Abstract
Using biochemical assays to determine the activation state of Rho-like GTPases, we show that the guanine nucleotide exchange factor Tiam1 functions as a specific activator of Rac but not Cdc42 or Rho in NIH3T3 fibroblasts. Activation of Rac by Tiam1 induces an epithelial-like morphology with functional cadherin-based adhesions and inhibits migration of fibroblasts. This epithelial phenotype is characterized by Rac-mediated effects on Rho activity. Transient PDGF-induced as well as sustained Rac activation by Tiam1 or V12Rac downregulate Rho activity. We found that Cdc42 also downregulates Rho activity. Neither V14Rho or N19Rho affects Rac activity, suggesting unidirectional signaling from Rac towards Rho. Downregulation of Rho activity occurs independently of Rac- induced cytoskeletal changes and cell spreading. Moreover, Rac effector mutants that are defective in mediating cytoskeleton changes or Jun kinase activation both downregulate Rho activity, suggesting that neither of these Rac signaling pathways are involved in the regulation of Rho. Restoration of Rho activity in Tiam1-expressing cells by expression of V14Rho results in reversion of the epithelioid phenotype towards a migratory, fibroblastoid morphology. We conclude that Rac signaling is able to antagonize Rho activity directly at the GTPase level, and that the reciprocal balance between Rac and Rho activity determines cellular morphology and migratory behavior in NIH3T3 fibroblasts.
Keywords: cell–cell adhesion, migration, Rho-like GTPases, Tiam1
Cell migration is an essential process during development and wound healing. Aberrations in signaling pathways involved in the regulation of cell migration, cell–cell, and cell–matrix interactions contribute to tumor invasion and metastasis. Family members of the Rho-like GTPases, including Cdc42, Rac1, and RhoA, have been recognized as key regulators of signal transduction pathways that mediate the distinct actin cytoskeleton changes required for cell migration (for review see Hall 1998). Rho-like GTPases function as molecular switches by cycling between an active, GTP-bound state and an inactive GDP-bound state. Cycling of Rho-like GTPases is tightly regulated by associated proteins. Guanine nucleotide exchange factors (GEFs) stimulate the exchange of bound GDP for GTP, leading to activation of the GTPases and binding of downstream effectors. Inactivation is promoted by GTPase-activating proteins (GAPs) that stimulate the intrinsic GTP hydrolysis rate of Rho-like proteins (for review see Van Aelst and D'Souza-Schorey 1997). GEFs are characterized by the catalytic Dbl homology (DH) domain, which is COOH terminally flanked by a Pleckstrin homology (PH) domain. PH domains have been shown to bind to specific phosphoinositol lipids as well as to proteins (for review see Shaw 1996). Some GEFs like Tiam1, an activator of Rac1 (Habets et al. 1994; Michiels et al. 1995), its homologues, STEF (Hoshino et al. 1999) and SIF (Sone et al. 1997), RasGRF (Shou et al. 1992), and Fgd1 (Pasteris et al. 1994), carry a second PH domain. For RasGRF and Tiam1, it has been shown that this second, NH2-terminal PH domain mediates localization at the cell membrane (Buchsbaum et al. 1996; Michiels et al. 1997).
By microinjection studies, the effects on the cytoskeleton by the distinct GTPases have been well documented in quiescent Swiss 3T3 fibroblasts. Rho activation leads to assembly of stress fibers and focal contacts, which are specific structures mediating adhesion to the extracellular matrix (Ridley and Hall 1992). Activation of Cdc42 and Rac results in the formation of filopodia and lamellipodia, respectively. Moreover, both Cdc42 and Rac induce focal complexes, adhesion structures that are distinct from focal contacts (Ridley et al. 1992; Kozma et al. 1995; Nobes and Hall 1995). In Swiss 3T3 fibroblasts, the Rho-like GTPases were found to be sequentially activated in a linear cascade, where activation of Cdc42 leads to activation of Rac and subsequently activation of Rho (Ridley et al. 1992; Nobes and Hall 1995). However, the effects of Rho-like proteins on the cytoskeleton and their sequence of activation seem to be dependent on the cell type studied. In epithelial cells, activation of Rac promotes E-cadherin–mediated cell–cell adhesion and, therefore, inhibits migration of epithelial cells (Hordijk et al. 1997). Activities of both Rac and Rho GTPases are required for the formation of adherent junctions (Braga et al. 1997; Takaishi et al. 1997; Zhong et al. 1997). Depending on the cell substrate, Rac may also promote migration of epithelial cells, as shown for T47D and MDCK-f3 cells on collagen (Keely et al. 1997; Sander et al. 1998). In neuronal N1E-115 cells, activation of Rac-induced cell spreading and extension of neuritelike structures, a process that was promoted by a laminin substrate (Van Leeuwen et al. 1997). In contrast, activation of Rho induced cell rounding and neurite retraction (Jalink et al. 1994). Cells expressing activated V12Rac were refractory to Rho-mediated cell rounding, suggesting that activities of Rac and Rho GTPases oppose each other in neuronal cells (Kozma et al. 1997; Van Leeuwen et al. 1997).
Thus far, the activation of specific Rho-like GTPases has been deduced from the appearance of specific cytoskeletal changes in cells. With respect to the transient activation state of Rho-like GTPases, it would be favorable to measure their activation during the actual signal transmission, instead of being limited to effectors that allow a phenotypic readout of the signal. Recently, we have used a biochemical assay to monitor the direct activation state of Rac, taking advantage of the specific binding of the Rac and Cdc42 downstream effector Pak to GTP, but not GDP, loaded Rac and Cdc42 (Sander et al. 1998). Here, we have used a similar assay to monitor the activation of Rho, using the Rho downstream effector, Rhotekin (Reid et al. 1996). Simultaneous analysis of Rac and Rho activities allows us to address the relationship between the activation state of both GTPases at the molecular level, and to get insight into the regulation of Rac- and Rho-dependent phenotypes. We have studied the consequences of Rac activation in NIH3T3 fibroblasts with respect to cell migration and the activation of Rho. Stable expression of Tiam1 in NIH3T3 cells results in inhibition of fibroblast migration, and induces an epithelial-like morphology characterized by increased cadherin-based cell–cell adhesion. We demonstrate that Tiam1 specifically activates Rac but not Cdc42 or Rho in NIH3T3 fibroblasts. Unexpectedly, signaling by constitutively active mutants of Cdc42 and Rac as well as PDGF-mediated Rac activation leads to downregulation of endogenous Rho activity. Rac-mediated inactivation of Rho occurs downstream of Rac. In contrast, receptor-mediated or sustained activation of Rho did not affect Rac activity. Our results indicate a unidirectional signaling from Rac to Rho, leading to downregulation of Rho activity in NIH3T3 fibroblasts, thereby causing an epithelioid, nonmigratory phenotype. Restoration of Rho activity by expression of constitutively active V14Rho in C1199Tiam1-expressing cells resulted in a fibroblastoid phenotype associated with migratory behavior, indicating that the reciprocal balance of Rac and Rho activities determines the cellular phenotype.
Materials and Methods
Cells and Culture Conditions
NIH3T3 fibroblast cells were cultured in DME supplemented with 10% newborn calf serum (Gibco-BRL Life Technologies). For serum starvation, cells were cultured for 24–36 h in DME supplemented with 0.2% newborn calf serum. Stable cell lines expressing the hemagglutinin (HA) epitope–tagged proteins C1199Tiam1 and C580Tiam1 (encoding the 1,199– and 580–COOH-terminal amino acids of Tiam1) or the Myc epitope–tagged GTPases were generated by retroviral transduction (Sander et al. 1998) and selected with neomycin or zeocin.
Constructs and Retroviral Transductions
HA-tagged C1199Tiam1, C580Tiam1, and Myc-tagged V12Rac have been described (Hordijk et al. 1997; Sander et al. 1998) and were cloned into an LZRS-IRES-Neo retroviral vector, a modified LZRS vector (Kinsella and Nolan 1996), conferring neomycin resistance. Myc-tagged RhoV14 and N19Rho were cloned as an EcoRI fragment in LZRS-IRES-Zeo that confers zeocin resistance. Myc-tagged L61Rac, L61RacA37, and L61RacC40 were cloned from pRK5 (Lamarche et al. 1996) as a ClaI-NotI fragment into the SfuI and NotI sites from LZRS-IRES-Neo. The Myc-tagged fast cycling F28LCdc42 mutant (Lin et al. 1997) was generated by a standard PCR procedure and cloned into the EcoRI site of LZRS-IRES-Neo. Activated Pak1E423 was provided by G. Bokoch (The Scripps Research Institute, La Jolla, CA) and J. Chernoff (Fox Chase Cancer Center, Philadelphia, PA) and cloned as a SalI-NotI fragment into LZRS-IRES-Neo. To produce retroviruses, Phoenix packaging cells (Kinsella and Nolan 1996) were transfected with retroviral constructs encoding Tiam1 or GTPase proteins as described (Michiels et al. 1995; Sander et al. 1998; Stam et al. 1998). Transductants were selected in DME containing neomycin (0.8 mg/ml), zeocin (0.2 mg/ml), or a combination of both drugs in case of doubly transduced cell lines. Expression of proteins in the pool of transduced cells was analyzed by Western blot, as described by Stam et al. 1997.
Association and Dissociation Assays
Dissociation assays were performed as described (Hordijk et al. 1997). The cells were scraped in culture medium and suspended by repeated pipetting. To demonstrate calcium sensitivity of cell–cell adhesion, calcium in the medium was chelated by the addition of 4 mM EGTA for 3 h before the dissociation assay. The number of particles (cell clusters) was counted and divided by the number of total cells (Np/Nc). For association assays, cells were scraped off the dish and suspended in medium by repeated pipetting to single cells. Dissociated cells were allowed to associate for 1 h in culture medium while rotating (37°C, 5% CO2). The number of particles was counted and divided by the number of total cells (Np/Nc).
Immunofluorescence
Cells grown on glass coverslips were proceeded as described Stam et al. 1998. C1199Tiam1 and C580Tiam1 were stained with anti–DH antibody (Habets et al. 1994) or mAbs against the HA tag (12CA5; Boehringer Mannheim), N- and P-cadherins with the monoclonal Pan-cadherin antibody (Sigma Chemical Co.), vinculin with the monoclonal hVinc-1 (Sigma Chemical Co.), and α-, β-, and γ-catenin and p120CAS were stained with respective mAbs from Transduction Laboratories.
Migration Assays
For the scratch assay, cells were nearly grown to confluency before 12 h serum starvation. The cell monolayer was wounded with a plastic pipet tip, generating a wound spanning ∼15–20 cells. Migration of cells was judged by monitoring the closure of the wound over a period of 12 h in medium containing 0.2% newborn calf serum.
Cell migration assays were performed using transwell migration chambers (diam, 6.5 mm; pore size, 8 μm; Costar Corp.) coated on both sides of the membrane with fibronectin (10 μg/ml; Sigma Chemical Co.) in PBS for 12 h at 4°C. The coated filters were rinsed once with PBS and placed into the lower chamber containing medium supplemented with 20 ng/ml PDGF (Calbiochem-Novabiochem). Trypsinization of cells was kept to a minimum time to preserve the presence of cell surface adhesion molecules as much as possible. 70,000 cells were added to the upper compartment of the transwell chamber and allowed to migrate to the underside of the top chamber for 2–3 h. Nonmigrated cells on the upper membrane were removed with a cotton swab, and migrated cells attached to the bottom surface of the membrane were fixed for 10 min in methanol, stained with Giemsa, and counted.
Production of GST-PAK-CRIB Domain (PAK-CD) and GST-C21 Fusion Proteins
Cloning of the GST-PAK-CD fusion protein (encompassing amino acids 56–141 from PAK1B), containing the Rac and Cdc42 binding region from human PAK1B, has been described (Sander et al. 1998). GST-C21 has been described by Reid et al. 1996, Reid et al. 1999 and contains the NH2-terminal 90 amino acids, representing the Rho binding domain, from the Rho effector protein Rhotekin. Escherichia coli BL21 cells transformed with the GST-PAK-CD construct were grown at 37°C, cells transformed with the GST-C21 construct were grown at 30°C to OD600 0.3. Expression and purification of recombinant proteins has been described (Sander et al. 1998).
GTPase Activity Assays
GTPase activity assays were performed as described (Sander et al. 1998). In brief, lysates of NIH3T3 cells were prepared and incubated with bacterially produced GST-PAK-CD or GST-C21 fusion proteins, bound to glutathione-coupled Sepharose beads. The beads and proteins bound to the fusion protein were washed in an excess of lysis buffer, eluted in Laemmli sample buffer, and analyzed for bound Cdc42, Rac1, or RhoA molecules by Western blot using antibodies against Cdc42 (rabbit polyclonal antibody from Santa Cruz Biotechnology), Rac1 (mAb from Transduction Laboratories or when indicated Upstate Biotechnology Inc.) or RhoA (mAb from Santa Cruz Biotechnology).
