Abstract
The syndecan family of four transmembrane heparan sulfate proteoglycans binds a variety of soluble and insoluble extracellular effectors. Syndecan extracellular domains (ectodomains) can be shed intact by proteolytic cleavage of their core proteins, yielding soluble proteoglycans that retain the binding properties of their cell surface precursors. Shedding is accelerated by PMA activation of protein kinase C, and by ligand activation of the thrombin (G-protein–coupled) and EGF (protein tyrosine kinase) receptors (Subramanian, S.V., M.L. Fitzgerald, and M. Bernfield. 1997. J. Biol. Chem. 272:14713–14720). Syndecan-1 and -4 ectodomains are found in acute dermal wound fluids, where they regulate growth factor activity (Kato, M., H. Wang, V. Kainulainen, M.L. Fitzgerald, S. Ledbetter, D.M. Ornitz, and M. Bernfield. 1998. Nat. Med. 4:691–697) and proteolytic balance (Kainulainen, V., H. Wang, C. Schick, and M. Bernfield. 1998. J. Biol. Chem. 273:11563–11569). However, little is known about how syndecan ectodomain shedding is regulated.
To elucidate the mechanisms that regulate syndecan shedding, we analyzed several features of the process that sheds the syndecan-1 and -4 ectodomains. We find that shedding accelerated by various physiologic agents involves activation of distinct intracellular signaling pathways; and the proteolytic activity responsible for cleavage of syndecan core proteins, which is associated with the cell surface, can act on unstimulated adjacent cells, and is specifically inhibited by TIMP-3, a matrix-associated metalloproteinase inhibitor. In addition, we find that the syndecan-1 core protein is cleaved on the cell surface at a juxtamembrane site; and the proteolytic activity responsible for accelerated shedding differs from that involved in constitutive shedding of the syndecan ectodomains. These results demonstrate the existence of highly regulated mechanisms that can rapidly convert syndecans from cell surface receptors or coreceptors to soluble heparan sulfate proteoglycan effectors. Because the shed ectodomains are found and function in vivo, regulation of syndecan ectodomain shedding by physiological mediators indicates that shedding is a response to specific developmental and pathophysiological cues.
Keywords: cellular stress, heparan sulfate, mitogen-activated protein kinase, protein tyrosine, kinase, proteoglycan
Introduction
The extracellular domains (ectodomains) of ∼1% of membrane-anchored proteins can be released from the cell surface by endogenous proteolytic cleavage in a process known as ectodomain shedding (Hooper et al. 1997; Kiessling and Gordon 1998; Werb and Yan 1998; Hooper and Turner 1999). These proteins are structurally and functionally diverse and include the following: cytokines, growth factors and their receptors, ectoenzymes, cell adhesion molecules, and other transmembrane proteins, such as transforming growth factor-α, tumor necrosis factor (TNF)-α, TNF receptors, interleukin 6 receptor, angiotensin-converting enzyme, L-selectin, the Fas ligand, and the β-amyloid precursor protein. Shedding liberates ectodomains as soluble molecules and can reduce their concentration at the cell surface. Phorbol esters, such as PMA, are the best characterized shedding agonists, but ectodomain shedding can also be induced by other agents, including calcium ionophores, chemotactic peptides, cytokines, and growth factors (Hooper et al. 1997; Subramanian et al. 1997; Desdouits-Magnen et al. 1998; Jones et al. 1999). Shed ectodomains are detected in vivo in various body fluids, and their levels are often increased by tissue injury and in certain disease states (Ehlers and Riordan 1991; Gearing and Newman 1993; Abraham and Klagsbrun 1996; Hooper et al. 1997; Hooper and Turner 1999).
Ectodomain shedding appears to contribute to diverse pathophysiological events such as host defense, wound healing, arthritis, and Alzheimer's disease, but how shedding is regulated remains largely unknown (Kiessling and Gordon 1998; Hooper and Turner 1999; Merlos-Suarez and Arribas 1999). The cleavage process, variously ascribed to sheddases, secretases, or convertases, has several shared features, leading to the proposal of a common shedding system (Arribas et al. 1996; Mullberg et al. 1997). Cleavage is enhanced by phorbol ester activation of protein kinase C (PKC), is generally close to the extracellular face of the plasma membrane, and is sensitive to peptide hydroxamate metalloproteinase (MP) inhibitors (Hooper et al. 1997; Hooper and Turner 1999).
The intact ectodomains of each mammalian syndecan and the single Drosophila syndecan are constitutively shed from cultured cells (Kim et al. 1994; Spring et al. 1994) as part of normal cell surface HSPG turnover (Yanagishita and Hascall 1992; Yanagishita 1998). The syndecans are a family of transmembrane heparan sulfate proteoglycans (HSPGs) which, together with the lipid-linked glypicans, are the major source of HS at cell surfaces (Bernfield et al. 1992, Bernfield et al. 1999). All adhesive cells express at least one syndecan, and most express multiple syndecans (Kim et al. 1994). The core proteins of each of these four distinct gene products place the HS chains distal from the plasma membrane. Each syndecan contains at its COOH terminus a short and highly homologous cytoplasmic domain with serine and tyrosine residues at conserved positions.
By way of their HS chains, syndecans bind a wide variety of soluble and insoluble ligands, such as follows: extracellular matrix components, cell adhesion molecules, growth factors, cytokines, proteinases and proteinase inhibitors, lipid metabolism proteins, and microbial pathogens (Bernfield et al. 1992; Carey 1997; Bernfield et al. 1999). Syndecans facilitate the formation of signaling complexes by acting as coreceptors, concentrating and presenting ligands to the cell surface receptors, or internalizing them via endocytosis, thus, modulating ligand activities (Bernfield et al. 1999).
Because the HS chains of the cell surface and shed syndecans can bind the same ligands, syndecan ectodomain shedding is a mechanism for producing soluble HSPG effectors that can compete for the same ligands as their cell surface counterparts. Shedding of syndecan-1 and -4 can be accelerated via receptor activation (e.g., thrombin and EGF family members) and by direct action of proteases (e.g., plasmin and thrombin; Subramanian et al. 1997). These ectodomains are in fluids accumulating following injury and inflammation (Subramanian et al. 1997; Kato et al. 1998), but not in normal human plasma (Subramanian et al. 1997). The soluble syndecan-1 ectodomain potently inhibits heparin-mediated FGF-2 mitogenicity (Kato et al. 1998), which is consistent with studies indicating that the shed ectodomains can inhibit cell proliferation (Mali et al. 1994; Forsten et al. 1997; Dhodapkar and Sanderson 1999), and binds neutrophil-derived elastase and cathepsin G, reducing the action of their physiological inhibitors (Kainulainen et al. 1998). These activities are consistent with a role for the soluble syndecan ectodomains in the response to tissue injury.
While syndecan ectodomain shedding is known to be activated by physiological stimulants (Subramanian et al. 1997) and the ectodomains are being ascribed pathophysiological roles, little is known about how their release from the cell surface is regulated. Therefore, we analyzed several features of the process that sheds the syndecan-1 and -4 ectodomains. We find that syndecan shedding is regulated at multiple levels, based on the following findings: (1) that in addition to proteases and receptor ligands, agents that mediate cellular responses to stress accelerate shedding; (2) shedding accelerated by various physiological agents involves activation of distinct intracellular signaling pathways; (3) the proteolytic activity responsible for cleavage of syndecan core proteins is associated with the cell surface, and is a TIMP-3–sensitive MP that can act on unstimulated adjacent cells; (4) the syndecan-1 core protein is cleaved on the cell surface at a juxtamembrane site; and (5) the proteolytic activity responsible for accelerated shedding differs from that involved in constitutive shedding. These results demonstrate the existence of highly regulated mechanisms that convert syndecans from cell surface receptors or coreceptors to soluble HSPG effectors. Regulation of shedding by physiological mediators suggests that syndecan ectodomains are shed in response to specific developmental and pathophysiological cues. Now soluble, the shed syndecan ectodomains likely have roles in morphogenesis, tissue repair, and host defense. Preliminary reports of this study have been presented in abstract form (Fitzgerald, M.L., J.-S. Chun, and M. Bernfield, American Society of Cell Biology. 1994. 1813 (Abstr.); Fitzgerald, M.L., and M. Bernfield, American Society of Cell Biology. 1997. 2286 (Abstr.); Fitzgerald, M.L., Z. Wang, and M. Bernfield, American Society of Cell Biology. 1998. 326 (Abstr.)).
Materials and Methods
Materials and Chemicals
Ceramide (d-erythro-Sphingosine, N-Acetyl), EDTA, 1,10 phenanthroline, phosphoramidon, actinonin, benzamidine, E-64, leupeptin, pepstatin A, colchicine, cytochalasin D, wortmannin, sphingomyelinase, sorbitol, and MTT (tetrazolium salt) were purchased from Sigma Chemical Co.; LY 294002 from BioMol Research Labs; paraformaldehyde (16%) from Electron Microscopy Sciences; recombinant human (rHu) EGF from Intergen; PMA, 4α-phorbol-12, 13-didecanoate (4αPDD), thrombin receptor agonist peptide (TRAP), PMSF, staurosporine, bisindolylmaleimide I, genistein, PD 98059, and SB 202190 were purchased from Calbiochem-Novabiochem. Tyrphostin A25 and methyl 2,5-dihydroxycinnamate were purchased from Toronto Research Chemicals, and peptide hydroxamates BB-2116, BB-1101, and control compound BB-3861 were gifts from Dr. Alan Drummond (British Biotech Pharmaceuticals Ltd, Oxford, UK). Recombinant human (rHu) TIMP-1, -2, and -3 were expressed and purified from NSO mouse myeloma cells (Apte et al. 1995; Murphy and Willenbrock 1995). All three inhibitors were active site–titrated against a standard preparation of stromelysin, and calibrated using an inhibitor standard to confirm that they were >95% functional protein as previously described (Murphy and Willenbrock 1995).
Immunochemicals
Antibodies specific to syndecan ectodomains included the rat mAb 281-2 against the mouse syndecan-1 ectodomain (Jalkanen et al. 1985), rabbit polyclonal antisera MSE-4 against the recombinant mouse syndecan-4 ectodomain (Kim et al. 1994; Subramanian et al. 1997), and the mouse mAb DL-101 against the recombinant human syndecan-1 ectodomain (Kainulainen et al. 1998). HRP-conjugated donkey anti–rat IgG, HRP-goat anti–rabbit IgG, HRP-goat anti–mouse IgG, and FITC-conjugated streptavidin were purchased from Jackson ImmunoResearch Laboratories, Inc., or Organon/Cappel.
Production of Full-length Syndecan-1 Fusion Constructs with Wild-type or Mutated Juxtamembrane (JM) Domains
Expression vectors for the synthesis of full-length syndecan-1 with both wild-type and mutated juxtamembrane domains were used for transient transfection shedding assays. A full-length expression vector in which the HindIII fragment containing the full-length murine syndecan-1 from LK444 (Kato et al. 1995) was inserted into the HindIII site of pcDNA3 (Invitrogen Corp.). The correct orientation was confirmed by restriction mapping, and this expression vector was designated syn1-WTJM.
To obtain a putative uncleavable syndecan-1 mutant, the JM domain of syndecan-1 spanning from Gln238 to Gln252 was replaced with the amino acid sequence of the corresponding JM domain of human CD4 (CD4-JM) (Hodge et al. 1991) using PCR with pfu DNA polymerase. The following oligonucleotides were used: the human CD4 JM region containing nucleotides 1,225–1,269 of the human CD4 cDNA was amplified using oligo A, 5′-CAGCCCCCGGTGGACGTCAAGGTCTGCCC-3′, and oligo B, 5′GACACCTCCCAGCACGGCCATTGGCGCAC-3′. The 5′ region of syndecan-1 containing nucleotides 214–953 of the murine syndecan-1 cDNA (Saunders et al. 1989) was amplified using oligos C, 5′-CACAAGCTTCCCGCCGCCGGTCTG-3′, and D, 5′-GGGCAGAACCTTGACGTCCACCGGGGGCTG-3′. The 3′ region of syndecan-1 containing nucleotides 995–1,379 was amplified using oligos E, 5′-CTTCTAGGCGGATCCCAAAGGAGG-3′, and F, 5′-GTGCAGCCAATGGCCGTGCTGGGAGGTGTC-3′. The three fragments were gel-purified and used to construct the putative uncleavable construct using PCR with pfu DNA polymerase. The resulting PCR product was subcloned into a pCR3 vector (Invitrogen Corp.) and sequenced. The syndecan-1/CD4-JM fragment was subcloned into the HindIII and BamHI sites of pcDNA3 and designated as syn1-CD4JM.
