After much speculation (Abrams 1999; McCall and Steller 1997; Meier and Evan 1998), work described in this issue of The Journal of Cell Biology and in a recent issue of Proceedings of the National Academy of Sciences of the United States of America (Igaki et al. 2000) unveils the long anticipated, missing piece of the apoptosome in flies. On page 703 in this issue, in a paper by Colussi et al., Kumur, Richardson, and colleagues characterize the first Drosophila members of the Bcl-2 gene family whose function is important for programmed cell death (PCD). The founding member of this gene family was identified as the proto-oncogene upregulated by t(14;18) translocations in B cell follicular lymphomas. Since this discovery, and its link to the regulation of cell death, the Bcl-2 family of proteins has grown to include more than twenty members of death-suppressing and -promoting molecules found in the genomes of worms, mammals, viruses, and now flies (Gross et al. 1999; Vander Heiden and Thompson 1999).
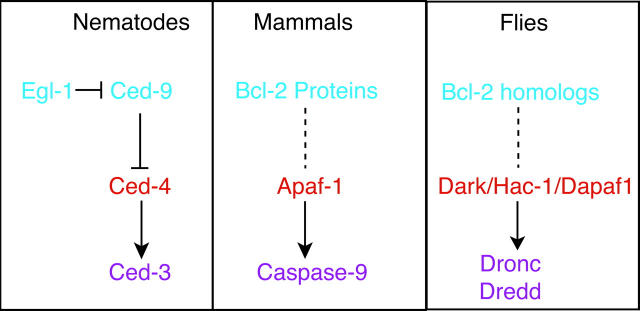
Central components of the apoptosis machinery in worms, mammals and flies are schematized in Fig. 1. In C. elegans, both Ced-3 and Ced-4 are required for all PCD during worm development. Ced-3 encodes a founding member of the caspase family (cysteine proteases) while Ced-4 promotes the activation of Ced-3 through direct physical interaction. The upstream death regulator, Ced-9, protects cells from death by forming a complex with Ced-4, thus preventing the activation of Ced-3 by Ced-4. A pro-apoptotic protein with limited Bcl-2 similarity, Egl-1, can interact with Ced-9 to derepress Ced-4 and permit activation of Ced-3 (reviewed in Metzstein et al. 1998; Gumienny et al. 1999; Horvitz 1999).
Figure 1.
Conserved elements of the cell death machinery in nematodes, mammals and flies are presented in schematic form. The order of gene action is based on precedents established in the nematode, C. elegans.
In mammals the Ced-4 homologue, Apaf-1, together with cytochrome c released from mitochondria, promotes oligomerization and activation of the mammalian apical caspase, caspase-9. Activated caspase-9 subsequently processes downstream effector caspases (e.g., caspase-3) thus initiating a caspase cascade leading to apoptosis. In this pathway, the Bcl-2 family of proteins is thought to act upstream of caspase activation, perhaps regulating the initial phase of caspase processing, while in other situations (e.g., death receptor signaling), Bcl-2 proteins may function to amplify or propagate an already initiated caspase cascade. The Bcl-2 related proteins can form homo- and hetero-dimers through their conserved domains (BH domains). This ability to dimerize, and the relative abundance of the pro- and anti-apoptotic members, are thought to be important determinants regulating the propensity of a given cell to convert death signals into an apoptotic response (reviewed in Adams and Cory 1998; Gross et al. 1999; Vander Heiden and Thompson 1999).