Results
Tiam1/Rac Signaling Induces an Epithelial-like Morphology of NIH3T3 Cells by Promoting N- and P-Cadherin–mediated Adhesion
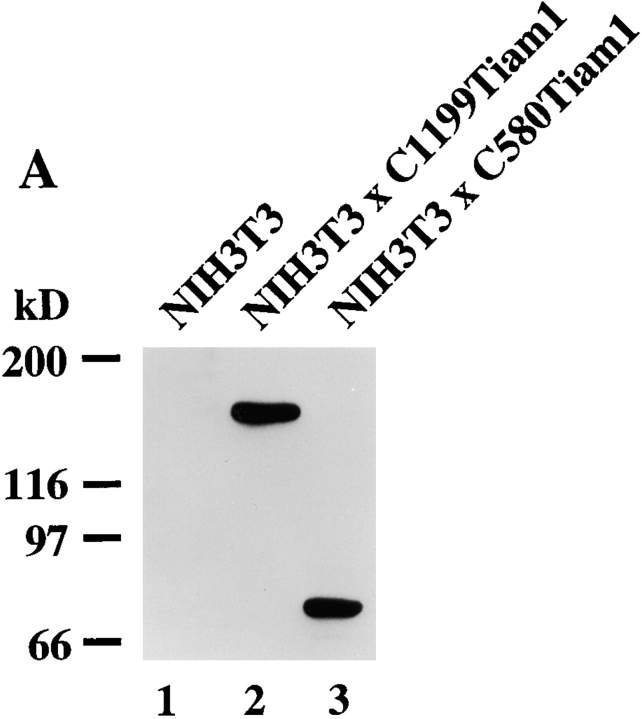
To study the molecular basis of the morphological transformation of NIH3T3 fibroblasts upon expression of Tiam1 (van Leeuwen et al. 1995), we generated NIH3T3 cell lines stably expressing C1199- and C580Tiam1 cDNAs by retroviral transduction (Fig. 1 A). Stable pools of cells expressing C1199Tiam1 grew in small groups of flat, spread cells and displayed an epithelial-like morphology. The C1199Tiam1 protein was predominantly localized at the plasma membrane and induced extensive membrane ruffling. Moreover, C1199Tiam1 was also enriched at the sites of cell–cell contact, which was accompanied by increased actin polymerization (Fig. 1 B). We previously showed that Tiam1-induced Rac activation promotes E-cadherin–mediated cell–cell adhesion in epithelial cells (Hordijk et al. 1997; Sander et al. 1998). Therefore, we studied whether the Tiam1-induced epithelial-like morphology in NIH3T3 fibroblasts was due to the establishment of cadherin-based cell–cell adhesions. NIH3T3 fibroblasts do not express E-cadherin, but instead express the family members N- and P-cadherin (Reynolds et al. 1996). In the C1199Tiam1-expressing cells, N-cadherin and P-cadherin (stained with a Pan-cadherin antibody; Fig. 1 B) as well as the cadherin-associated catenins, such as α-, β-, and γ-catenin (not shown) and p120CAS (Fig. 1 B) localized to sites of cell–cell contact. The adhesion-related proteins were to some extent also detected in Tiam1-induced membrane ruffles (Fig. 1 B).
Figure 1.
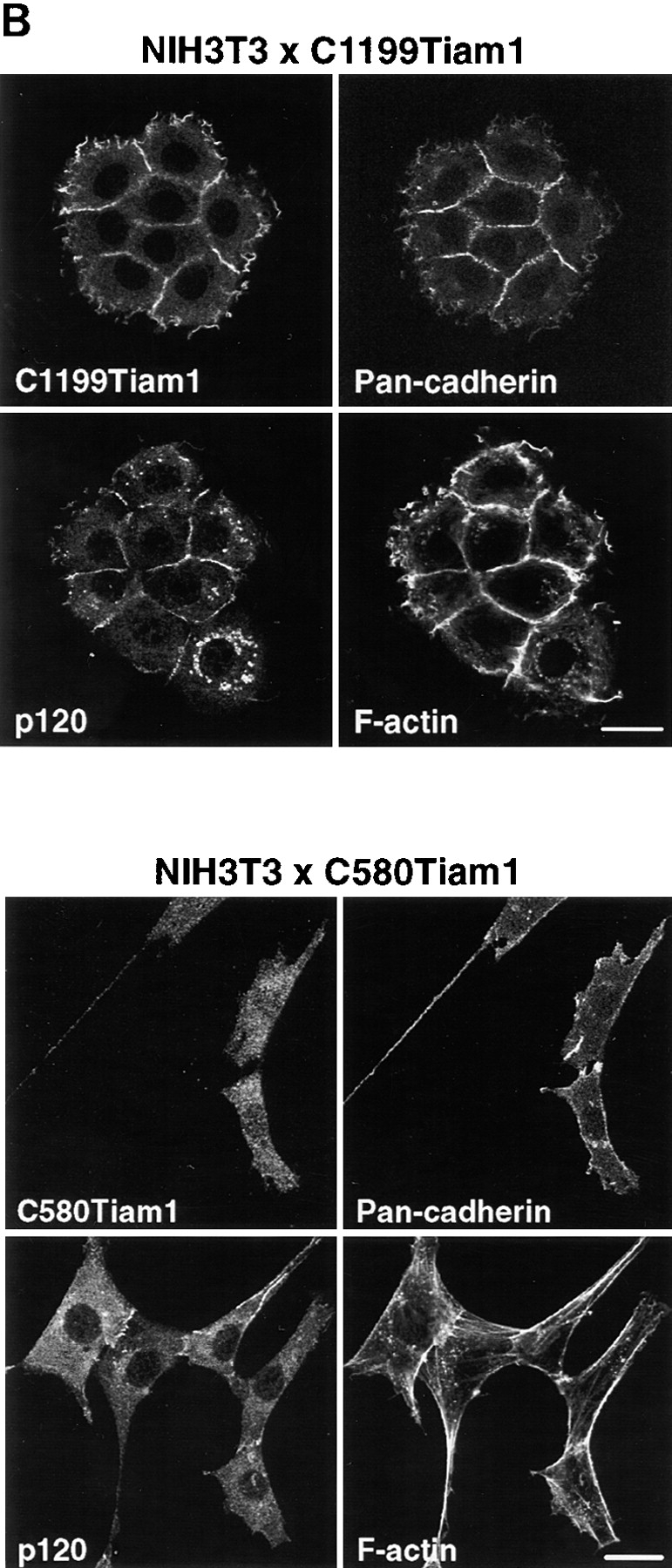
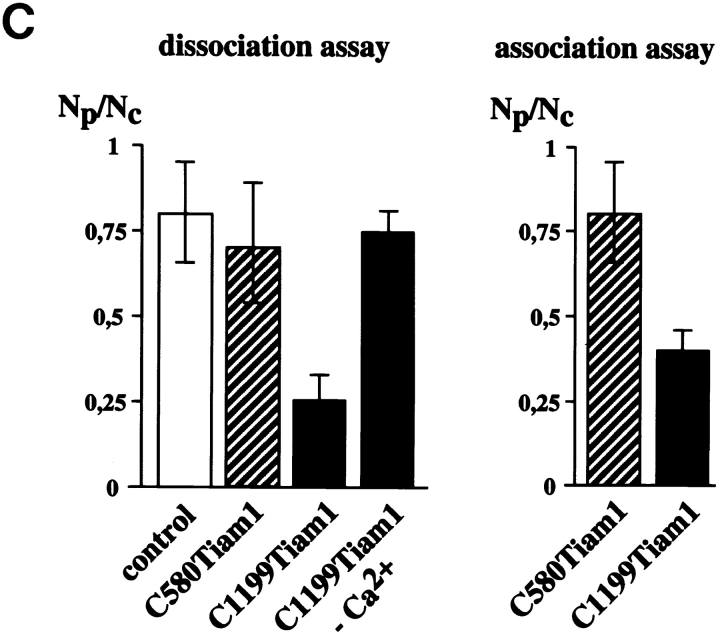
C1199Tiam1 induces an epithelial-like morphology in NIH3T3 cells by increasing N- and P-cadherin-based cell–cell adhesion. (A) Western blot using the Tiam1-specific C16 antibody of C1199- or C580Tiam1 immunoprecipitated with anti–HA antibody from the respective stable NIH3T3 cell lines. (B) NIH3T3 cells stably expressing C1199- or C580Tiam1 were stained for Tiam1, N- and P-cadherin (stained with a Pan-cadherin antibody), p120CAS, and F-actin. C1199Tiam1 localizes to sites of cell–cell contact and to membrane ruffles, whereas C580Tiam1 localizes to the cytoplasm. Cadherins as well as p120CAS localize to sites of cell–cell contact that were induced by C1199Tiam1. Bars, 25 μm. (C) Quantification of cell–cell adhesion of control NIH3T3 cells and cell lines expressing C1199- or C580Tiam1 determined by dissociation (in the presence and absence of Ca2+) and association assays and expressed as numbers of particles (cell clusters) per total number of cells (Np/Nc). Each bar represents the mean ± SD of triplicate assays. Shown is one representative example of three independent experiments.
In contrast, the phenotype of cells expressing the C580Tiam1 protein was not different from untransfected control cells (Fig. 1 B). C580Tiam1, lacking the NH2-terminal PH domain as a membrane localization signal (Michiels et al. 1997), was predominantly cytoplasmic and did not result in phenotypic changes such as ruffling or increased actin polymerization between neighboring cells (Fig. 1 B). Cells expressing the nonfunctional C580Tiam1 protein displayed no relocalization of components of cadherin adhesion complexes (Fig. 1 B), indicating that membrane localization of Tiam1 is required to induce the recruitment of cadherins and members of the cadherin complex to sites of cell–cell contact.
To demonstrate a functional increase in the strength of cell–cell adhesion induced by Tiam1, we used dissociation and association assays. For dissociation studies, subconfluently grown control NIH3T3 cells or cells expressing C1199- or C580Tiam1 were scraped off the dish, suspended by repeated pipetting, and the number of cell aggregates was counted. Expression of C1199Tiam1 increased the number and size of aggregates considerably when compared with C580Tiam1, indicative for increased cell–cell adhesion. Formation of aggregates by expression of C1199Tiam1 was calcium-dependent, supporting a role for cadherin-based cell–cell adhesions (Fig. 1 C). In the reverse experiment, NIH3T3 cells expressing either Tiam1 protein were suspended by pipetting to single cells and subsequently rotated in cell culture medium to allow the formation of cell–cell contacts. Quantification of the cell aggregates that reformed showed that C1199Tiam1 but not C580Tiam1 induced cell aggregation, indicating that C1199Tiam1 promotes adhesion between cells (Fig. 1 C). Taken together, these data indicate that C1199Tiam1-induced Rac activation leads to recruitment of cadherin-based adhesion complexes to sites of cell–cell contact that results in a functional increase of cell–cell adhesion and an epithelial-like morphology of NIH3T3 fibroblasts. We could not detect changes in protein expression of cadherins or associated complex members in the Tiam1-expressing cell lines compared with wild-type fibroblasts (data not shown). Most likely, the Tiam1/Rac–mediated increase in cell–cell adhesions is due to increased actin polymerization at cell–cell contacts, which facilitates the formation of functional cadherin-based adhesions, similarly as found in epithelial cells (Braga et al. 1997; Hordijk et al. 1997; Sander et al. 1998).
Activation of Endogenous Rac Requires Membrane Localization of the GEF Tiam1
C580Tiam1 still contains the catalytic DH domain, but does not localize at the cell membrane and does not induce an epithelial-like morphology, including membrane ruffling. To study whether C580Tiam1 is able to activate Rac, we compared the activation of endogenous Rac in cells expressing C1199- or C580Tiam1 (Fig. 2 A). Cell lysates expressing either one of the Tiam1 proteins were incubated with a fusion protein of glutathione-S-transferase (GST) and the CRIB domain of the Rac/Cdc42 effector molecule Pak (GST-PAK-CD). Activated, i.e., GTP-loaded Rac bound to GST-PAK-CD, was analyzed by Western blot with an antibody directed against Rac1. C1199Tiam1 strongly activated endogenous Rac, compared with control NIH3T3 cells (Fig. 2 A, lanes 1 and 2). Expression of the cytoplasmic C580Tiam1 containing the catalytic DH domain only weakly stimulated Rac activity (Fig. 2 A, lane 3). This indicates that efficient activation of endogenous Rac by Tiam1 requires membrane localization of the GEF.
Figure 2.
