Abstract
Most Apicomplexan parasites, including the human pathogens Plasmodium, Toxoplasma, and Cryptosporidium, actively invade host cells and display gliding motility, both actions powered by parasite microfilaments. In Plasmodium sporozoites, thrombospondin-related anonymous protein (TRAP), a member of a group of Apicomplexan transmembrane proteins that have common adhesion domains, is necessary for gliding motility and infection of the vertebrate host. Here, we provide genetic evidence that TRAP is directly involved in a capping process that drives both sporozoite gliding and cell invasion. We also demonstrate that TRAP-related proteins in other Apicomplexa fulfill the same function and that their cytoplasmic tails interact with homologous partners in the respective parasite. Therefore, a mechanism of surface redistribution of TRAP-related proteins driving gliding locomotion and cell invasion is conserved among Apicomplexan parasites.
Keywords: gliding motility, cell invasion, Apicomplexan parasites, thrombospondin-related anonymous protein, micronemal protein 2
Many Apicomplexan protozoan parasites have life cycle stages that actively invade target cells. Most of these invasive stages are also mobile by means of gliding motility, a form of substrate-dependent locomotion during which, in contrast to crawling motility, the moving cell maintains a fixed shape. Gliding motility and cell invasion, which depend on microfilaments in the parasite (Dobrowolski and Sibley 1996), are thought to result from a capping activity of parasite surface ligands. After parasite attachment to the host cell, a tight junction initially forms between the apical tip of the parasite and the host cell membrane and is progressively redistributed to the posterior pole of the parasite as it moves into the target cell (Aikawa et al. 1978; Jensen and Edgar 1978; Pimenta et al. 1994). In addition, a capping activity is suggested by the observation that invasive stages are able to redistribute molecules bound to their surface, such as antibodies or cationized ferritin, and shed them from their posterior pole (Vanderberg 1974; Dubremetz et al. 1985; Speer et al. 1985). Based on these phenomena, it has been proposed that forward locomotion on the substrate and penetration into a host cell result from the backward translocation of parasite ligands bound to their substrate/cell receptors (Russell and Sinden 1981; Russell 1983; King 1988).
In Plasmodium (malaria) sporozoites, the parasite form that infects the salivary glands of the mosquito vector and the liver of the mammalian host, thrombospondin-related anonymous protein (TRAP) (Robson et al. 1988) is a candidate ligand for interaction with host cell or substrate receptors. TRAP is a type 1 transmembrane protein that carries two adhesive domains in its extracellular portion, an A-type domain first described in von Willebrand factor and a motif similar to the type 1 repeat of thrombospondin (TSR). As shown in Table , a putative TRAP paralog has been identified in the ookinete stage of Plasmodium (CTRP) (Trottein et al. 1995; Yuda et al. 1999) and putative orthologs have been identified in the Apicomplexan parasites Toxoplasma (micronemal protein 2, MIC2) (Wan et al. 1997), Eimeria (Etp100) (Tomley et al. 1991; Pasamontes et al. 1993) and Cryptosporidium (TRAPC1) (Spano et al. 1998). These proteins carry various numbers of A-type and TSR domains, but their cytoplasmic tails do not exhibit primary sequence homology. Some of these proteins (TRAP, MIC2, and Etp100) have been localized to the parasite micronemes, secretory vesicles of the apical complex that release their content at the anterior pole of the parasite. Gene disruption experiments have shown that TRAP(−) sporozoites do not display gliding motility and do not infect the salivary glands of the mosquito vector and the liver of the mammalian host (Sultan et al. 1997).
Table 1.
TRAP and Its Putative Paralog and Orthologs in Apicomplexan Parasites
| Protein | |||||
|---|---|---|---|---|---|
| Parasite | Name | EC domain | Cytoplasmic tail | ||
| Genus | Stage | A-DOM | TSR | ||
| Plasmodium | Sporozoite | TRAP | 1 | 1 | YNFIAGSSAAAMAGAAPF**VMA***KGIVNQFKLP**NWN |
| Ookinete | CTRP | 6 | 7 | YNTLNGGTPHNSNMFNVNNGII***N**FVIANPMWN | |
| Toxoplasma | Tachyzoite | MIC2 | 1 | 5 | YHYYLSSSVGSPSAIYA**GATKVVM***KTLVPV***SMWME |
| Eimeria | Sporozoite | Etp100 | 1 | 5 | YGLSGGSAAAATAGAVMTAGTSNAAVKSLISAGQSMWAS |
| Cryptosporidium | Sporozoite | TRAPC1 | — | 5 | LFLIGGRSGQ****TNYQYFQSSATLQSYVQIGPSQNWAS |
In this report, we tested the hypothesis that TRAP acts as a link connecting the parasite cortical microfilaments and the matrix/host cell surface. By introducing various modifications in the cytoplasmic tail of TRAP, we provide genetic evidence that surface-associated TRAP is directly responsible for sporozoite gliding and cell invasion. The cytoplasmic tail of TRAP is interchangeable with that of Toxoplasma gondii MIC2, which has been shown to undergo anterior to posterior redistribution during parasite penetration into target cells (Carruthers and Sibley 1997). Furthermore, amino acid substitutions suggest two functions mediated by the cytoplasmic tail of TRAP: anterior to posterior redistribution and posterior shedding of the protein, both functions crucial for sporozoite gliding motility and host cell invasion.
