Abstract
AIRE is a transcriptional activator that directs the ectopic expression of many tissue-specific genes in medullary thymic epithelial cells, which plays an important role in the negative selection of autoreactive T cells. However, its mechanism of action remains poorly understood. In this study, we found that AIRE regulates the step of elongation rather than initiation of RNA polymerase II. For these effects, AIRE bound and recruited P-TEFb to target promoters in medullary thymic epithelial cells. In these cells, AIRE activated the ectopic transcription of insulin and salivary protein 1 genes. Indeed, by chromatin immunoprecipitation, we found that RNA polymerase II was already engaged on these promoters but was unable to elongate in the absence of AIRE. Moreover, the genetic inactivation of cyclin T1 from P-TEFb abolished the transcription of AIRE-responsive genes and led to lymphocytic infiltration of lacrimal and salivary glands in the CycT1−/− mouse. Our findings reveal critical steps by which AIRE regulates the transcription of genes that control central tolerance in the thymus.
Among genetic factors implicated in autoimmunity, the autoimmune regulator (AIRE) is an important transcriptional activator that mediates central tolerance in the thymus. Loss-of-function mutations in the human Aire gene lead to the development of autoimmune polyendocrinopathy syndrome type 1 (APECED), which is manifested by the destruction of mostly endocrine glands (31). AIRE is expressed mainly in the thymus but is also present in low levels in lymph nodes, spleen, and fetal liver (16, 31). In the thymus, its expression is restricted to a subpopulation of medullary thymic epithelial cells (mTECs) (16). Indeed, mTECs express ectopically a number of tissue-restricted antigens (9, 19), which is abolished in AIRE−/− mice (4). This absence leads to a defect in negative selection of autoreactive T cells in the thymus (3, 26) and autoimmunity in the periphery, which is manifested by the infiltration of autoreactive T cells in many tissues and the expression of autoantibodies in the periphery (4, 40).
AIRE is a 55-kDa nuclear protein. It has several functional domains, such as the N-terminal homogenously staining region, a functional bipartite nuclear localization signal, a putative DNA-binding SAND (Sp100, AIRE, NucP41/75, and DEAF-1) domain (5, 10), two plant homeodomain (PHD)-type Zn2+ fingers separated by a proline-rich region, and four LXXLL nuclear receptor motifs (1, 15). When fused to a heterologous DNA-binding domain, AIRE can activate transcription in transient-expression assays (37). Because it binds preferentially to GG repeats and AT-rich sequences (24, 39), its interactions with DNA could be rather promiscuous (reviewed in references 28 and 36). The first PHD may also function as an E3 ubiquitin ligase (47). Finally, analyses of AIRE−/− mice revealed that mouse insulin 2 (Ins2), salivary protein 1 (Spt1), casein α, and several hundred other genes are regulated by AIRE (4). Interestingly, some of these genes are localized in chromosomal clusters (9, 19).
Eukaryotic transcription starts with the recruitment of RNA polymerase II (RNAPII) to start sites of transcription. This process requires DNA-bound activators, general transcription factors, chromatin remodeling machinery, and RNAPII. This recruitment leads to the formation of the preinitiation complex (PIC) (reviewed in reference 30). At this stage, transcription is initiated but further elongation is blocked by the negative transcription elongation factor (N-TEF), which contains the DRB sensitivity-inducing factor and the negative elongation factor (reviewed in reference 35). To enable efficient elongation and cotranscriptional processing of primary transcripts, positive transcription elongation factor b (P-TEFb) must be recruited to the PIC. It counteracts N-TEF and prepares RNAPII for elongation. P-TEFb is a heterodimer of a C-type cyclin (CycT1, CycT2, or CycK) and cyclin-dependent kinase 9 (Cdk9). P-TEFb phosphorylates N-TEF and the C-terminal domain of RNAPII, and this change enables RNAPII to transition from abortive to productive elongation. In cells, P-TEFb is found in two molecular complexes. In the small complex, the catalytically active P-TEFb associates with activators and RNAPII. In the catalytically inactive large complex, P-TEFb binds 7SK snRNA and hexamethylene bis-acetamide inducible protein 1 (HEXIM1) (reviewed in reference 35). Thus, HEXIM1 is a specific inhibitor of P-TEFb.
Transcriptional activators can be divided into three groups: type I (e.g., Sp1 and CTF), which stimulate initiation; type IIA (e.g., Tat from human immunodeficiency virus), which stimulate predominantly elongation; and type IIB (VP16, class II transactivator [CIITA], and NF-κB, among others), which stimulate both initiation and elongation of transcription (6). Moreover, type I and type IIA activators can synergize with one another but not with type IIB activators. Synergy occurs from concerted actions of factors stimulating two different steps in transcription: initiation and elongation (6). In this study, we wanted to determine how AIRE regulates transcription in mTECs.
MATERIALS AND METHODS
Plasmids.
Plasmid targets and effectors were described previously (34, 46). pMycAIRE, pFlagAIRE, and insulin reporters were generous gifts from P. Peterson (16) and M. German (33), respectively. We also moved AIRE cDNA into the retroviral expression vector pBABE-puro (pBABE.AIRE).
Chemicals and immunoreagents.
Trichostatin A (TSA) and anti-Flag M2 (αFlag M2) agarose beads were from Sigma (St. Louis, MO). αMyc, αCdk9, αAIRE, αCycT1, and αRNAPII were from Santa Cruz Biotechnology (Santa Cruz, CA); αGAPDH (GAPDH is glyceraldehyde-3-phosphate dehydrogenase) was from Ambion (Austin, TX); αHEXIM1 was from Antibody Solutions (Mountain View, CA); and αAIRE 6.1 was from J. Pitkänen.
Cell culture and transient-expression studies.
HeLa-MAGI, 1C6, and Phoenix-ampho cells were grown and transfected as described in the supplemental material.
Activation of endogenous genes.
1C6 cells were grown, transfected, and analyzed as described in the supplemental material.
GST pulldowns, immunoprecipitations, and Western blotting.
Glutathione S-transferase (GST) pulldowns and immunoprecipitations were performed essentially as described previously (20). In brief, 20 μg of GST or GST fusion proteins was incubated with cell lysates, reacted with beads, and washed four times with lysis buffer. Bound proteins were eluted by being boiled in sodium dodecyl sulfate sample buffer, resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 12% gel, and analyzed by Western blotting using αAIRE 6.1 antibody. For immunoprecipitation, cells were lysed and immunoprecipitated with αFlag M2 agarose beads (Sigma) overnight. Bound proteins were eluted and separated by SDS-PAGE and analyzed by immunoblotting with αCycT1, αCdk9, αCdk7, and αFlag M2 antibodies.
Knockdown of HEXIM1 by use of siRNA and chloramphenicol acetyltransferase (CAT) reporter assays.
In brief, 1C6 cells were transfected with specific or negative-control scrambled small interfering RNA (siRNA) (mock siRNA) by use of Lipofectamine 2000 (Invitrogen, Carlsbad, CA), analyzed for the expression of HEXIM1, and later transfected with plasmid effectors and targets. Further details are provided in the supplemental material.
