Abstract
The Sleeping Beauty (SB) transposon represents an important vehicle for in vivo gene delivery because it can efficiently and stably integrate into mammalian genomes. In this report, we examined transposon expression in human cells using a novel nonselective fluorescence-activated cell sorter-based method and discovered that SB integrates ∼20 times more frequently than previously reported within systems that were dependent on transgene expression and likely subject to postintegrative gene silencing. Over time, phenotypic analysis of clonal integrants demonstrated that SB undergoes additional postintegrative gene silencing, which varied based on the promoter used for transgene expression. Molecular and biochemical studies suggested that transposon silencing was influenced by DNA methylation and histone deacetylation because both 5-aza-2′-deoxycytidine and trichostatin A partially rescued transgene silencing in clonal cell lines. Collectively, these data reveal the existence of a multicomponent postintegrative gene silencing network that efficiently targets invading transposon sequences for transcriptional silencing in mammalian cells.
Transposable elements play an important role in evolution which is demonstrated by an estimated 50 genes within the human genome that are transposon derived, including genes vital to our adaptive immunity (16) and DNA repair (33). Amazingly, sequences recognized as derived from transposable elements account for approximately 45% of the overall human genome (31). Transposable elements can be broken down into two main groups: those which rely on an RNA intermediate for remobilization (retro-elements) and those which are remobilized directly as DNA (DNA transposons). Fossils of one group of DNA transposons, the Tc1/mariner type, are found throughout nature. Remnants, which have been deactivated through the accumulation of mutations over time (21, 22), have been isolated from a variety of organisms, including fish (22), Xenopus laevis (30, 51), insects (49), Caenorhabditis elegans (48), Drosophila melanogaster (47), and humans (43, 52). Recognizing the many potential uses of an active and efficient DNA transposon system, Ivics et al. reconstructed a functional Tc1/mariner-like element from inactive transposon remnants found in fish and named it Sleeping Beauty (SB) (21). Shortly following its description, our group established the possible utility of this system as a means to genetically modify somatic tissues in adult mammals to treat animal models of human disease (40, 57, 59).
DNA transposons of the Tc1/mariner type contain a simple structure in which the only components required for transposition are inverted repeats (IRs), which flank the DNA to be transposed, and Sleeping Beauty transposase. As with other DNA transposons, SB transposition occurs through a “cut and paste” mechanism mediated by binding to the IRs of Sleeping Beauty transposase, which can be supplied either in cis from an autonomous element or in trans from a nonautonomous element. The excision and integration steps are mediated by the transposase catalytic core, which shares the DDE motif that is found in many evolutionarily related recombinase proteins, including the V(D)J recombinase and retrovirus integrases (21, 46). On a genome-wide level, transposon integration occurs without preference for transcriptional activity of target sites (61).
In this study, we examined SB integration using a nonselective system. In doing so, we discovered SB's integrative potential in somatic mammalian cells to be 41 to 52%, which is significantly higher than the 2 to 3% previously reported using earlier SB systems (13, 64). Moreover, through molecular and phenotypic analysis of clonal cell populations, we discovered that in a vector-dependent manner SB-mediated integrations undergo progressive gene silencing in human cells. Biochemical and functional analyses of the silenced integrants revealed a potential mechanistic role for DNA methylation and histone deacetylation in transposon silencing. Collectively, this work suggests that postintegrative gene silencing may be an underappreciated obstacle to transposon-based clinical gene therapy programs and provides the necessary framework by which to uncover the molecular mechanisms responsible for transposon silencing in mammals.
MATERIALS AND METHODS
Plasmids.
The cis-vector pT/RSV-eYFP.CMV-SB was made by ClaI-XmaI insertion of a PCR-amplified Rous sarcoma virus (RSV)-enhanced yellow fluorescent protein (eYFP) fragment into pT/MCS (59) (generating pT/RSV-eYFP), followed by insertion of a cytomegalovirus (CMV)-driven SB transposase expression cassette into the plasmid backbone. The control vector pRSV-eYFP.CMV-SB was the same as pT/RSV-eYFP.CMV-SB except the inverted repeats had been removed. To accomplish this we first excised the transposon sequence from pT/RSV-eYFP.CMV-SB by utilizing the flanking KpnI and XbaI restriction enzyme sites. Extended primers containing KpnI and XbaI overhangs were then used to PCR amplify the intervening sequence between the two IRs, and this product was then ligated back into the plasmid backbone from the original digestion, resulting in the creation of pRSV-eYFP.CMV-SB.
To create the cis-acting vector pT/EF1α-eGFP.CMV-SB, we used PCR to amplify the core elongation factor 1 alpha (EF1α) promoter (40), utilizing primers that added a 5′ SpeI site and a 3′ HindIII site, so that the product could be ligated with a SpeI/HindIII-treated pCpG-mcs vector (Invivogen). We then amplified the newly created EF1α-small intron sequence and cloned it into pBGT103 (5), creating an EF1α-small intron-enhanced green fluorescent protein (eGFP) expression cassette, which was then used to replace the RSV-eYFP expression cassette in pT/RSV-eYFP.CMV-SB, producing pT/EF1α-eGFP.CMV-SB.
The cis-acting vector pT/dmEF1α-dmGFP.CMV-SB was created in a similar manner as pT/EF1α-eGFP.CMV-SB; however, we amplified the dmEF1α-small intron sequence directly from pCpG-mcs and then inserted it adjacent to dmGFP in a dmGFP-containing version of pBGT103 (5). The resulting dmEF1α-intron-dmGFP expression cassette was then used to replace the RSV-eYFP expression cassette of pT/RSV-eYFP.CMV-SB, which created the vector pT/dmEF1α-dmGFP.CMV-SB, a cis-acting vector with a CpG-less transposon expression cassette.
Cell culture and transfections.
We obtained HeLa cells from ATCC, which were maintained under normal tissue culture conditions (37°C, 5% CO2) in Dulbecco's modified Eagle's medium (Mediatech) supplemented with l-glutamine (Gibco-BRL/Invitrogen), penicillin-streptomycin (Gibco-BRL/Invitrogen), and fetal bovine serum (10%). Transfections were performed according to the supplied company protocol using a mixture of 5 to 10 μg of plasmid DNA and Superfect (Qiagen), after which cells from each transfection mixture were cultured under normal tissue culture conditions.
FACS and flow cytometry.
For studies involving fluorescence-activated cell sorting (FACS), HeLa cells were transiently transfected with 5 to 10 μg of cis-acting plasmid DNA constructs encoding fluorescent-based reporter genes and then trypsinized and pelleted 2 to 3 days later for single-cell sorting. Samples were resuspended in phosphate-buffered saline to a concentration of 2 to 3 million cells/ml and single-cell sorted into 96-well plates at the Stanford FACS facility using the Vantage Vanford or Vantage SE/DiVa Vantoo FACS machine. Surviving cells (35 to 40% overall viability) were grown to confluence and then passaged repeatedly (approximately 1:20 every 5 days) on 6-cm-diameter plates for a period of 10 weeks to ensure complete loss of episomal plasmid DNA. This resulted in the production of 450 individual cell lines for subsequent analyses. For flow cytometry studies, HeLa cells were trypsinized 24 h (for trichostatin A [TSA] studies) and 96 h (for 5-aza-2′-deoxycytidine [5-AzaC] studies) posttreatment, pelleted, and resuspended in phosphate-buffered saline at a concentration of 2 to 3 million cells/ml. Samples were stored on ice and analyzed using a Becton Dickinson FACSCalibur system.