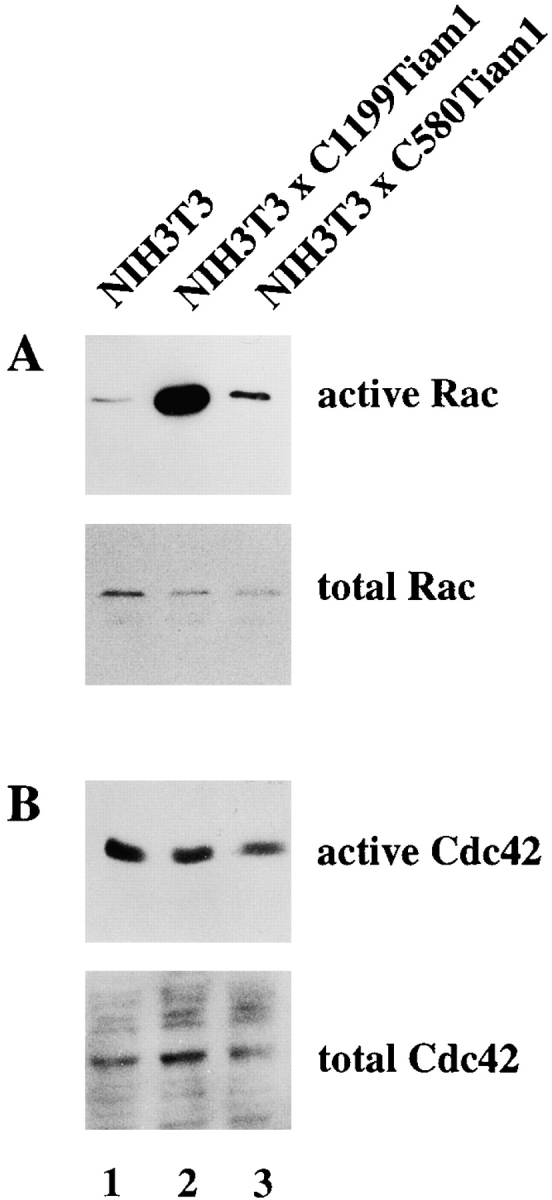
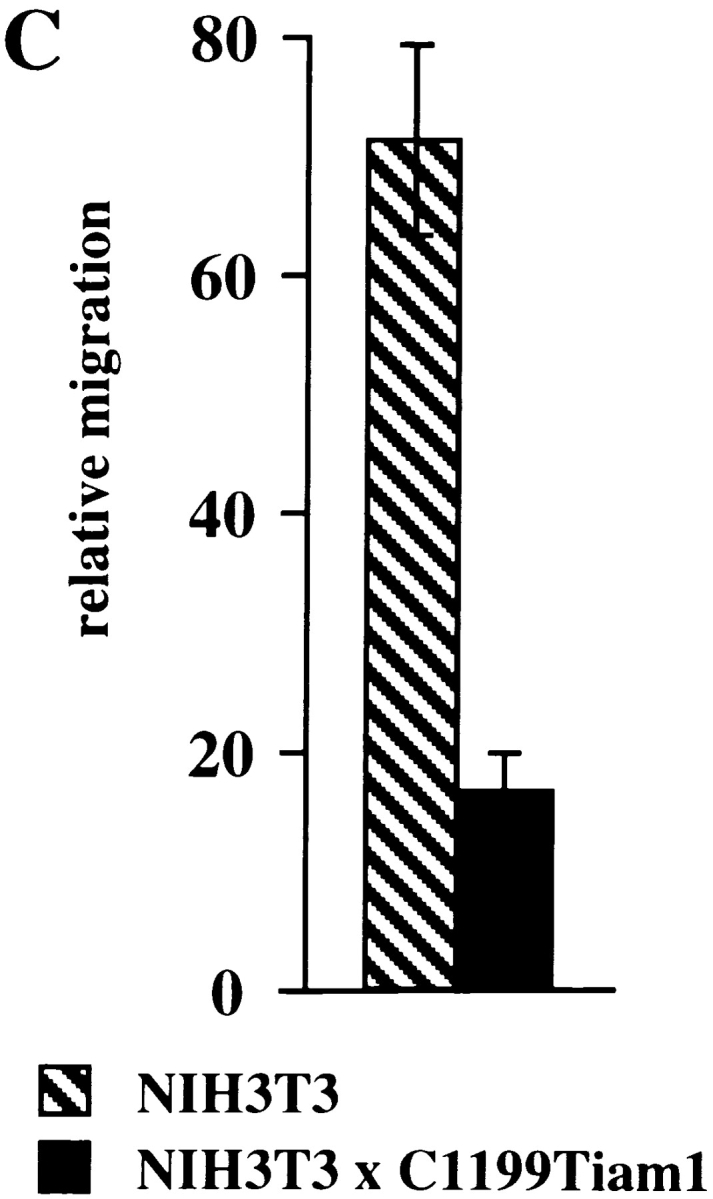
Activation of endogenous Rac by Tiam1 requires membrane localization of the GEF and inhibits migration of NIH3T3 fibroblasts. (A) Activation of endogenous Rac in control NIH3T3 cells or cells expressing C1199- or C580Tiam1. Lysates of the indicated cell lines (from 3 × 106 cells) were incubated with GST-PAK-CD and the bound Rac molecules were detected by Western blot. Aliquots of the respective lysates serve as controls for analyzing total amounts of Rac protein. (B) Tiam1 is a specific activator of Rac and not Cdc42. Lysates of control or Tiam1-expressing NIH3T3 cells were incubated with GST-PAK-CD and the precipitated molecules probed with antibodies against Cdc42. (C) Migration rates of 7 × 104 control or C1199Tiam1-expressing NIH3T3 cells per well coated with fibronectin were determined towards 20 ng/ml PDGF contained in the lower chamber of the transwell. Each bar represents the mean ± SD of triplicate migration assays. Shown is one representative example of three independent experiments.
In addition to Rac, Cdc42 also has been implicated in the formation of cell–cell adhesions in epithelial cells (Kuroda et al. 1997). To determine whether the observed Tiam1-induced cell–cell adhesion in fibroblasts could involve activation of Cdc42, GST-PAK-CD precipitates were incubated with an antibody directed against Cdc42. Cdc42 activity was not increased by either Tiam1 protein (Fig. 2 B). These data demonstrate that in NIH3T3 fibroblasts, Tiam1 functions as a specific activator of Rac and not Cdc42. We conclude that activation of Rac and not Cdc42 is responsible for the morphological transformation and increased cell–cell adhesion of Tiam1-expressing NIH3T3 cells.
Tiam1/Rac Signaling Inhibits Migration of NIH3T3 Cells
In addition to promoting cadherin-mediated cell–cell adhesion in fibroblasts, C1199Tiam1 also induced increased spreading of fibroblasts when compared with control cells (Fig. 1 B). Enhanced cell spreading was found on different substrates including vitronectin, fibronectin, collagen I, and laminin 1 (data not shown). This is consistent with findings in neuronal and lymphoid cells, where Rac was shown to promote integrin-mediated cell spreading and adhesion (van Leeuwen et al. 1995; D'Souza-Schorey et al. 1998).
To study the effect of Tiam1-mediated Rac activation on migration, we quantified the migratory behavior of control and C1199Tiam1-expressing fibroblast cells using a transwell invasion assay. Expression of C1199Tiam1 resulted in inhibition of fibroblast migration through filters coated with fibronectin as compared with the control cells (Fig. 2 C). Fibroblasts expressing C580Tiam1, which does not activate Rac (Fig. 2 A), showed a migration rate similar to control cells (data not shown). This indicates that the migratory behavior of fibroblasts is inhibited by Tiam1-mediated Rac activation, similarly as found in epithelial cells (Sander et al. 1998).
Tiam/Rac–induced Cell–Cell Adhesion Requires Rho Activity
Upon serum starvation, C1199Tiam1-expressing NIH3T3 cells lost their epithelial-like morphology. The cadherin-mediated cell–cell contacts were largely disassembled and many cells acquired a polarized, sickle-shaped phenotype (Fig. 3 A). As determined in scratch assays, these serum-deprived cells did not migrate (data not shown). Addition of serum partly restored the epithelial-like morphology within 3–5 h, the cells appeared more round again and began to re-establish cell–cell contacts (Fig. 3 A). Growth factors such as LPA, PDGF, insulin, and bradykinin were tested for their capability to substitute for serum to restore the epithelial-like phenotype of the serum-starved C1199Tiam1-expressing NIH3T3 cells. From all growth factors tested, only LPA was capable of partly restoring the epithelial-like morphology, similar to serum (Fig. 3 B). The C1199Tiam1 protein was localized to membrane ruffles that were also restored in the presence of LPA. Serum or LPA had to be present for a few hours before restoration of the epithelial-like phenotype was observed. LPA is a major component in serum and a potent activator of Rho (Ridley and Hall 1992). To substantiate the role of Rho in the maintenance of the epithelial-like phenotype, Rho was inactivated by treating C1199Tiam1-expressing NIH3T3 cells grown in the presence of serum with C3 transferase toxin. Similar to serum-starved cells (Fig. 3 A), these cells became sickle-shaped and lost their epithelial-like morphology including most membrane ruffles (Fig. 3 B). From these data we conclude that Rho activity is required for both cadherin-mediated cell–cell adhesion and membrane ruffles.
Figure 3.