Cell Lines and Culture Conditions
Nonadherent suspension cell lines (plasma cells P3X63 Ag8.653 [murine] and ARK [human]; Ridley et al. 1993) stimulated with PMA were used for immunofluorescence and cell mixing experiments. Adherent cells (NMuMG epithelial and SVEC4-10 endothelial) were used to assay shedding agonists. PMA was used in serum-free media and incubations were performed for 15–30 min. Other agonists were assayed in media containing 1% FCS and routinely incubated for 2 or 14 h when constitutive shedding was measured. COS-7 African green monkey kidney cells were used for cleavage site analysis.
Nonadherent cells were cultured in RPMI 1640 medium containing glucose at 4.5 g/l (Mediatech), supplemented with 10% heat-inactivated FCS. Adherent cells were cultured in DME containing glucose at 4.5 g/l (Mediatech), and supplemented with 10% (SVEC4-10) or 7.5% (NMuMG) FCS (Intergen). COS-7 cells were cultured in DME containing glucose at 1 g/l, and supplemented with 10% FCS. Cells were cultured in a humidified incubator containing 5% CO2.
Cell Surface Labeling and Fluorescence Analysis of Syndecan-1
P3X63 cells were incubated with 20 μg/ml biotinylated mAb 281-2 for 30 min on ice (conditions that prevent shedding) with gentle agitation, washed twice by centrifugation at 200 g at 4°C to remove unbound mAb, and incubated (106 cells/tube) for 30 min at 37°C with or without 0.5 μM PMA. All washes and incubations were done in serum-free RPMI 1640 media. After treatment, cells were fixed in 4% paraformaldehyde in PBS for 15 min at 4°C, washed in PBS, and incubated with FITC-conjugated streptavidin for 30 min at room temperature. Cells labeled with FITC-streptavidin only were included as controls for nonspecific staining. Cells were washed in PBS, mounted in ProLong Antifade (Molecular Probes, Inc.), and viewed on a Zeiss Axiophot microscope equipped with epifluorescence and a 100× PlanApo oil immersion objective. Samples were photographed with Kodak TMAX 400 film exposed at ASA 800.
Shedding Assays
Adherent cells were cultured to confluency in 96-well tissue culture plates for PMA treatment and constitutive shedding assays, or in 6-well plates for treatment with other agonists. Nonadherent cells were routinely transferred to 1.5-ml microcentrifuge tubes (∼106 cells/tube) for PMA treatment, as described below. Shedding assays were performed essentially as previously described (Subramanian et al. 1997). In brief, at the time of the assay, culture media were replaced with fresh media containing the indicated test agents in the absence of serum (PMA treatment) or in the presence of 1% FCS (2- and 14-h assays). Cells were incubated at 37°C for 15–30 min (PMA treatment), 2 h (other agonists), or 14 h (constitutive shedding). After incubations, cells were examined by phase microscopy for survival and morphology, and the conditioned media were harvested for dot blot analysis. The 96-well plate assays were performed in triplicate using 100 μl of media per well; 90 μl of media was used for dot blot analysis. The 6-well plate assays contained 600 μl of media per well, which was divided into three equal portions for the dot blot analysis. To evaluate changes in cell number after EGF and TRAP treatment, in some assays, cells were trypsinized and counted with a hemocytometer after incubation. For assays using hyperosmolarity, ceramide, and sphingomyelinase, cell viability was measured using the tetrazolium salt (MTT) conversion assay (Hansen et al. 1989). Within each experiment, there was no significant difference in cell number or viability after the different treatments. All assays were performed at least twice.
To determine whether the proteolytic activity responsible for shedding is soluble or membrane-associated, P3X63 cells were coincubated for 15 min at 37°C with human ARK cells that had been pretreated with or without 0.5 μM PMA for 15 min at 37°C and washed twice with serum-free media. Cells were mixed together in 1.5-ml microcentrifuge tubes or separated from each other by a Transwell insert (6.5 mm; Costar Corp.) containing a polycarbonate filter with 0.4-μm pores. In both the cell mixing and Transwell assays, a constant number of P3X63 cells (5 × 106) and a varying number of ARK cells were used. Conditioned media were divided into three equal portions and analyzed by dot blot analysis using mAb 281-2 to detect syndecan-1 shed from the untreated P3X63 cells. The same assay was also done using PMA-treated P3X63 cells and untreated ARK cells, but the conditioned media were analyzed by dot blot using mAb DL-101 to detect human syndecan-1.
To determine whether the putative uncleavable mutant ectodomain is shed in response to PMA treatment, wild-type plasmids (syn1-WTJM) and plasmids containing the syn1-CD4JM mutant were transiently transfected into COS-7 cells using Lipofectamine (Life Technologies, Inc.). Cells were cultured in 100-mm tissue culture plates (Falcon) for transient transfections. Cells were trypsinized 24 h after transfection and replated in 6-well plates for shedding assays at 36–48 h after transfection. Cell surface expression of syndecan-1 was confirmed by immunocytochemistry using mAb 281-2. The two constructs were shown to be expressed at equivalent levels by dot blot analysis of supernatants collected after trypsinization of cells to remove cell surface syndecan-1, as previously described (Subramanian et al. 1997).
Dot Immunoassay
Specificity and quantitation of the dot immunoassay for shed syndecan-1 and -4 ectodomains has been described previously (Subramanian et al. 1997). In brief, the conditioned media were diluted in buffer A (0.15 M NaCl buffered to pH 4.5 with 50 mM sodium acetate, and with 0.1% Triton X-100), and applied to cationic polyvinylidine difluoride–based membranes (Immobilon-N; Millipore) under mild vacuum in an immunodot apparatus (V&P Scientific). By acidifying the samples in buffer A, only highly anionic molecules in the conditioned media, such as proteoglycans, are retained by the cationic Immobilon-N membrane while most proteins are cationic at this pH and pass through the membrane. The membranes were washed twice with buffer A, blocked for 1 h with Blotto (3% Carnation instant nonfat dry milk, 0.15 M NaCl in 10 mM Tris, pH 7.4), incubated with 1.12 μg/ml of mAb 281-2, 3.6 μg/ml mAb DL-101, or a 1:1,000 dilution of MSE-4 antiserum, washed with TBS containing 0.3% Tween 20, and incubated with a 5,000-fold dilution of HRP-conjugated anti-rat, anti-mouse, or anti-rabbit IgG, respectively. All antibodies were diluted in Blotto with 0.3% Tween 20.
Detection was by the ECL system (Amersham) as described by the manufacturer. Results were quantified by scanning the exposed X-ray film with a Hewlett Packard Scan Jet IVC running the DeskScan II software package, and using area measurements from NIH Image (V. 1.57 or 1.60) software. Experimental values were within the linear range of the assay. Results are expressed as the amount of syndecan shed in relative absorbance units (AU). AU varied between experiments, in part, because of differences in exposure times and because of differences in treatment parameters. Thus, AUs cannot be compared between experiments. Each point represents the mean ± SD of triplicate determinations. Statistical significance was calculated using the t test with the InStat biostatistic program (P < 0.01).
Results
This study extends our previous work showing that shedding of syndecan ectodomains can be accelerated by physiological agonists (Subramanian et al. 1997). We have now studied regulation of this shedding using a pharmacological approach under defined conditions (PMA in serum-free medium for 15–30 min and other agonists in 1% FCS for 2 h) and have identified several aspects, including the proteolytic activity, involved in its regulation.
Inhibition of Tyrosine Kinase Activity Prevents Shedding Accelerated by PMA, Receptor Activation, and Cellular Stress
We previously showed that the tyrosine kinase inhibitor, genistein, blocked shedding of syndecan-1 and -4 ectodomains accelerated by receptor activation, suggesting that tyrosine phosphorylation is involved in accelerated shedding (Subramanian et al. 1997). Using NMuMG cells, we have now asked whether shedding accelerated by PMA, TRAP, and cellular stress (e.g., ceramide and hyperosmolarity) involves tyrosine kinase activity.
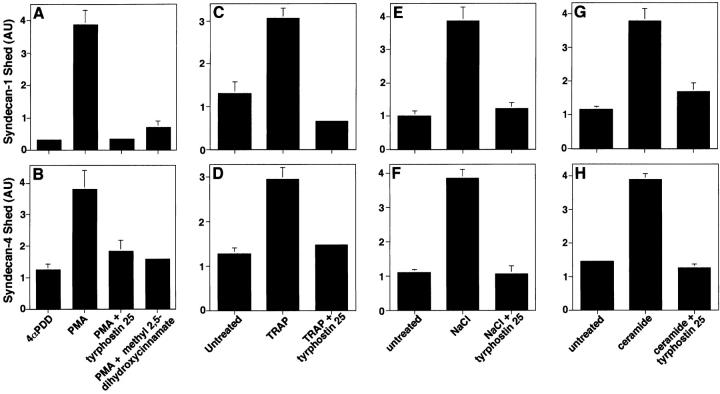
Phorbol esters, PKC activators known to enhance ectodomain shedding of a diverse group of cell surface proteins (Hooper et al. 1997), rapidly (5 min) accelerate shedding of syndecan-1 and -4 ectodomains from a variety of cultured cells (Fitzgerald, M.L., J.-S. Chun, and M. Bernfield, American Society of Cell Biology. 1994. 1813 (Abstr.); Subramanian et al., 1997). Both tyrphostin A25 and methyl 2,5 dihydroxycinnamate, protein tyrosine kinase inhibitors, prevented the PMA-accelerated shedding of syndecan-1 (Fig. 1 A) and syndecan-4 (Fig. 1 B) ectodomains.
Figure 1.
Accelerated shedding of syndecan-1 and -4 depends on protein tyrosine kinase activity. (A and B) NMuMG epithelial cells were incubated with 4αPDD (0.5 μM), an inactive PMA analogue or PMA for 30 min and in the presence or absence of tyrphostin A25 (5 μg/ml) or methyl 2,5, dihydroxycinnamate (5 μg/ml). NMuMG cells were incubated with or without the following: (C and D) 100 μM TRAP, or (E and F) 700 mOsm NaCl, or (G and H) 100 μM ceramide for 2 h in the presence or absence of tyrphostin A25 (5 μg/ml). Conditioned media were harvested and applied to cationic Immobilon-N membranes for dot blot analysis using ECL detection. Syndecan-1 (A, C, E, and G) and syndecan-4 (B, D, F, and H) ectodomains were detected by ECL using mAb 281-2 and MSE-4 antiserum, respectively. Results are expressed as the amount of syndecan ectodomain shed in AU quantified by densitometric scanning and analyzed with NIH image software. Each point represents the mean ± SD of triplicate determinations. For each shedding agonist assayed, incubation with inhibitor alone had no effect on the level of shedding compared with the untreated control.
The thrombin receptor (also known as proteinase-activated receptor–1 [Dery and Bunnett 1999; Dery et al. 1998]) is activated by the 14–amino acid thrombin receptor agonist peptide (TRAP), which has no inherent proteolytic activity (Troyer et al. 1992; Dery and Bunnett 1999). Tyrphostin A25 inhibited TRAP-accelerated shedding of syndecan-1 (Fig. 1 C) and syndecan-4 (Fig. 1 D) ectodomains, as previously reported for SVEC4-10 cells (Subramanian et al. 1997). Because syndecan-1 and -4 ectodomains are in the fluids that surround injured tissues (Subramanian et al. 1997), we reasoned that cellular stress may accelerate shedding. We tested whether hyperosmolarity and the stress response agonist, ceramide, accelerate syndecan ectodomain shedding. Shedding of syndecan-1 and -4 ectodomains was accelerated by treatment of NMuMG cells with 700 mOsm NaCl (Fig. 1E and Fig. F) and ceramide (Fig. 1G and Fig. H). This shedding was also inhibited by tyrphostin A25. Similar results were observed when SVEC4-10 cells were assayed. Sphingomyelinase (1 U/ml), which produces ceramide, sorbitol (700 mOsm), NaCl (as low as 400 mOsm), and heat shock (42°C) also accelerated shedding of syndecan-1 and -4 ectodomains (data not shown). These results indicate that shedding of syndecan-1 and -4 ectodomains can be accelerated by agents that mediate cellular responses to stress (Rosette and Karin 1996; Verheij et al. 1996) and, therefore, implicate the c-Jun NH2-terminal/stress–activated protein kinase (JNK/SAPK) pathways in accelerated shedding (Kyriakis and Avruch 1996). Thus, shedding of syndecan ectodomains accelerated by PMA, thrombin receptor activation, and cellular stress involves protein tyrosine kinase (PTK) activity. Cell morphology and viability were not affected during these assays, except for minimal cell retraction under hypertonic conditions and in the presence of ceramide.