How do the mammalian Bcl-2 proteins function to regulate cell death? Since human Bcl-2 can partially reverse cell death defects found in Ced-9 mutant worms, Ced-9 and Bcl-2 are thought to share at least some functional properties (Vaux et al. 1992; Hengartner and Horvitz 1994). Thus, one proposed mechanism draws on possible functional analogies to the Ced-9 protein, which physically binds to and inhibits Ced-4. Consistent with this hypothesis, some Bcl-2 proteins were found to associate with Apaf-1 (Hu et al. 1998; Inohara et al. 1998; Pan et al. 1998; Song et al. 1999), leading to speculation that Bcl-2 proteins could regulate apoptosis by interfering with Apaf-1-dependent caspase-9 activation. This is an attractive model, easily reconciled with the relationship between Egl-1, Ced-9, Ced-4, and Ced-3 in C. elegans. However, when native Bcl-2 proteins were directly examined, the predicted associations with Apaf-1 were not found, raising doubts as to whether the Apaf-1/Bcl-2 overexpression studies truly reflect the physiological condition (Moriishi et al. 1999). Alternative models for Bcl-2 function have also developed over the years. Some members of the Bcl-2 family are either resident proteins of mitochondrial membranes or transit from the cytosol to mitochondria in association with an apoptotic signal. Together with a resemblance to bacterial toxins and their ability to form channels in lipid bilayers, it has been suggested that Bcl-2 proteins also function to regulate the flow of caspase-activating substances, such as cytochrome c and apoptosis-inducing factor (AIF), from the mitochondria to the cytosol by forming and/or regulating pores on mitochondrial membranes (Green and Reed 1998, Gross et al. 1999). This picture is consistent with the ability of Bcl-2 to suppress the release of cytochrome c from isolated mitochondria (Kluck et al. 1997; Yang et al. 1997) and a reported association with the mitochondrial voltage-dependent anion channel (Shimizu et al. 1999). Although we have learned a great deal since their discovery more than a decade ago, the precise mechanism(s) by which these proteins exert their function remains both an elusive and intensely controversial issue. Given that evolutionary perspectives often provide clues to the nature of molecular function, an implicit undertone coloring this debate is whether (or to what extent) the anti-apoptotic Bcl-2 proteins actually share biochemical functions with Ced-9. Accordingly, the newly identified Bcl-2 homologues from a distantly related genetic model promise additional avenues to help resolve this problem.
Colussi et al. 2000 identified Debcl (death executioner Bcl-2 homologue) and a second Bcl-2 homologue from a database search of the Drosophila genome. These two Drosophila proteins share a high degree of similarity, and among the published Bcl-2 members, they are most similar to Bok. Both contain BH1, BH2, and BH3 domains as well as a hydrophobic transmembrane region. Interaction profiling studies demonstrated that Debcl can bind to most of the mammalian anti-apoptotic family members (e.g., Bcl-2, Bcl-xL, etc.), but not to pro-apoptotic members (such as Bik, Bax, and Bak, etc.). In cell culture and in transgenic animals, directed expression of Debcl provokes extensive cell death which required an intact BH3 domain and was suppressible by the virally derived caspase inhibitor, p35. In the embryo, the appearance of Debcl RNA correlates with PCD at many stages and in various tissues. Colussi et al. 2000 also used the RNA interference technique, a relatively new method of blocking gene expression, to validate a pro-apoptotic function for Debcl and demonstrate a requirement for this gene during embryonic cell death. A concurrent paper by Igaki et al. 2000 characterizes the same gene, which they refer to as Drob-1. They used a clone that is 25 residues longer at the NH2 terminus and found that Drob-1, like Debcl, was pro-apoptotic. In related studies, they also uncovered a requirement for the carboxyl hydrophobic transmembrane region and found that, like its mammalian counterparts, Drob-1 localized to mitochondria when expressed in cultured cells. While the two groups studying this gene agree on its expression profile and its pro-death properties, there are significant discrepancies as well. For instance, whereas the Debcl group contends that the gene does not include a BH4 domain, the Drob-1 group contends that it does. The issue might be more than academic since it would be the first example of a pro-apoptotic member of the family that contains a BH4 motif (outside of Bclxs). Another discrepancy is that although expression of Debcl/Drob-1 provoked caspase activation, Colussi et al. 2000 found that p35 was able to suppress accompanying cell death (in cultured cells and the animal) whereas Igaki et al. 2000 report that it was not (only cultured cells were tested). The differential effects of p35 in the two studies might be due to the slightly different clones that were used or the differing expression levels obtained in the two labs. Other possibilities include the induction of p35-insensitive caspases or the activation of caspase-independent events that lead to cell death. Again, given the importance of caspase-independent events associated with killing by pro-death Bcl-2 genes in mammalian cells (Xiang et al. 1996; McCarthy et al. 1997), reconciling these differences will be more than simply an academic exercise.