Southern blot analysis.
Following 10 weeks of repeated passaging, cells from each of 450 different clonal cell lines were trypsinized and genomic DNA was prepared, following phenol-chloroform extraction and ethanol precipitation. We digested 15 to 20 μg of genomic DNA from each sample with restriction endonucleases NcoI (a transposon single cutter which cleaves at the 3′ end of the transgene promoter) and SpeI (which does not cleave within the transposon but aids in gel migration for Southern blot analysis) and then separated the resulting DNA fragments on a 0.8% agarose gel before transferring them to a Hybond membrane (Amersham) using 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). Blots were then hybridized in Church buffer (1 mM EDTA, 0.5 M NaPO4, pH 7.2, 7% sodium dodecyl sulfate, 1% bovine serum albumin) with an [α-32P]dCTP-labeled probe corresponding to the respective transposon-encoded transgene (eYFP, eGFP, or dmGFP), washed, and imaged using a Personal Molecular Imager FX (Bio-Rad).
5-AzaC and TSA treatments.
Six-cm-diameter plates were seeded with experimental and control cell lines at a confluence of about 2 to 3% to permit ongoing cell division during 5-AzaC treatment. Approximately 24 h postseeding, 5-AzaC (5 μM final concentration) was added to the cells for a period of 4 days (with medium and inhibitor being changed every 24 h), after which each sample was analyzed by flow cytometry. For TSA treatments, 6-cm-diameter plates were seeded with experimental and control cell lines at a confluence of about 10%. After 24 h of growth, TSA (100 ng/ml, final concentration) was added to the cells for a total of 24 h (medium and inhibitor were changed once at 12 h) and then the cells were analyzed by flow cytometry.
RESULTS
Nonselective approach for investigating postintegrative transposon silencing in mammalian cells.
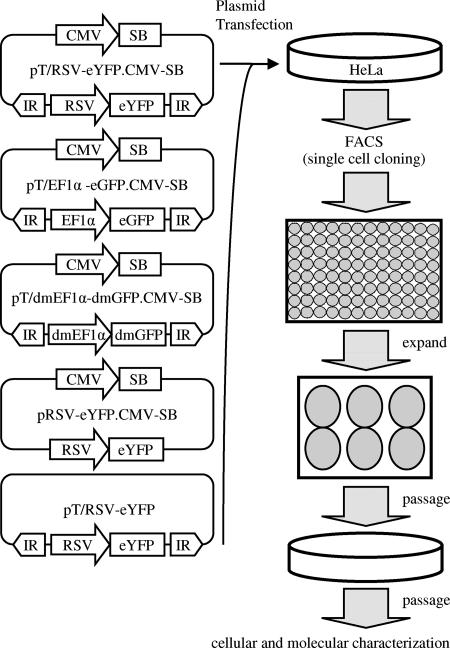
An analysis of integration and transgene expression at the single-locus level is essential to achieving a clear understanding of the basic biology of SB and to assessing its true potential for clinical gene therapy applications. To achieve this end, we engineered a series of novel SB vectors that permit the generation of clonal cell lines containing single-copy transposon integrations in the absence of genetic (antibiotic-based) selection (Fig. 1). The pT/RSV-eYFP.CMV-SB, pT/EF1α-eGFP.CMV-SB, and pT/dmEF1α-dmGFP.CMV-SB vectors are cis-acting SB vectors that contain an SB transposon encoding an eYFP or eGFP reporter gene variant under the control of either the RSV long terminal repeat promoter or the constitutively active human EF1α-derived promoter (40). The dmEF1α and dmGFP genes encoded by pT/dmEF1α-dmGFP.CMV-SB do not contain any CpG motifs and were included here to test what effects, if any, variations in promoter, transgene, and/or CpG content might have on the integration efficiency and expression of integrated SB elements. To ensure high-frequency codelivery of the transposon-transposase activities, all three cis-acting vectors contained within the plasmid backbone an SB transposase expression cassette driven by the CMV promoter. We also generated two important control vectors that are incapable of SB-mediated integration due to a deficiency in either (i) transposon inverted repeats (pRSV-eYFP.CMV-SB) or (ii) a source of SB transposase (pT/RSV-eYFP).
FIG. 1.
Experimental approach. HeLa cells were transfected with either pT/RSV-eYFP.CMV-SB, pT/EF1α-eGFP.CMV-SB, pT/dmEF1α-dmGFP.CMV-SB, pRSV-eYFP.CMV-SB (-IR control), or pT/RSV-eYFP (-SB control) and grown under normal conditions. After 48 to 72 h, eYFP-positive (or eGFP- or dmGFP-positive) cells were single-cell sorted (via FACS) into individual wells of 96-well plates and grown until colony formation. Colonies were picked and diluted into six-well plates and then established cell lines were maintained long term in 6-cm culture dishes. After >50 cell divisions, cell lines were characterized.
To generate clonal cell lines, we transiently transfected each of these vectors into HeLa cells and incubated them for 48 to 72 h to permit SB-mediated transposition into host cell chromosomes. Under these experimental conditions, the vast majority of fluorescent signal intensity at this time should be derived from episomal-based forms of the transfected plasmids, thereby permitting separation and cloning of individually transfected cells via FACS analysis. Transfected cells were trypsinized, and single eYFP-, eGFP-, and dmGFP-positive cells were distributed to each well of a 96-well plate in the presence of appropriate growth medium. In an effort to avoid a sorting bias in the initial stages of our studies, we gated cells such that the decision for plating single cells was determined only by whether it was positive or negative, without any consideration for the relative level of reporter gene expression. After FACS, the individually dispensed cells were expanded into cell lines and passaged repeatedly on 6-cm-diameter plates for a period of 10 weeks to ensure loss of any episomal plasmid. In this manner, we generated a total of 450 different clonal cell lines for subsequent molecular analyses (Table 1).
TABLE 1.
Summary of Sleeping Beauty integration efficiency
| Construct | No. of individual cell lines created | No. of cell lines containing integration(s) | Integration efficiency (%) |
|---|---|---|---|
| pT/RSV-eYFP.CMV-SB | 85 | 38 | 47 |
| pT/EF1α-eGFP.CMV-SB | 61 | 25 | 41 |
| pT/dmEF1α-dmGFP.CMV-SB | 202 | 105 | 52 |
| pRSV-eYFP.CMV-SB | 46 | 3 | 6.5 |
| pT/RSV-eYFP | 56 | 0 | 0 |
Analysis of SB-mediated integration.
