Introduction
Immunoglobulin (Ig) gene rearrangements in developing B lymphocytes generate a diverse repertoire of B cells, each with a distinct specificity for antigen. This immunoglobulin is initially expressed in a membrane form that serves as an antigen receptor, the B cell antigen receptor (BCR), on the surface of the B cell. The BCR serves key roles in directing the activation of B cells that make antibodies reactive to infecting microbes and viruses, and also in tolerizing B cells capable of making antibodies reactive to self-components. Although BCR engagement is typically required for a mature B cell to leave the quiescent state and become activated to proliferate and differentiate into an antibody-secreting plasma cell, when the BCR is engaged on an immature B cell, the cell makes one or more responses that either change the specificity of the cell (“receptor editing”), lead to its rapid death (“clonal deletion”) or reduce its responsiveness to the BCR (“clonal anergy”). These responses serve to prevent self-reactive B cells from secreting autoantibodies in most cases. B cell activation also requires a variety of other signals coming from cytokines and cell-bound stimulatory factors made by helper T cells. In this review, we discuss the role of lipid rafts in the activation of B cells. Our major focus is on the role of lipid rafts in the signaling by the BCR and how this is impacted by other cell surface receptors of B cells that either promote or inhibit B cell activation. In addition, we discuss the role of lipid rafts in endocytosis of antigen via the BCR, a process that is essential for interactions with T cells that provide key helper signals for complete activation and differentiation of B cells into antibody-secreting plasma cells. Finally, we describe recent insights into how the actin cytoskeleton might regulate lipid raft dynamics, and discuss scenarios wherein the loss of certain components of BCR signaling from lipid rafts might contribute to the development and/or progression of human diseases.
BCR signaling and membrane rafts
The BCR is a complex between a membrane-bound Ig (mIg) molecule and a disulfide-linked heterodimer of two polypeptides responsible for signaling and endocytosis, called Igα and Igβ. Membrane and secreted forms of Ig are generated by differential splicing events that incorporate or leave out exons encoding a transmembrane domain and a very short cytoplasmic domain at the C-terminus of the Ig heavy chain. The resulting mIg must assemble with an Igα/Igβ heterodimer in order to leave the endoplasmic reticulum and traffic to the cell surface. Igα and Igβ each contain an amino acid motif that is also found in other activating receptors of T cells, natural killer cells, and phagocytes, called the Immunoreceptor Tyrosine-based Activation Motif (ITAM), the consensus sequence of which is D/ExxYxxL/Ix7YxxL/I. The most widely accepted model of ITAM signaling holds that receptor engagement leads to 1) phosphorylation of both tyrosines of the ITAM by Src-family tyrosine kinases, 2) recruitment of the cytosolic protein tyrosine kinases Syk or ZAP-70 to the phosphorylated ITAMs by binding of their tandem SH2 domains to the two phosphoYxxL/I halves of the ITAM, 3) increases in activity of Syk or ZAP-70, the latter expressed primarily in T cells and natural killer cells, resulting from ITAM binding and/or tyrosine phosphorylation of these kinases, perhaps by Src-family kinases, and 4) phosphorylation of adaptor molecules and signaling enzymes by Syk or ZAP-70, and perhaps in some cases by Src-family kinases. The localization of Src-family kinases in the membrane and their basal activity likely promotes BCR ITAM and subsequent phosphorylations at a low level prior to ligand binding to the BCR, a process referred to as tonic signaling (1); these signaling reactions are countered by protein tyrosine phosphatases, perhaps including SHP-1 (2).
Ligand-induced clustering of the BCR greatly increases ITAM phosphorylation, Syk recruitment and downstream signaling, which represents the way in which the information about ligand binding is transmitted to the inside of the cell. Exactly how BCR binding to antigen increases the biochemical sequence of events outlined above is not fully understood, although the clustering of BCR ITAMs and their association with active Syk and Src-family kinases, which also bind to phosphorylated ITAMs via their SH2 domains (3), can keep them in the phosphorylated state. This likely amplifies signaling of clustered BCRs beyond the low level of tonic signaling.
It has been hypothesized that lipid rafts, also called “membrane rafts”, facilitate amplification of BCR signaling after ligand binding. According to a recent definition, membrane rafts are small (10–200 nm in diameter), heterogenous, highly dynamic, sterol- and sphingolipid-enriched domains that compartmentalize cellular processes. Small rafts can sometimes be stabilized to form larger platforms through protein-protein and protein-lipid interactions (4). Prior to stimulation, the unbound BCR is present in the non-membrane raft fraction of the plasma membrane, whereas BCR clustering rapidly leads to BCR association with the membrane raft fraction, as determined by biochemical isolation following cold non-ionic detergent extraction of the cells and sucrose-density gradient fractionation (5). Co-localization of clustered BCR molecules with membrane rafts has been directly visualized by fluorescence microscopy both in cell lines and in primary B cells stimulated with antigen (6). In this study, membrane rafts were visualized either by staining the ganglioside GM1 or by use of a fusion protein containing green fluorescent protein (GFP) fused to the N-terminal 24 amino acids of Lyn, which contains the N-terminal myristoylation and palmitoylation sequences of this Src-family kinase but lacks its protein-protein interaction and kinase domains and thus does not form any known associations with other proteins. This study also demonstrated that Syk was recruited to BCR clusters associated with membrane rafts and that cellular protein tyrosine phosphorylation was enhanced at regions of the membrane enriched in membrane rafts. These results suggest that BCR signaling is enhanced upon its association with membrane rafts.
Recently these studies were extended by use of fluorescence resonance energy transfer (FRET), which detects very close molecular interactions in living cells. With this technique, it was observed that the BCR associates with membrane rafts within seconds of being clustered (7). In these studies, the FRET donor was cyan fluorescent protein (CFP) linked to the C-terminus of Igα and the FRET acceptor was yellow fluorescent protein (YFP) fused to the first sixteen amino acids of Lyn, which is a reporter for the membrane rafts that contain Src-family kinases, similar to the Lyn-based reporter described above. Interestingly, FRET was not seen between Igα and a membrane reporter containing the C-terminal isoprenylation motif of Rho, indicating that the latter is not localized to the membrane rafts that associate with clustered BCRs. Similarly, a membrane reporter derived from the N-terminus of Src, which lacks the palmitoylation site found in Lyn, also did not exhibit an increase in FRET upon BCR clustering. The FRET between the N-terminal Lyn-YFP reporter and the BCR appeared to precede the increase in intracellular calcium induced by BCR signaling, suggesting that it reflects a very early event in the BCR signaling cascade. Interestingly, this FRET was blocked by the Src-family tyrosine kinase inhibitor PP2, indicating that phosphorylation of ITAMs by Src-family tyrosine kinases in some way induces association of the BCR with membrane rafts. The mechanism by which this occurs is not yet defined, but since it has been shown that Lyn, Fyn and Blk associate with phosphorylated BCR ITAMs (3), and these kinases are constitutively associated with membrane rafts, an attractive idea is that after these kinases phosphorylate BCR ITAMs, they bind to the phosphorylated BCR, and induce its association with membrane rafts. It should be noted that the translocation of the BCR to membrane rafts, as assessed by biochemical isolation of detergent-resistant membrane rafts, was not blocked by the same Src-family kinase inhibitor (8). The explanation for this discrepancy between the requirement for Src-kinase activity for BCR association with membrane rafts as assessed by biochemical isolation vs. FRET is not clear.
