Abstract
Matrix GLA protein (MGP), a γ-carboxyglutamic acid (GLA)–rich, vitamin K–dependent and apatite-binding protein, is a regulator of hypertrophic cartilage mineralization during development. However, MGP is produced by both hypertrophic and immature chondrocytes, suggesting that MGP's role in mineralization is cell stage–dependent, and that MGP may have other roles in immature cells. It is also unclear whether MGP regulates the quantity of mineral or mineral nature and quality as well. To address these issues, we determined the effects of manipulations of MGP synthesis and expression in (a) immature and hypertrophic chondrocyte cultures and (b) the chick limb bud in vivo. The two chondrocyte cultures displayed comparable levels of MGP gene expression. Yet, treatment with warfarin, a γ-carboxylase inhibitor and vitamin K antagonist, triggered mineralization in hypertrophic but not immature cultures. Warfarin effects on mineralization were highly selective, were accompanied by no appreciable changes in MGP expression, alkaline phosphatase activity, or cell number, and were counteracted by vitamin K cotreatment. Scanning electron microscopy, x-ray microanalysis, and Fourier-transform infrared spectroscopy revealed that mineral forming in control and warfarin-treated hypertrophic cell cultures was similar and represented stoichiometric apatite. Virally driven MGP overexpression in cultured chondrocytes greatly decreased mineralization. Surprisingly, MGP overexpression in the developing limb not only inhibited cartilage mineralization, but also delayed chondrocyte maturation and blocked endochondral ossification and formation of a diaphyseal intramembranous bone collar. The results show that MGP is a powerful but developmentally regulated inhibitor of cartilage mineralization, controls mineral quantity but not type, and appears to have a previously unsuspected role in regulating chondrocyte maturation and ossification processes.
Keywords: chondrocytes, matrix GLA protein, mineralization, ossification, limb development
Mineralization of the extracellular matrix is a cell-mediated process that is required for normal development and function of skeletal and dental tissues. For example, during endochondral bone formation hypertrophic chondrocytes direct the deposition of hydroxyapatite crystals in the extracellular matrix (Boyde and Shapiro 1980; Boskey et al. 1992). Once mineralized, the cartilage matrix is invaded by bone and bone marrow progenitor cell populations; this is followed by initiation of osteoblast differentiation, deposition of apatitic mineral, and replacement of mineralized hypertrophic cartilage with bone (Fell 1925; Hall 1971; Haines 1975). Maintenance of normal bone structure and function is subsequently dependent upon a critical balance between mineral deposition and resorption. Indeed, an imbalance in either of these processes can lead to pathological conditions. In osteoporosis, resorption exceeds deposition, leading to bone mass and mineral losses (Manolagas and Jilka 1995); in atherosclerosis, the vessel-associated plaques often contain apatite crystals and may display a histological appearance similar to that of lamellar bone, contributing to tissue stiffness and loss of normal resiliency (Bostrom et al. 1995).
Given the importance of the mineralization process in health and disease, it is not surprising that there is intense scrutiny of the cellular and molecular mechanisms regulating apatite deposition. Two extracellular matrix proteins, matrix GLA protein (MGP) and osteocalcin, have attracted much research interest (Price 1985; Hale et al. 1988; Hauschka et al. 1989; Luo et al. 1995; Neugebauer et al. 1995; Ducy et al. 1996; Boskey et al. 1998; Munroe et al. 1999). These proteins belong to a large family that includes blood coagulation factors (Furie and Furie 1988). All members of the family contain glutamyl groups, some of which are posttranslationally modified by a vitamin K–dependent γ-carboxylase into γ-carboxyglutamic acid (GLA) residues (Price 1985; Suttie 1985). In both MGP and osteocalcin, the GLA residues promote binding of calcium and phosphate ions. A combination of charge and lattice geometry facilitates adsorption of calcium atoms into the hydroxyapatite crystals (Poser and Price 1979; Hauschka et al. 1989). During development, MGP and osteocalcin preferentially accumulate in mineralized cartilage and bone (Mark et al. 1988; Price 1989; Strauss et al. 1990; Barone et al. 1991; Dohi et al. 1992; Neugebauer et al. 1995). These findings have led to the long held view that MGP and osteocalcin must serve important regulatory roles in the mineralization of skeletal and dental tissues (Bronckers et al. 1985; Price 1985; Hauschka et al. 1989).
Pharmacological and genetic approaches have been used to gain insights into these regulatory roles. For example, treatment of pregnant rats with the vitamin K antagonist, warfarin (Suttie 1985), reduces GLA residue synthesis in the fetus and newborn and causes severe skeletal malformations. These defects include disorganization and excessive mineralization of growth plate and nasal septum cartilages, precocious closure of the growth plate, growth retardation, and abnormalities in, or absence of, ossification centers (Price et al. 1982; Price 1989; Feteih et al. 1990). A similar prenatal exposure to warfarin is associated with embryopathy and skeletal defects in humans (Hall et al. 1980). Recent genetic studies have shown that mice lacking a functional MGP gene are viable, but exhibit increased calcification of growth plate cartilage, short stature, osteopenia, and fractures (Luo et al. 1997). The MGP-deficient mice die around two months of age as a result of excessive abnormal calcification of their arteries leading to blood vessel rupture. In a related study, ablation of the osteocalcin gene was also found to be compatible with life (Ducy et al. 1996). Though viable, fertile, and seemingly normal during early postnatal life, the osteocalcin-null mice exhibit excessive bone formation. Mutations in the human MGP gene cause Keutel syndrome, a disorder characterized by excessive cartilage calcification (Munroe et al. 1999). The above pharmacological and genetic studies have led to the intriguing conclusion that, despite their abundance in skeletal tissues and their ability to bind calcium and apatite, MGP and osteocalcin may actually serve as inhibitors of cartilage mineralization and bone formation, respectively.
There are additional intriguing and unanswered questions regarding MGP function. First, the protein is not only present in the mineralizing zone of the growth cartilage, but is also present in articular cartilage and certain nonskeletal tissues. Yet, interference with MGP function in vivo by warfarin treatment or ablation of the MGP gene does not lead to widespread random mineralization. Excessive mineralization is seen only at specific sites, such as the growth cartilage and arteries (Price et al. 1982; Luo et al. 1997). This finding raises the possibility that MGP function in mineralization may depend on the stage of development of the responding cells or the nature of the responding cells. A second question is whether MGP mainly determines the quantity of mineral deposited or whether it influences the type and quality of mineral. Last, it is not known whether overexpression of MGP simply causes effects opposite to those seen after warfarin treatment or MGP gene ablation, or whether there are additional effects indicative of other functions of the protein. In the present study, we have examined these three questions. We show that MGP is a developmentally regulated modulator of chondrocyte matrix mineralization and mainly controls mineral quantity but not quality. We show also that constitutive MGP expression in the chick limb in vivo not only inhibits cartilage mineralization, but also blocks chondrocyte maturation and intramembranous and endochondral ossification.
Materials and Methods
Cell Cultures
Immature and hypertrophic chondrocytes were isolated from the caudal one-third portion and the cephalic core portion of day 17–18 chick embryo sterna, respectively (Pacifici et al. 1991b; Iwamoto et al. 1993a,Iwamoto et al. 1993b). Tissue fragments were pretreated for 2 h at 37°C with 0.25% trypsin to eliminate perichondrial and blood cells, and were then treated with a fresh 0.25% trypsin/0.1% collagenase enzyme mixture for an additional 2–3 h. The resulting cell populations were filtered, recovered by centrifugation, and plated at a density of 1.3 × 106 cells/60-mm tissue culture dish or 2 × 105 cells/22-mm well (12 well plates). Cells were grown continuously, without subculturing, for ∼2 wk in monolayer. During the first 2 d, cultures received 4 U/ml of testicular hyaluronidase to minimize cell detachment (Leboy et al. 1989). Cultures were fed every other day with Dulbecco's modified high glucose Eagle's medium containing 10% fetal calf serum, 2 mM l-glutamine, and 50 U/ml penicillin and streptomycin (complete medium) (Pacifici et al. 1991a).
To allow cells to deposit mineral, confluent cultures received complete medium supplemented with 5 mM β‴glycerophosphate to serve as a phosphate source and with 25 μg/ml ascorbic acid or 35 nM all-trans-retinoic acid to serve as mineralization cofactors (Leboy et al. 1989; Iwamoto et al. 1993a). Complete medium supplemented with ascorbate and β-glycerophosphate was termed medium A, and complete medium supplemented with retinoic acid and β-glycerophosphate was termed medium B. Mineral was revealed by staining with alizarin red (McGee-Russel 1958). When indicated, cultures were treated with 3–10 μM vitamin K, warfarin, or a combination of both biochemicals. Stock solutions (1,000×) of warfarin and vitamin K3 were made in saline, whereas vitamin K1 and K2 stock solutions were made in 95% ethanol. Untreated cultures received equal volumes of vehicle. During treatment, the medium was changed daily.
Determination of Alkaline Phosphatase (APase) and Calcium Content
The APase activity of the cell layer was measured using p-nitrophenyl phosphate (pNP) as a substrate (Pacifici et al. 1991b). Cells were scraped from the dish, recovered by centrifugation, and resuspended in ice-cold 0.9% NaCl in 3 mM Tris-HCl, pH 7.4. One half of each cell suspension was centrifuged for 1 min in a microfuge (10,000 g) and the resulting cell pellet was solubilized in 0.9% NaCl and 0.2% Triton X-100. Samples were clarified by centrifugation for 5 min and the supernatants were mixed with one volume of 1 M Tris-HCl, pH 9.0, containing 1 mM pNP and 1 mM MgCl2. The reaction was stopped by addition of 0.25 vol of 1 N NaOH, and hydrolysis of pNP was monitored as a change in absorbance at 410 nm. The remaining half of each cell suspension was used to determine DNA content (Johnson-Wint and Hollis 1982).
