Abstract
Older adults who are at risk of developing influenza illness, have a low level of influenza virus-stimulated cytotoxic T lymphocyte (CTL) activity as measured by an assay of granzyme B (GrB). The purpose of this study was to determine whether aging affected memory CTL populations identified by GrB expression in influenza virus-stimulated peripheral blood mononuclear cells (PBMC). The expression and activity of GrB increased with virus stimulation over five days of culture. Virus-specific CD8 effector T cells with the phenotype, GrB+CD62Lhigh CD8 TCM, were found to be the source of the early CTL response to influenza virus. Comparing the CD8 T cell response in 5-day PBMC cultures of 161 adult subjects, the response of GrB+CD62Lhigh CD8 TCM lymphocytes in older individuals was significantly lower than in younger adults after viral stimulation (p<0.001). The increase in the proportion of CD28nullCD8 T cells in fresh PBMC negatively correlated with the proportion GrB+CD62Lhigh CD8 TCM lymphocytes in virus-stimulated PBMC. Thus, the increase in CD28nullCD8 T cells with age may contribute to the limited CTL response to influenza vaccination and diminished protection in older adults.
Keywords: T cell, Influenza, Granzyme B, Cytotoxicity
1. Introduction
The diminished protection offered by influenza vaccination in older adults is well-recognized (Glezen et al., 2000; Thompson et al., 2004). The reduced protection is believed to be due to poor stimulation of cellular immunity (Powers et al., 1993; McElhaney et al., 2006) and risk for influenza illness in vaccinated older adults is associated with low levels of granzyme (GrB), a key cytolytic mediator of the CTL response to influenza (McElhaney et al., 2001; 2006; Schmidt et al., 2004). This current study focused on age-related changes in the virus-specific memory CTL (CD8+) response to influenza that may explain the increased risk of influenza illness in older adults.
Antigen-specific memory T cells have the capacity to mediate, accelerate and provide a vigorous response to secondary viral challenge. Memory T cells can be resolved into two major subsets based on their expression of lymph node homing receptors (CD62L and CCR7), referred to as central memory (CD62LhighCCR7+) (TCM) and effector (CD62LlowCCR7−) memory (TEM) T cells (Sallusto et al., 1999; Lefrancois et al., 2002). TCM cells are predominantly found in lymphoid tissues, whereas TEM cells are found in both lymphoid and peripheral tissues. Analysis of the response to systemic lymphocytic choriomeningitis virus (LCMV) infection in the mouse showed that CD8 TCM cells respond more vigorously to secondary challenge than TEM cells (Wherry et al., 2003). The results of Sendai virus infection in mouse lung showed that TEM cells generated a recall response that was at least as strong as that mediated by TCM cells (Roberts et al., 2004). The relationship between TCM and TEM is a recent hotly debated topic. Early studies indicated that the TCM precursors had the capacity to become fully functional effector TEM cells after secondary challenge (Wherry et al., 2003; Roberts et al., 2005) Some studies suggested that a TEM →T CM transition was possible, both during the acute response and in long-term memory (Bouneaud et al., 2005; Roberts et al., 2005). Recently, the diverse T cell receptor profiles detected with influenza tetramers suggested that the CD62Lhigh TCM cells constitute a relatively stable pool that is not maintained by conversion from the CD62Llow population (Kedzierska et al., 2006). Others suggest that the TEM and TCM subsets segregate immediately into different lineages from the time of primary antigen challenge, but the conversion between CD62Lhigh and CD62Llow T cells was never directly demonstrated (Marzo et al., 2005). CD62L expression alone is not sufficient to define distinct CTL functional subpopulations of memory CD8 T cell subsets (Jackson et al., 2005). If the TCM and TEM are distinct cell lineages, their functions, especially for TCM, remains undetermined. These studies were based on mouse models and there was very little information about how these memory T cell subsets contribute to the response to influenza virus in people (Schwaiger et al., 2003).
