Abstract
Background
Based on data from large multicentre US trials, the National Institute for Health and Clinical Excellence (NICE) is advocating a stepped-care model for the management of depression, with ‘case management’ or ‘collaborative care’ for selected patients in primary care.
Aim
To conduct a pilot study examining the use of graduate mental health workers case managing depressed primary care NHS patients.
Design of study
A randomised controlled trial comparing usual GP care with or without case management over 16 weeks of acute antidepressant drug treatment.
Setting
Three primary care practices in the North East of England.
Method
Patients with depression, aged 18–65 years, who had failed to adequately respond to antidepressant treatment, were randomised to the two treatments. Assessments were made at baseline, 12, and 24 weeks using a combination of observer and self ratings.
Results
Randomisation of 62 patients required screening of 1073 potential patients. There was little difference in outcome between the two treatment arms but a gradual improvement in symptoms over time was seen. Client satisfaction was assessed as high across both treatments.
Conclusion
While this pilot study confirmed the integrity of the study protocol and the suitability of the outcome measures and randomisation procedure, it raises questions regarding the practicality of recruitment and feasibility of the intervention. It would be crucial to address these issues prior to the implementation of a large multi-centre randomised controlled trial.
Keywords: case management, collaborative care, community mental health services, depression, graduate mental health workers, primary care
INTRODUCTION
Depression requires a chronic disease-management model utilising elements of ‘collaborative care’ or ‘case management’,1 and this is recommended by the National Institute for Health and Clinical Excellence (NICE).2 However, previous research in this area has mainly been conducted in the US,3 and has used experienced professionals, such as mental health nurses, psychiatrists, or clinical psychologists, to deliver collaborative care. The NHS Plan produced by the Department of Health described the recruitment of 1000 graduate primary mental health workers,4 and it has been argued that these individuals should be utilised in providing case management of depressed patients.1 The nature of these primary care workers, who are most commonly psychology graduates without any previous specific clinical training, has been described previously.5,6 In essence, such individuals work generically and are significantly cheaper to employ than clinically-trained individuals such as community psychiatric nurses. While NICE has suggested that some degree of enhanced care be available for all patients with depression, given the vast numbers of such patients this may not be practical.7 Alternatively, care has to be more targeted. This paper hypothesises that it is most cost-effective to target case management at patients who show either little or no response to first-or second-line antidepressants, or only achieve partial remission of symptoms.8,9
Prior to a proposed large multicentre randomised controlled trial, a pilot study was conducted of such primary care workers in the management of patients who have failed to achieve remission of symptoms despite a minimum of an 8-week trial with an antidepressant. The pilot study objectives included the development of the study protocol, an examination of the acceptability of the intervention, and the feasibility of recruitment and obtaining outcome data that could be used to calculate the sample size needed for a larger randomised controlled trial, in line with published recommendations.10
METHOD
It is recommended that a sample size of 30 to 50 is sufficient for a pilot study.11 However, to focus on the objectives of exploring both the feasibility of recruitment and the practicalities of the intervention in clinical practice, a set of inclusion and exclusion criteria was applied to an entire population covered by three general practices in the north east of England (combined population: 23 217). All suitable patients were offered the intervention. Using the practice databases, all patients aged 18–65 years who were currently prescribed an antidepressant and had been on this for at least 8 weeks were identified. All those not meeting the exclusion criteria of secondary care mental health involvement, a recorded diagnosis of personality disorder, an organic brain disorder, alcohol or drug dependency, pregnancy, or learning disability, received a letter inviting their participation. This was followed up by a telephone call in which an initial screening appointment was arranged. Patients who met ICD–10 (International Classification of Diseases–10) diagnostic criteria for a depressive illness, suffering from a moderate to severe episode (using the Mini-International Neuropsychiatric Interview),12 and scoring at least 14 on the Hamilton Depression Rating Scale (HDRS17),13 indicating that they were not in remission,14 and also providing written informed consent to take part, proceeded with a baseline assessment.