ChIP.
1C6 and 1C6.AIRE cells were grown on 15-cm plates. They were subjected to chromatin immunoprecipitation (ChIP) by use of a ChIP assay kit (Upstate Biotechnology, Charlottesville, VA) according to the manufacturer's instructions. Lysates were sonicated at power 4 four times for 10 s each by use of a Sonic Dismembrator model 100 (Fisher Scientific, Pittsburgh, PA) to shear genomic DNA. The average size of sheared fragments was 300 to 500 bp. We used the following antibodies from Santa Cruz: αAIRE, αRNAPII, αCycT1, and αCdk9. A portion (2 μl) of the extracted DNA was amplified in 30-μl reactions by use of ExTaq polymerase (Takara, Shiga, Japan) for 24 to 36 cycles, integrated over that range, and normalized to input DNA as described previously (51). Further details are provided in the supplemental material.
Generation of CycT1−/− mice, histopathology, and clinical scoring.
Details of the generation and characterization of CycT1−/− mice are provided in the supplemental material.
RESULTS
AIRE is a type IIA activator and promotes transcriptional elongation.
To investigate the mechanism of action of AIRE, we used a well-established assay for differentiating between transcriptional initiation and elongation (43). We chose three artificial plasmid targets, which differ by ability to recruit and load RNAPII to the promoter and thus to initiate and/or elongate transcription. The plasmid target pG5CAT contains five Gal4 DNA-binding sites (upstream activation sequences [UASs]) placed in front of the E1b TATA promoter linked to the CAT reporter gene (Fig. 1A, pG5CAT) (43). pG5CAT recruits RNAPII inefficiently and thus cannot initiate or elongate transcription. The second plasmid target, pG6SpCAT, contains six UASs and three Sp1 binding sites placed in front of the E1b TATA promoter and the CAT gene (Fig. 1A, pG6SpCAT) (45). The third plasmid target, pG6TARCAT, contains six UASs positioned upstream of the κB sites in the human immunodeficiency virus type 1 long terminal repeat, which also transcribes the transactivation response (TAR) RNA stem loop and the CAT gene (45). Three Sp1 sites load and position RNAPII on these two promoters, but they require an enhancer to recruit P-TEFb for the transition to the elongation phase of transcription and the expression of the CAT gene (45). We used these plasmid targets to determine whether AIRE stimulates the initiation and/or elongation of transcription.
FIG. 1.
AIRE cooperates with initiation factors to activate transcription. (A) (Top) Schematic representations of plasmid targets used in CAT assays. pG5CAT contains the CAT gene and five repeats of the GAL4 DNA-binding site (5×UAS) in front of the E1b TATA box (T). pG6SpCAT contains a complete promoter of three Sp1 sites (3×Sp1) and the E1b TATA box in addition to six UASs and the CAT gene. pG6TARCAT contains a TAR RNA structure in addition to the elements present in pG6SpCAT. pA represents the polyadenylation signal. (Bottom) AIRE requires the presence of initiation factors to activate transcription in 1C6 and HeLa-MAGI cells. Cells expressed the indicated plasmid targets alone or with AIRE (lanes 3, 4, 6, 8, 10, 12, and 14) and GalDBD (lanes 2, 4, 11, and 12). Expression levels of AIRE and GalDBD, as determined by Western blotting, are presented below the CAT data. Levels of endogenous GAPDH were determined by Western blotting to validate input for each sample. Error bars denote standard errors of the means of three independent experiments. (B) AIRE activates transcription in a dose-dependent manner. 1C6 cells expressed pG6SpCAT and increasing amounts of AIRE (0.1, 0.25, and 0.8 μg in lanes 2, 3, and 4, respectively). Expression levels of AIRE and GAPDH, as determined by Western blotting, are presented below the CAT data. Error bars denote standard errors of the means of three independent experiments. (C) AIRE induces the elongation of transcription. (Top) Primer combinations for the amplification of primary transcripts. Primers 1 and 2 amplify TAR and thus all transcripts (ST). Primers 1 and 3 amplify only the LT. nt, nucleotides. (Bottom) Total RNA was extracted from HeLa-MAGI cells coexpressing pG6TARCAT and AIRE or GalDBD and was analyzed by RT-qPCR. The Gal.CycT1 chimera was used as the positive control. Data are representative of three independent experiments.
To test whether AIRE can activate transcription in different cell types, we used the human epithelial HeLa-MAGI (49) and mouse 1C6 mTEC (22) cell lines, which do not express AIRE (47). Importantly, 1C6 cells were derived from primary mTECs and were never immortalized with the help of an oncogene or telomerase (22). As presented in Fig. 1A with pG5CAT, where no Sp1 sites are present, AIRE did not activate transcription (Fig. 1A, lanes 3 and 10). Indeed, neither AIRE nor the Gal4 DNA-binding domain protein from positions 1 to 147 (GalDBD) could activate transcription when coexpressed with this plasmid target alone (Fig. 1A, lanes 2, 3, 10, and 11). Of interest, GalDBD contains a weak activation domain (27), which behaves as a type I activator. In sharp contrast, when coexpressed with AIRE, GalDBD activated transcription 50-fold (Fig. 1A, lanes 4 and 12). Importantly, AIRE did not have to be tethered artificially to DNA for these effects. Therefore, our results suggest that AIRE promotes the elongation of transcription.
To confirm this observation, we used a cooperativity assay (43) with pG6SpCAT, where AIRE should synergize with Sp1. Indeed, in the presence of Sp1 sites, AIRE activated transcription 30- and 15-fold over background levels in HeLa-MAGI and 1C6 cells, respectively (Fig. 1A, lanes 5, 6, 13, and 14). AIRE also activated transcription of the pG6TARCAT reporter gene equivalently (Fig. 1A, lane 8). Thus, the presence of TAR did not alter the effects of AIRE on plasmid targets in cells. Importantly, the expression of GalDBD alone did not activate transcription of pG6SpCAT or pG6TARCAT reporter genes (data not presented). Furthermore, the exogenous expression of increasing amounts of AIRE resulted in a dose-dependent increase of transcriptional activation (Fig. 1B, lanes 2, 3, and 4). Thus, AIRE appears to function as a type IIA activator, which is unable to recruit RNAPII and assemble the PIC but can act synergistically with type I activators to stimulate transcriptional elongation.
To determine if AIRE affects the movement of RNAPII, we used an established assay for transcriptional elongation, which looks for promoter-proximal short transcripts (ST) and elongated long transcripts (LT) by use of quantitative reverse transcription-PCR (RT-qPCR) approaches (Fig. 1C) (21). Since AIRE behaved identically in 1C6 and HeLa-MAGI cells (Fig. 1A), we used the latter cells for our studies. We analyzed RNA samples from cells coexpressing pG6TARCAT with AIRE, the Gal.CycT1 chimera, or GalDBD as positive or negative controls, respectively. The specificity of our primers and the ability to isolate ST or LT were demonstrated previously, where levels of steady-state mRNA also correlated directly with RNase protection and nuclear run-on data (2). Only ST corresponding to TAR were observed when GalDBD was coexpressed with pG6TARCAT (Fig. 1C, lane 1). In sharp contrast, the Gal.CycT1 fusion protein, which served as the positive control (46), and AIRE promoted the elongation of ST to LT (Fig. 1C, lanes 2 and 3). These results demonstrate that AIRE enables RNAPII to elongate.
