Abstract
Antagonist affinity measurements have traditionally been considered important in characterizing the cell-surface receptors present in a particular cell or tissue. A central assumption has been that antagonist affinity is constant for a given receptor–antagonist interaction, regardless of the agonist used to stimulate that receptor or the downstream response that is measured. As a consequence, changes in antagonist affinity values have been taken as initial evidence for the presence of novel receptor subtypes. Emerging evidence suggests, however, that receptors can possess multiple binding sites and the same receptor can show different antagonist affinity measurements under distinct experimental conditions. Here, we discuss several mechanisms by which antagonists have different affinities for the same receptor as a consequence of allosterism, coupling to different G proteins, multiple (but non-interacting) receptor sites, and signal-pathway-dependent pharmacology (where the pharmacology observed varies depending on the signalling pathway measured).
Introduction
The measurement of antagonist affinity has traditionally been an important feature of the characterization of cell surface receptors [1–5] and has been used to identify novel receptor subtypes [1,3,6,7]. A central assumption of this approach is that antagonist affinity is constant for a given receptor–antagonist interaction, regardless of the agonist used to stimulate that receptor or the downstream response that is measured [6]. However, there is emerging evidence that receptors can have multiple binding sites and that the same receptor can exhibit different antagonist affinity measurements under different experimental conditions [8–13]. It might, therefore, be timely to ‘rethink’ this basic concept in pharmacology.
Classical analysis of functional agonist–antagonist interactions
Analysis of logarithmic concentration–response curves has long been used to evaluate the nature of the competitive interactions between agonists and antagonists from functional measurements and particularly so in the case of G-protein-coupled receptors (GPCRs) (Figure 1). The standard approach is the construction of full agonist concentration–response curves in the absence and presence of fixed concentrations of antagonist. The extent of the parallel rightward shift in the position of the agonist concentration–response curve is then used to calculate the antagonist affinity directly (assuming competitive antagonism) or (if various antagonist concentrations have been used) to construct a Schild plot, which will have a slope of one if the interaction is competitive (Figure 1).
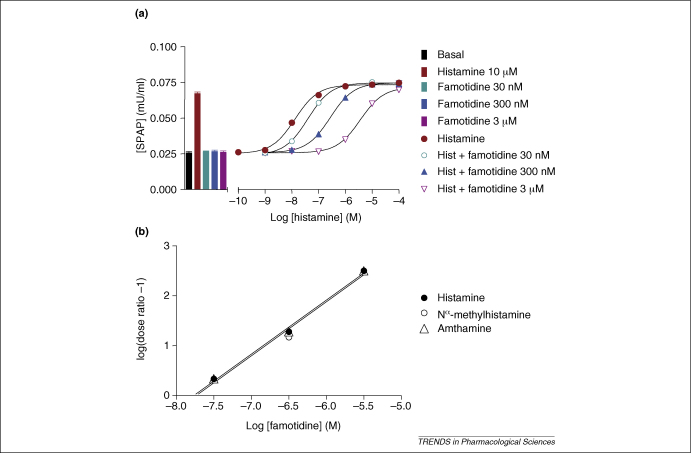
Figure 1.
Antagonism of histamine H2 receptor responses. (a) Antagonism of histamine-stimulated CRE (cAMP response element) gene transcription, mediated through the H2 receptor, by increasing concentrations of the H2 antagonist famotidine in CHO cells expressing the human H2 receptor. Gene transcription was measured by using a secreted placental alkaline phosphatase (SPAP) reporter gene. (b) Schild plots of the famotidine antagonism of H2 responses stimulated by histamine, Nα-methylhistamine and amthamine. The x-axis intercept gives the −log Kb value. Data are from Ref. [16] and J.G.B. (unpublished observations).
The principal assumptions made in these calculations are (i) that there is only one binding site; (ii) that the agonist and the antagonist are competing at this same site; and (iii) that, for a given level of response, the agonist occupancy of the receptors will be identical in the presence and absence of antagonist [1]. This latter assumption is required to take into account the two fundamental properties of an agonist: namely, affinity and efficacy, which contribute to the final measured response [14,15]. Provided that the agonist–antagonist interaction with the receptor is competitive, then the ratio of agonist concentrations required to give the same-sized functional response in the presence and absence of antagonist is equal to 1 + BKb, where B is the concentration of antagonist and Kb is the antagonist affinity constant [1] (Figure 1). For a given receptor, the Kb value derived from this relationship should be independent of the agonist used to stimulate the receptor and the level in the receptor signalling cascade at which responses are measured. For example, in the case of the histamine H2 receptor, affinity constants for the H2 antagonist famotidine are the same whether histamine, amthamine or Nα-methylhistamine are used as H2 receptor agonists [16] (Figure 1).
Allosteric antagonism
It has been accepted for some time that some GPCRs (most notably muscarinic, adenosine and CCR5 receptors) have more than one binding site [17–21]. Endogenous ligands bind to and activate the orthosteric site, whereas others, known as ‘allosteric ligands’, bind to a separate site or sites on the same receptor. As a consequence, both the orthosteric and allosteric sites can be occupied by ligands at the same time.