The proliferation and differentiation of effector T cells requires effective CTL activation and the co-stimulatory molecule, CD28. CD28 signal transduction serves primarily as an amplifier of the TCR signal. Recent studies showed that CD28null T cells accumulate with advancing age and loss of CD28 occurs more rapidly among CD8 T cells relative to CD4 T cells (Effros et al., 2005). Low antibody response to influenza vaccination in older adults is correlated with high frequencies of CD28null T cells (Goronzy et al., 2001; Saurwein-Teissl et al., 2002). The lack of CD28 potentially diminishes T cell responses to influenza vaccination and suggests that CD28 plays a critical role in the subsequent response to infection (Lumsden et al., 2000).
Current cytolytic assays do not distinguish effector memory CTL from other memory cells. Previous results have shown that the level of GrB is correlated with cytotoxicity in 51Cr-release assays of virus-stimulated human PBMC and is a very sensitive measure of functional CTL (McElhaney et al., 1996; Ewen et al., 2003). Therefore, GrB may be an accurate marker of the effector response to influenza virus challenge in CD8 T cells using flow cytometric methods (Rong et al., 2004).
This paper provides evidence that human CD8 TCM lymphocytes are the source of influenza-specific CD8 effector cells identified by the expression of GrB mRNA, the related enzymatic activity, and its association with degranulation of CD8 T cells in response to influenza virus. Further, it was postulated that, there is an age-related decline in the influenza-specific memory T cell response to influenza. Human PBMC stimulated in vitro with live influenza virus was used as the model to characterize the interaction between CD8 T cells (CTL) and influenza virus, and age-related changes in this interaction. An age-related difference in the CTL response to influenza virus and the effect of influenza vaccination on CD8 T cell subsets, particularly in GrB+CD62Lhigh CD8 TCM cells, has been identified. Further, TCM are stimulated at an early stage of influenza virus stimulation. GrB+CD62Lhigh CD8 T lymphocytes appear within 20 hours of virus stimulation and contribute to the expansion of the effector CD8 T cell subset in PBMC cultures. In older adults, the increase in the proportion of CD28null CD8 T cells may limit the CTL response to influenza and influenza vaccination.
2. Materials and Methods
2.1. Human subjects and procedures
Between June 2005 and November 2006, 29 younger adults (mean age, 29 years older; range, 22–40 years older) and 130 older adults (mean age, 74 years older; range, 60–94 years older) were recruited for this study through written, informed consent. The Institutional Review Board of the University Connecticut Health Center approved the protocol and informed consent document. All subjects had received the 2004–05 influenza vaccine in the previous year. In a subset of the study cohort, 20 younger (mean age, 31 years older; range, 22–40 years older) and 144 older (mean age, 75 years older; range, 60–94 years older) adults were vaccinated with the 2005–06 licensed influenza vaccine (Aventis Pasture Inc.) containing 15 μg each of A/California/7/2004 (H3N2)-like, A/New Caledonia/20/99 (H1N1)-like, and B/Shanghai/361/2002-like antigens. Younger adults defined as age 20–40 years older and without any underlying chronic conditions, were enrolled in the study as controls for the ‘normal’ response to influenza vaccination. Older adult subjects were defined as age 60 years and older and characterized according to underlying medical diagnoses, medications and performance on the Six-Minute Walk Test (SMWT) (Bittner et al., 1993). Venous blood samples were collected in the summer of 2005, or just prior to and four weeks following vaccination in the fall of 2005. Volunteers who refused vaccination or had not been vaccinated in the previous year, had an egg allergy, had a previous severe reaction to the vaccine, or had an acute illness in the 2-week period before vaccination were excluded.