AIRE binds P-TEFb in vitro and in cells.
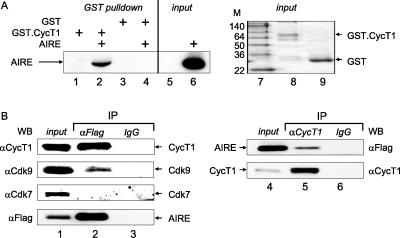
As type IIA activators, including Tat, recruit P-TEFb to promote transcriptional elongation, we wanted to test whether AIRE also binds and recruits P-TEFb to the transcriptional machinery. First, we performed GST pulldown assays using the GST.CycT1 chimera or GST alone, which was incubated with lysates of HeLa-MAGI cells expressing AIRE. As shown in Fig. 2A, lane 2, AIRE bound the GST.CycT1 chimera. Since no interaction between GST alone and AIRE was detected, this binding was specific (Fig. 2A, lane 4). The inputs of AIRE, the GST.CycT1 chimera, and GST alone are also presented (Fig. 2A, lanes 6, 8, and 9, respectively).
FIG. 2.
AIRE binds CycT1 in vitro and in cells. (A) AIRE binds the GST.CycT1 chimera in vitro. Binding reactions were performed between GST and the GST.CycT1 chimera, which were expressed in Escherichia coli, and AIRE, which was expressed in HeLa-MAGI cells. (Left) Lanes 1 to 4 contain specific pulldowns, and lane 6 contains 10% of the input AIRE protein. (Right) Input of GST proteins, which were visualized by Coomassie blue staining of SDS-PAGE. Molecular size markers (in kilodaltons) are given in lane M. (B) AIRE interacts with the endogenous CycT1 and Cdk9 proteins in 1C6 cells. The Flag epitope-tagged AIRE protein (lanes 1 to 6) was expressed in 1C6 cells. Total cell lysates were immunoprecipitated (IP) with αFlag M2 agarose beads (lane 2), αCycT1 (lane 5), or mouse IgG (lanes 3 and 6) antibodies and examined for the presence of CycT1, Cdk9, Cdk7, and AIRE by Western blotting (WB) with αCycT1, αCdk9, αCdk7, and αFlag antibodies, respectively. Lanes 1 and 4 represent 10% of the input of indicated proteins.
Next, the endogenous CycT1 and Cdk9 proteins could be coprecipitated with AIRE from cells. We expressed the Flag epitope-tagged AIRE protein in 1C6 cells. Lysates were immunoprecipitated with αFlag M2 or normal mouse immunoglobulin G (IgG) antibodies as the negative control, and immunoprecipitation products were subjected to Western blotting with αCycT1 or αCdk9 antibody. Whereas no CycT1 or Cdk9 was coimmunoprecipitated by mouse IgG antibodies (Fig. 2B, lane 3), both CycT1 and Cdk9 bound AIRE (Fig. 2B, lane 2). We further confirmed this interaction by immunoprecipitating CycT1 from cell lysates with αCycT1 antibodies and Western blotting for AIRE. Whereas no binding was observed with normal rabbit IgG antibodies (Fig. 2B, lane 6), AIRE bound CycT1 (Fig. 2B, lane 5). In contrast, Cdk7, which is not in the P-TEFb complex, did not bind AIRE (Fig. 2B, lane 2, Cdk7). In Fig. 2B, lanes 1 and 4 present inputs of the indicated proteins.
To confirm that AIRE and CycT1 are expressed in the same subcellular compartments, we also performed colocalization studies of AIRE and CycT1. We expressed the Myc epitope-tagged AIRE protein in 1C6 cells grown on coverslips and performed double immunostaining with αMyc and αCycT1 antibodies. AIRE and CycT1 were expressed and colocalized in a speckled pattern in the nuclei of 1C6 cells (see Fig. S1 in the supplemental material, middle, colocalization). Although all AIRE colocalized with P-TEFb, since it was expressed at physiological levels, the majority of P-TEFb remained free (see Fig. S1 in the supplemental material, middle). We also observed the colocalization of AIRE with the splicing factor SC35, which localizes to sites of active transcription and was shown previously to colocalize with CycT1 (17 and data not presented). We conclude that AIRE binds and colocalizes with P-TEFb in cells.
HEXIM1 inhibits transcriptional activity of AIRE.
A large fraction of P-TEFb is bound by HEXIM1 and 7SK snRNA in the inactive large complex (reviewed in reference 35). Thus, increased levels of HEXIM1 block the activity of P-TEFb and should therefore decrease the transcriptional activity of AIRE. To test this hypothesis, we coexpressed pG6SpCAT, AIRE, and increasing amounts of HEXIM1 in 1C6 cells. When AIRE was coexpressed with pG6SpCAT alone, the CAT activity increased 11-fold (Fig. 3A, lane 3). Importantly, the coexpression of increasing amounts of HEXIM1 (ratios between amounts of plasmids coding for AIRE and HEXIM1 increased from 8:1 to 4:1 and 2:1, respectively) resulted in a dose-dependent decrease of this activity (Fig. 3A, compare lanes 4, 5, and 6). Expression levels of AIRE and HEXIM1 are presented below the bar graph. Also, to ensure that equivalent amounts of lysates were loaded for each sample, levels of GAPDH were determined with αGAPDH antibodies (Fig. 3A, bottom).
FIG. 3.
HEXIM1 inhibits the transcriptional activity of AIRE. (A) Increased levels of HEXIM1 block AIRE-mediated transcription from pG6SpCAT. 1C6 cells coexpressed AIRE and increasing amounts of HEXIM1 (0.125, 0.25, and 0.5 μg). CAT assays were performed 24 h after transfection. CAT activity of the plasmid target alone is given as 1 (white bar). Black bars represent activation (n-fold) by AIRE (lane 3). In the presence of increasing amounts of HEXIM1, the activity of AIRE decreases (lanes 4 to 6). Below the bar graphs are presented levels of AIRE, HEXIM1, and GAPDH as determined by Western blotting. Error bars denote standard errors of the means of three independent experiments. (B) Depletion of endogenous HEXIM1 protein increases the transcriptional activity of AIRE. 1C6 cells were transfected with mock siRNA (lanes 1 and 2) or siRNA-Hex1 (lanes 3 and 4). The next day, cells were cotransfected with pG6SpCAT and the plasmid encoding MycAIRE (lanes 2 and 4) or the empty plasmid vector as the control (lanes 1 and 3). After an additional 24 h, CAT assays were performed. Amounts of AIRE, endogenous HEXIM1, and GAPDH proteins after siRNA treatment were assessed by immunoblotting and are presented below the bar graph. Error bars denote standard errors of the means of three independent experiments.
