBCD for 30 min are shown.
A distinctly different solubility pattern was observed for members of group B (claudin-1, -4, and -7) (Fig. 2B). Only traces of these three proteins were recovered in large complexes in either TX-100 or CHAPS extracts. In TX-100 extracts, more than 90% of each of these three proteins was retrieved in the smallest complexes (HSS) and removal of CH had no effect on their distribution among the three fractions. The nearly complete solubilization of group B proteins by TX-100 does not exclude the possibility that they may reside in CH enriched DRMs. In fact, it has been suggested that the basic unit of a DRM may consist of a single protein surrounded by a sphingolipid/CH-rich lipid shell [24, 42], yielding a structure that would be likely to remain in the supernatant after centrifugation at 107,600 g. It is conceivable that such minimal DRM subunits are associated with each other in the native membrane to form aggregates that are disrupted completely by TX-100, but only partially by CHAPS. Evidence supporting the latter idea derives from the strikingly different solubility profile of these group B proteins in CHAPS extracts, where a sizeable fraction of each was associated with aggregates of intermediate size that was, at least in part, sensitive to the removal of CH (Fig. 2B). Since removal of CH altered the solubility of these TJ proteins in CHAPS lysates, it suggests that they, like the group A TJ proteins, are also components of a CH enriched DRM. In OG lysates, group B proteins were retrieved almost exclusively in the HSS fraction. The fact that they do not appear to form large complexes in either TX-100 or OG suggests that their link to cytoplasmic protein aggregates is more tenuous than those TJ proteins in group A.
To address the question of whether the TJ proteins retrieved in the LSP might result from incomplete solubilization of the plasma membrane, these experiments were repeated with detergent concentrations of TX-100 and CHAPS that were increased 3-fold. This increased the protein to detergent ratio from 1:6 to over 1:20 (Table 1). In addition, we probed for the transferrin receptor, a non-DRM associated protein [29]. As shown in Fig. 2E, increasing the detergent concentration had little effect on the distribution of TJ proteins among the three fractions. In contrast, none of the transferrin receptor was observed in the LSP in either detergent regardless of the concentration employed (Fig. 2F). Electron microscopy conducted on glutaraldehyde and osmium tetroxide fixed LSP and HSP obtained from 1 or 3.25% Triton X-100 and from 1.2% or 4% CHAPS preparations showed amorphous material and nuclear fragments that were similar in appearance irrespective of the concentration or the type of detergent used. No intact membranes or vesicles were seen (data not shown).
The lipid binding proteins (caveolin-1 and annexin-II) and the cytoplasmic proteins (ZO-1 and actin) were also distributed among the three fractions in a detergent dependent manner (Fig. 2C and D). Reducing cell CH had no effect upon the distribution of caveolin-1 and annexin-II among these three fractions, which may be explained by the fact that both of these proteins are localized to the inner lipid leaflet of the plasma membrane where they would be less accessible to the cell impermeant MBCD [36, 37]. Caveolin-1 was found predominantly in the HSP fraction containing intermediate size complexes, while annexin II was retrieved mainly in the small aggregate, HSS fraction. The solubility of ZO-1 in TX-100 and its CH dependence was most similar to those TJ proteins of group A, suggesting that the links between this TJ scaffold protein and group A TJ proteins were partially preserved. It is conceivable that binding sites in the long C-terminal domains of occludin, claudin-2 and -3 (255, 42, and 31 amino acids in length, respectively) facilitate their interaction with peripheral TJ proteins. The shorter cytoplasmic domains of claudins-1, -4, and -7 (22, 21 and 21 amino acids in length, respectively) may be linked to cytoskeletal/ scaffolding proteins by weaker interactions that are readily disrupted by TX-100. However, further studies are required to rigorously establish the mechanisms by which integral TJ proteins interact and bind to members of the TJ scaffold proteins.