Participants were randomised to one of two conditions: case management from graduate primary care mental health workers in addition to treatment as usual from their GP, or GP usual treatment only. Randomisation codes were generated by an independent researcher and stored at a remote site. Subjects were balanced between the two treatment options in blocks of 10. All patients received a prescription for an alternative antidepressant within a week of their baseline assessment, regardless of the condition they had been assigned to. GPs were instructed to prescribe an antidepressant of their choice, in line with NICE guidelines.2 Case management consisted of six contacts over a 16-week period; these were held in private consultation rooms at the surgeries (weeks 1, 4, and 16) or over the telephone (weeks 2, 6, and 10). At weeks 4 and 10 the mental health worker could recommend an increase in medication dosage to the GP, where appropriate and minimal supportive counselling was provided throughout. However, no formal psychotherapeutic techniques were permitted. Mental health workers received 2 days' training delivered by two consultant psychiatrists, and operated according to a written protocol to ensure consistency of care. Weekly supervision was available from two specialist registrars in psychiatry, as was telephone consultation as and when required.
How this fits in
Previous work, mainly from the US, suggests that case management, or collaborative care, improves the outcome of depressed patients managed in primary care. This pilot study explores the feasibility of such a study using graduates as case managers in the UK NHS. The results raise questions not just regarding the feasibility of a large randomised controlled trial but also the implementation of such case management of depression within the NHS.
A battery of assessments was conducted at baseline, 12, and 24 weeks, by a research psychologist blind to the treatment randomisation of the patients. The primary outcome measure was participants' scores on the Beck Depression Inventory (BDI).15 A number of secondary measures were also conducted including the HDRS17, the Montgomery–Asberg Depression Rating Scale (MADRS),16 standard observer-rated depression rating scales, and the Social Adaptation Self-evaluation Scale (SASS) to assess the functional outcome of patients.17 A measure of patient satisfaction was obtained at week 24 using the Client Satisfaction Questionnaire (CSQ).18 Adjustments in medication (changes in dose, switch of medication, or stopping due to response) were recorded over the course of the 24-week treatment period.
Analysis was conducted using SPSS version 11. An intention-to-treat analysis was conducted, with last observation carried forward, using an analysis of variance with factors of treatment (case management versus usual treatment), and time (baseline, week 12, week 24). All data are quoted as means ± standard deviations. Adjustments in medication were only compared for completers (to ensure an equal period of time in which adjustments could take place) using a two-tailed Fisher's exact test.
RESULTS
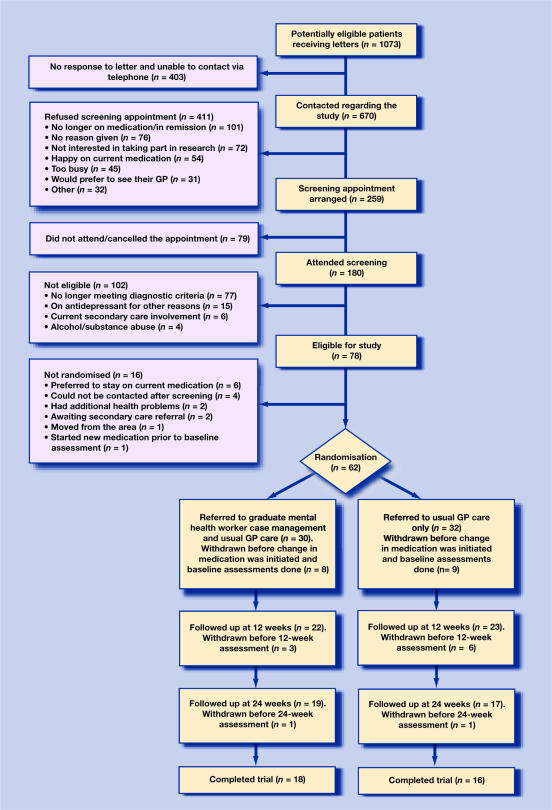
Figure 1 provides a full breakdown of the recruitment process and maps the flow of participants over the course of the study. From a total of 1073 patients, a sample of 45 patients was used for analysis (4% of the initial sample). Following baseline assessments, four out of 22 patients in the case-management group and seven out of 23 in the usual care group were lost to follow up. In those remaining in the study, there was 100% adherence with the intervention.
Figure 1.
Consort diagram of patient flow through trial.