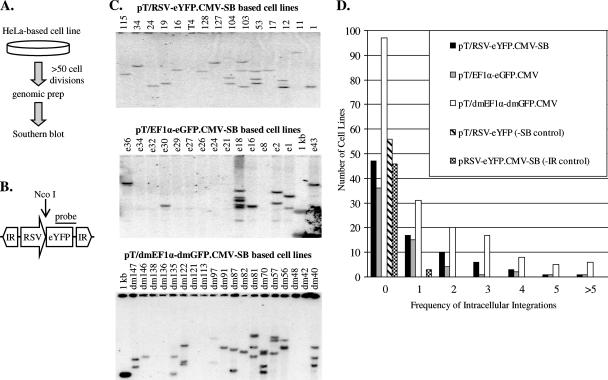
Each of the 450 newly isolated cell lines was screened for stable transposon integration via Southern blot analysis in order to (i) verify loss of episomal plasmid DNA and (ii) determine the number of integrations per cell line (Fig. 2). As summarized in Table 1, all three cis-acting SB vectors showed an integration efficiency of 41% to 52%, which is significantly higher than the 2 to 3% value previously reported within first-generation SB systems (13, 64). These data suggest that a large proportion of SB elements may undergo postintegrative gene silencing in mammalian cells, events which would ordinarily go undetected in screening methods that rely on antibiotic selection and thus require persistent transposon expression. Importantly, control vectors lacking either SB inverted repeats (pRSV-eYFP.CMV-SB) or a source of SB transposase (pT/RSV-eYFP) yielded integration efficiencies of approximately 0 to 6.5%. These data suggest that the elevated integration frequencies observed with each of the experimental transposon vectors is the result of SB-mediated DNA transposition. Additionally, the integration frequencies of the cis-acting vectors did not follow a Poisson distribution (Fig. 2D), further verifying that integration within our system occurs through a specific SB-mediated integration mechanism and not via random integration.
FIG. 2.
Characterization of SB-mediated integration events. (A) After growing the cells for over 50 cell divisions posttransfection, genomic DNA was isolated from each cell line for Southern blot analysis. (B) Genomic DNA was digested with NcoI and SpeI prior to gel electrophoresis. (C) Southern blot analysis was performed on each digested genomic preparation, using a reporter gene-specific probe. Representative Southern blots are shown for cell lines created from the constructs pT/RSV-eYFP.CMV-SB (top), pT/EF1α-eGFP.CMV-SB (middle), and pT/dmEF1α-dmGFP.CMV-SB (bottom). (D) The integration frequencies of the eYFP-, eGFP-, and dmGFP-containing transposons were determined by Southern blot analysis and plotted. The x axis depicts the frequency of intracellular integrations, which is the number of unique integrations within a cell line, while the y axis shows how many different cell lines contain a specific number of unique integrations.
Quantitative analysis of transposon gene expression.
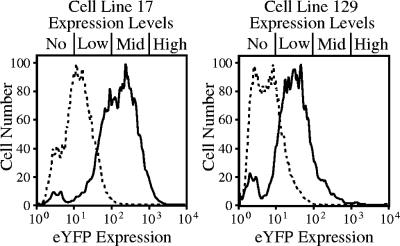
We analyzed cell lines that contained a single integration event over time via flow cytometry in order to define the expression profile associated with each cell line. At 12 weeks posttransfection, all three cis-acting vectors experienced similar levels of transgene silencing, ranging from 13 to 23% (Table 2). These data suggest that differences in promoter (e.g., RSV versus EF1α), transgene (e.g., eYFP versus eGFP), or CpG content (e.g., eGFP versus dmGFP) were unlikely to significantly influence the degree of SB transposon silencing in the soma at this time point. Cell lines were followed for an additional 30 weeks in order to determine whether the observed degree of silencing would remain stable or progress over time. Interestingly, all 17 single integration cell lines created from pT/RSV-eYFP.CMV-SB experienced an increase in postintegrative gene silencing, with 71% showing total extinction at week 42 compared to 23% at week 12 (Table 3). Histogram plots of two representative cell lines are shown in Fig. 3. In contrast to what we observed with the pT/RSV-eYFP.CMV-SB transposon, single-integration cell lines created with either pT/EF1α-eGFP.CMV-SB or pT/dmEF1α-dmGFP.CMV-SB did not undergo a significant increase in gene silencing between 12 and 42 weeks (Table 2). Collectively, our data indicate that SB-mediated transposition events can be subject to progressive postintegrative gene silencing but that the amount of long-term silencing is influenced by the transgene promoter.
TABLE 2.
Summary of transgene expression
| Construct | No. of cell lines containing a single integration | % Cell lines expressing constructa (wks posttransfection) |
|---|---|---|
| pT/RSV-eYFP.CMV-SB | 17 | 77 (12) |
| 29 (42) | ||
| pT/EF1α-eGFP.CMV-SB | 15 | 87 (12) |
| 87 (42) | ||
| pT/dmEF1α-dmGFP.CMV-SB | 31 | 87 (12) |
| 83 (42) |
A cell line is defined as an expressing cell line if ≥5% of the total cells were found within a low, mid, or high region.
TABLE 3.
Expression profiles for pT/RSV-eYFP.CMV-SB
| Cell line no. | Expression profile ata:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Wk 12
|
Wk 42
|
|||||||
| No | Low | Mid | High | No | Low | Mid | High | |
| 1 | 9 | 17 | 22 | 51 | 99 | 1 | 0 | 0 |
| 16 | 29 | 70 | 1 | 0 | 86 | 14 | 0 | 0 |
| 17 | 1 | 32 | 65 | 2 | 37 | 62 | 1 | 0 |
| 19 | 95 | 5 | 0 | 0 | 100 | 0 | 0 | 0 |
| 24 | 95 | 5 | 0 | 0 | 100 | 0 | 0 | 0 |
| 26 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 |
| 34 | 97 | 3 | 0 | 0 | 99 | 1 | 0 | 0 |
| 37 | 85 | 13 | 2 | 0 | 55 | 45 | 0 | 0 |
| 50 | 99 | 1 | 0 | 0 | 100 | 0 | 0 | 0 |
| 115 | 93 | 7 | 0 | 0 | 100 | 0 | 0 | 0 |
| 116 | 42 | 57 | 1 | 0 | 97 | 3 | 0 | 0 |
| 118 | 71 | 29 | 0 | 0 | 99 | 1 | 0 | 0 |
| 127 | 96 | 4 | 0 | 0 | 100 | 0 | 0 | 0 |
| 129 | 12 | 79 | 9 | 1 | 73 | 27 | 0 | 0 |
| 140 | 51 | 46 | 3 | 0 | 69 | 30 | 1 | 0 |
| 142 | 65 | 33 | 2 | 0 | 100 | 0 | 0 | 0 |
| 162 | 100 | 0 | 0 | 0 | 98 | 2 | 0 | 0 |
Flow cytometry data were graphed on dot plots which had been divided into four regions (no, low, mid, and high) based on the level of transgene expression (see Fig. 3).
FIG. 3.
The site of integration regulates transgene expression. At 12 and 42 weeks posttransfection, cell lines were analyzed by flow cytometry and plotted in graphs containing predivided regions (no, low, mid, or high). The “no” regions contain cells in which the transposon's transgene does not express or has been silenced. The other regions contain cells that are positive for transgene expression.
Postintegrative DNA methylation.
