Abstract
Histone deacetylase 6 (HDAC6) is a cytoplasmic deacetylase that uniquely catalyzes α-tubulin deacetylation and promotes cell motility. However, the mechanism underlying HDAC6-dependent cell migration and the role for microtubule acetylation in motility are not known. Here we show that HDAC6-induced global microtubule deacetylation was not sufficient to stimulate cell migration. Unexpectedly, in response to growth factor stimulation, HDAC6 underwent rapid translocation to actin-enriched membrane ruffles and subsequently became associated with macropinosomes, the vesicles for fluid-phase endocytosis. Supporting the importance of these associations, membrane ruffle formation, macropinocytosis, and cell migration were all impaired in HDAC6-deficient cells. Conversely, elevated HDAC6 levels promoted membrane ruffle formation with a concomitant increase in macropinocytosis and motility. In search for an HDAC6 target, we found that heat shock protein 90 (Hsp90), another prominent substrate of HDAC6, was also recruited to membrane ruffles and macropinosomes. Significantly, inhibition of Hsp90 activity suppressed membrane ruffling and cell migration, while expression of an acetylation-resistant Hsp90 mutant promoted ruffle formation. Our results uncover a surprising role for HDAC6 in actin remodeling-dependent processes and identify the actin cytoskeleton as an important target of HDAC6-regulated protein deacetylation.
Proper cell migration is critical for normal development as well as physiological processes such as wound healing, whereas its deregulation is a key factor contributing to tumor invasion and metastasis. Cell migration is often initiated by extracellular growth factors that activate receptor tyrosine kinase-dependent signaling, leading to extensive actin remodeling and the formation of unique structures termed membrane ruffles (18). These hallmark structures are important for cell motility and for invasive migration of cells through the extracellular matrix (51). Elucidating the molecular network that regulates and connects actin remodeling to cell migration is therefore fundamentally important.
Rac1, a member of the Rho family small GTPases, plays a critical role in actin remodeling and motility (5). In response to growth factor stimulation, Rac1 activates WAVE and the Arp2/3 multiprotein complex, which induces branching of actin filaments, resulting in membrane ruffling at the leading edge (lamellipodia or peripheral ruffles) and at the dorsal surface (dorsal ruffles). The induction of membrane ruffles is uniquely accompanied by a specialized form of endocytosis, fluid phase endocytosis, also termed macropinocytosis (14, 40). Distinct from other forms of endocytosis, macropinocytosis is initiated when extended membrane ruffles fuse with the plasma membrane, which generates large endocytic vesicles (>0.1 μm) termed macropinosomes (10, 52). Macropinocytosis provides an efficient route for taking up extracellular macromolecules and nutrients. As is the case for cell migration, macropinocytosis in most cell types is stimulated by growth factors (10, 52). In fact, macropinocytosis and directed cell movement are often correlated. For example, oncogenic Ras, Src, and phosphoinositide 3-kinase (PI3K) promote both macropinocytosis and motility (1, 24, 49, 53, 55). A constitutively active p21-activated kinase 1 (PAK1), a key effector for Rac1, stimulates actin membrane ruffle formation and macropinocytosis, with a concomitant increase in directed cell migration (14). Analysis of fibroblasts derived from WAVE2 knockout (KO) mice demonstrates deficiency in ruffle formation, macropinocytosis, and motility (51). Together, these observations reveal that macropinocytosis and cell motility are functionally connected and are subjected to the control of growth factor and oncogenic signaling. However, unlike the well-studied clathrin-dependent or caveola-mediated endocytosis, the regulation of macropinocytosis is still poorly understood.
We have previously shown that HDAC6, a cytoplasmic member of the histone deacetylase family, promotes growth factor-induced cell motility (22). This unexpected finding is in stark contrast to the well-established function of the histone deacetylase family in histone acetylation-dependent chromatin remodeling and gene transcription (12). Overexpression of HDAC6 in fibroblasts results in two prominent phenotypes: global microtubule deacetylation and an increase in cell motility (22). Indeed, HDAC6 uniquely possesses a microtubule deacetylase activity (22, 31, 60). However, the mechanism by which HDAC6 regulates cell motility remains poorly understood. Although the microtubule network is a critical element in cell migration, whether microtubule deacetylation controlled by HDAC6 is the primary factor driving cell motility is not known. This has become an important issue, since α-tubulin is not the exclusive substrate of HDAC6. Another prominent HDAC6 substrate recently identified is the molecular chaperone heat shock protein 90 (Hsp90). HDAC6-catalyzed deacetylation of Hsp90 is required for its full chaperone activity, which has been shown to regulate multiple signaling pathways (29, 33, 34). Identifying the relevant substrate is critical for elucidating how the protein deacetylase HDAC6 regulates cell motility and will likely uncover novel mechanisms that connect growth factor signaling to the cell migration machinery.
In this article, we present evidence that HDAC6-induced microtubule deacetylation is not sufficient to regulate cell migration. Rather, HDAC6 and its substrate Hsp90 regulate cell motility and macropinocytosis by promoting actin remodeling-dependent membrane ruffle formation. We show that in response to growth factor stimulation, both HDAC6 and Hsp90 are recruited to membrane ruffles and macropinosomes and that functional HDAC6 and Hsp90 are required for efficient Rac1 activation, ruffle formation, macropinocytosis, and cell motility. Our study identifies HDAC6 as a new regulatory component in growth factor-induced actin remodeling, macropinocytosis, and cell migration.
MATERIALS AND METHODS
Cell lines.
Mouse embryonic fibroblasts (MEFs) obtained from E14.5 wild-type and HDAC6 KO embryos were immortalized by stably expressing a dominant-negative p53 mutant (pCMVDD), following a previously described protocol (48). MEFs were maintained and propagated in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) and 10% fetal calf serum at 37°C with 5% CO2. Stable MEF lines were created through retrovirus-mediated gene transfer using pPABE-puro plasmids containing full-length HDAC6, HDAC6-mut, or HDAC6-ΔBUZ. These constructs have been described before (27) and are either green fluorescent protein (GFP) tagged or FLAG tagged.
Antibodies and reagents.
Anti-mHDAC6 antibody was generated by injecting rabbits with recombinant glutathione S-transferase (GST)-mouse HDAC6 (amino acids 991 to 1149), followed by affinity purification. The following antibodies were also used in this study: anti-human HDAC6 (H-300; Santa Cruz Biotechnology, Inc.), anti-GFP (Roche), anti-SirT2 (Cell Signaling), anti-Rac1 (BD Bioscience), anti-p150glued (BD Bioscience), anti-acetyl-α-tubulin (from M. Yoshida, RIKEN, for immunoblotting or from Sigma-Aldrich for immunostaining), anti-tyrosinated tubulin (from J. C. Bulinski, Columbia University), and anti-Hsp90 (25). Antiactin (AC-15), anti-FLAG (M2), anti-polyglutamylated tubulin, and antitransferrin antibodies; geldanamycin; transferrin-biotin; and ExtrAvidin-horseradish peroxidase were from Sigma-Aldrich. Platelet-derived growth factor BB (PDGF-BB) was from Sigma-Aldrich or Upstate Cell Signaling Solutions. F-Actin was stained with phalloidin-Alexa Fluor 647, phalloidin-rhodamine, or phalloidin-Alexa Fluor 488 (Molecular Probes). Dextran (70 kDa)-tetramethylrhodamine (lysine fixable; Molecular Probes), dextran (70 kDa)-Texas Red (Molecular Probes), and dextran (70 kDa)-fluorescein isothiocyanate (FITC) powder (Fluka) were used in macropinocytosis assays. The transfection reagents FuGene6 and Lipofectamine LTX were from Roche and Invitrogen separately.
Immunofluorescence microscopy.