The data was subjected to a mixed-design ANOVA. No significant main effect of treatment was detected on the primary outcome measure, the BDI (F[1,43] = 0.2, P = 0.65) and there was no interaction between treatment type and time (F[1,43] = 1.0, P = 0.32). However the ANOVA confirmed that there was a significant difference in BDI scores over time (F[1,43] = 22.1 P<0.01). Data from the secondary measures also failed to detect any significant treatment effects. The data in Table 1 show that the participants' scores improved over time, with those in the case-management group scoring slightly better on the BDI, HDRS17, MADRS and SASS than those in the usual treatment group. However, of those patients who completed the study, those who were case managed were more likely to have had their medication adjusted (16 out of 18 patients) compared with those with usual care (5 out of 16; P<0.01).
Table 1.
Primary and secondary outcomes at baseline, 12 and 24 week follow up.
| Outcome measure | Usual care mean ± SD | Case management, mean ± SD |
|---|---|---|
| BDI | ||
| Baseline | 26.2 ± 11.9 | 26.4 ± 10.5 |
| 12 weeks | 20.5 ± 12.7 | 19.2 ± 11.3 |
| 24 weeks | 18.3 ± 14.0 | 15.1 ± 10.9 |
| HDRS17 | ||
| Baseline | 18.1 ± 4.0 | 19.1 ± 4.7 |
| 12 weeks | 12.3 ± 5.7 | 12.9 ± 6.9 |
| 24 weeks | 11.3 ± 7.4 | 10.9 ± 7.8 |
| MADRS | ||
| Baseline | 24.3 ± 6.9 | 26.8 ± 6.6 |
| 12 weeks | 16.8 ± 10.3 | 16.5 ± 10.5 |
| 24 weeks | 14.3 ± 12.4 | 13.2 ± 12.0 |
| SASS | ||
| Baseline | 29.0 ± 9.9 | 28.3 ± 10.2 |
| 12 weeks | 30.5 ± 9.3 | 30.5 ± 11.6 |
| 24 weeks | 29.9 ± 10.5 | 32.6 ± 12.4 |
Intention-to-treat analysis. BDI = Beck Depression Inventory. HDRS17 = Hamilton Depression Rating Scale-17 item. MADRS = Montgomery-Asberg Depression Rating Scale. SASS = Social Adaptation Self-evaluation Scale.
Patients receiving case management showed high levels of satisfaction with their treatment, but this was also true of those receiving GP care alone. Results showed a mean CSQ score of 14 versus 15 for controls (where lower scores represent greater satisfaction).
DISCUSSION
Summary of main findings
The aim of this pilot study was to provide specific information to inform the future design of a randomised controlled trial of case management of depression in primary care using graduate primary care mental health workers. A recent meta-analysis suggests that the effect size of case management found in 6-month studies from the US is around 0.25.3 To detect such an effect in a study similar to the current one, with a power of 80% and a significance level of P<0.05, 215 patients would be required. The authors' experience suggests that they would need to be recruited from over 5000 primary care patients being prescribed antidepressants, and a total population of around 120 000. These estimates are based on a randomised controlled trial in which individual patients, as opposed to primary care practices, are the units of randomisation. It has been argued that randomisation should be at the practice level,19 and this would lead to the need for an even larger population.
This pilot study generated interesting findings with regard to the recruitment of participants and the acceptability of the intervention. Figure 1 shows that from a large sample of patients thought to be eligible at the start of the study, only 17% made it to an initial screening appointment, and 4% to initiation of treatment post randomisation. Large numbers of potential participants were ‘lost’ at each stage in the recruitment process. Previous research has shown that typically depressed patients are reluctant to become engaged in research projects.20 Although this featured among the reasons given by patients for not wishing to engage in the current study (18% of those refusing screening), the main hindrance to recruitment was that patients were getting better or were content with the treatment they were receiving from their GP. The CSQ data supported this view, with high levels of satisfaction found across conditions. The difficulty of contacting many patients to arrange screening may have related to a reluctance to take part in research, or the fact that the subjects were well and at work. This latter contention is supported by the observation that the main reason for exclusion at screening was that patients had recovered. Likewise, a significant proportion of those who refused screening gave reasons that indicated they may have recovered. Such factors would affect implementation of case management into clinical practice as much as conducting a randomised controlled trial.
