Abstract
Recent evidence has shown that the activation of receptor tyrosine kinases is not only dependent on binding of their ligands but in addition requires adhesion molecules as coreceptors. We have identified CD44v6 as a coreceptor for c-Met in several tumor and primary cells. The CD44v6 ectodomain is required for c-Met activation, whereas the cytoplasmic tail recruits ERM proteins and the cytoskeleton into a signalosome complex. Here we demonstrate that c-Met (and hepatocyte growth factor and Gab1) is haploinsufficient in a cd44−/− background, as the cd44−/−; met+/− (and cd44−/−; hgf+/− and cd44−/−; gab1+/−) mice die at birth. They have impaired synaptic transmission in the respiratory rhythm-generating network and alterations in the phrenic nerve. These results are the first genetic data showing that CD44 and c-Met collaborate in vivo and that they are involved in synaptogenesis and axon myelination in the central and peripheral nervous systems.
After ligand binding, receptor tyrosine kinases (RTKs) are activated and induce physiological events such as proliferation, migration, differentiation, or apoptosis (reviewed in reference 25). For the majority of RTKs, the activation step was thought to be exclusively dependent on binding of the ligand, although for members of the fibroblast growth factor receptor (FGF-R) family an involvement of a coreceptor, namely heparansulfate modified proteins (HSPG), has long been proposed (39, 44). In this case it is believed that the ligand has to bind to the HSPG and the complex then activates the receptor. This concept of a coreceptor function for RTKs has expanded recently and appears to have a much broader relevance. A variety of adhesion molecules such as N-cadherin, N-CAM, or integrins can interact with FGF-Rs, vascular endothelial growth factor receptor, GFRα1, or c-Met to facilitate or enhance receptor activation (2, 5, 21, 34, 36). Particularly interesting is the family of CD44 adhesion molecules. CD44s or CD44v3 isoforms collaborate with epidermal growth factor receptors (29, 30). We have shown that CD44v6 acts as a coreceptor for c-Met. We have demonstrated that the expression of CD44 isoforms containing exon v6 is a prerequisite for c-Met activation by its ligand hepatocyte growth factor (HGF) in several tumor cells and in primary cells (19). CD44v6, HGF, and c-Met form a ternary complex and the CD44v6 extracellular portion is required for c-Met activation, whereas the cytoplasmic tail recruits ERM proteins (ezrin, radixin, and moesin) and the cytoskeleton into a signalosome complex that mediates signal transduction (19, 20).
The experiments performed on cancer cell lines suggest a collaboration between CD44v6 and c-Met in the metastatic process. The expression of CD44v6 in otherwise nonmetastatic tumor cells rendered these cells responsive to HGF (19) and made them metastatic (7). Data obtained from knockout mice seem to contradict that such a collaboration occurs in animals. The c-Met (and the HGF) knockout mice are embryonic lethal (1, 26, 37). They die between embryonic day 12.5 (E12.5) and E16.5 from a placental defect. The migration of the myogenic precursors is impaired so that organs like the tongue, the diaphragm, or the limbs are not formed. In addition, the liver is severely damaged. This is in striking contrast to the CD44 knockout mice. Although the activation of c-Met in primary keratinocytes is strictly dependent on CD44v6 (19) and limb outgrowth relies on CD44v3 heparansulfated isoforms (29), the CD44-null mice showed no overt phenotype during development. They showed only mild abnormalities in myeloid progenitor migration, bone marrow colonization (27), and lack of homing of lymphocytes to lymph nodes or to the thymus (22).
An explanation for this striking difference between the CD44 knockout mice and the c-Met and HGF knockout mice could be that the function(s) of CD44 is substituted by another protein in the CD44-null mice (no other CD44-related protein has so far been detected). This hypothesis is strongly supported by the data obtained for another type of “knockout” mouse in which CD44 was downregulated by means of CD44 antisense sequences expressed under the control of the keratinocyte K5 promoter (10). Accordingly, these mice do not express CD44 in the keratinocytes. Surprisingly and in clear contrast to the total CD44 knockout, the newborn mice have severe skin alterations, such as a delay in wound healing, in local inflammatory responses, and in hair regrowth. Since the K5 promoter is turned on around day 10 during embryogenesis, we assume that CD44 functions can be substituted during early embryogenesis whereas at later times (when the K5 promoter becomes active) CD44 can no longer be substituted and knocking down of CD44 is then detrimental for the animals. This notion is confirmed by the fact that the only overt phenotypes observed in the CD44 total knockout mice are visible at the adult stage; for example, the maintenance of postpartum lactation is impaired (45).
Here we test the relevance of CD44/c-Met cooperation in vivo and the substitution of the CD44v6 coreceptor function in CD44 knockout mice. We performed crossing experiments between CD44 knockout mice and met (or hgf or gab1) heterozygotes and asked whether the cd44−/−; met+/− (or hgf+/− or gab1+/−) mice show haploinsufficiency and therefore a phenotype. hgf and gab1 crosses are included, since both knockout mice have a phenotype similar to the c-Met knockout mouse (1, 24, 26, 37). For HGF, the ligand for c-Met, this was expected. Gab1 is a docking protein (reviewed in reference 12) that mediates most of the signaling pathways from c-Met (17, 40) as it recruits Grb2, the p85 subunit of PI3K, phospholipase C1, and SHP-2 to the receptor. The similarity of the knockout of Gab1 with c-Met underlines the importance of this interaction.
The c-met (or hgf or gab1) heterozygotes in a cd44+/+ background (and in cd44+/−, shown here) develop normally and show no overt phenotype. In striking contrast, the heterozygotes in a cd44−/− background show lethality at birth with a penetrance reaching 70%. The animals cannot breathe and their lungs are not inflated. This defect results most likely from an impaired synaptic transmission in the brain stem respiratory rhythm-generating network and from alterations in the phrenic nerve that innervates the diaphragm. This haploinsufficiency of met (or hgf or gab1) in the cd44−/− mice can only be explained if CD44 and c-Met/HGF/Gab1 cooperate in vivo. We give here the first genetic evidence that these molecules collaborate in embryogenesis.