2.2. Preparation of human peripheral blood mononuclear cells (PBMC) and stimulation with virus
PMBC were prepared from heparinized whole blood (35cc) by Histopaque-1077 (Sigma-Aldrich) gradient purification, treated with RBC Lysis Solution (Bio-Rad) to remove red blood cells, washed, resuspended in AIM-V media (Gibco Laboratories, Grand Island, NY), and diluted to a concentration of 2 ×106/ml. One ml of suspended cells was added into each well of a 48-well multiplate (Nalge Nunc), stimulated with influenza virus A/Wyoming/03/2003 [A/H3N2] (or other influenza strains as noted) for 20 hours or 5 days using a multiplicity of infection (MOI) = 2 (4 ×106/ml TCID50/ml) and incubated in a humidified atmosphere at 37°C in 5% CO2.
2.3. RNA preparation and RT-PCR
The level of expression l of GrB and Perf (perforin) mRNA was compared in fresh and virus-stimulated human PBMC using GAPDH as a positive control. Human GrB primers were 5′-AAGACGACTTCGTGCT-3′ and 5′-CAGATTCGCACTTTCGATC-3′. Human Perf primers were 5′-ACGCAAATTCGCAAACT-3′ and 5′-GGATTAGCGTGTAAACCC-3′. Primers to the human housekeeping gene, GAPDH, were 5′-GTCGGAGTCAACGGAT-3′ and 5′-CCACGACGTACTCAGC-3′. Total RNA from PBMC samples were prepared with Qiagen RNeasy Mini Kit. 1μg total RNA per sample was used for the reverse transcriptions (Bio-Rad iScript cDNA Synthesis Kit), followed by 35 cycles of PCR (Clontech Advantage 2 Kit), and detection on 1.5% agarose gel stained with ethidium bromide. Real-Time PCR was carried out in a Bio-Rad iCycler with the Bio-Rad SYBR Green PCR kit.
2.4. Detection of intracellular GrB activity
Human PBMC (1.5 ×10 6 cells/ml in AIM-V media) were stimulated with virus (MOI=2) for 20 hours and 5 days and PBMC lysates were prepared and analyzed by the GrB assay using the Ac-IEPD-pNA (paranitroanilide) substrate according to previously described methods (McElhaney et al., 2006). GrB activity was standardized in the assay using an YT cell lysate and calculated as A405 per mg. protein in the BCA Protein Assay Kit (Pierce).
2.5. Antibodies and flow cytometry
CD28 expression on CD8 T-cells was measured on fresh PBMC. All other fluorescent antibody staining for flow cytometry was performed on virus-stimulated PBMC in culture. Antibodies were purchased from BD Pharmingen including: anti-CD8-Percp, anti-Perf-PE, anti-CD62L-APC, anti-CD28-PE, anti-CD107b-FITC and anti-CD69-APC-Cy7. Anti-CD3-PE-Cy7 was purchased from eBioscience. Fv17 single chain anti-GrB antibody (scFv GrB) (Rong et al., 2004) was labeled with FITC (Sigma) or APC (Dojindo Molecular Technologies, Inc.). Influenza APC-Pentamer (HLA-A0201/GILGFVFTL) was purchased from ProImmune. Cells were prepared for flow cytometry as previously described (McElhaney et al., 2006). Briefly, cells (0.5–1×106) were incubated with surface Abs, washed with colder 0.2%BSA/PBS before and after fixing with 2% paraformaldehyde and then resuspended in colder permeabilization buffer (0.3% saponin, 5% normal human serum PBS). Following scFv GrB intracellular staining, cells were washed with 0.1% saponin and 0.2%BSA/PBS, resuspended in 0.2%BSA/PBS, transferred to FACS tubes for data acquisition on the BD LSR II. 30,000 events per each sample were counted and analyzed using Flow Jo software (Tree Star).
2.6. Virus rechallenge
Virus-stimulated PBMC were rechallenged with live virus to evaluate the cytolytic response of GrB+ CD8 T cells by flow cytometry, using cell-surface expression of CD107b as an indicator of degranulation (Betts et al., 2003). PBMC (2×106/ml) were stimulated with influenza virus A/Wyoming/03/2003 [A/H3N2] (MOI=2) for 5 days. The cells were collected, washed once with PBS, resuspended in 1ml AIM-V medium containing 1μg/ml anti-CD107b antibody and separated into two wells. A/Wyoming/03/2003 [A/H3N2] (MOI=2) was added to one well and the other well was used as a control. Culture plates were incubated in a humidified atmosphere at 37°C in 5% CO2 for 4 hours, followed surface staining of CD3 and CD8 and intracellular staining of GrB for flow cytometry.