Inhibition of Protein Kinase C Activity Prevents Shedding Accelerated by PMA and Cellular Stress but Not by Receptor Activation
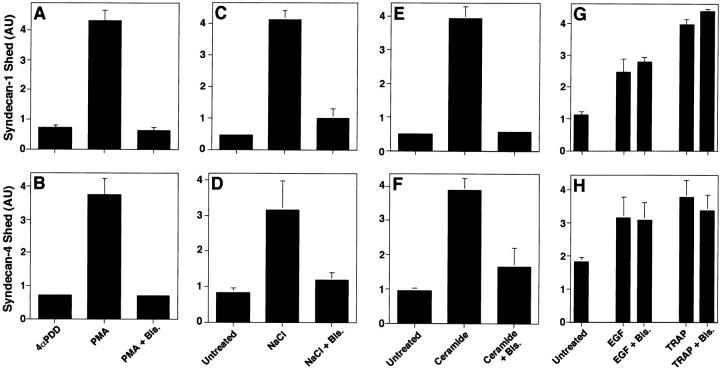
To determine whether syndecan ectodomain shedding accelerated by PMA, thrombin, and EGF receptor activation, or cellular stress involves PKC activity, we tested whether bisindolylmaleimide I, a potent and selective inhibitor of PKC (Toullec et al. 1991), affected shedding. Bisindolylmaleimide I completely blocked PMA-accelerated shedding of syndecan-1 (Fig. 2 A) and -4 (Fig. 2 B). Shedding of syndecan-1 and -4 accelerated by both hyperosmolarity (Fig. 2C and Fig. D) and ceramide (Fig. 2E and Fig. F) were also inhibited by bisindolylmaleimide I. In contrast, neither TRAP- nor EGF-accelerated shedding of syndecan-1 or -4 (Fig. 2G and Fig. H) were affected by bisindolylmaleimide I. Similar results were observed using SVEC4-10 cells. Thus, inhibition of PKC activity prevents shedding of syndecan-1 and -4 ectodomains accelerated by PMA and cellular stress, but does not affect thrombin or EGF receptor–activated shedding. These results indicate that different agonists activate distinct intracellular signaling pathways to accelerate syndecan shedding.
Figure 2.
Shedding accelerated by PMA and stress but not by receptor activation depends on protein kinase C activity. NMuMG cells were incubated (A and B) for 30 min with PMA or 4αPDD (0.5 μM). NMuMG cells were incubated (C and D) for 2 h with or without the following: 700 mOsm NaCl, or (E and F) 100 μM ceramide, or (G and H) 10 ng/ml EGF, or 100 μM TRAP. Each agonist was assayed in the absence or presence of bisindolylmaleimide I (Bis., 1 μM). Conditioned media were assayed for syndecan-1 (A, C, E, and G) and syndecan-4 (B, D, F, and H) ectodomains by dot blot analysis as in Fig. 1. Quantitation was done as in Fig. 1, and each point represents the mean ± SD (n = 3). For each shedding agonist assayed, incubation with inhibitor alone had no effect on the level of shedding compared with the untreated control.
Inhibition of MAP Kinase Activity Prevents Shedding Accelerated by Receptor Activation but Not by PMA
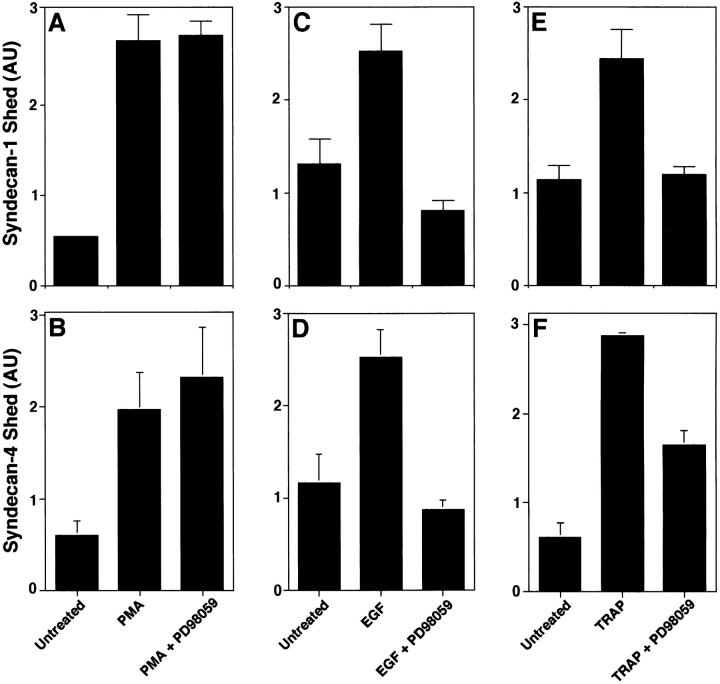
We asked whether inhibitors known to target mitogen-activated protein kinase (MAP kinase) signaling systems affect shedding. We tested whether the specific MAP kinase inhibitors, PD98059 and SB202190, affected shedding. PD98059 is a selective inhibitor of MAP kinase kinase, which blocks the subsequent phosphorylation and activation of ERK MAP kinase in vitro and in vivo (Alessi et al. 1995; Dudley et al. 1995). PD98059 (at ≤50 μM) did not inhibit PMA-accelerated shedding of syndecan-1 (Fig. 3 A) or syndecan-4 (Fig. 3 B) ectodomains from NMuMG cells, but did block the EGF-accelerated (Fig. 3C and Fig. D) and TRAP-accelerated (Fig. 3E and Fig. F) shedding of these ectodomains. PD98059 does not affect cell viability at concentrations up to 100 μM (Desdouits-Magnen et al. 1998), and no change in cell morphology was seen during the assays. SB202190 is a specific inhibitor of the p38 MAP kinase pathway both in vitro and in vivo (Lee et al. 1994; Cuenda et al. 1995). SB202190 (at ≤50 μM) did not inhibit PMA- or receptor-activated shedding of syndecan-1 or -4 (data not shown). These results suggest that both stress- and receptor-activated, but not PMA-accelerated shedding involve MAP kinase activity.
Figure 3.
Inhibition of MAP kinase activity prevents shedding accelerated by receptor activation but not by PMA. NMuMG cells were incubated (A and B) for 30 min with or without 0.5 μM PMA, or (C and D) for 2 h with or without 10 ng/ml EGF, or (E and F) for 2 h with or without 100 μM TRAP. Each agonist was assayed in the absence or presence of PD98059 (20 μM). Conditioned media were analyzed by dot blot for syndecan-1 (A, C, and E) and syndecan-4 (B, D, and F) ectodomains as in Fig. 1. Quantitation was done as in Fig. 1 and each point represents the mean ± SD (n = 3). For each shedding agonist assayed, incubation with inhibitor alone had no effect on the level of shedding compared with the untreated control.
PMA Causes Syndecan Ectodomain Shedding from the Cell Surface
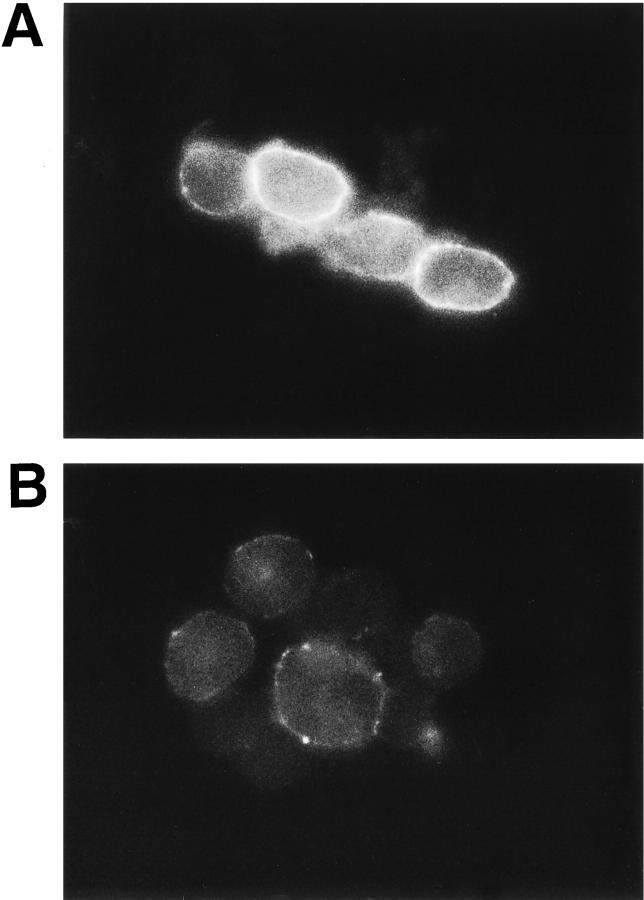
P3X63 cells, a mouse plasma cell line, express abundant cell surface syndecan-1, have barely detectable levels of intracellular syndecan-1, and shed syndecan-1 at a low constitutive rate. Immunoprecipitation and dot blot analysis of P3X63 cell lysates and conditioned media showed that PMA treatment causes cell surface syndecan-1 to be rapidly lost with the concomitant appearance of the shed syndecan-1 ectodomains in the conditioned media (Fitzgerald, M.L., J.-S. Chun, and M. Bernfield, American Society of Cell Biology. 1994. 1813 (Abstr.)). We have now labeled syndecan-1 at the surface of these cells with biotinylated mAb 281-2 at 4°C to avoid constitutive shedding. Bright fluorescence and occasional punctate stain was seen at the cell surface (Fig. 4 A). After PMA treatment of these cells, the syndecan-1 stain was reduced after 5 min and was completely lost after 30 min, except for some residual punctate stains (Fig. 4 B). To confirm that shedding is from the cell surface, we asked whether trypsinization of unlabeled P3X63 cells at 4°C, which removes cell surface syndecan-1 quantitatively (Jalkanen et al. 1987), prevents subsequent PMA-induced shedding. No syndecan-1 ectodomain was detected in the conditioned media after PMA treatment of trypsinized cells (data not shown). Moreover, pretreatment of cells with cytochalasin D (1 μg/ml) or colchicine (50 μM) does not inhibit PMA-accelerated shedding, and antibody-induced cross-linking of cell surface syndecan-1 actually prevents accelerated shedding (data not shown). Thus, accelerated syndecan-1 ectodomain shedding is a cell surface event.
Figure 4.
Syndecan ectodomains are shed by proteolytic cleavage at the cell surface. Live P3X63 mouse plasma cells were incubated for 30 min at 4°C with biotinylated mAb 281-2 against the syndecan-1 ectodomain before treatment with or without 0.5 μM PMA for 30 min at 37°C. Cells were fixed and labeled with FITC-avidin for immunofluorescent detection of cell surface syndecan-1 on untreated (A) or PMA-treated (B) cells.