MATERIALS AND METHODS
Mouse strains, genotyping.
cd44−/− mice (27) were backcrossed with C57BL/6 mice for 10 generations and then used for crossings with met+/−, gab1+/−, or hgf+/− mice (1, 26, 37) that had also been backcrossed with C57BL/6 mice. Genotyping using mouse tails was performed with the following primers in the PCR: for met inactivation, NEO1 (5′CTTGCGTGCAATCCATCTTGTTCAATG3′) and MET1 (5′CACTGAGCCCAGAAGAGCTAGTGG3′); for endogenous met, MET2 (5′GTACACTGGCTTGTACAATGTACAGTTG3′) and MET3 (5′CTTTTTCAATAGGGCATTTTGGCTGTG3′); for gab1 inactivation, NEO2 (5′TTGTTTTTCGAGCTTCAAGGTTCAT3′) and GAB1 (5′CCCTTTGTGGATGGCTTCTTTGT3′); for endogenous gab1, GAB1 and GAB2 (5′TTCTTGGCATGATCGTTTTTGTAA3′); for hgf inactivation, NEO1 and HGF1 (5′CCCGCAGAGGTATATTGTGTTGTCC3′); for endogenous hgf, HGF1 and HGF2 (5′CTGTTCCTGATACACCTGTTGGCAC3′); for cd44 inactivation, CD441 (5′CGCAGGTGTATTCCATGTGG3′) and NEO3 (5′ACGTTGTCACTGAAGCGGG3′); and for endogenous cd44, CD441 and CD443 (5′ACTGATATGACCCTAATGGCTTCC3′).
Histology.
Newborn mice were sacrificed, fixed in Bouin's solution for 24 h and embedded in paraffin. Serial sagittal sections of 6 μm were stained with hematoxylin and eosin. The histological analysis was performed by Frimorfo, Fribourg, Switzerland.
In situ hybridization.
HGF and cMet cDNAs were a kind gift from Carmen and Walter Birchmeier, MDC, Berlin, Germany (32). The CD44 cDNA probe corresponds to positions 1 to 690 of rat cDNA (7). This region has 98% homology with the mouse sequence. Mouse brains were prepared, fixed for 16 h in 4% paraformaldehyde (PFA), and embedded in paraffin. In situ hybridization on 6-μm sections was performed with a digoxigenin-labeled RNA probe synthesized with a Dig RNA labeling kit (Roche, Mannheim, Germany). The probes were used at a concentration of 1 μg/ml. Hybridization conditions have been described previously (31). Hybridized RNA was detected with alkaline phosphatase-coupled digoxigenin antibodies according to the manufacturer's protocol (Roche).
Analysis of motoneurons in the facial nucleus and nucleus ambiguus.
We determined the number of motoneuron cell bodies in the facial nucleus and nucleus ambiguus of cd44−/−; met+/− double mutant and cd44+/−; met+/− control mice that were delivered by cesarean section at E19. Similar analyses were performed with 3-week-old mice applying established techniques (18). Animals were perfused transcardially with 4% PFA in 0.1 M phosphate-buffered saline at pH 7.4. The brain stem containing the facial nucleus and nucleus ambiguus was dissected, and 7-μm paraffin sections were prepared. After Nissl staining, motoneurons were counted in every fifth section, and the raw counts were corrected for split nuclei as has been described previously (13).
Phrenic nerves.
We analyzed phrenic nerve fibers in E19 and 3-week-old cd44−/−; met+/− mice as well as in cd44+/−; met+/− and cd44+/−; met+/+ control mice. Animals were perfused as described above. The phrenic nerves were then dissected, and in 3-week-old mice, the proximal and distal parts of the nerve were separately processed. The nerves were postfixed for 3 h in 0.1 M sodium cacodylate buffer containing 4% PFA and 2% glutaraldehyde and kept overnight in sodium cacodylate buffer containing only 4% PFA. After osmification and dehydration, all samples were embedded in Spurr's medium. From phrenic nerves of 3-week-old mice, semithin (1-μm) cross sections were cut with a diamond knife on an ultramicrotome. Sections were then stained with azur-methylene blue. The number of intact myelinated fibers was determined from photographs taken from nerve cross sections under a Zeiss light microscope equipped with a Zeiss HRC digital camera. For morphometric analysis of E19 phrenic nerve axons, we prepared ultrathin (∼80-nm) sections from the resin-embedded phrenic nerves, floated on Formvar-coated single-slot nickel grids, contrasted with uranyl acetate and lead citrate (23). Sections were then analyzed in a LEO 912 AB transmission electron microscope (Zeiss SMT, Oberkochen, Germany). High-resolution overview images of nerve cross sections were acquired using a slow-scan charge-coupled-device camera system and the multiple image alignment function of the Universal TEM Imaging Platform ITEM (Soft Imaging System, Münster, Germany). These images allow detailed qualitative and quantitative analyses of entire nerve cross sections. As a measure for axon size, the circumference of axons was determined and used to calculate axonal diameter.
Electrophysiological recordings in brain stem slices.
All electrophysiological analyses were performed in a blinded manner on brain stem neurons of mice. Slices containing the pre-Bötzinger complex (preBötC) from late embryonic littermate mice (E18 to E19) were used for whole-cell recordings (46). Briefly, the bath solution in all experiments consisted of (in mM) 118 NaCl, 3 KCl, 1.5 CaCl2, 1 MgCl2, 25 NaHCO3, 1 NaH2PO4, and 5 glucose at pH 7.4, which was aerated with 95% O2 and 5% CO2 and kept at 28°C. The pipette solution for patch-clamp recording contained (in mM) 140 K-gluconate (for glutamatergic postsynaptic current [PSC]) or 140 KCl (for GABAergic/glycinergic PSC), 1 CaCl2, 10 EGTA, 2 MgCl2, 4 Na3ATP, 0.5 Na3GTP, 10 HEPES, pH 7.3. Spontaneous GABAergic/glycinergic and glutamatergic PSCs were recorded from neurons of the preBötC in 10 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) or 1 μM strychnine and 1 μM bicuculline, respectively. Spontaneous miniature GABAergic/glycinergic and glutamatergic PSCs (miniature inhibitory PSCs [mIPSCs] and miniature excitatory PSCs [mEPSCs]) were recorded as described above, but in the presence of 0.5 μM tetrodotoxin. Generally, signals with amplitudes of at least two times above the background noise were selected, and the statistical significance was tested in each experiment. In all tested animals, there were no significant differences in the noise levels between different genotypes. The PSCs were amplified and filtered by a four-pole Bessel filter at a corner frequency of 2 kHz and digitalized at a sampling rate of 5 kHz using the DigiData 1200B interface (Axon Instruments). Data acquisition and analysis were done using commercially available software (pClamp 9 and AxoGraph 4.6 [Axon Instruments] and Prism 4 software [GraphPad]).
Statistical analysis.
Statistical data are expressed as means ± standard errors of the mean (SEM). The statistical significance of the differences between means was assessed with Student t tests (InStat; GraphPad Software Inc.). The level of significance was set at P of <0.05.
Statistical analysis of the motoneuron and axon counts was performed using a two-tailed test, and P values of ≤0.05 were considered significant.
Statistical analysis of size distribution of axonal profiles was performed using the Mann-Whitney U test.
RESULTS
cd44−/−; met+/− (or hgf+/− or gab1+/−) animals die at birth.