An interesting feature of the FRET observed between the BCR and the membrane raft reporter is that it peaked after just 20 seconds and decreased to an intermediate value soon thereafter. This behavior was not affected by addition of latrunculin A to block actin polymerization. The decrease in FRET after its initial rise could reflect a change in the nature of the membrane environment or alternatively could reflect a loss of FRET due to the build up of multiprotein signaling complexes at the receptor and consequent crowding out of the FRET reporter. Although FRET studies have substantial advantages in terms of avoiding the potential artifacts associated with detergent extraction methods, and have excellent spatial and temporal resolution, clearly some of the phenomena demonstrated by FRET need to be explained in terms of what protein-protein or protein-lipid associations are responsible for which changes in FRET.
Membrane rafts have been especially associated with activation of the transcription factor NF-κB by the BCR in B cells and by the TCR in T cells (9). Activation of NF-κB in response to many receptors typically involves a common final step in which the I-κB kinase (IKK) complex phosphorylates the inhibitory subunit I-κB, inducing the latter’s ubiquitinylation and degradation and releasing NF-κB to translocate to the nucleus and activate transcription. Upstream events differ between different classes of receptors, however, and the BCR and TCR uniquely signal to this pathway via the scaffold molecule caspase recruitment domain membrane-associated guanylate kinase protein 1 (CARMA1). Signaling-induced conversion of CARMA1 from an inactive to active conformation or oligomeric state nucleates a large protein complex between CARMA1, Bcl-10 and MALT1, which then recruits other components of the NF-κB pathway, including TRAF2 and TRAF6. BCR signaling induces assembly of this signaling complex via protein kinase Cβ-mediated phosphorylation of CARMA1 (9). This complex appears to form at the plasma membrane preferentially in regions of coalesced membrane rafts (10, 11). Extraction of cholesterol with methyl β-cyclodextrin to disrupt membrane rafts, however, apparently does not prevent NF-κB activation via CARMA1 (12), suggesting that this signaling event can occur outside membrane rafts with reasonable efficiency.
Co-receptors for BCR signaling
An important feature of lymphocyte antigen receptor signaling is the participation of additional receptors on the cell surface that may also interact with the same ligand and contribute to lymphocyte activation. These receptors are referred to as co-receptors since they cooperate with the antigen receptors to promote biological responses. For example, T cells express either of two co-receptors, CD4 or CD8, which bind to conserved regions of class II or class I MHC molecules, respectively. When the TCR contacts an MHC-peptide complex that matches the specificity of the co-receptor on that type of T cell, a trimolecular complex is formed between the MHC-peptide complex, the TCR and the CD4 or CD8 co-receptor. The co-receptor helps to stabilize the binding of the TCR to its ligand, and in addition it provides a complementary signaling function. CD4 and CD8 are bound to the Src-family kinase Lck, and therefore co-receptor engagement with the MHC brings Lck next to the cytoplasmic ITAMs of that TCR, where it can efficiently phosphorylate them. B cells have two types of co-receptors, those that promote BCR signaling analogously to CD4 and CD8 in T cells, and those that decrease BCR signaling and for that reason are often called inhibitory co-receptors.
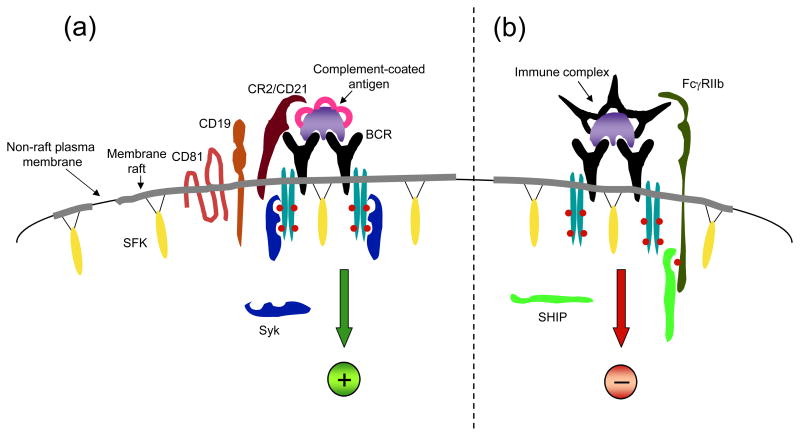
An important positive-acting co-receptor for B cells is complement receptor 2 (CR2, also called CD21), which is present on the cell surface in a complex with two other transmembrane proteins CD19 and CD81. When a protein antigen has complement fragments derived from C3b attached to it, indicating recognition of its status as foreign by elements of the immune system, B cell activation is enhanced by up to 10,000-fold (13). In this circumstance, the CR2 subunit binds to the complement fragment attached to the antigen and co-clusters the CR2/CD19/CD81 complex adjacent to the BCR (Fig. 1a). The CD19 subunit plays a critical role in promoting BCR signaling by virtue of its adaptor molecule function. Multiple tyrosines within the cytoplasmic tail of CD19 become phosphorylated when the CR2 complex is clustered with the BCR and these tyrosines serve as binding sites for phosphatidylinositol 3′-kinase (PI 3′-kinase), for Lyn, and for Vav, which is a guanine nucleotide exchange factor for Rac. Of these, the two binding sites for PI 3′-kinase are especially important for promoting B cell activation (14, 15). Production of phosphatidylinositol 3,4,5-trisphosphate (PIP3) by PI 3′-kinase is essential to many of the BCR signaling reactions. In addition to activating Akt, PIP3 recruits the PH domain-containing proteins Btk and phospholipase Cγ2 (PLCγ2), leading to phosphatidylinositol 4,5-bisphosphate (PIP2) hydrolysis. PIP2 hydrolysis by PLCγ2 generates the second messengers, inositol 1,4,5 trisphosphate (IP3) and diacylglycerol. As in other cell types, IP3 induces calcium elevation by acting on IP3-receptors in the endoplasmic reticulum and inducing release of calcium. Once the ER has fully released its calcium, it sends an unidentified signal to the plasma membrane that opens the store-operated calcium channels, which in B cells are in some way regulated by the B cell-specific tetraspanin protein CD20 that also associates with membrane rafts (16).
Figure 1. BCR activation and feedback inhibition occur in membrane rafts.
(a) Upon binding to complement-coated antigen, the BCR associates with membrane rafts, and comes in proximity to Src family kinases (SFKs) that phosphorylate ITAM tyrosine residues (red filled circles) in the Igα/Igβ chains. Phosphorylated ITAMs recruit Syk which phosphorylates many signaling substrates resulting in B cell activation. The co-receptor CR2/CD21 recognizes the complement component of the antigen and associates with CD19 and CD81, which provide an adaptor function and promote membrane raft association, respectively. (b) Feedback inhibition of B cell responses occurs upon immune complex-mediated co-ligation of the BCR with the inhibitory FcγRIIB receptor. In this circumstance, FcγRIIB is phosphorylated on ITIM tyrosine by the SFK Lyn and recruits SHIP which is a cytosolic inositol phosphatase that abrogates PI 3′-kinase and Ras signaling, thus dampening B cell activation.
The other second messenger resulting from PIP2 hydrolysis is diacylglycerol, a membrane-bound second messenger that activates various protein kinase C isoforms and also RasGRP, a guanine nucleotide exchange factor for Ras (17, 18). Protein kinase C isoforms are responsible for activation of the transcription factor NF-κB, as described above, among other events (9).
The CD81 subunit of the CR2 complex is also important for BCR signaling. CD81 is a tetraspan protein and its function in the CR2 co-receptor complex appears to be to stabilize association of the BCR with membrane rafts and in that way promote prolonged signaling (19). Interestingly, the CR2 complex does not appear to associate with membrane rafts prior to engagement, as judged by the detergent solubilization approach. When the BCR and the CR2 complex are co-clustered, BCR partitioning into the detergent-insoluble membrane raft fraction is prolonged. The ability of the CR2 complex to promote prolonged association of the BCR with membrane rafts has been confirmed by FRET measurements using the N-terminal Lyn-YFP FRET acceptor (7). This property of the CR2 complex is dependent on the inclusion of CD81 in the complex, since CD81-deficient B cells fail to promote BCR association with membrane rafts and a chimeric form of CD19 that does not associate with CD81 is similarly ineffective (20). Interestingly, co-ligation of the BCR and the CR2 complex also causes a rapidly inducible palmitoylation of CD81. The inducibly palmitoylated CD81 molecules are strongly enriched in the detergent-insoluble membrane raft fraction of cells, suggesting that the inducible palmitoylation of CD81 is important for maintaining co-ligated BCR and CR2 in membrane rafts.