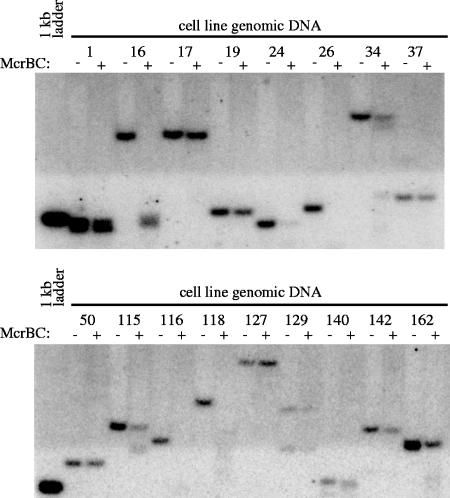
Here, we investigated possible mechanisms involved in long-term postintegrative transposon silencing, focusing initially on the potential role of CpG dinucleotide methylation. To investigate DNA methylation of transposon sequences, we first utilized the endonuclease McrBC, which cleaves DNA only in the presence of CpG methylation. Genomic DNA samples from the single-integration pT/RSV-eYFP.CMV-SB-derived cell lines were isolated approximately 36 to 40 weeks post-FACS and cleaved with NcoI and SpeI restriction endonucleases, which resulted in a band containing the eYFP transgene, right IR, and a genomic fragment whose size depended on the location of an adjoining SpeI or NcoI site elsewhere in the HeLa cell genome. These samples were then split and digested in the presence or absence of McrBC before analysis by Southern blot hybridization using a probe against eYFP. Using this approach, we found that the majority of integrated transposons experienced at least some level of DNA methylation, with 53% (9/17) of the cell lines (16, 24, 26, 34, 115, 116, 118, 142, and 162) showing significant digestion in the presence of McrBC (Fig. 4).
FIG. 4.
Analysis of DNA methylation via McrBC digestion. (A) Genomic DNA was isolated from each single-integration cell line created from pT/RSV-eYFP.CMV-SB and digested by NcoI and SpeI and then in the presence (+) or absence (−) of McrBC (which cleaves in the presence of CpG methylation). After digestion, the samples were examined via Southern blot analysis using a reporter-specific probe.
To investigate possible functional consequences of this methylation, we studied what effect, if any, the DNA methyltransferase inhibitor 5-AzaC had on the expression of these transposon-encoded transgenes. 5-AzaC is frequently used to assess the role of DNA methylation in a given cellular process and functions during DNA synthesis by covalently attaching to cellular methyltransferase enzymes, thereby blocking subsequent methylation of newly synthesized DNA (4). Since 5-AzaC functions only in dividing cells, we added it to subconfluent cells and grew them in the presence of the inhibitor for 4 days, at which time we analyzed each cell line for eYFP expression by flow cytometry. As negative controls we included wild-type HeLa cells and mock-treated samples for each cell line.
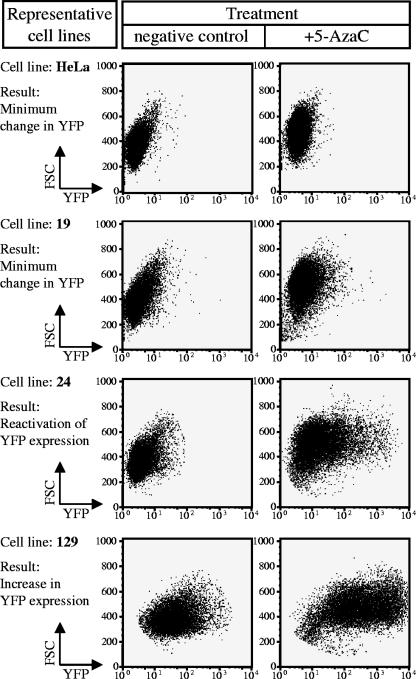
After 5-AzaC treatment, all 17 single-integration cell lines (except cell line 19) created using pT/RSV-eYFP.CMV-SB experienced a significant increase in transgene expression (Fig. 5). As summarized in Table 4, not only did we observe an increase in expression in cell lines that were already eYFP positive (cell lines 1, 16, 17, 37, 116, 118, 129, 140, 142, and 162), but we also observed reactivation within silenced cell lines (cell lines 24, 26, 34, 50, 115, and 127). These data indicate that in the context of integrated SB elements, DNA methylation can play a functional role in postintegrative transgene repression.
FIG. 5.
DNA methylation plays a role in transgene expression. The role of CpG dinucleotide methylation in transgene regulation was investigated by testing the single-integration cell lines (created from pT/RSV-eYFP.CMV-SB) with 5-AzaC, a methyltransferase inhibitor. Representative dot plots are shown for the three categories of cell line responses. The first category (represented by cell line 19) contains silenced cell lines which showed only a minimal response to 5-AzaC. The second category (represented by cell line 24) contains silenced cell lines that were reactivated in the presence of 5-AzaC. The third category (represented by cell line 129) contains transgene-expressing cell lines in which there was an increase in expression in the presence of 5-AzaC.
TABLE 4.
Summary of transgene responses to 5-AzaC and TSAa
| Construct | Cell line(s) with indicated 5-AzaC response
|
Cell line(s) with indicated TSA response
|
||||
|---|---|---|---|---|---|---|
| Minimal | Reactivation | Increase | Minimal | Reactivation | Increase | |
| pT/RSV-eYFP.CMV-SB | 19 | 24, 26, 34, 50, 115, 127 | 1, 16, 17, 37, 116, 118, 140, 142, 162, 129 | 26 | 19, 24, 34, 50, 115, 127 | 1, 16, 17, 129, 140, 142, 162, 37, 116, 118 |
A region must contain >5% of the total cells within a cell line to be considered positive. Reactivation requires that ≥95% of the cells within a cell line were initially found within the “no” region prior to treatment but in posttreatment one or more of the low, mid, or high regions became positive.
Histone deacetylation within the SB system.
To ascertain whether or not histone deacetylation might also contribute to postintegrative transgene repression, we made use of the histone deacetylase inhibitor TSA. We added TSA to the medium of subconfluent pT/RSV-eYFP.CMV-SB cell lines and continued to grow the cells for 24 h, followed by analysis of eYFP expression via flow cytometry. As negative controls we included wild-type HeLa cells and mock-treated samples for each cell line.
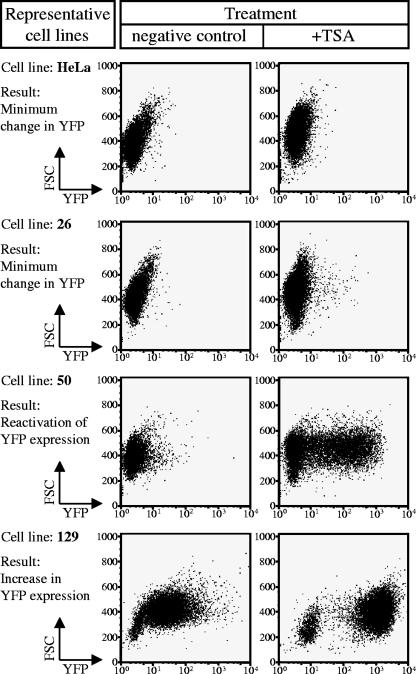
Similar to the 5-AzaC experiments, most cell lines treated with TSA experienced an increase in eYFP expression (Fig. 6; summarized in Table 4). We observed a significant increase in transposon expression in many of the cell lines (1, 16, 17, 129, 142, 37, 116, 118, 140, and 162) and detected reactivation in four previously silenced cell lines (19, 24, 34, 50, 115, and 127). Only cell line 26 showed a negligible increase in eYFP expression in the presence of TSA. These results indicate that histone deacetylation may also contribute to postintegrative transposon silencing.
FIG. 6.