In order to test whether c-Met is haploinsufficient in the context of CD44 knockout mice, we crossed cd44+/−; met+/− mice that were healthy and showed no phenotype with cd44−/−; met+/+ mice (Table 1). cd44−/−; met+/− mice were born with the expected Mendelian ratio (36 out of 152 progenies). However, shortly after birth, 70% of these animals had breathing difficulties and died. Similar experiments were performed with hgf as well as gab1 heterozygote mice. In the case of hgf, 208 progenies were analyzed, and 60% of cd44−/−; hgf+/− mice died. In the case of the gab1 crossing (143 progenies), 69% of the cd44−/−; gab1+/− animals did not survive (Table 1). In contrast, all animals that were heterozygous for cd44 and for met, hgf, or gab1 survived and developed normally.
TABLE 1.
c-Met haploinsufficiency in the context of CD44
| Genotype and test parameter | No. of progeny | No. of cd44−/−; x+/− (%) | No. dead (%) | No. with lungs that sank (%) |
|---|---|---|---|---|
| Cross of cd44−/−; x+/+ and cd44+/−; x+/− | ||||
| Survival | ||||
| x = met | 152 | 36 (24) | 25 (70) | |
| x = hgf | 208 | 56 (27) | 33 (60) | |
| x = gab1 | 143 | 32 (22) | 22 (69) | |
| Lung assay | ||||
| x = met | 85 | 21 (25) | 16 (76) | |
| x = hgf | 97 | 23 (23) | 12 (52) | |
| x = gab1 | 84 | 21 (25) | 13 (62) | |
| Backcross of cd44−/−; x+/− and cd44−/−; x+/+ | ||||
| Survival, x = met | 55 | 13 (24) | 9 (69) | |
| Lung assay, x = met | 63 | 19 (30) | 8 (42) |
These results imply that the three molecules used in the crossings are involved in the same pathways. Their haploinsufficiency in the context of CD44-null mice shows that these molecules are connected to CD44 in wild-type animals.
A possible explanation for the survival of 30% of the cd44−/−; met+/− mice could be an upregulation of c-Met in the surviving animals. Therefore, we determined the amount of c-Met in liver lysates by Western blot analysis. Livers were isolated from animals at birth. From the same animals, the lungs were tested for floating on water (see below). Livers of the cd44−/−; met+/+ mice show higher amounts of c-Met compared to amounts for the cd44−/−; met+/− mice, irrespective of whether the lungs of cd44−/−; met+/− animals sank or floated on water (data not shown). Thus the survival of these animals appears not to be due to an upregulation of c-Met.
The cd44−/−; met+/− animals that survived developed in all respects normally and in particular were fertile. To evaluate whether these animals gained an unrelated genetic alteration that accounts for their survival, we crossed them with cd44−/−; met+/+ animals and monitored again the phenotypes of progenies. The majority of the cd44−/−; met+/− progenies also died at birth, similar to the first crosses (Table 1).
cd44−/−; met+/− animals die at birth from a lung defect.
To analyze phenotypic changes that might account for the haploinsufficiency, an overall histological analysis of whole embryos was performed. Serial sagittal sections through entire embryos were done, followed by hematoxylin and eosin staining. Two control animals (cd44+/−; met+/−) were compared to two cd44−/−; met+/− animals that showed breathing difficulty at birth. The gross anatomy of the most vital organs, such as heart, kidney, as well as the overall structure of the brain, did not show any obvious abnormalities that might explain the lethal phenotype. The lungs of the two cd44−/−; met+/− mice, however, showed multifocal atelectasis that was obviously caused by a primary asphyxia (Fig. 1A).
FIG. 1.
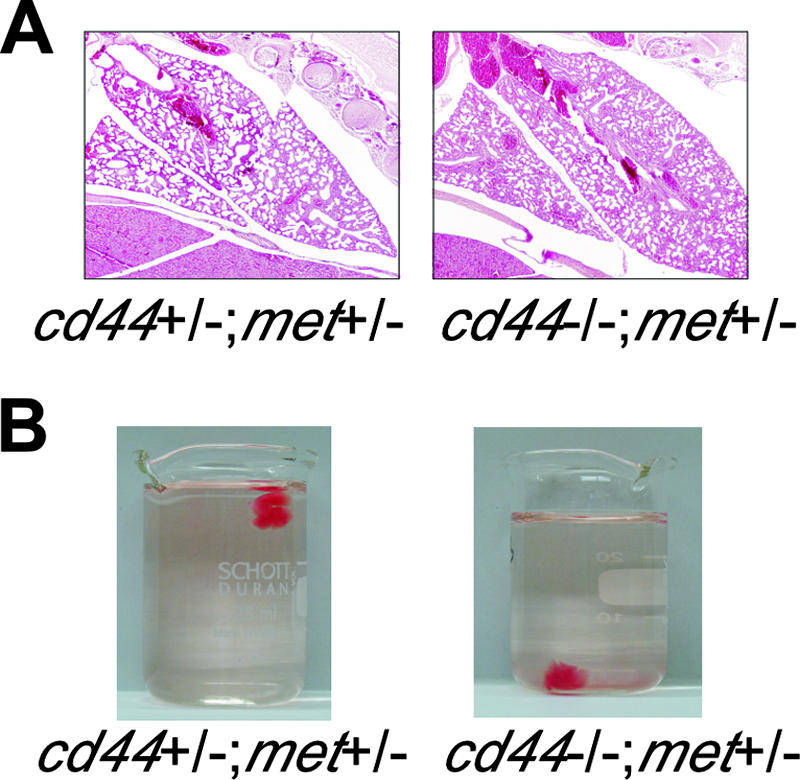
A lung defect in cd44−/−; met+/− mice. (A) Histology of lungs obtained shortly after birth from mice with the indicated genotypes. (B) Floating test for lungs on water.
We next performed a simple test to demonstrate whether the lungs were inflated. The lungs of newborn animals were removed and deposited in flasks containing water. We reasoned that the lungs from the cd44−/−; met+/− mice that eventually would die should sink. In 76% of the cd44−/−; met+/− animals, the lungs sank, indicating that they were not inflated (Table 1 and Fig. 1B). All the control lungs floated. The percentage of cd44−/−; met+/− animals affected in the floating assay corresponded well to the percentage of animals that died (Table 1).
Nerve fiber morphology in the phrenic nerve is changed in the cd44−/−; met+/− mice.