The best understood negative co-receptor of B cells is the inhibitory Fc receptor FcγRIIB (21). When antigen is present in a complex with antigen-specific IgG molecules, it can bind both the BCR and FcγRIIB and co-cluster them (Fig. 1b). This results in phosphorylation of a single tyrosine in the cytoplasmic tail of FcγRIIB in a sequence also seen in other immune inhibitory receptors and referred to as the Immunoreceptor Tyrosine-based Inhibitory Motif (ITIM). This phosphorylated ITIM then binds to the SH2 domain-containing inositol phosphatase (SHIP), which converts PIP3 to phosphatidylinositol 3,4-bisphosphate, thereby attenuating most features of PI 3′-kinase signaling. FcγRIIB appears to localize to membrane rafts upon co-ligation with the BCR (22), although how this effect is mediated is not known. The significance of this association will be discussed in a later section.
Developmental regulation of BCR signaling
The participation of membrane rafts in BCR signaling is developmentally regulated. During early development of B cells in the bone marrow, ITAM signaling is required for the transition between pro-B cells, which are rearranging the Ig heavy chain locus to create a functional heavy chain, and pre-B cells, which express a heavy chain and are rearranging the Ig light chain loci. Once an Ig heavy chain protein is produced as a consequence of in-frame VDJ recombination, it pairs with two proteins expressed only in developing B cell precursors, collectively called the surrogate light chain, and with the Igα/Igβ signaling heterodimer to form a membrane-bound complex referred to as the pre-BCR. The pre-BCR is thought to signal constitutively (1), but there is some evidence for a pre-BCR ligand on bone marrow stromal cells that may further enhance signaling. In any case, it has been shown that the pre-BCR associates with membrane rafts in B cell precursors as it induces tonic signaling (23). The pro-B cell to pre-B cell transition, which is characterized by changes in expression of cell surface markers, by ability to proliferate in response to the cytokine interleukin 7, and by a change in the targeting of the V(D)J recombinase away from the Ig heavy chain locus and to the Ig light chain loci, can be promoted artificially by membrane-targeted fusion proteins containing ITAM sequences, which are presumed to generate a low level of tonic signaling (1). Interestingly, these ITAM-containing fusion proteins are effective whether they are targeted to the liquid-disordered or to the liquid-ordered membrane raft fraction of the plasma membrane, suggesting that ITAM signaling in B cell precursors can occur outside of membrane rafts as well as inside them.
Once successful rearrangement has occurred at one of the Ig light chain loci, leading to expression of the BCR, the cell is referred to as an immature B cell if it is in the bone marrow and as a transitional B cell after it has left the bone marrow for the spleen and before it has matured to a long-lived mature B cell. Studies with B lymphoma cell lines of the immature or transitional phenotype and with primary immature B cells have observed that BCR clustering in these cells does not result in its co-localization with membrane rafts (24, 25), although substantial BCR signaling can be generated in these cells. Thus, it appears that significant BCR signaling can occur outside of membrane rafts, although in mature B cells there is a correlation between strength of signaling and localization in membrane rafts, as described above. Recently, it has been found that immature B cells have a lower content of cholesterol in their plasma membrane than do mature B cells (26). This is a fascinating observation and it suggests that cholesterol content of B cells is regulated during maturation in the spleen, perhaps to alter either the magnitude of BCR signaling or the relative amounts of different signaling reactions. Further studies will be required to address this issue, but it was shown that supplementation of membrane cholesterol in immature B cells up to the level present in mature B cells, using methyl β-cyclodextrin-cholesterol complexes, was sufficient to cause clustered BCRs to localize with membrane rafts in these immature B cells, demonstrating that the failure of the BCR to associate with membrane rafts was due to the lower cholesterol content of the plasma membrane in immature B cells. Whether an increase in the membrane cholesterol content of immature B cells in vivo would alter the outcome of signaling and the fate of these cells remains to be seen.
Membrane rafts in BCR-dependent antigen uptake and antigen presentation
While the BCR serves to recognize antigen and transduces an activation signal for B cell expansion, it is also responsible for delivering the bound antigen to specific compartments within the cell where the antigen is processed into peptides that are loaded onto MHC class II (MHC-II) molecules, which are then trafficked to the cell surface for presentation to antigen-specific helper T cells. Accumulating evidence suggests that the initial endocytosis of the antigen and MHC-II-associated presentation of antigenic peptides to T cells are both coordinated within membrane raft domains.
Following internalization, the antigen-BCR complexes are delivered to early endosomes from where they move to late endosomes. The Igα/Igβ components of the BCR were shown to be necessary and sufficient for the initial internalization as well as for sorting to the late endosomes (27). Igβ-mutant receptors are retained in early endosomes, whereas those containing only Igβ were shown to go straight from early endosomes to terminal lysosomes and undergo degradation without productive loading of peptides onto MHC-II (27, 28). The contribution of Igβ ITAM residues was further examined more recently in vivo by exchanging the ITAM tyrosines for alanines by gene targeting. The resulting mice (IgβAA) showed a normal development for all B cell subtypes, except for B1 cells which were significantly reduced. Purified B cells from the IgβAA mice showed highly decreased steady state and ligand-mediated BCR internalization. BCR cross-linking resulted in diminished Src family and Syk activation, but elevated and prolonged signaling with respect to Ca2+ flux, total tyrosine phosphorylation, and Akt and Erk activation. This study concluded that the Igβ component of the BCR is responsible for setting a threshold for signaling by regulating receptor internalization, which terminates signaling (29).
The Igα chain of the BCR complex also participates in its internalization. Proper sorting of the BCR-antigen complexes requires the recruitment of the tyrosine kinase Syk to phosphorylated ITAMs on Igα (30, 31). Interestingly, both the ITAM tyrosines (29) and the non-ITAM tyrosines Y176 and Y204 of Igα participate in coordinating the internalization signals. Receptors bearing tyrosine to phenylalanine mutations in the Igα chain are still internalized but do not co-localize with MHC-II-rich internal compartments and could not facilitate antigen presentation to T cells (32). The requirement for both ITAM and non-ITAM tyrosine residues on Igα for efficient and productive internalization of the BCR complexes is further supported by extensive mutational analysis reported in a recent study (33).
While the BCR complex contains sufficient signals for its own internalization and sorting, recent reports suggest that B cells can endocytose via two pathways, internalization via clathrin-coated pits and via a clathrin-independent pathway involving membrane rafts. A significant amount of clathrin heavy chain was found to be constitutively associated with membrane rafts in B cells (34, 35), and it becomes tyrosine phosphorylated upon BCR engagement in a Src family kinase-dependent manner (34). Furthermore, antigen uptake is largely dependent on the association of clathrin with membrane rafts and its phosphorylation at these sites. Chicken DT40 B cells conditionally deficient in clathrin heavy chain showed a marked reduction in their BCR-mediated uptake of antigen. Disruption of membrane rafts by treatment with nystatin caused a reduction similar to that seen with loss of clathrin, whereas treatment with latrunculin A to disrupt the actin cytoskeleton led to a 50% loss in antigen internalization. These data suggest that clathrin, actin and membrane rafts are needed for the most complete endocytosis of BCR-bound antigen, but argue that internalization can still proceed with at least two of these three components intact. However, membrane rafts and the actin cytoskeleton cannot support internalization independently. A complete block in endocytosis of the BCR resulted in increased BCR signaling as assessed by sustained tyrosine phosphorylation and Erk phosphorylation, further supporting the idea that internalization is a means of signal attenuation as well as a means to promote antigen presentation to helper T cells (36). Therefore, the view that has emerged from these studies is that BCR ligation drives its association with membrane rafts already bearing the clathrin heavy chain and enriched in Src family kinases. Both the BCR and clathrin get phosphorylated in this confined space, the BCR localizes to clathrin-coated pits and internalizes along with membrane rafts (34, 37).