Immunostaining was performed essentially as described previously (15, 22). Specifically, MEFs were cultured on glass coverslips in serum-free medium for 15 h, followed by treatment with PDGF-BB at 50 ng/ml. Cells were fixed in 4% paraformaldehyde- made in phosphate-buffered saline (PBS) for 15 min at room temperature. In macropinocytosis assays, cells containing dextran-loaded macropinosomes were fixed for at least 2 h with freshly prepared 4% paraformaldehyde-PBS. Cells were examined on a spinning-disk confocal microscope (Olympus ZX-70) equipped with an ORCA ER charge-coupled-device camera using a 60×/1.4-numerical-aperture oil objective. Brightness and contrast of the images were adjusted in Photoshop CS (Adobe Systems Incorporated).
Macropinocytosis assay.
Macropinocytosis assays were performed as previously described (11) with some modifications. Briefly, serum-starved MEFs were stimulated with PDGF (50 ng/ml) in culture medium in the presence of 3.0 mg/ml dextran (70 kDa)-FITC for 45 min (or 1.0 mg/ml dextran (70 kDa)-Texas Red for 30 min). Cells were rinsed with prechilled PBS and detached using 0.05% trypsin-0.02% EDTA-Na2 (GIBCO) at 4°C. Cells were then washed and resuspended in 5 mg/ml bovine serum albumin (BSA) (Sigma-Aldrich)-PBS before being analyzed by fluorescence-activated cell sorting (FACS). Signals from wortmannin-treated (100 nM; 30-min pretreatment) samples were subtracted to eliminate dextran uptake from nonmacropinocytic endocytosis. Propidium iodide (1 μg/ml; Molecular Probes) was used to exclude dead cells in FACS analysis.
Endocytosis of transferrin-biotin.
Wild-type and HDAC6 KO MEFs were serum starved for 1.5 h in DMEM, and the cells were then incubated with 25 μg/ml transferrin-biotin in 5 mg/ml BSA-DMEM at 25°C for different periods of time. Cells were rinsed with ice-cold PBS, washed once with ice-cold 2 N acetate-2 M NaCl solution (pH 2.5, 2 min), and lysed in 1% Triton X-100-PBS. Transferrin-biotin in the cell lysates was analyzed using enzyme-linked immunosorbent assays with goat antitransferrin antibody-coated plates and ExtrAvidin-horseradish peroxidase.
Transwell migration assay.
A transwell migration assay in Boyden chambers (Costar) was performed as outlined previously (22). Briefly, serum-starved MEFs were detached by trypsinization (5 min at 37°C) from the master plates and washed/resuspended in 7.5% BSA-PBS (GIBCO). After pretreatment (described in figure legends), 35,000 (in 100 μl) cells were seeded in fibronectin (Roche)-coated upper chambers and were allowed to migrate through the 8.0-μm pores at 37°C for 135 min toward the lower chambers, which were supplied with 500 μl complete medium. Reagents for treatment were included in both upper and lower chambers during the assay. Upon completion of the assay, cells remaining on the upper side of the membranes that separate the upper and lower chambers were removed by mopping with a cotton swab. Membranes were stained with Coomassie blue, cut out, and examined on a Leitz Axioscope microscope using a 10× objective. Migrated cells were quantified from five fields of the membrane, including the center and positions corresponding to 3, 6, 9, and 12 o'clock, but away from the edge of the membrane. Numbers of cells migrated at these positions were combined. All experiments were performed in duplicate.
Rac1-GTP pull-down assay.
The Rac1 activation assay was preformed as described previously (42, 43). Briefly, PDGF-BB treated MEFs were lysed in Rac1-GTP binding buffer (50 mM Tris-HCl, pH 7.4, 100 mM NaCl, 10 mM MgCl2, 1% NP-40, 10% glycerol, protease inhibitor cocktail (Sigma-Aldrich), 1 mM Na3VO4, 5 mM NaF, and 1 mM dithiothreitol). The cell lysates were incubated with 30 μg immobilized GST-PAK1-CRIB for 1 h at 4°C. Bound Rac1-GTP was examined by immunoblotting using monoclonal anti-Rac1 antibody.
RESULTS
HDAC6 catalyzed deacetylation of microtubules alone is not sufficient to support cell migration.
Cell migration is driven and sustained by dynamic yet coordinated rearrangements of actin and microtubule structures (41, 56). The microtubule network is posttranslationally modified by acetylation, which has been suggested to modify microtubule stability (31, 37, 54). We have previously demonstrated that ectopically expressed HDAC6 promotes NIH 3T3 cell migration, with markedly decreased microtubule acetylation (22). However, it is unclear whether HDAC6 regulates cell migration solely through deacetylation of microtubules and whether microtubule deacetylation alone is sufficient to drive cell migration.
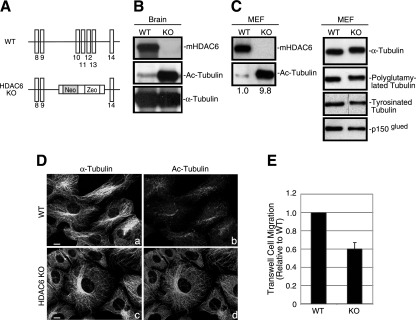
In order to investigate this issue, we generated HDAC6 KO mice, in which the genomic sequence covering exons 10 to 13 of HDAC6 was deleted by homologous recombination (Fig. 1A). Figure 1B and C verify that HDAC6 was absent from tissues or MEFs derived from HDAC6 KO mice. Importantly, α-tubulin acetylation was dramatically elevated in HDAC6-deficient tissues and MEFs by about 10-fold as determined densitometrically and normalized against total α-tubulin, which is not affected by HDAC6 status (Fig. 1C). Of note, the majority of α-tubulin from HDAC6-deficient MEFs migrated slower in a sodium dodecyl sulfate-polyacrylamide gel than those isolated from wild-type MEFs (Fig. 1C), suggesting that most α-tubulin subunits are acetylated in the absence of HDAC6. In contrast, tubulin glutamylation and tubulin detyrosination (16, 39) are not affected appreciably by HDAC6 deficiency (Fig. 1C, right panel). Furthermore, in line with the biochemical data, immunofluorescence microscopy revealed that the entire microtubule network in HDAC6-null cells was stained positive for acetylated α-tubulin (Fig. 1D, d) while in wild-type cells only subpopulations of the microtubule filaments were positive (Fig. 1D, b). The level of another putative microtubule deacetylase, SirT2 (35), was not affected in HDAC6 KO cells (Fig. 2B), indicating that SirT2 likely does not play a dominant role as an α-tubulin deacetylase in fibroblasts. Importantly, as shown in Fig. 1E, loss of HDAC6 resulted in a defect in cell motility. These results unambiguously establish that HDAC6 is the major enzyme catalyzing the deacetylation of microtubules and is required for efficient cell motility.
FIG. 1.
Accumulation of hyperacetylated microtubules in HDAC6 KO tissues and MEFs. (A) A schematic diagram of the targeting strategy with mouse HDAC6 genomic sequences. Exons (vertical bars) 10 to 13 were replaced by a targeting vector containing a neomycin (Neo) and zeocin (Zeo) cassette, resulting in the disruption of the first catalytic domain of HDAC6. The sequences are not drawn to scale. (B and C) Immunoblotting analysis of brain samples (B) or MEFs (C) from wild-type (WT) or HDAC6 KO mice revealed the absence of HDAC6 protein and significantly elevated tubulin acetylation in HDAC6 KO animals. The relative amount of acetylated α-tubulin (Ac-Tubulin) is provided under panel C. p150glued was shown as a loading control. (D) Wild-type (a and b) and HDAC6 KO (c and d) MEFs were immunostained with antibodies for acetylated α-tubulin (b and d) and α-tubulin (a and c). Note that almost the entire microtubule network in HDAC6 KO MEFs was stained positive for acetylated α-tubulin. Bar = 10 μm. (E) Cell motility of wild-type and KO MEFs was analyzed by Boyden chamber cell migration assays. Average values with standard errors of the means from four independent experiments are shown.