For calcium determination, cultures in multiwell plates were rinsed three times with 0.9% NaCl in 10 mM Tris-HCl, pH 7.4, and extracted with 0.5 ml/well of 1 N HCl for 30 min at room temperature. The calcium concentration in the extract was determined by atomic absorption spectrophotometry (Perkin-Elmer 2380) in the presence of 0.1% LaCl3 (Iwamoto et al. 1993a).
Northern Blot Analysis
Total RNAs were isolated from chondrocyte cultures as described (Pacifici et al. 1991b; Iwamoto et al. 1993b). For Northern blot analysis, 10 μg of total RNA denatured by glyoxalation was fractionated on 1% agarose gels and transferred to Hybond-N membrane. Blots were stained with 0.04% methylene blue to verify that each sample had been transferred efficiently. Blots were hybridized in 6× SSC, 5× Denhardt's solution, 100 μg/ml sheared salmon sperm DNA, 2% SDS, and 50% formamide at 45°C overnight with 32P-labeled cDNA MGP probe. A 306-bp full-length chick MGP cDNA clone was prepared in the pTA vector by reverse transcriptase PCR (RT-PCR), using primers designed on published sequence (Wiedemann et al. 1998). The EcoRI-BamHI insert was excised and labeled with [32P]CTP by random priming. Blots were exposed to Kodak x-ray Bio-Max films at −70°C for different lengths of time to insure linearity of exposure.
Fourier Transform Infrared (FT-IR) Analysis
This procedure was carried out as described (Iwamoto et al. 1993b; Rey et al. 1995; Gadaleta et al. 1996). Cultures were rinsed three times with 0.9% NaCl in 10 mM Tris-HCl, pH 7.4, freeze-dried at −60°C, and stored dessicated. Samples were analyzed by FT-IR spectroscopy using a Nicolet Magna–IR spectrometer operated in the diffuse reflectance mode. Each sample was gently milled in an agate mortar and layered on KBr at a KBr:sample ratio of 300:1 (wt/wt). Routinely, 300 interferograms were collected at 4 cm−1 resolution; the background was subtracted using a nonmineralized chondrocyte preparation. Spectra were co-added and the resulting interferograms were fourier transformed; second derivative spectra (1,200–500 cm−1) were obtained using a software package (Omnic). Resolution factors are presented in the legend of each figure.
Scanning Electron Microscopy (SEM) and X-ray Microanalysis
Chondrocyte monolayer cultures were processed for these analyses as described previously (Harrison et al. 1995). In brief, cultures were rinsed with PBS, fixed with 1:1 dilution of Karnovsky's fixative for 30 min, postfixed with full strength Karnovsky for 1 h, and then dehydrated by incubation in 10, 30, 50, 70, 80, 90, 95, and 100% ethanol (15 min each); incubation with 100% ethanol was repeated twice. After removal of the last ethanol, the cells were covered with Freon 113; the dishes were sealed with parafilm in which a few holes were punctured with a needle, and were allowed to dry slowly under a hood overnight. A circle ∼2 cm in diameter was cut from each dish and mounted by colloidal silver paint onto an aluminum stub and cells were carbon sputter–coated. Samples were viewed using a JEOL T330A scanning electron microscope and were simultaneously examined by energy dispersive x-ray microanalysis using Kevex hardware (Kevex Corp.) with Quantex 5 software.
RCAS Construction and Room Temperature–PCR (RT-PCR) Analysis
The entire coding sequence of chick MGP (Wiedemann et al. 1998) was amplified by RT-PCR (see below) and subcloned into the RCAS(A) retroviral vector (Hughes et al. 1987). The resulting plasmid (RCAS-MGP) was resequenced to verify sequence fidelity. Recombinant plasmid DNA was transfected into chick embryo fibroblasts by a FuGENE6 transfection reagent (Boehringer Mannheim). Recombinant viral particles released into the medium were concentrated using a centrifugation concentrator and used to infect freshly isolated chondrocytes (Iwamoto et al. 1993b).
For determination of efficiency of viral infection, cultured chondrocytes were detached by trypsinization and replated onto poly-l-lysine–coated dishes at a density of 2 × 106 cells/ml. After 15 min, adhered cells were washed with PBS twice, fixed with 3.7% neutralized formaldehyde solution for 5 min, and permeabilized by incubation with PBS containing 0.5% Triton X-100 for 15 min. Cells were incubated with hybridoma culture supernatant containing AMV-3C2, a mouse mAb against the gag protein of avian myeloblastosis virus and recognizing cells harboring avian Rous sarcoma or leukemia virus. Detection of bound antibody was obtained by the ABC method using Histofine kits (Nichirei).
RT-PCR was carried out as described (Enomoto-Iwamoto et al. 1998). 1 μg of whole cellular RNA was reverse transcribed by Superscript reverse transcriptase (GIBCO BRL) and random hexamers. After reverse transcription, samples were amplified for the indicated number of cycles using Elongase (GIBCO BRL) at the following conditions: 10 s at 95°C for denaturation and 1 min at 60°C for annealing and extension. The sequences of chick MGP primers were based on the report by Wiedemann et al. 1998 and were: 5′ GAGTGAGGCACAGCAAGAGACA 3′ and 5′ AGGAGGTTACCCTGAGGTCCAA 3′, generating a 402-bp fragment. The chick cytoplasmic β-actin primers were as follows: 5′ AAGGAGAAGCTGTGCTACGTCG 3′ and 5′ CTTCTGCATCCTGTCAGCAATG 3′, generating a 309-bp fragment (Kost et al., 1983).
Implantations in Chick Embryo Limb Buds and In Situ Hybridization
Chick embryo fibroblasts transfected with RCAS-MGP or control insertless RCAS plasmids were grown to confluency in monolayer. Small cell layer fragments, ∼200–300 μm2, were scraped off the culture dish surface and were microsurgically implanted in the mesenchyme at the distal region of the leg bud of Hamburger-Hamilton stage 22–23 chick embryos (Koyama et al. 1996). Eggs were returned to the incubator and allowed to develop further. At the indicated time points, embryos were removed from eggs, sacrificed, and examined visually or under a dissecting scope to determine the gross effects of experimental manipulations on limb development. Control and experimental limbs were removed, fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned. Sections were processed for general histology by staining with hematoxylin and eosin stains and for histochemical detection of mineral by staining with 0.5% alizarin red S solution, pH 4.0, for 5 min at room temperature.
Companion continuous 5-μm sections were processed for in situ hybridization using 35S-labeled riboprobes as described previously (Koyama et al. 1996) to determine the gene expression patterns of MGP, type II collagen, and type X collagen. The cDNA clones used were the following: a full-length chick MGP clone prepared by RT-PCR (see above), the 0.8-kb chick type II collagen clone pDLr2, and the 0.197-kb chick type X collagen clone pDLr10 (Leboy et al. 1989).
Results
Warfarin Effects on Mineralization in Cultured Chondrocytes
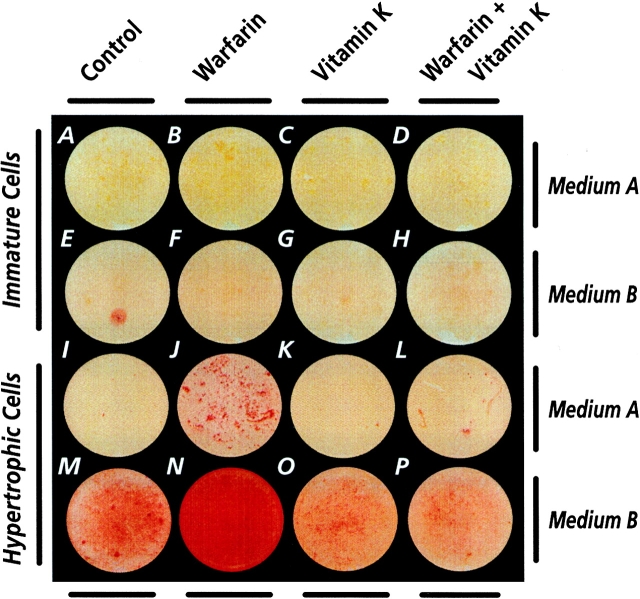
In the first set of experiments, we determined whether interference with endogenous MGP function by treatment with the vitamin K antagonist, warfarin, causes excessive matrix mineralization regardless of the stage of maturation of chondrocytes or whether the effects are developmentally regulated. To this end, confluent multiwell cultures of immature and hypertrophic chick embryo sternal chondrocytes were treated with 10 μM warfarin for 4 d in medium containing supplements required for mineral deposition in culture, namely the phosphate donor β-glycerophosphate and the collagen synthesis cofactor ascorbic acid (hereafter called medium A). As controls, companion cultures were treated with warfarin plus vitamin K1 or vitamin K1 alone, or were left untreated. To detect mineralization, cultures were stained with alizarin red or subjected to cell layer–associated calcium content analysis. We found that warfarin treatment had no appreciable effects on mineralization in immature chondrocyte cultures (Fig. 1A and Fig. B); these cultures all contained ∼1–1.5 μg/μg DNA of cell layer–associated calcium. However, the warfarin treatment markedly stimulated mineralization of the hypertrophic cultures (Fig. 1I and Fig. J); cell-associated calcium content increased from ∼3.3 ± 0.4 μg/μg DNA in control to 11.2 ± 2.1 μg/μg DNA in warfarin-treated cultures. The warfarin stimulation of mineralization was counteracted by cotreatment with vitamin K1 (Fig. 1 L), attesting to the specificity of warfarin effects. Vitamin K1 alone had no obvious effects in either hypertrophic (Fig. 1 K) or immature cultures (Fig. 1C and Fig. D). Vitamin K2 and K3 gave identical results (not shown); thus, the three vitamins were used interchangeably hereafter.
Figure 1.