In another study, anergic B cells (which are continually binding their antigen) were reported to have an enhanced rate of BCR endocytosis, which was blocked by depleting membrane cholesterol with methyl β-cyclodextrin, suggesting that membrane rafts play a critical role in BCR internalization in these B cells (12). Further evidence for at least two pathways of BCR internalization has recently been presented. In this study, the pathway of BCR endocytosis, as indicated by its sensitivity to membrane raft- and actin-disrupting agents and its dependence on Src and Syk family kinase signaling, was shown to be governed by the nature of the ligand. The internalization of anti-Ig antibody/BCR complexes was dependent on Src and Syk family kinase signaling, the integrity of the actin cytoskeleton and membrane rafts, and these complexes were delivered to early and recycling endosomes. In contrast, the internalization of a model antigen/BCR complex was independent of signaling, membrane rafts and actin and these complexes trafficked to late endosomes, and were targeted for proteolytic processing (38, 39).
A membrane raft-localized transmembrane protein, the linker for activation of B cells/non-T cell activation linker (LAB/NTAL) (40, 41) also appears to play an important role in BCR internalization. BCR cross-linking led to co-internalization of LAB/NTAL along with the receptor and it is the C-terminal tail of LAB/NTAL that is responsible for this effect. Mouse B cells deficient in LAB/NTAL showed a reduction in the ligation-dependent uptake of the BCR (42). To the extent that LAB/NTAL is important for BCR internalization, its localization to membrane rafts may contribute to the importance of these domains for BCR-mediated antigen uptake.
Taken together, the available data suggest that BCR endocytosis is a dynamic and complex process comprised of at least two mechanistically distinct pathways for internalization, and includes additionally regulated steps that control delivery to late endosomes where antigen can be processed and loaded onto MHC-II molecules. Resolution of molecular features of this process will depend on getting a closer look at early events that govern the intracellular fate of the BCR and its association with components of membrane rafts.
The endocytosed BCR is delivered to intracellular compartments where its bound antigen is proteolyzed into antigenic peptides. The peptides are then loaded onto MHC-II molecules, and traffic out to the surface of the B cell from where they can be surveyed by CD4+ T cells bearing the cognate T cell receptors (TCRs). Biochemical fractionation and fluorescence microscopy experiments indicate that not only are the MHC-II molecules constitutively associated with membrane rafts, they are also loaded with antigenic peptides in a concentrated membrane raft environment (43). Indeed, our proteomic analysis of purified membrane rafts from the human B cell line Ramos confirmed that MHC-II molecules associate with membrane rafts and showed that this association is unaffected by ligation of the BCR (35). Constitutive association of MHC-II with membrane rafts has also been observed in human tonsil B cells, in transformed B cell lines, and in human monocytes (44). While it is unclear where in the biosynthetic pathway MHC-II molecules get associated with membrane rafts, both microscopic and biochemical determinations suggest that half of the cell surface MHC-II is associated with membrane rafts. The functional relevance of MHC-II association with membrane rafts is suggested by the fact that raft-associated MHC-II/peptide complexes concentrate in the immunological synapse (IS) and facilitate antigen presentation to T cells. Pharmacological disruption of membrane rafts on the antigen presenting B cells abrogated their recruitment to the IS and T cell activation. However, this was only true under conditions when the numbers of MHC-II/peptide complexes were limiting, indicating that the role of membrane rafts is to concentrate rare MHC-peptide complexes at the IS for recognition by T cells (45). Consistent with this hypothesis, confocal imaging of the B cell side of the IS has revealed that membrane rafts are rapidly enriched in the IS upon B:T conjugation, and that this is an actin-dependent phenomenon. In addition, fluorescence recovery after photobleaching (FRAP) and fluorescence loss in photobleaching (FLIP) experiments showed that the membrane raft proteins in the IS are highly dynamic and rapidly exchange with other membrane compartments of the B cell (46).
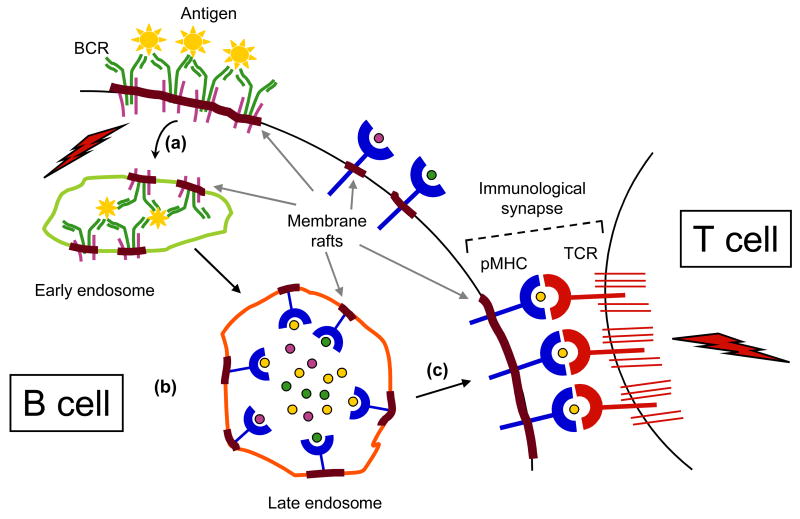
Thus, it appears that lipid rafts play a significant role in the entire journey of an antigen, starting with its initial uptake through the BCR, trafficking through intracellular MHC-II-loading compartments, and finally its presentation to T cells in the immunological synapse (Fig. 2).
Figure 2. Membrane rafts in BCR endocytosis and antigen presentation.
(a) The cross-linked BCR is endocytosed along with bound antigen and membrane rafts. (b) Early endosomes fuse with late endosomes for antigen processing and loading of antigenic peptides onto MHC-II molecules within the membrane raft milieu. (c) Peptide-MHC (pMHC) complexes traffic to the surface and are displayed in association with membrane rafts. Specific peptide-MHC complexes are presented within membrane rafts to cognate TCRs in an immunological synapse (IS) with T cells. Membrane rafts are depicted by grey arrows, and signal transduction via BCR and TCR is indicated by lightning signs.
Cytoskeletal regulation of membrane raft dynamics
As mentioned above, BCR cross-linking induces fusion of smaller membrane raft units to generate membrane raft patches that ultimately coalesce into stable, micron-sized rafts (6). Similar membrane raft coalescence also occurs in other cell types upon interaction of a number of cell surface receptors with their ligands. While the exact mechanism of this large-scale coalescence is unclear, it has been suggested that the actin-based cytoskeleton plays a role in the regulation of membrane raft dynamics (47, 48).