Materials and Methods
Construction of Targeting Plasmids
All insertion plasmids used in this study are derivatives of the previously described plasmid pINCO (Nunes et al. 1999), which contains a DHFR-TS mutant, pyrimethamine-resistance gene, and a targeting sequence consisting of the distal part of TRAP lacking nucleotides (nt) 1–67 and 1.4 kb of downstream sequence. The 3′ end of TRAP bearing the TΔL deletion was generated by PCR using 5′ primer P008 (5′ CGCGAAGCTTCTGAATGTTCTACTACATGTGACAATG 3′), which hybridizes from nt 736 of TRAP onwards, and 3′ primer P011 (5′ CGCTTAATTAACGCTACTTCCTGCTATAAAATTATAACC 3′), which hybridizes at the 3′ end of TRAP and introduces a stop codon as well as a PacI restriction site (bolded). The resulting PCR product was then cloned into plasmid pCRScriptSK, yielding plasmid pΔL1. A linker encompassing the first 24 bp of the TRAP 3′ UTR, including the natural XbaI site located 18 bp from the stop codon, was then cloned downstream from the TRAP coding sequence in plasmid pΔL1 using PacI and EcoRI adaptors, creating plasmid pΔL2. Further 3′ UTR, borne by a XbaI–XmnI 1.4-kb fragment, was then cloned downstream from the linker in plasmid pΔL2 digested with XbaI and EcoRV, yielding plasmid pMutΔL. The HincII–AflII internal portion of plasmid pMutΔL, which extends from nt 1150 of TRAP to 0.6 kb 3′ to its stop codon, was then used to replace its wild-type counterpart in plasmid pINCO, giving rise to plasmid pTΔL. The 3′ end of TRAP bearing the TΔS deletion was generated by PCR using 5′ primer P017 (5′ GAATGGAGTGAATGTTCTACTACATGTG 3′), which hybridized from nt 738 of TRAP onwards, and 3′ primer P012 (5′ CGCTTAATTAACAACAATACCCTTTTCATCATCTGC 3′) that hybridizes at the 3′ end of TRAP and introduces a stop codon as well as a PacI restriction site (bolded). The resulting PCR product was then cloned into plasmid pCRScriptSK, yielding plasmid pΔS1. The 3′ UTR of TRAP, borne by the PacI-KpnI fragment of plasmid pMutΔL, was further cloned into plasmid pΔS1, yielding plasmid pMutΔS. The HincII-AflII internal portion of plasmid pMutΔS was then used to replace its wild-type counterpart in plasmid pINCO, giving rise to plasmid pTΔS. The exchanged fragments in plasmids pTΔS and pTΔL were sequenced and confirmed to differ from the corresponding wild-type fragment only by the desired mutation.
The DNA encoding the cytoplasmic tail of MIC2 was amplified from the XhoI fragment of BAC G11-11 (Wan et al. 1997) using 5′ primer P27 (5′ AAAACTGCAGGATCCCCATCCGCGGAGATAG 3′) and 3′ primer P28 (5′ TGCTCTAGATATATATGTTTATTAAAATTACTCCATCCACATATCACTATCG 3′), which contain a PstI and a XbaI site, respectively (bolded). The resulting PCR fragment was digested with PstI and XbaI and cloned into plasmid pMutΔS digested with the same enzymes, yielding plasmid pMut-MIC2. The AgeI-AflII internal portion of plasmid pMut-MIC2 was sequenced, confirmed to contain the expected sequence, and used to replace its wild-type counterpart in plasmid pINCO, giving rise to plasmid pTMIC.
Both TRYP and ACID mutations were generated using 5′ primer P017 (5′ GAATGGAGTGAATGTTCTACTACATGTG 3′) and 3′ primer SK01 for the TRYP mutation (5′ TCATCTAGATATATATGTTTATTAAAATTAGCTAGCGTCATTATCTTCAGGTAATTTAAACT- GCTC 3′) or SK02 for the ACID mutation (5′ TCATCTAGATATATATGTTTATTAAAATTAGTTCCAGGCATTGCTAGCAGG- TAATTTAAACTGCTC 3′). Both 3′ primers contain an XbaI site, and a mutation-tagging NheI site (bolded). PCR fragments were cloned into plasmid pCRScript, excised as an AgeI-XbaI insert and cloned into plasmid pMutΔS digested with AgeI and XbaI. The AgeI-AflII fragments of the resulting plasmids that encompassed the mutations were sequenced, verified to differ from their wild-type counterpart only by the desired mutations, and used to replace the corresponding fragment in plasmid pINCO, yielding plasmids pTRYP and pACID.
Parasite Transfection and Phenotypic Analysis
Parasite transformation and selection was performed as described (Ménard and Janse 1997; Waters et al. 1997). Anopheles stephensi mosquitoes were fed on infected young rats and sporozoites dissected out at days 14–18 postfeeding. Preparation of sporozoites from the various mosquito compartments was as described (Sultan et al. 1997). For immunofluorescence assays, sporozoites were incubated in RPMI–3% BSA on ice for 3 h, pelleted, and resuspended in 0.5% BSA/PBS containing primary antibody at 1:50. After 30 min at 37°C, sporozoites were pelleted, washed three times with PBS, and air dried in wells on glass IFA slides. For permeabilized staining, some wells were incubated again with primary antibody after drying. Revelation was performed with anti–rabbit IgG-FITC (Kirkegaard & Perry Laboratories, Inc.) at 1:40 in 0.5% BSA/PBS for 30 min at 37°C, slides were washed three times with PBS and mounted. For cell invasion assays, ∼105 HepG2 cells were seeded in eight chamber slides and grown to semiconfluency. Sporozoites (∼15,000/well) were added, incubated 2 h at 37°C, and washed off. After 48 h, parasite exoerythrocytic forms (EEF) were revealed as described (Sultan et al. 1999) using primary antibody against parasite HSP70, which is expressed only in maturing liver stages.