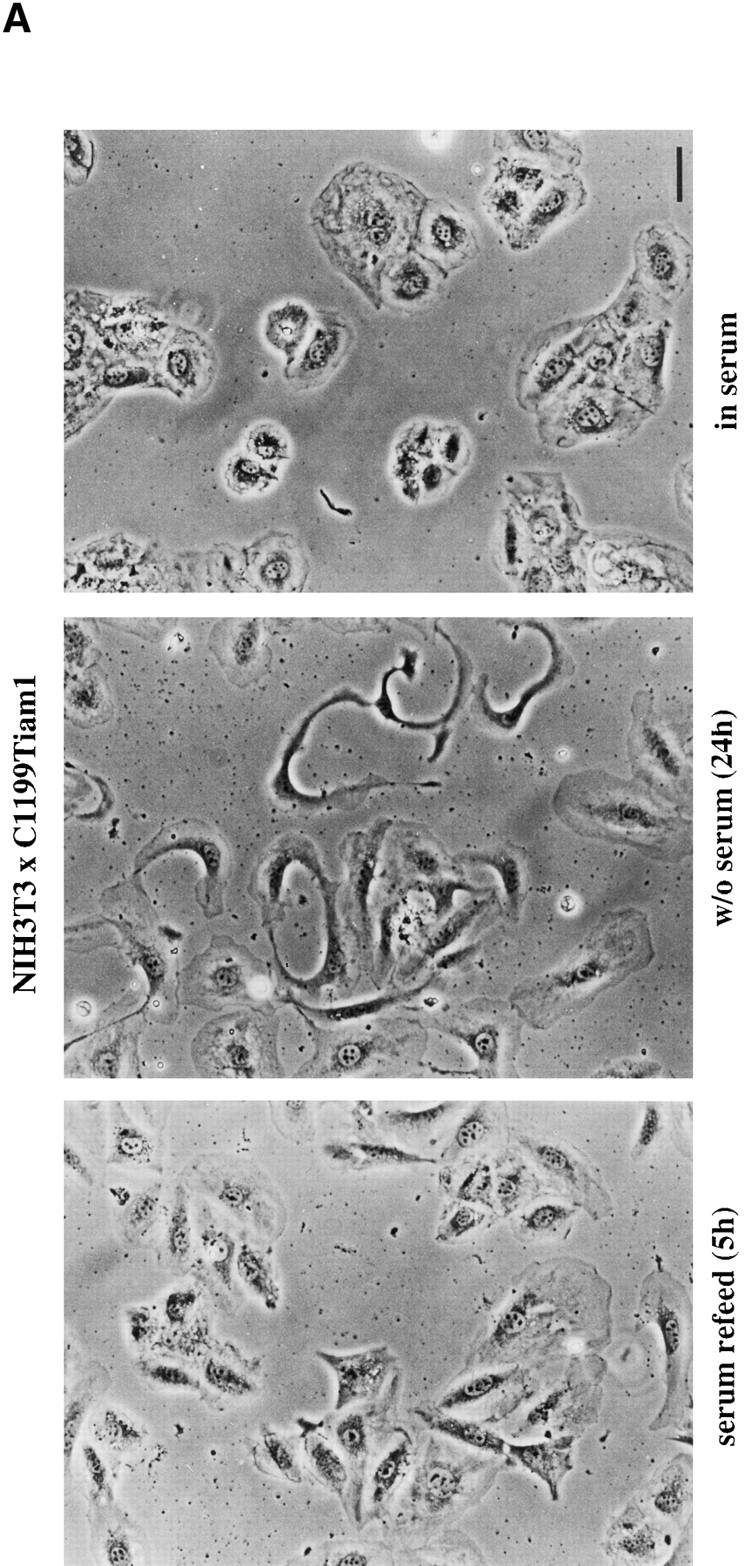
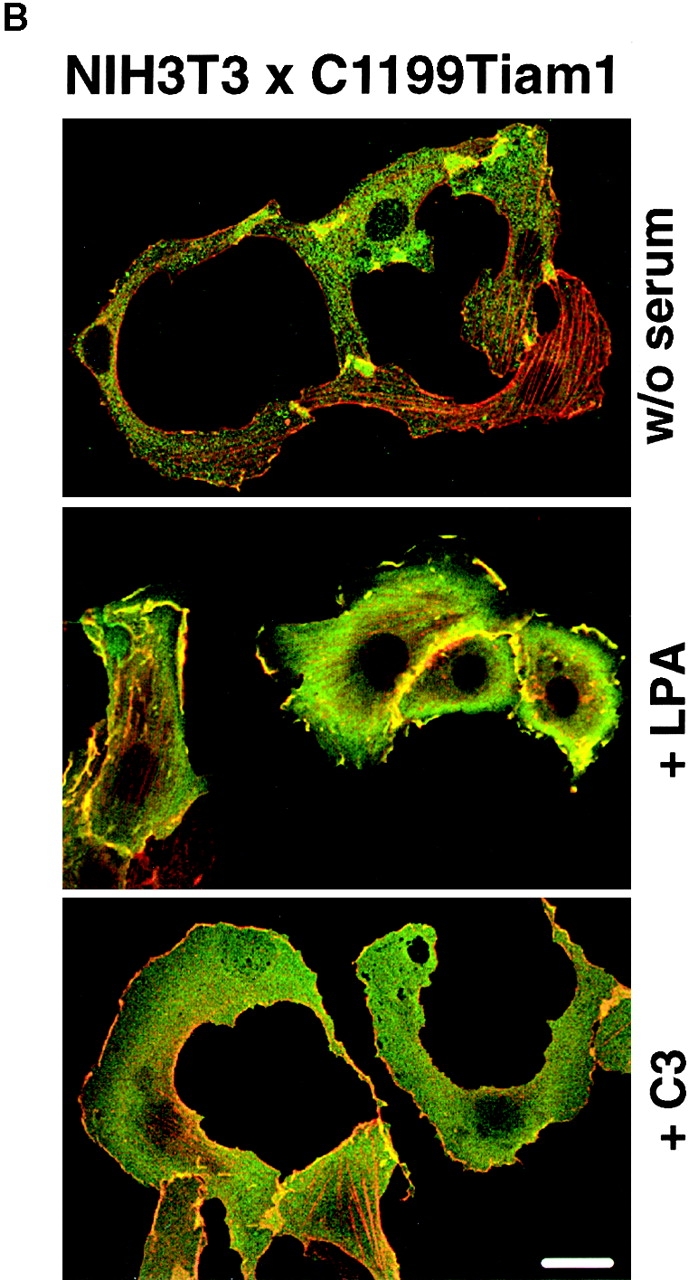
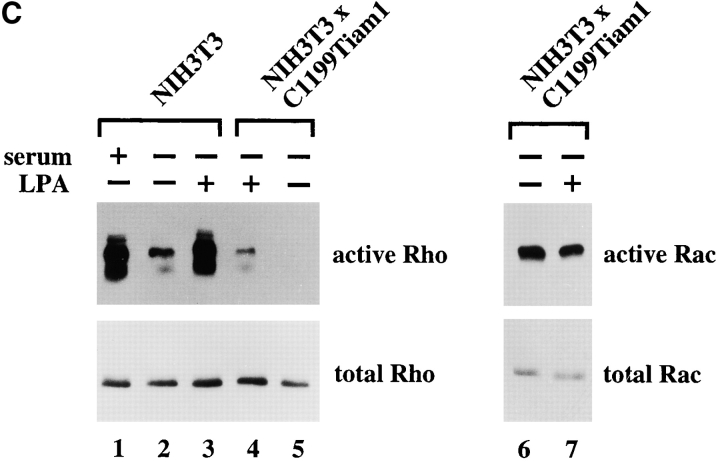
Basal Rho activity is required for the maintenance of Tiam1/Rac–induced cell–cell contacts in NIH3T3 fibroblasts. (A) Serum is required for Tiam1-induced cell–cell contacts. Phase-contrast pictures of NIH3T3 fibroblasts expressing C1199 Tiam1 showing cells grown in the presence of serum, after 24 h serum starvation, and 5 h after addition of serum to 24 h serum-starved cells. Bar, 10 μM. (B) Rho activity is required for Tiam1-induced cell–cell contacts. C1199Tiam1-expressing NIH3T3 fibroblasts were stained for Tiam1 (DH antibody, green) and F-actin (red) after 24 h of serum starvation, 24 h of serum starvation followed by addition of 5 μM LPA (8 h), and treatment for 12 h with 30 μg/ml C3 transferase toxin (in the presence of serum). Bar, 25 μM. (C) Cell lysates (from 5 × 106 cells) of control and C1199Tiam1-expressing NIH3T3 fibroblasts were assayed for Rho and Rac activities. Cells were grown in serum (lane 1) or 24 h serum-starved (lanes 2–7) before stimulation with LPA (5 μM, 15 min) as indicated. Lysates were incubated with GST-C21 (lanes 1–5) or GST-PAK-CD (lanes 6 and 7), and bound GTPases were analyzed with antibodies against RhoA or Rac1.
LPA-mediated Rho Activation
To assess activation of endogenous Rho protein by LPA, we used the Rho binding domain (GST-C21) of the Rho effector Rhotekin fused to GST (Reid et al. 1996, Reid et al. 1999). As expected, control fibroblasts showed a significant downregulation of Rho activity upon 24 h serum starvation (Fig. 3 C, lanes 1 and 2). LPA (lane 3) or serum (not shown) treatment of these cells led to complete restoration of the Rho activity. Similarly, LPA led to the activation of endogenous Rho (lanes 5 and 4) but not Rac protein (lanes 6 and 7) in serum-starved C1199Tiam1-expressing fibroblasts. These biochemical data are consistent with the established link between LPA-mediated receptor stimulation and subsequent activation of Rho (Ridley and Hall 1992).
However, comparing control and Tiam1-expressing fibroblasts, we found that in serum-starved C1199Tiam1-expressing cells almost no Rho activity was detectable (compare lanes 5 and 2), whereas C1199Tiam1-expressing cells grown in the presence of serum still showed some Rho activity (data not shown and see Fig. 4). Similarly, Rho activation after stimulation with LPA was much lower in C1199Tiam1-expressing cells than the activation of Rho by LPA in control cells (compare Fig. 3, lanes 4 and 3). Thus, expression of C1199Tiam1 resulted in decreased Rho activity and a desensitized stimulation by LPA.
Figure 4.
Rac activation downregulates Rho activity. (A) Cell lysates (from 3 × 106 cells) of control, C1199Tiam1- or C580Tiam1-expressing NIH3T3 fibroblasts were assayed for Rho activities. Lysates were incubated with GST-C21 and bound Rho was analyzed with antibodies against RhoA. (B) Cell lysates of control, C1199Tiam1- or V12Rac-expressing NIH3T3 fibroblasts were assayed for Rho activities, similar as in A. Aliquots of total lysates were probed with anti–Rho antibodies to control for total amounts of Rho protein or with anti–Myc (9E10) antibodies to control expression of V12Rac.
This suggests, that sustained activation of Rac by Tiam1 resulted in downmodulation of Rho activity. Complete inactivation of Rho, either by the absence of serum (Fig. 3 A) or C3 transferase treatment (Fig. 3 B), gave rise to sickle-shaped C1199Tiam1-expressing cells. The phenotypic switch because of complete inactivation of Rho could be restored by LPA- or serum-mediated activation of Rho, indicating that the maintenance of the Rac-induced epithelial-like morphology of NIH3T3 cells requires at least a basal Rho activity.
Rac Inactivates Rho by Downstream Signaling
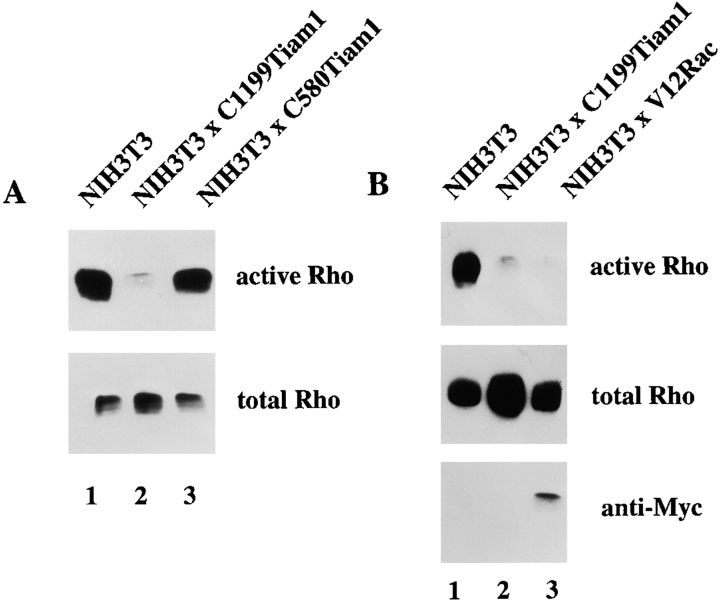
The different levels of Rho activities in serum-starved control and C1199Tiam1-expressing cells (Fig. 3 C) suggested that the phenotype of C1199Tiam1-expressing cells, characterized by an epithelial-like morphology and inhibited migration, might not only be determined by activation of Rac, but also by downregulation of Rho signaling. Therefore, we determined in more detail the effect of Tiam1-mediated Rac activation on the activity state of endogenous Rho protein. In the presence of serum, expression of C1199Tiam1 caused a strong decrease of Rho activity, compared with control cells (Fig. 4 A, lanes 1 and 2). Expression of C580Tiam1 resulted in a minor decrease of Rho activity, consistent with the little activation of Rac found to be induced by this protein (Fig. 4 A, lane 3). Similar to C1199Tiam1, also constitutively active V12Rac resulted in downregulation of endogenous Rho activity (Fig. 4 B), indicating that inhibition of Rho activity occurred downstream of Rac.
It could be argued that downregulation of Rho is a consequence of Rac-induced cellular spreading, which is accompanied by cytoskeletal changes rather than by Rac-mediated signaling events. To address this issue, we measured Rho activity of attached and suspended cells in relation to Rac activation. V12Rac-expressing cells showed a similar degree of Rac activity, independent from attached or suspended conditions (Fig. 5 A). Irrespective of cell adhesion, these cells also showed a strong downregulation of Rho activity when compared with the control cells (Fig. 5 B). This argues that the downregulation of Rho activity is not due to cytoskeletal rearrangements caused by Rac-induced cell spreading, but rather the result of signaling events downstream of Rac.
Figure 5.
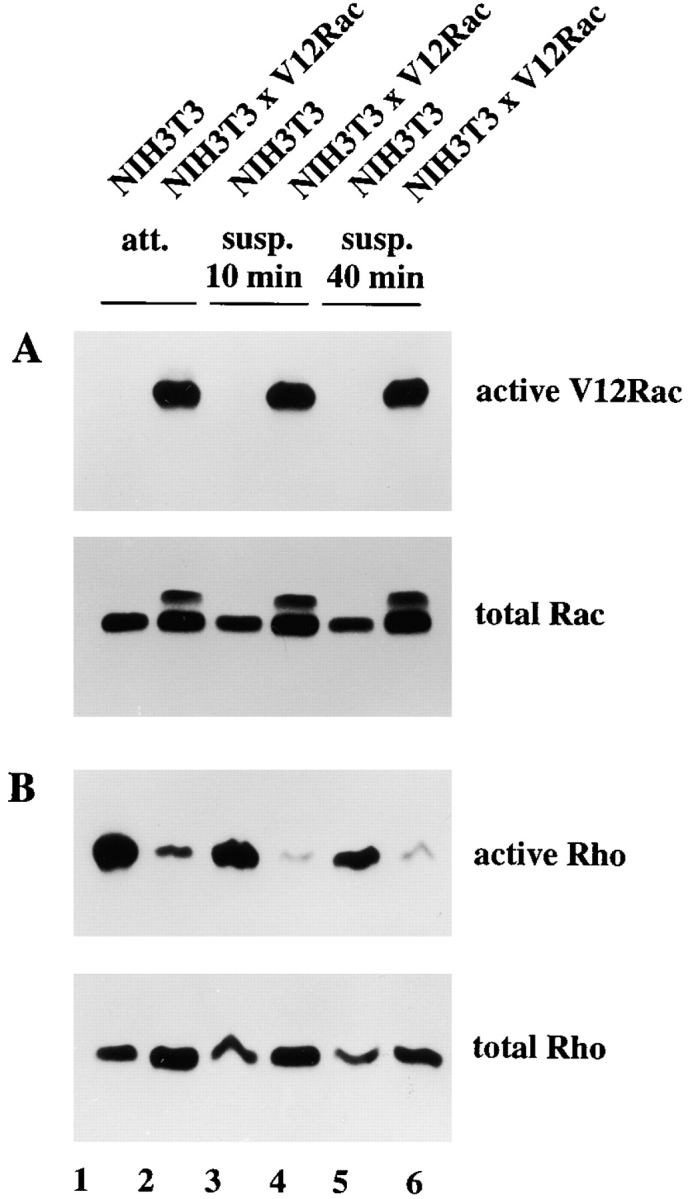
Downregulation of Rho activity by Rac signaling. (A) Cell lysates (from 3 × 106 cells) of control and V12Rac-expressing NIH3T3 fibroblasts were assayed for Rac and Rho activities. Lysates from attached cells (lanes 1 and 2) or trypsinized cells kept in suspension for 10 min (lanes 3 and 4) or 40 min (lanes 5 and 6) were incubated with GST-PAK-CD and bound Rac was analyzed with antibodies against Rac. Aliquots of total lysates were probed with antibodies against Rac to control for total amounts of Rac protein. Note that Myc-tagged V12Rac exhibits a slightly lower electrophoretic mobility as endogenous Rac. (B) Lysates, similar as described in A, were assayed for Rho activities by incubating with GST-C21.