Use of higher resolution imaging tools such as single fluorescent molecule video imaging (SFVI), high-speed single particle tracking (SPT) using colloidal gold probes, and optical trapping, which allows one to move the gold particle-tagged molecules in the living cell membrane, have led to the suggestion that the plasma membrane is divided into submicron-sized compartments, and that the diameter of these compartments varies from 30 to 230 nm. Phospholipid molecules can move to adjacent compartments by undergoing “hop diffusion”, and the average residency time for single molecules within these compartments varies by cell type falling within a broad range of 1–17 ms. Data from optical trapping and FRAP experiments initially led to the membrane skeleton “fence” model that proposed that the actin cytoskeleton forms a meshwork underneath the plasma membrane, and that transmembrane proteins protruding into the cytoplasm collide with the cytoskeleton causing them to be temporarily confined within the skeletal mesh. These interactions create confinement zones. An examination of the effect of membrane skeleton, extracellular matrix, extracellular domains of membrane proteins, and membrane raft domains on hop diffusion subsequently led to the “anchored-protein picket model” whereby various transmembrane proteins anchored directly or indirectly to the membrane skeleton (fence) act as rows of pickets (the transmembrane proteins serve as posts for the fence, hence termed pickets) that serve as diffusion barriers and effectively block free diffusion of phospholipid molecules and non-anchored proteins due to steric hindrance and hydrodynamic friction (49, 50). Thermal fluctuations causing dissociation of actin filaments from membrane attachments are postulated to result in temporary breaks in the barriers, and allow hop diffusion of molecules between adjacent compartments.
The use of biophysical methods to observe diffusion of receptors in the absence or presence of ligands revealed that ligand-induced receptor oligomers exhibit considerably slower diffusion than do the monomers, and that their confinement time within a compartment is significantly increased (51). Based on these findings it was expected that cell stimulation-induced membrane raft coalescence would proceed with smaller membrane rafts fusing to form bigger entities, which would then become trapped within compartments and not continue to consolidate. Furthermore, the hydrodynamic friction is expected to be higher in the membrane raft regions due to their ordered nature, further limiting their movement. In B cells, however, binding of antigen to the BCR induces coalescence of membrane rafts within minutes until most or all of them aggregate at one pole of the cell along with the BCR cap (6).
Interestingly, BCR stimulation leads to a rapid global depolymerization of actin followed by new actin polymerization. It was postulated that actin depolymerization and/or remodeling could promote an increase in BCR signaling. Indeed, actin depolymerization was shown to enhance BCR signaling, with increase in sustained Ca2+ elevation, phosphorylation of Erk and activation of transcription factors including NFAT and NF-κB as well as increased BCR and membrane raft clustering. Blockade of actin depolymerization had the opposite effect (52). Apparently, the actin depolymerization breaks down the diffusion barriers and allows both ligand-clustered BCR complexes and membrane rafts to coalesce and be mobilized to one pole of the cell. These data suggest that the actin cytoskeleton actively keeps the membrane rafts in a dispersed state in the absence of BCR stimulation to limit tonic signaling to a low level. Thus, BCR-induced remodeling of the actin cytoskeleton may serve to increase the strength and duration of BCR signals facilitating efficient B cell activation.
Recent studies have provided insight into the molecular mechanism of coupling plasma membrane rafts with the actin cytoskeleton. We employed a tandem mass spectrometry and isotope-coded affinity tag (ICAT)-based proteomics approach to examine changes in the protein composition of membrane rafts during B cell activation as a means to identify positive and negative regulators of membrane raft coalescence (35). This approach led to the identification of several proteins that change their relative abundance in purified membrane raft fractions in a BCR stimulation-dependent manner. A majority of these proteins are known to act as modifiers of the actin cytoskeleton, including the ERM family member ezrin, the non-muscle myosin Myh9, and the myosin regulatory light chain. While Myh9 and myosin regulatory light chain inducibly associated with membrane rafts, ezrin was found to dissociate from these domains. Consistent with the latter observation, BCR stimulation resulted in a transient dephosphorylation of a conserved threonine residue in the C-terminal actin-binding domain of ezrin. The phosphorylation of threonine at this site acts as a switch that controls the open and closed conformation of ezrin and other ERM family members, and their ability to cross-link the membrane to actin filaments (53). Indeed, BCR ligation causes ezrin to dissociate from the membrane rafts and from actin filaments. The association of ezrin with membrane rafts in unstimulated B cells is mediated at least in part by binding to the raft-resident transmembrane protein PAG and this association is decreased following BCR ligation. These observations suggest that, in its open conformation, ezrin, together with PAG, constitutes either a diffusion barrier or a tether that extends from the membrane to the actin cytoskeleton and limits the mobility of membrane components over large distances, and that BCR stimulation induces dephosphorylation of ezrin, its release from PAG and the membrane, and a loss of the tethers or barriers that keep membrane rafts in a dispersed state (35).
To test this hypothesis, mutants of ezrin with constitutive actin binding ability were fused to one of two transmembrane domains that would target the chimeric protein to membrane rafts or to the non-raft region of the plasma membrane. These ezrin chimeric proteins were expressed in a B cell line, and their effect on BCR-induced membrane raft coalescence was examined. Both mutant ezrin chimeric proteins were shown to block large-scale coalescence of membrane rafts as well as BCR capping (35). The ezrin chimeras may prevent large-scale membrane raft coalescence either by tethering membrane rafts to the cytoskeleton irreversibly (raft-targeted ezrin) or by creating more stable diffusion barriers and trapping them irreversibly within compartments (raft-targeted and/or raft-excluded ezrin) (Fig. 3).
Figure 3. Ezrin and the actin cytoskeleton regulate B cell membrane raft dynamics.
(a) Membrane rafts are small, dynamic structures dispersed randomly on the cell surface, and tethered to the cortical actin cytoskeleton by protein pickets composed of the raft-associated protein PAG and the linker protein ezrin. In the absence of BCR stimulation, ezrin is phosphorylated (red filled circles) on T567 and exists in its open conformation. The ezrin-based pickets may also create membrane compartments (black rectangle) in which protein and lipid components of membrane rafts are trapped with limited diffusion between adjacent compartments. The Src family kinases (SFKs) are pre-associated with the membrane rafts, while the BCR is excluded from these domains in the absence of antigen resulting in low tonic signaling. (b) Oligomerization of the BCR by multivalent antigen results in its association with membrane rafts, increased proximity to SFKs and greatly amplified signal transduction. Among the effects of BCR signaling is dephosphorylation of ezrin on T567 with concomitant dissociation from PAG and actin, resulting in a break in the diffusion barriers, allowing coalescence of individual membrane rafts into bigger entities. At later times (not shown), ezrin becomes rephosphorylated and again binds to membrane rafts. These reconnections may allow for active actin-myosin-based movement of large rafts to one pole of the cell.
Further support for the idea that ezrin-based tethers and/or traps may keep protein components of the plasma membrane relatively immobile over large distances comes from a recent report that used single particle tracking to demonstrate that the cystic fibrosis transmembrane conductance regulator (CFTR) is immobilized in the plasma membrane via its interactions with the EBP50-ezrin linker that couples it to the actin cytoskeleton. Mutations in CFTR, EBP50 or ezrin that resulted in uncoupling of CFTR from the actin skeleton relieved this immobility (54). Therefore, the ability of ezrin and related proteins to tether the membrane to the cortical actin meshwork may be a generalized mechanism by which cells regulate the dynamics of their membrane components including membrane rafts.
It is important to note that a number of studies addressing the significance of membrane rafts in cellular activation have resorted to the use of pharmacological inhibitors such as methyl β–cyclodextrin, which disrupts these domains by depleting them of cholesterol which is an essential component of membrane rafts. Methyl β–cyclodextrin can have a generalized disruptive effect on both the plasma membrane and actin cytoskeleton that is not restricted to membrane rafts, so it is increasingly considered a less than ideal tool (55, 56). Thus, the membrane-targeted ezrin mutants that we reported may provide a useful new genetic tool to allow manipulation of membrane rafts to examine their roles in B cell activation as well as in other cellular processes.