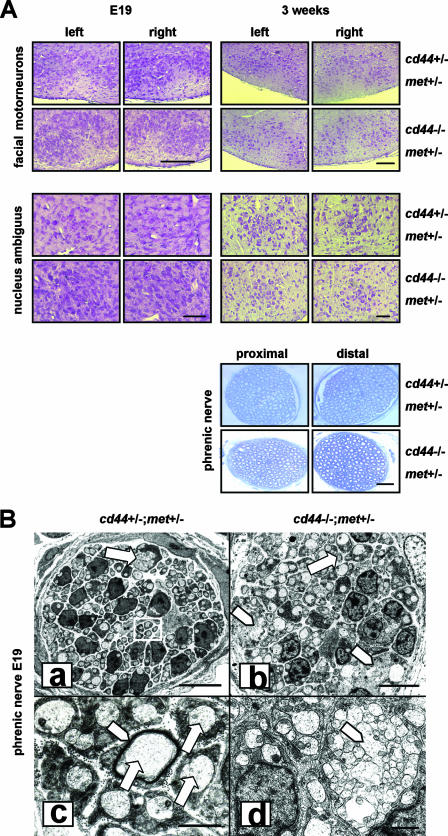
Explanations for the lethal asphyxia in cd44−/−; met+/− mice might be either pathological alterations of the peripheral organs along the airway, such as lung, diaphragm, etc., or neuronal impairments in the peripheral and/or central nervous system related to respiration. The histological analysis revealed that the general anatomy of the intercostal muscles and the muscle fibers of the diaphragm was normal (data not shown). We determined the number of motoneuron cell bodies in the facial nucleus and nucleus ambiguus in mice at E19. We examined four animals of each genotype. We could not find any difference in motoneuron numbers between cd44−/−; met+/− and control mice in the facial nucleus or in the nucleus ambiguus. Also, the morphology of motoneurons appeared similar (see example in Fig. 2A). Similar results were obtained with 3-week-old cd44−/−; met+/− mice (that survived) and cd44+/−; met+/− control mice.
FIG. 2.
Motoneurons in facial nucleus and nucleus ambiguus and nerve fibers in phrenic nerves. (A) Nissl-stained paraffin sections of motoneurons in the facial nucleus and nucleus ambiguus of the brain stem of E19 and 3-week-old mice with the indicated genotypes and semithincross sections of the phrenic nerves of 3-week-old mice. Scale bars for facial motoneurons equal 500 μm, for motoneurons of the nucleus ambiguous, 200 μm, and for phrenic nerves, 50 μm. (B) Digitally generated overview (a, b) and high-magnification images (c, d) of phrenic nerve cross sections of E19 mice. In phrenic nerves of cd44+/−; met+/− mice (a, c), a 1:1 ratio of Schwann cell to axon is very frequent, as indicated in the boxed area in panel a and by arrows in panel c. Occasionally, early myelination is found (white arrowhead in panel c). Bundles of axons that are in direct apposition and surrounded by a common Schwann cell sheath (arrow in panel a) are comparatively rare. In phrenic nerves of cd44−/−; met+/− mice, a 1:1 Schwann cell-to-axon ratio is found only occasionally (arrow in panel b), while bundles are more often seen (arrowheads in panels b and d). Scale bars for panels a and b, 5 μm; for c and d, 1 μm.
In the phrenic nerve, however, we detected differences. Electron microscopy of phrenic nerve cross sections of E19 control mice showed that most of the axons were completely encircled and were thus segregated from each other by Schwann cells (Fig. 2B, panels a and c). Individual Schwann cell profiles were surrounded by a basal lamina and collagen (Fig. 2B, panel c). In isolated Schwann cell profiles, 54% ± 4.5% ensheathed one axon (Fig. 2B, panels a and c), 40% ± 3.13% ensheathed 2 to 4 axons, and 5% ± 1.98% ensheathed 5 to 10 axons. Only 0.5% ± 1.8% of isolated Schwann cell profiles were found to ensheath bundles of axons in which more than 10 axons were in close apposition without intervening Schwann cell processes (Fig. 2B, panel a). Occasionally, we observed that myelination was just starting (Fig. 2B, panel c).
In contrast, in cross sections of phrenic nerves of cd44−/−; met+/− mice individual Schwann cell profiles appeared to ensheath, on average, more axons than in control mice (Fig. 2B, panel b). Significantly less Schwann cell profiles than in controls ensheathed only one axon (28.0% ± 6.0%; P ≤ 0.001), 43.0% ± 1.5% ensheathed 2 to 4 axons, and 24.0% ± 5.6% ensheathed 5 to 10 axons. The number of Schwann cells associated with bundles of axons without intervening Schwann cell processes was increased to 5.0% ± 0.6% (Fig. 2B, panels b and d). Moreover, axons that were ensheathed by a single Schwann cell profile showed comparatively large variations in size, while in controls they usually displayed similar diameters. The number of segregated axons (axons completely ensheathed by Schwann cell profiles) in phrenic nerves of cd44−/−; met+/− mice was significantly reduced by 20% (Table 2) (192 ± 15, n = 4, compared to 240 ± 12, n = 5, in cd44+/−; met+/− or cd44−/−; met+/+ mice). In addition the percentage of segregated axons with a diameter size between 0.75 to 1.00 μm was 14.96% ± 1.41% in cd44−/−; met+/− mice compared to 27.41% ± 4.36% in control mice (Table 2). In contrast, the percentage of small (<0.75-μm) and large (>1.00-μm) caliber axons was only slightly increased in cd44−/−; met+/− mice (Table 2).
TABLE 2.
Phrenic nerves at E19
| Mouse type | No. of segregated axons | Fiber distribution (%)
|
||
|---|---|---|---|---|
| 0.75-1 μm | <0.75 μm | >1 μm | ||
| cd44−/−; met+/+ | 240 ± 12 | 27.41 ± 4.63 | 53.72 ± 1.32 | 18.85 ± 7.8 |
| cd44−/−; met+/− | 192 ± 15 | 14.96 ± 1.41 | 60.73 ± 2.35 | 24.28 ± 2.23 |
In summary, these data suggest that the mechanisms for axonal segregation by Schwann cells, myelination, and axon size distribution are impaired in cd44−/−; met+/− mice. Interestingly, in cd44−/−; met+/− mice that survived we could not find any change in axon numbers or Schwann cell morphology in the proximal or distal phrenic nerves (Fig. 2A).
Severe impairment of overall network activity in preBötC neurons in cd44−/−; met+/− mice.
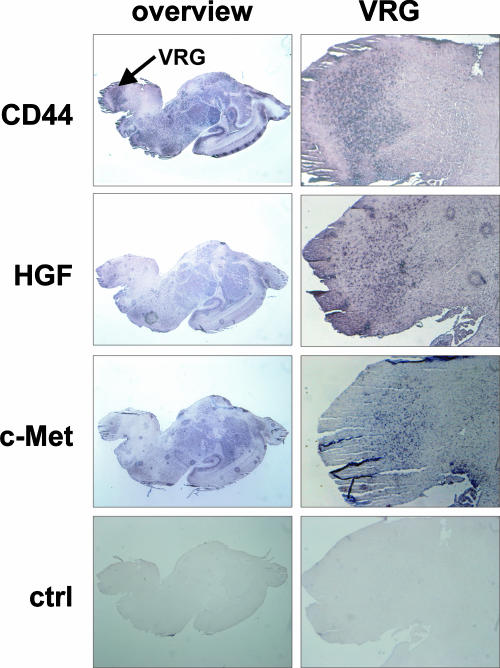
Another possibility that might cause a lethal phenotype is a failure in the central respiratory control system, as was shown previously (15, 38). We therefore performed in situ hybridization experiments to test the expression of the respective proteins in the central respiratory system. Indeed, our results revealed coexpression of CD44, c-Met, and HGF in the ventral respiratory group within the brain stem (Fig. 3), suggesting that these molecules might cooperate. Thus it is possible that alterations in these tissues in the cd44−/−; met+/− mice could have an impact on the central respiratory system.