Ligands binding to the allosteric site can alter the binding of ligands to the orthosteric site and have traditionally been considered not to induce a receptor response on their own. Thus, a positive allosteric regulator binds to the allosteric site and increases the binding affinity of an orthosteric ligand, causing the concentration–response curve of an orthosteric agonist to move leftward to lower agonist concentrations. A negative allosteric regulator decreases the binding of the orthosteric ligand such that either the orthosteric agonist concentration–response curve will be shifted to higher agonist concentrations (i.e. rightward) or the maximum response will be decreased (depending on the signalling efficiency of the orthosteric site). In this situation, the allosteric ligand is effectively acting as an allosteric antagonist.
The nature of the antagonism produced by an allosteric antagonist is therefore very different from the classic competitive antagonism described earlier (where the agonist and antagonist compete for binding to the same orthosteric site), which has several important consequences. When all of the allosteric sites are occupied, the orthosteric agonist concentration–response curve cannot be shifted any further to the right and will reach a limiting value. This can lead to nonlinear Schild plots and to incomplete displacement of radioligands from their specific binding sites [10].
The effect of the allosteric antagonist can vary markedly depending on the agonist under study [10]. For example, the M2 muscarinic allosteric ligand eburnamonine enhances the agonist affinity of pilocarpine at the orthosteric site, has no net effect on the binding affinity of arecaidine propargyl ester, but reduces the agonist affinity of arecoline [10,22]. In addition, the magnitude of the shift of agonist affinities varies not only between agonists but also with different allosteric ligands, even at the same allosteric site [10]. Thus, each ‘allosterically occupied’ form of the receptor can be considered to represent a conformationally altered receptor that will have its own unique set of ligand affinities and efficacies [10,21,22].
Different G-protein-coupled states of GPCRs
Increasing evidence indicates that GPCRs can couple to more than one G protein, raising the possibility that different agonists can direct signalling from the receptor to specific signalling cascades as a consequence of their relative affinities for different G-protein-coupled states of the same receptor [13,23,24]. This possibility was first realized for the 5-HT2C receptor, which in Chinese hamster ovary (CHO) cells can couple to two different signalling pathways (phosphoinositide hydrolysis and arachidonic acid release) with a different rank order of agonist efficacies [23]. It is equally possible, however, that antagonists might differ in their affinity for these G-protein-specific states of the receptor, which will be manifest in both agonist and signalling-pathway-dependent pharmacology.
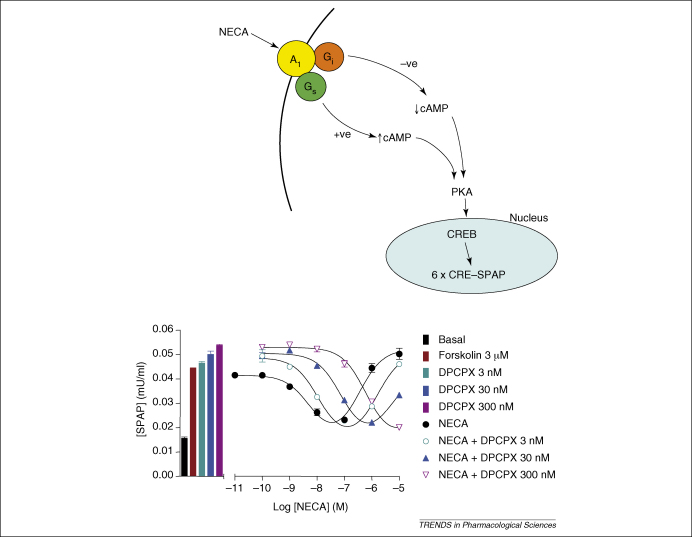
When expressed in CHO cells, the human adenosine A1 receptor couples to both Gi and Gs proteins [24,25]. When antagonist affinity measurements were made at both the Gi- and Gs-coupled forms of the A1 receptor, however, the antagonist affinities were found to be constant, regardless of the signalling cascade that was monitored (Gi or Gs) or the level at which the signalling cascade was evaluated (cAMP or CRE-mediated gene expression) [26] (Figure 2). Thus, in this study the fundamental pharmacological concept that antagonist affinities are indeed constant seems to hold true for the A1 receptor.
Figure 2.
5′-N-ethylcarboxamidoadenosine (NECA)-induced gene transcription mediated by the human A1 adenosine receptor. (a) Gi and Gs signalling pathways from the adenosine A1 receptor to CRE-mediated gene transcription in CHO cells expressing the human A1-receptor. Abbreviations: CREB, CRE-binding protein; PKA, protein kinase A. 6 × CRE–SPAP signifies a SPAP reporter gene containing six CRE elements. (b) Concentration–response curves for the effect of the agonist NECA on forskolin-stimulated CRE gene transcription in the presence and absence of increasing concentrations of DPCPX. Data are from Ref. [26].