FIG. 2.
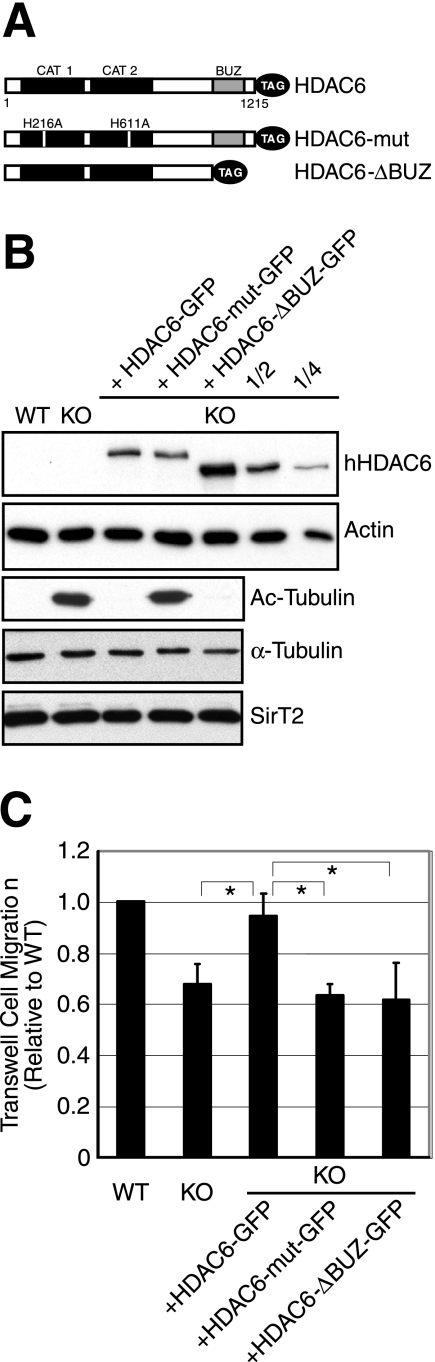
Both enzymatic activity and the BUZ domain of HDAC6 are required to rescue motility defects of HDAC6 KO cells. (A) Schematic representation of the human HDAC6 constructs used in this study. The constructs are tagged either with GFP or with FLAG. Boxes in black, catalytic domains; boxes in gray, BUZ domain. H216A and H611A are histidine-to-alanine point mutations that inactivate the deacetylase activity of HDAC6. (B) Wild-type, HDAC6 KO, and HDAC6 KO MEFs stably expressing the human HDAC6 constructs were analyzed for the level of HDAC6 in the cells using an anti-human HDAC6 antibody (upper panel). In addition, the HDAC6-ΔBUZ-GFP sample was also serially diluted by two- and fourfold for analysis. Total and acetylated α-tubulin and SirT2 levels were examined using corresponding antibodies. (C) Quantification of cell migration using Boyden chamber assays for cell lines described for panel B. Each bar represents an average value plus standard error of the mean from four independent experiments. *, t < 0.05 in two-tailed and paired t test.
We next determined whether tubulin hyperacetylation is the key to the migration defect observed in the HDAC6-deficient cells. Specifically, we asked whether cell motility could be restored in HDAC6 KO MEFs if hyperacetylated microtubules were deacetylated. To this end, we stably reconstituted HDAC6 KO MEFs with the following HDAC6 constructs (Fig. 2A): GFP-tagged wild-type HDAC6 (HDAC6-GFP), a catalytically inactive mutant (HDAC6-mut-GFP), and a mutant that lacks the C-terminal ubiquitin-binding BUZ domain (HDAC6-ΔBUZ-GFP). The BUZ domain, also known as PAZ and ZnF-UBP (21, 46), is essential for HDAC6 to process misfolded proteins and regulate Hsp90 activity (27, 29). As expected, reexpression of wild-type HDAC6-GFP but not HDAC6-mut-GFP reduced acetylated α-tubulin to the level in wild-type cells (Fig. 2B). Interestingly, HDAC6-ΔBUZ-GFP was also fully competent to deacetylate microtubules (Fig. 2B). Therefore, the BUZ domain is not required for HDAC6 deacetylase activity toward α-tubulin.
We next examined the motility behavior of the HDAC6-deficient MEFs and the reconstituted lines in Boyden chamber migration assays. As shown in Fig. 2C, reexpression of wild-type HDAC6 restored cell migration whereas catalytically inactive HDAC6-mut-GFP was unable to do so. Unexpectedly, the HDAC6-ΔBUZ-GFP mutant failed to promote cell migration despite its higher expression level (Fig. 2B) and the ability to potently induce α-tubulin deacetylation. These results demonstrate that both deacetylase activity and the ubiquitin-binding BUZ domain of HDAC6 are required to promote cell migration. Furthermore, global deacetylation of microtubules per se is not sufficient to enhance cell motility.
HDAC6 regulates membrane ruffle formation.
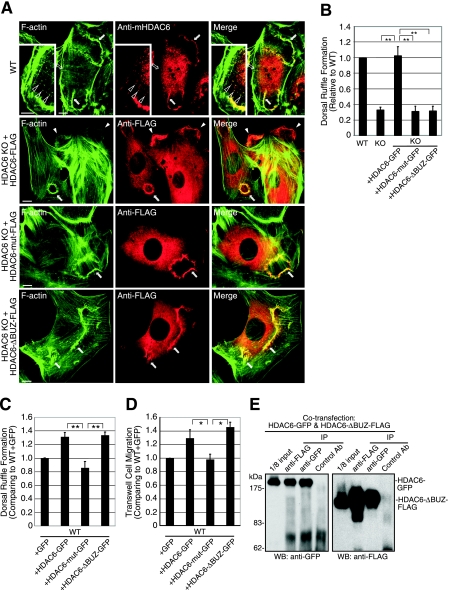
Since microtubule deacetylation alone is not sufficient to promote cell migration, we searched for other potential effectors of HDAC6. We first focused on the actin cytoskeleton since it plays a critical role in cell migration, often in concert with the microtubule network with which HDAC6 interacts. Upon stimulation with PDGF-BB, cells quickly formed ruffles at both the dorsal surface and the periphery of the cells (Fig. 3A). Remarkably, we found that HDAC6 quickly translocated to these ruffles, as revealed by antibodies against either endogenous HDAC6 (Fig. 3A, top panel), or ectopically expressed HDAC6-FLAG (Fig. 3A, second panel from top). This association of HDAC6 to actin membrane ruffles is specific, since HDAC6 did not colocalize with stress fibers (Fig. 3A, top panel). Furthermore, careful examination revealed that HDAC6 was also concentrated at F-actin spots (Fig. 3A, insert of top panel), which have been proposed to serve as precursor structures for dorsal membrane ruffles (30). Interestingly, both HDAC6-mut-FLAG and HDAC6-ΔBUZ-FLAG mutants were also able to associate with membrane ruffles (Fig. 3A, lower two panels). These results reveal that HDAC6 becomes rapidly and selectively concentrated at actin-enriched membrane ruffles in response to growth factor stimulation.
FIG. 3.