FIG. 3.
In situ hybridization of brain tissue. In situ hybridization of sagittal brain sections of mouse pups at birth with CD44, HGF, and c-Met probes. VRG indicates the ventral respiratory group. An overview of the whole brain section is shown in the left panels, the area of the VRG is shown in higher magnification in the right panels. Hybridization with sense CD44 mRNA is shown in the lower panels (ctrl). Hybridization with sense HGF and c-Met mRNA gave similar results.
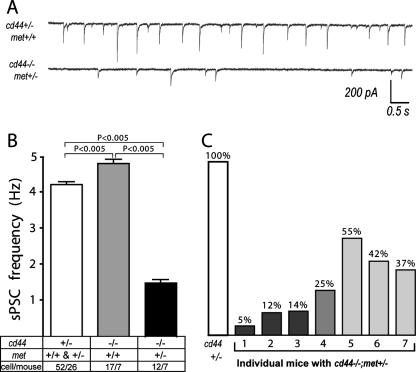
We therefore started a series of electrophysiological experiments to find possible failures in the central respiratory control system that might cause the lethal phenotype in cd44−/−; met+/− mice. We first monitored the overall spontaneous postsynaptic currents (sPSCs) using whole-cell recordings in preBötC neurons. These currents represent the general activity level of the respiratory network. The activity in preBötC neurons was diminished in cd44−/−; met+/− mice compared to that in the control littermates (Fig. 4A and B). It is interesting to note that the activity levels in the preBötCs of individual cd44−/−; met+/− mice were heterogenous. Four of seven mice showed an activity level that was lower than 25% of the control level (mice no. 1 to 4 in Fig. 4C). In three of seven mice the activity levels ranged between 37% to 55% of the level in control mice (mice no. 5 to 7 in Fig. 4C).
FIG. 4.
Severe impairment of overall network activity in preBötC neurons in cd44−/−; met+/− mice. (A) Representative recordings of sPSCs in brain stem preBötC neurons. (B) Average frequency of sPSCs. Data are expressed as means ± SEM. Statistical significance of differences between means was assessed with Student t tests (InStat; GraphPad Software Inc.). The level of significance was set at P of <0.05. The numbers indicate the number of neurons/mouse. (C) The sPSC average frequency of cd44+/− mice in panel B was set to 100% and was compared to the sPSC frequency of individual cd44−/−; met+/− mice.
Severe impairment of synaptic transmission in preBötC neurons in cd44−/−; met+/− mice.
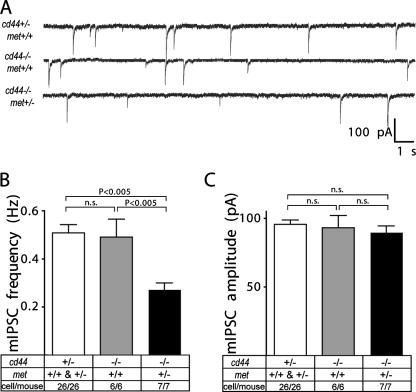
To further characterize the failure in the respiratory network, we next analyzed the inhibitory and excitatory PSCs in the cd44−/−; met+/− mice in comparison with their littermates. The frequency of glycinergic and GABAergic miniature mIPSCs in preBötC neurons was significantly decreased in cd44−/−; met+/− mice (Fig. 5A and B). In contrast, the amplitudes of mIPSCs were not changed (Fig. 5C).
FIG. 5.
Severe impairment of inhibitory synaptic transmission in preBötC neurons in cd44−/−; met+/− mice. (A) Representative recordings of miniature glycinergic and GABAergic IPSCs (mIPSCs) in brain stem preBötC neurons. (B and C) Average frequency (B) and amplitude (C) of mIPSCs. Data are expressed as means ± SEM. Statistical significance of differences between means was assessed with Student t tests. The level of significance was set at P of <0.05; n.s., not significant. The numbers indicate the number of neurons/mouse.
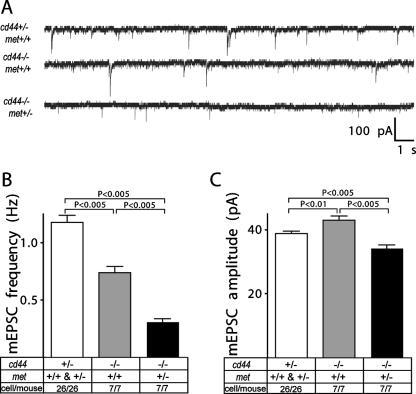
The mEPSCs were measured using whole-cell recordings in preBötC neurons. It is striking that the frequency of glutamatergic mEPSCs in preBötC neurons was already decreased in cd44−/−; met+/+ mice, while they were even further decreased in cd44−/−; met+/− mice (Fig. 6A and B). The amplitude of mEPSCs was only moderately changed between littermates with different genotypes (Fig. 6C). Thus, these data demonstrated that homozygous null mutation of CD44 showed a significant effect on excitatory transmission that became even more severe by additional heterozygous mutation of c-Met.
FIG. 6.
Severe impairment of excitatory synaptic transmission in preBötC neurons in cd44−/− mice. (A) Representative recordings of miniature glutamatergic EPSCs (mEPSCs) in brain stem preBötC neurons. (B and C) Average frequency (B) and amplitude (C) of miniature glutamatergic EPSCs. Data are expressed as means ± SEM. Statistical significance of differences between means was assessed with Student t tests. The level of significance was set at P of <0.05. The numbers indicate the number of neurons/mouse.
In conclusion, these experiments show that the haploinsufficiency of c-Met in the CD44-null background is based on a defect in synaptic transmission in the preBötC, suggesting that in the CD44-expressing animals c-Met and CD44 cooperate in synaptogenesis.
DISCUSSION
Previous data have demonstrated that CD44v6 acts as a coreceptor for c-Met in transformed as well as primary cells (14, 19, 20). An important question remained as to whether CD44 is essential for c-Met function in vivo. The data presented here, that the majority of met+/− mice in a CD44-null background did not survive in contrast to met+/+ or met+/− mice in a CD44-positive background, are the first genetic evidence that the coreceptor CD44 is essential for the function of c-Met during embryogenesis in vivo.