Histone deacetylation plays a role in transgene expression. The role of chromatin structure in transgene regulation was investigated by testing the single-integration cell lines (created from pT/RSV-eYFP.CMV-SB) with TSA, a deacetylase inhibitor. Representative dot plots are shown for the three categories of cell line responses. The first category (represented by cell line 26) contains silenced cell lines which showed only a minimal response to TSA. The second category (represented by cell line 50) contains silenced cell lines that were reactivated in the presence of TSA. The third category (represented by cell line 129) contains transgene-expressing cell lines in which there was an increase in expression in the presence of TSA.
Recovery and mapping of transposon insertion sites.
In an effort to gain more insight into the events leading to postintegrative gene silencing, we investigated the genomic features associated with silenced and nonsilenced transposons in pT/RSV-eYFP.CMV-SB-derived cell lines. To do this, we used linker-mediated PCR to isolate the genomic sequences immediately flanking the transposon (7) from two pools of genomic DNA collected at week 12: (i) one derived from 10 expressing cell lines, and (ii) one derived from 7 silenced clones. Although we were only able to unambiguously map five clones from the expressing pool and two from the silenced pool, all five integrations from the pool of high-expressing clones mapped to within 2 kb of a known or predicted gene (Table 5), perhaps indicating that within a nonselective system transposons located within close proximity of endogenous genes may have a greater likelihood of maintaining expression upon genomic insertion. Of the two silenced clones we successfully mapped, one mapped >2 kb from the nearest predicted gene, while the other localized within the highly expressed (23) dihydropyrimidine dehydrogenase gene and may have been silenced due to transcriptional interference (50), as has been seen within retroviruses (15, 34, 35). While these data do provide some much-needed preliminary insight into these regulatory events, the limited sample size is much too low to make any definitive conclusions herein. Future work in the field using more high-throughput screening methods should thus prove invaluable in better clarifying this important issue.
TABLE 5.
Analysis of Sleeping Beauty integration sites and transgene expression
| Sequence | Chromosome locus | Nearest transcribed region | Wk 12 expression state |
|---|---|---|---|
| TAGGTGACTTATT GAAATGAAAAG | 1p21.3 | 0 kb, within DPYD gene | Silenced |
| TAGTTTCAGTCAAGTTTTGAGGTG | 19q13.41 | >2 kb from nearest predicted gene | Silenced |
| TATATAGTATGCTGTAATTGCTAA | 3p25.2 | 0 kb, within RAF-1 gene | Expressed |
| TACACAGTAGAAGTTATCTAAAAA | 11q22.3 | 0 kb, within predicted gene GENSCAN00000034075 | Expressed |
| TAACATAAAAATTTACCATTTTGG | 6p22.3 | 0 kb, within estimated transcript ENSESTT00000084856 | Expressed |
| TATAAGATGCATTTTTTTCCTCAA | 8q12.2 | 2 kb from estimated transcript ENSESTT00000082392 | Expressed |
| TAGGTAGGGAAGAGTGCCAAAT | 8p12 | 0 kb, within known transcript ENST00000340497 | Expressed |
DISCUSSION
In this report, we have developed a novel nonselective, FACS-based method to measure the frequency of Sleeping Beauty transposon integration, independent of its expression, in cultured human cells. Using this unique approach, we demonstrate that SB's true integrative potential is up to 25 times higher than previously reported selection-based estimates (i.e., 41 to 52% versus 2 to 3%). In addition, we have identified the existence of an ill-defined postintegrative gene regulatory network that efficiently targets some invading transposon sequences for transcriptional silencing in mammalian cells.
Our work indicates that postintegrative gene silencing can play a role in limiting long-term SB-based expression and suggests that at least two types of potential silencing “triggers” may be involved. In the case of “contextual” silencing, the expression of the integrated transposon is predominantly influenced by the regional chromosomal sequences to which it is now confined. This mode of gene silencing has also been reported within the context of retrovirus-based systems (3, 8, 24, 25, 34, 35, 42, 54, 62), although the underlying mechanisms involved remain under investigation. In theory, however, contextual silencing could originate from integration of the transposon into regions that are inherently restrictive to gene expression (e.g., heterochromatin). Alternatively, if a transposon were to integrate into a highly active region of the chromosome, it is possible that such an event could also initiate a signaling cascade that culminated in the selective repression of the invading sequence via RNA interference or through transcriptional interference. In the present study, we described our preliminary analyses of three different cis-acting vectors and found similar levels of silencing after a 12-week period, a result that is highly consistent with a “cargo-independent” mode of transposon silencing. Although the results of the integration site analyses performed herein are also consistent with this contextual model, future large-scale investigations will be needed to firmly establish the link between integration local and transposon expression levels.
As indicated by the detailed work with pT/RSV-eYFP.CMV-SB, a second class of silencing triggers exists which is dependent on the presence of specific sequences and/or structural elements within the transposon itself. One such “intrinsic” element that may serve to flag the transposon sequence as foreign (and thereby target it for silencing) is DNA that is rich in CpG dinucleotides. Indeed, within the pT/RSV-eYFP.CMV-SB-derived cell lines, we not only observed a general trend towards increased transposon CpG methylation and progressive transgene silencing but also we observed that many silenced clones could be reactivated in the presence of the DNA methyltransferase inhibitor 5-AzaC. These experiments not only indicate that DNA methylation may play a functional role in postintegrative gene silencing of transposons but also they indicate on biochemical and functional levels that the observed gene silencing was not due to genetic loss or recombination.
Other groups have also recently shown that integrated transposon sequences can become methylated; however, these studies were more limited in that they did not correlate DNA methylation to transgene expression (44, 45). Others have also discovered that under certain conditions and due to their high level of CpG dinucleotides, eGFP variants appear especially susceptible to CpG methylation (5). Although the exact role CpG methylation plays in transcriptional silencing is not presently clear, a wealth of data suggests that DNA methyltransferases can inhibit transcription both directly (20, 28, 37) and indirectly via interaction with the histone deacetylases involved in chromatin condensation (6, 10-12, 28). Although CpG methylation often correlates with the transcriptional silencing of retroviral sequences (8, 19, 25, 32, 37, 38, 53-55, 62), our study is the first to establish a similar correlation within a mammalian transposon system. As such, our findings indicate that DNA methylation may play a general role in some host cell mechanisms which recognize and then silence invading genetic sequences, which is collaborated by data from a variety of biological systems. For instance, Drosophila lack a DNA methylation system and are unable to effectively repress transposon activity, and as a result 50 to 85% of all spontaneous Drosophila mutations are caused by transposon insertions, compared to less than 1% of human mutations (9, 36, 63). In addition, the suppression of DNA methylation in plants (18, 26, 41), Dictyostelium (29), and mammalian germ cells (2) has been shown to lead to reactivation of endogenous transposon sequences, suggesting that CpG methylation-based silencing mechanisms (and perhaps others) are evolutionarily conserved, presumably because they play an integral role in minimizing the damage caused by invading genetic parasites.
Another epigenetic modification that may contribute to the transcriptional repression of integrated transposons is the deacetylation of chromatin-bound histones. Using the histone deacetylase inhibitor TSA, we have found that postintegrative transposon silencing can occur in a histone deacetylation-dependent manner. Similar findings have been reported from the retroviral field (17, 25, 33, 38, 39, 54, 55).