Immunoelectron Microscopy
Samples were fixed for 30 min at 4°C with 1% formaldehyde, 0.5% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4. Fixed samples were washed, dehydrated, and embedded in LR White resin (Polysciences Inc.) as described previously (Aikawa and Atkinson 1990). Thin sections were blocked in PBS containing 5% wt/vol nonfat dry milk and 0.01% vol/vol Tween 20 (PBTM). Grids were then incubated with primary antibodies diluted 1:50 to 1:300 in PBTM for 2 h at room temperature. After washing, grids were incubated for 1 h in 15-nm gold-conjugated goat anti–rabbit IgG (Amersham Life Sciences), diluted in 1:20 in PBS containing 1% wt/vol bovine serum albumin and 0.01% vol/vol Tween 20 (PBTB), rinsed with PBTB, and fixed with glutaraldehyde to stabilize the gold particles. Samples were stained with uranyl acetate and lead citrate, and examined in an electron microscope (CEM902; Carl Zeiss, Inc.).
Antibodies
Polyclonal antibodies against the TRAP repeats (antirepeats) were obtained using a recombinant polypeptide corresponding to residues 263–428 of TRAP, and polyclonal antibodies against the TRAP cytoplasmic tail (antitail) using the synthetic peptide corresponding to D586DE to DND604 of the TRAP tail.
Results and Discussion
Deletions in the Cytoplasmic Tail of TRAP Do Not Alter Surface Presentation of the Protein, but Impair Its Function
We generated Plasmodium berghei sporozoites that produced TRAP proteins lacking the cytoplasmic tail. Sequence comparison of the cytoplasmic tails of the TRAP proteins sequenced so far (from six plasmodial species; Robson et al. 1988, Robson et al. 1997; Rogers et al. 1992b; Templeton and Kaslow 1997) reveals that the 14 carboxy-terminal residues are the most highly conserved (Fig. 1 A). We thus created sporozoites whose TRAP lacked the 14 or 37 carboxy-terminal residues, named TΔS and TΔL, respectively. For this, modifications in the single-copy TRAP gene were introduced via a single recombination event promoted by targeting insertion plasmids (Nunes et al. 1999). As shown in Fig. 1 B, homologous integration of these targeting plasmids generate two TRAP copies: the first is full-length, bears the mutation, and is flanked by expression sequences, whereas the second lacks the 5′ part of the gene and promoter sequences.
Figure 1.
TRAP cytoplasmic tail mutants and gene targeting strategy. (A) Schematic representation of the TRAP protein and amino acid sequences (one letter code), of the cytoplasmic tails of Plasmodium berghei TRAP and TRAP recombinants. CoTRAP indicates the residues conserved in at least five of six plasmodial TRAP sequenced to date (+ indicates E or D). In the TRAP recombinants shown below, the amino acid substitutions or heterologous exchange are underlined. Hatched boxes represent the leader sequence and the transmembrane domain. (B) Generation of the TRAP mutations by insertion mutagenesis in P. berghei. The wild-type (Wt), single-copy TRAP is targeted with an insertion plasmid whose targeting sequence contains the deletion/mutation (*) and is linearized upstream from the mutation (crossover); thin lines, TRAP untranslated region; open box, TRAP coding region; thick lines, bacterial plasmid and DHFR-TS resistance cassette. The recombinant locus (Rec. locus) expected to result from plasmid integration that preserves the mutation is shown. Below are the restriction maps of the 3′ end of the TRAP gene in the first duplicate of the recombinant clones. The nucleotide and the amino acid sequences tagging the mutations are indicated, and the corresponding restriction sites italicized in the sequence and the map. P, PstI; Pa, PacI; X, XbaI; B, BamHI; N, NheI. (C) The first TRAP duplicate of recombinant parasites were amplified by PCR using primer O1 and T7, which annealed upstream from the region of homology and to the vector sequence, respectively, and digested with restriction enzymes. See the restriction maps and mutation-tagging restriction sites in B.
Three such plasmids, in which the targeting sequence was wild type (WT) or contained the TΔS- or the TΔL-encoding mutations (plasmids pINCO, pTΔS, and pTΔL, respectively), were independently transformed into WT merozoites. One parasite clone from each transformation experiment, named INCO (integration control), TΔS, and TΔL, was selected in rats by limiting dilution. Southern hybridization demonstrated that parasites in each clone had a single copy of the corresponding plasmid integrated into chromosomal TRAP, as depicted in Fig. 1 B (data not shown). The first TRAP duplicate, which should contain the mutation present in the targeting plasmid, was specifically amplified by PCR using primers O1 and T7 (Fig. 1 B) and analyzed by restriction digestion. The PCR products obtained from TΔS and TΔL parasites contained the corresponding deletion tagged with a PacI site, as shown in Fig. 1 C.