Neither occludin nor claudin-1 is covalently bound to radiolabeled, photoactivatable CH after UV irradiation
That the distribution of the six integral TJ proteins, among the three fractions isolated by differential centrifugation in TX-100 or CHAPS, was altered by CH depletion suggested that they might reside in membrane domains in which they are intimately associated with CH. To test this hypothesis MDCK II cells, pre-loaded with radiolabeled [3α-3H]6-azi-5α-cholestan-3β-ol (photoactivatable CH) [7], were subjected to UV irradiation. Under these conditions any protein closely associated with plasma membrane CH via non-covalent interactions should become covalently linked to the radiolabeled sterol. In a parallel experiment, cells pre-loaded with [3H]-choline and 10-azi-stearic acid (10-ASA), were similarly irradiated to crosslink proteins bound via non-covalent interactions to choline containing membrane lipids, including sphingomyelin and/or phosphatidyl choline. Lysates prepared in RIPA buffer were immunoprecipitated with antibodies that recognize either occludin (group A) or claudin-1 (group B) and subjected to Western blot analysis. The blots were either probed with antibody for the relevant protein, or subjected to autoradiography. As a positive control, caveolin-1, a plasma membrane protein that resides in CH, sphingolipid-rich microdomains in the inner lipid leaflet and is known to react with photoactivatable CH and sphingolipid [7], was immunoprecipitated from the lysates and similarly analyzed. The data in Fig. 4A show that immunoprecipitated occludin and claudin-1 were readily detected in Western blots, however, no corresponding radiolabeled band was observed despite development of the autoradiogram for up to six weeks. Both Western blotting and autoradiography, by contrast, detected caveolin-1. Despite MBCD-induced shifts in their distribution among the three fractions isolated by differential centrifugation, if these TJ proteins reside in CH and sphingolipid-containing DRMs, their presence there is not dependent on a direct and intimate association with CH or choline-containing lipids.
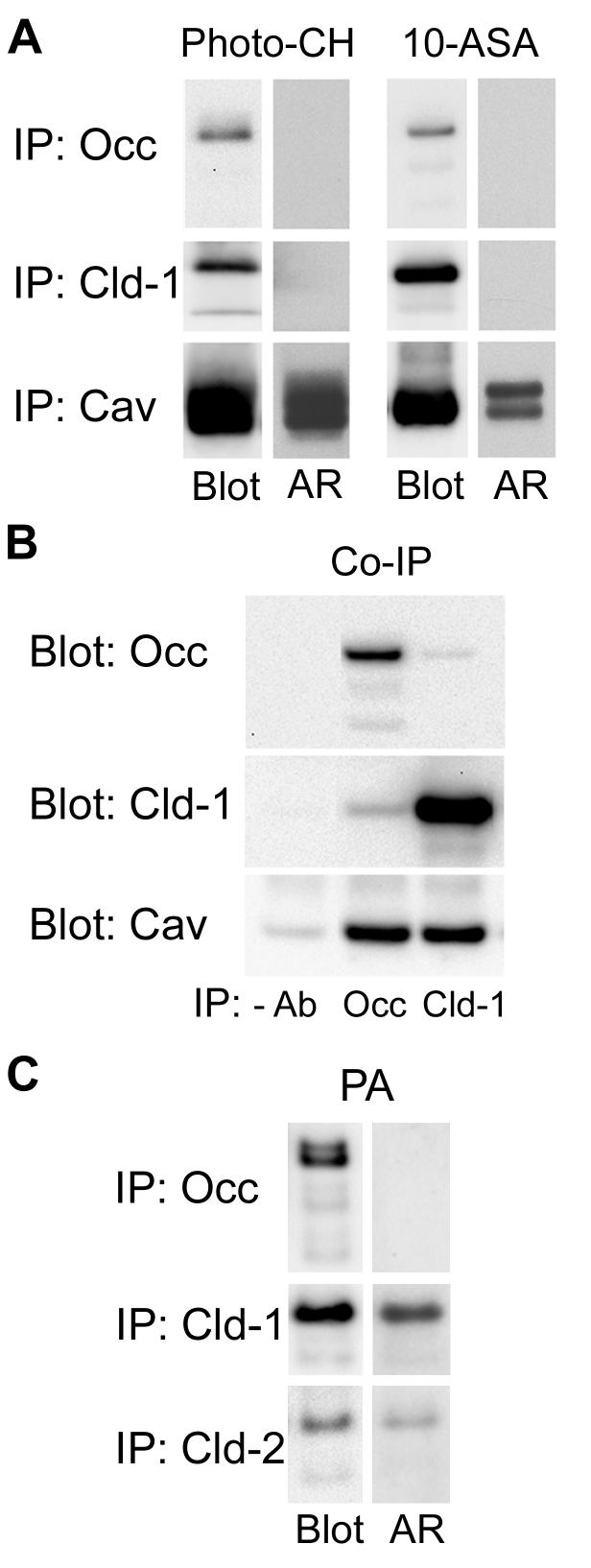
Figure 4.