Because transcriptional effects depend on the small complex (reviewed in reference 35), the depletion of endogenous HEXIM1 protein from cells increases this pool of active P-TEFb (23). As a result, the effects of AIRE should be increased. To address this hypothesis, levels of HEXIM1 were decreased by siRNA against HEXIM1 (siRNA-Hex1) and effects of AIRE measured in 1C6 cells. In these cells, negative-control scrambled siRNA (mock siRNA) (Fig. 3B, lanes 1 and 2) or specific siRNA-Hex1 (Fig. 3B, lanes 3 and 4) was coexpressed with AIRE and pG6SpCAT (Fig. 3B, lanes 2 and 4) or the empty plasmid vector as the control (Fig. 3B, lanes 1 and 3). The use of siRNA-Hex1 has been validated previously (23). CAT assays were performed after 36 h. When mock siRNA was used, AIRE activity increased 10-fold (Fig. 3B, compare lanes 1 and 2). Critically, when levels of HEXIM1 were reduced extensively by siRNA-Hex1, AIRE activity was increased by 60% in comparison to activity when mock siRNA was used (Fig. 3B, compare lanes 2 and 4). Western blotting with αHEXIM1 antibodies revealed that whereas amounts of HEXIM1 were reduced with siRNA-Hex1 (Fig. 3B, middle blot, lanes 3 and 4), the expression of AIRE and GAPDH remained unaffected (Fig. 3B, top and bottom blots, lanes 3 and 4). These data strengthen the connection between AIRE and P-TEFb for its transcriptional effects.
AIRE activates transcription from the human Ins promoter and induces expression of mouse Ins2 and Spt1 genes.
In mTECs of AIRE−/− mice, the expression of the mouse Ins2 gene is decreased (4). To extend our findings to this relevant target in cells, we examined whether AIRE can activate transcription from the human Ins promoter. To this end, we coexpressed AIRE and this promoter from positions −339 to +50 linked to the firefly luciferase reporter gene (HIP339) (33) in 1C6 cells. Renilla luciferase readings were used to normalize the relative firefly luciferase activity of each sample. As presented in Fig. 4A, cells expressing the exogenous AIRE protein increased the luciferase activity 52-fold over background levels (Fig. 4A). Expression levels of AIRE and GAPDH are presented below the bar graph. These data demonstrate that AIRE also activates the transcription of the Ins gene.
FIG. 4.
AIRE activates the transcription of Ins and Spt1 genes in mTECs. (A) AIRE activates transcription from the human Ins promoter. 1C6 cells coexpressed AIRE and the plasmid target containing the human Ins promoter linked to the firefly luciferase gene, and luciferase activity was measured. Renilla luciferase readings were used to normalize the firefly luciferase activity of each sample for all transfections. The expression of AIRE was confirmed by Western blotting and is presented below the bar graph. Error bars denote standard errors of the means of three independent transfections. (B) AIRE activates expression of Ins2 and Spt1 genes in mTECs. RNA was extracted from 1C6 cells transiently expressing AIRE (lanes 1 to 4) or the empty plasmid vector (lanes 5 to 8) and treated additionally with 100 mM TSA for 12 h or from AIRE.1C6 cells that stably expressed AIRE (lane 9). Semiquantitative RT-PCR (fourfold serial dilution) analyses of several tissue-specific genes were performed. PCR was carried out using gene-specific primers as indicated. (C) AIRE mRNA levels in mTECs. RNA was isolated from 1C6 cells (lane 1), primary (1°) mTECs (lane 2), and transiently (lane 3) or stably (lane 4) transfected 1C6 cells, and RT-qPCR was performed with primers specific for AIRE. mRNA levels were normalized to actin.
To determine if AIRE activates the expression of its endogenous target genes in 1C6 cells, we performed semiquantitative RT-PCR analyses with fourfold serial dilutions of cDNA from 1C6 cells, which expressed AIRE or the empty plasmid vector. As presented in Fig. 4B, the two previously reported genes which are dependent on AIRE, those encoding Ins2 and Spt1, were transcribed only in the presence of AIRE (Fig. 4B, Ins2 and Spt1, compare lanes 1 to 4 and lanes 5 to 8). Importantly, the expression of c-reactive protein (CRP), a tissue-specific antigen which was reported previously to be independent of AIRE (4), and the expression of actin were independent of AIRE (Fig. 4B, CRP and actin, compare lanes 1 to 4 and lanes 5 to 8). Although we added TSA in our transient-expression assays, as suggested by others (8), TSA by itself had no effect on the expression of these genes (Fig. 4B, compare AIRE versus the vector control). Moreover, we created the AIRE.1C6 cell line, which expresses AIRE stably, and obtained identical results in the absence of TSA (Fig. 4B, lane 9). Importantly, mRNA levels of AIRE expression were comparable between AIRE.1C6 and primary mTECs (Fig. 4C, lanes 2, 3, and 4). This finding extends previously published results obtained with AIRE−/− mice (4) and confirms that AIRE regulates the expression of Ins2 and Spt1 genes in mTECs.
AIRE recruits P-TEFb to Ins2 and Spt1 promoters and enables RNAPII to elongate.
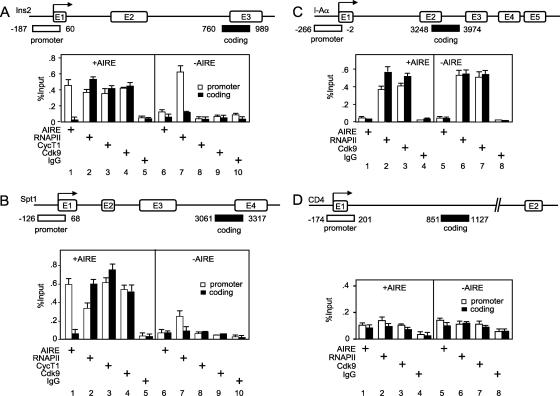
To determine if AIRE recruits P-TEFb to promoters of AIRE-responsive genes, we performed ChIP followed by quantitative PCR (ChIP/qPCR) assays (51) with 1C6 cells expressing AIRE or the empty plasmid vector. Formaldehyde-cross-linked chromatin extracts were prepared, and these extracts were immunoprecipitated with specific αAIRE, αRNAPII, αCycT1, and αCdk9 antibodies. Immunoprecipitated DNA was amplified by PCR using primers specific for the promoters and coding regions of two AIRE-responsive genes, Ins2 and Spt1 (Fig. 5A and B), and two AIRE-independent genes, mouse major histocompatibility complex (MHC) class II (I-Aα) and CD4 (Fig. 5C and D). We chose MHC class II because it is expressed in mTECs independently of AIRE. In contrast, CD4 is not expressed in mTECs, so no component of the transcriptional machinery should be present on its promoter. Data were normalized to input DNA (Fig. 5).
FIG. 5.