A further conclusion drawn from our experiments is that the CD44 function for c-Met is substituted by another protein in the CD44 knockout mice and that this protein does not function as efficiently as CD44. This idea that functions of CD44 might be substituted in the CD44 knockout mouse is well documented. Ablation of CD44 expression in the skin shows a drastic phenotype that is not at all observed in total CD44 knockout mice (10). From this observation two conclusions can be drawn, (i) CD44 functions are substituted in CD44 total knockout animals and (ii) a substitution cannot occur at later times in embryogenesis, namely at day 10 when the expression of CD44 in the skin is turned off (10). In addition Rhamm, a receptor for hyaluronic acid, has been shown to take over a function of CD44 in the CD44 knockout mice. In the absence of CD44, Rhamm is able to react with hyaluronan and triggers an enhanced inflammatory response to collagen-induced arthritis (16). Furthermore, we have recently identified ICAM-1 as a protein that takes over the function of CD44 as a coreceptor for c-Met in human hepatoma cells and in the liver of CD44-null mice (our unpublished data).
That the decreased concentration of collaborating molecules might lead to haploinsufficiency was already successfully demonstrated in the case of Gab1 knockout mice (24). In gab1−/− embryos, the migration of muscle precursor cells into the limbs and the diaphragm is strongly reduced but not completely blocked. This is in contrast to the hgf−/− and met−/− mice and also to the gab1−/−; met+/− mice where the phenotype is much more drastic. In the context of the Gab1 knockout mice, c-Met is haploinsufficient because it cannot transmit some crucial signals (24). Another example of haploinsufficiency in signaling pathways is the rescue of embryonic lethality of pten+/− mice by grb2 heterozygosity (4).
In general, there might be many different causes for the lethal phenotype of the met+/− mice in the CD44-null background. One reason might be failures in the organogenesis of one or several peripheral organs. The gross anatomy of the most vital organs, such as heart, lung, kidney, as well as the overall structure of the brain, did not show any obvious abnormalities that might explain the lethal phenotype.
Although the gross anatomy of the lung seems to be intact, the lungs of the lethal met+/− mice in the CD44-null background were never inflated. As all organs and tissues along the airways were intact, there are no physical or aerodynamic reasons why respiratory movement and gas exchanges never take place in these animals. This suggests that the lethal phenotype was caused by failures in neuronal activity.
The respiratory rhythm is generated by a brain stem respiratory network. Among other parts of the brain, the preBötC in the brain stem is an essential component of the central respiratory rhythm-generating network. The presented data show that the overall network activity of the preBötC is strongly compromised. The reduction of network activity varies between different cd44−/−; met+/− animals from 45 to 95% of the level in control animals. Previous studies of neurexin and neuroligin triple knockout mice (15, 38) demonstrated that a reduction of network activity of >45% was necessary to cause visible irregularities in the resting ventilation activity, and only a reduction of >75% caused a life-threatening failure of ventilation. Thus, the different level in the reduction of overall network activity could explain the partial penetrance of the defect in the cd44−/−; met+/− mice.
The detailed analysis of the defect in the preBötC showed that met heterozygotes in the CD44-null background have a decrease of both synaptic excitation and inhibition. Interestingly, the glutamatergic synaptic excitation in the CD44 knockout mice is already severely decreased but no change is observed in glycinergic and GABAergic synaptic inhibition nor in the overall synaptic activity. Within the respiratory network, the synaptic inhibition is essential to generate a stable and regular respiratory rhythm, and therefore the overall network activity is mainly influenced by synaptic inhibition. Thus it seems that the impairment of the synaptic inhibition is the main cause of the decrease of the overall synaptic activity in the cd44−/−; met+/− animals.
It is noteworthy that although there is a strong decrease in the frequency of the miniature excitatory and inhibitory transmission in cd44−/−; met+/− animals, their amplitude was only moderately compromised. This suggests that both the excitatory and inhibitory synaptic reductions are most likely of presynaptic nature. As the overall structure within the brain stem network was not dramatically changed, as judged from the histology of the region, these data would suggest either a reduced number of neurons and/or synapses in the brain stem preBötC region.
In addition to the disturbances in the preBötC, in cd44−/−; met+/− mice a significant reduction in the number of axons that were segregated for myelination and a change in their size distribution were observed in the phrenic nerve that innervates the diaphragm. Axonal signals are thought to be of crucial importance for early stages in myelination (for an example, see reference 28). A function of HGF/c-Met in the development of motoneurons has been described at stages corresponding to the period of naturally occurring cell death (between E13 and E20) (42), where they might act as motoneuronal survival factors (6). HGF also promotes neural induction (33) and axon outgrowth (43). Interestingly, HGF acts preferentially on a subset of limb-innervating motoneurons within the cervical spinal cord (42), a population of motoneurons that extends long axons. In addition, HGF has been shown to act as a chemoattractant that guides developing axons to their target (6). A reason for the change in axon morphology and for the delay in reaching the 1:1 axon/Schwann cell ratio required for myelination (3) in the phrenic nerve might therefore be that the capacity of motoneurons to sustain outgrowth of functionally adequate axons is impaired. Indeed, the phrenic nucleus is a column of motoneurons extending from the third to the sixth cervical cord segment that also makes long axons and therefore might be preferentially affected. Also, the migration defect of muscle precursor cells in c-Met- or HGF-null mice (1, 26, 37) seems to be due to the loss of a function of HGF as a chemoattractant since ectopic expression of HGF in transgenic animals leads to ectopic muscle formation (35). However, the morphology of the diaphragm and of the intercostal muscles was normal, indicating that a defect of muscle cell migration does not contribute to the haploinsufficiency in the cd44−/−; met+/− mice.
Alternatively or additionally, changes in myelination may be caused by impaired differentiation of Schwann cells. HGF can stimulate Schwann cell mitosis via c-Met (11). However, the function of HGF or c-Met for Schwann cell differentiation has never been investigated in knockout mice because HGF and c-Met knockout mice die early during development, before Schwann cells start to differentiate. The effects of the cd44 deletion in met heterozygote mice provide the first genetic evidence that HGF/c-Met signaling plays a physiologically relevant role for development of functional axons that innervate the diaphragm and possibly also other muscles that contribute to respiration.
Taken together, our data suggest that the presence of the coreceptor CD44 is essential for the function of c-Met in the synaptogenesis and axon/nerve fiber development in the peripheral and central nervous systems.
Although the haploinsufficiency of c-Met in the CD44-null background undoubtedly shows that in wild-type mice c-Met and CD44 cooperate, it does not allow any conclusion as to whether this cooperation is direct or indirect and what the underlying mechanism is. From our previous studies using cell lines, we suggest that during embryogenesis specific isoforms of CD44 containing exon v6 also act as coreceptors for c-Met. Indeed CD44 variant expression has been shown on axons and neurons in the central nervous system (9) and HGF acts via c-Met on central nervous system neurons (8, 41). The suggestion that CD44 acts as a coreceptor for c-Met during embryogenesis is further strengthened by our findings that in carcinoma cells lacking CD44v6, ICAM-1 fulfills the coreceptor function for c-Met, and that in CD44-null mice this new coreceptor for c-Met substitutes for the CD44 function in liver (our unpublished results).