A. MDCK II cell monolayers were incubated with either [3H]-photo-CH or [3H]-choline and 10-ASA as described in Materials and Methods. Following photo-activation, occludin, claudin-1 and caveolin-1 were sequentially immunoprecipitated (IP) in RIPA buffer. Dried PVDF membranes were subjected to autoradiography (AR) and probed for the respective immunoprecipitated protein (Blot). Whereas [3H]-photo-CH and [3H]-photo-PC prominently labeled caveolin-1, neither occludin nor claudin-1 were labeled. B. Occludin or claudin-1 was immunoprecipitated from lysates in 1% CHAPS, 0.05% SDS and the blots were probed with anti-occludin, anti-claudin-1 or anti-caveolin-1. Caveolin-1 is co-immunoprecipitated by both anti-occludin and anti-claudin-1. C. MDCK II cell monolayers were metabolically labeled with [3H]-palmitic acid (PA) as described in Materials and Methods. Occludin, claudin-1 and claudin-2 were sequentially immunoprecipitated. Dried PVDF membranes were subjected to autoradiography (AR) and probed for the respective immunoprecipitated protein (Blot). [3H]-palmitate labels claudin-1 and -2 but not occludin.
In an earlier study, occludin was reported to co-immunoprecipitate with caveolin-1 [13], which is readily cross-linked to the photoactivatable CH analog used in the current study. Using lysates prepared under conditions that preserve protein-protein interactions, we confirmed that a fraction of caveolin-1 co-immunoprecipitates with occludin and showed further that caveolin-1 also co-immunoprecipitates with claudin-1 (Fig. 4B). Since in the experiments with photoactivatable CH, the individual proteins (occludin, claudin-1 and caveolin-1) were immunoprecipitated under conditions (RIPA buffer) that disrupt protein-protein interactions, it remains to be determined whether the caveolin-1 that co-immunoprecipitates with occludin and claudin-1 has CH bound to it. That we were unable to demonstrate photo-cholesterol binding to the TJ proteins examined may be the result of insufficient contact between TJ proteins and photo-cholesterol. Alternatively, because of the tightly packed organization of TJ proteins in TJ strands, they may be inaccessible to MBCD bound photo-cholesterol.
Claudin-1 and -2 are palmitoylated but occludin is not
Incorporation of claudin-14 into TJs has recently been shown to be dependent on its palmitoylation [15], a post-translational modification that may increase the affinity of integral membrane proteins for DRMS [21],. To determine whether occludin has a similar requirement, we metabolically labeled MDCK II cells with [3H]-palmitic acid. Autoradiographs prepared from Western blots of occludin, claudin-1 and -2 immunoprecipitates showed that claudin-1 and -2 were clearly labeled with [3H]-palmitic acid, while occludin was not (Fig. 4C). These results indicate that, in contrast to the claudins, occludin does not require palmitoylation for its insertion into the TJ or for its association with DRMs.
The buoyant density profile of TJ proteins is detergent dependent
Given the fact that a. claudins-1 and -2 from MDCK cells are recovered in low-density, TX-100 insoluble membrane fractions [43], and b. that the solubility patterns of TJ proteins in TX-100 and CHAPS are altered following CH depletion (Figs. 1 and 2), the possibility remains that these and other TJ proteins are components of CH-rich microdomains.
To investigate this possibility further, cells incubated with or without MBCD for either 30 min or 2 h, were lysed in TX-100 or CHAPS, centrifuged briefly at low speeds to remove interfering insoluble protein aggregates, and the resulting LSS loaded onto discontinuous OptiPrep density gradients. Western blotting and densitometric analysis of each fraction was used to compare the distribution of TJ and selected non-TJ proteins in the gradient with that of CH, a component of DRMs. It should be noted that for technical reason it was necessary to subject all lysates to a low speed spin prior to loading them onto the OptiPrep gradient. Since, as noted above, a larger proportion of some TJ proteins (occludin, claudin-2) are recovered in the LSP of control cells than in the LSP from cells treated with MBCD, the amount of TJ proteins loaded onto the gel is, therefore, less for control lysates that those from MBCD treated cells (Fig. 1). For that reason the pixel intensity values of a given protein, obtained by densitometry of Western blots, were plotted as a percent of the sum of pixel intensities for that protein in the 10 fractions. This enabled us to directly compare density profiles for each protein in MBCD treated and control preparations.