HDAC6 translocates to actin membrane ruffles and is required for efficient ruffle formation. (A) Wild-type (WT; top panel) or HDAC6 KO MEFs that stably express human HDAC6-FLAG, HDAC6-mut-FLAG, or HDAC6-ΔBUZ-FLAG were stimulated with PDGF-BB (50 ng/ml for 8 min) and stained with phalloidin-Alexa Fluor 488 for F-actin (green) or with an antibody for HDAC6 or FLAG. Endogenous HDAC6 (red, anti-mHDAC6) and FLAG-tagged HDAC6 (red, anti-FLAG) are both detected at dorsal (filled arrows) and peripheral (filled arrowheads, second panel from top) membrane ruffles. Open arrows indicate stress fibers (top panel). Insert in top panel: an enlarged image shows the concentration of HDAC6 at F-actin-containing spots (open arrowheads). Bar = 10 μm. (B) Quantification of PDGF-BB-induced dorsal ruffle formation in various cell types. The graph represents cells with dorsal circular ruffles. Approximately 500 cells were examined from each group. Results are averages plus standard errors of the means. n = 5 for WT and KO cells; n = 4 for reconstituted cells. **, t < 0.01 in two-tailed t test. (C) Wild-type MEFs that stably express GFP control, HDAC6-GFP, HDAC6-mut-GFP, or HDAC6-ΔBUZ-GFP were examined for dorsal ruffle formation as for panel B. Each bar represents the average value plus standard error of the mean from four independent experiments. **, t < 0.01 in a two-tailed and paired t test. (D) Cell lines described for panel C were tested in Boyden chamber migration assays, and relative motility values are graphed. Each bar represents the average value plus standard deviation from three independent experiments with duplicate wells. *, t < 0.05 in a two-tailed and paired t test. (E) 293T cells were cotransfected with HDAC6-ΔBUZ-FLAG and HDAC6-GFP and processed for immunoprecipitation using anti-FLAG, anti-GFP, or control mouse antibody. The precipitates were analyzed by immunoblotting using anti-GFP (left panel) or anti-FLAG (right panel) antibody to show mutual association of HDAC6-ΔBUZ and the full-length HDAC6.
To determine if association of HDAC6 with membrane ruffles is functionally significant, we asked whether HDAC6 regulates the formation of membrane ruffles. We focused on dorsal ruffles since they are prominent structures and can be unambiguously identified. As shown in Fig. 3B, significantly fewer cells from the HDAC6 KO group formed circular dorsal ruffles than their wild-type counterparts in response to PDGF treatment, indicating that HDAC6 is required for efficient membrane ruffle formation. Importantly, this defect was rescued by stable expression of wild-type HDAC6-GFP. In contrast, neither HDAC6-mut-GFP nor HDAC6-ΔBUZ-GFP rescued the defect (Fig. 3B). In conclusion, similar to the case with cell motility (Fig. 2C), HDAC6 requires both its deacetylase activity and BUZ domain to promote membrane ruffle formation.
To further assess the activity of HDAC6 in membrane ruffle formation and cell motility, we determined whether overexpression of HDAC6 in wild-type MEFs enhances membrane ruffle formation. As shown in Fig. 3C and D, HDAC6-GFP, but not enzymatically inactive HDAC6-mut-GFP, indeed promoted both membrane ruffling and cell migration. To our surprise, in contrast to its behavior in HDAC6-deficient cells, overexpression of HDAC6-ΔBUZ-GFP in wild-type MEFs stimulated both ruffle formation and cell motility. The unexpected activity of the HDAC6-ΔBUZ mutant is likely caused by complex formation between HDAC6-ΔBUZ and the full-length wild-type HDAC6, as indicated by their coimmunoprecipitation (Fig. 3E), which possibly targets the HDAC6-ΔBUZ mutant to relevant substrates. Together, these results establish that HDAC6 can promote growth factor-induced membrane ruffle formation and this activity is tightly correlated with its ability to promote cell motility.
HDAC6 regulates macropinocytosis.
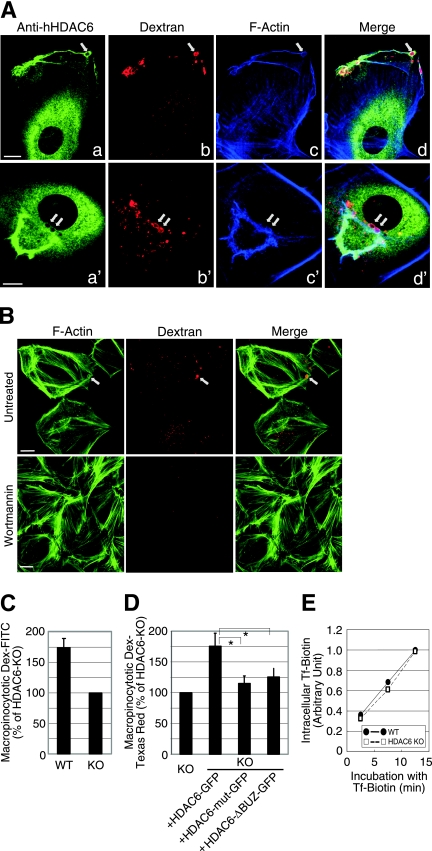
It has been suggested that membrane ruffling is functionally linked to macropinocytosis (52). In characterizing HDAC6 subcellular localization in response to PDGF stimulation, we observed an association of HDAC6 with large intracellular vesicles (Fig. 4). Interestingly, these vesicles were often continuous with or in close vicinity to both peripheral (Fig. 4A, a to d) and dorsal (Fig. 4A, a′ to d′) membrane ruffles. We concluded that these vesicles were macropinosomes based on several criteria. First, these vesicles were typically larger than 0.2 μm in diameter and positive for fluorescently labeled dextran, which is taken up by cells via a macropinocytic mechanism (Fig. 4A, b and b′). Second, these vesicles were encased with F-actin, another hallmark of macropinosomes (Fig. 4A, c and c′). Last, generation of these large dextran-containing vesicles was abolished by treatment with the PI3K inhibitor wortmannin (Fig. 4B), which prevents macropinosome formation (2, 4, 8).
FIG. 4.
HDAC6 regulates macropinocytosis. (A) Dorsal ruffle formation and macropinocytic uptake were visualized in HDAC6 KO MEFs that stably express human HDAC6-FLAG. The cells were stimulated with 20 ng/ml PDGF-BB in the presence of 3 mg/ml dextran (70 kDa)-tetramethylrhodamine for 10 min. Triple labeling using anti-hHDAC6 and phalloidin-Alexa Fluor 647 revealed colocalization of HDAC6 (green) and F-actin (blue) at the dextran-positive macropinosomes (red). Arrows point to macropinosomes. Bar = 10 μm. (B) Wild-type MEFs were treated with 100 nM wortmannin or vehicle control for 30 min at 37°C. Cells were stimulated with PDGF-BB in the presence of Dextran-tetramethylrhodamine and wortmannin (or vehicle) for 10 min. The cells were then fixed and stained for F-actin using phalloidin-Alexa Fluor 488. Wortmannin significantly inhibited macropinocytosis of dextran. The arrow indicates a macropinosome. Bar = 10 μm. (C) Fluorocytometric analysis revealed a significant decrease in macropinocytosis of dextran (Dex)-FITC in HDAC6 KO MEFs. Values are averages plus standard errors of the means from four independent experiments. (D) HDAC6 KO MEFs and KO cells stably expressing human HDAC6 constructs as indicated were assessed for the uptake of dextran (70 kDa)-Texas Red as described for panel C. Values are averages plus standard errors of the means from four independent experiments. *, t < 0.05 in two-tailed and paired t test. (E) Transferrin uptake remained unaffected in HDAC6 KO MEFs. Biotinylated transferrin was incubated with wild-type or KO MEFs, and internalized transferrin at different time points was measured as described in Materials and Methods. Average values from two independent experiments with quadruple wells are shown.
The association of HDAC6 with membrane ruffles and macropinosomes prompted us to investigate whether HDAC6 indeed regulates macropinocytosis. To this end, wild-type and HDAC6-deficient MEFs were incubated with dextran-FITC in the presence of PDGF, and the uptake of fluorescent dextran was measured by FACS analysis. In comparison to their wild-type counterparts, HDAC6 KO cells took up markedly less fluorescent dextran (Fig. 4C). Importantly, reexpression of wild-type, but not catalytically inactive HDAC6 or the ΔBUZ mutant, in HDAC6 KO cells significantly restored the uptake of dextran (Fig. 4D). These results demonstrate that HDAC6 is necessary for efficient macropinocytosis. In contrast, the constitutive internalization of transferrin via clathrin-dependent endocytosis was comparable in wild-type and HDAC6-null cells (Fig. 4E), suggesting HDAC6 specifically regulates macropinocytosis.