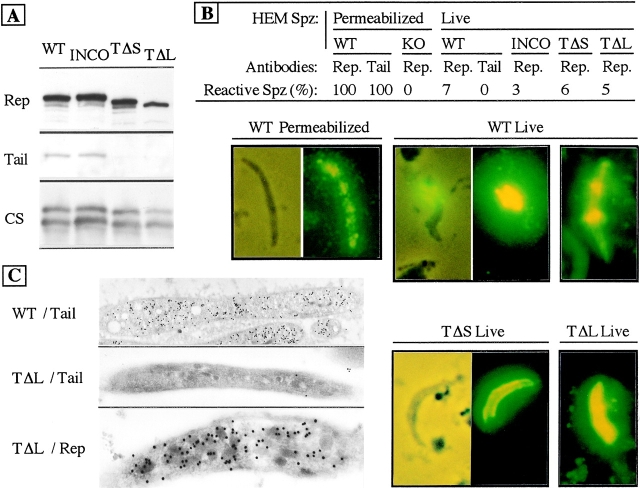
Mutant parasite clones, created at the red blood cell stage, were then transmitted to Anopheles stephensi mosquitoes. Sporozoites are formed inside oocysts in mosquito midguts and are released in the hemolymph, the fluid that bathes the mosquito body cavity. TRAP production was assessed in sporozoites collected from mosquito midguts; i.e., freshly released from, or still within, oocysts. Sporozoite extracts were subjected to Western blot using antibodies directed against the TRAP extracellular repeats (antirepeats) or the TRAP cytoplasmic tail (antitail). As shown in Fig. 2 A, wild-type and INCO sporozoites produced similar amounts of TRAP. TΔS and TΔL sporozoites expressed comparable amounts of their TRAP truncate that, as expected, did not react with antitail antibodies.
Figure 2.
TRAP truncates are correctly expressed and targeted to the sporozoite surface. (A) Western blot analysis of midgut sporozoite extracts. Sporozoites were collected from midguts of mosquitoes dissected at day 16 postfeeding. Crude extracts from ∼105 sporozoites of each population were separated by SDS-PAGE and transferred to a membrane that was probed successively with polyclonal antibodies to the TRAP repeats (Rep), the TRAP cytoplasmic tail (Tail), and Mabs 3D11 to the repeats of the circumsporozoite protein (CS). (B) Immunofluorescence assays of sporozoites collected from the hemolymph of infected mosquitoes. Mosquitoes infected with WT P. berghei, the INCO, TΔS, or TΔL clones, or the TRAP knockout (KO) REP line (Sultan et al. 1997) were dissected at days 14–16 postfeeding, permeabilized or processed live, and stained using antibodies to the TRAP repeats (Rep.) or cytoplasmic tail (Tail). Typical fluorescence patterns are shown. A small proportion of live WT, INCO, TΔS, and TΔL sporozoites displayed a cap-like fluorescence frequently limited to one sporozoite pole. WT live sporozoites occasionally displayed a bright, ring-like pattern around a portion of the sporozoite body. (C) Immunolocalization of WT TRAP and of the TΔL truncate on ultrathin sections of the corresponding sporozoites using antibodies to the TRAP repeats (Rep.) or cytoplasmic tail (Tail). Using TRAP repeat antibodies, full-length TRAP and the TΔL truncate showed an identical distribution, frequently over most of the sporozoite length and associated with electron-dense micronemes.
The presence of TRAP and TRAP truncates on the sporozoite surface was then examined by immunofluorescence. Sporozoites collected from mosquito hemolymph were used because, as described below, TΔS and TΔL sporozoites did not invade the salivary glands. In the WT and INCO clone, virtually all sporozoites permeabilized before staining strongly reacted with antirepeats as well as antitail antibodies, in a typical patchy pattern (Fig. 2 B). However, when staining was performed with live parasites, only ∼5% of sporozoites in both populations reacted with antirepeats, but not antitail, antibodies. Fluorescence patterns mostly resembled caps covering the sporozoite body to various extents, frequently over less than half its length. In addition, a ring-type pattern could be observed in a small number of WT sporozoites. In the TΔS and TΔL clones, ∼5% of the live sporozoites also strongly reacted with antirepeats antibodies and displayed the cap-type fluorescence pattern (Fig. 2 B), indicating that the cytoplasmic tail of TRAP is dispensable for surface exposure of the protein. To determine the subcellular localization of TRAP, WT and TΔL sporozoites were examined by immunoelectron microscopy with antirepeats and antitail antibodies (Fig. 2 C). Using antitail antibodies, WT, but not TΔL, sporozoites were labeled. However, micronemal localization was not unambiguous with these antibodies. Labeling with antirepeats antibodies showed association of TRAP with the micronemes in both TΔL (Fig. 2 C) and WT (data not shown) sporozoites. This subcellular localization was similar to that previously described for Plasmodium falciparum TRAP/SSP-2 (Rogers et al. 1992a), indicating that the TΔL truncate was correctly targeted to the parasite micronemes.