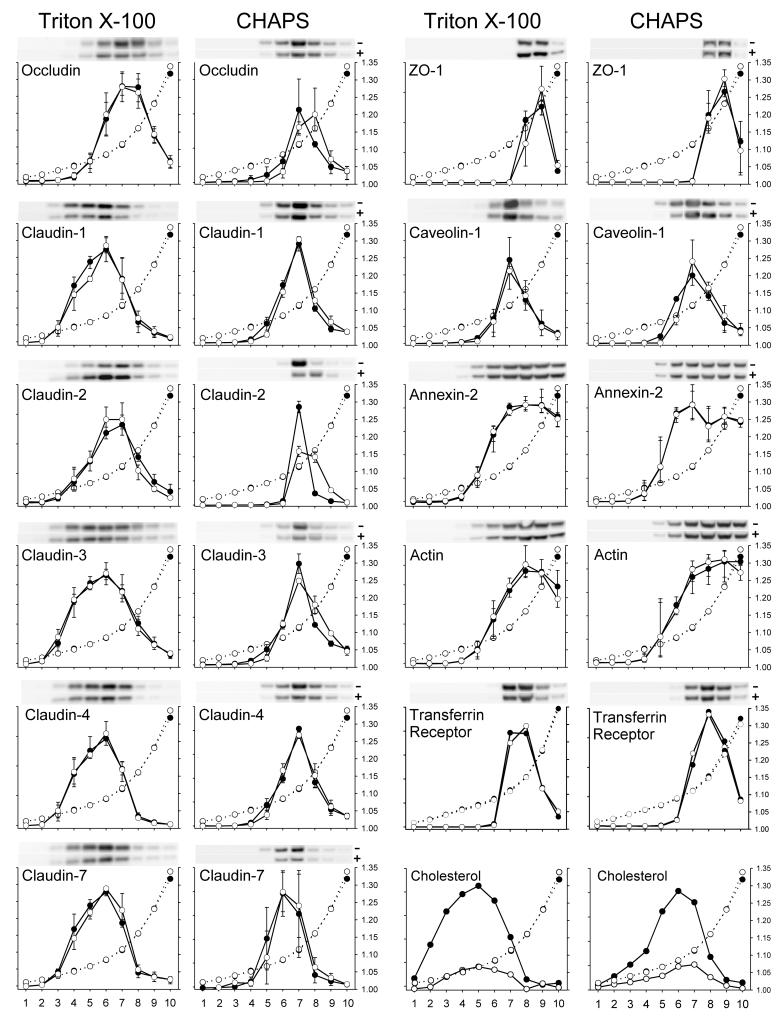
Claudin-1, -2, -3, -4 and -7 from TX-100 lysates of control cells were distributed primarily among fractions 3 - 8 with peak amounts detected in fraction 6 (d ∼1.08 g/ml). By contrast, occludin was detected in higher density fractions (fractions 5-9) with peak amounts present in fraction 7 (d∼1.12 g/ml) (Fig. 5). The distribution of DRMs bearing TJ proteins in TX-100 lysates is quite broad indicating their variable densities. Caveolin-1, an oligomerized protein that binds CH, was concentrated in fewer fractions (6-9) with a peak in fraction 7. Interestingly, the density profiles of CH containing DRMs were also distributed broadly throughout the gradient with peak levels in fractions less dense (d∼1.06 g/ml) than those bearing TJ proteins. Based on their overlap with the CH density profile, the DRMs containing claudins 1, 3, 4, and 7 were located in fractions that contained approximately 60% of the CH, while occludin, claudin-2 and caveolin-1 were restricted to more dense fractions that overlapped with approximately 20, 40 and 20%, respectively, of the total CH. Surprisingly, despite a reduction in cell CH levels by ca. 50% and 75% following incubations with MBCD for 30 min and 2 h, respectively, there was no change in the density profiles of the proteins, suggesting either that MBCD was ineffective in reducing CH in DRMS bearing TJ proteins or that the removal of CH had little or no effect on their density.
Figure 5.
Densitometric data of 10 fractions each, obtained from discontinuous OptiPrep step gradients of control MDCK II cells (black circles) or MDCK II cells treated with 10 mM MBCD for 2h (white circles). The cells were lysed in either 1% TX-100 or 1.2% CHAPS. Lysates were prepared and OptiPrep gradients were centrifuged as described in Materials and Methods. Fractions, 0.5 ml, were collected beginning from the top of the gradient and prepared for Western blotting as described in Materials and Methods. Parallel gradients were run for CH determinations and to determine the refractive index for each fraction (white circles and dotted lines). Density in g/ml is indicated on the right hand Y-axis, while fraction number is indicated on the X-axis. The percent of a particular protein in each fraction is plotted on the left Y-axis and was estimated by dividing the pixel intensity of that protein in that fraction by the sum of its pixel intensities in all 10 fractions. All data obtained from TX-100 extracts are the mean and range bars of three independent experiments, whereas the data from the CHAPS extracts are derived from two independent experiments. Data from the transferrin receptor, a non-DRM associated protein, are included for comparison.