Hsp90 regulates membrane ruffle formation and cell migration.
The finding that global microtubule deacetylation induced by the HDAC6-ΔBUZ mutant is not sufficient to promote membrane ruffling, macropinocytosis, and cell motility (Fig. 2, 3, and 4) suggests that HDAC6 might work through additional substrates and mechanisms. One such candidate is Hsp90, a molecular chaperone implicated in many signaling cascades (34). To test this hypothesis, we first examined the subcellular distribution of Hsp90 by immunofluorescence microscopy. Hsp90 is largely present in the cytoplasm without a distinct pattern. However, upon PDGF treatment, Hsp90 also translocated to membrane ruffles, where it colocalized with HDAC6 and actin (Fig. 5A, a to f). Similar to HDAC6, Hsp90 was also found concentrated at punctate F-actin structures (Fig. 5A, d to f) and nascent macropinosomes (Fig. 5A, g to i) but not at stress fibers. To determine whether Hsp90 could be functionally linked to these actin reorganization-associated cellular activities, we utilized geldanamycin, a specific Hsp90 inhibitor (50). As shown in Fig. 5B and C, brief treatment with geldanamycin significantly reduced PDGF-activated dorsal ruffle formation and motility in wild-type MEFs. Consistent with our finding that hyperacetylated Hsp90 still retains some chaperone activity (29, 33), geldanamycin treatment in HDAC6-null cells could further reduce actin ruffle formation (Fig. 5D). Together, these findings show that Hsp90, similar to HDAC6, is required for growth factor-induced membrane ruffling and cell migration.
FIG. 5.
Hsp90 regulates membrane ruffle formation and cell migration. (A) Colocalization of Hsp90 (b, e, and h) with HDAC6 (a) or F-actin (d and g) in wild-type MEFs stimulated with 50 ng/ml PDGF-BB for 8 min. Filled arrowheads indicate ruffles. Open arrowheads in d to f point to Hsp90 (red)-containing F-actin (green) spots. Open arrowheads in g to i indicate colocalization of Hsp90 (red) and F-actin (green) at the macropinosomes. Bar = 10 μm. (B) Inhibition of PDGF-induced dorsal ruffle formation in wild-type MEFs was dose responsive to geldanamycin treatment (2 h at 3 μM or 10 μM). Bars represent average values plus standard errors of the means; n = 4. DMSO, dimethyl sulfoxide (C) Cells pretreated with either vehicle or geldanamycin at 1 μM or 5 μM for 2 h were analyzed for motility. The average value from two independent experiments with duplicate wells is shown. Note that geldanamycin treatment did not induce cytotoxicity under these experimental conditions. (D) Wild-type (WT) and HDAC6 KO MEFs were pretreated with 9 μM geldanamycin (GA) for 2.5 h before they were induced to form ruffles by 50 ng/ml PDGF-BB (8 min at 37°C). Cells are stained with phalloidin-rhodamine for F-actin. At least 500 cells of each cell type were examined. Cells forming dorsal ruffles were scored. Average values plus standard errors of the means from four independent experiments are graphed. (E) HDAC6 KO MEFs were transfected with the FLAG-tagged wild type, mutant human Hsp90α constructs, or a control plasmid as indicated. Cells were stimulated with 50 ng/ml PDGF-BB and were stained with phalloidin-rhodamine for membrane ruffles and anti-FLAG to identify FLAG-tagged Hsp90α-expressing cells, respectively. Cells that formed dorsal ruffles were scored (average value plus standard error). At least 400 FLAG-positive and 800 nontransfected cells on the same cover glass were examined in four independent experiments. The dotted line indicates the level of ruffle formation in nontransfected cells. **, t < 0.01 in a two-tailed t test.
To further investigate whether Hsp90 acetylation plays an important role in membrane ruffle formation, we utilized two Hsp90 mutants with mutations at lysine 294, which has been recently identified as a critical acetylated residue in Hsp90α (45). Toward this end, we transfected HDAC6 KO MEFs with expression plasmids for FLAG-tagged Hsp90α-WT, Hsp90α-K294R (acetylation resistant), and Hsp90α-K294Q (acetylation mimic), and we assessed membrane ruffle formation. As shown in Fig. 5E, expression of Hsp90α-K294R markedly increased membrane ruffles, while the acetylation-mimicking Hsp90α-K294Q had no effect. The expression of Hsp90α-WT increased ruffle formation very slightly. These results strongly support the idea that deacetylation of K294 is necessary for Hsp90α to regulate actin remodeling.
HDAC6 and Hsp90 regulate Rac1 activity.
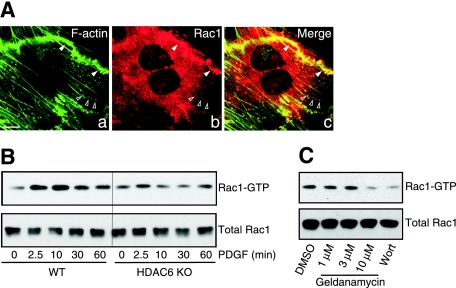
Rac1 is a critical regulatory factor in ruffle formation and cell migration (19, 38, 58). Similar to HDAC6 and Hsp90, Rac1 also translocated to membrane ruffles (Fig. 6A, a to c) and F-actin dots, which strongly suggested the possibility that HDAC6 and Hsp90 might regulate Rac1 activity. To examine Rac1 activation in wild-type and HDAC6 KO cells, we measured the binding of intracellular Rac1-GTP (the active form of Rac1) to GST-PAK1-CRIB (Cdc42/Rac interactive binding domain) (43). As shown in Fig. 6B, after PDGF stimulation, Rac1 from wild-type cells was activated and able to associate with GST-PAK1-CRIB. In contrast, Rac1 activation was markedly reduced in HDAC6 KO MEFs. Consistent with the hypothesis that HDAC6 functions in conjunction with Hsp90, treatment of cells with geldanamycin also significantly reduced the activation of Rac1 (Fig. 6C). Taken together, these data indicate that HDAC6 and Hsp90 are required for full activation of Rac1 and consequently for efficient membrane ruffle formation and cell migration.
FIG. 6.
Functional HDAC6 and Hsp90 are required for efficient Rac1 activation. (A) Wild-type MEFs were stimulated with 50 ng/ml PDGF-BB for 8 min. Colocalization of Rac1 (b) with F-actin (a) was observed at dorsal ruffle (filled arrowheads) and F-actin dots (open arrowheads) using anti-Rac1 antibody and phalloidin-Alexa Fluor 488. Bar = 10 μm. (B) Rac1 activation in wild-type (WT) and HDAC6 KO MEFs was evaluated by GST-PAK1-CRIB pull-down assay. A diminished Rac1 activation in response to PDGF-BB treatment was observed in HDAC6 KO MEFs. (C) Pretreatment of wild-type MEFs with vehicle control, wortmannin (Wort) (100 nM for 30 min as another control), or geldanamycin at indicated concentrations for 2 h led to reduced Rac1 activation by PDGF-BB treatment (50 ng/ml for 10 min) in a GST-PAK1-CRIB pull-down assay. Note that wortmannin treatment inhibits PI3K function and Rac1 activation (23, 44). DMSO, dimethyl sulfoxide.
DISCUSSION
The identification of HDAC6 as a microtubule-associated deacetylase has revealed an unexpected link between histone deacetylase-regulated protein acetylation and biological processes independent of histone modification (22). In this study, we discovered a surprising activity of HDAC6 in the regulation of membrane ruffle formation, macropinocytosis, and cell motility, thus identifying HDAC6 as a new regulatory component in actin remodeling.