Acknowledgments
We are thankful to Walter Birchmeier, MDC Berlin-Buch, for fruitful suggestions and discussions and to Selma Huber and the members of the animal facility for their help in animal experiments.
This work was supported by grants of the Deutsche Krebshilfe (Förderschwerpunktprogramm: Zelladhäsion, Invasion und Metastasierung), the Deutsche Forschungsgemeinschaft (SPP 1190), the Deutsche Forschungsgemeinschaft through the DFG-Research Center for Molecular Physiology of the Brain (W.Z.), and SFB 487 (M.S.) and SFB 581 (E.A., B.H., and M.S.).
Footnotes
Published ahead of print on 8 October 2007.
REFERENCES
- 1.Bladt, F., D. Riethmacher, S. Isenmann, A. Aguzzi, and C. Birchmeier. 1995. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature 376:768-771. [DOI] [PubMed] [Google Scholar]
- 2.Cavallaro, U., J. Niedermeyer, M. Fuxa, and G. Christofori. 2001. N-CAM modulates tumour-cell adhesion to matrix by inducing FGF-receptor signalling. Nat. Cell Biol. 3:650-657. [DOI] [PubMed] [Google Scholar]
- 3.Court, F. A., L. Wrabetz, and M. L. Feltri. 2006. Basal lamina: Schwann cells wrap to the rhythm of space-time. Curr. Opin. Neurobiol. 16:501-507. [DOI] [PubMed] [Google Scholar]
- 4.Cully, M., A. Elia, S. H. Ong, V. Stambolic, T. Pawson, M. S. Tsao, and T. W. Mak. 2004. grb2 heterozygosity rescues embryonic lethality but not tumorigenesis in pten+/− mice. Proc. Natl. Acad. Sci. USA 101:15358-15363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dejana, E., M. G. Lampugnani, O. Martinez-Estrada, and G. Bazzoni. 2000. The molecular organization of endothelial junctions and their functional role in vascular morphogenesis and permeability. Int. J. Dev. Biol. 44:743-748. [PubMed] [Google Scholar]
- 6.Ebens, A., K. Brose, E. D. Leonardo, M. G. Hanson, Jr., F. Bladt, C. Birchmeier, B. A. Barres, and M. Tessier-Lavigne. 1996. Hepatocyte growth factor/scatter factor is an axonal chemoattractant and a neurotrophic factor for spinal motor neurons. Neuron 17:1157-1172. [DOI] [PubMed] [Google Scholar]
- 7.Günthert, U., M. Hofmann, W. Rudy, S. Reber, M. Zöller, I. Hauβmann, S. Matzku, A. Wenzel, H. Ponta, and P. Herrlich. 1991. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell 65:13-24. [DOI] [PubMed] [Google Scholar]
- 8.Hamanoue, M., N. Takemoto, K. Matsumoto, T. Nakamura, K. Nakajima, and S. Kohsaka. 1996. Neurotrophic effect of hepatocyte growth factor on central nervous system neurons in vitro. J. Neurosci. Res. 43:554-564. [DOI] [PubMed] [Google Scholar]
- 9.Kaaijk, P., S. T. Pals, F. Morsink, D. A. Bosch, and D. Troost. 1997. Differential expression of CD44 splice variants in the normal human central nervous system. J. Neuroimmunol. 73:70-76. [DOI] [PubMed] [Google Scholar]
- 10.Kaya, G., I. Rodriguez, J. L. Jorcano, P. Vassalli, and I. Stamenkovic. 1997. Selective suppression of CD44 in keratinocytes of mice bearing an antisense CD44 transgene driven by a tissue-specific promoter disrupts hyaluronate metabolism in the skin and impairs keratinocyte proliferation. Genes Dev. 11:996-1007. [DOI] [PubMed] [Google Scholar]
- 11.Krasnoselsky, A., M. J. Massay, M. C. DeFrances, G. Michalopoulos, R. Zarnegar, and N. Ratner. 1994. Hepatocyte growth factor is a mitogen for Schwann cells and is present in neurofibromas. J. Neurosci. 14:7284-7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu, Y., and L. R. Rohrschneider. 2002. The gift of Gab. FEBS Lett. 515:1-7. [DOI] [PubMed] [Google Scholar]
- 13.Masu, Y., E. Wolf, B. Holtmann, M. Sendtner, G. Brem, and H. Thoenen. 1993. Disruption of the CNTF gene results in motor neuron degeneration. Nature 365:27-32. [DOI] [PubMed] [Google Scholar]
- 14.Matzke, A., P. Herrlich, H. Ponta, and V. Orian-Rousseau. 2005. A five-amino-acid peptide blocks Met- and Ron-dependent cell migration. Cancer Res. 65:6105-6110. [DOI] [PubMed] [Google Scholar]
- 15.Missler, M., W. Zhang, A. Rohlmann, G. Kattenstroth, R. E. Hammer, K. Gottmann, and T. C. Sudhof. 2003. Alpha-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature 423:939-948. [DOI] [PubMed] [Google Scholar]
- 16.Nedvetzki, S., M. Walmsley, E. Alpert, R. O. Williams, M. Feldmann, and D. Naor. 1999. CD44 involvement in experimental collagen-induced arthritis (CIA). J. Autoimmun. 13:39-47. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen, L., M. Holgado-Madruga, C. Maroun, E. D. Fixman, D. Kamikura, T. Fournier, A. Charest, M. L. Tremblay, A. J. Wong, and M. Park. 1997. Association of the multisubstrate docking protein Gab1 with the hepatocyte growth factor receptor requires a functional Grb2 binding site involving tyrosine 1356. J. Biol. Chem. 272:20811-20819. [DOI] [PubMed] [Google Scholar]
- 18.Oppenheim, R. W., S. Wiese, D. Prevette, M. Armanini, S. Wang, L. J. Houenou, B. Holtmann, R. Gotz, D. Pennica, and M. Sendtner. 2001. Cardiotrophin-1, a muscle-derived cytokine, is required for the survival of subpopulations of developing motoneurons. J. Neurosci. 21:1283-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orian-Rousseau, V., L. Chen, J. P. Sleeman, P. Herrlich, and H. Ponta. 2002. CD44 is required for two consecutive steps in HGF/c-Met signaling. Genes Dev. 16:3074-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orian-Rousseau, V., H. Morrison, A. Matzke, T. Kastilan, G. Pace, P. Herrlich, and H. Ponta. 2007. Hepatocyte growth factor-induced Ras activation requires ERM proteins linked to both CD44v6 and F-actin. Mol. Biol. Cell 18:76-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paratcha, G., F. Ledda, and C. F. Ibanez. 2003. The neural cell adhesion molecule NCAM is an alternative signaling receptor for GDNF family ligands. Cell 113:867-879. [DOI] [PubMed] [Google Scholar]
- 22.Protin, U., T. Schweighoffer, W. Jochum, and F. Hilberg. 1999. CD44-deficient mice develop normally with changes in subpopulations and recirculation of lymphocyte subsets. J. Immunol. 163:4917-4923. [PubMed] [Google Scholar]