In CHAPS lysates, the density profiles of TJ proteins differed in several respects from those in TX-100 (Fig. 5). Most striking was our observation that DRMs bearing TJ proteins and CH were shifted to denser fractions with peak amounts of all TJ proteins and caveolin-1 being located in fraction 7 (d∼1.12). In addition, these DRMs were distributed over 3-4 fractions, suggesting that they are less polydisperse than those in TX-100 lysates that span 7-8 fractions. The fact that TJ proteins were shifted to higher densities in CHAPS as compared to TX-100 may reflect differences in the propensity of these two detergents to extract or solubilize proteins and lipids from membranes [28] Since TX-100 more effectively extracts proteins than lipids from DRMs and CHAPS has the opposite effect, the lipid/protein ratio of the residual DRMS would be expected to be higher and density lower in TX-100 than in CHAPS. In CHAPS extracts, the distribution of DRMs containing TJ proteins and caveolin-1 overlapped more closely with those containing CH, than those present in TX-100. Except for occludin and claudin-2 and -3, the buoyant density properties of TJ proteins in CHAPS were relatively unaffected by CH depletion. However, after 2h of MBCD treatment, there was some broadening in the distribution of fractions containing the group A proteins (occludin, claudin-2 and -3) with a slight shift to higher density fractions. Since there is some degradation of TJ proteins after 2 h of MBCD treatment these experiments were repeated using cells treated with MBCD for only 30 min, a time when no degradation is detected. In these preparations the tendency of occludin, claudin-2 and -3 to shift to higher densities was much less pronounced (data not shown). The density profile of the transferrin receptor is displaced toward higher density proteins as expected for a non-DRM associated protein.
In a parallel set of experiments OG lysates wee analyzed by density gradient centrifugation. This detergent’s ability to release all TJ proteins from the LSP (Fig. 3) allowed us to load equal amounts of TJ proteins from control and MBCD treated cells onto the gradient after a brief low speed spin. The distribution of the TJ proteins in the Optiprep gradient as well as the peak densities were virtually identical to those observed with TX-100 lysates (data not shown).
Figure 3.
Densitometric data derived from control MDCK II cells (black bar), treated for 30 min (light gray bars) with 10 mM MBCD and lysed in 1.75% OG. In contrast to TX-100 and CHAPS lysates, virtually no group A TJ proteins are detected as large aggregates in the LSP, while the distribution of group B TJ proteins is similar to that observed in TX-100 and CHAPS lysates. Furthermore, in contrast to TX-100 and CHAPS preparations, incubation with 10 mM MBCD did not alter the distribution of these proteins in the different fractions.
Rapid extraction of membrane CH is associated with transient tyrosine and threonine phosphorylation of occludin
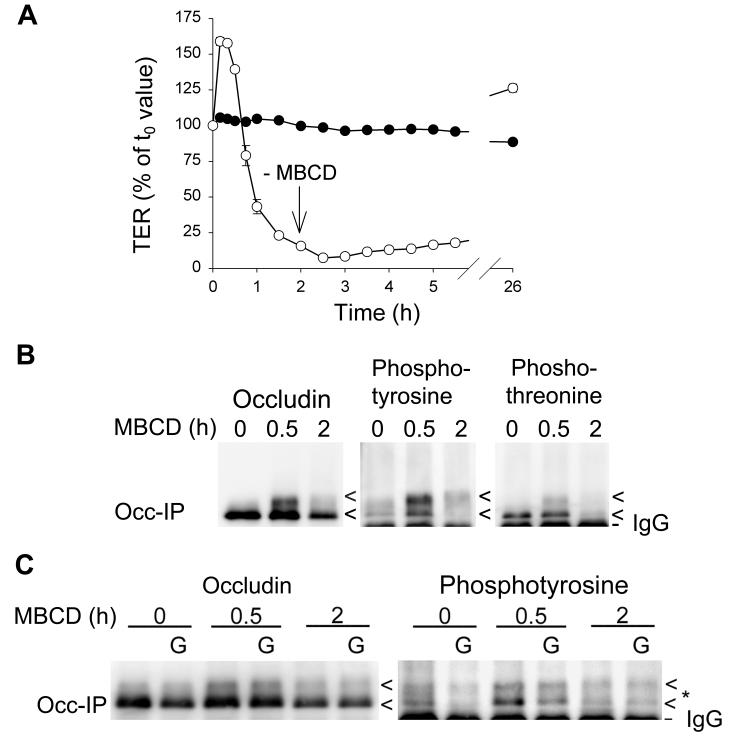