We then examined the phenotype of recombinant sporozoites (Table ). Similar numbers of sporozoites were found in the midguts of mosquitoes infected with the three parasite populations. However, dramatically fewer sporozoites were found associated with the salivary glands of mosquitoes infected with TΔS or TΔL parasites than with INCO or WT parasites. This indicated that the former, like TRAP(−) sporozoites (Sultan et al. 1997), did not infect salivary glands. Sporozoite infectivity to the vertebrate host was estimated by injecting sporozoites intravenously into rats and measuring the prepatent period of erythrocytic infection. Whereas WT and INCO sporozoites induced erythrocytic infections with similar prepatent periods, TΔS and TΔL sporozoites were not infective. Microscopic examination of sporozoites deposited on glass slides revealed that WT and INCO sporozoites glided with similar speed (∼2 μm/s) and pattern, but that TΔS or TΔL sporozoites did not exhibit typical gliding (TΔS sporozoites displayed a limited locomotion described below). Sporozoite invasion into mammalian cells was examined by immunostaining of EEF of the parasite that developed within hepatoma HepG2 cells. Whereas WT and INCO sporozoites generated similar numbers of EEF, no EEF was detected in cells incubated with either TΔS or TΔL sporozoites. Therefore, the cytoplasmic tail of TRAP is dispensable for surface presentation of the protein, but is essential for sporozoite gliding motility and cell invasion.
Table 2.
Phenotype of Wild-Type and Recombinant P. berghei Sporozoites
| Parasite population | No. sporozoites/infected mosquito | Typical gliding | Prep. period of infection in rats | No. EEF in HepG2 cells | |||||
|---|---|---|---|---|---|---|---|---|---|
| MG | HEM | SG | HEM | SG | HEM | SG | HEM | SG | |
| % | % | d | d | ||||||
| WT | 17,000 | 1,000 | 19,000 | 21 | 82 | 5.2 | 3 | 10 | 260 |
| INCO | 20,000 | 900 | 16,000 | 17 | 72 | 5 | 3 | 21 | 310 |
| TΔS | 19,000 | ND | 400 | 0 | 0 | — | — | 0 | 0 |
| TΔL | 23,000 | ND | 1,000 | 0 | 0 | — | — | 0 | 0 |
| TMIC | 18,000 | ND | 17,000 | ND | 75 | ND | 3 | 23 | 290 |
| TRYP | 20,000 | 4,000 | 2,000 | 0 | 0 | — | — | 0 | 0 |
| ACID | 21,000 | 4,500 | 800 | 0 | 0 | — | — | 0 | 0 |
Mosquitoes were dissected at days 14–18 after feeding, and sporozoites in the midguts (MG), the hemolymph (HEM), or associated with the salivary glands (SG) of mosquitoes were counted (average from at least 200 mosquitoes in three independent feeding experiments). To assess gliding motility, sporozoites were kept 2 h in 3% BSA at 4°C and allowed to glide on uncoated microscope slides. Sporozoite infectivity to rats was assessed by injecting 15,000 HEM or SG sporozoites into Sprague-Dawley rats and measuring the prepatent (prep.) period of infection; i.e., the number of days between injection and detection of erythrocytic stages of the parasite (average from six experiments; —, no infection). Sporozoite invasion was assessed by incubating 15,000 HEM or SG sporozoites with HepG2 cells for 2 h, and counting EEF 40 h later (average from four experiments).
The Cytoplasmic Tails of Plasmodium TRAP and Toxoplasma MIC2 Are Functionally Homologous
MIC2 is a TRAP-related protein expressed by tachyzoites of Toxoplasma gondii (Table ). MIC2 is first secreted at the apical pole of the parasite upon attachment to the host cell, and is then translocated to the posterior pole during parasite penetration into the cell (Carruthers and Sibley 1997). We tested the hypothesis that TRAP and MIC2 have similar functional properties by generating a TRAP variant, named TMIC, in which the cytoplasmic tail was substituted by that of MIC2 starting at the TΔL deletion site (Fig. 1 A). The insertion plasmid that contained the sequence encoding the tail switch, pTMIC, was transformed into WT merozoites, and one resistant clone, TMIC, was selected. Southern blot hybridization confirmed that a single copy of the plasmid was integrated at the TRAP locus in TMIC parasites (data not shown), and PCR amplification of the first TRAP duplicate confirmed the presence of the additional BamHI site tagging the exchange (Fig. 1 C). To rule out the possibility that a discontinuous gene conversion event had preserved the BamHI site (located at the junction of the TRAP and MIC2 sequences; see Fig. 1 B) but corrected downstream heterologies, the sequence of two independent PCR products generated with primers O1 and T7 was determined. Both sequences contained the desired exchange.
As shown in Table , TMIC sporozoites behaved similarly to WT or INCO sporozoites in all tests performed. Most strikingly, TMIC sporozoites glided at the same average speed and followed a similar circular pattern than WT or INCO sporozoites (Fig. 3 A). In addition, TMIC sporozoites were as infectious to the rodent host as WT sporozoites. In all rodent infection experiments, Southern hybridization indicated that the blood stages of the parasite induced by TMIC sporozoites still contained the TMIC recombinant locus (data not shown), with no trace of WT TRAP that could have been recreated via plasmid excision. We conclude that the cytoplasmic tail of MIC2, despite little primary amino acid sequence similarity to the TRAP cytoplasmic tail, can function in its place during gliding locomotion and cell invasion by malaria sporozoites. The cytoplasmic tails of these proteins must then interact with homologous partners in the respective Apicomplexan host.
Figure 3.