In relation to the framework provided by Lancaster, et al,10 this pilot has fulfilled additional objectives. For those patients who entered the study there was a high degree of satisfaction with the intervention as evidenced by the high rate of adherence. The study protocol was implemented without difficulty. Specialist supervision was available as necessary at an appropriate level for the complexity of the patients' needs. The outcome measures selected were appropriate, particularly the BDI, which was sensitive enough to detect clinical changes in individuals over time. No ethical problems were noted, and the randomisation procedure was satisfactory.
With regard to outcomes, there was no significant difference in the mean scores of both the primary and secondary symptom rating scales in each group, as can be expected in a pilot study. This does not exclude a difference in either direction. However, case-managed patients were significantly more likely to have their medication altered over the course of a 24-week period of time, demonstrating that the intervention did affect the management patients received.
Strengths and limitations of the study
Previously, most studies of case management of depression in primary care have been conducted in the US, with highly skilled case managers. The great strength of the current study is that it tests an intervention that is much more feasible in the context of the UK NHS, using graduate primary case mental health workers and treatment recommendations in line with NICE guidelines. Three entire GP practices were included in the study, and exclusion criteria were minimised as much as possible, in line with how the intervention would operate in practice. However, like many studies in this area, this pilot investigation excluded depressed patients with severe personality disorders and drug and alcohol problems. This is a potential weakness, since these are the very patients with whom GPs need most assistance, and hence they may be most likely to be referred for case management.
Comparison with existing literature
A cluster randomised controlled trial of primary care mental health workers in the UK has recently been published. This involved similar workers although they were trained to a higher level than in the current study.6 The primary outcome of this study was patient satisfaction and, as in the current study, patients valued the intervention, although, again as found here, there was no effect of case management on symptom scores.6 Another recent UK study of collaborative care in older patients found benefit in reducing depressive symptoms, although this involved the use of more highly-trained case managers (community psychiatric nurses).21
While it is difficult to draw any conclusions from the primary outcome measure of a pilot study, it does appear that the effect size was less than reported in a meta-analysis of 6-month studies from the US.3 This could be due to differences in the model of collaborative care that was used in the present study compared to those previously reported. Alternatively, there is evidence that usual care in different countries can vary significantly in its effect size, with UK studies often showing a larger response than those in the US.22 This can lead to a smaller difference between usual care and the response to the active intervention. The small number of patients on antidepressants eligible for recruitment into the study is not inconsistent with US studies with similar entry criteria.23
Implications for future research
This study has been the first of its kind to look at the effectiveness of graduate primary care mental health workers providing case management of depressed patients in UK primary care. The pilot has revealed important issues regarding the feasibility of conducting a larger randomised controlled trial with this population due to the difficulties in the uptake of participants and the predicted number that would be required to ensure sufficient power. In addition, the suitability of such workers in the provision of collaborative care for depressed patients in the form undertaken in this study needs to be questioned. Since there are now sufficient numbers of primary care mental health workers in post it may be possible to carry out a naturalistic study whereby comparisons are made between primary care patients who are routinely case managed by such individuals, and those who are not. This may be a more appropriate method for generating data assessing the cost-effectiveness of such workers. However, the feasibility of graduate primary care mental health worker-based collaborative care in NHS primary care settings has to be questioned.
Acknowledgments
The authors would like to thank all those GPs and primary care staff who assisted in undertaking this study. We would also like to thank all of the patients who took part.