A Cell Surface–associated Proteolytic Activity Is Responsible for Accelerated Shedding
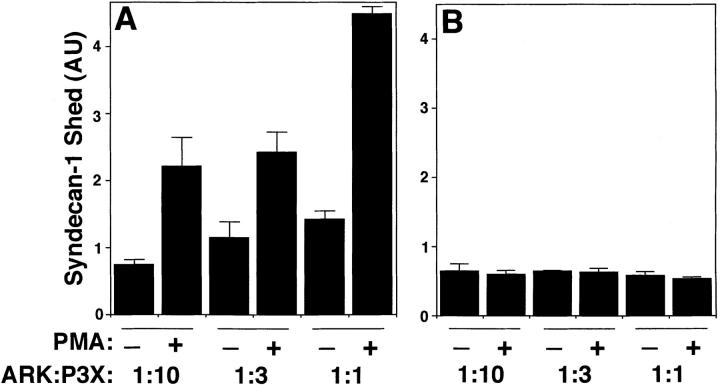
We next evaluated whether the proteolytic activity responsible for cleavage of the core protein and shedding of the ectodomain is soluble or membrane-associated. For these assays, PMA-treated human ARK cells and untreated mouse P3X63 cells were either mixed together or separated from each other by a Transwell membrane. In both assays, a constant number of P3X63 cells and a varying number of ARK cells were used. Human and mouse syndecan-1 ectodomains were distinguished using mAb DL-101 and 281-2, respectively. When cells were mixed to allow cell–cell contact, the PMA-treated ARK cells accelerated shedding of the syndecan-1 ectodomain from the untreated P3X63 cells (Fig. 5 A). However, when a Transwell membrane separated the cells, the PMA-treated ARK cells did not accelerate shedding from the untreated P3X63 cells (Fig. 5 B). Similar results were observed when the same experiments were performed using PMA-treated P3X63 cells and untreated ARK cells (data not shown). These results indicate that the proteolytic activity responsible for shedding is cell-associated rather than soluble. Further, because this proteolytic activity can act on adjacent cells, it likely acts at the cell surface.
Figure 5.
A membrane-associated proteolytic activity is responsible for accelerated shedding. P3X63 cells were coincubated for 15 min with human ARK cells that had been pretreated with or without PMA for 15 min and washed twice with serum-free media. Cells were (A) mixed together or (B) separated from each other by a Transwell membrane. A constant number of P3X63 cells (5 × 106) and a varying number of ARK cells were used. Conditioned media were analyzed by dot blot using mAb 281-2 to detect syndecan-1 shed from the surface of P3X63 cells. Quantitation was done as in Fig. 1 and each point represents the mean ± SD (n = 3).
PMA-accelerated Shedding of Syndecan-1 Ectodomains Is Due to Cleavage at a Juxtamembrane Site in the Core Protein
We previously showed that the GAG-free syndecan-1 and -4 ectodomain core proteins, released from cultured cells, were the same size regardless of whether shed constitutively, accelerated by PMA, or receptor activation, or by direct plasmin or thrombin treatment (Subramanian et al. 1997). Based on the relative molecular mass, which was determined by SDS-PAGE analysis of the shed ectodomains and the transmembrane proteoglycans (Jalkanen et al. 1987; Rapraeger et al. 1987; Subramanian et al. 1997), we predicted that cleavage occurs at a juxtamembrane site in the core protein ectodomain.
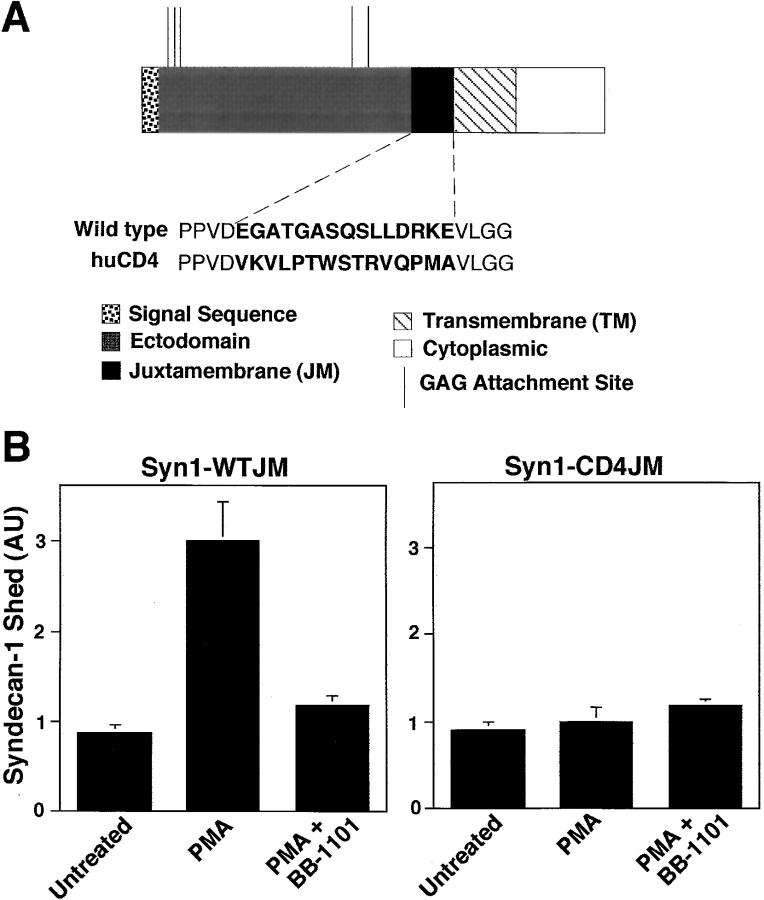
We constructed a putative uncleavable syndecan-1 mutant by replacing 15 amino acids of the wild-type syndecan-1 juxtamembrane domain with the corresponding amino acid sequence of the human CD4 T cell transmembrane antigen (Fig. 6 A), and tested whether the mutant was resistant to PMA-accelerated shedding. CD4 does not undergo PMA-accelerated shedding (Wang et al. 1987; Shin et al. 1991), suggesting that CD4 is not cleaved by the proteolytic activity that sheds syndecan-1. Whereas PMA treatment of COS-7 cells transfected with wild-type syndecan-1 (syn1-WTJM) released the soluble ectodomain, an activity inhibited by a peptide hydroxamate (cf. below), PMA did not accelerate release of syndecan-1 from cells transfected with the syn1-CD4 juxtamembrane mutant (Fig. 6 B). Similar results were observed using CHO cells and HT-1080 human fibrosarcoma cells (data not shown). Thus, the core protein is cleaved in the juxtamembrane region within 15 amino acids from the cell surface.
Figure 6.
PMA-accelerated shedding of syndecan-1 ectodomains results from cleavage at a juxtamembrane site in the core protein. (A) Schematic depiction of wild-type (WT) syndecan-1 and the CD4-juxtamembrane (JM) mutant syndecan-1. The JM region of the ectodomain is shown both with the WT amino acid sequence and with the CD4-JM domain mutant sequence (bold face). (B) COS-7 cells were transiently transfected with either the WT syndecan-1 or the CD4-JM mutant. Cells were incubated with or without PMA (0.5 μM for 15 min) in the absence or presence of peptide hydroxamate BB-1101 (1 μM). Syndecan-1 ectodomains in the conditioned media were assayed by dot blot analysis and quantitation was done as in Fig. 1. Each point represents the mean ± SD (n = 3).
Hydroxamate Inhibitors of Metalloproteinases Prevent Accelerated Shedding
Because the accelerated shedding of a variety of cell surface protein ectodomains has been shown to require MP activity (Hooper et al. 1997), we examined a panel of MP inhibitors for their effect on PMA-accelerated shedding of syndecan-1. We found that the transition metal ion chelators, EDTA and 1,10 phenanthroline, and the hydroxamic acid–based MP inhibitor actininon (Sayama et al. 1995), blocked PMA-accelerated shedding of syndecan-1 ectodomains from P3X63 and SVEC4-10 cells in a dose-dependent manner, but phosphoramidon, a zinc chelator specific for thermolysin, did not affect accelerated shedding (Fitzgerald, M.L., and M. Bernfield, American Society of Cell Biologists. 1997. 2286 (Abstr.)). Inhibitors of aspartic, cysteine, and serine proteinases (pepstatin A, E-64, benzamidine, leupeptin, and PMSF) did not block PMA-accelerated shedding (data not shown).
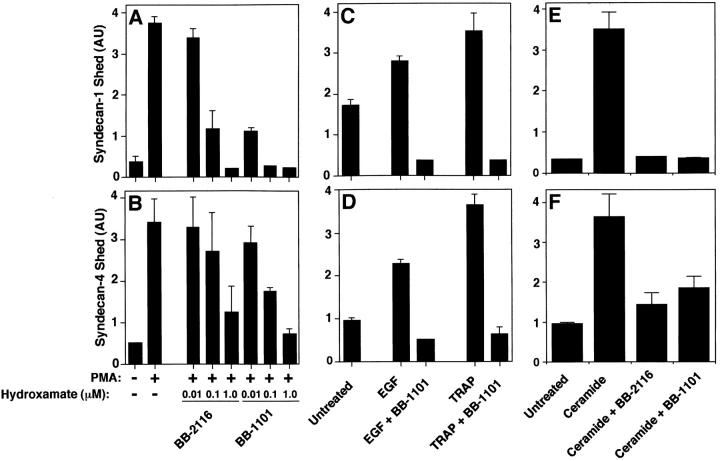
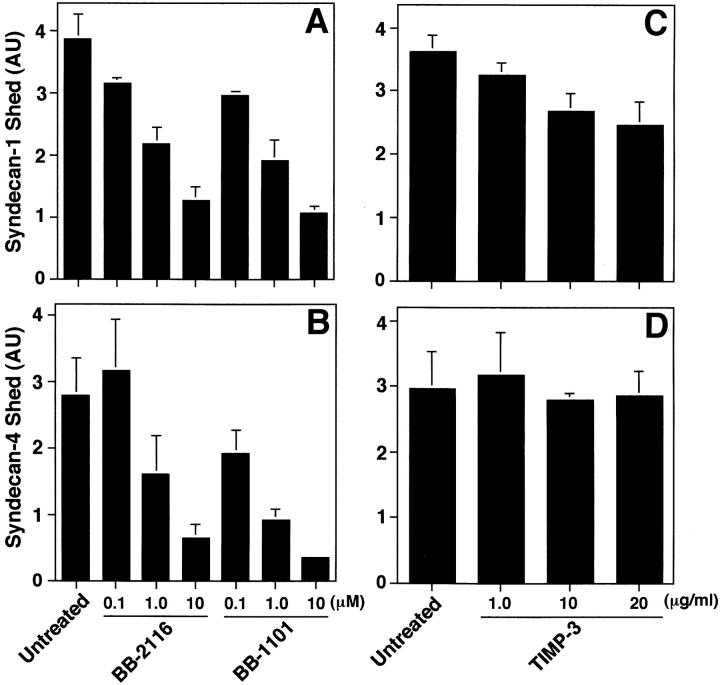
Thus, we tested the effects of hydroxamic acid-based MP inhibitors, initially developed to inhibit shedding of pro-TNF-α (Odake et al. 1994; Sayama et al. 1995; Wojtowicz-Praga et al. 1997; Pratt et al. 1998) on syndecan-1 and -4 shedding accelerated by PMA, receptor activation, and cellular stress. The peptide hydroxamates BB-2116 and BB-1101 reduced the shedding of syndecan-1 (Fig. 7 A) and syndecan-4 (Fig. 7 B) ectodomains from PMA-treated NMuMG cells in a concentration-dependent manner without affecting cell morphology or viability; the IC50 was ∼0.1 μM. Similar results were observed when P3X63 and SVEC4-10 cells were used (data not shown). EGF-, TRAP-, and ceramide-accelerated shedding of syndecan-1 (Fig. 7C and Fig. E) and syndecan-4 (Fig. 7D and Fig. F) ectodomains were also inhibited by hydroxamate BB-1101. The control hydroxamate compound BB-3861, which lacks the zinc-binding domain, had no effect on syndecan shedding at concentrations up to 10 μM (data not shown). These results suggest that syndecan-1 and -4 shedding, accelerated by each of the agonists tested, depends on metalloproteinase activity.
Figure 7.
Accelerated shedding is inhibited by peptide hydroxamates. (A and B) NMuMG cells were incubated with or without PMA (0.5 μM for 30 min) in the presence or absence of peptide hydroxamate BB-2116 or BB-1101 at the indicated concentration. (C and D) SVEC4-10 cells were incubated for 2 h with or without 10 ng/ml EGF or 100 μM TRAP in the presence or absence of 1 μM BB-1101. (E and F) NMuMG cells were incubated for 2 h with or without 100 μM ceramide in the presence or absence of 1 μM BB-1101. Syndecan-1 (A, C, and E) and syndecan-4 (B, D, and F) ectodomains in the conditioned media were assayed by dot blot analysis as in Fig. 1. Quantitation was done as in Fig. 1 and each point represents the mean ± SD (n = 3). For each shedding agonist assayed, incubation with inhibitor alone (1 μM) reduced the level of shedding compared with the untreated control.