Gliding phenotypes of wild-type and mutant sporozoites. (A) Time-lapse micrographs of sporozoites gliding on uncoated glass slides. Sporozoites were collected from the hemolymph of infected mosquitoes at days 14–16 postfeeding and kept 2 h in 3% BSA at 4°C before microscopic examination. The parasite population and a schematic representation of the TRAP cytoplasmic tail are shown at left. Wild-type, INCO, and TMIC gliding sporozoites described circular patterns and completed one circle in ∼20 s. ACID, TRYP, and TΔS gliding sporozoites described a “pendulum” movement covering one third of a circle and going back to the starting position, repeated several times (shown here with an ACID sporozoite). Numbers indicate seconds. (B) Immunofluorescence using TRAP antirepeat antibodies of trails left behind WT sporozoites gliding over glass slides. Note in the top panels (phase + immunofluorescence at left) the presence of a “ring” of TRAP around the middle portion of the sporozoite. Unlike the glycosylphosphatidylinositol-anchored CS protein of Plasmodium sporozoites that is uniformly deposited in the trail (Stewart and Vanderberg 1988), TRAP found in the trail displays a periodic intensity pattern reminiscent of the nonuniform expression of TRAP on the sporozoite surface (see also Fig. 2 B).
Amino Acid Substitutions in the TRAP Cytoplasmic Tail Modify the Sporozoite Gliding Phenotype
Although their primary sequence is not conserved, the cytoplasmic tails of the TRAP-related proteins have two common features (Table ). They are rich in acidic residues (18–30%) and contain a tryptophan as the penultimate or antepenultimate residue. To test the contribution of these residues, we created two additional TRAP mutants. One had the carboxy-terminal WN residues modified to AS, named TRYP, and one had the last three acidic residues ED[N]D modified to AS[N]A, named ACID (Fig. 1 A). Parasite clones TRYP and ACID were selected after transformation with the targeting plasmids containing the corresponding mutation, pTRYP and pACID. Each clone was confirmed by Southern hybridization (data not shown) and PCR analysis (Fig. 1 C) to contain the expected TRAP recombinant locus, with a mutated (NheI-tagged) first TRAP duplicate. Surface expression of the TRYP and ACID variants was confirmed by IFA using antirepeat antibodies (data not shown). In both TRYP and ACID sporozoites, cap-like structures were seen that were similar to those in WT, TΔS, and TΔL sporozoites. Ring-type patterns, however, seemed less intense in TRYP and ACID sporozoites than in the WT. However, since the ring-type patterns showed some variability in WT sporozoites themselves (Fig. 2 B and 3 B), the significance of this difference remains questionable.
As shown in Table , both TRYP and ACID sporozoites were not infective to the mosquito salivary glands or the rodent liver, nor did they invade HepG2 cells in vitro. Surprisingly, the gliding phenotype of TRYP and ACID sporozoites was not abolished but drastically modified, and identical to the gliding pattern of TΔS sporozoites. Whereas ∼10% of WT hemolymph sporozoites typically described circles at a constant speed, the same proportion of hemolymph sporozoites in the TRYP, ACID, and TΔS clones all displayed an identical phenotype of “pendulum” gliding. This new phenotype consisted in repeated cycles of (a) gliding over one third of a circle, (b) stopping for usually 1–2 s, and (c) moving back to the original position (Fig. 3 A). This phenotype was not observed with TΔL or TRAP knock-out sporozoites, indicating that it depends on the cytoplasmic tail of TRAP. Importantly, it was not due to low-level expression (as verified with TΔS sporozoites) or mistargeting of the TRAP variant (since their surface presentation was not affected), and thus appeared to be a direct consequence of the modifications.
In WT sporozoites, TRAP is released on the substrate during gliding locomotion (Fig. 3 B). Labeling of TRAP on the substrate revealed a periodic intensity pattern reminiscent of the nonuniform expression of TRAP on the sporozoite surface. Also, TRAP released on the substrate is detected by antirepeats, but not antitail, antibodies (data not shown), suggesting that TRAP release occurs after cleavage of the protein. Therefore, the pendulum phenotype may be explained by the sporozoite capacity to translocate TRAP to its posterior tip, making it glide over one third of a circle (corresponding approximately to the sporozoite length), and incapacity to release TRAP accumulated at its posterior pole, making it stop. The TRAP cytoplasmic tail could therefore have a bifunctional character; the membrane proximal part would be required for protein translocation along the cortical microfilaments, whereas the distal part would contain a signal for TRAP release at the posterior pole, probably by proteolytic cleavage.
Whatever the molecular basis for the pendulum phenotype, it demonstrates a direct role of TRAP as a transmembrane link in gliding motility (and cell invasion). The critical role of the conserved tryptophan and acidic residues also indicates a similar role for TRAP-related proteins.
Concluding Remarks
The results presented here show that TRAP and structurally related proteins constitute a family of functionally homologous bridging proteins involved in parasite gliding motility and cell penetration. The interchangeability of the TRAP and MIC2 cytoplasmic tails suggests the conservation of the molecular machinery that drives protein redistribution. The cytoplasmic tails of these proteins may interact with some component of the cortical microfilament system, possibly a motor protein. Recent evidence suggests that myosin colocalizes with actin underneath the parasite plasma membrane in a circumferential pattern and powers motility and cell invasion by Toxoplasma gondii tachyzoites (Dobrowolski et al. 1997). Favorable signals on the parasite surface may trigger interaction between the cytoplasmic tail of the TRAP-related protein with myosin, and their translocation along submembranous actin filaments. In addition, if our interpretation of the pendulum phenotype is correct (i.e., if the distal part of the TRAP cytoplasmic tail and the conserved tryptophan are necessary for protein release), then the product(s) necessary for releasing the bridging protein may also be conserved in these parasites.