In addition to CH playing a direct role in the physical organization of TJs, it may also function indirectly by regulating kinases and phosphatases that are involved in regulating the phosphorylation of occludin and/or other proteins required for TJ structure and function. Functionally, the addition of 10 mM MBCD to MDCK II cell monolayers results in a rise in TER during the first hour, which then slowly declines to near zero by 2 h. If MBCD is removed at that time, TER returns to values equal to or greater than control cultures (Fig. 6A) [12]. During the first 30 min of CH depletion, when TER is rising, diffuse, higher molecular weight bands of occludin were observed on Western blots (Fig. 6B, C). We hypothesized that these might represent phospho-occludin. We, therefore, probed occludin immunoblots obtained after 0, 0.5 and 2 h of MBCD treatment, using antibodies that recognized phosphotyrosine, phosphothreonine or phosphoserine. Phosphotyrosine and lesser amounts of phosphothreonine were detected in occludin at 0.5 h, but no staining for phosphoserine was observed. After 2 h of MBCD treatment, levels of phosphotyrosine and phosphothreonine were reduced (Fig. 6B). To determine the contribution of a tyrosine kinase, MBCD treatment was conducted with and without Genistein, an inhibitor of tyrosine kinase. The presence of Genistein reduced, but did not eliminate completely, the level of phosphotyrosine staining (Fig. 5C), suggesting that a tyrosine kinase is activated upon rapid removal of membrane CH. However, the increase in TER, observed within 0.5 h of adding MBCD, was unaffected by Genistein (data not shown), indicating that the increase in tyrosine phosphorylation of occludin is unrelated to the increase in TER or alternatively that transient phosphorylation of threonine residues (Fig. 6B) is sufficient to elevate TER. Surprisingly, incubation of MBCD treated monolayers with either of the protein kinase inhibitors, staurosporine or H7, resulted in an increase in both TER and phosphorylated occludin (data not shown), suggesting the possibility that the activity of a phosphorylation-dependent phosphatase was inhibited. Further studies will be required to identify the precise enzymes that are involved.
Figure 6.
A. Sequential TER measurements of MDCK II cell monolayers that were treated with 10 mM MBCD for 2 h (white circles). The control monolayers (black circles) underwent medium changes at the same time that MBCD was either added or removed from the experimental monolayers. TER falls to near zero within 2 h of adding MBCD. Within 24 h of removing MBCD, TER returns to control levels. B. Occludin was immunoprecipitated from untreated monolayers or from monolayers incubated for 0.5 or 2 h with 10 mM MBCD and the immunoprecipitates were subjected to Western blotting. The Western blots were probed with anti-occludin, anti-phosphotyrosine or anti-phosphothreonine antibodies. C. Monolayers of MDCK II cells were treated with 10 mM MBCD without or with 100 μM Genistein (G) for 0, 0.5 or 2 h. Occludin was then immunoprecipitated and Western blots were probed using either anti-occludin (loading control) or anti-phosphotyrosine antibodies. TER data are the mean +/- SD of triplicate inserts. Data in Fig. 6A and B are representative of three independent experiments and the data in Fig 6C are from a single experiment.
In conclusion, the rapid removal of membrane cholesterol has a significant, but reversible effect on the physical and functional properties of integral TJ proteins. While claudins-1 and-2, but not occludin, are palmitoylated, none of the integral TJ proteins examined were in sufficiently intimate contact to bind [3H]-photoactivatable CH. However, reducing membrane CH had little effect on the buoyant density profiles of the TJ-bearing DRMs supporting the view that in the native membrane, these proteins are in DRMs that are inaccessible to MBCD, or that their extractability by the detergents used is unaffected by reducing CH levels.