Micrographs of multiwell cultures of immature (A–H) and hypertrophic (I–P) stained with alizarin red to reveal mineralization. Confluent cultures in medium A or medium B were treated with 10 μΜ ϕαρφαριν ≲Β< Φ< Ξ< ανδ Ν≳< +″ μM vitamin K3 (C, G, K, and O), and both warfarin and vitamin K (D, H, L, and P) for 4 d. Companion control cultures received vehicle alone (A, E, I, and M).
To insure that the differential responses above were specific, reflected the cells' potentials to mineralize, and were not influenced by the type of mineralization cofactors used, similar confluent immature and hypertrophic cultures were grown for 4 d in medium supplemented with β‴γλυψ∈ροπηοσπηατ∈ ανδ ρ∈τινοιψ αψιδ ≲η∈ρ∈αφτ∈ρ ψαλλ∈δ μ∈διθμ Β≳> Ιν ∈αρλι∈ρ στθδι∈σ< ϕ∈ σηοϕ∈δ τηατ ρ∈τινοιψ αψιδ ισ α στρονγ∈ρ στιμθλατορ οφ μιν∈ραλιζατιον‴ρ∈λατ∈δ αψτιωιτι∈σ ιν ψηονδροψυτ∈σ ≲Ιϕαμοτο ∈τ αλ>< +′′×α≳> Ψθλτθρ∈σ ιν μ∈διθμ Β ϕ∈ρ∈ τρ∈ατ∈δ ϕιτη +″ mM warfarin, 10 mM vitamin K, or both as above. Even in this medium, warfarin treatment failed to stimulate matrix mineralization in immature cultures (Fig. 1, E–H), but did so in hypertrophic cultures (Fig. 1M and Fig. N). Calcium content increased from 14.4 ± 2.7 μg/μg DNA in control hypertrophic cultures to 32.6 ± 4.2 μg/μg DNA after warfarin treatment. This stimulation was counteracted by cotreatment with vitamin K (Fig. 1 P). Interestingly, the substantial mineralization seen in control hypertrophic cultures (Fig. 1 M) was partially counteracted by treatment with vitamin K (Fig. 1 O), which decreased the cell layer-associated calcium content to 9.1 6 1.8 μg/μg DNA.
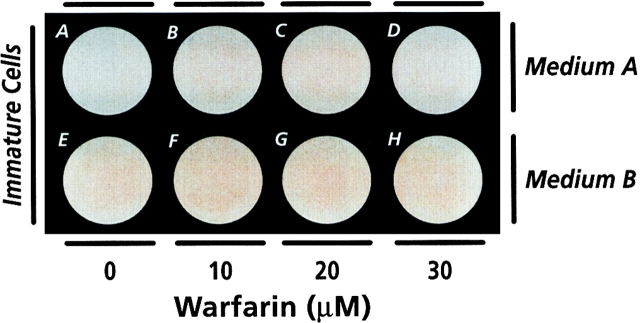
To investigate whether higher doses of warfarin or longer treatment periods could actually induce mineralization in immature cultures, cultures in medium A and B were treated with 10, 20, or 30 μM warfarin for 4 d; parallel cultures were treated for 8 d. We found that neither the higher warfarin concentrations used (Fig. 2, A–H) nor the longer treatment period (not shown) resulted in mineralization of immature chondrocyte cultures.
Figure 2.
Micrographs of confluent immature cultures grown in medium A or B, treated for 4 d with 10, 20, or 30 μM warfarin, and stained with alizarin red.
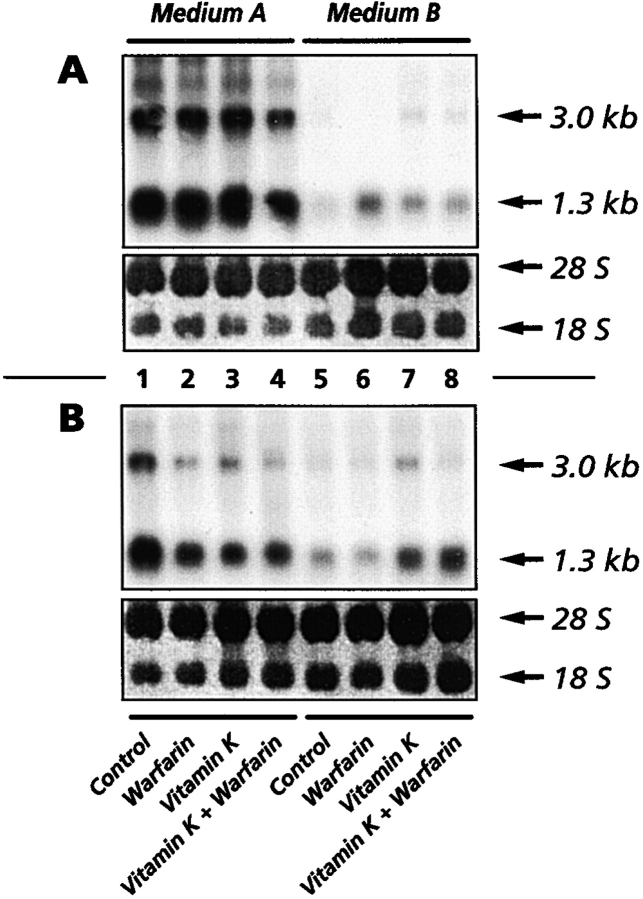
To determine the relationship between mineralization responses and endogenous MGP gene expression, cultures of immature and hypertrophic chondrocytes in medium A or medium B were treated as above and were processed for Northern blot analyses. We found that both immature and hypertrophic cultures contained obvious amounts of the 1.3- and 3.0-kb MGP RNAs (Fig. 3A and Fig. B, respectively) (Wiedemann et al. 1998). Cultures in medium A contained two to threefold more MGP transcripts (Fig. 3A and Fig. B, lane 1) than cultures in medium B (Fig. 3A and Fig. B, lane 5). However, these basal levels of expression were not significantly affected by treatment with warfarin, vitamin K, or warfarin plus vitamin K (Fig. 3, A–B, lanes 1–8).
Figure 3.
Northern blot analysis of MGP gene expression. Confluent immature (A) and hypertrophic (B) cultures in medium A or medium B were treated for 4 d with warfarin, vitamin K, or both. Blots (10 μg RNA/lane) were stained with methylene blue to reveal the ribosomal bands and to verify equal loading and transfer efficiency. Blots were hybridized with a 32P-labeled MGP probe and exposed to x-ray films. The two main 3.0- and 1.3-kb MGP transcripts are indicated.
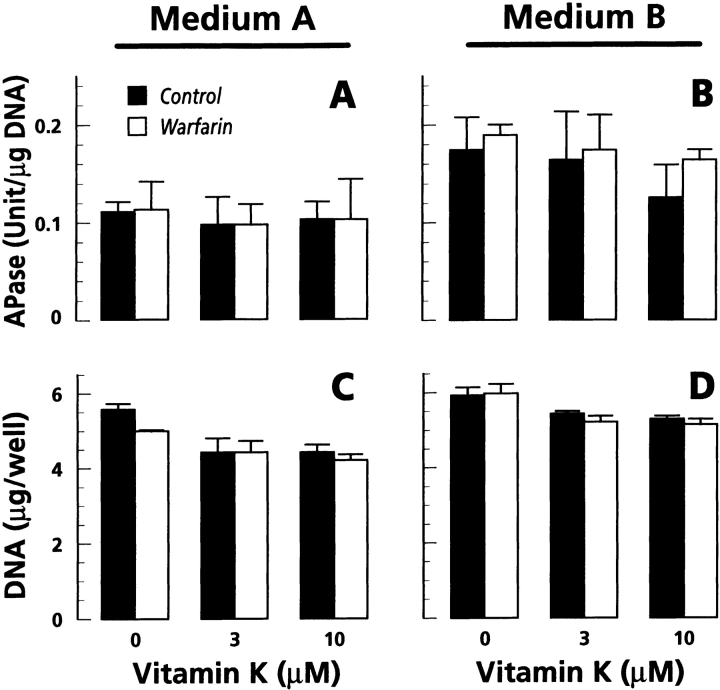
Since APase is an enzyme whose activity is closely associated with the mineralization process, we determined the activity of the phosphatase in control and warfarin-treated hypertrophic cultures. We found no change in APase activity per cell as a result of warfarin and vitamin K treatments or cotreatments in either medium A or medium B (Fig. 4A and Fig. B). This analysis also showed that warfarin treatment had no effect on cell number (Fig. 4C and Fig. D). Thus, MGP function in mineralization as revealed by warfarin interference depends on the stage of chondrocyte maturation, does not appear to be related to MGP expression levels, and does not involve changes in APase activity.
Figure 4.
Histograms showing levels of APase activity and DNA content in hypertrophic chondrocyte cultures. Confluent cultures grown in medium A (A and C) and medium B (B and D) were left untreated (solid bars) or were treated with 10 μM warfarin (open bars) for 4 d in the absence or presence of indicated doses of vitamin K. After treatment, aliquots of the cell layer were used for determination of APase activity and DNA content.