This concept might not, however, apply to all GPCRs and GPCR–G-protein complexes; indeed, studies of large libraries of ligands and multiple signalling pathways might be required to confirm or to refute it. Likewise, antagonist affinity can vary for the monomeric GPCR as compared with a dimeric or multimeric complex. Similarly, if a complex is held in a scaffold (whether or not this scaffold signals to different G proteins), then the antagonist affinity might vary depending on the make-up of the specific complex or scaffold in each cell type.
Because it is becoming increasing clear that specific GPCR–G-protein signalling complexes seem to be compartmentalized in microdomains within the same cell [27,28], single-molecule approaches might be required to determine antagonist affinities in specific microdomains of single living cells [29,30].
Non-interacting conformations of the same GPCR
The large number of ligands available for the β-adrenoceptor family of GPCRs has enabled detailed studies to be performed on these receptors. For example, there is now strong evidence that the human β1 adrenoceptor has at least two ligand-binding sites, each with unique pharmacological properties [31–33]. These sites are distinguishable by the ability of β-antagonists to show markedly different antagonist affinities dependent on the agonist being used to stimulate the β1 adrenoceptor [31].
Initial evidence for multiple binding sites on the β1 adrenoceptor came from detailed studies with CGP 12177. This compound is a high-affinity neutral antagonist of the classical ‘catecholamine’-binding site of the β1 adrenoceptor; however, at higher concentrations it produces an agonist response that is relatively resistant to antagonism by other classical β-antagonists [34–36]. The unique pharmacological properties of the agonist actions of CGP 12177 in producing cardiostimulant effects in the heart first led to the suggestion that a novel β4 adrenoceptor was responsible for these effects [37]. However, the loss of this ‘β4’ activity in β1 adrenoceptor knockout mice, coupled with the demonstration of ‘β4’ pharmacology in cells transfected with only the β1 adrenoceptor, led to the acceptance that the β1 adrenoceptor has an obligatory role in the expression of this unexpected pharmacological profile [35,38].
Several studies have now examined in detail the ability of different ligands to interact with the high-affinity ‘catecholamine site’ and the low-affinity ‘CGP 12177 site’ [8,11,35,36]. Classical catecholamines such as isoprenaline and adrenaline act as agonists of the catecholamine site, whereas CGP 12177, LY 362884 and carvedilol have agonist actions at the secondary CGP 12177 site [8,11,35,36,39]. In addition, some compounds (e.g. alprenolol and pindolol) have agonist actions at both sites [8].
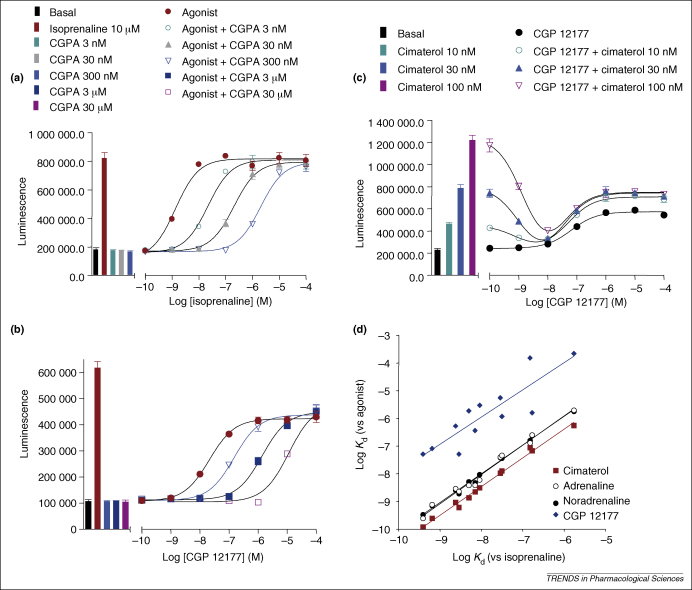
The agonist dependence of the antagonist affinity estimates is perhaps best illustrated by a correlation plot of the values obtained for a range of different antagonist affinities when several different ligands were used as agonists [8,11] (Figure 3). Although all antagonists have low affinity for the CGP 12177 site, the rank order of affinities varies between the two sites; for example, some compounds (e.g. atenolol) differ by up to 1000-fold in their affinities for the two sites, whereas others (e.g. ICI 118551) differ by only a factor of 10 [11]. Schild analysis of the ability of the β1 adrenoceptor antagonist CGP 20712A to antagonise the responses at both sites indicated that both interactions are perfectly described by a competitive interaction yielding a Schild slope of 1 [8] (Figure 3a,b). This finding strongly suggests that the two sites are completely separate and non-interacting. Furthermore, because CGP 12177 is a high-affinity antagonist of the catecholamine site (site 1), binding of CGP 12177 to the two sites of the receptor can be observed in a single experiment.
Figure 3.