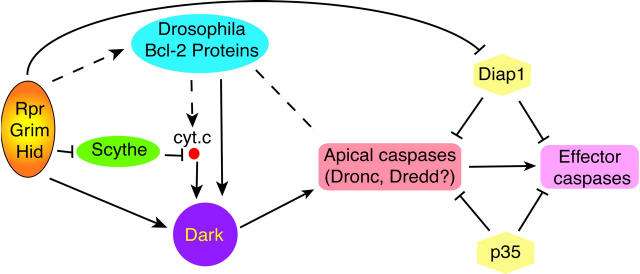
How does Debcl/Drob-1 fit into our current view of cell death genetics in flies? Although it is far too early for a well-focused picture, the similarities to pro-death Bcl-2 genes combined with epistasis data connecting Debcl to existing players of the Drosophila cell death pathway suggests a tentative molecular order for gene action (see Colussi et al. 2000, and Fig. 2). Debcl-associated death phenotypes were sensitive to the dosage of DIAP1 and Dark (the APAF-1/Ced4 ortholog) indicating that the protein functions upstream, or parallel to, the action of these genes. Expression of Debcl/Drob-1 also provoked caspase activation which (at least in the animal) was reversed by the broad-spectrum caspase inhibitor, p35. In contrast, cell killing by Debcl was insensitive to the dosage of the death activators Rpr, Grim, and Hid, suggesting that the protein either functions downstream or parallel to these genes. While the pathways in Fig. 2 (and Colussi et al. 2000) offer a reasonable interpretation of the current data, the usual caution and caveats apply since the position of the fly Bcl-2 proteins is largely based upon dominant phenotypes resulting from directed overexpression studies. Nevertheless, given the attention these genes are likely to receive, we can expect rigorous testing of the model for years to come. In this regard, the isolation of null mutations in these genes and the identification of an anti-apoptotic ortholog are perhaps the highest priorities.
Figure 2.
Programmed cell death pathways in Drosophila. In Drosophila, all embryonic PCD requires the activities of three closely linked genes, Rpr, Grim, and Hid. Expression of these apoptosis regulators initiates multiple downstream pathways to activate caspases and kill cells. Rpr, Grim, and Hid may induce formation of an apoptosome complex consisting of cytochrome c (cyt. c), Dark (Kanuka et al. 1999; Rodriguez et al. 1999; Zhou et al. 1999), and apical caspases such as Dronc (Loretta et al. 1999) and Dredd (Chen et al. 1998), which in turn promotes caspase activation and propagation of proteolytic activity to downstream, effector caspases (Abrams 1999). Alterations in cyt. c (Varkey et al. 1999) could be regulated by Scythe, a protein that binds all three death activators (Thress et al. 1999, Thress et al. 1998). Rpr, Grim, and Hid also engage caspases via one or more Dark-independent pathways. These involve derepression of native caspase inhibitors such as Diap1 (Wang et al. 1999). The pro-apoptotic Bcl-2 proteins (Drob-1/Debcl) probably function downstream of Rpr, Grim, and Hid and might directly engage caspases or function through Dark/cyt. c to propagate death signals. Currently, no pro-survival Bcl-2 gene has been reported in Drosophila.