Analysis of Mineral
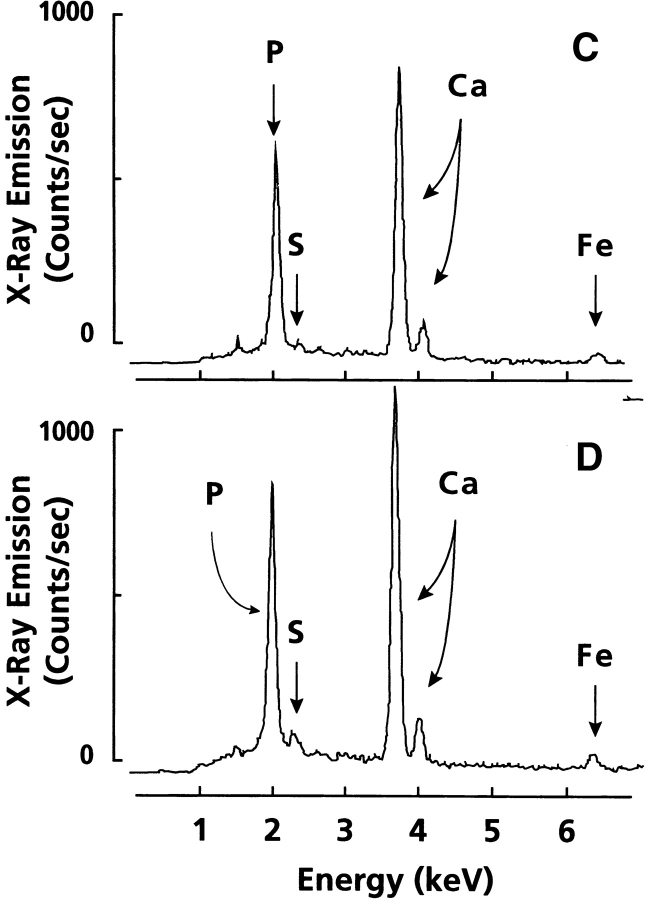
We determined next whether MGP regulates only the quantity of mineral deposited by cells in their matrix or may also affect mineral nature and overall quality. Thus, we compared the mineral deposited by control and warfarin-treated hypertrophic chondrocytes by SEM, x-ray microanalysis, and FT-IR spectroscopy. Cells used for these experiments were grown in medium B because they formed more mineral than cells in medium A (Fig. 1). When viewed by SEM, many crystals were observed on, and to be associated with, the cell surface in both control and warfarin-treated cells (Fig. 5A and Fig. B). When examined at a higher magnification, each crystal displayed a typical multibead organization, with beads averaging 200–400 nm in diameter (Fig. 5 B, inset). After SEM, multiple microscopic fields were subjected to x-ray microanalysis. Prominent phosphorus and calcium peaks were produced by crystals in both cultures (Fig. 5C and Fig. D). Integration of the peaks showed that the calcium/phosphorus molar ratio was ∼1.3–1.4, thus, resembling that of apatite in hypertrophic cartilage (Boyde and Shapiro 1980; Boskey et al. 1992).
Figure 5.
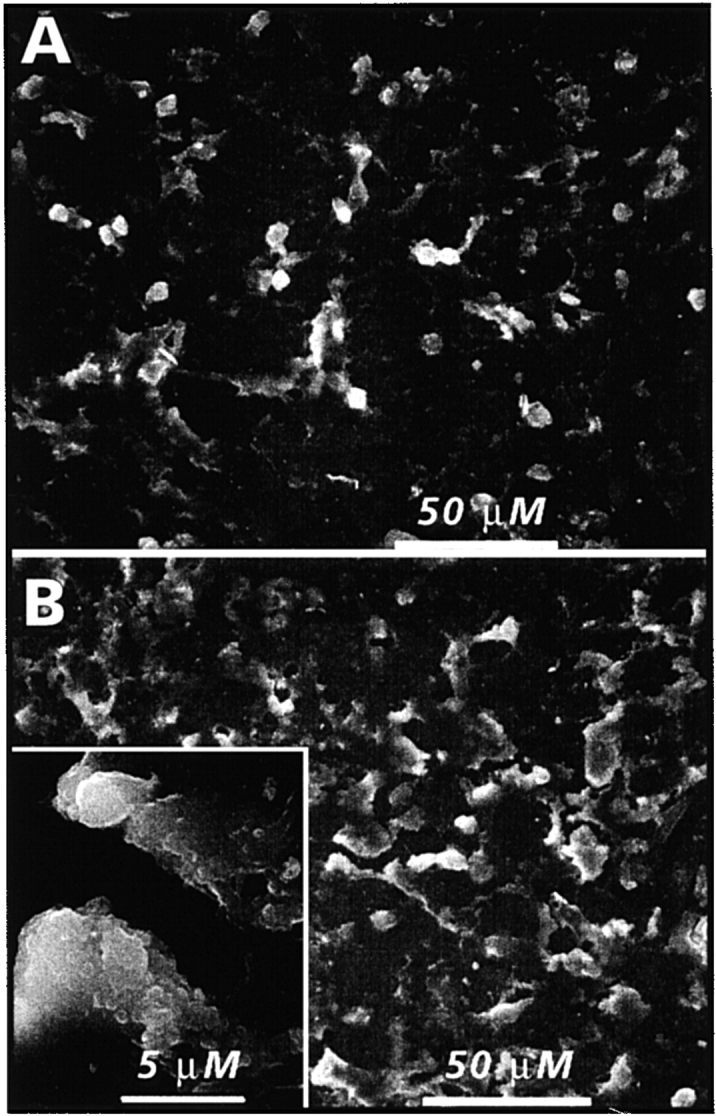
Scanning electron micrographs of chondrocyte cultures (A and B) and x-ray element microanalysis of mineral present (C and D). Confluent hypertrophic cultures in medium B were treated with 10 μM warfarin for 4 d (B and D) or were left untreated (A and C), and were then processed for SEM and x-ray analyses. Note the numerous mineral crystal deposits present in both cultures (A and B). Inset in B is a higher magnification view of one such deposit revealing its multibead structure. X-ray analysis of crystals (C and D) shows that they contain prominent phosphorus (P) and calcium (Ca) peaks in ratios typical of biologic apatite. The position of sulfur (S) and iron (Fe) is indicated.
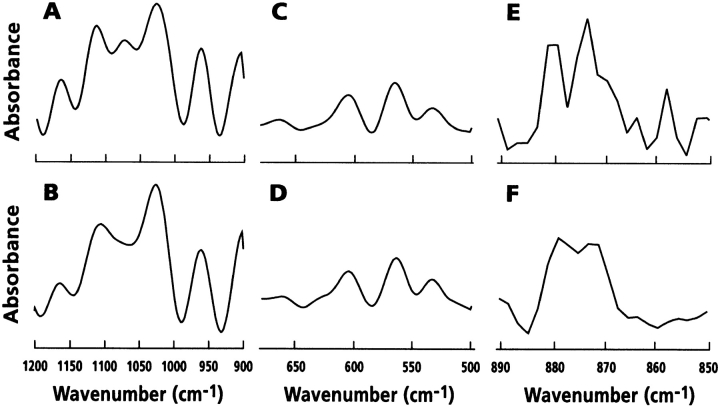
To examine the mineral composition in greater detail, mineral samples from control and warfarin-treated cultures were examined by FT-IR spectroscopy (Fig. 6). The samples elicited similar second derivative spectra in the v1 v3 phosphate region 1,200–950 cm−1 (Fig. 6A and Fig. B), with clear peaks at 1,160, 1,102, 1,028, 962, and 906 cm−1 that are characteristic of stoichiometric hydroxyapatite. Likewise, similar second derivative spectra were observed in the v4 phosphate domain 650–500 cm−1 (Fig. 6C and Fig. D) with strong peaks at 606 and 565 cm−1 attributable to phosphate ions in apatite, and in the v2 carbonate region 890–850 cm−1 (Fig. 6E and Fig. F). Bands at 880, 875, and 872 cm−1 in the carbonate region indicated that CO3 had at least in part substituted for OH and PO4, and that carbonate apatite was present in the mineralized cultures. Thus, qualitatively similar mineral is deposited by hypertrophic chondrocytes in the absence or presence of warfarin. In both cases, the mineral has morphological, organizational, and biochemical characteristics of apatite crystals displaying relatively low crystallinity, typical of mineralizing cells in culture as well as young bone tissue in vivo (Roufosse et al. 1984; Rey et al. 1991; Iwamoto et al. 1993b; Rey et al. 1995).
Figure 6.
FT-IR analysis of mineral. Confluent hypertrophic chondrocyte cultures were left untreated (A, C, and E) or were treated for 4 d with 10 μM warfarin for 4 d (B, D, and F) in medium B. After treatment, cell layers were processed for mineral harvest and samples were analyzed by FT-IR.
MGP Overexpression in Cultured Chondrocytes
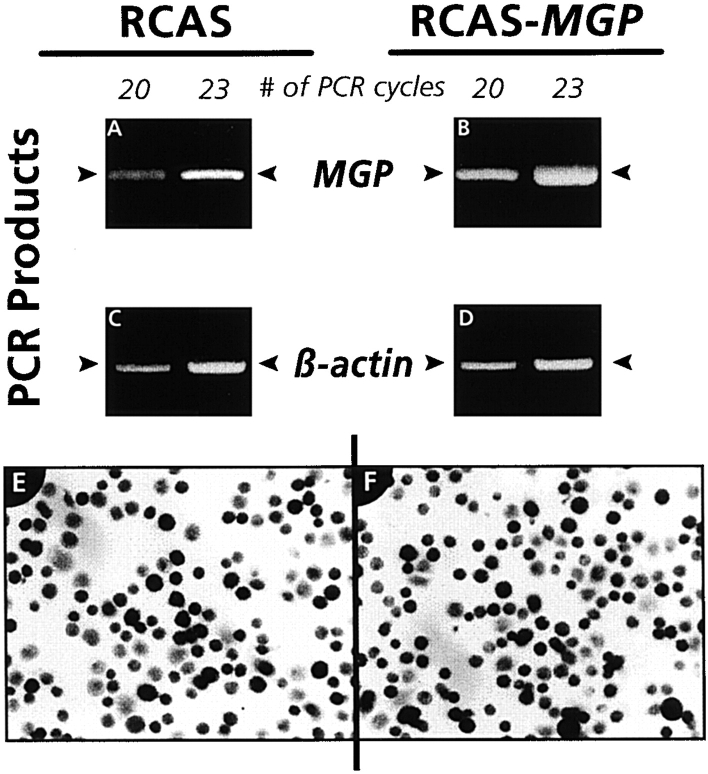
We asked next whether the amount of MGP expressed by hypertrophic chondrocytes is a factor influencing the mineralization potential of the cells. To address this question, we constructed an RCAS-based expression vector (Hughes et al. 1987) encoding full-length chick MGP and used the resulting viral particles (RCAS-MGP) to infect freshly isolated hypertrophic chondrocyte populations. As a control, companion cells were infected with insertless RCAS viral particles. Cultures were grown for ∼1 wk until confluent, and were processed for RT-PCR to estimate the levels of MGP gene expression and for immunocytochemistry to determine the percentage of virally infected cells in control and RCAS-MGP cultures, using an antibody to the viral structural protein p19. We found that MGP gene expression was significantly higher in RCAS-MGP cultures (expressing both endogenous and virally encoded MGP) (Fig. 7 B) than in control cultures (expressing endogenous MGP only) (Fig. 7 A). Housekeeping actin gene expression was identical in both cell populations (Fig. 7C and Fig. D). Immunocytochemistry revealed that ∼60% of the cells in both control and RCAS-MGP cultures were virally infected (Fig. 7E and Fig. F, respectively).