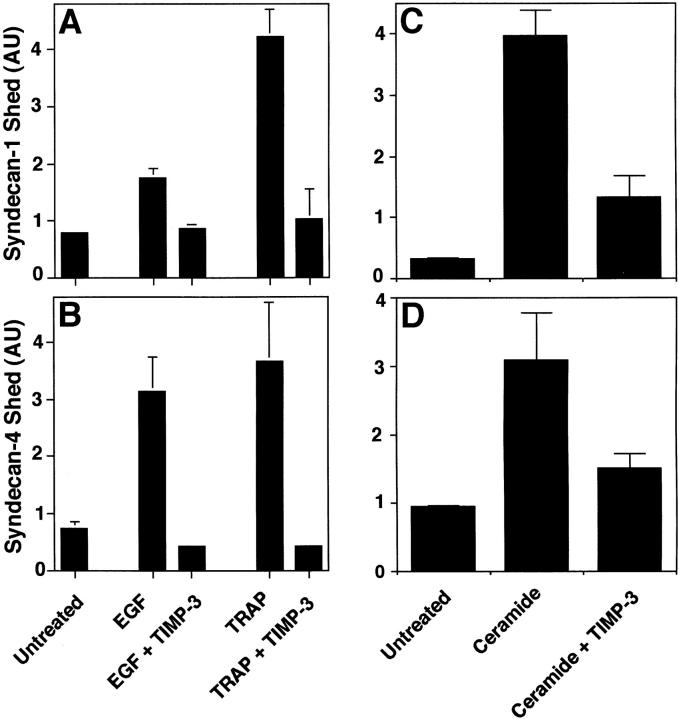
The Matrix-associated Metalloproteinase Inhibitor TIMP-3 Prevents Accelerated Shedding
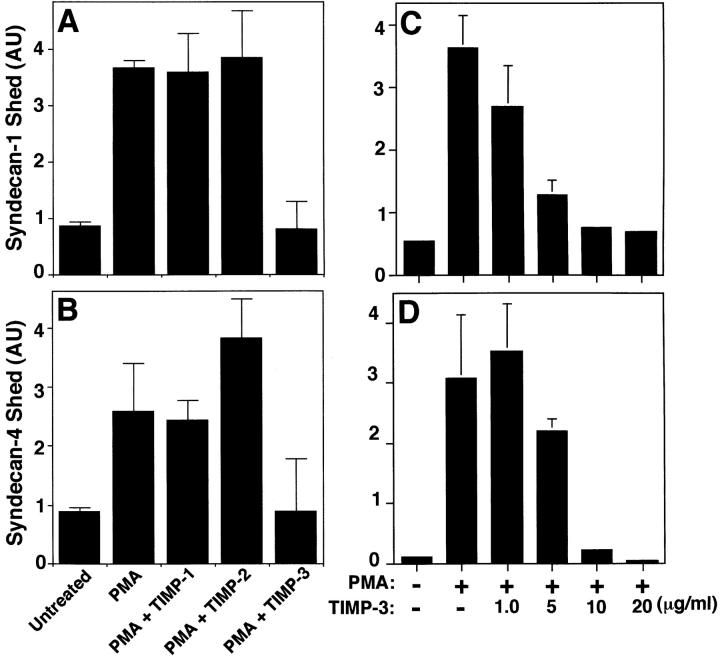
Because the peptide hydroxamate MP inhibitors prevented syndecan-1 and -4 ectodomain shedding, we tested members of the tissue inhibitor of metalloproteinase (TIMP) family of secreted MMP inhibitors (Murphy and Willenbrock 1995; Anand-Apte et al. 1996; Gomez et al. 1997). TIMP family members have distinct expression patterns (Leco et al. 1994), form complexes with different progelatinases (Murphy and Willenbrock 1995; Gomez et al. 1997; Butler et al. 1999b; Murphy et al. 1999), and differ in their ability to bind heparin (Butler et al. 1999b). PMA-accelerated shedding of both syndecan-1 and -4 ectodomains from NMuMG cells (Fig. 8, A and B) is specifically inhibited by TIMP-3; TIMP-1 and TIMP-2 had no effect on shedding at concentrations up to 20 μg/ml. Similar results were observed when P3X63 and SVEC4-10 cells were used (data not shown). TIMP-3 inhibition was concentration-dependent; the IC50 was ∼5 μg/ml, and PMA-accelerated shedding was completely prevented in the presence of 20 μg/ml (∼0.8 μM) TIMP-3 (Fig. 8C and Fig. D). To determine whether shedding accelerated by physiological agonists is also inhibited by TIMP-3, we tested the effect of TIMP-3 on EGF-, TRAP-, and ceramide-accelerated shedding of syndecan-1 and -4 ectodomains. TIMP-3 reduced EGF and TRAP receptor–activated shedding from SVEC4-10 cells (Fig. 9A and Fig. B), and ceramide-induced shedding of these syndecans from NMuMG cells (Fig. 10C and Fig. D). Thus, shedding accelerated by PMA and a variety of physiological agonists requires a TIMP-3–sensitive MP activity.
Figure 8.
PMA-accelerated shedding is specifically inhibited by TIMP-3. (A and B) NMuMG cells were treated with PMA (0.5 μM for 30 min) in the presence or absence of 20 μg/ml TIMP-1, -2, or -3. (C and D) NMuMG cells were treated with or without PMA (0.5 μM for 30 min) in the presence or absence of TIMP-3 at the indicated concentration. Syndecan-1 (A and C) and syndecan-4 (B and D) ectodomains in the conditioned media were assayed by dot blot analysis as in Fig. 1. Quantitation was done as in Fig. 1 and each point represents the mean ± SD (n = 3). For each shedding agonist assayed, treatment with TIMP-1, -2, or -3 alone (20 μM) had no effect on the level of shedding compared with the untreated control.
Figure 9.
Receptor- and stress-activated shedding is inhibited by TIMP-3. (A and B) SVEC4-10 cells were treated with or without 10 ng/ml EGF or 100 μM TRAP, or (C and D) NMuMG cells were treated with or without 100 μM ceramide for 2 h in the presence or absence of 20 μg/ml TIMP-3. The syndecan-1 (A and C) and syndecan-4 (B and D) ectodomains in the conditioned media were assayed by dot blot analysis as in Fig. 1. Quantitation was done as in Fig. 1 and each point represents the mean ± SD (n = 3). For each shedding agonist assayed, treatment with TIMP-1, -2, or -3 alone (20 μM) had no effect on the level of shedding compared with the untreated control.
Figure 10.
Constitutive shedding of syndecan-1 and -4 is inhibited by peptide hydroxamates but not by TIMP-3. SVEC4-10 cells were incubated for 14 h in the presence or absence of the indicated concentration of (A and B) peptide hydroxamate BB-2116 and BB-1101, or (C and D) TIMP-3. The syndecan-1 (A and C) and syndecan-4 (B and D) ectodomains in the conditioned media were assayed by dot blot analysis as in Fig. 1. Quantitation was done as in Fig. 1 and each point represents the mean ± SD (n = 3). TIMP-1 and TIMP-2 (20 μM) did not inhibit constitutive shedding in this assay, and treatment with the PTK, PKC, or MAPK inhibitors alone did not affect the level of shedding compared with untreated controls during the 2 h shedding assays.
Hydroxamates, but Not TIMP-3, Prevent Constitutive Shedding
All cultured cells studied shed syndecan ectodomains as part of normal turnover (Yanagishita and Hascall 1992; Kim et al. 1994; Yanagishita 1998). To evaluate whether this constitutive shedding differs from accelerated shedding, we tested the effects of the peptide hydroxamates and TIMP-3 on constitutive shedding from SVEC4-10 cells in 14-h assays. Peptide hydroxamates BB-2116 and BB-1101 reduced the level of syndecan-1 (Fig. 10 A) and syndecan-4 (Fig. 10 B) shedding in a concentration-dependent manner; the IC50 values were ∼10-fold higher than those observed for inhibition of PMA-accelerated shedding (Fig. 7). In contrast, increasing concentrations of TIMP-3 had no effect on syndecan-1 and -4 ectodomain shedding from SVEC4-10 cells (Fig. 10C and Fig. D). TIMP-1 and TIMP-2 also had no effect on constitutive shedding at concentrations up to 20 μg/ml, and similar results were observed when NMuMG cells were used (data not shown). These results suggest that constitutive shedding involves a metalloproteinase. However, this activity differs from the MP activity that mediates accelerated shedding.
Discussion
In this study, we demonstrate that the ectodomains of syndecan-1 and -4 are shed from the cell surface after activation of multiple intracellular signaling pathways by diverse physiological effectors. This shedding involves the activity of a cell surface metalloproteinase (sheddase) that is specifically inhibited by TIMP-3. Shedding of the syndecan-1 core protein involves cleavage in its juxtamembrane domain at the extracellular face of the plasma membrane. Finally, the enzymatic activity responsible for regulated shedding differs from that involved in constitutive shedding. Ectodomain shedding converts syndecans from cell surface receptors or coreceptors to soluble HS proteoglycan effectors. Because shedding is regulated by physiological mediators, the syndecan ectodomains can be shed in response to specific developmental and pathophysiological cues. Considering their large number of potential ligands, the shed syndecan ectodomains are likely to have roles in morphogenesis, tissue repair, and host defense.
Multiple Signaling Pathways Regulate Syndecan-1 and -4 Ectodomain Shedding
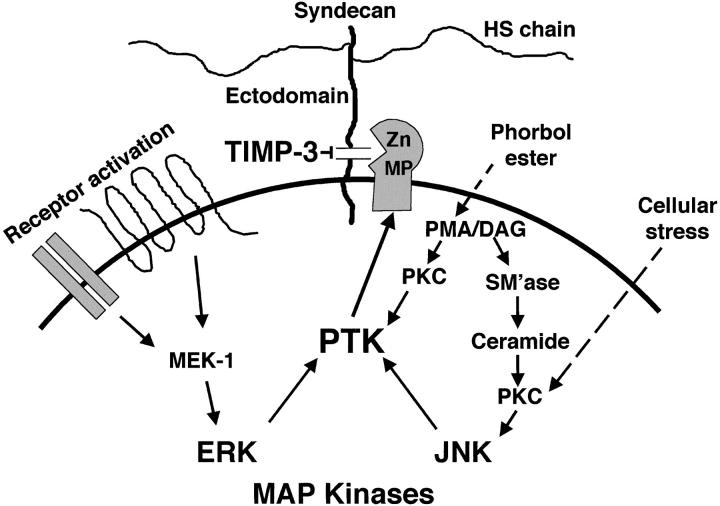
Diverse agonists were identified as accelerators of syndecan-1 and -4 ectodomain shedding (Fig. 11). Shedding accelerated by activation of the EGF and thrombin receptors correlates best with activation of the ERK MAP kinase pathway, but does not appear to involve PKC activation. In contrast, agonists associated with cellular stress (e.g., hyperosmolarity and ceramide) appear to require PKC activation upstream of the JNK/SAPK MAP kinase pathway. Inhibition of p38 MAP kinase activity by SB 202190 had limited effect on shedding accelerated by any of the agonists tested. Taken together, these results strongly correlate syndecan ectodomain shedding with the action of specific MAP kinase signaling pathways.
Figure 11.
Syndecan ectodomain shedding. The syndecan ectodomains can be shed intact by proteolytic cleavage of their core proteins, yielding soluble HSPGs that retain the binding properties of their cell surface counterparts. Shedding of syndecan-1 and -4 ectodomains from the cell surface is accelerated by PMA and a variety of physiological effectors (e.g., EGF family members, thrombin, hyperosmolarity, sphingomyelinase, and ceramide) via activation of multiple intracellular signaling pathways. These signals converge on a TIMP-3–sensitive cell surface metalloproteinase system that cleaves the core protein within 15 amino acids of the cell surface, and can act on unstimulated adjacent cells. Shedding is stimulated by tissue injury and these syndecan ectodomains are found in inflammatory fluids (Subramanian et al. 1997) where they modulate the activities of growth factors and proteinases (Kainulainen et al. 1998; Kato et al. 1998). See text for details.
Syndecan-1 and -4 ectodomains are also shed in response to phorbol ester activation of PKC. Thus, it is likely that physiological effectors that produce diacylglycerol would also accelerate shedding. In the cell types studied, shedding in response to phorbol esters does not apparently require MAP kinase activity, but this regulation may be cell type–specific. For example, whereas the phosphoinositide 3-kinase inhibitors, wortmannin and LY 294002, do not inhibit EGF and thrombin receptor–activated shedding in epithelial and endothelial cells, these effectively inhibit insulin-induced shedding in 3T3-L1 adipocytes (Fitzgerald, M., and M. Bernfield, unpublished results).