ACKNOWLEDGEMENTS
This study was supported by NIH grants HL25822 and HL36781. We thank C. Yeaman for his helpful advice in the use of OptiPrep gradients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: A novel integral membrane protein localizing at tight junctions. Journal of Cell Biology. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furuse M, Fujita K, Fujimoto K, Tsukita S. Claudin 1 and 2: Novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. Journal of Cell Biology. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nature Reviews Molecular Cell Biology. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 4.Staehelin LA. Further observations on the fine structure of freeze-cleaved tight junctions. Journal of Cell Science. 1973;13:763–786. doi: 10.1242/jcs.13.3.763. [DOI] [PubMed] [Google Scholar]
- 5.Vogelmann R, Nelson WJ. Fractionation of the epithelial apical junctional complex: Reassessment of protein distributions in different substructures. Molecular Biology of the Cell. 2005;16:701–716. doi: 10.1091/mbc.E04-09-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneeberger EE, Lynch RD. The tight junction: A multifunctional complex. AJP: Cell Physiology. 2004;286:C1213–C1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 7.Thiele C, Hannah MJ, Fahrenholz F, Huttner WB. Cholesterol binds to synaptophysin and is required for biogenesis of synaptic vesicles. Nature Cell Biology. 2000;2:42–49. doi: 10.1038/71366. [DOI] [PubMed] [Google Scholar]
- 8.Schneeberger EE, Lynch RD, Kelly CA, Rabito CA. Modulation of tight junction formation in clone 4 MDCK cells by fatty acid supplementation. AJP: Cell Physiology. 1988;254:C432–C440. doi: 10.1152/ajpcell.1988.254.3.C432. [DOI] [PubMed] [Google Scholar]
- 9.Calderon V, Lazaro A, Contreras RG, Shoshani L, Flores-Maldonado C, Gonzalez-Mariscal L, Zampighi G, Cereijido M. Tight junctions and the experimental modifications of lipid content. Journal of Membrane Biology. 1998;164:59–69. doi: 10.1007/s002329900393. [DOI] [PubMed] [Google Scholar]
- 10.Lynch RD, Tkachuk LJ, Ji X, Rabito CA, Schneeberger EE. Depleting cell cholesterol alters calcium-induced assembly of tight junctions by monolayers of MDCK cells. European Journal of Cell Biology. 1993;60:21–30. [PubMed] [Google Scholar]
- 11.Stankewich MC, Francis SA, Vu QU, Schneeberger EE, Lynch RD. Alterations in cell cholesterol content modulate of calcium induced tight junction assembly by MDCK cells. Lipids. 1996;31:817–828. doi: 10.1007/BF02522977. [DOI] [PubMed] [Google Scholar]
- 12.Francis SA, Kelly JM, McCormack JM, Rogers RA, Lai J, Schneeberger EE, Lynch RD. Rapid reduction of MDCK cell cholesterol by methyl-β-cyclodextrin alters steady state transepithelial electrical resistance. European Journal of Cell Biology. 1999;78:473–484. doi: 10.1016/s0171-9335(99)80074-0. [DOI] [PubMed] [Google Scholar]
- 13.Nusrat A, Parkos CA, Verkade P, Foley CS, Liang TW, Innis-Whitehouse W, Eastburn KK, Madara JL. Tight junctions are membrane microdomains. Journal of Cell Science. 2000;113:1771–1781. doi: 10.1242/jcs.113.10.1771. [DOI] [PubMed] [Google Scholar]
- 14.Lambert D, O’Neill CA, Padfield PJ. Depletion of Caco-2 cell cholesterol disrupts barrier function by altering the detergent solubility and distribution of specific tight junction proteins. Biochemical Journal. 2005;387:553–560. doi: 10.1042/BJ20041377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Itallie CM, Gambling TM, Carson JL, Anderson JM. Palmitoylation of claudins is required for efficient tight junction localization. Journal of Cell Science. 2005;118:1427–1436. doi: 10.1242/jcs.01735. [DOI] [PubMed] [Google Scholar]
- 16.Sakakibara A, Furuse M, Saitou M, Ando-Akatsuka Y, Tsukita Y. Possible involvement of phosphorylation of occludin in tight junction formation. Journal of Cell Biology. 1997;137:1393–1401. doi: 10.1083/jcb.137.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wessel D, Flugge UI. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Analytical Biochemistry. 1984;138:141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- 18.Goh EH, Krauth DK, Colles SM. Analysis of cholesterol and desmosterol in cultured cells without organic solvent extraction. Lipids. 1990;25:738–741. doi: 10.1007/BF02544043. [DOI] [PubMed] [Google Scholar]
- 19.Spector AA, Hoak JC. An improved method for the addition of long-chain free fatty acid to protein solutions. Analytical Biochemistry. 1969;32:297–302. doi: 10.1016/0003-2697(69)90089-x. [DOI] [PubMed] [Google Scholar]
- 20.Brown DA, London E. Functions of lipid rafts in biological membranes. Annual Review of Cell and Developmental Biology. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 21.Simons K, Toomre D. Lipid rafts and signal transduction. Nature Reviews Molecular Cell Biology. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 22.Simons K, Ikonen E. How cells handle cholesterol. Science. 2000;290:1721–1726. doi: 10.1126/science.290.5497.1721. [DOI] [PubMed] [Google Scholar]