- 23.Reynolds, E. S. 1963. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 17:208-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sachs, M., H. Brohmann, D. Zechner, T. Muller, J. Hulsken, I. Walther, U. Schaeper, C. Birchmeier, and W. Birchmeier. 2000. Essential role of Gab1 for signaling by the c-Met receptor in vivo. J. Cell Biol. 150:1375-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlessinger, J., and A. Ullrich. 1992. Growth factor signaling by receptor tyrosine kinases. Neuron 9:383-391. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt, C., F. Bladt, S. Goedecke, V. Brinkmann, W. Zschiesche, M. Sharpe, E. Gherardi, and C. Birchmeier. 1995. Scatter factor/hepatocyte growth factor is essential for liver development. Nature 373:699-702. [DOI] [PubMed] [Google Scholar]
- 27.Schmits, R., J. Filmus, N. Gerwin, G. Senaldi, F. Kiefer, T. Kundig, A. Wakeham, A. Shahinian, C. Catzavelos, J. Rak, C. Furlonger, A. Zakarian, J. J. Simard, P. S. Ohashi, C. J. Paige, J. C. Gutierrez-Ramos, and T. W. Mak. 1997. CD44 regulates hematopoietic progenitor distribution, granuloma formation, and tumorigenicity. Blood 90:2217-2233. [PubMed] [Google Scholar]
- 28.Sherman, D. L., and P. J. Brophy. 2005. Mechanisms of axon ensheathment and myelin growth. Nat. Rev. Neurosci. 6:683-690. [DOI] [PubMed] [Google Scholar]
- 29.Sherman, L., D. Wainwright, H. Ponta, and P. Herrlich. 1998. A splice variant of CD44 expressed in the apical ectodermal ridge presents fibroblast growth factors to limb mesenchyme and is required for limb outgrowth. Genes Dev. 12:1058-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sherman, L. S., T. A. Rizvi, S. Karyala, and N. Ratner. 2000. CD44 enhances neuregulin signaling by Schwann cells. J. Cell Biol. 150:1071-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sonnenberg, E., A. Godecke, B. Walter, F. Bladt, and C. Birchmeier. 1991. Transient and locally restricted expression of the ros1 protooncogene during mouse development. EMBO J. 10:3693-3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sonnenberg, E., D. Meyer, K. M. Weidner, and C. Birchmeier. 1993. Scatter factor/hepatocyte growth factor and its receptor, the c-met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. J. Cell Biol. 123:223-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Streit, A., C. D. Stern, C. Thery, G. W. Ireland, S. Aparicio, M. J. Sharpe, and E. Gherardi. 1995. A role for HGF/SF in neural induction and its expression in Hensen's node during gastrulation. Development 121:813-824. [DOI] [PubMed] [Google Scholar]
- 34.Suyama, K., I. Shapiro, M. Guttman, and R. B. Hazan. 2002. A signaling pathway leading to metastasis is controlled by N-cadherin and the FGF receptor. Cancer Cell 2:301-314. [DOI] [PubMed] [Google Scholar]
- 35.Takayama, H., W. J. La Rochelle, M. Anver, D. E. Bockman, and G. Merlino. 1996. Scatter factor/hepatocyte growth factor as a regulator of skeletal muscle and neural crest development. Proc. Natl. Acad. Sci. USA 93:5866-5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trusolino, L., A. Bertotti, and P. M. Comoglio. 2001. A signaling adapter function for alpha6beta4 integrin in the control of HGF-dependent invasive growth. Cell 107:643-654. [DOI] [PubMed] [Google Scholar]
- 37.Uehara, Y., O. Minowa, C. Mori, K. Shiota, J. Kuno, T. Noda, and N. Kitamura. 1995. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature 373:702-705. [DOI] [PubMed] [Google Scholar]
- 38.Varoqueaux, F., G. Aramuni, R. L. Rawson, R. Mohrmann, M. Missler, K. Gottmann, W. Zhang, T. C. Sudhof, and N. Brose. 2006. Neuroligins determine synapse maturation and function. Neuron 51:741-754. [DOI] [PubMed] [Google Scholar]
- 39.Vlodavsky, I., H. Q. Miao, B. Medalion, P. Danagher, and D. Ron. 1996. Involvement of heparan sulfate and related molecules in sequestration and growth promoting activity of fibroblast growth factor. Cancer Metastasis Rev. 15:177-186. [DOI] [PubMed] [Google Scholar]
- 40.Weidner, K. M., S. Di Cesare, M. Sachs, V. Brinkmann, J. Behrens, and W. Birchmeier. 1996. Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature 384:173-176. [DOI] [PubMed] [Google Scholar]
- 41.Wong, V., D. J. Glass, R. Arriaga, G. D. Yancopoulos, R. M. Lindsay, and G. Conn. 1997. Hepatocyte growth factor promotes motor neuron survival and synergizes with ciliary neurotrophic factor. J. Biol. Chem. 272:5187-5191. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto, Y., J. Livet, R. A. Pollock, A. Garces, V. Arce, O. deLapeyriere, and C. E. Henderson. 1997. Hepatocyte growth factor (HGF/SF) is a muscle-derived survival factor for a subpopulation of embryonic motoneurons. Development 124:2903-2913. [DOI] [PubMed] [Google Scholar]
- 43.Yang, X. M., and M. Park. 1993. Expression of the met/hepatocyte growth factor/scatter factor receptor and its ligand during differentiation of murine P19 embryonal carcinoma cells. Dev. Biol. 157:308-320. [DOI] [PubMed] [Google Scholar]
- 44.Yayon, A., M. Klagsbrun, J. D. Esko, P. Leder, and D. M. Ornitz. 1991. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell 64:841-848. [DOI] [PubMed] [Google Scholar]
- 45.Yu, W. H., J. F. Woessner, Jr., J. D. McNeish, and I. Stamenkovic. 2002. CD44 anchors the assembly of matrilysin/MMP-7 with heparin-binding epidermal growth factor precursor and ErbB4 and regulates female reproductive organ remodeling. Genes Dev. 16:307-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang, W., A. Rohlmann, V. Sargsyan, G. Aramuni, R. E. Hammer, T. C. Sudhof, and M. Missler. 2005. Extracellular domains of alpha-neurexins participate in regulating synaptic transmission by selectively affecting N- and P/Q-type Ca2+ channels. J. Neurosci. 25:4330-4342. [DOI] [PMC free article] [PubMed] [Google Scholar]