Interestingly, the silencing induced by elements intrinsic to the transposon (as described for pT/RSV-eYFP.CMV-SB) does not appear to uniformly apply to all SB transposons, since we observed little long-term change in transgene expression within the context of pT/EF1α-eGFP.CMV-SB- and pT/dmEF1α-dmGFP.CMV-SB-derived clones. We believe the lack of continued silencing within these two transposons was due mainly to our switch from the Rous sarcoma virus-derived promoter to the endogenous human EF1α promoter, since such a change has been previously documented to positively affect persistence of transgene expression (14). Several possibilities exist for why such a change in promoter could elicit such a strong alteration in long-term gene expression patterns. For example, the RSV promoter itself could be acting as a nucleating factor for silencing, or perhaps the RSV promoter is simply not strong enough to overcome the silencing effect of the high CpG dinucleotide concentration found within the pT/RSV-eYFP.CMV-SB transposon. The exact cause for the difference in silencing between our RSV and EF1α transposons was not fully elucidated within the context of this study. Nonetheless, our finding that some SB transposons are subject to high levels of postintegrative gene silencing remains an important consideration for future transposon studies.
Within our study, the transposons containing a virus-derived promoter (RSV) were silenced over time, and as a result had we employed an antibiotic selection scheme in measuring the system's integration efficiency, then we would have likely underestimated the true integration efficiency. Importantly, it is worth noting that previous estimates of SB's integration efficiency not only relied upon an antibiotic selection scheme but they also employed virus-derived promoters (cytomegalovirus [13] and simian virus 40 [64]) to drive expression of their respective antibiotic resistance markers, which may explain why these studies observed a much lower integration efficiency for SB than what was demonstrated within our study.
The Sleeping Beauty transposon system represents an increasingly important vehicle for in vivo gene delivery. At present, however, much of the effort directed towards successfully adapting this and other DNA transposons to a clinical setting have focused to a large extent on obtaining improved integration frequencies in target cells via mutation of transposon (donor) and/or transposase (helper) components (1, 13, 27, 60, 64). Our work presented herein suggests that SB's integration efficiency may not be as great a barrier as previously thought and implies that a greater emphasis on postintegrative regulatory mechanisms may ultimately prove more productive in perfecting SB for a clinical environment. First, more studies are required for identifying, and ultimately deleting and/or altering, all intrinsic silencing triggers embedded within the transposon while maintaining its integration properties. Second, extreme care should be applied when making the decision as to what promoter will be used, since our data indicate this may be one of the major determinants of persistence of transgene expression. And third, in order to alleviate the impact of contextual silencing entirely, it would be beneficial if researchers could also modify transposon systems in such a way as to promote integration into sites predetermined to be suitable for long-term expression (56, 58). Based on the degree of transposon silencing observed in our studies, all of these approaches, while experimentally challenging, are likely to prove essential in the optimization of transposons (and possibly retroviruses) for clinical gene therapy. Additionally, it will also be important to ensure that the maximization of transgene expression within the SB system does not affect expression of local endogenous sequences, which perhaps could be accomplished through the use of efficient poly(A) sequences to prevent 3′ readthrough and insulators to limit chromatin influences. Finally, while our study does identify postintegrative gene silencing as an area of transposon biology warranting further investigation, we remain entirely optimistic of Sleeping Beauty's therapeutic future, since we also demonstrated that SB has the ability to integrate at sufficiently high levels for applications within the field of gene therapy.
Acknowledgments
We thank Stanford University's FACS facility staff for their technical assistance and James Ellis for generously providing us with pBGT103-based constructs.
This work was supported by NIH HL 64274.
Footnotes
Published ahead of print on 15 October 2007.
REFERENCES
- 1.Baus, J., L. Liu, A. D. Heggestad, S. Sanz, and B. S. Fletcher. 2005. Hyperactive transposase mutants of the Sleeping Beauty transposon. Mol. Ther. 12:1148-1156. [DOI] [PubMed] [Google Scholar]
- 2.Bourc'his, D., and T. H. Bestor. 2004. Meiotic catastrophe and retrotransposon reactivation in male germ cells lacking Dnmt3L. Nature 431:96-99. [DOI] [PubMed] [Google Scholar]
- 3.Bushman, F., M. Lewinski, A. Ciuffi, S. Barr, J. Leipzig, S. Hannenhalli, and C. Hoffmann. 2005. Genome-wide analysis of retroviral DNA integration. Nat. Rev. Microbiol. 3:848-858. [DOI] [PubMed] [Google Scholar]
- 4.Christman, J. K. 2002. 5-Azacytidine and 5-aza-2′-deoxycytidine as inhibitors of DNA methylation: mechanistic studies and their implications for cancer therapy. Oncogene 21:5483-5495. [DOI] [PubMed] [Google Scholar]
- 5.Dalle, B., J. E. Rubin, O. Alkan, T. Sukonnik, P. Pasceri, S. Yao, R. Pawliuk, P. Leboulch, and J. Ellis. 2005. eGFP reporter genes silence LCR β-globin transgene expression via CpG dinucleotides. Mol. Ther. 11:591-599. [DOI] [PubMed] [Google Scholar]
- 6.Deplus, R., C. Brenner, W. A. Burgers, P. Putmans, T. Kouzarides, Y. de Launoit, and F. Fuks. 2002. Dnmt3L is a transcriptional repressor that recruits histone deacetylase. Nucleic Acids Res. 30:3831-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dupuy, A. J., K. Akagi, D. A. Largaespada, N. G. Copeland, and N. A. Jenkins. 2005. Mammalian mutagenesis using a highly mobile somatic Sleeping Beauty transposon system. Nature 436:221-226. [DOI] [PubMed] [Google Scholar]
- 8.Ellis, J. 2005. Silencing and variegation of gammaretrovirus and lentivirus vectors. Hum. Gene Ther. 16:1241-1246. [DOI] [PubMed] [Google Scholar]
- 9.Finnegan, D. J. 1992. Transposable elements. Curr. Opin. Genet. Dev. 2:861-867. [DOI] [PubMed] [Google Scholar]
- 10.Fuks, F. 2005. DNA methylation and histone modifications: teaming up to silence genes. Curr. Opin. Genet. Dev. 15:490-495. [DOI] [PubMed] [Google Scholar]
- 11.Fuks, F., W. A. Burgers, A. Brehm, L. Hughes-Davies, and T. Kouzarides. 2000. DNA methyltransferase Dnmt1 associates with histone deacetylase activity. Nat. Genet. 24:88-91. [DOI] [PubMed] [Google Scholar]