Membrane rafts and B cell-related disease
The importance of membrane rafts in B cell activation is further emphasized by recent reports that membrane rafts are co-opted by gene products of certain pathogens and that in certain disease conditions, critical regulators of BCR signaling lose their association with membrane rafts. The LMP2A gene product of the Epstein Barr virus (EBV) is expressed on the surface of resting B cells during latent infection by this virus. The intracellular amino terminal region of LMP2A contains 8 tyrosine residues, 2 of which (Y74 and Y85) are configured into an ITAM sequence. Upon phosphorylation of these residues, LMP2A binds Lyn and Syk tyrosine kinases and in this way is thought to deviate these proteins away from BCR signal transduction, preventing phosphorylation of key signaling molecules as well as Ca2+ flux (57). LMP2A constitutively resides in membrane rafts of EBV-transformed human B cell lines, and interestingly, blocks the entry of ligand-clustered BCRs into these domains. The mechanism of this effect is not known. LMP2A also inhibits downstream signaling events and internalization of the BCR. The molecular mechanisms for blockade of signaling and endocytosis appear to be different as a Y112 mutant of LMP2A that cannot associate with Lyn, still blocks BCR translocation to membrane rafts and associated signaling but does not affect cross-linking-induced BCR endocytosis (58). This observation suggests that BCR signaling and internalization are differentially regulated, and also that EBV has evolved separate mechanisms to block these important functions of the BCR.
Another EBV protein that is responsible for the maintenance and proliferation of latently infected B cells is the LMP-1 protein. LMP-1 is capable of activating signaling pathways resembling those of the TNF receptor family member CD40, including binding to TRAFs, TRADD and JAK3, and activating NF-κB, AP-1 and STAT-mediated transcription. Like ligand-stimulated CD40, LMP-1 localizes to membrane rafts and recruits TRAF3 into these domains (59). Targeting the C-terminal cytoplasmic domain of LMP-1 to membrane rafts results in constitutive signaling. Since the C-terminal domain of LMP-1 recruits TRAF3, this observation suggests that targeting TRAF3 to membrane rafts promotes its signaling (60).
Not only does membrane raft localization of viral proteins contribute to disease, alterations in the membrane raft localization of certain signaling molecules may also contribute to the initiation or severity of certain B cell-dependent autoimmune diseases. A study comparing a group of British patients with the autoimmune disease systemic lupus erythematosus (SLE) to normal individuals found that a majority of the SLE patients exhibited lower expression levels of Lyn as well as reduced association Lyn with membrane rafts. These patients also had an increased translocation of c-Cbl into membrane rafts. Cbl contains an E3 ubiquitin ligase activity and is an inhibitor of receptor-mediated tyrosine kinase signaling pathways (61). In B cells, it may induce ubiquitinylation and degradation of membrane raft-associated Lyn (62). As Lyn is important for the function of inhibitory receptors which decrease BCR signaling, as described above, it was suggested that reduced negative signaling in the membrane raft environment facilitates hyperactivation of B cells resulting in uncontrolled antibody responses to autoantigens.
Two independent studies that compared signaling mechanisms in a Japanese cohort of SLE patients with normal controls also provide evidence for the hypothesis that alterations in the sub-cellular localization of BCR signaling pathways may contribute to development of SLE. Both found that a single polymorphism in the FcγRIIB gene, a substitution of threonine for isoleucine at amino acid position 232, which lies in the transmembrane region of the protein, is preferentially associated with the SLE patients and that this mutation causes FcγRIIB to be excluded from membrane rafts in B cells. The functional impact of this change in membrane distribution was diminished inhibition by FcγRIIB of BCR-derived signals including PIP3 generation, Akt and PLCγ2 activation, and Ca2+ mobilization. The T232 form of FcγRIIB also had less phosphorylation on its ITIM tyrosine residues and recruited lower levels of the inositol phosphatase SHIP (63, 64). Together, the findings concerning the altered distribution of Lyn and FcγRIIB in membrane rafts indicate that, like positive signaling by the BCR in mature B cells, its feedback inhibition also occurs in a concentrated membrane raft environment.
Concluding remarks
Membrane rafts participate in many of the cell surface events involved in B cell activation, including signaling by the BCR, modulation of that signaling by co-receptors, signaling by CD40, endocytosis of antigen bound to the BCR and its routing to late endosomes to facilitate loading of antigen-derived peptides onto class II MHC molecules, routing of those peptide/MHC-II complexes to the cell surface, and their participation in antigen presentation to T cells. Moreover, in some cases, the involvement of membrane rafts in B cell activation is developmentally controlled. Thus, it is likely that membrane rafts play important roles in B cell activation at multiple stages by controlling the local concentrations of components that must act together and/or components that inhibit the process in question. Understanding the way in which membrane rafts affect particular events is, however, hampered by the limited numbers of ways that membrane rafts can be manipulated within viable B cells. Mutational approaches to target proteins of interest into or away from membrane rafts represent an attractive alternative to the cholesterol depletion approach and are beginning to provide some insight into these questions. Clearly many questions remain regarding the ways in which these microdomains modulate signaling and trafficking events within B cells and studies in the near future should help clarify these issues.
Acknowledgments
The authors would like to thank their colleagues for discussions. N.G. is the recipient of a K01 mentored research career development award (DK068292) from NIDDK. Research in the DeFranco lab is funded by a National Institutes of Health research grant from National Institute of Allergy and Infectious Diseases (R01 AI20038).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Monroe JG. ITAM-mediated tonic signalling through pre-BCR and BCR complexes. Nat Rev Immunol. 2006;6:283–94. doi: 10.1038/nri1808. [DOI] [PubMed] [Google Scholar]
- 2.Reth M, Brummer T. Feedback regulation of lymphocyte signaling. Nat Rev Immunol. 2004;4:269–77. doi: 10.1038/nri1335. [DOI] [PubMed] [Google Scholar]
- 3.Law DA, Chan VWF, Datta SK, DeFranco AL. B-cell antigen receptor motifs have redundant signalling capabilities and bind the tyrosine kinases PTK72, Lyn and Fyn. Curr Biol. 1993;3:645–57. doi: 10.1016/0960-9822(93)90062-s. [DOI] [PubMed] [Google Scholar]
- 4.Pike LJ. Rafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell Function. J Lipid Res. 2006;47:1597–8. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 5.Dykstra M, Cherukuri A, Sohn HW, Tzeng SJ, Pierce SK. Location is everything: lipid rafts and immune cell signaling. Annu Rev Immunol. 2003;21:457–81. doi: 10.1146/annurev.immunol.21.120601.141021. [DOI] [PubMed] [Google Scholar]