Microtubule acetylation has been observed for more than 20 years (36). However, its functional significance remains poorly understood. The dramatic increase in α-tubulin acetylation observed in HDAC6 KO brain tissue and MEFs provides the definitive proof that HDAC6 is a tubulin deacetylase in vivo. Remarkably, α-tubulin from HDAC6 KO MEFs demonstrates a slower mobility in sodium dodecyl sulfate-polyacrylamide gel than that from wild-type cells, which could be caused by an increase in the molecular weight and a change in the overall charge of α-tubulin by acetylation. This finding strongly indicates that the majority of the α-tubulin population has become acetylated in the absence of HDAC6. Immunostaining analyses also show that the entire microtubule network becomes acetylated in HDAC6-deficient MEFs. These results clearly show that at least in fibroblasts, HDAC6 is the dominant α-tubulin deacetylase, while the other putative tubulin deacetylase, SirT2, likely plays a minor role in microtubule acetylation. Acetylated microtubules are often associated with increased stability (31). Indeed, microtubules in HDAC6-deficient cells show reduced dynamics (54). However, in HDAC6 KO cells, we did not observe an apparent increase in detyrosinated tubulin (Fig. 1C, Polyglutamylated Tubulin), which is also associated with stable microtubules (57). A similar conclusion was reached in other studies (58, 59). These results suggest that acetylation and detyrosination are not necessarily coupled and they might affect microtubule stability via different mechanisms.
HDAC6 has been shown to regulate cell migration in response to serum growth factors (22). Overexpression of HDAC6 promotes cell motility, while pharmacological or genetic inactivation of HDAC6 inhibits it (17, 22) (Fig. 1 and 2). Since HDAC6-dependent cell motility is tightly correlated with the acetylation status of α-tubulin, the first substrate identified for HDAC6, we have thus speculated that microtubule acetylation is critical for cell motility. Surprisingly, our data clearly show that α-tubulin deacetylation and cell migration can be uncoupled. Although the BUZ domain-deleted HDAC6 mutant potently catalyzes α-tubulin deacetylation, it cannot promote cell migration (Fig. 2). Therefore, global deacetylation of α-tubulin is not sufficient to promote cell migration. Instead, several lines of evidence in this study identify the actin cytoskeleton as a main target of HDAC6 in cell motility. We found that HDAC6 becomes rapidly associated with the actin cytoskeleton in response to growth factor stimulation, concentrating at F-actin spots, membrane ruffles, and macropinosomes (Fig. 3 and 4). These dynamic structures have long been suggested to associate with cell motility (38). Supporting an important function of HDAC6 in these actin-remodeling processes, we showed that cells deficient in HDAC6 are defective in membrane ruffle formation, macropinocytosis, and cell migration. Conversely, overexpression of wild-type HDAC6 promotes actin membrane ruffle formation, macropinocytosis, and cell motility (Fig. 2, 3, and 4). These findings demonstrate a regulatory role for HDAC6 in growth factor-induced actin remodeling and provide a molecular mechanism by which HDAC6 controls cell motility.
Mechanistically, we found that Hsp90 and Rac1 are also concentrated at actin-associated membrane ruffles (Fig. 5 and 6). Thus, HDAC6 and its substrate Hsp90, as well as Rac1, a key factor in actin remodeling machinery, become colocalized in response to growth factor stimulation. These observations suggest the interesting possibility that HDAC6 might affect Rac1 activation (Fig. 6B) through regulation of Hsp90, whose full chaperone activity requires HDAC6 (3, 29, 33). Consistent with this notion, inactivation of either HDAC6 or Hsp90 dampens Rac1 activation (Fig. 6), resulting in defective membrane ruffling and cell motility (Fig. 2, 3, and 5). Although there are likely additional targets of HDAC6 in the actin remodeling machinery, our study suggests that HDAC6 contributes to actin remodeling, at least in part, by regulating Hsp90. This idea is strongly supported by our finding that the acetylation-resistant Hsp90α-K294R mutant, but not the acetylation-mimicking Hsp90α-K294Q mutant, could partially rescue the membrane ruffle defect in HDAC6-deficient MEFs (Fig. 5E). This conclusion is also consistent with the observation that the catalytically inactive HDAC6 mutant and HDAC6-ΔBUZ, both of which cannot bind and regulate Hsp90 (29), are also deficient in supporting actin remodeling (Fig. 2C, 3, and 4D).
Our study shows that HDAC6 is involved in the regulation of macropinocytosis. Of all forms of endocytosis, macropinocytosis is the least understood, but it is tightly linked to growth factor and oncogenic signaling (1, 26, 52, 55). In fact, induction of macropinosome formation is one of the first cellular effects observed for oncogenic Ras (5). In contrast to the case in normal cells, macropinocytosis is commonly deregulated and becomes constitutive in tumor cells. Deregulated macropinocytosis might enhance motility and facilitate uptake of nutrients for aggressive tumor growth. Our finding that HDAC6 is required for efficient macropinocytosis (Fig. 4) identifies HDAC6 as a novel regulator in this poorly characterized but important endocytic pathway. It is important to point out that both HDAC6 and actin are associated with nascent macropinosomes after they are internalized. This observation suggests that HDAC6 and the actin machinery might be involved in the intracellular trafficking of the vesicles. This is an intriguing possibility in light of our previous observation that HDAC6 associates with dynein motors and has the capacity to regulate protein transport (27). Interestingly, the ability of HDAC6 to regulate both protein transport and macropinocytosis (Fig. 4) requires the ubiquitin-binding BUZ domain, suggesting that HDAC6 regulates endocytosis through binding to ubiquitinylated proteins. Given the prominent role of ubiquitin modification in endocytic trafficking (20, 32), we speculate that HDAC6 might play additional roles in trafficking of the endocytic vesicles, thereby further affecting growth factor signaling and cell motility.
Our results uncover an important function for HDAC6 in signaling-dependent actin remodeling. It is worth noting that HDAC6 has been implicated in the formation of mDia-dependent podosomal rings (6, 13), an F-actin-enriched structure (7). HDAC6 was also reported to be present at another F-actin structure, the T-cell immunological synapse (47). Although those studies did not address a specific role for HDAC6 in podosomes or immune synapse formation, they are nonetheless consistent with the proposition that HDAC6 likely plays a role in a broad range of actin reorganization-dependent processes. It is important to point out that HDAC6 is not an essential gene. HDAC6 KO mice are viable and fertile and show modest behavioral abnormalities (Y. Kawaguchi and T. P. Yao, unpublished observation). The lack of prominent defects in animals deficient in HDAC6 might reflect the fact that actin reorganization and cell migration are redundantly controlled by multiple pathways under normal conditions (5). Nevertheless, given that oncogenic Ras, Src, and PI3K can all potently induce membrane ruffling, macropinocytosis, and cell migration (24, 49, 53), our findings underline the potential importance of HDAC6 in tumor development and metastasis. In this regard, inhibitors for histone deacetylases are potent antitumor agents. Although their antitumor activities have been mostly attributed to the regulation of gene expression, our finding that HDAC6 is capable of regulating cell migration and endocytosis suggests that histone deacetylase inhibitors could potentially elicit antitumor activity via a nongenomic mechanism.
Extensive work on histone deacetylases has firmly established their roles in chromatin remodeling and transcriptional regulation (12). The recent identification of a large number of nonhistone substrates, however, implicates novel functions for the histone deacetylase family in previously unexpected areas (9, 28). Our characterization of HDAC6 as an important regulatory factor in membrane ruffle formation, endocytosis, and cell migration is another surprising addition to the activities of histone deacetylases and suggests an important role for reversible acetylation in actin remodeling-dependent processes.