Footnotes
Address correspondence to John M. Abrams, Department of Cell Biology, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd., Dallas, TX 75390-9039. Tel.: (214) 648-9226. Fax: (214) 648-8694. E-mail: abrams@utsw.swmed.edu
References
- Abrams J.M. An emerging blueprint for apoptosis in Drosophila . Trends Cell Biol. 1999;9:435–440. doi: 10.1016/s0962-8924(99)01646-3. [DOI] [PubMed] [Google Scholar]
- Adams J.M., Cory S. The Bcl-2 protein familyarbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- Chen P., Rodriguez A., Erskine R., Thach T., Abrams J.M. Dredd, a novel effector of the apoptosis activators Reaper, Grim, and Hid in Drosophila . Dev. Biol. 1998;201:202–216. doi: 10.1006/dbio.1998.9000. [DOI] [PubMed] [Google Scholar]
- Colussi P.A., Quinn L.M., Huang D.C.S., Coombe M., Read S.H., Richardson H., Kumar S. Debcl, a proapoptotic Bcl-2 homologue, is a component of the Drosophila melanogaster cell death machinery. J. Cell Biol. 2000;148:703–714. doi: 10.1083/jcb.148.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D.R., Reed J.C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Gross A., McDonnell J.M., Korsmeyer S.J. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- Gumienny T.L., Lambie E., Hartwieg E., Horvitz H.R., Hengartner M.O. Genetic control of programmed cell death in the Caenorhabditis elegans hermaphrodite germline. Development. 1999;126:1011–1022. doi: 10.1242/dev.126.5.1011. [DOI] [PubMed] [Google Scholar]
- Hengartner M.O., Horvitz H.R. C. elegans cell survival gene ced-9 encodes a functional homolog of the mammalian proto-oncogene bcl-2. Cell. 1994;76:665–676. doi: 10.1016/0092-8674(94)90506-1. [DOI] [PubMed] [Google Scholar]
- Horvitz H.R. Genetic control of programmed cell death in the nematode Caenorhabditis elegans . Cancer Res. 1999;59:1701S–1706S. [PubMed] [Google Scholar]
- Hu Y., Benedict M.A., Wu D., Inohara N., Núñez G. Bcl-XL interacts with Apaf-1 and inhibits Apaf-1-dependent caspase-9 activation. Proc. Natl. Acad. Sci. USA. 1998;95:4386–4391. doi: 10.1073/pnas.95.8.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igaki T., Kanuka H., Inohara N., Sawamoto K., Núñez G., Okano H., Miura M. Drob-1, a Drosophila member of the Bcl-2/CED-9 family that promotes cell death. Proc. Natl. Acad. Sci. USA. 2000;97:662–667. doi: 10.1073/pnas.97.2.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inohara N., Gourley T.S., Carrio R., Muniz M., Merino J., Garcia I., Koseki T., Hu Y., Chen S., Núñez G. Diva, a Bcl-2 homologue that binds directly to Apaf-1 and induces BH3-independent cell death. J. Biol. Chem. 1998;273:32479–32486. doi: 10.1074/jbc.273.49.32479. [DOI] [PubMed] [Google Scholar]
- Kanuka H., Sawamoto K., Inohara N., Matsuno K., Okano H., Miura M. Control of the cell death pathway by Dapaf-1, a Drosophila Apaf-1/CED-4-related caspase activator. Mol. Cell. 1999;4:757–769. doi: 10.1016/s1097-2765(00)80386-x. [DOI] [PubMed] [Google Scholar]
- Kluck R.M., Bossywetzel E., Green D.R., Newmeyer D.D. The release of cytochrome c from mitochondria—a primary site for bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- Loretta D., Colussi P.A., Quinn L.M., Richardson H., Kumar S. DRONC, an ecdysone-inducible Drosophila caspase. Proc. Natl. Acad. Sci. USA. 1999;96:4307–4312. doi: 10.1073/pnas.96.8.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall K., Steller H. Facing death in the flygenetic analysis of apoptosis in Drosophila . Trends Genet. 1997;13:222–226. doi: 10.1016/S0168-9525(97)01126-8. [DOI] [PubMed] [Google Scholar]