Differential affinities of antagonists for the ‘catecholamine’ and ‘CGP 12177’ sites of the human β1 adrenoceptor expressed in CHO cells. (a,b) Effect of increasing concentrations of the selective β1 adrenoceptor antagonist CGP 20712A (CGPA) on isoprenaline-stimulated (a) and CGP-12177-stimulated (b) CRE-mediated luciferase expression. Note that the blue data points and line in (a) and (b) represent the same concentration of CGP 20712A (300 nM). (c) CRE–luciferase response to CGP 12177 in the absence and presence of fixed concentrations of cimaterol. (d) Correlation between the logarithm of the antagonist dissociation constant (log Kd) obtained for 12 antagonists with the agonist isoprenaline (x axis) and log Kd determined with the same 12 antagonists but with adrenaline, noradrenaline, cimaterol or CGP 12177 as the agonist (y axis). Data are from Refs [8,11].
Figure 3c shows that low concentrations of CGP 12177 inhibit cimaterol (a site-1 agonist), whereas higher concentrations of CGP 12177 stimulate an agonist response (through site 2). These effects cannot simply be explained by an allosteric regulation by CGP 12177 of the orthosteric binding site [8,11,34,35]. Also plotted in Figure 3 is the correlation of the antagonist affinity (log Kd) of 12 antagonists, determined in the presence of different agonists (Figure 3d). The log Kd value of the antagonists is the same whether isoprenaline, adrenaline or noradrenaline is used as the agonist (thus, the points are indistinguishable). The Kd values obtained for the same 12 antagonists when CGP 12177 is used as the agonist are clearly very different, and the rank order (seen as the pattern of scatter) does not parallel that of the catecholamines. Lastly, although the antagonist affinities measured when cimaterol is the agonist are in the same rank order, the values obtained are consistently higher than those obtained when the catecholamines are present. This observation raises the possibility that there might be more than two ‘sites’ on the β1 adrenoceptor, or at least more than one conformation of the catecholamine site.
The data obtained with the human β1 adrenoceptor firmly establish the concept that there might be additional ligand-binding sites on GPCRs that are separate from the classical orthosteric site and that can stimulate functional responses. These sites do not necessarily need to interact cooperatively and they might provide an alternative means by which cell signalling can be initiated with a completely different pharmacological profile and possibly by a completely different set of ligands to those of the orthosteric site. Furthermore, the data obtained with the β1 adrenoceptor also beg the question of how widespread this phenomenon is. Possibly, we have not found other agonist sites on other GPCRs because we have not looked for them or because our high-throughput screening strategies are too focused on the traditional view of the orthosteric site.
A detailed study of the human β3 adrenoceptor set out to test precisely this possibility. Early suggestions for multiple states of the human β3 adrenoceptor had been put forward to explain the apparently opposite relative potencies of β3 agonists obtained when evaluated by cAMP measurements in intact cells and when measured by radioligand binding studies in membrane preparations [40]. The differences in antagonist affinity observed at the β3 adrenoceptor were not as pronounced as those at the β1 adrenoceptor; however, some agonists were clearly inhibited more potently by antagonists than were other ligands [41]. Furthermore, ZD 7114 was shown to be able to activate two conformations or sites of the β3 adrenoceptor (analogous to alprenolol and pindolol at the β1 adrenoceptor) and alprenolol was found to be a neutral antagonist of one site while activating a second site (similar to CGP 12177 at the β1 adrenoceptor) [41].
Signalling-pathway-dependent pharmacology
As their name suggests, the main signalling pathways by which GPCRs have normally been thought to function is by activation of heterotrimeric G proteins. Recent studies, however, have provided evidence for G-protein-independent signalling by the β2 adrenoceptor, vasopressin V2, parathyroid hormone and angiotensin AT1 receptors [9,42–48]. The potential for GPCRs to form complexes with signalling proteins other than G proteins also raises the possibility that agonists and antagonists can discriminate between these complexes in terms of both binding affinity and efficacy. Thus, each downstream signalling pathway measured in a particular cell might have its own unique pharmacology depending on the pathway stimulated by each unique ligand–receptor conformation or complex involved.