AIRE recruits P-TEFb to Ins2 and Spt1 promoters and stimulates transcriptional elongation by RNAPII. 1C6 (−AIRE) and 1C6.AIRE (+AIRE) cells were analyzed. Formaldehyde-fixed and sonicated chromatin extracts were immunoprecipitated with the indicated antibodies. ChIP/qPCR was performed with the indicated primers to determine the amounts of DNA associated with immunoprecipitated proteins on promoters or coding sequences. The positions of primers are indicated in the diagrams above the graphs. We looked for the presence of AIRE, RNAPII, and subunits of P-TEFb on AIRE-responsive genes (A and B) and AIRE-nonresponsive genes (C and D). ChIP/qPCR with rabbit IgG antibodies was used as the negative control for specificity. All values are expressed relative to the control input DNA (% input) and represent experiments performed in triplicate, with errors indicated.
First, we looked for the presence of AIRE and RNAPII on the indicated chromatin regions of the Ins2 gene. Indeed, AIRE was recruited to the Ins2 promoter and was absent from its coding region (Fig. 5A, compare bars in lanes 1 and 6). In sharp contrast, RNAPII was already engaged on the Ins2 promoter whether or not AIRE was expressed (Fig. 5A, compare white bars in lanes 2 and 7). Although amounts of RNAPII on the Ins2 promoter were comparable, RNAPII was present on the Ins2 coding region only in the presence of AIRE (Fig. 5A, compare black bars in lanes 2 and 7). Thus, the presence of AIRE coincides with the elongation of RNAPII on this gene. Importantly, the same results were obtained when we analyzed the Spt1 locus. Indeed, AIRE was present on the Spt1 promoter (Fig. 5B, compare white bars in lanes 1 and 6) and RNAPII was present on the Spt1 coding region only in the presence of AIRE (Fig. 5B, compare black bars in lanes 2 and 7). These data correlate the presence of AIRE with the elongation of RNAPII on two AIRE-responsive genes in cells.
Next, we wanted to determine if the elongation of RNAPII was due to the recruitment of P-TEFb, so we looked for the presence of components of P-TEFb on the indicated chromatin regions of the Ins2 gene. We found that CycT1 and Cdk9 subunits of P-TEFb were found on the Ins2 promoter (Fig. 5A, compare white bars in lanes 3, 4, 8, and 9) and on the coding region (Fig. 5A, compare black bars in lanes 3, 4, 8, and 9) only in the presence of AIRE. Identical results were observed for the Spt1 gene (Fig. 5B, compare bars in lanes 3, 4, 8, and 9). Importantly, in the case of the I-Aα gene, whose expression is independent of AIRE but dependent on CIITA (reviewed in reference 41), RNAPII was present on the promoter and on the coding region whether or not AIRE was expressed (Fig. 5C, compare bars in lanes 2 and 6). It is known that CIITA also recruits P-TEFb to MHC class II promoters (20, 23). We observed that Cdk9 was also present on the I-Aα promoter and coding region independently of AIRE (Fig. 5C, compare bars in lanes 3 and 7). As an additional control, we analyzed the CD4 locus. Since this gene is not expressed in mTECs, neither RNAPII nor Cdk9 was present on the CD4 promoter or coding regions in 1C6 cells (Fig. 5D, compare bars in lanes 2, 3, 4, 6, 7, and 8). We conclude that the presence of AIRE correlates with the recruitment of P-TEFb and the elongation of RNAPII on two AIRE-responsive genes in cells.
AIRE-responsive genes are not expressed in the thymuses of CycT1−/− mice.
Next, we wanted to demonstrate that P-TEFb plays an important role in the transcription of AIRE-responsive genes in the organism. To this end, we generated mice with a severe depletion of CycT1 (see Fig. S2 in the supplemental material) in the thymus (Fig. 6A, top, lane 2) and other organs (see Fig. S3 in the supplemental material) by means of gene trap technology of embryonic stem cells (50). Interestingly, this ablation of CycT1 also resulted in decreased expression of HEXIM1 and Cdk9 (Fig. 6A, top, lane 2). Moreover, whereas levels of AIRE transcripts were not affected, those of Ins2 and Spt1 were decreased greatly in mTECs from CycT1−/− mice (Fig. 6A, bottom, compare bars in lanes 1, 2, 3, and 4).
FIG. 6.
CycT1−/− mice do not express AIRE-responsive genes in the thymus and display lymphocytic infiltration of lacrimal and salivary glands. (A) Absent expression of CycT1 in the thymus (see Fig. S3 in the supplemental material) parallels the lack of Spt1 and Ins2 transcripts in CycT1−/− mice. We assessed levels of CycT1, HEXIM1, Cdk9, and GAPDH in the thymuses from WT or CycT1−/− mice by Western blotting (top). Next, primary mTECs were isolated from the thymuses of WT or CycT1−/− mice, followed by isolation of total RNA and analyses by RT-qPCR with primers specific for AIRE, actin, Spt1, and Ins2 transcripts (bottom, l to 4). WT and CycT1−/− denote parental CycT1+/+ and genetically inactivated CycT1−/− mice, respectively. Amounts of RNA are expressed relative to those in WT mTECs, with standard errors from three independent measurements. (B) Lack of CycT1 in the mouse results in lymphocytic infiltration of lacrimal and salivary glands. Hematoxylin and eosin staining of formalin-fixed sections of lacrimal and salivary glands from 5- to 7-month-old WT and CycT1−/− mice is presented. Arrows point to lymphocytic infiltrates in these organs. Photographs were obtained at ×40 and ×100 (insets) magnifications. Below histological sections are presented relative infiltrations of salivary and lacrimal glands of up to 10 CycT1+/+ and CycT1+/− (WT) and 21 CycT1−/− mice. Scoring was performed blindly, as described previously (18).
Since AIRE−/− mice exhibited an autoimmune phenotype (4), which was manifested by lymphocytic infiltrates in many organs, we investigated if the same situation pertains to our CycT1−/− mice. Paraffin sections from eye, lung, stomach, spleen, ovary, and lacrimal and salivary glands were prepared, stained with hematoxylin and eosin dyes, and examined for the presence of lymphocytic infiltrates. Importantly, we detected such infiltration in lacrimal and salivary glands in CycT1−/− but not parental, wild-type (WT) mice (Fig. 6B, top right). Moreover, these infiltrations occurred in fewer than half of our CycT1−/− mice and were relatively mild (Fig. 6B, bottom) (18). In contrast, their WT littermates had little to no infiltration of these organs (Fig. 6B, bottom). We conclude that the inactivation of the CycT1 gene in the mouse has a severe impact on the expression of AIRE-responsive genes in mTECs and leads to lymphocytic infiltration in endocrine organs, which resembles the autoimmune phenotype of AIRE−/− mice.