The shedding accelerated by each agonist involves PTK activity. This activity could phosphorylate the syndecan core protein cytoplasmic domain, but because cells activated with PMA can cleave the syndecan ectodomains from unactivated adjacent cells, shedding does not apparently require such phosphorylation. Syndecan-1 and -4 shedding is accelerated after pervanadate inhibition of protein tyrosine phosphatases (Reiland et al. 1996; Fitzgerald, M., unpublished results), and this phosphorylation is undetectable or detected at low levels in resting cells (Ott and Rapraeger 1998) that shed syndecan ectodomains constitutively. Thus, the PTK activity may phosphorylate the cleaving enzyme(s) and/or other unidentified components of the shedding system.
In each assay, we tested the effect of the PKC, MAP kinase, or PTK inhibitor alone. In no case did the inhibitor affect the level of shedding compared with the untreated control. These data suggest that these inhibitors do not affect the synthesis, glycosylation, or transport of syndecans to the cell surface during the brief assay periods (30 min to 2 h). Each cell line tested responded nearly identical to each of the agonists of syndecan-1 and -4 ectodomain shedding, suggesting that the same process(es) mediate shedding of both HSPGs. Because multiple distinct intracellular pathways are involved, our results contrast with studies that suggest a common shedding system for a variety of proteins (Arribas et al. 1996; Mullberg et al. 1997). However, in concert with the proposed common shedding system, protein cleavage is at a juxtamembrane site, the sheddase does not appear to cleave a specific sequence, and shedding is inhibited by similar concentrations of peptide hydroxamates (Hooper et al. 1997; Hooper and Turner 1999). Identification of the enzyme(s) involved in shedding will be required to document the differences in these shedding mechanisms.
Syndecan Ectodomains Are Shed from the Cell Surface
Syndecan ectodomain shedding is a cell surface event. Fluorescently tagged syndecan-1 is lost from the cell surface within 30 min after PMA treatment of P3X63 cells, and pretrypsinization of cells to remove cell surface syndecan-1 prevents accelerated shedding, suggesting that the core protein cleavage site is at the extracellular face of the plasma membrane. Introduction of a 15–amino acid region from human CD4 into the corresponding juxtamembrane region of the syndecan-1 core protein ectodomain prevents accelerated shedding, indicating that the core protein cleavage site is within 15 amino acid residues of the plasma membrane. We are currently in the process of identifying the precise cleavage site.
The enzymatic activity responsible for this cleavage appears to be associated with the cell surface because contact with PMA-activated cells causes accelerated shedding from untreated cells. Because the syndecan and the cleaving activity are both linked to the plasma membrane, and shedding is blocked at 4°C (Jalkanen et al. 1987), shedding is also likely regulated by changes in membrane dynamics (e.g., fluidity and actin filament association).
Regulated Syndecan Ectodomain Shedding Is Mediated by a TIMP-3–sensitive Metalloproteinase
In each of the cell types tested, accelerated shedding of syndecan-1 and -4 was blocked by the peptide hydroxamates BB-2116 and BB-1101, which are compounds originally designed to inhibit zinc-dependent matrix MPs (MMPs) (Odake et al. 1994; Wojtowicz-Praga et al. 1997; Pratt et al. 1998). High concentrations of these compounds inhibit ectodomain shedding of a diverse group of transmembrane proteins (Hooper et al. 1997). Indeed, BB-2116 and BB-1101 inhibit accelerated syndecan shedding from each of the cell types at ∼100-fold greater concentrations than needed to inhibit MMP-1, soluble human fibroblast collagenase. These data suggest that the syndecan sheddase is an MP, but not an MMP.
The physiological MMP inhibitor, TIMP-3, but not the closely related inhibitors TIMP-1 and TIMP-2, also inhibits accelerated syndecan shedding. Between them, TIMP-1 and TIMP-2 can inhibit the activity of all known MMPs tested at the concentrations used in this study (Murphy and Willenbrock 1995), again suggesting that the syndecan sheddase is not a known MMP. TIMP-3 is unique among the TIMPs in that it contains a heparin binding motif (Butler et al. 1999a) by which it is largely bound to the extracellular matrix (Pavloff et al. 1992; Leco et al. 1994; Kishnani et al. 1995). Indeed, this binding appears to potentiate its inhibitory activity (Butler et al. 1999a). TIMP-3 is expressed by a large variety of tissues and cells (Reponen et al. 1995) and is induced by treatment of cells with cytokines, growth factors, and anti-inflammatory agents (Leco et al. 1994), indicating that it may regulate tissue remodeling.
The affinity of TIMP-3 for HS chains may expand its role in syndecan ectodomain shedding. Syndecan HS chains might localize TIMP-3 to the cell surface, the site of ectodomain shedding, thereby potentiating its inhibitory activity (Amour et al. 1998). Conversely, the HS chains on soluble syndecan ectodomains could reduce its ability to inhibit at the cell surface. Further, this modulation of TIMP-3 activity could affect the shedding of membrane-anchored proteins such as l-selectin, TNF-α, and the interleukin 6 receptor that are sensitive to TIMP-3 (Hargreaves et al. 1998; Borland et al. 1999).
Our results suggest that the syndecan sheddase is a zinc-dependent MP. The enzyme is not likely a known MMP because TIMP-1 or TIMP-2 does not inhibit shedding. Furthermore, the activity is associated with the cell surface, whereas most MMPs are secreted enzymes. However, the syndecan sheddase could be a member of the membrane-type (MT)-MMPs. Although at least four MT-MMP family members have been identified (Mattei et al. 1997), MT1-MMP and MT2-MMP can be excluded as possible candidates because they are inhibited by TIMP-2 (Will et al. 1996; Butler et al. 1997). Indeed, it is not clear whether TIMP-3 inhibits the syndecan sheddase directly or another enzyme in a proteinase cascade involved in syndecan shedding.
The ADAMs family of membrane-spanning proteins that combine features of both adhesion molecules and proteinases (Wolfsberg and White 1996; Blobel 1997; Black and White 1998; Izumi et al. 1998) are also candidates for the syndecan sheddase. The sole mammalian cell surface–associated metalloproteinase sheddase so far identified is ADAM 17 (TACE). TACE cleaves the ectodomain of TNF-α (Black et al. 1997; Moss et al. 1997), and several other structurally and functionally unrelated transmembrane proteins. However, PMA accelerates the shedding of syndecan-1 and -4 ectodomains from a TACE-deficient fibroblast cell line (Black et al. 1997), albeit at a reduced rate in comparison to P3X63, NMuMG and SVEC4-10 cells (Fitzgerald, M., unpublished results), indicating that TACE is not the syndecan sheddase.
Constitutive and Accelerated Syndecan Ectodomain Shedding Use Distinct Mechanisms
The enzymatic cleavage responsible for accelerated shedding appears to be distinct from that involved in constitutive shedding. The intact ectodomains of each mammalian syndecan and the single Drosophila syndecan are constitutively shed into the conditioned media of cultured cells (Kim et al. 1994; Spring et al. 1994) as part of normal cell surface HSPG turnover (Yanagishita and Hascall 1992; Yanagishita 1998). In contrast with accelerated ectodomain shedding, constitutive syndecan ectodomain shedding is not inhibited by TIMP-3 and its inhibition requires ∼10-fold greater peptide hydroxamate concentrations. These data suggest different proteolytic systems for accelerated and constitutive syndecan ectodomain shedding, consistent with distinct cellular roles for these processes.
Functional Significance of Ectodomain Shedding
Solubilization of the ectodomains appears to be the major consequence of syndecan shedding. For both constitutive and accelerated shedding, the ectodomains appear to be rapidly replaced on the cell surface (Subramanian et al. 1997; Kainulainen 1999). Many of the agents that accelerate shedding are involved in tissue injury, suggesting that the soluble ectodomains function during tissue repair and host defense.
Shed soluble syndecan ectodomains are found in inflammatory fluids (Subramanian et al. 1997) where they maintain proteolytic and growth factor balance. Indeed, the agents that accelerate syndecan ectodoman shedding are released and act during acute wound repair (Clark 1996). Tissue injury is accompanied by cellular stress, accumulation of plasma- and neutrophil-derived proteases (e.g., thrombin, plasmin, elastase, cathepsin G), and release of growth factors (e.g., EGF, heparin-binding epidermal growth factor-like growth factor, transforming growth factor-α), each of which accelerate syndecan shedding (Subramanian et al. 1997). This shedding is modulated by plasma-derived proteinase inhibitors (Kainulainen et al. 1998), and by TIMP-3. The soluble syndecan-1 ectodomain acts as a dominant-negative inhibitor of the proliferative response of cells to HB-EGF (Wang, H., and M. Bernfield, unpublished results) and FGF-2 (Kato et al. 1998), two of the major growth factors involved in wound repair (Flaumenhaft and Rifkin 1992; Marikovsky et al. 1993; Abraham and Klagsbrun 1996). Soluble syndecan ectodomains can also modify proteolytic activities at sites of inflammation by binding to neutrophil-derived proteases and reducing their interactions with their physiological inhibitors (Kainulainen et al. 1998). Thus, ectodomain shedding provides a mechanism for balancing the proteinase and growth factor activities needed for normal wound repair.
The action of the soluble ectodomains during tissue injury is transient. Tissue repair is accompanied by accumulation of fibrotic deposits of interstitial extracellular matrix components, including fibrillar collagens, fibronectin and tenascin (Clark 1996). These extracellular matrix component deposits bind the soluble syndecan ectodomains, thus, limiting their activity and effectively terminating their influence. Thus, shed syndecan ectodomains are newly defined mediators of inflammation. These findings suggest a novel physiologic role for the soluble syndecan ectodomains and new approaches to modulate the inflammatory process.
Accelerated syndecan shedding accompanies host cell invasion by certain microbial pathogens. In the case of Pseudomonas aeruginosa, the organism accelerates shedding to enhance its infectivity. The secreted virulence factor, LasA (staphylolysin), accelerates syndecan-1 shedding by activation of PTKs and a zinc-dependent MP (Park et al. 2000). The mechanism by which syndecan shedding enhances infectivity is unclear.
Syndecan-1 and -4 can function in the maintenance of epithelial morphology and the formation of focal contacts, respectively (Kato et al. 1995; Woods and Couchman 1998; Bernfield et al. 1999). These functions depend on the adhesion of these syndecans to the extracellular matrix as coreceptors for integrins. Integrin-mediated adhesion to the extracellular matrix is central to growth control, and could activate the same MAP kinase/ERK pathways as those responsible for accelerated shedding of these syndecan ectodomains (Thomas and Brugge 1997). Thus, syndecan ectodomain shedding via activation of MAP kinase pathways suggests the possible involvement of cell surface HSPG shedding in growth control, as suggested many years ago by Kraemer and Tobey 1972. This involvement could operate via the role of syndecans in controlling cell shape through their action on actin cytoskeleton organization and contractility (Burridge and Chrzanowska-Wodnicka 1996).
Syndecan ectodomain shedding is a highly regulated means of delivering soluble syndecan ectodomains to tissues. Additionally, syndecan ectodomains can be solubilized by the direct action of extracellular proteinases (e.g. plasmin, thrombin, elastase) (Subramanian et al. 1997). However, acceleration of syndecan ectodomain shedding by physiological mediators indicates that shedding is a response to specific developmental and pathophysiological cues. Indeed, regulated shedding likely accompanies normal development, as the soluble syndecan-1 ectodomain is found in early mouse embryos (Sutherland et al. 1991). Just as every adherent cell type expresses at least one of the syndecan family members at the cell surface (Kim et al. 1994), we hypothesize that all syndecan-expressing cells have a mechanism to regulate syndecan ectodomain shedding. A further understanding of these mechanisms should lead to devising molecules that selectively promote or prevent syndecan shedding and to their pharmacological use to modify inflammatory processes.
Note Added in Proof. After this paper was accepted, a publication appeared (EMBO (Eur. Mol. Biol. Organ.) J. 1999. 18:6962–6972) that demonstrated that TGFα and L-selectin are shed after activation of the ERK MAP kinase pathway, which is similar to the syndecan-1 and -4 shedding reported here.