- 6.Gupta N, DeFranco AL. Visualization of lipid raft dynamics and early signaling events during antigen receptor-mediated B cell activation. Mol Biol Cell. 2003;14:432–44. doi: 10.1091/mbc.02-05-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sohn HW, Tolar P, Jin T, Pierce SK. Flourescence resonance energy transfer in living cells reveals dynamic membrane changes in the initiation of B cell signaling. Proc Natl Acad Sci U S A. 2006;103:8143–8. doi: 10.1073/pnas.0509858103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng PC, Brown BK, Song W, Pierce SK. Translocation of the B cell antigen receptor into lipid rafts reveals a novel step in signaling. J Immunol. 2001;166:3693–701. doi: 10.4049/jimmunol.166.6.3693. [DOI] [PubMed] [Google Scholar]
- 9.Rawlings DJ, Sommer K, Moreno-Garcia ME. The CARMA1 signalosome links the signalling machinery of adaptive and innate immunity in lymphocytes. Nat Rev Immunol. 2006;6:799–812. doi: 10.1038/nri1944. [DOI] [PubMed] [Google Scholar]
- 10.Su TT, Guo B, Kawakami Y, Sommer K, Chae K, Humphries LA, et al. PKC-β controls IκB kinase lipid raft recruitment and activation in response to BCR signaling. Nat Immunol. 2002;3:780–6. doi: 10.1038/ni823. [DOI] [PubMed] [Google Scholar]
- 11.Gaide O, Favier B, Legler DF, Bonnet D, Brissoni B, Valitutt S, et al. CARMA1 is a critical lipid raft-associated regulator of TCR-induced NF-κB activation. Nature Immunol. 2002;3:836–43. doi: 10.1038/ni830. [DOI] [PubMed] [Google Scholar]
- 12.Blery M, Tze L, Miosge LA, Jun JE, Goodnow CC. Essential role of membrane cholesterol in accelerated BCR internalization and uncoupling from NF-κB in B cell clonal anergy. J Exp Med. 2006;203:1773–83. doi: 10.1084/jem.20060552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dempsey PW, Allison MED, Akkaraju S, Goodnow CC, Fearon DT. C3d of complement as a molecular adjuvant: Bridging innate and acquired immunity. Science. 1996;271:348–50. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Brooks S, Li X, Anzelon A, Rickert R, Carter R. The physiologic role of CD19 cytoplasmic tyrosines. Immunity. 2002;17:501–14. doi: 10.1016/s1074-7613(02)00426-0. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Carter R. CD19 regulates B cell maturation, proliferation, and positive selection in the FDC zone of murine splenic germinal centers. Immunity. 2005;22:749–61. doi: 10.1016/j.immuni.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Ayer LM, Lytton J, Deans JP. Store-operated cation entry mediated by CD20 in membrane rafts. J Biol Chem. 2003;278:42427–34. doi: 10.1074/jbc.M308802200. [DOI] [PubMed] [Google Scholar]
- 17.Coughlin JJ, Stang SL, Dower NA, Stone JC. RasGRP1 and RasGRP3 regulate B cell proliferation by facilitating B cell receptor-Ras signaling. J Immunol. 2005;175:7179–84. doi: 10.4049/jimmunol.175.11.7179. [DOI] [PubMed] [Google Scholar]
- 18.Aiba Y, Oh-hora M, Kiyonaka S, Kimura Y, Hijikata A, Mori Y, et al. Activation of RasGRP3 by phosphorylation of Thr-133 is required for B cell receptor-mediated Ras activation. Proc Natl Acad Sci U S A. 2004;47:16612–7. doi: 10.1073/pnas.0407468101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cherukuri A, Carter RH, Brooks S, Bornmann W, Finn R, Dowd CS, et al. B cell signaling is regulated by induced palmitoylation of CD81. J Biol Chem. 2004;279:31973–82. doi: 10.1074/jbc.M404410200. [DOI] [PubMed] [Google Scholar]
- 20.Cherukuri A, Shoham T, Sohn HW, Levy S, Brooks S, Carter RH, et al. The tetraspanin CD81 is necessary for partitioning of coligated CD19/CD21-B cell antigen receptor complexes into signaling active lipid rafts. J Immunol. 2004;172:370–80. doi: 10.4049/jimmunol.172.1.370. [DOI] [PubMed] [Google Scholar]
- 21.Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;290:84–9. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- 22.Aman MJ, Tosello-Trampont AC, Ravichandran K. FcγRIIB1/SHIP-mediated inhibitory signaling in B cells involves lipid rafts. J Biol Chem. 2001;279:46371–78. doi: 10.1074/jbc.M104069200. [DOI] [PubMed] [Google Scholar]
- 23.Guo B, Kato RM, Garcia-Lloret M, Wahl MI, Rawlings DJ. Engagement of the human pre-B cell receptor generates a lipid raft-dependent calcium signaling complex. Immunity. 2000;13:243–53. doi: 10.1016/s1074-7613(00)00024-8. [DOI] [PubMed] [Google Scholar]
- 24.Sandel PC, Gendelman M, Kelsoe G, Monroe JG. Definition of a novel cellular constituent of the bone marrow that regulates the response of immature B cells to B cell antigen receptor engagement. J Immunol. 2001;166:5935–44. doi: 10.4049/jimmunol.166.10.5935. [DOI] [PubMed] [Google Scholar]
- 25.Cherukuri A, Cheng PC, Pierce SK. The role of the CD19/CD21 complex in B cell processing and presentation of complement-tagged antigens. J Immunol. 2001;167:163–72. doi: 10.4049/jimmunol.167.1.163. [DOI] [PubMed] [Google Scholar]
- 26.Karnell FG, Brezski RJ, King LB, Silverman MA, Monroe JG. Membrane cholesterol content accounts for developmental differences in surface B cell receptor compartmentalization and signaling. J Biol Chem. 2005;280:25621–8. doi: 10.1074/jbc.M503162200. [DOI] [PubMed] [Google Scholar]
- 27.Clark MR, Massenburg D, Siemasko K, Hou P, Zhang M. B-cell antigen receptor signaling requirements for targeting antigen to the MHC class II presentation pathway. Curr Opin Cell Biol. 2004;16:382–7. doi: 10.1016/j.coi.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Siemasko K, Eisfelder BJ, Stebbins C, Kabak S, Sant AJ, Song W, et al. Igα and Igβ are required for efficient trafficking to late endosomes and to enhance antigen presentation. J Immunol. 1999;162:6518–25. [PubMed] [Google Scholar]
- 29.Gazumyan A, Reichlin A, Nussenzweig MC. Igβ tyrosine residues contribute to the control of B cell receptor signaling by regulating receptor internalization. J Exp Med. 2006;203:1785–94. doi: 10.1084/jem.20060221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cassard S, Salamero J, Hanau D, Spehner D, Davoust J, Fridman WH, et al. A tyrosine-based signal present in Igα mediates B cell receptor constitutive internalization. J Immunol. 1998;160:1767–73. [PubMed] [Google Scholar]
- 31.Lankar D, Briken V, Adler K, Weiser P, Cassard S, Blank U, et al. Syk tyrosine kinase and B cell antigen receptor (BCR) immunoglobulin-α subunit determine BCR-mediated major histocompatibility complex class II-restricted antigen presentation. J Exp Med. 1998;188:819–31. doi: 10.1084/jem.188.5.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siemasko K, Skaggs BJ, Kabak S, Williamson E, Brown BK, Song W, et al. Receptor-facilitated antigen presentation requires the recruitment of B cell linker protein to Igα. J Immunol. 2002;168:2127–38. doi: 10.4049/jimmunol.168.5.2127. [DOI] [PubMed] [Google Scholar]
- 33.Hou P, Araujo E, Zhao T, Zhang M, Massenburg D, Veselits M, et al. B cell antigen receptor signaling and internalization are mutually exclusive events. PLOS Biology. 2006;4:1147–58. doi: 10.1371/journal.pbio.0040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stoddart A, Dykstra ML, Brown BK, Song W, Pierce SK, Brodsky FM. Lipid rafts unite signaling cascades with clathrin to regulate BCR internalization. Immunity. 2002;17:451–62. doi: 10.1016/s1074-7613(02)00416-8. [DOI] [PubMed] [Google Scholar]