DISCUSSION
In this study, we found that AIRE acts as a type IIA activator that regulates the elongation phase of transcription. First, AIRE cooperated with initiation factors, such as Sp1, to elongate stalled transcripts. Second, AIRE bound CycT1 from P-TEFb. Third, the specificity of this interaction was confirmed by the inhibition of AIRE by HEXIM1. As a corollary, the depletion of HEXIM1 by siRNA led to the increased activity of AIRE. Fourth, AIRE activated the transcription of human and mouse Ins genes as well as the Spt1 gene in mTECs. Fifth, AIRE and P-TEFb were colocalized on Ins2 and Spt1 promoters and P-TEFb was found there only in the presence of AIRE, which was required for the elongation of RNAPII on these genes. Finally, the genetic inactivation of CycT1 led to loss of expression of AIRE-responsive genes and infiltration of lacrimal and salivary glands in the mouse. We conclude that by interacting with P-TEFb, AIRE stimulated transcriptional elongation of its target genes in mTECs.
Previously, AIRE was found to activate transcription via heterologous DNA tethering using the Gal.AIRE fusion proteins (14, 37, 38). In our study, we demonstrated that the free AIRE protein also acted synergistically with GalDBD or Sp1 to activate transcription. GalDBD has a weak activation domain (from positions 74 to 147), which can initiate but not elongate primary transcripts in higher eukaryotic cells (27). Sp1 interacts with general transcription and TATA binding protein-associated factors (reviewed in reference 30). This binding leads to the formation of the PIC at the start site of transcription and, in the presence of an activator, such as AIRE, to the elongation of transcription. Thus, AIRE is the first cellular type IIA activator besides the viral Tat protein, both of which promote transcriptional elongation.
In cells and synthesized in vitro, AIRE forms oligomers. They migrate as a 670-kDa complex, which could contain up to 12 AIRE proteins (14). Monomers of AIRE neither bind DNA in vitro (24, 39) nor activate transcription in cells (14). Of interest, most mutations in patients disrupt this oligomerization of AIRE (14, 32). Moreover, the homogenously staining region, SAND, and two PHD motifs are necessary for the formation of oligomers as well as the transcriptional activity of AIRE (5, 48). Thus, although discrete functional domains have not been defined, we were able to demonstrate that AIRE binds CycT1 and P-TEFb in vitro and in cells. AIRE and CycT1 also colocalized in cells. Furthermore, this interaction was confirmed functionally by its inhibition with HEXIM1, which is a specific inhibitor of P-TEFb (29). Of interest, both Tat and AIRE, two type IIA activators, proved to be exceedingly sensitive to the inhibition of P-TEFb. Finally, in chromatin, CycT1 and Cdk9 were present on promoters of AIRE-responsive genes only in the presence of AIRE.
We also demonstrated effects of AIRE on two known target genes in mTECs (4). Thus, the introduction of AIRE led to the expression of mouse Ins2 and Spt1 genes in 1C6 cells. By ChIP, this effect was on the elongation rather than the initiation of transcription of these genes and depended on the recruitment of P-TEFb. Our ChIP data also demonstrated that AIRE interacts with cis-acting sequences, and future studies will reveal further details of these DNA-protein interactions. Finally, the genetic inactivation of CycT1 led to the loss of expression of AIRE-responsive genes in the thymus and the lymphocytic infiltration of lacrimal and salivary glands. Since hematopoietic cells retained some expression of CycT1, these findings suggest that although central tolerance was compromised, inflammatory processes were preserved. Moreover, since only half of our CycT1−/− mice developed these infiltrates of lacrimal and salivary glands, this finding argues against a global defect in T cells leading to this autoimmune phenotype. Consistent with this notion, our CycT1−/− mice had normal numbers of B and T cells, with an appropriate ratio of CD4+ and CD8+ cells. These cells also lacked activation markers (data not presented). Thus, a combination of biochemical and genetic data indicate that P-TEFb is an important coactivator of AIRE.
In conclusion, we present a mechanism for the regulation of transcription by AIRE, which suggests that AIRE is a global activator that affects different target genes via the recruitment of P-TEFb. In prokaryotes, the mechanism of antitermination, i.e., the regulation of transcription at the stage of elongation, is well established and known to play a major role (13). However, such regulation in eukaryotic systems has been appreciated only recently. Indeed, RNAPII is engaged on many regulated but silent promoters in organisms from Drosophila melanogaster to humans (25). P-TEFb is also required for the transcription of many activated genes transcribed by RNAPII in cells (7). Of note, activators found on enhancers, such as CIITA, NF-κB, steroid hormone receptors, and c-Myc, all bind and recruit P-TEFb to their transcription units (reviewed in reference 35). An important difference is that unlike these type IIB activators, AIRE cannot initiate transcription and its interactions with DNA appear more promiscuous (24, 39). Thus, it is able to interact with more targets but has a greater requirement for a preassembled PIC. These features are expected to give it greater and lesser flexibilities in pluripotent and differentiated cells, where chromatin is relatively open and closed, respectively.
Some genes that are regulated by P-TEFb are likely to cluster. Indeed, DNA looping and interactions of distal enhancers and locus control regions with promoters are important for the expression of MHC class II and β-globin genes (12, 42), which are regulated at the level of transcriptional elongation. Moreover, one study found AIRE to be associated with such matrix attachment sites (44). Thus, global chromatin conformations are also likely to play a critical role in the regulation of genes by AIRE (9, 19). Different programs of gene expression would then depend only on the stage of differentiation of mTECs. To this end, it is interesting that populations of mTECs are highly heterogeneous in the thymus (11). They appear to emerge from pluripotent precursors and then differentiate so that individual populations express distinct sets of genes. In this scenario, AIRE can be viewed as a promiscuous activator that preys upon various differentiation profiles of mTECs to elicit central tolerance to as many tissue-restricted proteins as possible. However, further details of these effects of AIRE and P-TEFb in chromatin represent an important area for future study.
Supplementary Material
Acknowledgments
We thank members of the Peterlin lab as well as M. Anderson, J. Gardner, and J. DeVoss for helpful discussions and critical reading of the manuscript. We thank M. German, M. Kasai, J. Pitkänen, and P. Peterson for reagents.
This work was supported by a grant from the Nora Eccles Treadwell Foundation. I. Oven was partially supported by the Slovenian Research Agency, Republic of Slovenia.