Figure 7.
RT-PCR and immunocytological analyses of chondrocyte cultures. Freshly isolated cephalic sternal chondrocytes infected with control viral particles (RCAS) or viral particles encoding MGP (RCAS-MGP) were grown for ∼1 wk in medium A. Cultures were processed for RT-PCR analysis of MGP (A and B) and β-actin (C and D) expression or for immunocytochemical determination of virally infected cells (E and F), using an antibody to the viral protein p19. Note that infected cells are dark/black, whereas uninfected cells are light gray.
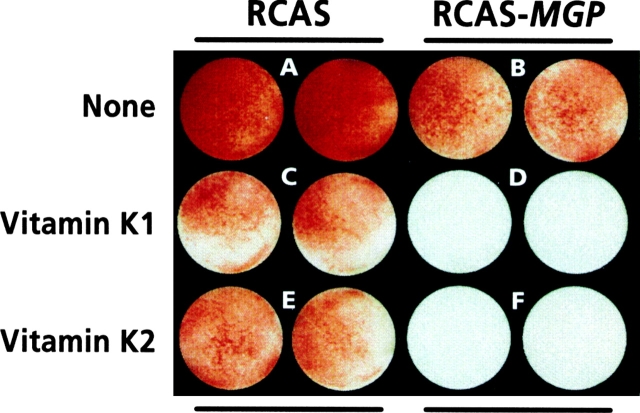
To determine the effects of MGP overexpression on mineralization, companion multiwell cultures were maintained in mineralization medium A or B for 7 d (rather than 4 d as in the experiments above) to induce optimal mineralization. At the end of this period, cultures (in duplicate) were stained with alizarin red to reveal mineral deposition. Control cultures were clearly stained by alizarin red (Fig. 8 A, RCAS), indicating that the cells had mineralized their matrix and that infection by insertless virus had no deleterious effect on mineralization. In comparison, cultures infected with the RCAS-MGP virus exhibited significantly reduced alizarin red staining (Fig. 8 B, RCAS-MGP). When control cultures were treated with 10 μM vitamin K1 or K2 for the last 7 d of culture, mineralization was also reduced (Fig. 8C and Fig. E, RCAS); indeed, when RCAS-MGP cultures were similarly treated with vitamin K, mineralization was completely prevented (Fig. 8D and Fig. F, RCAS-MGP).
Figure 8.
Histochemical determination of mineral in control (RCAS) and MGP-overexpressing (RCAS-MGP) cultures. Cultures similar to those described in Fig. 7 legend were grown for ∼2 wk until confluent. Cultures (in duplicate) were grown for an additional 7 d in medium A in the absence (none) or presence of 10 μM vitamin K1 or K2. At the end of the treatment, cultures were stained with alizarin red to reveal mineralization.
MGP Overexpression In Vivo
We asked next whether MGP overexpression in the developing chick limb would have similar inhibitory effects on the mineralization process of long bones. A small pellet of fibroblasts producing RCAS-MGP or control RCAS viruses was implanted in the vicinity of the mesenchymal condensations of tibia and fibula present in stage 22–23 (day 3.5–4.0) chick embryo hindlimb buds. We aimed to infect only these skeletal elements and to use uninfected femur and tarsal elements as an internal control. Embryos were reincubated until day 10–12, and longitudinal sections through the entire legs were prepared and processed for histology, histochemistry, and in situ hybridization. Analysis of alizarin red–stained sections from control day 12 embryo showed that the femur (fe), tibia (ti), and tarsal (ta) elements displayed their typical elongated morphology, and that their alizarin red staining diaphyses were undergoing mineralization and ossification (Fig. 9 A). In contrast, in RCAS-MGP–infected embryos, the tibia failed to stain with alizarin red (Fig. 9 B); unexpectedly, the tibia was also significantly shorter and broader than the control. These changes were limited to the tibia since femur and tarsal elements in the RCAS-MGP leg were normal and stained with alizarin red (Fig. 9 B). Higher magnification analysis of alizarin red–stained or hematoxylin/eosin–stained sections showed that the diaphysis of control tibia contained hypertrophic mineralizing cartilage (Fig. 9 E, arrowhead) and endochondral bone (not shown), was surrounded by a mineralized intramembranous bone collar (Fig. 9C and Fig. E, arrow), and was being invaded by marrow cells (Fig. 9 E, double arrow). In contrast, the diaphysis of RCAS-MGP tibia was entirely cartilaginous (Fig. 9D and Fig. F). There was no evidence of erosion or invasion by marrow cells, and there was a lack of a bone collar (Fig. 9 D, arrow). In addition, the diaphyseal chondrocytes were not fully hypertrophic (Fig. 9 F, arrowheads) and displayed average cell diameters smaller than those in the control (Fig. 9 E).
Figure 9.
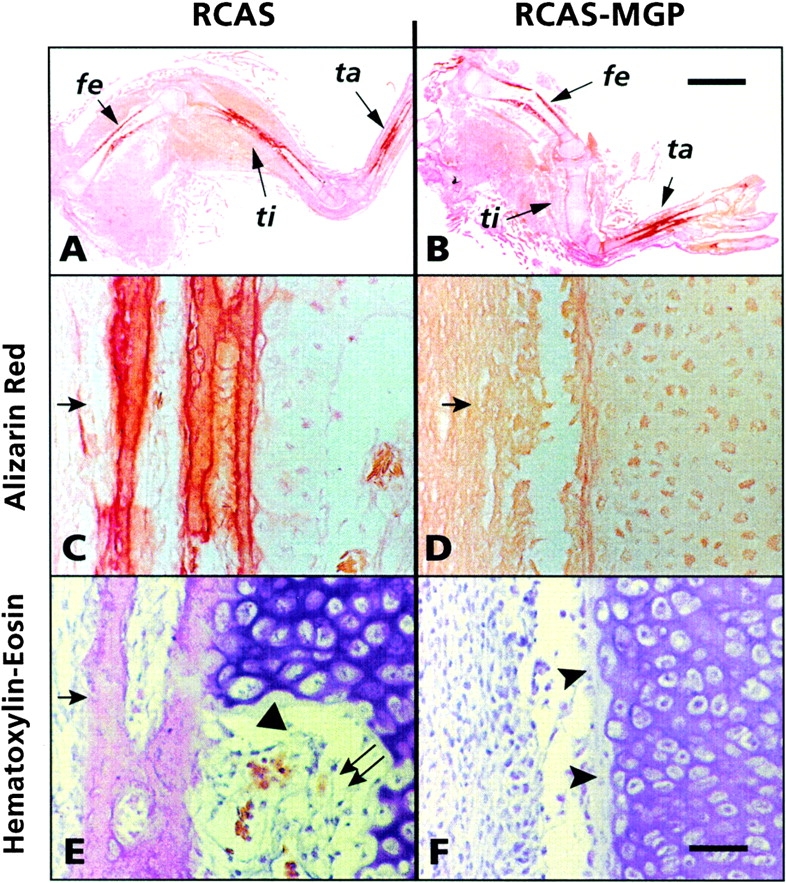
Histological analyses of the effects of MGP overexpression on limb long bone development. In the control limb, femur (fe), tibia (ti), and tarsal (ta) elements display normal elongated morphology (A). Their diaphyses stain positively with alizarin red (A), are surrounded by an intramembranous bone collar (C and E, arrow), and display fully hypertrophic chondrocytes (E, arrowhead) and invading marrow cells (E, double arrow). In RCAS-MGP limb, tibia is completely cartilaginous and does not stain with alizarin red (B); its diaphysis lacks both a bone collar (D, arrow) and fully hypertrophic chondrocytes (F, arrowheads). Bars (A and B) 2.5 mm; (C–F) 80 μm.
To confirm and extend these observations, we carried out in situ hybridization on similar longitudinal sections of day 12 control (Fig. 10, A–C) and RCAS-MGP (Fig. 10, D–F) tibia, using probes encoding cartilage characteristic type II and X collagens and MGP. It should be noted that, because of their different lengths, about one half of the control tibia is shown in Fig. 10A–C, whereas the entire RCAS-MGP tibia is shown in Fig. 10D–F. In control tibia (Fig. 10 A), transcripts encoding type II collagen were abundant in the epiphyseal articular layer and underlying growth plate containing proliferating, prehypertrophic, and hypertrophic chondrocytes; the transcripts were reduced in late posthypertrophic chondrocytes (Fig. 10 A), as reported previously (Leboy et al. 1988; Iyama et al. 1991). Transcripts encoding type X collagen, a product typical of hypertrophic chondrocytes, were abundant in hypertrophic and posthypertrophic chondrocytes (Fig. 10 B). MGP transcripts displayed more complex patterns reminiscent of those seen in mouse embryos (Luo et al. 1997; Komori et al. 1997); they were prominent in the articular layer (Fig. 10 C, arrow), in proliferating/prehypertrophic chondrocytes along the lateral side of tibia (Fig. 10 C, double arrowhead), and in posthypertrophic chondrocytes (Fig. 10 C, double arrow). Notably, MGP transcripts were essentially undetectable in the entire hypertrophic zone, either along its lateral side (Fig. 10 C, arrowhead) or its central region.