- 35.Gupta N, Wollscheid B, Watts JD, Scheer B, Aebersold R, DeFranco AL. Quantitative proteomic analysis of B cell lipid rafts reveals that ezrin regulates antigen receptor-mediated lipid raft dynamics. Nat Immunol. 2006;7:625–33. doi: 10.1038/ni1337. [DOI] [PubMed] [Google Scholar]
- 36.Stoddart A, Jackson AP, Brodsky FM. Plasticity of B cell receptor internalization upon conditional depletion of clathrin. Mol Biol Cell. 2005;16:2339–48. doi: 10.1091/mbc.E05-01-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng PC, Dykstra ML, Mitchell RN, Pierce SK. A role for lipid rafts in B cell antigen receptor signaling and antigen targeting. J Exp Med. 1999;190:1549–60. doi: 10.1084/jem.190.11.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Putnam MA, Moquin AE, Merrihew M, Outcalt C, Sorge E, Caballero A, et al. Lipid raft-independent B cell receptor-mediated antigen internalization and intracellular trafficking. J Exp Med. 2003;170:905–12. doi: 10.4049/jimmunol.170.2.905. [DOI] [PubMed] [Google Scholar]
- 39.Caballero A, Katkere B, Wen XY, Drake L, Nashar TO, Drake JR. Functional and structural requirements for the internalization of distinct BCR-ligand complexes. Eur J Immunol. 2006;36:3131–45. doi: 10.1002/eji.200636447. [DOI] [PubMed] [Google Scholar]
- 40.Brdicka T, Imrich M, Angelisova P, Brdickova N, Horvath O, Spicka J, et al. Non-T cell activation linker (NTAL): a transmembrane adaptor protein involved in immunoreceptor signaling. J Exp Med. 2002;196:1617–26. doi: 10.1084/jem.20021405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janssen E, Zhu M, Zhang W, Koonpaew S, Zhang W. LAB: a new membrane-associated adaptor molecule in B cell activation. Nat Immunol. 2003;4:117–23. doi: 10.1038/ni882. [DOI] [PubMed] [Google Scholar]
- 42.Mutch CM, Sanyal R, Unruh TL, Grigoriou L, Zhu M, Zhang W, et al. Activation-induced endocytosis of the raft-associated transmembrane adaptor protein LAB/NTAL in B lymphocytes: evidence for a role in internalization of the B cell receptor. Int Immunol. 2007;19:19–30. doi: 10.1093/intimm/dxl118. [DOI] [PubMed] [Google Scholar]
- 43.Poloso NJ, Roche PA. Association of MHC class II-peptide complexes with plasma membrane lipid microdomains. Curr Opin Immunol. 2004;16:103–7. doi: 10.1016/j.coi.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 44.Bouillon M, El Fakhry Y, Girouard J, Khalil H, Thibodeau J, Mourad W. Lipid raft-dependent and -independent signaling through HLA-DR molecules. J Biol Chem. 2003;278:7099–107. doi: 10.1074/jbc.M211566200. [DOI] [PubMed] [Google Scholar]
- 45.Anderson HA, Hiltbold EM, Roche PA. Concentration of MHC class II molecules in lipid rafts facilitates antigen presentation. Nat Immunol. 2000;1:156–62. doi: 10.1038/77842. [DOI] [PubMed] [Google Scholar]
- 46.Gordy C, Mishra S, Rodgers W. Visualization of antigen presentation by actin-mediated targeting of glycolipid-enriched membrane domains to the immune synapse of B cell APCs. J Immunol. 2004;172:2030–8. doi: 10.4049/jimmunol.172.4.2030. [DOI] [PubMed] [Google Scholar]
- 47.Edidin M. The state of lipid rafts: from model membranes to cells. Annu Rev Biophys Biomol Struct. 2003;32:257–83. doi: 10.1146/annurev.biophys.32.110601.142439. [DOI] [PubMed] [Google Scholar]
- 48.Kusumi A, Nakada C, Ritchie K, Murase K, Suzuki K, Murakoshi H, et al. Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules. Annu Rev Biophys Biomol Struct. 2005;34:351–78. doi: 10.1146/annurev.biophys.34.040204.144637. [DOI] [PubMed] [Google Scholar]
- 49.Fujiwara T, Ritchie K, Murakoshi H, Jacobson K, Kusumi A. Phospholipids undergo hop diffusion in compartmentalized cell membrane. J Cell Biol. 2002;157:1071–81. doi: 10.1083/jcb.200202050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kusumi A, Ike H, Nakada C, Murase K, Fujiwara T. Single-molecule tracking of membrane molecules: plasma membrane compartmentalization and dynamic assembly of raft-philic signaling molecules. Semin Immunol. 2005;17:3–21. doi: 10.1016/j.smim.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 51.Kusumi A, Sako Y. Cell surface organization by the membrane skeleton. Curr Opin Cell Biol. 1996;8:566–74. doi: 10.1016/s0955-0674(96)80036-6. [DOI] [PubMed] [Google Scholar]
- 52.Hao S, August A. Actin depolymerization transduces the strength of B-cell receptor stimulation. Mol Biol Cell. 2005;16:2275–84. doi: 10.1091/mbc.E04-10-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586–99. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- 54.Haggie PM, Kim JK, Lukacs GL, Verkman AS. Tracking of quantum dot-labeled CFTR shows near immobilization by C-terminal PDZ interactions. Mol Biol Cell. 2006;17:4937–45. doi: 10.1091/mbc.E06-08-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Munro S. Lipid rafts: elusive or illusive? Cell. 2003;115:377–88. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- 56.Kwik J, Boyle S, Fooksman D, Margolis L, Sheetz MP, Edidin M. Membrane cholesterol, lateral mobility, and the phosphatidylinositol 4,5-bisphosphate-dependent organization of cell actin. Proc Natl Acad Sci U S A. 2003;100:13964–9. doi: 10.1073/pnas.2336102100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Longnecker R, Miller CL. Regulation of Epstein-Barr virus latency by latent membrane protein 2. Trends Microbiol. 1996;4:38–42. doi: 10.1016/0966-842x(96)81504-6. [DOI] [PubMed] [Google Scholar]
- 58.Dykstra ML, Longnecker R, Pierce SK. Epstein-Barr virus coopts lipid rafts to block the signaling and antigen transport functions of the BCR. Immunity. 2001;14:57–67. doi: 10.1016/s1074-7613(01)00089-9. [DOI] [PubMed] [Google Scholar]
- 59.Brown KD, Hostager BS, Bishop GA. Differential signaling and tumor necrosis factor receptor-associated factor (TRAF) degradation mediated by CD40 and the Epstein-Barr virus oncoprotein latent membrane protein 1 (LMP1) J Exp Med. 2001;193:943–54. doi: 10.1084/jem.193.8.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaykas A, Worringer K, Sugden B. CD40 and LMP-1 both signal from lipid rafts but LMP-1 assembles a distinct, more efficient signaling complex. Embo J. 2001;20:2641–54. doi: 10.1093/emboj/20.11.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thien CB, Langdon WY. Negative regulation of PTK signalling by Cbl proteins. Growth Factors. 2005;23:161–7. doi: 10.1080/08977190500153763. [DOI] [PubMed] [Google Scholar]
- 62.Flores-Borja F, Kabouridis PS, Jury EC, Isenberg DA, Mageed RA. Decreased Lyn expression and translocation to lipid raft signaling domains in B lymphocytes from patients with systemic lupus erythematosus. Arthritis Rheum. 2005;52:3955–65. doi: 10.1002/art.21416. [DOI] [PubMed] [Google Scholar]
- 63.Kono H, Kyogoku C, Suzuki T, Tsuchiya N, Honda H, Yamamoto K, et al. FcγRIIB Ile232Thr transmembrane polymorphism associated with human systemic lupus erythematosus decreases affinity to lipid rafts and attenuates inhibitory effects on B cell receptor signaling. Hum Mol Genet. 2005;14:2881–92. doi: 10.1093/hmg/ddi320. [DOI] [PubMed] [Google Scholar]
- 64.Floto RA, Clatworthy MR, Heilbronn KR, Rosner DR, MacAry PA, Rankin A, et al. Loss of function of a lupus-associated FcγRIIb polymorphism through exclusion from lipid rafts. Nat Med. 2005;11:1056–8. doi: 10.1038/nm1288. [DOI] [PubMed] [Google Scholar]