- 12.Fuks, F., W. A. Burgers, N. Godin, M. Kasai, and T. Kouzarides. 2001. Dnmt3a binds deacetylases and is recruited by a sequence-specific repressor to silence transcription. EMBO J. 20:2536-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geurts, A. M., Y. Yang, K. J. Clark, G. Liu, Z. Cui, A. J. Dupuy, J. B. Bell, D. A. Largaespada, and P. B. Hackett. 2003. Gene transfer into genomes of human cells by the sleeping beauty transposon system. Mol. Ther. 8:108-117. [DOI] [PubMed] [Google Scholar]
- 14.Gill, D. R., S. E. Smyth, C. A. Goddard, I. A. Pringle, C. F. Higgins, W. H. Colledge, and S. C. Hyde. 2001. Increased persistence of lung gene expression using plasmids containing the ubiquitin C or elongation factor 1α promoter. Gene Ther. 8:1539-1546. [DOI] [PubMed] [Google Scholar]
- 15.Greger, I. H., F. Demarchi, M. Giacca, and N. J. Proudfoot. 1998. Transcriptional interference perturbs the binding of Sp1 to the HIV-1 promoter. Nucleic Acids Res. 26:1294-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hackett, P. B., S. C. Ekker, D. A. Largaespada, and R. S. McIvor. 2005. Sleeping beauty transposon-mediated gene therapy for prolonged expression. Adv. Genet. 54:189-232. [DOI] [PubMed] [Google Scholar]
- 17.He, J., Q. Yang, and L. J. Chang. 2005. Dynamic DNA methylation and histone modifications contribute to lentiviral transgene silencing in murine embryonic carcinoma cells. J. Virol. 79:13497-13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirochika, H., H. Okamoto, and T. Kakutani. 2000. Silencing of retrotransposons in Arabidopsis and reactivation by the ddm1 mutation. Plant Cell 12:357-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofmann, A., B. Kessler, S. Ewerling, A. Kabermann, G. Brem, E. Wolf, and A. Pfeifer. 2006. Epigenetic regulation of lentiviral transgene vectors in a large animal model. Mol. Ther. 13:59-66. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh, C. L. 1997. Stability of patch methylation and its impact in regions of transcriptional initiation and elongation. Mol. Cell. Biol. 17:5897-5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ivics, Z., P. B. Hackett, R. H. Plasterk, and Z. Izsvak. 1997. Molecular reconstruction of Sleeping Beauty, a Tc1-like transposon from fish, and its transposition in human cells. Cell 91:501-510. [DOI] [PubMed] [Google Scholar]
- 22.Izsvak, Z., Z. Ivics, and P. B. Hackett. 1995. Characterization of a Tc1-like transposable element in zebrafish (Danio rerio). Mol. Gen. Genet. 247:312-322. [DOI] [PubMed] [Google Scholar]
- 23.Johnson, M. R., K. Wang, J. B. Smith, M. J. Heslin, and R. B. Diasio. 2000. Quantitation of dihydropyrimidine dehydrogenase expression by real-time reverse transcription polymerase chain reaction. Anal. Biochem. 278:175-184. [DOI] [PubMed] [Google Scholar]
- 24.Jordan, A., D. Bisgrove, and E. Verdin. 2003. HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. EMBO J. 22:1868-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jordan, A., P. Defechereux, and E. Verdin. 2001. The site of HIV-1 integration in the human genome determines basal transcriptional activity and response to Tat transactivation. EMBO J. 20:1726-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kato, M., K. Takashima, and T. Kakutani. 2004. Epigenetic control of CACTA transposon mobility in Arabidopsis thaliana. Genetics 168:961-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keravala, A., D. Liu, E. R. Lechman, D. Wolfe, J. A. Nash, D. J. Lampe, and P. D. Robbins. 2006. Hyperactive Himar1 transposase mediates transposition in cell culture and enhances gene expression in vivo. Hum. Gene Ther. 17:1006-1018. [DOI] [PubMed] [Google Scholar]
- 28.Klose, R. J., and A. P. Bird. 2006. Genomic DNA methylation: the mark and its mediators. Trends Biochem. Sci. 31:89-97. [DOI] [PubMed] [Google Scholar]
- 29.Kuhlmann, M., B. E. Borisova, M. Kaller, P. Larsson, D. Stach, J. Na, L. Eichinger, F. Lyko, V. Ambros, F. Soderbom, C. Hammann, and W. Nellen. 2005. Silencing of retrotransposons in Dictyostelium by DNA methylation and RNAi. Nucleic Acids Res. 33:6405-6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lam, W. L., P. Seo, K. Robison, S. Virk, and W. Gilbert. 1996. Discovery of amphibian Tc1-like transposon families. J. Mol. Biol. 257:359-366. [DOI] [PubMed] [Google Scholar]
- 31.Lander, E. S., L. M. Linton, B. Birren, C. Nusbaum, M. C. Zody, J. Baldwin, K. Devon, K. Dewar, M. Doyle, W. FitzHugh, R. Funke, D. Gage, K. Harris, A. Heaford, J. Howland, L. Kann, J. Lehoczky, R. LeVine, P. McEwan, K. McKernan, J. Meldrim, J. P. Mesirov, C. Miranda, W. Morris, J. Naylor, C. Raymond, M. Rosetti, R. Santos, A. Sheridan, C. Sougnez, N. Stange-Thomann, N. Stojanovic, A. Subramanian, D. Wyman, J. Rogers, J. Sulston, R. Ainscough, S. Beck, D. Bentley, J. Burton, C. Clee, N. Carter, A. Coulson, R. Deadman, P. Deloukas, A. Dunham, I. Dunham, R. Durbin, L. French, D. Grafham, S. Gregory, T. Hubbard, S. Humphray, A. Hunt, M. Jones, C. Lloyd, A. McMurray, L. Matthews, S. Mercer, S. Milne, J. C. Mullikin, A. Mungall, R. Plumb, M. Ross, R. Shownkeen, S. Sims, R. H. Waterston, R. K. Wilson, L. W. Hillier, J. D. McPherson, M. A. Marra, E. R. Mardis, L. A. Fulton, A. T. Chinwalla, K. H. Pepin, W. R. Gish, S. L. Chissoe, M. C. Wendl, K. D. Delehaunty, T. L. Miner, A. Delehaunty, J. B. Kramer, L. L. Cook, R. S. Fulton, D. L. Johnson, P. J. Minx, S. W. Clifton, T. Hawkins, E. Branscomb, P. Predki, P. Richardson, S. Wenning, T. Slezak, N. Doggett, J. F. Cheng, A. Olsen, S. Lucas, C. Elkin, E. Uberbacher, M. Frazier, et al. 2001. Initial sequencing and analysis of the human genome. Nature 409:860-921. [DOI] [PubMed] [Google Scholar]
- 32.Lavie, L., M. Kitova, E. Maldener, E. Meese, and J. Mayer. 2005. CpG methylation directly regulates transcriptional activity of the human endogenous retrovirus family HERV-K(HML-2). J. Virol. 79:876-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee, S. H., M. Oshige, S. T. Durant, K. K. Rasila, E. A. Williamson, H. Ramsey, L. Kwan, J. A. Nickoloff, and R. Hromas. 2005. The SET domain protein Metnase mediates foreign DNA integration and links integration to nonhomologous end-joining repair. Proc. Natl. Acad. Sci. USA 102:18075-18080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewinski, M. K., D. Bisgrove, P. Shinn, H. Chen, C. Hoffmann, S. Hannenhalli, E. Verdin, C. C. Berry, J. R. Ecker, and F. D. Bushman. 2005. Genome-wide analysis of chromosomal features repressing human immunodeficiency virus transcription. J. Virol. 79:6610-6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewinski, M. K., and F. D. Bushman. 2005. Retroviral DNA integration—mechanism and consequences. Adv. Genet. 55:147-181. [DOI] [PubMed] [Google Scholar]
- 36.Lindsley, D. L., and G. G. Zimm. 1992. Genome of Drosophila melanogaster. Academic Press, San Diego, CA.