Figure 10.
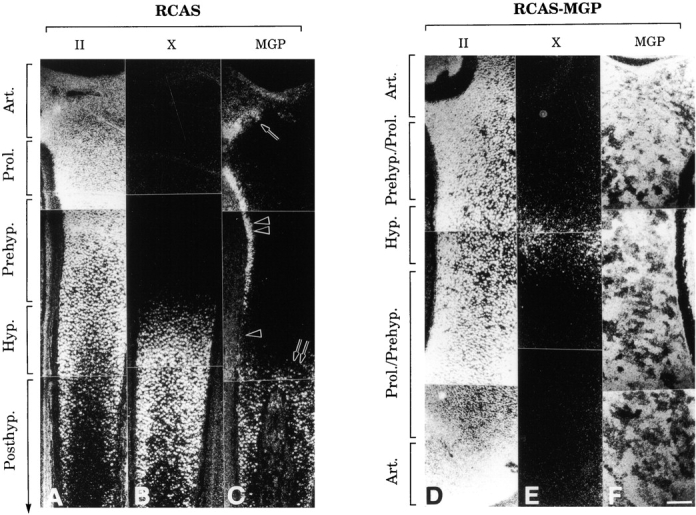
In situ hybridization analysis of gene expression patterns in control (A–C) and MGP-overexpressing (D–F) tibia. Only about half of the control tibia is shown in A-C, whereas the entire RCAS-MGP tibia is shown in D–F. Note in C the particularly strong MGP expression in articular layer (arrow), along the lateral side of the proliferative and prehypertrophic zones (double arrowheads), and in posthypertrophic zone (double arrow); barely detectable signal is seen throughout the hypertrophic zone (arrowhead). II, type II collagen; X, type X collagen. Art., articular layer; Prol., proliferative zone; Prehyp., prehypertrophic zone; and Posthyp., posthypertrophic zone. Bar, 250 μm.
However, in the RCAS-MGP tibia, the type II collagen transcripts were equally abundant from epiphysis to diaphysis (Fig. 10 D) and the type X collagen transcripts were quite scarce (Fig. 10 E). This indicated that the respective downregulation and upregulation of these two genes, normally associated with chondrocyte hypertrophy, had not occurred and that chondrocyte maturation had indeed been inhibited. As expected, MGP transcripts were very prominent and present throughout tibia and neighboring tissues, reflecting the presence of both endogenous and virally derived transcripts (Fig. 10 F).
Discussion
The results of the study provide clear evidence that MGP roles in endochondral ossification are under tight cellular control. In hypertrophic chondrocyte cultures, interference with GLA residue synthesis by warfarin treatment causes rapid and extensive mineralization; in immature chondrocyte cultures, a similar treatment does not cause mineralization. This differential response is not simply because of differences in MGP levels, since there is little difference in the levels of MGP gene expression in the two cell populations. We find also that mineral deposited by hypertrophic cells in the absence or presence of warfarin treatment is qualitatively similar and represents biological apatite. We infer from this observation that MGP mainly controls the degree of mineralization, rather than the quality or type of mineral deposited. Last, we find that virally driven overexpression of MGP in cultured hypertrophic chondrocytes causes a marked decrease in mineralization. This finding lends further strength to the inhibitor studies, and indicates that in mineralization-competent cells, the amount of MGP plays a critical role in the mineralization process. As might be expected, MGP overexpression in the limb bud in vivo causes a marked reduction in mineralization. However, we did not anticipate to also observe that such overexpression delays chondrocyte maturation, causes shortening and broadening of the skeletal elements, and blocks both endochondral and intramembranous ossifications. This data provides new evidence that MGP roles in skeletogenesis may extend beyond the regulation of mineralization.
GLA-containing Proteins and Mineralization
In our inhibitor studies, we examined the effects of warfarin on the mineralization of hypertrophic chondrocytes under two conditions, medium A and medium B. In earlier investigations, we showed that both conditions induce expression of many cellular activities associated with the terminal mineralization phase of maturation, including increased APase activity, decreased type X collagen gene expression, and shifts in energy metabolism (Leboy et al. 1989; Iwamoto et al. 1993b; Shapiro et al. 1994). We now show that in either condition, warfarin treatment does not elicit major changes in MGP expression, APase activity, or cell number. Thus, because the treatment enhances mineralization in both conditions, it is clear that the charged carboxyl groups by themselves serve as critical sites for the regulation of mineral deposition by MGP. A previous study showed that osteocalcin is expressed by hypertrophic sternal chondrocytes in culture (Neugebauer et al. 1995), suggesting that this protein and its carboxyl groups may also have contributed to the regulation of mineralization. Ongoing analyses of the absolute amounts, distribution, and timing of expression of osteocalcin and MGP should clarify the respective contributions of these proteins to mineralization in the hypertrophic chondrocyte cultures used here.
Effort was extended in evaluating the quality of the mineral formed in hypertrophic cell cultures. FT-IR analysis, SEM, x-ray microanalysis, and measurements of calcium/phosphate ratios all show that the mineral deposited in the presence of warfarin treatment is indistinguishable from that deposited in untreated cultures and represents normal carbonate apatite. The only difference observed is that much more mineral is deposited in warfarin-treated cultures, lending support to our conclusion that a critical role of MGP is to control the initiation of mineralization and the amount of mineral deposited. This conclusion correlates well with previous pharmacological, biochemical, and genetic studies (Price 1985; Romberg et al. 1986; Luo et al. 1997), indicating that MGP and other calcium-binding proteins act as strong inhibitors of the initiation phase of mineralization. Given the self-sustaining and self-promoting nature of the mineralization process, the presence of these strong inhibitors may be needed to restrict mineralization to the appropriate sites and times in the embryo and growing organism. Once the first seed crystals form, however, MGP and other calcium-binding proteins may have additional roles, including controlling size, rates of growth, directionality, and maturation of mineralization. A recent study has provided evidence that osteocalcin may have a role in mineral maturation in the growing animal (Boskey et al. 1998). These considerations and our findings reiterate the idea that mineralization is complex and that multiple mechanisms are needed to regulate it during embryogenesis and postnatal life.
How could MGP exert its control on the initiation phase of mineralization? Given its ability to bind calcium, the protein could simply serve as a competitor and limit the amount of calcium ions available to initiate calcification (Romberg et al. 1986). Mineralization is thought to initiate in matrix vesicles (Landis 1986; Genge et al. 1989; Anderson 1990; Kirsch and Wuthier 1994) and/or at focal sites of proteoglycan accumulation (Hunter 1987; Poole 1991). MGP has actually been found to be associated with matrix vesicles isolated from articular and growth plate cartilage (Loeser et al. 1992). In this location, MGP would be in an ideal topographical position to inhibit or compete with calcium influx into the vesicle's lumen and inhibit the vesicle's capability to initiate mineralization. Likewise, MGP could limit the amount of calcium ions available for binding to focally accumulated proteoglycans. It is important to note that there are additional mechanisms limiting mineralization. For example, we have shown previously that there actually are two structurally and functionally distinct types of matrix vesicles produced by chondrocytes (Kirsch et al. 1997). Others have shown that the focal accumulations of proteoglycans are restricted to hypertrophic cartilage and are not common in cartilages not involved in mineralization (Poole 1991). Thus, MGP is part of multiple mechanisms that serve to control mineralization and allow it to occur only at appropriate sites, times, and levels in the organism.
This conclusion correlates well with the phenotype of MGP-null mice (Luo et al. 1997). Lack of MGP was found to lead to excessive or ectopic mineralization of specific tissues and structures such as the growth plate, aorta, and tracheal rings. However, articular cartilage, a tissue rich in MGP (Hale et al. 1988), was not reported to be abnormally mineralized. Clearly, this tissue must possess multiple means by which mineralization is prevented, even in the absence of MGP. Multiple inhibitory means must also characterize the immature chondrocytes used in our study, which do not mineralize their matrix once treated with warfarin. On the other hand, susceptible tissues such as blood vessels must possess a strong potential to mineralize, and will do so simply in the absence of MGP. This would account for the relatively high frequency with which arteries undergo pathological calcification in conditions such as atherosclerosis (Bostrom et al. 1995).
MGP Gene Expression, Chondrocyte Maturation, and Ossification
Our in vivo data show that virally driven misexpression of MGP causes severe alterations of limb skeletogenesis. We find that the MGP-overexpressing tibia is shorter and wider than its normal counterpart, and the maturation process of growth plate chondrocytes is severely inhibited. In addition, the diaphysis fails to be invaded by bone and marrow progenitor cells, to undergo endochondral ossification, and to be surrounded by an intramembranous bone collar. Interestingly, it was found in MGP-null mice that the growth plates of limb skeletal elements are not only more mineralized than those present in control animals, but are also disorganized and lack characteristic chondrocyte columns (Luo et al. 1997). It is clear that absence or overexpression of MGP are both incompatible with the normal processes of chondrocyte maturation, cartilage mineralization, and ossifications.