Members of the TRAP family of proteins have been found in the malaria sporozoite and ookinete, as well as in invasive stages of Toxoplasma, Cryptosporidium, and Eimeria. An invasion system dependent on the redistribution of TRAP-related proteins, which contain various associations of A-type and TSR domains, may be restricted to penetration of Apicomplexan parasites into epithelial cells. For example, the bridging protein of the capping machinery used by the malaria merozoite for invading a red blood cell, which remains unknown, probably requires specialized cell-binding modules. However, the cytoplasmic tail of such protein might carry the features shared by members of the TRAP protein family. Further unraveling of the mechanisms of gliding motility and cell invasion by Apicomplexa should reveal new features of cell adhesion associated with force transduction and may identify common targets for blocking their infectivity.
Acknowledgments
We thank J.W. Ajioka for providing the MIC2 clone.
This work was supported by grants from Burroughs Wellcome Fund (New Initiative in Malaria Research), United Nations Development Programme/World Bank/World Health Organization Special Programme, the Karl-Enigk Foundation, and the National Institutes of Health (AI-43052 and AI-35827). S. Kappe is a recipient of the B. Levine fellowship in malaria vaccinology. R. Ménard is a recipient of the Burroughs Wellcome Fund Career Award in the Biomedical Sciences.
Footnotes
Stefan Kappe and Thomas Bruderer contributed equally to this work and should be considered co-first authors.
Abbreviations used in this paper: EEF, exoerythrocytic forms; MIC2, micronemal protein 2; nt, nucleotide; TRAP, thrombospondin-related anonymous protein; TSR, thrombospondin type 1 repeat; WT, wild type.
References
- Aikawa M., Atkinson C.T. Immunoelectron microscopy of parasites. Adv. Parasitol. 1990;29:151–214. doi: 10.1016/s0065-308x(08)60106-2. [DOI] [PubMed] [Google Scholar]
- Aikawa M., Miller L.H., Johnson J., Rabbege J. Erythrocyte entry by malarial parasites. A moving junction between erythrocyte and parasite. J. Cell Biol. 1978;77:72–82. doi: 10.1083/jcb.77.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers V.B., Sibley L.D. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur. J. Cell Biol. 1997;73:114–123. [PubMed] [Google Scholar]
- Dobrowolski J.M., Sibley L.D. Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell. 1996;84:933–939. doi: 10.1016/s0092-8674(00)81071-5. [DOI] [PubMed] [Google Scholar]
- Dobrowolski J.M., Carruthers V.B., Sibley L.D. Participation of myosin in gliding motility and host cell invasion by Toxoplasma gondii . Mol. Microbiol. 1997;26:163–173. doi: 10.1046/j.1365-2958.1997.5671913.x. [DOI] [PubMed] [Google Scholar]
- Dubremetz J.-F., Rodriguez C., Ferreira E. Toxoplasma gondiiredistribution of monoclonal antibodies on tachyzoites during host cell invasion. Exp. Parasitol. 1985;59:24–32. doi: 10.1016/0014-4894(85)90053-0. [DOI] [PubMed] [Google Scholar]
- Jensen J.B., Edgar S.A. Fine structure of penetration of cultured cells by Isospora canis sporozoites. J. Protozool. 1978;25:169–173. [Google Scholar]
- King C.A. Cell motility of Sporozoan protozoa. Parasitol. Today. 1988;4:315–319. doi: 10.1016/0169-4758(88)90113-5. [DOI] [PubMed] [Google Scholar]
- Ménard R., Janse C. Gene targeting in malaria parasites Ajioka J.W. Methodsa Companion to Methods in Enzymology—Analysis of Apicomplexan Parasites 1997. 148 157 Academic Press Inc; Orlando, FL: Vol. 13. [DOI] [PubMed] [Google Scholar]
- Nunes A., Thathy V., Bruderer T., Sultan A.A., Nussenzweig R.S., Ménard R. Subtle mutagenesis by ends-in recombination in malaria parasites. Mol. Cell. Biol. 1999;19:2895–2902. doi: 10.1128/mcb.19.4.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasamontes L., Hug D., Hümbelin M., Weber G. Sequence of a major Eimeria maxima antigen homologous to the Eimeria tenella microneme protein Etp100. Mol. Biochem. Parasitol. 1993;57:171–174. doi: 10.1016/0166-6851(93)90255-v. [DOI] [PubMed] [Google Scholar]
- Pimenta P.F., Touray M., Miller L. The journey of malaria sporozoites in the mosquito salivary gland. J. Eukaryot. Microbiol. 1994;41:608–624. doi: 10.1111/j.1550-7408.1994.tb01523.x. [DOI] [PubMed] [Google Scholar]