2.7. Statistics
The mean and standard error was used to describe the samples. Analyses were performed using SAS 9.1 (SAS Institute Inc.) and significant differences between the younger and older adults, virus strains, were assessed using ANOVA. Significant differences between pre-vaccination and 4-week post-vaccination samples were examined using the paired t test. The Pearson correlation coefficient was used to assess the correlation between different CD8 T cell subsets. All tests are two-sided and were reported as significant at the 95% level of confidence. All PBMC sample testing was blinded to the source of PBMC. Information on study participants was compiled only after all experiments were completed and the data were prepared for analysis.
3. Experimental results
3.1. Influenza virus stimulation increased the expression and activity of GrB in human PBMC
To evaluate the CTL response to influenza virus in PBMC cultures, the expression of GrB mRNA and the enzymatic activity of GrB were measured. RT-PCR results showed that the expression of GrB mRNA increased from a very weak band in fresh PBMC and gradually increasing over 6 hours, 20 hours and 5 days of virus stimulation. Under the same conditions, Perf showed a relatively high level of mRNA expression in both fresh and stimulated PBMC (Fig. 1A). The results of Real-Time RT-PCR confirmed that the expression of GrB mRNA in PBMC increased with virus stimulation (Fig. 1B). The enzymatic activity of GrB in lysates of virus-stimulated PBMC was measured by Ac-IEPD-pNA cleavage after 20 hours and 5 days in culture. The activity of GrB in fresh PBMC lysates was below the limits of detection in the assay. GrB levels showed a 3-fold increase in activity from 20 hours to 5 days in culture; similar levels of GrB activity were obtained with two different virus strains, A/Panama/2007/99 [A/H3N2] and A/New Caledonia/20/99 [A/H1N1] (Fig. 1C). The contrasting profiles of GrB and Perf mRNA expression were supported by flow cytometric studies showing that fresh PBMC contained a significant proportion of Perf+ CD8 T cells with no GrB while both Perf+GrB− and Perf+GrB+ CD8+T cells were observed in 5-day virus stimulated PBMC (Fig. 1D). These results suggested that GrB compared to Perf, is a more sensitive marker of CTL activation. Given that IEPD cleavage by GrB corresponds to cytolytic activity in virus-stimulated PBMC (Ewen et al., 2003), the GrB assay is a sensitive and simple assay to detect the immune response to influenza virus under both ex vivo (20 hours) and in vitro (5 day) conditions.
Fig. 1.