Rho-dependent Cytoskeletal Changes in Tiam1-expressing Cells
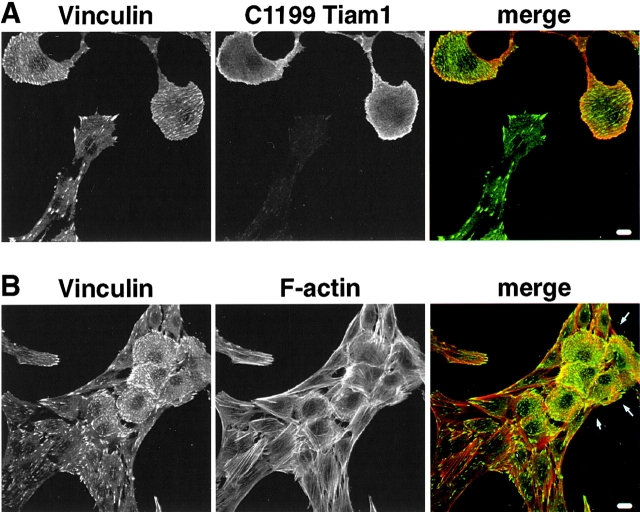
Although wild-type and Tiam1-expressing fibroblasts display different cell shapes and are, therefore, difficult to compare, we tried to address the corresponding cytoskeletal changes after LPA stimulation with respect to typical Rho-dependent cytoskeletal organization. We monitored the distribution of vinculin-containing adhesion complexes and actin stress fibers. For this, wild-type and C1199Tiam1-expressing cells were mixed and grown on coverslips. After serum starvation, the cells were subsequently stimulated with LPA or serum and analyzed by immunocytochemistry. After LPA or serum stimulation, wild-type fibroblasts are characterized by relatively high Rho activity, whereas Tiam1-expressing cells show decreased levels of Rho activity, as a result of Tiam1-mediated Rac activation (Fig. 3 C, lane 4). In wild-type NIH3T3 cells, we observed the typical Rho-induced pattern of pronounced punctuate spots of focal contacts containing vinculin at the end of thick, heavy bundles of stress fibers upon LPA (Fig. 6A and Fig. B) or serum (data not shown) stimulation. In C1199Tiam1-expressing cells (characterized by their roundish shape, see arrows), the actin fibers appeared finer and less bundled compared with wild-type cells (Fig. 6A and Fig. B). Vinculin-containing adhesion complexes were present at the cell periphery as well as small fine spots throughout the basal side of the cell (Fig. 6A and Fig. B). The larger spots at the cell periphery may correspond to the previously described Rac-induced focal complexes (Ridley et al. 1992; Rottner et al. 1999), and mostly did not overlap with the endpoints of stress fibers. The distribution of the small vinculin-containing complexes throughout the basal side of the cell followed the distribution of thin stress fibers (Fig. 6 B) and, therefore, most likely represent true Rho-induced focal contacts. The Rho-dependent cytoskeletal organization is, therefore, consistent with the low Rho activity observed after LPA or serum stimulation in Tiam1-expressing cells. Compared with wild-type fibroblasts, Tiam1-expressing cells exhibit less bundled and very thin stress fibers, which are accompanied by small focal contacts.
Figure 6.
Rho-dependent cytoskeletal organization in control and Tiam1-expressing fibroblasts. Wild-type and C1199Tiam1-expressing fibroblasts were mixed and grown on coverslips. Subsequently, cells were serum-starved for 24 h and stimulated with LPA (5 min). Under these conditions, wild-type cells show low Rac and high Rho activity, whereas Tiam1-expressing cells show high Rac and relatively low Rho activity. Cells were stained for vinculin and C1199Tiam1 (A) or vinculin and F-actin (B). C1199Tiam1-expressing cells are characterized by their flat round shape (see A). In (B), an island of flat-round C1199Tiam1-expressing cells is seen between wild-type NIH3T3 cells. Note that wild-type cells are characterized by pronounced focal adhesions at the end of thick, bundled stress fibers, whereas C1199Tiam1-expressing cells show thin, less bundled stress fibers and small focal contacts throughout the basal side of the cell. Bar, 10 μm.
Rac Downstream Pathways to Rho
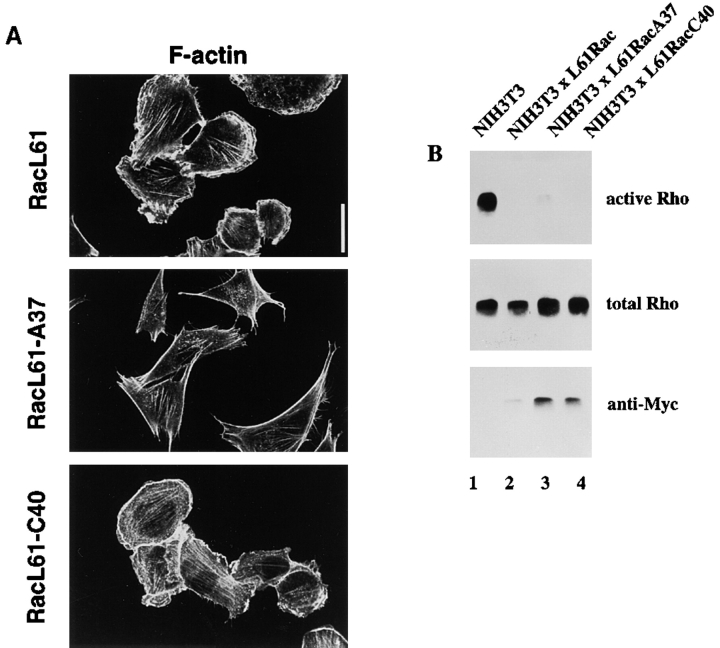
To get insight into the signaling events downstream of Rac responsible for downregulation of Rho activity, we generated NIH3T3 cells stably expressing the Rac effector mutants L61Rac, L61RacA37, and L61RacC40. Consistent with the findings in Swiss 3T3 fibroblasts (Lamarche et al. 1996), activated L61Rac induced similar phenotypic changes as activated V12Rac, including membrane ruffling and lamellae formation in NIH3T3 fibroblasts (Fig. 7 A). L61RacA37, which has been described to bind Pak1 and to activate the Jun kinase pathway, did not cause cytoskeletal rearrangements, whereas L61RacC40, which cannot bind Pak1 or activate the Jun kinase pathway, induces cytoskeletal reorganization similar to L61Rac. By assaying binding of the Rac effector mutants to GST-PAK-CD, we confirmed that L61Rac and L61RacA37 efficiently bound to GST-PAK-CD, whereas L61RacC40 did not (data not shown; Lamarche et al. 1996). To gain insight into the possible Rac effectors mediating the downregulation of Rho, we determined Rho activities in fibroblast lines expressing the Rac effector mutants (Fig. 7 B). Downregulation of Rho by L61Rac was comparable to V12Rac (Fig. 4 B and 7 B). Expression of both L61RacA37 and L61RacC40 resulted also in strong downregulation of Rho activity (Fig. 7 B, lanes 3 and 4), although L61RacA37 mediated this response somewhat less effectively than L61Rac and L61RacC40. A stable cell line expressing an activated mutant of Pak1, PakE423 (Sells et al. 1997), did not show downregulation of Rho activity (data not shown). This confirms the findings with the L61RacC40 effector mutant, and suggests that the Rac-mediated downregulation of Rho is not dependent on Pak1. Furthermore, on the bases of the L61RacA37 mutant, activation of the Jun kinase pathway seems not to be involved in signaling towards Rho. Similarly, Rac signaling pathways leading to reorganization of the cytoskeleton, do not play a role in inactivation of Rho, substantiating the conclusions drawn from the experiments with adherent and suspended cells.
Figure 7.
Both L61RacA37 and L61RacC40 effector mutants mediate Rho inactivation. (A) Immunofluorescence analysis of NIH3T3 fibroblasts stably expressing Myc-tagged L61Rac, L61RacA37, or L61RacC40. Changes in morphology and cytoskeleton organization are visualized by F-actin staining. Bar, 20 μm. (B) Cell lysates (from 3 × 106 cells) of control and L61Rac-, L61RacA37-, or L61RacC40-expressing NIH3T3 fibroblasts were assayed for Rho activity. Lysates were incubated with GST-C21 and bound Rho was analyzed with antibodies against Rho. Aliquots of total lysates were probed with antibodies against Rho or the Myc-epitope to control for total amounts of Rho proteins and expression of Myc-tagged Rac proteins.
Activation of Rho Does Not Affect Rac Activity
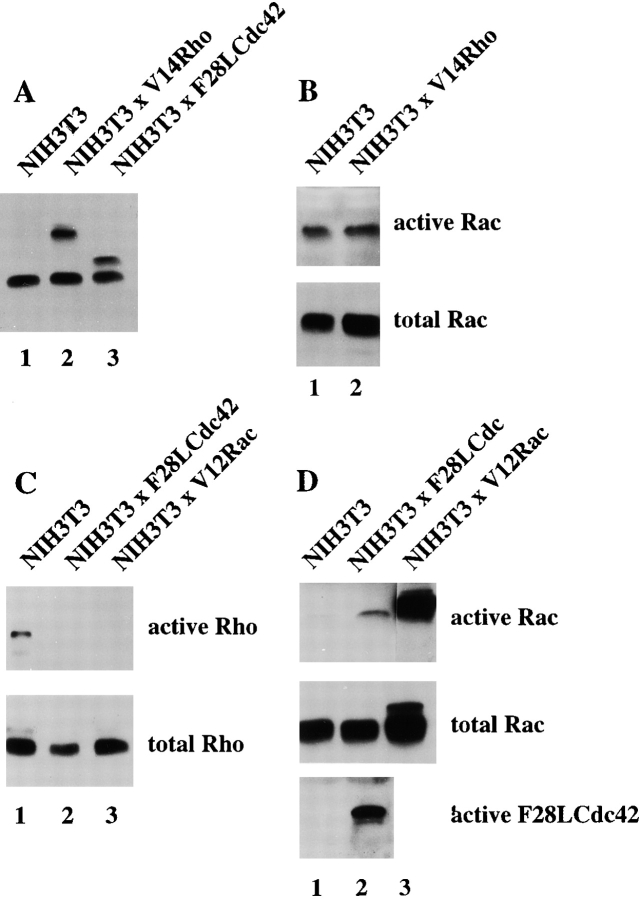
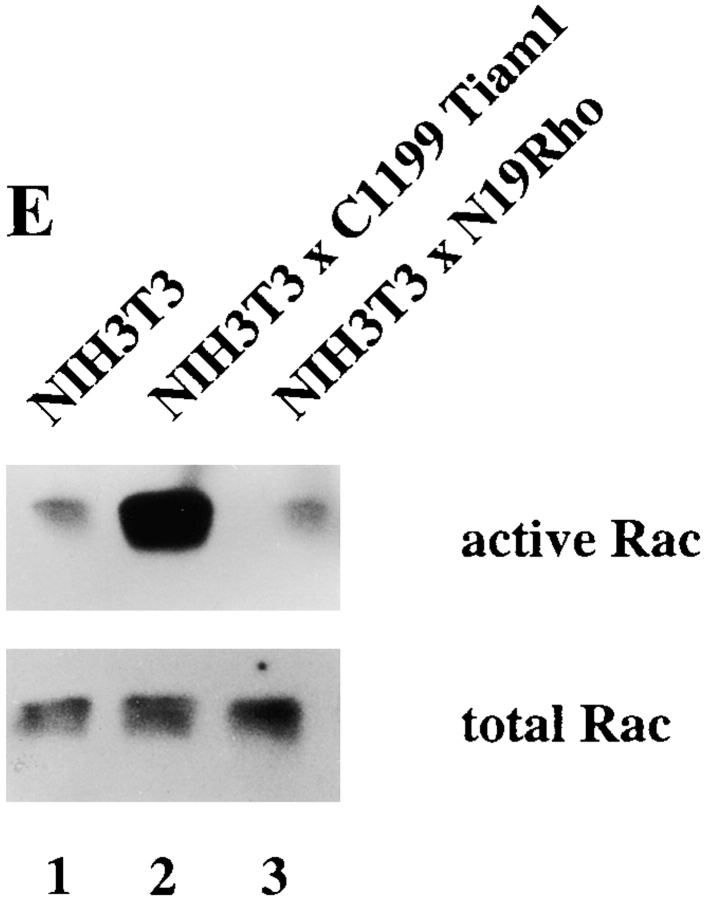
To address whether Rac and Rho regulate their activities in a reciprocal manner or whether downregulation of Rho activity is specifically mediated by Rac, we established an NIH3T3 cell line stably expressing Myc-tagged V14Rho (Fig. 8 A, lane 2). Rac activity in V14Rho-expressing cells was not significantly changed compared with control cells (Fig. 8 B), consistent with the equal Rac activities found in serum-starved Tiam1-expressing cells and cells stimulated with LPA (Fig. 3 C). Similarly, blocking Rho activity by dominant negative N19Rho (Fig. 8 E) or treatment of cells with the Rho-kinase inhibitor Y27632 (not shown) did not affect endogenous Rac activity. From these data, we conclude that V14Rho or LPA-mediated Rho activation does not result in downmodulation of Rac activity, and neither does dominant negative N19Rho result in upregulation of Rac activity. Apparently, modulation of Rho activity itself does not affect cellular Rac activity in NIH3T3 cells.