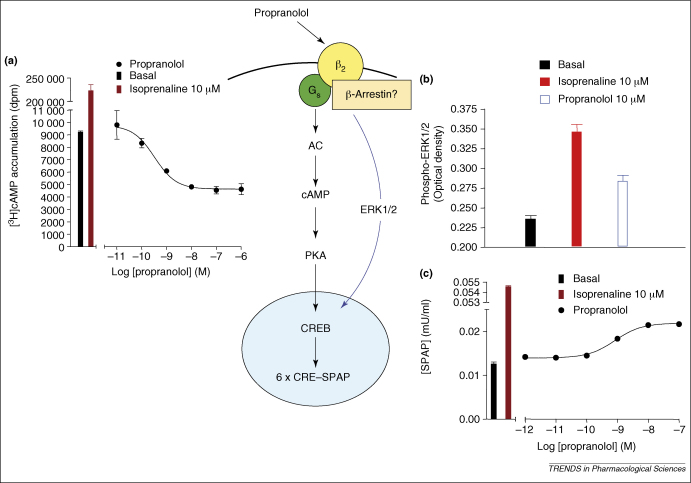
Some insight into the potential for the human β2 adrenoceptor to exist in different conformations (each with its own unique pharmacology) and to couple to distinct signalling pathways has been provided by detailed studies of the agonist and inverse agonist properties of propranolol on activation of the ERK1/2 mitogen-activated protein (MAP) kinase pathway and cAMP accumulation in CHO and human embryonic kidney 293 (HEK293) cells [9,43,49] (Figure 4). In the case of cAMP accumulation in CHO or HEK293 cells expressing the human β2 adrenoceptor, propranolol shows clear inverse agonist properties [9,43,49]. When ERK1/2 activation is measured in the same cells, however, propranolol behaves as a partial agonist. It also seems that stimulation of ERK1/2 phosphorylation by propranolol is independent of Gi or Gs protein activation [9,43]. Furthermore, it has been shown in HEK293 cells that ERK1/2 stimulation involves an interaction with β-arrestins [43]. These data suggest that ligand-specific conformations of the β2 adrenoceptor do indeed exist that can differentially activate distinct signalling pathways with very different pharmacologies. A similar observation has also been made for the murine β3 adrenoceptor, for which SR59230A is an antagonist of CL316243-mediated increases in cAMP accumulation in adipocytes, but is an agonist with greater efficacy than CL316243 for extracellular acidification in the same cells [50].
Figure 4.
Dual efficacy of propranolol on β2-adrenoceptor-mediated responses in CHO cells expressing the human β2 adrenoceptor. (a) Inverse agonist effect of propranolol on [3H]cAMP accumulation. 6 × CRE–SPAP signifies a SPAP reporter gene containing six CRE elements. (b) Agonist effects of isoprenaline and propranolol on levels of phosphorylated ERK1/2 monitored by an ELISA assay. (c) Agonist effect of propranolol on CRE-mediated gene transcription. Data are from Ref. [9].
It is therefore possible that antagonists might differ in their affinity for different GPCR–signalling protein complexes (e.g. Gs- and β-arrestin-coupled forms of the β2 adrenoceptor) and show agonist- and signalling-pathway-dependent affinities. In this respect, it is interesting that the affinity constants for different β2 adrenoceptor antagonists obtained in gene transcription studies in cells expressing the β2 adrenoceptor were found to differ by a factor of 10 depending on the competing agonist [51]. The exact mechanisms underlying this observation, however, remain to be established.
Other receptors with different ‘pharmacological’ antagonist affinities
Comparison of the antagonist affinities estimated from radioligand binding studies and those estimated from functional studies with the same ligands shows that there are major discrepancies in the affinities obtained at the β1 adrenoceptor. For example, binding of 3H-labelled CGP 12177 gives a receptor affinity for propranolol of 6.9 nM; when propranolol antagonises the CGP 12177 agonist response in functional assays, however, the affinity obtained is 363 nM [11,52]. This discrepancy arises because the low concentration of 3H-labelled CGP 12177 used in the binding assay is measuring site-1 binding (there is not sufficient 3H-labelled CGP 12177 present to occupy site 2), whereas the CGP 12177 agonist response is occurring at site 2 and therefore propranolol affinity at site 2 is being measured. Antagonist affinities measured in functional assays with site-1 agonists correlate well with the binding studies. Comparing similar data from other receptors is a means by which insight might be gained into how widespread the presence of multiple binding sites might be in GPCRs.
For example, the α1L adrenoceptor might represent an alternative conformation of the α1A adrenoceptor [53–55]. The α1L adrenoceptor in functional studies in native tissues has a unique pharmacological profile that is characterized by lower affinities for prazosin, WB 4101, 5-methylurapidil and S-niguldipine than would be predicted from studies of ligand binding to the α1A adrenoceptor [53,56,57]. Other ligands such as tamsulosin and indoramin, however, yield identical binding affinities between the two assays. Expression of the cloned human α1A adrenoceptor in CHO cells, and comparison of antagonist affinities between ligand binding studies and functional noradrenaline-stimulated inositol phosphate responses, has yielded α1A adrenoceptor (binding) and α1L adrenoceptor (functional) pharmacologies [53,54]. It is possible, therefore, that the presence of multiple binding sites on the α1A adrenoceptor (analogous to those on the human β1 and β3 adrenoceptor) or signalling-pathway-dependent α1A adrenoceptor conformations might explain these discrepancies.
Concluding remarks
It is now clear that, for many GPCRs, antagonist affinities can no longer be assumed to be always constant for a particular receptor. Antagonist affinities can vary depending on the agonist that they are counteracting, the presence or absence of allosteric ligands, the specific site on the GPCR through which they exert their effect, and the specific signalling pathway under consideration. The fact that antagonist drugs can have differential effects at different sites on the same receptor means that we should no longer simply think in terms of ‘class effects’ of receptor antagonists. This concept is particularly important when we consider ‘antagonist’ drugs that can manifest different effects on specific signalling cascades through a single receptor within the same cell.