Footnotes
Published ahead of print on 15 October 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Aasland, R., T. J. Gibson, and A. F. Stewart. 1995. The PHD finger: implications for chromatin-mediated transcriptional regulation. Trends Biochem. Sci. 20:56-59. [DOI] [PubMed] [Google Scholar]
- 2.Adams, M., L. Sharmeen, J. Kimpton, J. Romeo, J. Garcia, B. Peterlin, M. Groudine, and M. Emerman. 1994. Cellular latency in human immunodeficiency virus-infected individuals with high CD4 levels can be detected by the presence of promoter-proximal transcripts. Proc. Natl. Acad. Sci. USA 91:3862-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson, M. S., E. S. Venanzi, Z. Chen, S. P. Berzins, C. Benoist, and D. Mathis. 2005. The cellular mechanism of Aire control of T cell tolerance. Immunity 23:227-239. [DOI] [PubMed] [Google Scholar]
- 4.Anderson, M. S., E. S. Venanzi, L. Klein, Z. Chen, S. P. Berzins, S. J. Turley, H. von Boehmer, R. Bronson, A. Dierich, C. Benoist, and D. Mathis. 2002. Projection of an immunological self shadow within the thymus by the aire protein. Science 298:1395-1401. [DOI] [PubMed] [Google Scholar]
- 5.Bjorses, P., M. Pelto-Huikko, J. Kaukonen, J. Aaltonen, L. Peltonen, and I. Ulmanen. 1999. Localization of the APECED protein in distinct nuclear structures. Hum. Mol. Genet. 8:259-266. [DOI] [PubMed] [Google Scholar]
- 6.Blau, J., H. Xiao, S. McCracken, P. O'Hare, J. Greenblatt, and D. Bentley. 1996. Three functional classes of transcriptional activation domain. Mol. Cell. Biol. 16:2044-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chao, S.-H., K. Fujinaga, J. E. Marion, R. Taube, E. A. Sausville, A. M. Senderowicz, B. M. Peterlin, and D. H. Price. 2000. Flavopiridol inhibits P-TEFb and blocks HIV-1 replication. J. Biol. Chem. 275:28345-28348. [DOI] [PubMed] [Google Scholar]
- 8.Chin, R. K., J. C. Lo, O. Kim, S. E. Blink, P. A. Christiansen, P. Peterson, Y. Wang, C. Ware, and Y.-X. Fu. 2003. Lymphotoxin pathway directs thymic Aire expression. Nat. Immunol. 4:1121-1127. [DOI] [PubMed] [Google Scholar]
- 9.Derbinski, J., J. Gabler, B. Brors, S. Tierling, S. Jonnakuty, M. Hergenhahn, L. Peltonen, J. Walter, and B. Kyewski. 2005. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J. Exp. Med. 202:33-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibson, T. J., C. Ramu, C. Gemund, and R. Aasland. 1998. The APECED polyglandular autoimmune syndrome protein, AIRE-1, contains the SAND domain and is probably a transcription factor. Trends Biochem. Sci. 23:242-244. [DOI] [PubMed] [Google Scholar]
- 11.Gillard, G. O., and A. G. Farr. 2006. Features of medullary thymic epithelium implicate postnatal development in maintaining epithelial heterogeneity and tissue-restricted antigen expression. J. Immunol. 176:5815-5824. [DOI] [PubMed] [Google Scholar]
- 12.Gomez, J. A., P. Majumder, U. M. Nagarajan, and J. M. Boss. 2005. X box-like sequences in the MHC class II region maintain regulatory function. J. Immunol. 175:1030-1040. [DOI] [PubMed] [Google Scholar]
- 13.Greenblatt, J., J. R. Nodwell, and S. W. Mason. 1993. Transcriptional antitermination. Nature 364:401-406. [DOI] [PubMed] [Google Scholar]
- 14.Halonen, M., H. Kangas, T. Ruppell, T. Ilmarinen, J. Ollila, M. Kolmer, M. Vihinen, J. Palvimo, J. Saarela, I. Ulmanen, and P. Eskelin. 2004. APECED-causing mutations in AIRE reveal the functional domains of the protein. Hum. Mutat. 23:245-257. [DOI] [PubMed] [Google Scholar]
- 15.Heery, D. M., E. Kalkhoven, S. Hoare, and M. G. Parker. 1997. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature 387:733-736. [DOI] [PubMed] [Google Scholar]
- 16.Heino, M., P. Peterson, J. Kudoh, K. Nagamine, A. Lagerstedt, V. Ovod, A. Ranki, I. Rantala, M. Nieminen, J. Tuukkanen, H. S. Scott, S. E. Antonarakis, N. Shimizu, and K. Krohn. 1999. Autoimmune regulator is expressed in the cells regulating immune tolerance in thymus medulla. Biochem. Biophys. Res. Commun. 257:821-825. [DOI] [PubMed] [Google Scholar]
- 17.Herrmann, C. H., and M. A. Mancini. 2001. The Cdk9 and cyclin T subunits of TAK/P-TEFb localize to splicing factor-rich nuclear speckle regions. J. Cell Sci. 114:1491-1503. [DOI] [PubMed] [Google Scholar]
- 18.Jiang, W., M. S. Anderson, R. Bronson, D. Mathis, and C. Benoist. 2005. Modifier loci condition autoimmunity provoked by Aire deficiency. J. Exp. Med. 202:805-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnnidis, J. B., E. S. Venanzi, D. J. Taxman, J. P.-Y. Ting, C. O. Benoist, and D. J. Mathis. 2005. Chromosomal clustering of genes controlled by the aire transcription factor. Proc. Natl. Acad. Sci. USA 102:7233-7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanazawa, S., T. Okamoto, and B. M. Peterlin. 2000. Tat competes with CIITA for the binding to P-TEFb and blocks the expression of MHC class II genes in HIV infection. Immunity 12:61-70. [DOI] [PubMed] [Google Scholar]
- 21.Kao, S.-Y., A. F. Calman, P. A. Luciw, and B. M. Peterlin. 1987. Anti-termination of transcription within the long terminal repeat of HIV-1 by tat gene product. Nature 330:489-493. [DOI] [PubMed] [Google Scholar]
- 22.Kasai, M., K. Hirokawa, K. Kajino, K. Ogasawara, M. Tatsumi, E. Hermel, J. J. Monaco, and T. Mizuochi. 1996. Difference in antigen presentation pathways between cortical and medullary thymic epithelial cells. Eur. J. Immunol. 26:2101-2107. [DOI] [PubMed] [Google Scholar]
- 23.Kohoutek, J., D. Blazek, and B. M. Peterlin. 2006. Hexim1 sequesters positive transcription elongation factor b from the class II transactivator on MHC class II promoters. Proc. Natl. Acad. Sci. USA 103:17349-17354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar, P. G., M. Laloraya, C.-Y. Wang, Q.-G. Ruan, A. Davoodi-Semiromi, K.-J. Kao, and J.-X. She. 2001. The autoimmune regulator (AIRE) is a DNA-binding protein. J. Biol. Chem. 276:41357-41364. [DOI] [PubMed] [Google Scholar]
- 25.Lis, J. 1998. Promoter-associated pausing in promoter architecture and postinitiation transcriptional regulation. Cold Spring Harbor Symp. Quant. Biol. 63:347-356. [DOI] [PubMed] [Google Scholar]
- 26.Liston, A., S. Lesage, J. Wilson, L. Peltonen, and C. C. Goodnow. 2003. Aire regulates negative selection of organ-specific T cells. Nat. Immunol. 4:350-354. [DOI] [PubMed] [Google Scholar]