- Robson K.J.H., Hall J.R.S., Jennings M.W., Harris T.J.R., Marsh K., Newbold C.I., Tate V.E., Weatherall D.J. A highly conserved amino-acid sequence in thrombospondin, properdin, and in proteins from sporozoites and blood stages of a human malaria parasite. Nature. 1988;335:79–82. doi: 10.1038/335079a0. [DOI] [PubMed] [Google Scholar]
- Robson K.J.H., Naitza S., Barker G., Sinden R.E., Crisanti A. Cloning and expression of the thrombospondin related adhesive protein gene of Plasmodium berghei . Mol. Biochem. Parasitol. 1997;84:1–12. doi: 10.1016/s0166-6851(96)02774-0. [DOI] [PubMed] [Google Scholar]
- Rogers W.O., Malik A., Mellouk S., Nakamura K., Rogers M.D., Szarfman A., Gordon D.M., Nussler A.K., Aikawa M., Hoffman S.L. Characterization of Plasmodium falciparum sporozoite surface protein 2 Proc. Natl. Acad. Sci. USA. 89 1992. 9176 9180a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers W.O., Rogers M.D., Hedstrom R.C., Hoffman S.L. Characterization of the gene encoding sporozoite surface protein 2, a protective Plasmodium yoelii sporozoite antigen Mol. Biochem. Parasitol 53 1992. 45 52b [DOI] [PubMed] [Google Scholar]
- Russell D.G. Host cell invasion by Apicomplexaan expression of the parasite's contractile system? Parasitology. 1983;87:199–209. doi: 10.1017/s0031182000052562. [DOI] [PubMed] [Google Scholar]
- Russell D.G., Sinden R.E. The role of the cytoskeleton in the motility of coccidian sporozoites. J. Cell Sci. 1981;50:345–359. doi: 10.1242/jcs.50.1.345. [DOI] [PubMed] [Google Scholar]
- Spano F., Putignani L., Naitza S., Puri C., Wright S., Crisanti A. Molecular cloning and expression analysis of a Cryptosporidium parvum gene encoding a new member of the thrombospondin family. Mol. Biochem. Parasitol. 1998;92:147–162. doi: 10.1016/s0166-6851(97)00243-0. [DOI] [PubMed] [Google Scholar]
- Speer C.A., Wong R.B., Blixt J.A., Schenkel R.H. Capping of immune complexes by sporozoites of Eimeria tenella . J. Parasitol. 1985;71:33–42. [PubMed] [Google Scholar]
- Stewart M.J., Vanderberg J.P. Malaria sporozoites leave behind trails of circumsporozoite protein during gliding motility. J. Protozool. 1988;35:389–393. doi: 10.1111/j.1550-7408.1988.tb04115.x. [DOI] [PubMed] [Google Scholar]
- Sultan A.A., Thathy V., Frevert U., Robson K.J., Crisanti A., Nussenzweig V., Nussenzweig R.S., Ménard R. TRAP is necessary for gliding motility and infectivity of Plasmodium sporozoites. Cell. 1997;90:511–522. doi: 10.1016/s0092-8674(00)80511-5. [DOI] [PubMed] [Google Scholar]
- Sultan A.A., Thathy V., Nussenzweig V., Ménard R. Green fluorescent protein as a marker in Plasmodium berghei transformation. Infect. Immunol. 1999;67:2602–2606. doi: 10.1128/iai.67.5.2602-2606.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton T.J., Kaslow D.C. Cloning and cross–species comparison of the thrombospondin-related anonymous protein (TRAP) gene from Plasmodium knowlesi, Plasmodium vivax, and Plasmodium gallinaceum . Mol. Biochem. Parasitol. 1997;84:13–24. doi: 10.1016/s0166-6851(96)02775-2. [DOI] [PubMed] [Google Scholar]
- Tomley F.M., Clarke L.E., Kawazoe U., Dijkema R., Kok J.J. Sequence of the gene encoding an immunodominant microneme protein of Eimeria tenella . Mol. Biochem. Parasitol. 1991;49:277–288. doi: 10.1016/0166-6851(91)90071-d. [DOI] [PubMed] [Google Scholar]
- Trottein F., Triglia T., Cowman A.F. Molecular cloning of a gene from Plasmodium falciparum that codes for a protein sharing motifs found in adhesive molecules from mammals and Plasmodia. Mol. Biochem. Parasitol. 1995;74:129–141. doi: 10.1016/0166-6851(95)02489-1. [DOI] [PubMed] [Google Scholar]
- Vanderberg J.P. Studies on the motility of Plasmodium sporozoites. J. Protozool. 1974;21:527–537. doi: 10.1111/j.1550-7408.1974.tb03693.x. [DOI] [PubMed] [Google Scholar]
- Wan K.-L., Carruthers V.B., Sibley L.D., Ajioka J.W. Molecular characterization of an expressed sequence tag locus of Toxoplasma gondii encoding the micronemal protein MIC2. Mol. Biochem. Parasitol. 1997;84:203–214. doi: 10.1016/s0166-6851(96)02796-x. [DOI] [PubMed] [Google Scholar]
- Waters, A.P., A.W. Thomas, M.R. van Dijk, and C.J. Janse. 1997. Transfection of malaria parasites. In Methods: a Companion to Methods in Enzymology—Analysis of Apicomplexan Parasites. Vol. 13. Academic Press, Inc., Orlando, FL. 134–147. [DOI] [PubMed]
- Yuda M., Sawai T., Chinzei Y. Structure and expression of an adhesive protein-like molecule of mosquito invasive-stage malarial parasite. J. Exp. Med. 1999;189:1947–1952. doi: 10.1084/jem.189.12.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]