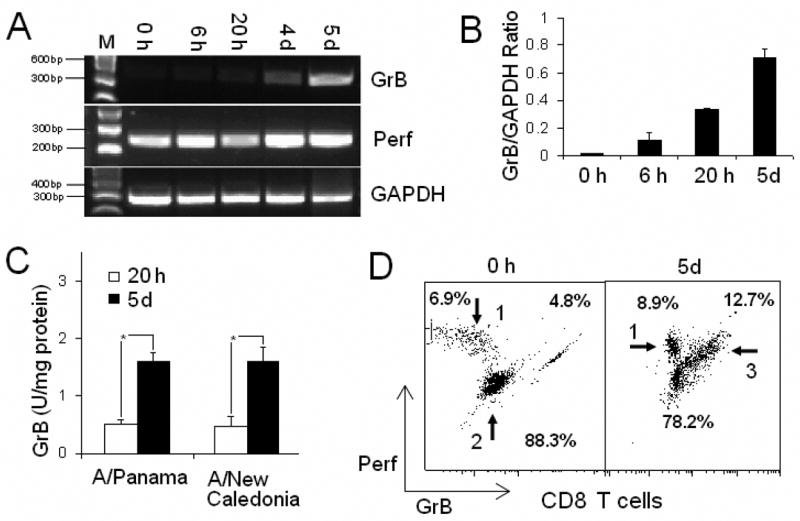
Influenza virus stimulation increased the expression of GrB mRNA and the activity of GrB in human PBMC. A, RT-PCR electrophoresis of GrB, Perf and the housekeeping gene GAPDH in human PBMC is shown. Human PBMC were fresh (0h) or stimulated with A/Panama/2007/99 [A/H3N2] for 6 hours (6h), 20 hours (20h), 4 days (4d) or 5 days (5d). The expression of GrB mRNA increased with influenza virus stimulation while the level of Perf mRNA expression remained high at all time points under the same conditions. B, Real-Time PCR confirmed the increase of GrB mRNA expression with virus stimulation. Mean ratio for the GrB/GAPDH copy number in PBMC stimulated under the same conditions, showed a significant increase over time (N=3, ANOVA, p<0.001). Error bars represent standard error. C, Mean GrB activity showed a significant 3-folder increase from 20 hours to 5 days in PBMC stimulated with influenza virus strains, A/Panama/2007/99 [A/H3N2] and A/New Caledonia/20/99 [A/H1N1](N=5, ANOVA, p<0.001), with no significant difference between the two virus strains. Error bars represent standard error. D, The proportion of Perf+ and Perf+GrB+ CD8 T cells increased with virus stimulation. Compared to fresh PBMC (0h), CD8 T cells in PBMC stimulated with A/Panama/2007/99 [A/H3N2] for 5 days (5d) contained a higher proportion of both Perf+GrB− (arrow 1) and Perf+GrB+ (arrow 3) CD8 T cells and a reciprocal lower proportion of PerflowGrB− (arrow 2).
3.2. GrB+CD62Lhigh CD8 TCM cells were the source of the early effector CTL response to influenza
Next the phenotypes of CD8 T cells induced by virus stimulation were examined through cell surface and intracellular staining. L-selectin (CD62L) is a cell surface marker of lymph node homing and memory T cells (Stamenkovic et al., 1995). GrB was chosen as the marker of a cytotoxic effector response, as compared to Perf, it was a more specific marker of CTL activation. Fluorescent anti-CD3 antibody was used to gate T lymphocytes from other cells. PBMC were stained for CD69 (early activation marker), CD62L, CD3, CD8 and GrB. After 20 hours of virus stimulation, most of the GrB+ CD8 T cells were CD69+ (Fig. 2A) ; unstimulated CD8 T cells did not express CD69 or GrB. In virus-stimulated PBMC, GrB+CD62Lhigh CD8 T cells were shown to be the main responding memory CD8 T cell subset as early as 4 hours through 17 hours of stimulation (Fig. 2B). At day 5, both CD62Lhigh and CD62Llow subsets of GrB+ CD8 T cells were observed while only a sparse population of GrB+CD62Llow CD8 T cells was observed in unstimulated PBMC (Fig. 2C). Compared to the response at 17 hours, there were a higher number of CD62L−GrB− CD8 T cells at 5 days. This observation may result from the response to virus infection, CD62L is lost during the proliferative response (Sallusto et al., 1999) and GrB is removed from the cell with degranulation. All GrB+ CD8 T cells were also Perf+ and displayed a “blast” like morphology with an increase in the forward and side-scatter profile related to the increase in cell size and cytoplasmic granularity, respectively. An ELISpot assay showed the responding cell clusters in PBMC cultures to be rich in IFN-γ after virus stimulation (Lindemann et al., 2006). The GrB+CD62Lhigh/low CD8 T cells expressed higher IFN-γ, were CD28+ and CD27+/−, and 0.2–0.5% of CD8 T cells in HLA-A2+ individuals were influenza tetramer+ (data not shown). Since virus-stimulated CD62Lhigh CD8 TCM cells contained Perf and GrB and expressed the activation marker, CD69, the phenotype of CD8 TCM cells was consistent with activated virus-specific CTL effectors. It was concluded that the GrB+CD62Lhigh CD8 TCM cells are the early source of virus-specific CTL effectors.