Acknowledgments
We thank Dmitriy Leyfer, Elena Shneider, and Mie Abe (Children's Hospital, Boston, MA) for excellent cell culture technical assistance.
This work was supported by National Institutes of Health predoctoral training grant 5T32NS07264 (to M.L. Fitzgerald), the Parker B. Francis Foundation Fellowship (to P.W. Park), NIH grants CA 28735 and HL56398 (to M. Bernfield), and the Wellcome Trust and the Arthritis Research Campaign, UK (to G. Murphy).
Footnotes
Abbreviations used in this paper: 4αPDD, 4α-phorbol-12, 13-didecanoate; AU, absorbance units; ECL, enhanced chemiluminescence; ERK, extracellular signal-related kinase; HSPG, heparan sulfate proteoglycan; JM, juxtamembrane; JNK, c-Jun NH2-terminal kinase; MAP kinase, mitogen-activated kinase; MMP, matrix metalloproteinase; MP, metalloproteinase; PKC, protein kinase C; PTK, protein tyrosine kinase; SAPK, stress-activated protein kinase; TIMP, tissue inhibitor of metalloproteinase; TNF, tumor necrosis factor; TRAP, thrombin receptor agonist peptide.
References
- Abraham J.A., Klagsbrun M. Modulation of wound repair by members of the fibroblast growth factor family. In: Clark R.A.F., editor. The Molecular and Cellular Biology of Wound Repair. Plenum Press; New York: 1996. pp. 195–248. [Google Scholar]
- Alessi D.R., Cuenda A., Cohen P., Dudley D.T., Saltiel A.R. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- Amour A., Slocombe P.M., Webster A., Butler M., Knight C.G., Smith B.J., Stephens P.E., Shelley C., Hutton M., Knauper V., Docherty A.J., Murphy G. TNF-alpha converting enzyme (TACE) is inhibited by TIMP-3. FEBS (Fed. Eur. Biochem. Soc.) Lett. 1998;435:39–44. doi: 10.1016/s0014-5793(98)01031-x. [DOI] [PubMed] [Google Scholar]
- Anand-Apte B., Bao L., Smith R., Iwata K., Olsen B.R., Zetter B., Apte S.S. A review of tissue inhibitor of metalloproteinases-3 (TIMP-3) and experimental analysis of its effect on primary tumor growth. Biochem. Cell Biol. 1996;74:853–862. doi: 10.1139/o96-090. [DOI] [PubMed] [Google Scholar]
- Apte S.S., Olsen B.R., Murphy G. The gene structure of tissue inhibitor of metalloproteinases (TIMP)-3 and its inhibitory activities define the distinct TIMP gene family [published erratum appears in J. Biol. Chem. 1996. 271:2874] J. Biol. Chem. 1995;270:14313–14318. doi: 10.1074/jbc.270.24.14313. [DOI] [PubMed] [Google Scholar]
- Arribas J., Coodly L., Vollmer P., Kishimoto T.K., Rose-John S., Massagué J. Diverse cell surface protein ectodomains are shed by a system sensitive to metalloproteinase inhibitors. J. Biol. Chem. 1996;271:11376–11382. doi: 10.1074/jbc.271.19.11376. [DOI] [PubMed] [Google Scholar]
- Bernfield M., Kokenyesi R., Kato M., Hinkes M.T., Spring J., Gallo R.L., Lose E.J. Biology of the syndecansa family of transmembrane heparan sulfate proteoglycans. Annu. Rev. Cell. Biol. 1992;8:365–393. doi: 10.1146/annurev.cb.08.110192.002053. [DOI] [PubMed] [Google Scholar]
- Bernfield M., Götte M., Park P.W., Reizes O., Fitzgerald M.L., Lincecum J., Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- Black R.A., White J.M. ADAMsfocus on the protease domain. Curr. Opin. Cell Biol. 1998;10:654–659. doi: 10.1016/s0955-0674(98)80042-2. [DOI] [PubMed] [Google Scholar]
- Black R.A., Rauch C.T., Kozlosky C.J., Peschon J.J., Slack J.L., Wolfson M.F., Castner B.J., Stocking K.L., Reddy P., Srinivasan S. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- Blobel C.P. Metalloprotease-disintegrinslinks to cell adhesion and cleavage of TNF-alpha and Notch. Cell. 1997;90:589–592. doi: 10.1016/s0092-8674(00)80519-x. [DOI] [PubMed] [Google Scholar]
- Borland G., Murphy G., Ager A. Tissue inhibitor of metalloproteinases-3 inhibits shedding of L-selectin from leukocytes. J. Biol. Chem. 1999;274:2810–2815. doi: 10.1074/jbc.274.5.2810. [DOI] [PubMed] [Google Scholar]
- Burridge K., Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu. Rev. Cell Dev. Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- Butler G.S., Will H., Atkinson S.J., Murphy G. Membrane-type-2 matrix metalloproteinase can initiate the processing of progelatinase A and is regulated by the tissue inhibitors of metalloproteinases. Eur. J. Biochem. 1997;244:653–657. doi: 10.1111/j.1432-1033.1997.t01-1-00653.x. [DOI] [PubMed] [Google Scholar]
- Butler G.S., Apte S.S., Willenbrock F., Murphy G. Human tissue inhibitor of metalloproteinases 3 interacts with both the N- and C-terminal domains of gelatinases A and B. Regulation by polyanions J. Biol. Chem 274 1999. 10846 10851a [DOI] [PubMed] [Google Scholar]
- Butler G.S., Hutton M., Wattam B.A., Williamson R.A., Knauper V., Willenbrock F., Murphy G. The specificity of TIMP-2 for matrix metalloproteinases can be modified by single amino acid mutations J. Biol. Chem 274 1999. 20391 20396b [DOI] [PubMed] [Google Scholar]
- Carey D.J. Syndecansmultifunctional cell-surface co-receptors. Biochem. J. 1997;327:1–16. doi: 10.1042/bj3270001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R.A.F. The Molecular and Cellular Biology of Wound Repair 1996. Plenum Press, ; New York: pp. 611 pp [Google Scholar]
- Cuenda A., Rouse J., Doza Y.N., Meier R., Cohen P., Gallagher T.F., Young P.R., Lee J.C. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS (Fed. Eur. Biochem. Soc.) Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- Dery O., Bunnett N.W. Proteinase-activated receptorsa growing family of heptahelical receptors for thrombin, trypsin and tryptase. Biochem. Soc. Trans. 1999;27:246–254. doi: 10.1042/bst0270246. [DOI] [PubMed] [Google Scholar]
- Dery O., Corvera C.U., Steinhoff M., Bunnett N.W. Proteinase-activated receptorsnovel mechanisms of signaling by serine proteases. Am. J. Physiol. 1998;274:C1429–C1452. doi: 10.1152/ajpcell.1998.274.6.C1429. [DOI] [PubMed] [Google Scholar]
- Desdouits-Magnen J., Desdouits F., Takeda S., Syu L.J., Saltiel A.R., Buxbaum J.D., Czernik A.J., Nairn A.C., Greengard P. Regulation of secretion of Alzheimer amyloid precursor protein by the mitogen-activated protein kinase cascade. J. Neurochem. 1998;70:524–530. doi: 10.1046/j.1471-4159.1998.70020524.x. [DOI] [PubMed] [Google Scholar]
- Dhodapkar M.V., Sanderson R.D. Syndecan-1 (CD 138) in myeloma and lymphoid malignanciesa multifunctional regulator of cell behavior within the tumor microenvironment. Leuk. Lymphoma. 1999;34:35–43. doi: 10.3109/10428199909083378. [DOI] [PubMed] [Google Scholar]
- Dudley D.T., Pang L., Decker S.J., Bridges A.J., Saltiel A.R. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc. Natl. Acad. Sci. USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers M.R.W., Riordan J.F. Membrane proteins with soluble counterpartsrole of proteolysis in the release of transmembrane proteins. Biochemistry. 1991;30:10065–10077. doi: 10.1021/bi00106a001. [DOI] [PubMed] [Google Scholar]
- Flaumenhaft R., Rifkin D.B. The extracellular regulation of growth factor action. Mol. Biol. Cell. 1992;3:1057–1065. doi: 10.1091/mbc.3.10.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsten K.E., Courant N.A., Nugent M.A. Endothelial proteoglycans inhibit bFGF binding and mitogenesis. J. Cell Physiol. 1997;172:209–220. doi: 10.1002/(SICI)1097-4652(199708)172:2<209::AID-JCP8>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Gearing A.J., Newman W. Circulating adhesion molecules in disease. Immunol. Today. 1993;14:506–512. doi: 10.1016/0167-5699(93)90267-O. [DOI] [PubMed] [Google Scholar]
- Gomez D.E., Alonso D.F., Yoshiji H., Thorgeirsson U.P. Tissue inhibitors of metalloproteinasesstructure, regulation and biological functions. Eur. J. Cell Biol. 1997;74:111–122. [PubMed] [Google Scholar]
- Hansen M.B., Nielsen S.E., Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Methods. 1989;119:203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- Hargreaves P.G., Wang F., Antcliff J., Murphy G., Lawry J., Russell R.G., Croucher P.I. Human myeloma cells shed the interleukin-6 receptorinhibition by tissue inhibitor of metalloproteinase-3 and a hydroxamate-based metalloproteinase inhibitor. Br. J. Haematol. 1998;101:694–702. doi: 10.1046/j.1365-2141.1998.00754.x. [DOI] [PubMed] [Google Scholar]
- Hodge T.W., Sasso D.R., McDougal J.S. Humans with OKT4-epitope deficiency have a single nucleotide base change in the CD4 gene, resulting in substitution of TRP240 for ARG240. Hum. Immunol. 1991;30:99–104. doi: 10.1016/0198-8859(91)90077-m. [DOI] [PubMed] [Google Scholar]
- Hooper N.M., Turner A.J. Membrane protein secretases. Biochem. Soc. Trans. 1999;27:211–257. doi: 10.1042/bst0270255. [DOI] [PubMed] [Google Scholar]
- Hooper N.M., Karran E.H., Turner A.J. Membrane protein secretases. Biochem. J. 1997;321:265–279. doi: 10.1042/bj3210265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y., Hirata M., Hasuwa H., Iwamoto R., Umata T., Miyado K., Tamai Y., Kurisaki T., Sehara-Fujisawa A., Ohno S., Mekada E. A metalloprotease-disintegrin, MDC9/meltrin-gamma/ADAM9 and PKC-delta are involved in TPA-induced ectodomain shedding of membrane-anchored heparin-binding EGF-like growth factor. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:7260–7272. doi: 10.1093/emboj/17.24.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalkanen M., Nguyen H., Rapraeger A., Kurn N., Bernfield M. Heparan sulfate proteoglycans from mouse mammary epithelial cellslocalization on the cell surface with a monoclonal antibody. J. Cell Biol. 1985;101:976–984. doi: 10.1083/jcb.101.3.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalkanen M., Rapraeger A., Saunders S., Bernfield M. Cell surface proteoglycan of mouse mammary epithelial cells is shed by cleavage of its matrix-binding ectodomain from its membrane-associated domain. J. Cell Biol. 1987;105:3087–3096. doi: 10.1083/jcb.105.6.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S.A., Novick D., Horiuchi S., Yamamoto N., Szalai A.J., Fuller G.M. C-reactive proteina physiological activator of interleukin 6 receptor shedding. J. Exp. Med. 1999;189:599–604. doi: 10.1084/jem.189.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kainulainen V. Syndecans in tissue injury. Turku Centre for Biotechnology. University of Turku, ; Turku, Finland: 1999. [Google Scholar]
- Kainulainen V., Wang H., Schick C., Bernfield M. Syndecans, heparan sulfate proteoglycans, maintain the proteolytic balance of acute wound fluids. J. Biol. Chem. 1998;273:11563–11569. doi: 10.1074/jbc.273.19.11563. [DOI] [PubMed] [Google Scholar]