It was shown previously in mouse embryo limb skeletal elements that the normal gene expression pattern of MGP is very complex (Komori et al. 1997; Luo et al. 1997); MGP expression is low in the proliferative zone of growth plate, strong in the prehypertrophic zone, low again in the hypertrophic zone, and strong again in the posthypertrophic mineralizing zone (Komori et al. 1997; Luo et al. 1997). We show here that the MGP gene expression pattern in chick growth plates is equally complex. The significance and biological consequences of these highly distinctive patterns of expression have remained largely unclear. In view of the results shown here and those in the MGP-null mouse study, it is conceivable that the MGP expression patterns may not only be related to mineralization, but may also have roles in chondrocyte and cartilage development. In particular, the downregulation of MGP gene expression in zones of the growth plate, and in the hypertrophic zone in particular, may actually be required for chondrocyte maturation and the emergence of hypertrophic chondrocytes. By maintaining constant MGP expression and preventing modulations of expression, the virally driven MGP misexpression may have blocked the cells at more immature stages and inhibited their further development into hypertrophic cells. The absence of fully hypertrophic chondrocytes would explain the lack of both endochondral ossification and invasion by bone and marrow progenitor cells, since only hypertrophic mineralizing cartilage can serve as a substrate for these processes. The absence of fully hypertrophic chondrocytes would also account for the reduction in skeletal element length since hypertrophy is the main determinant of longitudinal growth (Hunziker 1994). The lack of a diaphyseal intramembranous bone collar is harder to explain. It may be a direct consequence of widespread virally driven MGP expression acting on perichondrial tissues and inhibiting their differentiation into osteoblasts. Alternatively, it may be an indirect consequence of the failure of cartilage to become fully hypertrophic; it was suggested long ago that cartilage and perichondrial tissues are characterized by mutual interdependence and interactions, and that alterations in one tissue may have developmental repercussions in the other tissue (Fell 1925; Thorogood 1983). Whatever the explanation, the absence of an intramembranous bone collar would account for the increased width of the MGP-overexpressing skeletal element, given that the collar has a primary role in regulating the diameter of the shaft in developing long bones.
We do not know yet how MGP exerts influences on chondrocyte and cartilage maturation beyond its roles in mineralization. MGP may act via mechanisms independent of its ability to bind calcium and apatite, i.e., mechanisms unrelated to those involved in mineralization. Alternatively, MGP may again use its calcium binding ability to influence chondrocyte maturation. A previous study provides indirect support for the latter possibility. It was found that increases in extracellular calcium concentrations selectively induce expression of type X collagen in chondrocyte cultures (Bonen and Schmid 1991). Because type X collagen is a marker of maturing and hypertrophic chondrocytes, the finding indicates that extracellular calcium can favor the hypertrophic program. A similar increase in available calcium could occur in zones of the growth plate with low MGP expression, such as the hypertrophic zone; in other words, low MGP gene expression may signify increased free calcium available for cellular activities and for promotion and completion of the chondrocyte maturation program.
Acknowledgments
We thank Mr. G. Harrison (University of Pennsylvania Dental School) for help with the x-ray microanalysis, and Mrs. E. Golden (University of Pennsylvania Dental School) for help with cell cultures.
This work was supported by the National Institutes of Health grant AR40833 (to M. Pacifici) and a research grant from Eisai Pharmaceutical Co., Ltd. (to M. Iwamoto).
Footnotes
J.-Y. Suh's current address is Department of Periodontology, Kyunpook National University Dental School, 2-101 Dongin-dong, Joong-Gu, Taegu, Korea 700-422.
Abbreviations used in this paper: APase, alkaline phosphatase; FT-IR, Fourier transform infrared spectroscopy; GLA, γ‴carboxyglutamic acid; MGP, matrix GLA protein; pNP, p-nitrophenyl phosphate; RT-PCR, reverse transcriptase PCR; SEM, scanning electron microscopy.
References
- Anderson H.C. Biology of disease-mechanism of mineral formation in bone. In: Rubin E., Damjanov J., editors. Pathology Reviews. The Humana Press Inc; Clifton, NJ: 1990. pp. 13–23. [Google Scholar]
- Barone L.M., Owen T.A., Tassinari M.S., Bortell R., Stein G.S., Lian J.B. Developmental expression and hormonal regulation of the rat matrix GLA protein (MGP) gene in chondrogenesis and osteogenesis. J. Cell. Biochem. 1991;46:351–365. doi: 10.1002/jcb.240460410. [DOI] [PubMed] [Google Scholar]
- Bonen D.K., Schmid T.M. Elevated extracellular calcium concentrations induce type X collagen synthesis in chondrocyte cultures. J. Cell Biol. 1991;115:1171–1178. doi: 10.1083/jcb.115.4.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskey A.L., Stiner D., Doty S.B., Binderman I., Leboy P. Studies of mineralization in tissue cultureoptimal conditions for cartilage calcification. Bone Min. 1992;16:11–36. doi: 10.1016/0169-6009(92)90819-y. [DOI] [PubMed] [Google Scholar]
- Boskey A.L., Gadaleta S., Gundberg C., Doty S.B., Ducy P., Karsenty G. Fourier transform infrared microspectroscopic analysis of bones of osteocalcin-deficient mice provides insight into the function of osteocalcin. Bone. 1998;23:187–196. doi: 10.1016/s8756-3282(98)00092-1. [DOI] [PubMed] [Google Scholar]
- Bostrom K., Watson K.E., Stanford W.P., Demer L.L. Atherosclerotic calcificationrelation to developmental osteogenesis. Am. J. Cardiol. 1995;75:88B–91B. doi: 10.1016/0002-9149(95)80020-s. [DOI] [PubMed] [Google Scholar]
- Boyde A., Shapiro I.M. Energy dispersive X-ray elemental analysis of isolated epiphyseal growth plate cartilage fragments. Histochemistry. 1980;69:85–94. doi: 10.1007/BF00508369. [DOI] [PubMed] [Google Scholar]
- Bronckers A.L., Gay S., Dimuzio M.T., Butler W.T. Immunolocalization of gamma-carboxyglutamic acid containing proteins in developing rat bones. Collagen Rel. Res. 1985;5:273–281. doi: 10.1016/s0174-173x(85)80017-0. [DOI] [PubMed] [Google Scholar]
- Dohi Y., Ohgushi H., Tabata S., Yoshikawa T., Dohi K., Moriyama T. Osteogenesis associated with bone Gla protein gene expression in diffusion chambers by bone marrow cells with demineralized bone matrix. J. Bone Min. Res. 1992;7:1173–1180. doi: 10.1002/jbmr.5650071009. [DOI] [PubMed] [Google Scholar]
- Ducy P., Desbois C., Boyce B., Pinero G., Story B., Dunstan C., Smith E., Bonadio J., Goldstein S., Gundberg C., Bradley A., Karsenty G. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382:448–452. doi: 10.1038/382448a0. [DOI] [PubMed] [Google Scholar]
- Enomoto-Iwamoto M., Iwamoto M., Mukadai Y., Kawakami Y., Nohno T., Higuchi Y., Takemoto S., Ohuchi H., Noji S., Kurisu K. Bone morphogenetic protein signaling is required for maintenance of differentiated phenotype, control of proliferation, and hypertrophy in chondrocytes. J. Cell Biol. 1998;140:409–418. doi: 10.1083/jcb.140.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell H.B. The histogenesis of cartilage and bone in the long bones of the embryonic fowl. J. Morphol. Physiol. 1925;40:417–459. [Google Scholar]
- Feteih R., Tassinari M.S., Lian J.B. Effect of sodium warfarin on vitamin K-dependent proteins and skeletal development in the rat fetus. J. Bone Min. Res. 1990;5:885–894. doi: 10.1002/jbmr.5650050813. [DOI] [PubMed] [Google Scholar]
- Furie B., Furie B.C. The molecular basis of blood coagulation. Cell. 1988;53:505–518. doi: 10.1016/0092-8674(88)90567-3. [DOI] [PubMed] [Google Scholar]
- Gadaleta S.J., Camacho N.P., Mendelsohn R., Boskey A.L. Fourier transform infrared microscopy of calcified turkey leg tendon. Calcif. Tissue Int. 1996;58:17–23. doi: 10.1007/BF02509541. [DOI] [PubMed] [Google Scholar]
- Genge B.R., Wu L.N.Y., Wuthier R.E. Identification of phospholipid-dependent calcium-binding proteins as constituents of matrix vesicles. J. Biol. Chem. 1989;264:10917–10921. [PubMed] [Google Scholar]
- Haines R.W. The histology of epiphyseal union in mammals. J. Anat. 1975;120:1–25. [PMC free article] [PubMed] [Google Scholar]
- Hale J.E., Fraser J.D., Price P.A. The identification of matrix Gla protein in cartilage. J. Biol. Chem. 1988;263:5820–5824. [PubMed] [Google Scholar]
- Hall B.K. Histogenesis and morphogenesis of bone. Clin. Orthopaed. 1971;74:249–268. [PubMed] [Google Scholar]
- Hall J.G., Pauli R.M., Wilson K.M. Maternal and fetal sequelae of anticoagulation during pregnancy. Am. J. Med. 1980;68:122–140. doi: 10.1016/0002-9343(80)90181-3. [DOI] [PubMed] [Google Scholar]
- Harrison G., Shapiro I.M., Golub E.E. The phosphatidylinositol-glycolipid anchor on alkaline phosphatase facilitates mineralization initiation in vitro. J. Bone Min. Res. 1995;10:568–573. doi: 10.1002/jbmr.5650100409. [DOI] [PubMed] [Google Scholar]