Figure 8.
Interplay of Cdc42, Rac, and Rho activities. (A) Expression of Myc-tagged V14Rho and F28LCdc42 in established NIH3T3 cell lines. Western blot of cell lysates from control and V14Rho- and F28LCdc42-expressing cells using a mixture of anti–Rho and anti–Myc antibodies. (B) V14Rho does not affect endogenous Rac activity. Cell lysates (from 4 × 106 cells) of control and V14Rho-expressing NIH3T3 fibroblasts were assayed for Rac activity. Lysates were incubated with GST-PAK-CD and bound Rac was analyzed by Western blot with antibodies against Rac. Aliquots of total lysates were probed with antibodies against Rac to control for total amounts of Rac proteins. (C) Cdc42 inactivates Rho. Cell lysates (from 4 × 106 cells) of control, F28LCdc42- and V12Rac-expressing NIH3T3 fibroblasts were assayed for Rho activity. Lysates were incubated with GST-C21, and bound Rho was analyzed by Western blot with antibodies against Rho. Aliquots of total lysates were probed with antibodies against Rho to control for total amounts of Rho proteins. (D) F28LCdc42 is an activated Cdc42 mutant and activates Rac. Cell lysates (from 4 × 106 cells) of control and F28LCdc42 NIH3T3 fibroblasts were assayed for Cdc42 activity. After incubation with GST-PAK-CD, bound F28LCdc42 was analyzed by Western blot with antibodies recognizing the Myc-epitope tag and bound Rac with anti–Rac antibodies (Upstate Biotechnology Inc.). (E) Dominant negative N19Rho does not affect endogenous Rac activity. Cell lysates (from 4 × 106 cells) of control and N19Rho-expressing NIH3T3 fibroblasts were assayed for Rac activity and analyzed as in B.
Cdc42 Downregulates Rho Activity
To investigate the role of Cdc42 in the regulation of Rho activity, we established an NIH3T3 cell line expressing a Myc-tagged fast cycling Cdc42 mutant F28ACdc42 (Fig. 8 A, lane 3) that has been previously described by Lin et al. 1997. We used this fast cycling Cdc42 mutant because we were unable to obtain stable cell lines expressing V12Cdc42. F28ACdc42 downregulated Rho activity, similar to V12Rac (Fig. 8 C), indicating that both Rac and Cdc42 are capable of modulating Rho activity. F28ACdc42 clearly functioned as an activated mutant of Cdc42 and also activated endogenous Rac to some extent in NIH3T3 cells (Fig. 8 D, lanes 1 and 2), consistent with previous findings based on cytoskeletal changes in Swiss 3T3 fibroblasts (Nobes and Hall 1995). Therefore, downregulation of Rho activity by Cdc42 may either involve activation of Rac or occur independently of Rac, possibly by a downstream signaling pathway shared by Cdc42 and Rac.
Short Term Rac Activation by PDGF Results in Simultaneous Inactivation of Rho
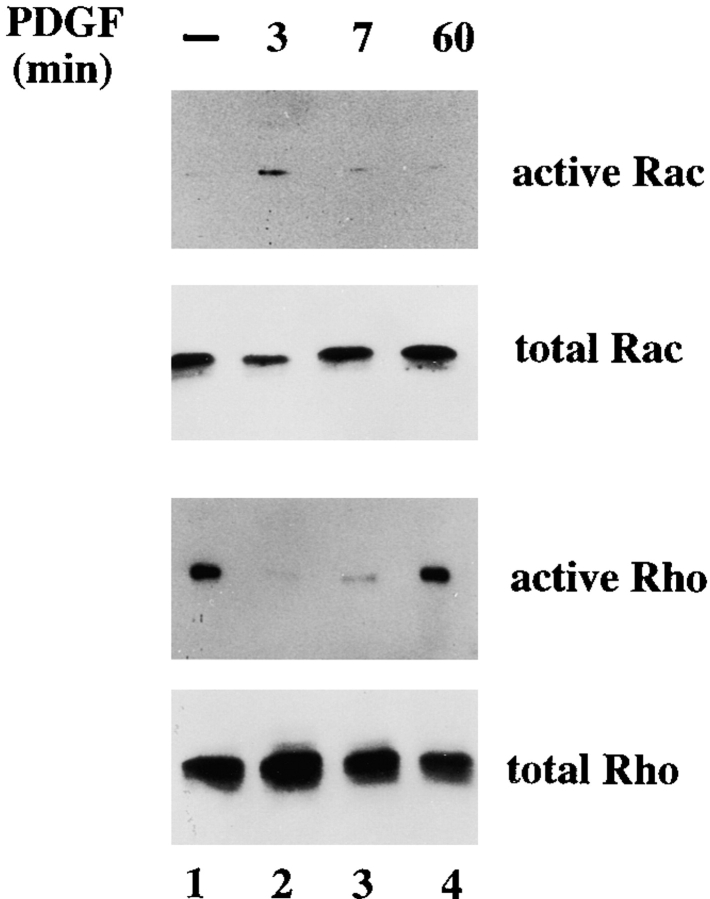
To address whether inactivation of Rho is a phenomenon restricted to sustained activation of Rac in established cell lines or whether growth factor–induced transient activation of Rac leads to downregulation of Rho, we stimulated serum-starved control NIH3T3 cells with PDGF. In agreement with the established link between PDGF-mediated receptor stimulation and Rac activation, PDGF activated Rac (Fig. 9) and not Cdc42 (data not shown) after 3 and 7 min of stimulation. PDGF-mediated activation of Rac was found to be a transient effect and was no longer detected 1 h after PDGF stimulation (Fig. 9). Simultaneously with activation of Rac, we found a time-dependent inactivation of Rho in response to PDGF stimulation (Fig. 9). Similarly to the activation of Rac, Rho activity returned to basal levels 1 h after PDGF stimulation. These data suggest that PDGF-mediated Rac activation also results in downregulation of Rho activity, as the kinetics of Rac activation correspond with inactivation of Rho.
Figure 9.
PDGF-mediated Rac activation simultaneously results in inactivation of Rho. 4 × 106 NIH3T3 cells were deprived of serum for 24 h, and then stimulated with 20 ng/ml PDGF for 0, 3, 7, or 60 min. Cell lysates were assayed for Rac and Rho activities and incubated either with GST-PAK-CD or GST-C21. Bound GTPases were analyzed by Western blot using specific Rac and Rho antibodies.
A Balance of Both Rac and Rho GTPase Activities Determines the Cellular Phenotype
Expression of Tiam1 or activated Rac in NIH3T3 fibroblasts resulted in immotile cells with established cell–cell contacts (Fig. 1 and Fig. 3). This phenotype is characterized by high Rac activity and downregulated Rho activity. Therefore, raising Rho activity exogenously in Tiam1-expressing cells should change the phenotype again towards fibroblastoid, motile cells. We generated an NIH3T3 cell line stably expressing C1199Tiam1 as well as V14Rho to test this hypothesis. Expression of C1199Tiam1 was not changed upon introduction of V14Rho (data not shown). Whereas C1199Tiam1-expressing cells grew in small epithelial-like islands of immotile cells, cells expressing Tiam1 as well as titrated amounts of V14Rho did not establish cell–cell contacts and exhibited a more motile, contractile phenotype (Fig. 10). Thus, restoration of Rho activity by V14Rho in Tiam1-expressing cells induces transition from an epithelioid to a more fibroblastoid phenotype. These data suggest that the balance of Rac and Rho activities determines the cellular phenotype and migratory behavior in NIH3T3 fibroblasts.
Figure 10.
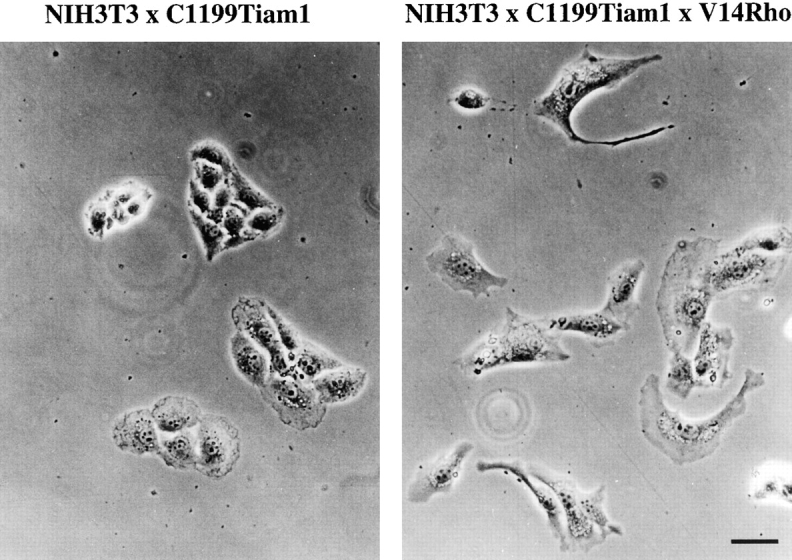
The reciprocal balance of Rac and Rho GTPase activities determines an epithelioid or fibroblastoid phenotype. NIH3T3 cells stably expressing C1199Tiam1 acquire an epithelioid phenotype, characterized by the formation of functional cell–cell contacts, because of activation of Rac and downregulation of Rho activity (Fig. 4). V14Rho-mediated restoration of Rho activity in C1199Tiam1-expressing cells reverts the epithelioid phenotype to a more fibroblastoid morphology associated with migratory behavior, indicating that the balance of both Rac and Rho activities determines cellular morphology. Bar, 10 μm.
Discussion
Stable expression of the GEF Tiam1 in NIH3T3 fibroblasts increases cell–substrate interactions and induces an epithelial-like morphology that is characterized by increased N- and P-cadherin–mediated cell–cell adhesion and inhibition of cell migration. This phenotypic transformation of NIH3T3 fibroblasts is due to Tiam1-mediated activation of Rac and Rac-mediated downregulation of Rho activity. Both sustained Cdc42 and Rac activation result in downregulation of endogenous Rho activity, demonstrating that Cdc42 and Rac antagonize Rho by regulating its GTP level. This crosstalk between Rac and Rho signaling pathways determines morphology and migratory behavior of NIH3T3 fibroblasts.
In epithelial cells, both Rac and Cdc42 GTPases promote the formation of E-cadherin–mediated cell–cell adhesions (Hordijk et al. 1997; Kuroda et al. 1997). We found that Tiam1-induced formation of cadherin-based cell–cell contacts in NIH3T3 cells is due to activation of Rac and not Cdc42, confirming that Tiam1 functions as a specific GEF for Rac in fibroblasts. In MDCK cells, expression of Tiam1 resulted in inhibition of cell migration by increasing E-cadherin–mediated adhesion (Hordijk et al. 1997; Sander et al. 1998). In fibroblasts, Tiam1-mediated Rac activation also resulted in strong inhibition of migration, by increasing cell–cell and cell–matrix adhesions.