Instead, we should explore the huge potential provided by multiple binding sites and divergent signalling cascades from the same GPCR, while at the same time exploiting quantitative analytical approaches and novel screening designs to re-evaluate how widespread these phenomena are.
Acknowledgements
J.G.B. is a Wellcome Trust Clinician Scientist. The research from the authors’ laboratories was funded by the Wellcome Trust, the Biotechnology and Biological Sciences Research Council, and the Medical Research Council.
References
- 1.Arunlakshama O., Schild H.O. Some quantitative uses of drug antagonists. Br. J. Pharmacol. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaddum J.H. The quantitative effects of antagonist drugs. J. Physiol. 1937;89:6–7. [Google Scholar]
- 3.Black J.W. Definition and antagonism of histamine H2 receptors. Nature. 1972;236:385–390. doi: 10.1038/236385a0. [DOI] [PubMed] [Google Scholar]
- 4.Hill S.J. G-protein-coupled receptors: past, present and future. Br. J. Pharmacol. 2006;147:S27–S37. doi: 10.1038/sj.bjp.0706455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rang H.P. The receptor concept: pharmacology's big idea. Br. J. Pharmacol. 2006;147:S9–S16. doi: 10.1038/sj.bjp.0706457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kenakin T. On the importance of the ‘antagonist assumption’ to how receptors express themselves. Biochem. Pharmacol. 1995;50:17–26. doi: 10.1016/0006-2952(95)00137-o. [DOI] [PubMed] [Google Scholar]
- 7.Black J.W. Comparison of some properties of pronethalol and propranolol. Br. J. Pharmacol. 1965;25:577–591. doi: 10.1111/j.1476-5381.1965.tb01782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker J.G. Agonist actions of ‘β-blockers’ provide evidence for two agonist activation sites on the human β1-adrenoceptor. Mol. Pharmacol. 2003;63:1312–1321. doi: 10.1124/mol.63.6.1312. [DOI] [PubMed] [Google Scholar]
- 9.Baker J.G. Agonist and inverse agonist actions of ‘β-blockers’ at the human β2-adrenoceptor provide evidence for agonist-directed signalling. Mol. Pharmacol. 2003;64:1357–1369. doi: 10.1124/mol.64.6.1357. [DOI] [PubMed] [Google Scholar]
- 10.Kenakin T. Allosteric modulators: the new generation of receptor antagonist. Mol. Interv. 2004;4:222–229. doi: 10.1124/mi.4.4.6. [DOI] [PubMed] [Google Scholar]
- 11.Baker J.G. Site of action of β-ligands at the human β1-adrenoceptor. J. Pharmacol. Exp. Ther. 2005;313:1163–1171. doi: 10.1124/jpet.104.082875. [DOI] [PubMed] [Google Scholar]
- 12.Nelson C.P., Challiss R.A.J. ‘Phenotypic’ pharmacology: the influence of cellular environment on G protein-coupled receptor antagonist and inverse agonist pharmacology. Biochem. Pharmacol. 2007;73:737–751. doi: 10.1016/j.bcp.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Urban J.D. Functional selectivity and classical concepts of quantitative pharmacology. J. Pharmacol. Exp. Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- 14.Stephenson R.P. Modification of receptor theory. Br. J. Pharmacol. Chemother. 1956;11:379–393. doi: 10.1111/j.1476-5381.1956.tb00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Black J.W., Leff P. Operational models of pharmacological agonists. Proc. R. Soc. Lond. B. Biol. Sci. 1983;220:141–162. doi: 10.1098/rspb.1983.0093. [DOI] [PubMed] [Google Scholar]
- 16.Baker J.G., Hill S.J. Antagonist affinity measurements at the histamine H2 receptor are independent of the agonist used. Proceedings of the British Pharmacological Society. 2005 http://www.pA2online.org/abstracts/Vol3Issue4abst096P.pdf [Google Scholar]
- 17.Christopoulos A., Kenakin T. G protein-coupled receptor allosterism and complexing. Pharmacol. Rev. 2002;54:323–374. doi: 10.1124/pr.54.2.323. [DOI] [PubMed] [Google Scholar]
- 18.Lazareno S. Thiochrome enhances acetylcholine affinity at muscarinic M4 receptors: receptor subtype selectivity via cooperativity rather than affinity. Mol. Pharmacol. 2004;65:257–266. doi: 10.1124/mol.65.1.257. [DOI] [PubMed] [Google Scholar]
- 19.Soudijn W. Allosteric modulation of G protein-coupled receptors: perspective and recent developments. Drug Discov. Today. 2004;9:752–758. doi: 10.1016/S1359-6446(04)03220-9. [DOI] [PubMed] [Google Scholar]
- 20.Birdsall N.J.M., Lazareno S. Allosterism at muscarinic receptors: ligands and mechanisms. Mini-Rev. Med. Chem. 2005;5:523–543. doi: 10.2174/1389557054023251. [DOI] [PubMed] [Google Scholar]