Fig. 2.
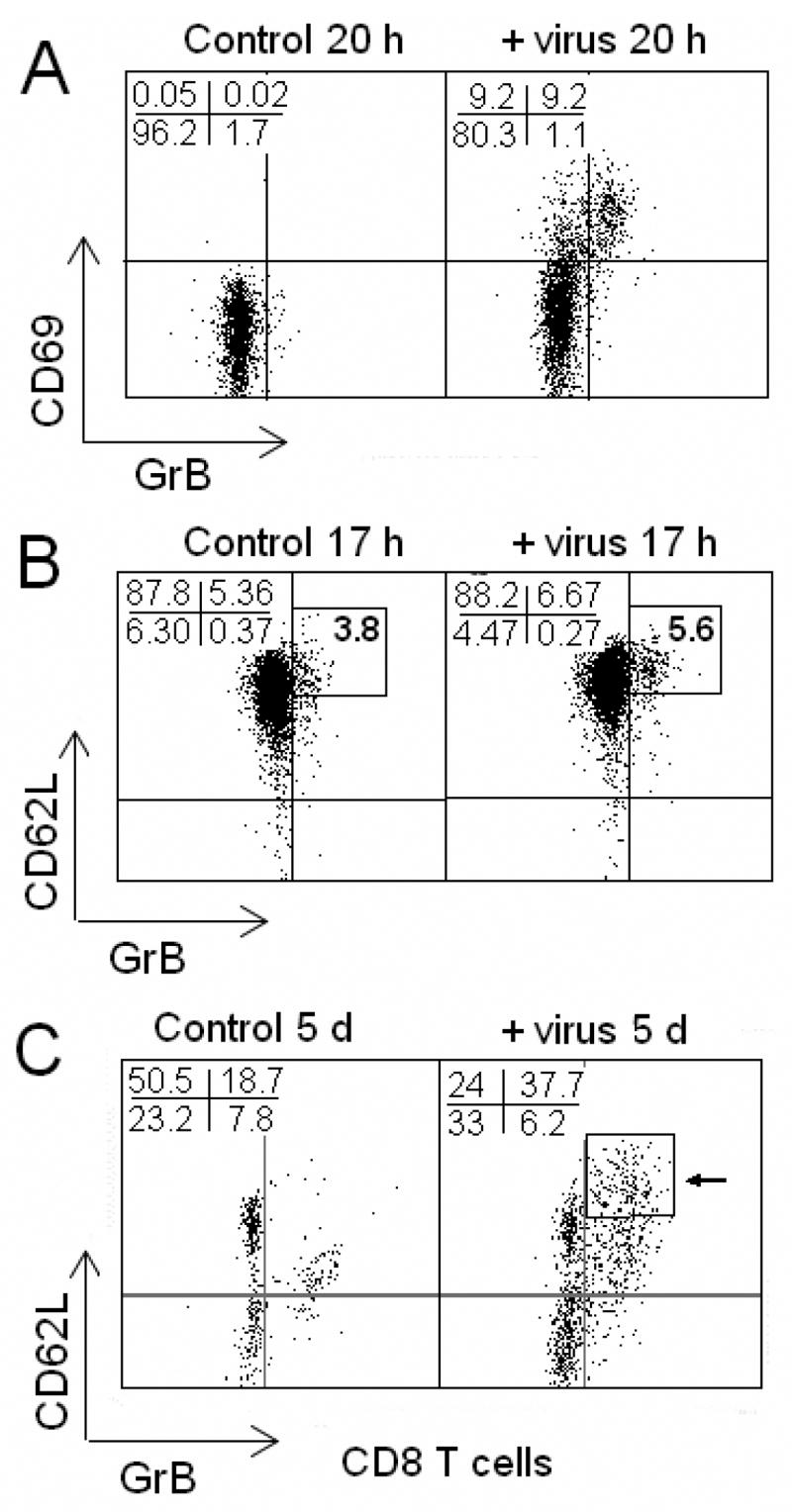
Influenza virus stimulates an increase in GrB+ CD8 T cell frequency, especially GrB+ CD62Lhigh CD8 T cells. Human PBMC stimulated with or without A/Wyoming/03/2003 [A/H3N2] for 20 hours or 5 days. A, GrB+ CD8 T cells appeared after stimulation with virus for 20 hours. All GrB+ CD8 T cells expressed the early activation marker, CD69. B, GrB+ CD8 T cells were CD62Lhigh after stimulation of PBMC with A/Wyoming/03/2003 [A/H3N2] for 17 hours. C, GrB+ CD8 T cells increased after stimulation with influenza virus for 5 days, particularly in the CD62Lhigh (arrow) CD62Llow subsets. Greater than 90% of GrB+ CD8 T cells were CD62Lhigh/low. Numbers represent the percentages of total CD3+CD8 T cells.