The formation of Rac-induced cell–cell contacts does not exclude a requirement for Rac in migratory responses, as was shown previously by us and others (Ridley et al. 1995; Anand-Apte et al. 1997; Keely et al. 1997; Shaw et al. 1997; Sander et al. 1998). In epithelial cells, we showed earlier that Rac may influence cell migration in a positive or negative fashion, predominantly because of its effect on cell–cell adhesion (Sander et al. 1998). V12Rac has been shown to increase haptotaxis towards an immobilized collagen gradient in NIH3T3 cells (Sells et al. 1999). Besides using an inducible expressing system, these variant results might originate from similar effects on cell–cell adhesion, which is largely determined by cell density applied in the migration assay. As established in epithelial MDCK cells, high cell densities favor cell–cell adhesion and inhibit migration, whereas increased Rac-mediated migration is only seen at low cell densities (Sander et al. 1998). In addition, preservation of cell surface adhesion proteins such as cadherins during the trypsinization procedures may account for differences in the formation of cell–cell adhesions and, therefore, affect migratory behavior.
The morphology and migratory behavior of Tiam1-expressing fibroblasts seems to be determined by the crosstalk of Rac and Rho proteins. V12Rac or Tiam1-mediated Rac activation resulted in a substantial reduction of Rho activity, leading to an epithelial-like morphology. However, complete inactivation of Rho activity by serum starvation or treatment with C3 transferase toxin results in disruption of cell–cell contacts in Tiam1-expressing fibroblasts. Most likely, this reflects a requirement for a basal Rho activity in the maintenance of Rac-induced cell–cell contacts. Similarly, both Rac and Rho activities have been shown to be required for cell–cell adhesion in epithelial cells (Braga et al. 1997; Takaishi et al. 1997; Zhong et al. 1997). LPA partly restored the epithelial-like morphology in serum-starved fibroblasts. We have ruled out that LPA exerts its effect by activation of Rac, and confirmed that LPA functions as a potent activator of endogenous Rho protein. However, LPA-mediated activation of Rho occurred within minutes after stimulation and preceded the restoration of the epithelioid phenotype by hours. In addition to Rho activation, LPA may stimulate other signaling pathways (Stam et al. 1998) required for phenotypic restoration.
Rac-induced inactivation of Rho occurred downstream of Rac and was found in attached as well as suspended cells. Moreover, L61RacA37, which is unable of inducing the typical Rac-mediated cytoskeletal changes, still causes downregulation of Rho. This makes it unlikely that inactivation of Rho is a secondary consequence of Rac-induced spreading and cytoskeletal rearrangements, but rather reflects a signaling event by Rac. Inactivation of Rho activity was mediated by both Rac effector mutants, L61RacA37 and L61RacC40. Moreover, an activated mutant of the Rac downstream effector Pak1 was not effective in downregulation of Rho activity. This suggests that Pak1 and signaling pathways that stimulate Jun kinase activity or cytoskeletal changes (Joneson et al. 1996; Lamarche et al. 1996) are not involved in the regulation of Rho activity by Rac. However, this does not exclude the possibility that other Pak-like kinases participate in downregulating Rho activity.
In Swiss 3T3 fibroblasts, activated V12Rac or PDGF-mediated Rac activation was shown to induce the accumulation of actin in membrane ruffles followed by the delayed Rho-dependent formation of stress fibers, suggesting activation of Rho by Rac (Ridley et al. 1992). However, our data show inactivation of Rho activity by Rac in NIH3T3 fibroblasts. We found a similar inactivation of Rho by Rac in epithelial MDCK cells (Zondag, G.C.M., E.E. Evers, J.P. ten Klooster, L. Janssen, and J.G. Collard, manuscript submitted for publication) and in Cos-7 cells (Reid et al. 1999). Our findings are in agreement with a recent study in Swiss 3T3 cells, where the authors concluded that Rac and Rho activities are mutually antagonistic by monitoring the formation of Rac and Rho-dependent adhesion structures. After inhibition of the p160 Rho kinase, Rottner et al. 1999 observed a shift from focal contacts to focal complexes, which is accompanied by enhanced membrane ruffling. The authors suggested that inhibition downstream of Rho leads to activation of Rac. However, by direct measuring of the GTPase activities, our results suggest a unidirectional signaling from Rac towards Rho. Up- or downregulation of Rho activity, using either V14Rho, LPA, N19Rho, or the p160 Rho kinase inhibitor Y27632, did not affect GTP loading of Rac. Therefore, it is likely that the cytoskeletal changes that occur after inhibition of a Rho downstream effector originate from a shifted balance in the Rac and Rho pathways rather than from a direct, Rho-mediated modulation of Rac activity. In addition to the direct regulation of GTPases shown here, crosstalk between Rac and Rho downstream effector pathways can occur, as was shown for Pak1 regulating myosin light chain phosphorylation (Sanders et al. 1999; Sells et al. 1999).
We found that downregulation of Rho by Rac or Cdc42 was not restricted to stable cell lines showing sustained activation of Cdc42 or Rac. A transient increase in Rac activation induced by PDGF, similarly resulted in a transient decrease in Rho activity, suggesting a coupling of both Rac and Rho activities upon PDGF receptor stimulation. However, we cannot exclude that Rac and Rho activities are regulated by separate pathways initiated by stimulation of the PDGF receptor. Stimulation of other receptors for growth factors results in different activation patterns of Rho and Rac proteins (Sander, E.E., J.P. ten Klooster, S. van Delft, A. van der Kammen, and J.G. Collard, unpublished results), thereby mediating different cellular responses depended on the type of receptor stimulated.
Similar mechanisms of opposing Rac and Rho activities as seen in fibroblasts seem to operate in other cell types as well, as deduced from cytoskeletal changes specific for Rac and Rho. Stimulation of C3H10T1/2 murine fibroblasts with EGF results in the formation of membrane ruffles, indicative of Rac activation, and at the same time in the dissolution of F-actin stress fibers, indicative of Rho inactivation (Chang et al. 1995). In neuronal N1E-115 cells, both Rac and Rho GTPases have been proposed to counteract each other's activities (Kozma et al. 1997; Van Leeuwen et al. 1997, Van Leeuwen et al. 1999). Rac activation promotes a loss of contractility associated with increased cell spreading, whereas activation of Rho by LPA promotes contraction and cell rounding (Van Leeuwen et al. 1997). These phenotypic changes have been attributed to Rac-induced myosin heavy chain phosphorylation (Van Leeuwen et al. 1999). Moreover, Rac has been suggested to negatively influence Rho activity since Rac-expressing neuronal cells are refractile to LPA stimulation, and expression of V14Rho is able to overcome this defect (Van Leeuwen et al. 1997). This is consistent with our biochemical data, demonstrating that Rac mediates downregulation of Rho activity and that activation of Rac inhibits LPA-mediated stimulation of Rho, indicating the desensitization of receptor-mediated Rho activation by Rac (Fig. 3 C). In fibroblasts, this Rac-mediated downregulation of Rho activity in Tiam1-expressing cells is reflected in a fine network of thin stress fibers, which are less bundled and correspond to small focal contacts, when compared with wild-type cells (Fig. 6). Upon integrin-mediated cell substrate adhesion, Rac and Rho activities might similarly have opposing roles. Rho activity in fibroblasts plated on fibronectin is low within approximately the first 15 min of cell spreading, followed by an increase of Rho activity (Ren et al. 1999). The initial low Rho activity could be the result of Rac activation, as Rac is activated early upon integrin-mediated adhesion (Price et al. 1998).
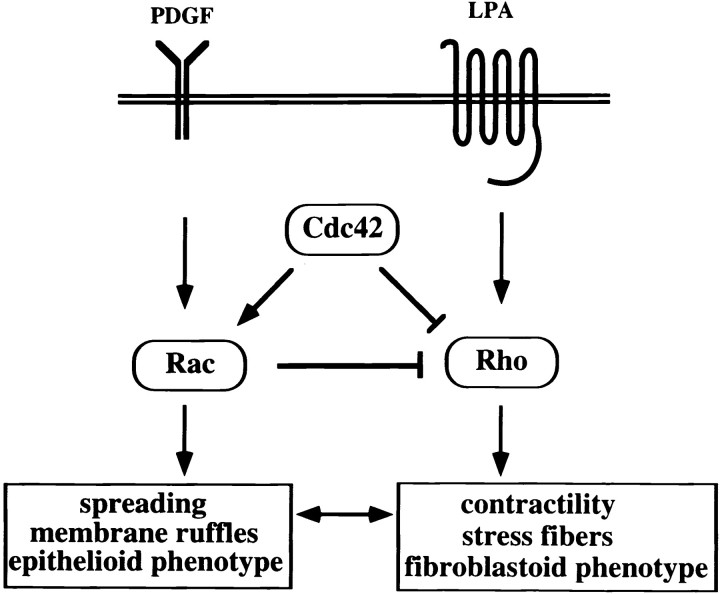
Rac-mediated downregulation of Rho occurred downstream of Rac. This signal regulates Rho activity upstream of Rho since low levels of GTP-bound Rho were found. The biochemical analysis of the actual GTPase activities in NIH3T3 fibroblasts supports a model (Fig. 11) that Rac and Rho signaling antagonize each other to control the cellular phenotype and migratory behavior. Activation of Rac induces cell spreading, resulting in an epithelioid phenotype. Activation of Rho promotes a more fibroblastoid, motile phenotype because of enhanced contractility by the formation of stress fibers. Note that for an epithelioid as well as for a migratory phenotype, the activities of both Rac and Rho GTPases are required, but the balance of their reciprocal activities determines the cellular morphology and the migratory behavior. At the molecular level, this is controlled by the negative regulation of Rho activity involving Cdc42 and Rac signaling. Activity of the distinct GTPases might be regulated locally in cells in response to extracellular stimuli, allowing cellular migration by coordinated activation/inactivation of Rho-like proteins. Our current research is focusing on the signaling pathways downstream of Cdc42 and Rac involved in downregulation of Rho. Complex formation and/or activity of regulators of Rho, including GAP, GEF, and GDI proteins, might be altered. p190RhoGAP may be a good candidate involved in Rac-mediated downregulation of Rho, as p190 becomes phosphorylated on tyrosine after EGF stimulation (Chang et al. 1995) and β1 integrin signaling (Nakahara et al. 1998). A PKC-mediated pathway could also play a role, since in Swiss 3T3 fibroblast, LPA-induced stress fiber formation is inhibited by short pretreatment with PDGF or the phorbol ester PMA. Stress fibers induced by microinjected V14Rho are not sensitive to PMA treatment (Ridley and Hall 1994). In light of the present data, Rac might activate a PMA-sensitive isoform of PKC, thereby negatively regulating Rho activity. As phosphorylation of the myosin light chain by Rho contributes to increased contractility (Amano et al. 1996; Kimura et al. 1996), a consequence of Rac-induced downregulation of Rho might be the inhibition of myosin light chain phosphorylation leading to loss of cellular contraction and allowing cell spreading. Taken together, we conclude that the molecular crosstalk of different Rho family GTPases, specifically Rac-mediated downregulation of Rho activity, determines cellular morphology and migratory behavior of NIH3T3 fibroblasts.
Figure 11.
Model showing the crosstalk of Rho-like GTPases regulating cell spreading and contractility. Constitutively active V12Rac or PDGF receptor-mediated Rac activation results in inactivation of Rho activity. Cdc42 similarly mediates inactivation of Rho, either by activation of Rac or by signaling directly to Rho. LPA stimulation activates Rho, but does not affect Rac activity. Moreover, in cells exhibiting high Rac activity (Tiam1- or V12Rac-expressing cells), LPA-mediated activation of Rho is desensitized. The Rac-mediated regulation of Rho activity might involve GAP, GEF or GDI proteins, as Rho-GTP levels are reduced by activation of Rac.
Acknowledgments
We thank A. Hall for the gift of the Rac effector mutants; J. Chernoff and G. Bokoch for providing Pak1 constructs; L. Oomen and N. Ong for preparing figures; and Drs. T. Reid, L. Price, and F. Van Leeuwen (all from the Netherlands Cancer Institute, Division of Cell Biology) for stimulating discussions.
This work is supported by grants of the Dutch Cancer Society and The Netherlands Scientific Research Organization to J.G. Collard. E.E. Sander is supported by a fellowship from the Deutsche Forschungsgemeinschaft (SA 763/1-1).
Footnotes
Abbreviations used in this paper: DH, dbl homology; GAP, GTPase-activating protein; GEF, guanine nucleotide exchange factor; GST, glutathione-S-transferase; HA, hemagglutinin; HGF, hepatocyte growth factor; PAK-CD, PAK-CRIB domain; PH, Pleckstrin homology.