- Hauschka P., Lian J.B., Cole D.C., Gundberg C.M. Osteocalcin and matrix Gla proteinvitamin K-dependent proteins in bone. Physiol. Rev. 1989;69:990–1047. doi: 10.1152/physrev.1989.69.3.990. [DOI] [PubMed] [Google Scholar]
- Hughes S.H., Greenhouse J.J., Petropoulos C.J., Sutrave P. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J. Virol. 1987;61:3004–3012. doi: 10.1128/jvi.61.10.3004-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter G.K. An ion-exchange mechanism of cartilage cacification. Connect. Tissue Res. 1987;16:111–120. doi: 10.3109/03008208709001999. [DOI] [PubMed] [Google Scholar]
- Hunziker E.B. Mechanisms of longitudinal bone growth and its regulation by growth plate chondrocytes. Microsc. Res. Tech. 1994;28:505–519. doi: 10.1002/jemt.1070280606. [DOI] [PubMed] [Google Scholar]
- Iwamoto M., Golden E.B., Adams S.L., Noji S., Pacifici M. Responsiveness to retinoic acid changes during chondrocyte maturation Exp. Cell Res. 205 1993. 213 224a [DOI] [PubMed] [Google Scholar]
- Iwamoto M., Shapiro I.M., Yagami K., Boskey A.L., Leboy P.S., Adams S.L., Pacifici M. Retinoic acid induces rapid mineralization and expression of mineralization-related genes in chondrocytes Exp. Cell Res 207 1993. 413 420b [DOI] [PubMed] [Google Scholar]
- Iyama K.-I., Ninomiya Y., Olsen B.R., Linsenmayer T.F., Trelstad R.L., Hayashi M. Spatiotemporal pattern of type X collagen gene expression and collagen deposition in embryonic chick vertebrae undergoing endochondral ossification. Anat. Rec. 1991;229:462–472. doi: 10.1002/ar.1092290405. [DOI] [PubMed] [Google Scholar]
- Johnson-Wint B., Hollis S. A rapid in situ deoxyribonucleic acid assay for determining cell number in culture and tissue. Anal. Biochem. 1982;122:338–344. doi: 10.1016/0003-2697(82)90292-5. [DOI] [PubMed] [Google Scholar]
- Kirsch T., Wuthier R.E. Stimulation of calcification of growth plate cartilage matrix vesicles by binding to type I and X collagens. J. Biol. Chem. 1994;269:11462–11469. [PubMed] [Google Scholar]
- Kirsch T., Nah H.-D., Shapiro I.M., Pacifici M. Regulated production of mineralization-competent matrix vesicles in hypertrophic chondrocytes. J. Cell Biol. 1997;137:1149–1160. doi: 10.1083/jcb.137.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori T., Yagi H., Nomura S., Yamaguchi A., Sasaki K., Deguchi K., Shimizu Y., Bronson R.T., Gao Y.-H., Inada M. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- Kost T.A., Theodorakis N., Hughes S.H. The nucleotide sequence of the chick cytoplasmic beta-actin gene. Nucleic Acids Res. 1993;11:8287–8301. doi: 10.1093/nar/11.23.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama E., Leatherman J.L., Noji S., Pacifici M. Early chick limb cartilaginous elements possess polarizing activity and express hedgehog-related morphogenetic factors. Dev. Dyn. 1996;207:344–354. doi: 10.1002/(SICI)1097-0177(199611)207:3<344::AID-AJA11>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Landis W.J. A study of calcification of the leg tendons from the domestic turkey. J. Ultrastruct. Mol. Struct. Res. 1986;94:217–238. doi: 10.1016/0889-1605(86)90069-8. [DOI] [PubMed] [Google Scholar]
- Leboy P.S., Shapiro I.M., Uschmann B.D., Oshima O., Lin D. Gene expression in mineralizing chick epiphyseal cartilage. J. Biol. Chem. 1988;263:8515–8520. [PubMed] [Google Scholar]
- Leboy P.S., Vaias L., Uschmann B., Golub E., Adams S.L., Pacifici M. Ascorbic acid induces alkaline phosphatase, type X collagen, and calcium deposition in cultured chick chondrocytes. J. Biol. Chem. 1989;264:17281–17286. [PubMed] [Google Scholar]
- Loeser R., Carlson C.S., Tulli H., Jerome W.G., Miller L., Wallin R. Articular-cartilage matrix γ-carboxyglutamic acid-containing protein. Biochem. J. 1992;282:1–6. doi: 10.1042/bj2820001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G., D'Souza R., Hogue D., Karsenty G. The matrix Gla protein gene is a marker of the chondrogenic cell lineage during mouse development. J. Bone Min. Res. 1995;10:325–334. doi: 10.1002/jbmr.5650100221. [DOI] [PubMed] [Google Scholar]
- Luo G., Ducy P., McKee M.D., Pinero G.J., Loyer E., Behringer R.R., Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- Manolagas S.C., Jilka R.L. Mechanisms of diseasebone marrow, cytokines, and bone remodeling. Emerging insights into the pathogenesis of osteoporosis. N. Engl. J. Med. 1995;332:305–311. doi: 10.1056/NEJM199502023320506. [DOI] [PubMed] [Google Scholar]
- Mark M.P., Butler W.T., Prince C.W., Finkelman R.D., Ruch J.-V. Developmental expression of 44-kDa bone phosphoprotein (osteopontin) and bone γ-carboxyglutamic acid (Gla)-containing protein (osteocalcin) in calcifying tissues of rat. Differentiation. 1988;37:123–136. doi: 10.1111/j.1432-0436.1988.tb00804.x. [DOI] [PubMed] [Google Scholar]
- McGee-Russel S.M. Histochemical method for calcium. J. Histochem. Cytochem. 1958;6:22–42. doi: 10.1177/6.1.22. [DOI] [PubMed] [Google Scholar]
- Munroe P.B., Plgunturk R.O., Fryns J.-P., Maldergem L.V., Ziereisen F., Yuksel B., Gardiner R.M., Chung E. Mutations in the gene encoding the human matrix Gla protein cause Keutel syndrome. Nat. Genet. 1999;21:142–144. doi: 10.1038/5102. [DOI] [PubMed] [Google Scholar]
- Neugebauer B.M., Moore M.A., Broess M., Gerstenfeld L.C., Hauschka P.V. Characterization of structural sequences in the chicken osteocalcin gene; expression of osteocalcin by maturing osteoblasts and by hypertrophic chondrocytes in vitro. J. Bone Min. Res. 1995;10:157–163. doi: 10.1002/jbmr.5650100122. [DOI] [PubMed] [Google Scholar]
- Pacifici M., Golden E.B., Adams S.L., Shapiro I.M. Cell hypertrophy and type X collagen synthesis in cultured articular chondrocytes Exp. Cell Res 192 1991. 266 270a [DOI] [PubMed] [Google Scholar]
- Pacifici M., Golden E.B., Iwamoto M., Adams S.L. Retinoic acid treatment induces type X collagen gene expression in cultured chick chondrocytes Exp. Cell Res. 195 1991. 38 46b [DOI] [PubMed] [Google Scholar]
- Poole A.R. The growth platecellular physiology, cartilage assembly and mineralization. In: Hall B., Newman S., editors. CartilageMolecular Aspects. CRC Press; Boca Raton, FL: 1991. pp. 179–211. [Google Scholar]
- Poser J.W., Price P.A. A method for decarboxylation of γ-carboxy-glutamic acid in proteins. Properties of the decarboxylated γ-carboxyglutamic acid protein from calf bone. J. Biol. Chem. 1979;254:431–436. [PubMed] [Google Scholar]
- Price P.A. Vitamin K-dependent formation of bone Gla protein (osteocalcin) and its function. Vitam. Horm. 1985;42:65–108. doi: 10.1016/s0083-6729(08)60061-8. [DOI] [PubMed] [Google Scholar]
- Price P.A. Gla-containing proteins of bone. Conn. Tissue Res. 1989;21:51–60. doi: 10.3109/03008208909049995. [DOI] [PubMed] [Google Scholar]
- Price P.A., Williamson M.K., Haba T., Dell R.B., Lee W.S. Excessive mineralization with growth plate closure in rats on chronic warfarin treatment. Proc. Natl. Acad. Sci. USA. 1982;79:7736–7738. doi: 10.1073/pnas.79.24.7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey C., Shimizu M., Collins B., Glincher M.J. Resolution–enhanced Fourier transform infrared spectroscopy study of the environment of phosphate ion in the early deposits of a solid phase caclium phosphate in bone and enamel and their evolution with age. Investigations in the v3 PO4 domain. Calcif. Tissue Int. 1991;49:383–388. doi: 10.1007/BF02555847. [DOI] [PubMed] [Google Scholar]
- Rey C., Kim H.-M., Gerstenfeld L., Glimcher M.J. Structural and chemical characteristics and maturation of the calcium-phosphate crystals formed during the calcification of the organic matrix synthesized by chicken osteoblasts in cell culture. J. Bone Min. Res. 1995;10:1577–1588. doi: 10.1002/jbmr.5650101020. [DOI] [PubMed] [Google Scholar]
- Romberg R.W., Werness P.G., Riggs B.L., Mann K.G. Inhibition of hydroxyapatite crystal growth by bone-specific and other calcium-binding proteins. Biochemistry. 1986;25:1176–1180. doi: 10.1021/bi00353a035. [DOI] [PubMed] [Google Scholar]
- Roufosse A.H., Aue W.P., Roberts J.E., Glimcher M.J., Griffin R.G. Investigation of the mineral phases of bone by solid-state phosphorus-31 magic angle sample spinning nuclear magnetic resonance. Biochemistry. 1984;23:6115–6120. doi: 10.1021/bi00320a033. [DOI] [PubMed] [Google Scholar]
- Shapiro I.M., Debolt K., Hatori M., Iwamoto M., Pacifici M. Retinoic acid induces a shift in the energetic state of hypertrophic chondrocytes. J. Bone Min. Res. 1994;9:1229–1237. doi: 10.1002/jbmr.5650090813. [DOI] [PubMed] [Google Scholar]
- Strauss P.G., Closs E.I., Schmidt J., Erfle V. Gene expression during osteogenic differentiation in mandibular condyles in vitro. J. Cell Biol. 1990;110:1369–1378. doi: 10.1083/jcb.110.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttie J.W. Vitamin K-dependent carboxylase. Annu. Rev. Biochem. 1985;54:459–477. doi: 10.1146/annurev.bi.54.070185.002331. [DOI] [PubMed] [Google Scholar]
- Thorogood P. Morphogenesis of cartilage. In: Hall B.K., editor. Cartilage. Vol. 2 Academic Press; New York: 1983. pp. 223–254. [Google Scholar]
- Wiedemann M., Trueb B., Belluoccio D. Molecular cloning of avian matrix Gla protein. Biochim. Biophys. Acta. 1998;1395:47–49. doi: 10.1016/s0167-4781(97)00155-3. [DOI] [PubMed] [Google Scholar]