- 23.Scheiffele P, Roth MG, Simons K. Interaction of influenza virus haemagglutinin with sphingolipid-cholesterol membrane domains via its transmembrane domain. EMBO Journal. 1997;16:5501–5508. doi: 10.1093/emboj/16.18.5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson RGW, Jacobson K. A role for lipid shells in targeting proteins to caveolae, rafts and other lipid domains. Science. 2002;296:1821–1825. doi: 10.1126/science.1068886. [DOI] [PubMed] [Google Scholar]
- 25.Lewis PM, Dunn MP, McMahon JA, Logan M, Martin JF, St-Jacques B, McMahon AP. Cholesterol modification of sonic hedgehog is required for long-range signaling activity and effective modulation of signaling by Ptc1. Cell. 2001;105:599–612. doi: 10.1016/s0092-8674(01)00369-5. [DOI] [PubMed] [Google Scholar]
- 26.Pike LJ. Lipid rafts: heterogeneity on the high seas. Biochemistry Journal. 2004;378:281–292. doi: 10.1042/BJ20031672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown DA, London E. Structure and function of sphingolipid and cholesterol-rich membrane rafts. Journal of Biological Chemistry. 2000;275:17221–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- 28.Banerjee P, Joo JB, Buse JT, Dawson G. Differential solubilization of lipids along with membrane proteins by different classes of detergents. Chemistry and Physics of Lipids. 1995;77:65–78. doi: 10.1016/0009-3084(95)02455-r. [DOI] [PubMed] [Google Scholar]
- 29.Schuck S, Honsho M, Ekroos K, Shevchenko A, Simons K. Resistance of cell membranes to different detergents. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:5795–5800. doi: 10.1073/pnas.0631579100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hjelmeland LM. A nondenaturing zwitterionic detergent for membrane biochemsitry: Design and synthesis. Proceedings of the National Academy of Sciences of the United States of America. 1980;77:6368–6370. doi: 10.1073/pnas.77.11.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melkonian KA, Chu T, Tortorella LB, Brown DA. Characterization of proteins in detergent-resistant membrane complexes from Madin-Darby canine kidney epithelial cells. Biochemistry. 1995;34:16161–16170. doi: 10.1021/bi00049a031. [DOI] [PubMed] [Google Scholar]
- 32.Simons K, C. VWL. Model systems, lipid rafts, and cell membranes. Annual Review of Biophysics and Biomolecular Structure. 2004;33:269–295. doi: 10.1146/annurev.biophys.32.110601.141803. [DOI] [PubMed] [Google Scholar]
- 33.Lichtenberg D, Goni FM, Heerklotz H. Detergent-resistant membranes should not be identified with membrane rafts. Trends in Biochemical Sciences. 2005;30:430–436. doi: 10.1016/j.tibs.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Roper K, Corbeil D, Huttner WB. Retention of prominin in microvilli reveals distinct cholesterol-based lipid micro-domains in the apical plamsa membrane. Nature Cell Biology. 2000;2:582–592. doi: 10.1038/35023524. [DOI] [PubMed] [Google Scholar]
- 35.Stevenson BR, Siliciano JD, Mooseker JD, Goodenough DA. Identification of ZO-1: A high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. Journal of Cell Biology. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murata M, Peranen J, Schreiner R, Wieland F, Kurzchalia TV, Simons K. VIP21/caveolin is a cholesterol-binding protein. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:10339–10343. doi: 10.1073/pnas.92.22.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harder T, Kellner R, Parton RG, Gruenberg J. Specific release of membrane-bound annexin II and cortical cytoskeletal elements by sequestration of membrane cholesterol. Molecular Biology of the Cell. 1997;8:533–545. doi: 10.1091/mbc.8.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gerke V, Moss SE. Annexins: from structure to function. Physiological Reviews. 2002;82:331–371. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- 39.Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. Journal of Biological Chemistry. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- 40.Shogomori H, Brown DA. Use of detergents to study membrane rafts: The good, the bad and the ugly. Biological Chemistry. 2003;384:1259–1263. doi: 10.1515/BC.2003.139. [DOI] [PubMed] [Google Scholar]
- 41.Fanning AS, Anderson JM. PDZ domains: fundamental building blocks in the organization of protein complexes at the plasma membrane. Journal of Clinical Investigation. 1999;103:767–772. doi: 10.1172/JCI6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chamberlain LH. Detergents as tools for the purification and classification of lipid rafts. FEBS Letters. 2004;559:1–5. doi: 10.1016/s0014-5793(04)00050-x. [DOI] [PubMed] [Google Scholar]
- 43.Yeaman C, Grindstaff KK, Nelson WJ. Mechanism of recruiting Sec6/8 (exocyst) complex to the apical junctional complex during polarization of epithelial cells. Journal of Cell Science. 2004;117:559–570. doi: 10.1242/jcs.00893. [DOI] [PMC free article] [PubMed] [Google Scholar]