- 21.Watson C. The CCR5 receptor-based mechanism of action of 873140, a potent allosteric non competitive HIV entry inhibitor. Mol. Pharmacol. 2005;67:1268–1282. doi: 10.1124/mol.104.008565. [DOI] [PubMed] [Google Scholar]
- 22.Jakubik J. Positive cooperativity of acetylcholine and other agonists with allosteric ligands on muscarinic acetycholine receptors. Mol. Pharmacol. 1997;52:172–179. doi: 10.1124/mol.52.1.172. [DOI] [PubMed] [Google Scholar]
- 23.Berg K.A. Effect pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Mol. Pharmacol. 1998;54:94–104. [PubMed] [Google Scholar]
- 24.Cordeaux Y. Influence of receptor number on functional responses elicited by agonists acting at the human adenosine A1 receptor: evidence for signaling pathway-dependent changes in agonist potency and relative intrinsic activity. Mol. Pharmacol. 2000;58:1075–1084. doi: 10.1124/mol.58.5.1075. [DOI] [PubMed] [Google Scholar]
- 25.Cordeaux Y. Coupling of the human A1 adenosine receptor to different heterotrimeric G-proteins: evidence for agonist-specific G-protein activation. Br. J. Pharmacol. 2004;143:705–714. doi: 10.1038/sj.bjp.0705925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker J.G., Hill S.J. A comparison of the antagonist affinities for the Gi- and Gs-coupled states of the human adenosine A1-receptor. J. Pharmacol. Exp. Ther. 2007;320:218–228. doi: 10.1124/jpet.106.113589. [DOI] [PubMed] [Google Scholar]
- 27.Ostrom R.S., Insel P.A. The evolving role of lipid rafts and caveolae in G protein-coupled receptor signaling: implications for molecular pharmacology. Br. J. Pharmacol. 2004;143:235–245. doi: 10.1038/sj.bjp.0705930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Insel P.A. Caveolae and lipid rafts: G protein-coupled receptor signaling microdomains in cardiac myocytes. Ann. N. Y. Acad. Sci. 2005;1047:166–172. doi: 10.1196/annals.1341.015. [DOI] [PubMed] [Google Scholar]
- 29.Briddon S.J. Quantitative analysis of the formation and diffusion of A1-adenosine receptor–antagonist complexes in living cells. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4673–4678. doi: 10.1073/pnas.0400420101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Middleton R.J. New fluorescent adenosine A1-receptor agonists which allow quantification of ligand-receptor interactions in microdomains of single living cells. J. Med. Chem. 2007;50:782–793. doi: 10.1021/jm061279i. [DOI] [PubMed] [Google Scholar]
- 31.Granneman J.G. The putative β4-adrenergic receptor is a novel state of the β1-adrenergic receptor. Am. J. Physiol. Endocrinol. Metab. 2001;280:E199–E202. doi: 10.1152/ajpendo.2001.280.2.E199. [DOI] [PubMed] [Google Scholar]
- 32.Molenaar P. The ‘state’ of β-adrenoceptors. Br. J. Pharmacol. 2003;140:1–2. doi: 10.1038/sj.bjp.0705420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arch J.R. Do low-affinity states of β-adrenoceptors have roles in physiology and medicine? Br. J. Pharmacol. 2004;143:517–518. doi: 10.1038/sj.bjp.0705991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pak M.D., Fishman P.H. Anomalous behaviour of CGP 12177A on β1-adrenergic receptors. J. Recept. Signal Transduct. Res. 1996;16:1–23. doi: 10.3109/10799899609039938. [DOI] [PubMed] [Google Scholar]
- 35.Konkar A.A. Aryloxypropanolamine and catecholamine ligand interactions with the β1-adrenergic receptor: evidence for interaction with distinct conformations of β1-adrenergic receptors. J. Pharmacol. Exp. Ther. 2000;294:923–932. [PubMed] [Google Scholar]
- 36.Lowe M.D. Comparison of the affinity of β-blockers for the two states of the β1-adrenoceptor in ferret ventricular myocardium. Br. J. Pharmacol. 2002;135:451–461. doi: 10.1038/sj.bjp.0704450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaumann A.J., Molenaar P. Modulation of human cardiac function through 4 β-adrenoceptor populations. Naunyn Schmiedebergs Arch. Pharmacol. 1997;355:667–681. doi: 10.1007/pl00004999. [DOI] [PubMed] [Google Scholar]
- 38.Kaumann A.J. Abolition of (−)-CGP 12177-evoked cardiostimulation in double β1/β2-adrenoceptor knockout mice. Obligatory role of β1-adrenoceptors for putative β4-adrenoceptor pharmacology. Naunyn Schmiedebergs Arch. Pharmacol. 2001;363:87–93. doi: 10.1007/s002100000336. [DOI] [PubMed] [Google Scholar]