Funding body
This study was supported via an independent investigator led award from Wyeth Laboratories
Ethics committee
Ethical approval was granted by The Newcastle and North Tyneside Local Research Ethics Committee 1 (Reference number: 2003/216)
Competing interests
R Hamish McAllister-Williams and Peter L Cornwall have received a speaker's honorarium and support to attend academic meetings from a number of pharmaceutical companies including Wyeth Laboratories and other manufacturers of antidepressant medications
Discuss this article
Contribute and read comments about this article on the Discussion Forum: http://www.rcgp.org.uk/bjgp-discuss
REFERENCES
- 1.Tylee A, Walters P. We need a chronic disease management model for depression in primary care. Br J Gen Pract. 2007;57(538):348–350. [PMC free article] [PubMed] [Google Scholar]
- 2.National Institute for Clinical Excellence. Depression: management of depression in primary and secondary care. London: NICE; 2004. Clinical Guideline 23. [Google Scholar]
- 3.Gilbody S, Bower P, Fletcher J, et al. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med. 2006;166(21):2314–2321. doi: 10.1001/archinte.166.21.2314. [DOI] [PubMed] [Google Scholar]
- 4.Department of Health. The NHS Plan: a plan for investment, a plan for reform. London: The Stationery Office; 2000. [Google Scholar]
- 5.England E, Lester H. Implementing the role of the primary care mental health worker: a qualitative study. Br J Gen Pract. 2007;57(536):204–211. [PMC free article] [PubMed] [Google Scholar]
- 6.Lester H, Freemantle N, Wilson S, et al. Cluster randomised controlled trial of the effectiveness of primary care mental health workers. Br J Gen Pract. 2007;57(536):196–203. [PMC free article] [PubMed] [Google Scholar]
- 7.McAllister-Williams RH. NICE guidelines for the management of depression. Br J Hosp Med (Lond) 2006;67(2):60–61. doi: 10.12968/hmed.2006.67.2.20461. [DOI] [PubMed] [Google Scholar]
- 8.Cornwall PL, Scott J. Partial remission in depressive disorders. Acta Psychiatr Scand. 1997;95(4):265–271. doi: 10.1111/j.1600-0447.1997.tb09630.x. [DOI] [PubMed] [Google Scholar]
- 9.Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 10.Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract. 2004;10(2):307–312. doi: 10.1111/j..2002.384.doc.x. [DOI] [PubMed] [Google Scholar]
- 11.Browne RH. On the use of a pilot sample for sample size determination. Stat Med. 1995;14(17):1933–1940. doi: 10.1002/sim.4780141709. [DOI] [PubMed] [Google Scholar]
- 12.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33. [PubMed] [Google Scholar]
- 13.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6(4):278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 14.Paykel ES, Freeling P, Hollyman JA. Are tricyclic antidepressants useful for mild depression? A placebo controlled trial. Pharmacopsychiatry. 1988;21(1):15–18. doi: 10.1055/s-2007-1014639. [DOI] [PubMed] [Google Scholar]
- 15.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 16.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 17.Bosc M, Dubini A, Polin V. Development and validation of a social functioning scale, the Social Adaptation Self-evaluation Scale. Eur Neuropsychopharmacol. 1997;7(Suppl 1):S57–70. doi: 10.1016/s0924-977x(97)00420-3. [DOI] [PubMed] [Google Scholar]
- 18.Larsen DL, Attkisson CC, Hargreaves WA, Nguyen TD. Assessment of client/patient satisfaction: development of a general scale. Eval Program Plann. 1979;2(3):197–207. doi: 10.1016/0149-7189(79)90094-6. [DOI] [PubMed] [Google Scholar]
- 19.Gilbody S, Whitty P. Improving the delivery and organisation of mental health services: beyond the conventional randomised controlled trial. Br J Psychiatry. 2002;180:13–18. doi: 10.1192/bjp.180.1.13. [DOI] [PubMed] [Google Scholar]
- 20.Wells KB, Sherbourne C, Schoenbaum M, et al. Impact of disseminating quality improvement programs for depression in managed primary care: a randomized controlled trial. JAMA. 2000;283(2):212–220. doi: 10.1001/jama.283.2.212. [DOI] [PubMed] [Google Scholar]
- 21.Chew-Graham CA, Lovell K, Roberts C, et al. A randomised controlled trial to test the feasibility of a collaborative care model for the management of depression in older people. Br J Gen Pract. 2007;57(538):364–370. [PMC free article] [PubMed] [Google Scholar]
- 22.Burns T, Catty J, Watt H, et al. International differences in home treatment for mental health problems. Results of a systematic review. Br J Psychiatry. 2002;181:375–382. doi: 10.1192/bjp.181.5.375. [DOI] [PubMed] [Google Scholar]
- 23.Katon W, Von Korff M, Lin E, et al. Stepped collaborative care for primary care patients with persistent symptoms of depression: a randomized trial. Arch Gen Psychiatry. 1999;56(12):1109–1115. doi: 10.1001/archpsyc.56.12.1109. [DOI] [PubMed] [Google Scholar]