- 37.Lorincz, M. C., D. R. Dickerson, M. Schmitt, and M. Groudine. 2004. Intragenic DNA methylation alters chromatin structure and elongation efficiency in mammalian cells. Nat. Struct. Mol. Biol. 11:1068-1075. [DOI] [PubMed] [Google Scholar]
- 38.Lorincz, M. C., D. Schubeler, and M. Groudine. 2001. Methylation-mediated proviral silencing is associated with MeCP2 recruitment and localized histone H3 deacetylation. Mol. Cell. Biol. 21:7913-7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merezak, C., M. Reichert, C. Van Lint, P. Kerkhofs, D. Portetelle, L. Willems, and R. Kettmann. 2002. Inhibition of histone deacetylases induces bovine leukemia virus expression in vitro and in vivo. J. Virol. 76:5034-5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mikkelsen, J. G., S. R. Yant, L. Meuse, Z. Huang, H. Xu, and M. A. Kay. 2003. Helper-Independent Sleeping Beauty transposon-transposase vectors for efficient nonviral gene delivery and persistent gene expression in vivo. Mol. Ther. 8:654-665. [DOI] [PubMed] [Google Scholar]
- 41.Miura, A., S. Yonebayashi, K. Watanabe, T. Toyama, H. Shimada, and T. Kakutani. 2001. Mobilization of transposons by a mutation abolishing full DNA methylation in Arabidopsis. Nature 411:212-214. [DOI] [PubMed] [Google Scholar]
- 42.Mohamedali, A., F. Moreau-Gaudry, E. Richard, P. Xia, J. Nolta, and P. Malik. 2004. Self-inactivating lentiviral vectors resist proviral methylation but do not confer position-independent expression in hematopoietic stem cells. Mol. Ther. 10:249-259. [DOI] [PubMed] [Google Scholar]
- 43.Oosumi, T., W. R. Belknap, and B. Garlick. 1995. Mariner transposons in humans. Nature 378:672. [DOI] [PubMed] [Google Scholar]
- 44.Park, C. W., B. T. Kren, D. A. Largaespada, and C. J. Steer. 2005. DNA methylation of Sleeping Beauty with transposition into the mouse genome. Genes Cells 10:763-776. [DOI] [PubMed] [Google Scholar]
- 45.Park, C. W., J. Park, B. T. Kren, and C. J. Steer. 2006. Sleeping Beauty transposition in the mouse genome is associated with changes in DNA methylation at the site of insertion. Genomics 88:204-213. [DOI] [PubMed] [Google Scholar]
- 46.Plasterk, R. H., Z. Izsvak, and Z. Ivics. 1999. Resident aliens: the Tc1/mariner superfamily of transposable elements. Trends Genet. 15:326-332. [DOI] [PubMed] [Google Scholar]
- 47.Sanchez-Gracia, A., X. Maside, and B. Charlesworth. 2005. High rate of horizontal transfer of transposable elements in Drosophila. Trends Genet. 21:200-203. [DOI] [PubMed] [Google Scholar]
- 48.Schukkink, R. F., and R. H. Plasterk. 1990. TcA, the putative transposase of the C. elegans Tc1 transposon, has an N-terminal DNA binding domain. Nucleic Acids Res. 18:895-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shao, H., and Z. Tu. 2001. Expanding the diversity of the IS630-Tc1-mariner superfamily: discovery of a unique DD37E transposon and reclassification of the DD37D and DD39D transposons. Genetics 159:1103-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shearwin, K. E., B. P. Callen, and J. B. Egan. 2005. Transcriptional interference—a crash course. Trends Genet. 21:339-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sinzelle, L., N. Pollet, Y. Bigot, and A. Mazabraud. 2005. Characterization of multiple lineages of Tc1-like elements within the genome of the amphibian Xenopus tropicalis. Gene 349:187-196. [DOI] [PubMed] [Google Scholar]
- 52.Smit, A. F., and A. D. Riggs. 1996. Tiggers and DNA transposon fossils in the human genome. Proc. Natl. Acad. Sci. USA 93:1443-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Swindle, C. S., H. G. Kim, and C. A. Klug. 2004. Mutation of CpGs in the murine stem cell virus retroviral vector long terminal repeat represses silencing in embryonic stem cells. J. Biol. Chem. 279:34-41. [DOI] [PubMed] [Google Scholar]
- 54.Swindle, C. S., and C. A. Klug. 2002. Mechanisms that regulate silencing of gene expression from retroviral vectors. J. Hematother. Stem Cell Res. 11:449-456. [DOI] [PubMed] [Google Scholar]
- 55.Taniguchi, Y., K. Nosaka, J. Yasunaga, M. Maeda, N. Mueller, A. Okayama, and M. Matsuoka. 2005. Silencing of human T-cell leukemia virus type I gene transcription by epigenetic mechanisms. Retrovirology 2:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu, S. C., Y. J. Meir, C. J. Coates, A. M. Handler, P. Pelczar, S. Moisyadi, and J. M. Kaminski. 2006. piggyBac is a flexible and highly active transposon as compared to Sleeping Beauty, Tol2, and Mos1 in mammalian cells. Proc. Natl. Acad. Sci. USA 103:15008-15013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yant, S. R., A. Ehrhardt, J. G. Mikkelsen, L. Meuse, T. Pham, and M. A. Kay. 2002. Transposition from a gutless adeno-transposon vector stabilizes transgene expression in vivo. Nat. Biotechnol. 20:999-1005. [DOI] [PubMed] [Google Scholar]
- 58.Yant, S. R., Y. Huang, B. Akache, and M. A. Kay. 7 March 2007, posting date. Site-directed transposon integration in human cells. Nucleic Acids Res. 35:e50. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yant, S. R., L. Meuse, W. Chiu, Z. Ivics, Z. Izsvak, and M. A. Kay. 2000. Somatic integration and long-term transgene expression in normal and haemophilic mice using a DNA transposon system. Nat. Genet. 25:35-41. [DOI] [PubMed] [Google Scholar]
- 60.Yant, S. R., J. Park, Y. Huang, J. G. Mikkelsen, and M. A. Kay. 2004. Mutational analysis of the N-terminal DNA-binding domain of sleeping beauty transposase: critical residues for DNA binding and hyperactivity in mammalian cells. Mol. Cell. Biol. 24:9239-9247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yant, S. R., X. Wu, Y. Huang, B. Garrison, S. M. Burgess, and M. A. Kay. 2005. High-resolution genome-wide mapping of transposon integration in mammals. Mol. Cell. Biol. 25:2085-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yao, S., T. Sukonnik, T. Kean, R. R. Bharadwaj, P. Pasceri, and J. Ellis. 2004. Retrovirus silencing, variegation, extinction, and memory are controlled by a dynamic interplay of multiple epigenetic modifications. Mol. Ther. 10:27-36. [DOI] [PubMed] [Google Scholar]
- 63.Yoder, J. A., C. P. Walsh, and T. H. Bestor. 1997. Cytosine methylation and the ecology of intragenomic parasites. Trends Genet. 13:335-340. [DOI] [PubMed] [Google Scholar]
- 64.Zayed, H., Z. Izsvak, O. Walisko, and Z. Ivics. 2004. Development of hyperactive sleeping beauty transposon vectors by mutational analysis. Mol. Ther. 9:292-304. [DOI] [PubMed] [Google Scholar]