Acknowledgments
We thank Phil Leders for his support in the generation of HDAC6-deficient mice. We thank John Collard for the generous gift of the GST-PAK1-Crib construct, Brad Scroggins and Len Neckers for Hsp90 constructs, and J. Chloe Bulinski, David Toft, and Minoru Yohsida for antibodies. We thank Tomasa Barrientos and Todd Cohen for critically reading the manuscript.
Y.-S.G. is a recipient of a Department of Defense Postdoctoral Traineeship Award (W81XWH-05-1-0573). This work was supported by American Cancer Society (RSG-03-147-01-CSM) and the Department of Defense (W81XWH-04-01-0555) grants to T.-P.Y., who is a Leukemia and Lymphoma Society Scholar.
Footnotes
Published ahead of print on 15 October 2007.
REFERENCES
- 1.Amyere, M., B. Payrastre, U. Krause, P. Van Der Smissen, A. Veithen, and P. J. Courtoy. 2000. Constitutive macropinocytosis in oncogene-transformed fibroblasts depends on sequential permanent activation of phosphoinositide 3-kinase and phospholipase C. Mol. Biol. Cell 11:3453-3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araki, N., M. T. Johnson, and J. A. Swanson. 1996. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J. Cell Biol. 135:1249-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bali, P., M. Pranpat, J. Bradner, M. Balasis, W. Fiskus, F. Guo, K. Rocha, S. Kumaraswamy, S. Boyapalle, P. Atadja, E. Seto, and K. Bhalla. 2005. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. J. Biol. Chem. 280:26729-26734. [DOI] [PubMed] [Google Scholar]
- 4.Barker, S. A., K. K. Caldwell, A. Hall, A. M. Martinez, J. R. Pfeiffer, J. M. Oliver, and B. S. Wilson. 1995. Wortmannin blocks lipid and protein kinase activities associated with PI 3-kinase and inhibits a subset of responses induced by Fc epsilon R1 cross-linking. Mol. Biol. Cell 6:1145-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bar-Sagi, D., and A. Hall. 2000. Ras and Rho GTPases: a family reunion. Cell 103:227-238. [DOI] [PubMed] [Google Scholar]
- 6.Bershadsky, A. D., C. Ballestrem, L. Carramusa, Y. Zilberman, B. Gilquin, S. Khochbin, A. Y. Alexandrova, A. B. Verkhovsky, T. Shemesh, and M. M. Kozlov. 2006. Assembly and mechanosensory function of focal adhesions: experiments and models. Eur. J. Cell Biol. 85:165-173. [DOI] [PubMed] [Google Scholar]
- 7.Buccione, R., J. D. Orth, and M. A. McNiven. 2004. Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nat. Rev. Mol. Cell Biol. 5:647-657. [DOI] [PubMed] [Google Scholar]
- 8.Clague, M. J., C. Thorpe, and A. T. Jones. 1995. Phosphatidylinositol 3-kinase regulation of fluid phase endocytosis. FEBS Lett. 367:272-274. [DOI] [PubMed] [Google Scholar]
- 9.Cohen, T., and T. P. Yao. 2004. AcK-knowledge reversible acetylation. Sci. STKE 2004:pe42. [DOI] [PubMed] [Google Scholar]
- 10.Conner, S. D., and S. L. Schmid. 2003. Regulated portals of entry into the cell. Nature 422:37-44. [DOI] [PubMed] [Google Scholar]
- 11.de Baey, A., and A. Lanzavecchia. 2000. The role of aquaporins in dendritic cell macropinocytosis. J. Exp. Med. 191:743-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Ruijter, A. J., A. H. van Gennip, H. N. Caron, S. Kemp, and A. B. van Kuilenburg. 2003. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem. J. 370:737-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Destaing, O., F. Saltel, B. Gilquin, A. Chabadel, S. Khochbin, S. Ory, and P. Jurdic. 2005. A novel Rho-mDia2-HDAC6 pathway controls podosome patterning through microtubule acetylation in osteoclasts. J. Cell Sci. 118:2901-2911. [DOI] [PubMed] [Google Scholar]
- 14.Dharmawardhane, S., A. Schurmann, M. A. Sells, J. Chernoff, S. L. Schmid, and G. M. Bokoch. 2000. Regulation of macropinocytosis by p21-activated kinase-1. Mol. Biol. Cell 11:3341-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao, Y., and E. Sztul. 2001. A novel interaction of the Golgi complex with the vimentin intermediate filament cytoskeleton. J. Cell Biol. 152:877-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gundersen, G. G., M. H. Kalnoski, and J. C. Bulinski. 1984. Distinct populations of microtubules: tyrosinated and nontyrosinated alpha tubulin are distributed differently in vivo. Cell 38:779-789. [DOI] [PubMed] [Google Scholar]
- 17.Haggarty, S. J., K. M. Koeller, J. C. Wong, C. M. Grozinger, and S. L. Schreiber. 2003. Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc. Natl. Acad. Sci. USA 100:4389-4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall, A. 1993. Ras-related proteins. Curr. Opin. Cell Biol. 5:265-268. [DOI] [PubMed] [Google Scholar]
- 19.Hall, A. 1998. Rho GTPases and the actin cytoskeleton. Science 279:509-514. [DOI] [PubMed] [Google Scholar]
- 20.Hicke, L., and R. Dunn. 2003. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu. Rev. Cell Dev. Biol. 19:141-172. [DOI] [PubMed] [Google Scholar]
- 21.Hook, S. S., A. Orian, S. M. Cowley, and R. N. Eisenman. 2002. Histone deacetylase 6 binds polyubiquitin through its zinc finger (PAZ domain) and copurifies with deubiquitinating enzymes. Proc. Natl. Acad. Sci. USA 99:13425-13430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hubbert, C., A. Guardiola, R. Shao, Y. Kawaguchi, A. Ito, A. Nixon, M. Yoshida, X. F. Wang, and T. P. Yao. 2002. HDAC6 is a microtubule-associated deacetylase. Nature 417:455-458. [DOI] [PubMed] [Google Scholar]
- 23.Innocenti, M., E. Frittoli, I. Ponzanelli, J. R. Falck, S. M. Brachmann, P. P. Di Fiore, and G. Scita. 2003. Phosphoinositide 3-kinase activates Rac by entering in a complex with Eps8, Abi1, and Sos-1. J. Cell Biol. 160:17-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang, K., J. Sun, J. Cheng, J. Y. Djeu, S. Wei, and S. Sebti. 2004. Akt mediates Ras downregulation of RhoB, a suppressor of transformation, invasion, and metastasis. Mol. Cell. Biol. 24:5565-5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson, J. L., and D. O. Toft. 1994. A novel chaperone complex for steroid receptors involving heat shock proteins, immunophilins, and p23. J. Biol. Chem. 269:24989-24993. [PubMed] [Google Scholar]
- 26.Kasahara, K., Y. Nakayama, I. Sato, K. Ikeda, M. Hoshino, T. Endo, and N. Yamaguchi. 2007. Role of Src-family kinases in formation and trafficking of macropinosomes. J. Cell Physiol. 211:220-232. [DOI] [PubMed] [Google Scholar]
- 27.Kawaguchi, Y., J. J. Kovacs, A. McLaurin, J. M. Vance, A. Ito, and T. P. Yao. 2003. The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell 115:727-738. [DOI] [PubMed] [Google Scholar]
- 28.Kim, S. C., R. Sprung, Y. Chen, Y. Xu, H. Ball, J. Pei, T. Cheng, Y. Kho, H. Xiao, L. Xiao, N. V. Grishin, M. White, X. J. Yang, and Y. Zhao. 2006. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol. Cell 23:607-618. [DOI] [PubMed] [Google Scholar]
- 29.Kovacs, J. J., P. J. Murphy, S. Gaillard, X. Zhao, J. T. Wu, C. V. Nicchitta, M. Yoshida, D. O. Toft, W. B. Pratt, and T. P. Yao. 2005. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol. Cell 18:601-607. [DOI] [PubMed] [Google Scholar]