References
- Anand-Apte B., Zetter B.R., Viswanathan A., Qiu R.-G., Cheng J., Ruggieri R., Symons M. Platelet-derived growth factor and fibronectin-stimulated migration are differentially regulated by the Rac and extracellular signal-regulated kinase pathways. J. Biol. Chem. 1997;272:30688–30692. doi: 10.1074/jbc.272.49.30688. [DOI] [PubMed] [Google Scholar]
- Amano M., Ito M., Kimura K., Fukata Y., Chihara K., Nakano T., Matsuura Y., Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J. Biol. Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Braga V.M.M., Machesky L.M., Hall A., Hotchin N.A. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell–cell contacts. J. Cell Biol. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum R., Telliez J.B., Goonesekera S., Feig L.A. The N-terminal pleckstrin, coiled-coil, and IQ domains of the exchange factor Ras-GRF act cooperatively to facilitate activation by calcium. Mol. Cell. Biol. 1996;16:4888–4896. doi: 10.1128/mcb.16.9.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J.H., Satinder G., Settleman J., Parsons S.J. c-Src regulates the simultaneous rearrangement of actin cytoskeleton, p190RhoGAP, and p120RasGAP following epidermal growth factor stimulation. J. Cell Biol. 1995;130:355–368. doi: 10.1083/jcb.130.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza-Schorey C., Boettner B., van Aelst L. Rac regulates integrin-mediated spreading and increased adhesion of T lymphocytes. Mol. Cell. Biol. 1998;18:3936–3946. doi: 10.1128/mcb.18.7.3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habets G.G.M., Scholtes E.H.M., Zuydgeest D., van der Kammen R.A., Stam J.C., Berns A., Collard J.G. Identification of an invasion-inducing gene, Tiam-1, that encodes a protein with homology to GDP-GTP exchangers for Rho-like proteins. Cell. 1994;77:537–549. doi: 10.1016/0092-8674(94)90216-x. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hordijk P.L., ten Klooster J.P., van der Kammen R.A., Michiels F., Oomen L.C., Collard J.G. Inhibition of invasion of epithelial cells by Tiam1-Rac signaling. Science. 1997;278:1464–1466. doi: 10.1126/science.278.5342.1464. [DOI] [PubMed] [Google Scholar]
- Hoshino M., Sone M., Fukata M., Kuroda S., Kaibuchi K., Nabeshima Y., Hama C. Identification of the stef gene that encodes a novel guanine nucleotide exchange factor specific for Rac1. J. Biol. Chem. 1999;274:17837–17844. doi: 10.1074/jbc.274.25.17837. [DOI] [PubMed] [Google Scholar]
- Jalink K., van Corven E.J., Hengeveld T., Morii N., Narumiya S., Moolenaar W.H. Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J. Cell Biol. 1994;126:801–810. doi: 10.1083/jcb.126.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joneson T., McDonough M., Bar-Sagi D., van Aelst L. RAC regulation of actin polymerization by a pathway distinct from Jun kinase. Science. 1996;274:1374–1376. doi: 10.1126/science.274.5291.1374. [DOI] [PubMed] [Google Scholar]
- Keely P.J., Westwick J.K., Whitehead I.P., Der C.J., Parise L.V. Cdc42 and Rac1 induce integrin-mediated cell motility and invasiveness through PI(3)K. Nature. 1997;390:632–635. doi: 10.1038/37656. [DOI] [PubMed] [Google Scholar]
- Kimura K., Ito M., Amano M., Chihara K., Fukata Y., Nakafuku M., Yamamori B., Feng J., Nakano T., Okawa K., Iwamatsu A., Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Kinsella T.M., Nolan G.P. Episomal vectors rapidly and stably produce high-titer recombinant retrovirus. Hum. Gene Ther. 1996;7:1405–1413. doi: 10.1089/hum.1996.7.12-1405. [DOI] [PubMed] [Google Scholar]
- Kozma R., Ahmed S., Best A., Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol. Cell. Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozma R., Sarner S., Ahmed S., Lim L. Rho family GTPases and neuronal growth cone remodelingrelationship between increased complexity induced by Cdc42Hs, Rac1 and acetylcholine and collapse induced by RhoA and lysophosphatidic acid. Mol. Cell Biol. 1997;17:1201–1211. doi: 10.1128/mcb.17.3.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda S., Fukata M., Fujii K., Nakamura T., Izwa I., Kaibuchi K. Regulation of cell-cell adhesion of MDCK cells by Cdc42 and Rac1 small GTPases. Biochem. Biophys. Res. Commun. 1997;17:430–435. doi: 10.1006/bbrc.1997.7675. [DOI] [PubMed] [Google Scholar]
- Lamarche N., Tapon N., Stowers L., Burbelo P.D., Aspenström P., Bridges T., Chant J., Hall A. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell. 1996;87:519–529. doi: 10.1016/s0092-8674(00)81371-9. [DOI] [PubMed] [Google Scholar]
- Lin R., Bagrodia S., Cerione R., Manor D. A novel Cdc42Hs mutant induces cellular transformation. Curr. Biol. 1997;7:794–797. doi: 10.1016/s0960-9822(06)00338-1. [DOI] [PubMed] [Google Scholar]
- Michiels F., Habets G.G.M., Stam J.C., van der Kammen R.A., Collard J.G. A role for Rac in Tiam1-induced membrane ruffling and invasion. Nature. 1995;375:338–340. doi: 10.1038/375338a0. [DOI] [PubMed] [Google Scholar]
- Michiels F., Stam J.C., Hordijk P.L., van der Kammen R.A., Ruuls-Van Stalle L., Feltkamp C.A., Collard J.G. Membrane localization of Tiam1 is required for Rac-mediated membrane ruffling and JNK activation. J. Cell Biol. 1997;137:387–398. doi: 10.1083/jcb.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara H., Mueller S.C., Nomizu M., Yamada Y., Yeh Y., Chen W.T. Activation of β1 integrin signaling stimulates tyrosine phosphorylation of p190RhoGAP and membrane-protrusive activities at invadopodia. J. Biol. Chem. 1998;273:9–12. doi: 10.1074/jbc.273.1.9. [DOI] [PubMed] [Google Scholar]
- Nobes C.D., Hall A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Pasteris N.G., Cadle A., Logie L.J., Porteous M.E., Schwartz C.E., Stevenson R.E., Glover T.W., Wilroy R.S., Gorski J.L. Isolation and characterization of the faciogenital dysplasia (Aarskog-Scott syndrome) genea putative Rho/Rac guanine nucleotide exchange factor. Cell. 1994;79:669–678. doi: 10.1016/0092-8674(94)90552-5. [DOI] [PubMed] [Google Scholar]
- Price L.S., Leng J., Schwartz M.A., Bokoch G. Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol. Biol. Cell. 1998;9:1863–1871. doi: 10.1091/mbc.9.7.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid T., Furuyashiki T., Ishizaki T., Watanabe G., Watanabe N., Fujisawa K., Morii N., Madaule P., Narumiya S. Rhotekin, a new putative target for Rho bearing homology to a serine/threonine kinase, PKN, and rhophilin in the rho-binding domain. J. Biol. Chem. 1996;271:13556–13560. doi: 10.1074/jbc.271.23.13556. [DOI] [PubMed] [Google Scholar]
- Reid T., Bathoorn A., Ahmadian M.R., Collard J.G. Identification and characterization of hPEM-2, a guanine nucleotide exchange factor specific for Cdc42. J. Biol. Chem. 1999;274:33587–33593. doi: 10.1074/jbc.274.47.33587. [DOI] [PubMed] [Google Scholar]
- Ren X.-D., Kiosses W.B., Schwartz M.A. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A.B., Daniel J.M., Mo Y.-Y., Wu J., Zhang Z. The novel catenin p120CAS binds classical cadherins and induces an unusual morphological phenotype in NIH3T3 fibroblasts. Exp. Cell Res. 1996;225:328–337. doi: 10.1006/excr.1996.0183. [DOI] [PubMed] [Google Scholar]
- Ridley A.J., Hall A. The small GTP-binding protein Rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley A.J., Hall A. Signal transduction pathways regulating Rho-mediated stress fibre formationrequirement for a tyrosine kinase. EMBO (Eur. Mol. Biol. Organ.) J. 1994;13:2600–2610. doi: 10.1002/j.1460-2075.1994.tb06550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley A.J., Paterson H.F., Johnston C.L., Diekmann D., Hall A. The small GTP-binding protein Rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Ridley A.J., Comoglio P.M., Hall A. Regulation of scatter factor/hepatocyte growth factor responses by Ras, Rac and Rho in MDCK cells. Mol. Cell Biol. 1995;15:1110–1122. doi: 10.1128/mcb.15.2.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottner K., Hall A., Small J.V. Interplay between Rac and Rho in the control of substrate dynamics. Curr. Biol. 1999;9:640–648. doi: 10.1016/s0960-9822(99)80286-3. [DOI] [PubMed] [Google Scholar]
- Sander E.E., van Delft S., ten Klooster J.P., Reid T., van der Kammen R.A., Michiels F., Collard J.G. Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes either cell–cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J. Cell Biol. 1998;143:1385–1398. doi: 10.1083/jcb.143.5.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders L.C., Matsamura F., Bokoch G.M., de Lanerolle P. Inhibition of myosin light chain kinase by p21-activated kinase. Science. 1999;283:2083–2085. doi: 10.1126/science.283.5410.2083. [DOI] [PubMed] [Google Scholar]
- Sells E., Knaus U.G., Bagrodia S., Ambrose D.M., Bokoch G.M. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr. Biol. 1997;7:202–210. doi: 10.1016/s0960-9822(97)70091-5. [DOI] [PubMed] [Google Scholar]
- Sells M.A., Boyd J.T, Chernoff J. P21-activated kinase 1 (Pak1) regulates cell motility in mammalian fibroblasts. J. Cell Biol. 1999;145:837–849. doi: 10.1083/jcb.145.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G. The pleckstrin homology domainan intriguing multifunctional protein module. Bioessays. 1996;18:35–46. doi: 10.1002/bies.950180109. [DOI] [PubMed] [Google Scholar]
- Shaw L.M., Rabinovitz I., Wang H.H.-F., Toker A., Mercurio A.M. Activation of phosphoinositide 3-OH kinase by the α6β4 integrin promotes carcinoma invasion. Cell. 1997;19:949–960. doi: 10.1016/s0092-8674(00)80486-9. [DOI] [PubMed] [Google Scholar]
- Shou C., Farnsworth C.L., Neel B.G., Feig L.A. Molecular cloning of cDNAs encoding a guanine-nucleotide-releasing factor for Ras p21. Nature. 1992;358:351–354. doi: 10.1038/358351a0. [DOI] [PubMed] [Google Scholar]
- Sone M., Hoshino M., Suzuki E., Kuroda S., Kaibuchi K., Nakagoshi H., Saigo K., Nabeshima Y., Hama C. Still life, a protein in synaptic terminals of Drosophila homologous to GDP-GTP exchangers. Science. 1997;275:543–547. doi: 10.1126/science.275.5299.543. [DOI] [PubMed] [Google Scholar]
- Stam J.C., Sander E.E., Michiels F., van Leeuwen F.N., Kain H.E.T., van der Kammen R.A., Collard J.G. Targeting of Tiam1 to the plasma membrane requires the cooperative function of the N-terminal pleckstrin homology domain and an adjacent protein interaction domain. J. Biol. Chem. 1997;272:28447–28454. doi: 10.1074/jbc.272.45.28447. [DOI] [PubMed] [Google Scholar]
- Stam J.C., Michiels F., van der Kammen R.A., Moolenaar W.H., Collard J.G. Invasion of T-lymphoma cellscooperation between Rho family GTPases and lysophospholipid receptor signaling. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:4066–4074. doi: 10.1093/emboj/17.14.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaishi K., Sasaki T., Kotani H., Nishioka H., Takai Y. Regulation of cell–cell adhesion by Rac and Rho small G proteins in MDCK cells. J. Cell Biol. 1997;139:1047–1059. doi: 10.1083/jcb.139.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Aelst L., D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- Van Leeuwen F.N., van der Kammen R.A., Habets G.G.M., Collard J.G. Oncogenic activity of Tiam1 and Rac1 in NIH3T3 cells. Oncogene. 1995;11:2215–2221. [PubMed] [Google Scholar]
- Van Leeuwen F.N., Kain H.E.T., van der Kammen R.A., Michiels F., Kranenburg O.W., Collard J.G. The guanine nucleotide exchange factor Tiam1 affects neuronal morphologyopposing roles for the small GTPases Rac and Rho. J. Cell Biol. 1997;139:797–807. doi: 10.1083/jcb.139.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leeuwen F.N., van Delft S., Kain H.E.T., van der Kammen R.A., Collard J.G. Rac regulates phosphorylation of the myosin-II heavy chain, actinomyosin disassembly and cell spreading. Nat. Cell Biol. 1999;1:242–248. doi: 10.1038/12068. [DOI] [PubMed] [Google Scholar]
- Zhong C., Kinch S.M., Burridge K. Rho-stimulated contractility contributes to the fibroblastic phenotype of Ras-transformed epithelial cells. Mol. Biol. Cell. 1997;8:2329–2344. doi: 10.1091/mbc.8.11.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]