- Kato M., Saunders S., Nguyen H., Bernfield M. Loss of cell surface syndecan-1 causes epithelia to transform into anchorage-independent mesenchyme-like cells. Mol. Biol. Cell. 1995;6:559–576. doi: 10.1091/mbc.6.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M., Wang H., Kainulainen V., Fitzgerald M.L., Ledbetter S., Ornitz D.M., Bernfield M. Physiological degradation converts the soluble syndecan-1 ectodomain from an inhibitor to a potent activator of FGF-2. Nat. Med. 1998;4:691–697. doi: 10.1038/nm0698-691. [DOI] [PubMed] [Google Scholar]
- Kiessling L.L., Gordon E.J. Transforming the cell surface through proteolysis. Chem. Biol. 1998;5:R49–R62. doi: 10.1016/s1074-5521(98)90056-4. [DOI] [PubMed] [Google Scholar]
- Kim C.W., Goldberger O.A., Gallo R.L., Bernfield M. Members of the syndecan family of heparan sulfate proteoglycans are expressed in distinct cell-, tissue-, and development-specific patterns. Mol. Biol. Cell. 1994;5:797–805. doi: 10.1091/mbc.5.7.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishnani N.S., Staskus P.W., Yang T.T., Masiarz F.R., Hawkes S.P. Identification and characterization of human tissue inhibitor of metalloproteinase-3 and detection of three additional metalloproteinase inhibitor activities in extracellular matrix. Matrix Biol. 1995;14:479–488. doi: 10.1016/0945-053x(95)90005-5. [DOI] [PubMed] [Google Scholar]
- Kraemer P.M., Tobey R.A. Cell cycle–dependent desquamation of heparan sulfate from the cell surface. J. Cell. Biol. 1972;55:713–717. doi: 10.1083/jcb.55.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis J.M., Avruch J. Sounding the alarmprotein kinase cascades activated by stress and inflammation. J. Biol. Chem. 1996;271:24313–24316. doi: 10.1074/jbc.271.40.24313. [DOI] [PubMed] [Google Scholar]
- Leco K.J., Khokha R., Pavloff N., Hawkes S.P., Edwards D.R. Tissue inhibitor of metalloproteinases-3 (TIMP-3) is an extracellular matrix-associated protein with a distinctive pattern of expression in mouse cells and tissues. J. Biol. Chem. 1994;269:9352–9360. [PubMed] [Google Scholar]
- Lee J.C., Laydon J.T., McDonnell P.C., Gallagher T.F., Kumar S., Green D., McNulty D., Blumenthal M.J., Heys J.R., Landvatter S.W. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- Mali M., Andtfolk H., Miettinen H.M., Jalkanen M. Suppression of tumor cell growth by syndecan-1 ectodomain. J. Biol. Chem. 1994;269:27795–27798. [PubMed] [Google Scholar]
- Marikovsky M., Breuing K., Liu P.Y., Eriksson E., Higashiyama S., Farber P., Abraham J., Klagsbrun M. Appearance of heparin-binding EGF-like growth factor in wound fluid as a response to injury. Proc. Natl. Acad. Sci. USA. 1993;90:3889–3893. doi: 10.1073/pnas.90.9.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattei M.G., Roeckel N., Olsen B.R., Apte S.S. Genes of the membrane-type matrix metalloproteinase (MT-MMP) gene family, MMP14, MMP15, and MMP16, localize to human chromosomes 14, 16, and 8, respectively. Genomics. 1997;40:168–169. doi: 10.1006/geno.1996.4559. [DOI] [PubMed] [Google Scholar]
- Merlos-Suarez A., Arribas J. Mechanisms controlling the shedding of transmembrane molecules. Biochem. Soc. Trans. 1999;27:243–246. doi: 10.1042/bst0270243. [DOI] [PubMed] [Google Scholar]
- Moss M.L., Jin S.L., Milla M.E., Bickett D.M., Burkhart W., Carter H.L., Chen W.J., Clay W.C., Didsbury J.R., Hassler D. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha [published erratum appears in Nature. 1997. 386:738] Nature. 1997;385:733–736. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- Mullberg J., Rauch C.T., Wolfson M.F., Castner B., Fitzner J.N., Otten-Evans C., Mohler K.M., Cosman D., Black R.A. Further evidence for a common mechanism for shedding of cell surface proteins. FEBS (Fed. Eur. Biochem. Soc.) Lett. 1997;401:235–238. doi: 10.1016/s0014-5793(96)01480-9. [DOI] [PubMed] [Google Scholar]
- Murphy G., Willenbrock F. Tissue inhibitors of matrix metalloendopeptidases. Methods Enzymol. 1995;248:496–510. doi: 10.1016/0076-6879(95)48032-3. [DOI] [PubMed] [Google Scholar]
- Murphy G., Stanton H., Cowell S., Butler G., Knauper V., Atkinson S., Gavrilovic J. Mechanisms for pro matrix metalloproteinase activation. Apmis. 1999;107:38–44. doi: 10.1111/j.1699-0463.1999.tb01524.x. [DOI] [PubMed] [Google Scholar]
- Odake S., Morita Y., Morikawa T., Yoshida N., Hori H., Nagai Y. Inhibition of matrix metalloproteinases by peptidyl hydroxamic acids. Biochem. Biophys. Res. Commun. 1994;199:1442–1446. doi: 10.1006/bbrc.1994.1392. [DOI] [PubMed] [Google Scholar]
- Ott V.L., Rapraeger A.C. Tyrosine phosphorylation of syndecan-1 and -4 cytoplasmic domains in adherent B82 fibroblasts. J. Biol. Chem. 1998;273:35291–35298. doi: 10.1074/jbc.273.52.35291. [DOI] [PubMed] [Google Scholar]
- Park P.W., Pier G.B., Preston M.J., Fitzgerald M.L., Goldberger O., Bernfield M. Syndecan-1 shedding is enhanced by LasA, a secreted virulence factor of Pseudomonas aeruginosa . J. Biol. Chem. 2000;275:3057–3064. doi: 10.1074/jbc.275.5.3057. [DOI] [PubMed] [Google Scholar]
- Pavloff N., Staskus P.W., Kishnani N.S., Hawkes S.P. A new inhibitor of metalloproteinases from chickenChIMP-3. A third member of the TIMP family. J. Biol. Chem. 1992;267:17321–17326. [PubMed] [Google Scholar]
- Pratt L.M., Beckett R.P., Bellamy C.L., Corkill D.J., Cossins J., Courtney P.F., Davies S.J., Davidson A.H., Drummond A.H., Helfrich K. The synthesis of novel matrix metalloproteinase inhibitors employing the Ireland-Claisen rearrangement. Bioorg. Med. Chem. Lett. 1998;8:1359–1364. doi: 10.1016/s0960-894x(98)00218-2. [DOI] [PubMed] [Google Scholar]
- Rapraeger A., Jalkanen M., Bernfield M. Integral Membrane Proteoglycans as Matrix ReceptorsRole in Cytoskeleton and Matrix Assembly at the Epithelial Cell Surface 1987. Academic Press, Inc., ; New York, NY: pp. 129–154 pp [Google Scholar]
- Reiland J., Ott V.L., Lebakken C.S., Yeaman C., McCarthy J., Rapraeger A.C. Pervanadate activation of intracellular kinases leads to tyrosine phosphorylation and shedding of syndecan-1. Biochem. J. 1996;319:39–47. doi: 10.1042/bj3190039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reponen P., Leivo I., Sahlberg C., Apte S.S., Olsen B.R., Thesleff I., Tryggvason K. 92-kDa type IV collagenase and TIMP-3, but not 72-kDa type IV collagenase or TIMP-1 or TIMP-2, are highly expressed during mouse embryo implantation. Dev. Dyn. 1995;202:388–396. doi: 10.1002/aja.1002020408. [DOI] [PubMed] [Google Scholar]
- Ridley R.C., Xiao H., Hata J., Woodliff J., Epstein J., Sanderson R.D. Expression of syndecan regulates human myeloma plasma cell adhesion to type I collagen. Blood. 1993;81:767–774. [PubMed] [Google Scholar]
- Rosette C., Karin M. Ultraviolet light and osmotic stressactivation of the JNK cascade through multiple growth factor and cytokine receptors. Science. 1996;274:1194–1197. doi: 10.1126/science.274.5290.1194. [DOI] [PubMed] [Google Scholar]
- Saunders S., Jalkanen M., O'Farrell S., Bernfield M. Molecular cloning of syndecan, an integral membrane proteoglycan. J. Cell Biol. 1989;108:1547–1556. doi: 10.1083/jcb.108.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayama K., Goto Y., Iguchi T., Takeda Y., Matsuzawa A. Effects of an antibiotic protease inhibitor, actinonin on the growth within collagen gels of non-metastatic and metastatic mouse mammary tumors of the same origin. Cancer Lett. 1995;94:171–177. doi: 10.1016/0304-3835(95)03847-p. [DOI] [PubMed] [Google Scholar]
- Shin J., Dunbrack R.L., Jr., Lee S., Strominger J.L. Phosphorylation-dependent down-modulation of CD4 requires a specific structure within the cytoplasmic domain of CD4. J. Biol. Chem. 1991;266:10658–10665. [PubMed] [Google Scholar]
- Spring J., Paine-Saunders S.E., Hynes R.O., Bernfield M. Drosophila syndecanconservation of a cell surface heparan sulfate proteoglycan. Proc. Natl. Acad. Sci. USA. 1994;91:3334–3338. doi: 10.1073/pnas.91.8.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S.V., Fitzgerald M.L., Bernfield M. Regulated shedding of syndecan-1 and -4 ectodomains by thrombin and growth factor receptor activation. J. Biol. Chem. 1997;272:14713–14720. doi: 10.1074/jbc.272.23.14713. [DOI] [PubMed] [Google Scholar]
- Sutherland A.E., Sanderson R.D., Mayes M., Siebert M., Calarco P.G., Bernfield M., Damsky C.H. Expression of syndecan, a putative low affinity fibroblast growth factor receptor, in the early mouse embryo. Development. 1991;113:339–351. doi: 10.1242/dev.113.1.339. [DOI] [PubMed] [Google Scholar]
- Thomas S.M., Brugge J.S. Cellular functions regulated by Src family kinases. Annu. Rev. Cell. Dev. Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- Toullec D., Pianetti P., Coste H., Bellevergue P., Grand-Perret T., Ajakane M., Baudet V., Boissin P., Boursier E., Loriolle F. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J. Biol. Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- Troyer D., Padilla R., Smith T., Kreisberg J., Glass W., II. Stimulation of the thrombin receptor of human glomerular mesengial cells by Ser-Phe-Leu-Leu-Arg-Asn-Pro-Asn-Asp-Lys-Tyr-Glu-Pro-Phe peptide. J. Biol. Chem. 1992;267:20126–20131. [PubMed] [Google Scholar]
- Verheij M., Bose R., Lin X.H., Yao B., Jarvis W.D., Grant S., Birrer M.J., Szabo E., Zon L.I., Kyriakis J.M. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- Wang P.T., Bigby M., Sy M.S. Selective down modulation of L3T4 molecules on murine thymocytes by the tumor promoter, phorbol 12-myristate 13-acetate. J. Immunol. 1987;139:2157–2165. [PubMed] [Google Scholar]
- Werb Z., Yan Y. A cellular striptease act. Science. 1998;282:1279–1280. doi: 10.1126/science.282.5392.1279. [DOI] [PubMed] [Google Scholar]
- Will H., Atkinson S.J., Butler G.S., Smith B., Murphy G. The soluble catalytic domain of membrane type 1 matrix metalloproteinase cleaves the propeptide of progelatinase A and initiates autoproteolytic activation. Regulation by TIMP-2 and TIMP-3. J. Biol. Chem. 1996;271:17119–17123. doi: 10.1074/jbc.271.29.17119. [DOI] [PubMed] [Google Scholar]
- Wojtowicz-Praga S.M., Dickson R.B., Hawkins M.J. Matrix metalloproteinase inhibitors. Invest. New Drugs. 1997;15:61–75. doi: 10.1023/a:1005722729132. [DOI] [PubMed] [Google Scholar]
- Wolfsberg T.G., White J.M. ADAMs in fertilization and development. Dev. Biol. 1996;180:389–401. doi: 10.1006/dbio.1996.0313. [DOI] [PubMed] [Google Scholar]
- Woods A., Couchman J.R. Syndecanssynergistic activators of cell adhesion. Trends Cell Biol. 1998;8:189–192. doi: 10.1016/s0962-8924(98)01244-6. [DOI] [PubMed] [Google Scholar]
- Yanagishita M. Cellular catabolism of heparan sulfate proteoglycans. TIG (Trends Genet) 1998;10:57–63. [Google Scholar]
- Yanagishita M., Hascall V.C. Cell surface heparan sulfate proteoglycans. J. Biol. Chem. 1992;267:9451–9454. [PubMed] [Google Scholar]