- 39.Joseph S.S. Binding (−)3H-CGP 12177 at two sites in recombinant human β1-adrenoceptors and interaction with β-blockers. Naunyn Schmiedebergs Arch. Pharmacol. 2004;369:525–532. doi: 10.1007/s00210-004-0884-y. [DOI] [PubMed] [Google Scholar]
- 40.Arch J.R. β3-adrenoceptor agonists: potential pitfalls and progress. Eur. J. Pharmacol. 2002;440:99–107. doi: 10.1016/s0014-2999(02)01421-8. [DOI] [PubMed] [Google Scholar]
- 41.Baker, J.G. (2005b) Evidence for a secondary state of the human β3-adrenoceptor. Mol. Pharmacol. 68, 1645–1655 [DOI] [PubMed]
- 42.Seta K. AT1 receptor mutant lacking heterotrimeric G protein coupling activates the Src–Ras–ERK pathway without nuclear translocation of ERKs. J. Biol. Chem. 2002;277:9268–9277. doi: 10.1074/jbc.M109221200. [DOI] [PubMed] [Google Scholar]
- 43.Azzi M. β-arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors. Proc. Natl. Acad. Sci. U. S. A. 2003;100:11406–11411. doi: 10.1073/pnas.1936664100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei H. Independent β-arrestin2 and G-protein-mediated pathways for angiotensin II activation of extracellular signal regulated kinase 1 and 2. Proc. Natl. Acad. Sci. U. S. A. 2003;100:10782–10787. doi: 10.1073/pnas.1834556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terrillon S., Bouvier M. Receptor-activity-independent recruitment of β-arrestin2 reveals specific signalling modes. EMBO J. 2004;23:3950–3961. doi: 10.1038/sj.emboj.7600387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gesty-Palmer D. Distinct β-arrestin- and G protein-dependent pathways for parathyroid hormone receptor-stimulated ERK1/2 activation. J. Biol. Chem. 2006;281:10856–10864. doi: 10.1074/jbc.M513380200. [DOI] [PubMed] [Google Scholar]
- 47.Shenoy S.K. β-arrestin-dependent, G protein-independent ERK1/2 activation by the β2 adrenergic receptor. J. Biol. Chem. 2006;281:1261–1273. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
- 48.Charest P.G. The V2 vasopressin receptor stimulates ERK 1/2 activity independently of heterotrimeric G protein signalling. Cell. Signal. 2007;19:32–41. doi: 10.1016/j.cellsig.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 49.Galandrin S., Bouvier M. Distinct signalling profiles of β1 and β2 adrenergic receptor ligands toward adenylyl cyclase and mitogen-activated protein kinase reveals the pluridimensionality of efficacy. Mol. Pharmacol. 2006;70:1575–1584. doi: 10.1124/mol.106.026716. [DOI] [PubMed] [Google Scholar]
- 50.Hutchinson D.S. Evidence for pleiotropic signalling at the mouse β3-adrenoceptor revealed by SR59230A [3-(2-ehtylphenoxy)-1-(1,S)-1,2,3,4-tetrahydronaph-1-ylamino]-2S-2-propanol oxylate. J. Pharmacol. Exp. Ther. 2005;312:1064–1074. doi: 10.1124/jpet.104.076901. [DOI] [PubMed] [Google Scholar]
- 51.Baker, J.G. et al. (2003c) Influence of agonist efficacy and receptor phosphorylation on antagonist affinity measurements: differences between second messenger and reporter gene responses. Mol. Pharmacol. 64, 679–688 [DOI] [PubMed]
- 52.Baker J.G. The selectivity of β-adrenoceptor antagonists at the human β1, β2 and β3 adrenoceptors. Br. J. Pharmacol. 2005;144:317–322. doi: 10.1038/sj.bjp.0706048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ford A.P.D.W. Pharmacological pleiotropism of the human recombinant α1A-adrenoceptor: implications for α1-adrenoceptor classification. Br. J. Pharmacol. 1997;121:1127–1135. doi: 10.1038/sj.bjp.0701207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Daniels D.V. Human cloned α1A-adrenoceptor isoforms display α1L-adrenceptor pharmacology in functional studies. Eur. J. Pharmacol. 1999;370:337–343. doi: 10.1016/s0014-2999(99)00154-5. [DOI] [PubMed] [Google Scholar]
- 55.Blue D.R. Pharmacological characteristics of Ro 115-1240, a selective α1A/α1L-adrenoceptor partial agonist: a potential therapy for stress urinary incontinence. BJU Int. 2004;93:162–170. doi: 10.1111/j.1464-410x.2004.04577.x. [DOI] [PubMed] [Google Scholar]
- 56.Flavahan N.A., Vanhoutte P.M. α1-Adrenoceptor subclassification in vascular smooth muscle. Trends Pharmacol. Sci. 1986;7:347–349. [Google Scholar]
- 57.Muramatsu I. Pharmacological subclassification of α1-adrenoceptors in vascular smooth muscle. Br. J. Pharmacol. 1990;99:197–201. doi: 10.1111/j.1476-5381.1990.tb14678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]