- McCarthy N.J., Whyte M., Gilbert C.S., Evan G.I. Inhibition of ced-3/ice-related proteases does not prevent cell death induced by oncogenes, DNA damage, or the bcl-2 homologue bak. J. Cell Biol. 1997;136:215–227. doi: 10.1083/jcb.136.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier P., Evan G. Dying like flies. Cell. 1998;95:295–298. doi: 10.1016/s0092-8674(00)81760-2. [DOI] [PubMed] [Google Scholar]
- Metzstein M.M., Stanfield G.M., Horvitz H.R. Genetics of programmed cell death in C. elegans—past, present and future. Trends Genet. 1998;14:410–416. doi: 10.1016/s0168-9525(98)01573-x. [DOI] [PubMed] [Google Scholar]
- Moriishi K., Huang D.C.S., Cory S., Adams J.M. Bcl-2 family members do not inhibit apoptosis by binding the caspase activator Apaf-1. Proc. Natl. Acad. Sci. USA. 1999;96:9683–9688. doi: 10.1073/pnas.96.17.9683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan G., O'Rourke K., Dixit V.M. Caspase-9, Bcl-XL, and Apaf-1 form a ternary complex. J. Biol. Chem. 1998;273:5841–5845. doi: 10.1074/jbc.273.10.5841. [DOI] [PubMed] [Google Scholar]
- Rodriguez A., Oliver H., Zou H., Chen P., Wang X.D., Abrams J.M. Dark is a Drosophila homologue of Apaf-1/CED-4 and functions in an evolutionarily conserved death pathway. Nat. Cell Biol. 1999;1:272–279. doi: 10.1038/12984. [DOI] [PubMed] [Google Scholar]
- Shimizu S., Narita M., Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- Song Q., Kuang Y., Dixit V.M., Vincenz C. Boo, a novel negative regulator of cell death, interacts with Apaf-1. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:167–178. doi: 10.1093/emboj/18.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thress K., Evans E.K., Kornbluth S. Reaper-induced dissociation of a Scythe-sequestered cytochrome c-releasing activity. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:5486–5493. doi: 10.1093/emboj/18.20.5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thress K., Henzel W., Shillinglaw W., Kornbluth S. Scythe—a novel reaper-binding apoptotic regulator. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:6135–6143. doi: 10.1093/emboj/17.21.6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden M.G., Thompson C.B. Bcl-2 proteinsregulators of apoptosis or of mitochondrial homeostasis? Nat. Cell Biol. 1999;1:E209–E216. doi: 10.1038/70237. [DOI] [PubMed] [Google Scholar]
- Varkey J., Chen P., Jemmerson R., Abrams J.M. Altered cytochrome c display precedes apoptotic cell death in Drosophila . J. Cell Biol. 1999;144:701–710. doi: 10.1083/jcb.144.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaux D.L., Weissman I.L., Kim S.K. Prevention of programmed cell death in Ceanorhabditis elegans by human bcl-2. Science. 1992;258:1955–1957. doi: 10.1126/science.1470921. [DOI] [PubMed] [Google Scholar]
- Wang S.L., Hawkins C.J., Yoo S.J., Muller H.A.J., Hay B.A. The Drosophila caspase inhibitor DIAP1 is essential for cell survival and is negatively regulated by HID. Cell. 1999;98:453–463. doi: 10.1016/s0092-8674(00)81974-1. [DOI] [PubMed] [Google Scholar]
- Xiang J.L., Chao D.T., Korsmeyer S.J. Bax-induced cell death may not require interleukin 1-beta-converting enzyme-like proteases. Proc. Natl. Acad. Sci. USA. 1996;93:14559–14563. doi: 10.1073/pnas.93.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Liu X.S., Bhalla K., Kim C.N., Ibrado A.M., Cai J.Y., Peng T.I., Jones D.P., Wang X.D. Prevention of apoptosis by bcl-2—release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- Zhou L., Song Z.W., Tittel J., Steller H. HAC-1, a Drosophila homolog of APAF-1 and CED-4 functions in developmental and radiation-induced apoptosis. Mol. Cell. 1999;4:745–755. doi: 10.1016/s1097-2765(00)80385-8. [DOI] [PubMed] [Google Scholar]