- 30.Krueger, E. W., J. D. Orth, H. Cao, and M. A. McNiven. 2003. A dynamin-cortactin-Arp2/3 complex mediates actin reorganization in growth factor-stimulated cells. Mol. Biol. Cell 14:1085-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuyama, A., T. Shimazu, Y. Sumida, A. Saito, Y. Yoshimatsu, D. Seigneurin-Berny, H. Osada, Y. Komatsu, N. Nishino, S. Khochbin, S. Horinouchi, and M. Yoshida. 2002. In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. EMBO J. 21:6820-6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mukhopadhyay, D., and H. Riezman. 2007. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science 315:201-205. [DOI] [PubMed] [Google Scholar]
- 33.Murphy, P. J., Y. Morishima, J. J. Kovacs, T. P. Yao, and W. B. Pratt. 2005. Regulation of the dynamics of hsp90 action on the glucocorticoid receptor by acetylation/deacetylation of the chaperone. J. Biol. Chem. 280:33792-33799. [DOI] [PubMed] [Google Scholar]
- 34.Neckers, L., and S. P. Ivy. 2003. Heat shock protein 90. Curr. Opin. Oncol. 15:419-424. [DOI] [PubMed] [Google Scholar]
- 35.North, B. J., B. L. Marshall, M. T. Borra, J. M. Denu, and E. Verdin. 2003. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol. Cell 11:437-444. [DOI] [PubMed] [Google Scholar]
- 36.Piperno, G., and M. T. Fuller. 1985. Monoclonal antibodies specific for an acetylated form of alpha-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J. Cell Biol. 101:2085-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piperno, G., M. LeDizet, and X. J. Chang. 1987. Microtubules containing acetylated alpha-tubulin in mammalian cells in culture. J. Cell Biol. 104:289-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raftopoulou, M., and A. Hall. 2004. Cell migration: Rho GTPases lead the way. Dev. Biol. 265:23-32. [DOI] [PubMed] [Google Scholar]
- 39.Regnard, C., S. Audebert, Desbruyeres, P. Denoulet, and B. Edde. 1998. Tubulin polyglutamylase: partial purification and enzymatic properties. Biochemistry 37:8395-8404. [DOI] [PubMed] [Google Scholar]
- 40.Ridley, A. J., H. F. Paterson, C. L. Johnston, D. Diekmann, and A. Hall. 1992. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70:401-410. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez, O. C., A. W. Schaefer, C. A. Mandato, P. Forscher, W. M. Bement, and C. M. Waterman-Storer. 2003. Conserved microtubule-actin interactions in cell movement and morphogenesis. Nat. Cell Biol. 5:599-609. [DOI] [PubMed] [Google Scholar]
- 42.Sander, E. E., J. P. ten Klooster, S. van Delft, R. A. van der Kammen, and J. G. Collard. 1999. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J. Cell Biol. 147:1009-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sander, E. E., S. van Delft, J. P. ten Klooster, T. Reid, R. A. van der Kammen, F. Michiels, and J. G. Collard. 1998. Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes either cell-cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J. Cell Biol. 143:1385-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scita, G., P. Tenca, E. Frittoli, A. Tocchetti, M. Innocenti, G. Giardina, and P. P. Di Fiore. 2000. Signaling from Ras to Rac and beyond: not just a matter of GEFs. EMBO J. 19:2393-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scroggins, B. T., K. Robzyk, D. Wang, M. G. Marcu, S. Tsutsumi, K. Beebe, R. J. Cotter, S. Felts, D. Toft, L. Karnitz, N. Rosen, and L. Neckers. 2007. An acetylation site in the middle domain of Hsp90 regulates chaperone function. Mol. Cell 25:151-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seigneurin-Berny, D., A. Verdel, S. Curtet, C. Lemercier, J. Garin, S. Rousseaux, and S. Khochbin. 2001. Identification of components of the murine histone deacetylase 6 complex: link between acetylation and ubiquitination signaling pathways. Mol. Cell. Biol. 21:8035-8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serrador, J. M., J. R. Cabrero, D. Sancho, M. Mittelbrunn, A. Urzainqui, and F. Sanchez-Madrid. 2004. HDAC6 deacetylase activity links the tubulin cytoskeleton with immune synapse organization. Immunity 20:417-428. [DOI] [PubMed] [Google Scholar]
- 48.Shaulian, E., A. Zauberman, D. Ginsberg, and M. Oren. 1992. Identification of a minimal transforming domain of p53: negative dominance through abrogation of sequence-specific DNA binding. Mol. Cell. Biol. 12:5581-5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sossey-Alaoui, K., X. Li, T. A. Ranalli, and J. K. Cowell. 2005. WAVE3-mediated cell migration and lamellipodia formation are regulated downstream of phosphatidylinositol 3-kinase. J. Biol. Chem. 280:21748-21755. [DOI] [PubMed] [Google Scholar]
- 50.Stebbins, C. E., A. A. Russo, C. Schneider, N. Rosen, F. U. Hartl, and N. P. Pavletich. 1997. Crystal structure of an Hsp90-geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell 89:239-250. [DOI] [PubMed] [Google Scholar]
- 51.Suetsugu, S., D. Yamazaki, S. Kurisu, and T. Takenawa. 2003. Differential roles of WAVE1 and WAVE2 in dorsal and peripheral ruffle formation for fibroblast cell migration. Dev. Cell 5:595-609. [DOI] [PubMed] [Google Scholar]
- 52.Swanson, J. A., and C. Watts. 1995. Macropinocytosis. Trends Cell Biol. 5:424-428. [DOI] [PubMed] [Google Scholar]
- 53.Timpson, P., G. E. Jones, M. C. Frame, and V. G. Brunton. 2001. Coordination of cell polarization and migration by the Rho family GTPases requires Src tyrosine kinase activity. Curr. Biol. 11:1836-1846. [DOI] [PubMed] [Google Scholar]
- 54.Tran, A. D., T. P. Marmo, A. A. Salam, S. Che, E. Finkelstein, R. Kabarriti, H. S. Xenias, R. Mazitschek, C. Hubbert, Y. Kawaguchi, M. P. Sheetz, T. P. Yao, and J. C. Bulinski. 2007. HDAC6 deacetylation of tubulin modulates dynamics of cellular adhesions. J. Cell Sci. 120:1469-1479. [DOI] [PubMed] [Google Scholar]
- 55.Veithen, A., P. Cupers, P. Baudhuin, and P. J. Courtoy. 1996. v-Src induces constitutive macropinocytosis in rat fibroblasts. J. Cell Sci. 109:2005-2012. [DOI] [PubMed] [Google Scholar]
- 56.Watanabe, T., J. Noritake, and K. Kaibuchi. 2005. Regulation of microtubules in cell migration. Trends Cell Biol. 15:76-83. [DOI] [PubMed] [Google Scholar]
- 57.Webster, D. R., G. G. Gundersen, J. C. Bulinski, and G. G. Borisy. 1987. Differential turnover of tyrosinated and detyrosinated microtubules. Proc. Natl. Acad. Sci. USA 84:9040-9044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.West, M. A., A. R. Prescott, E. L. Eskelinen, A. J. Ridley, and C. Watts. 2000. Rac is required for constitutive macropinocytosis by dendritic cells but does not control its downregulation. Curr. Biol. 10:839-848. [DOI] [PubMed] [Google Scholar]
- 59.Zhang, Y., B. Gilquin, S. Khochbin, and P. Matthias. 2006. Two catalytic domains are required for protein deacetylation. J. Biol. Chem. 281:2401-2404. [DOI] [PubMed] [Google Scholar]
- 60.Zhang, Y., N. Li, C. Caron, G. Matthias, D. Hess, S. Khochbin, and P. Matthias. 2003. HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo. EMBO J. 22:1168-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]