- 27.Ma, J., and M. Ptashne. 1987. Deletion analysis of GAL4 defines two transcriptional activating segments. Cell 48:847-853. [DOI] [PubMed] [Google Scholar]
- 28.Mathis, D., and C. Benoist. 2004. Back to central tolerance. Immunity 20:509-516. [DOI] [PubMed] [Google Scholar]
- 29.Michels, A. A., A. Fraldi, Q. Li, T. E. Adamson, F. Bonnet, V. T. Nguyen, S. C. Sedore, J. P. Price, D. H. Price, L. Lania, and O. Bensaude. 2004. Binding of the 7SK snRNA turns the HEXIM1 protein into a P-TEFb (CDK9/cyclin T) inhibitor. EMBO J. 23:2608-2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naar, A. M., B. D. Lemon, and R. Tjian. 2001. Transcriptional coactivator complexes. Annu. Rev. Biochem. 70:475-501. [DOI] [PubMed] [Google Scholar]
- 31.Nagamine, K., P. Peterson, H. S. Scott, J. Kudoh, S. Minoshima, M. Heino, K. J. Krohn, M. D. Lalioti, P. E. Mullis, S. E. Antonarakis, K. Kawasaki, S. Asakawa, F. Ito, and N. Shimizu. 1997. Positional cloning of the APECED gene. Nat. Genet. 17:393-398. [DOI] [PubMed] [Google Scholar]
- 32.Notarangelo, L. D., C. Mazza, C. Forino, E. Mazzolari, and F. Buzi. 2004. AIRE and immunological tolerance: insights from the study of autoimmune polyendocrinopathy candidiasis and ectodermal dystrophy. Curr. Opin. Allergy Clin. Immunol. 4:491-496. [DOI] [PubMed] [Google Scholar]
- 33.Ohneda, K., H. Ee, and M. German. 2000. Regulation of insulin gene transcription. Semin. Cell Dev. Biol. 11:227-233. [DOI] [PubMed] [Google Scholar]
- 34.Ouchida, R., M. Kusuhara, N. Shimizu, T. Hisada, Y. Makino, C. Morimoto, H. Handa, F. Ohsuzu, and H. Tanaka. 2003. Suppression of NF-kappaB-dependent gene expression by a hexamethylene bisacetamide-inducible protein HEXIM1 in human vascular smooth muscle cells. Genes Cells 8:95-107. [DOI] [PubMed] [Google Scholar]
- 35.Peterlin, B. M., and D. H. Price. 2006. Controlling the elongation phase of transcription with P-TEFb. Mol. Cell 23:297-305. [DOI] [PubMed] [Google Scholar]
- 36.Peterson, P., J. Pitkanen, N. Sillanpaa, and K. Krohn. 2004. Autoimmune polyendocrinopathy candidiasis ectodermal dystrophy (APECED): a model disease to study molecular aspects of endocrine autoimmunity. Clin. Exp. Immunol. 135:348-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pitkanen, J., V. Doucas, T. Sternsdorf, T. Nakajima, S. Aratani, K. Jensen, H. Will, P. Vahamurto, J. Ollila, M. Vihinen, H. S. Scott, S. E. Antonarakis, J. Kudoh, N. Shimizu, K. Krohn, and P. Peterson. 2000. The autoimmune regulator protein has transcriptional transactivating properties and interacts with the common coactivator CREB-binding protein. J. Biol. Chem. 275:16802-16809. [DOI] [PubMed] [Google Scholar]
- 38.Pitkanen, J., A. Rebane, J. Rowell, A. Murumagi, P. Strobel, K. Moll, M. Saare, J. Heikkila, V. Doucas, A. Marx, and P. Peterson. 2005. Cooperative activation of transcription by autoimmune regulator AIRE and CBP. Biochem. Biophys. Res. Commun. 333:944-953. [DOI] [PubMed] [Google Scholar]
- 39.Purohit, S., P. G. Kumar, M. Laloraya, and J. X. She. 2005. Mapping DNA-binding domains of the autoimmune regulator protein. Biochem. Biophys. Res. Commun. 327:939-944. [DOI] [PubMed] [Google Scholar]
- 40.Ramsey, C., O. Winqvist, L. Puhakka, M. Halonen, A. Moro, O. Kampe, P. Eskelin, M. Pelto-Huikko, and L. Peltonen. 2002. Aire deficient mice develop multiple features of APECED phenotype and show altered immune response. Hum. Mol. Genet. 11:397-409. [DOI] [PubMed] [Google Scholar]
- 41.Reith, W., and B. Mach. 2001. The bare lymphocyte syndrome and the regulation of MHC expression. Annu. Rev. Immunol. 19:331-373. [DOI] [PubMed] [Google Scholar]
- 42.Sawado, T., J. Halow, M. A. Bender, and M. Groudine. 2003. The beta-globin locus control region (LCR) functions primarily by enhancing the transition from transcription initiation to elongation. Genes Dev. 17:1009-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Southgate, C. D., and M. R. Green. 1991. The HIV-1 Tat protein activates transcription from an upstream DNA-binding site: implications for Tat function. Genes Dev. 5:2496-2507. [DOI] [PubMed] [Google Scholar]
- 44.Tao, Y., R. Kupfer, B. J. Stewart, C. Williams-Skipp, C. K. Crowell, D. D. Patel, S. Sain, and R. I. Scheinman. 2006. AIRE recruits multiple transcriptional components to specific genomic regions through tethering to nuclear matrix. Mol. Immunol. 43:335-345. [DOI] [PubMed] [Google Scholar]
- 45.Taube, R., K. Fujinaga, J. Wimmer, M. Barboric, and B. M. Peterlin. 1999. Tat transactivation: a model for the regulation of eukaryotic transcriptional elongation. Virology 264:245-253. [DOI] [PubMed] [Google Scholar]
- 46.Taube, R., X. Lin, D. Irwin, K. Fujinaga, and B. M. Peterlin. 2002. Interaction between P-TEFb and the C-terminal domain of RNA polymerase II activates transcriptional elongation from sites upstream or downstream of target genes. Mol. Cell. Biol. 22:321-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uchida, D., S. Hatakeyama, A. Matsushima, H. Han, S. Ishido, H. Hotta, J. Kudoh, N. Shimizu, V. Doucas, K. I. Nakayama, N. Kuroda, and M. Matsumoto. 2004. AIRE functions as an E3 ubiquitin ligase. J. Exp. Med. 199:167-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Villasenor, J., C. Benoist, and D. Mathis. 2005. AIRE and APECED: molecular insights into an autoimmune disease. Immunol. Rev. 204:156-164. [DOI] [PubMed] [Google Scholar]
- 49.Vodicka, M. A., W. C. Goh, L. I. Wu, M. E. Rogel, S. R. Bartz, V. L. Schweickart, C. J. Raport, and M. Emerman. 1997. Indicator cell lines for detection of primary strains of human and simian immunodeficiency viruses. Virology 233:193-198. [DOI] [PubMed] [Google Scholar]
- 50.Wurst, W., J. Rossant, V. Prideaux, M. Kownacka, A. Joyner, D. P. Hill, F. Guillemot, S. Gasca, D. Cado, A. Auerbach, et al. 1995. A large-scale gene-trap screen for insertional mutations in developmentally regulated genes in mice. Genetics 139:889-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu, P., S. H. Baek, E. M. Bourk, K. A. Ohgi, I. Garcia-Bassets, H. Sanjo, S. Akira, P. F. Kotol, C. K. Glass, M. G. Rosenfeld, and D. W. Rose. 2006. Macrophage/cancer cell interactions mediate hormone resistance by a nuclear receptor derepression pathway. Cell 124:615-629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.