3.3. GrB+ CD8 T cells (including GrB+CD62Lhigh cells) can degranulate and contribute to the response to influenza virus infection
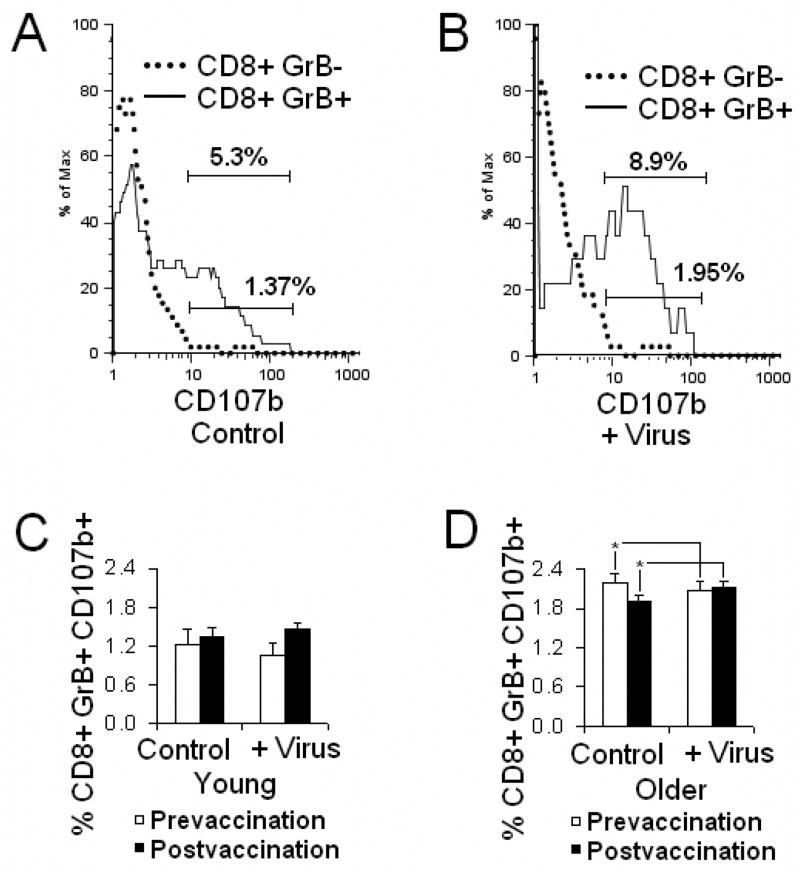
The accumulation of granular membrane proteins (CD107a and CD107b) on the cell surface of responding antigen-specific T cells provides a positive marker of CTL degranulation. CD107a and CD107b appear on the cell surface as early as 30 minutes following stimulation of CD8 T cells in primary cell cultures, and reached a maximum by 4 hours (Betts et al., 2003). CD107b on the cell surface and intracellular GrB were dually expressed in the CD8 T cell subset (data not shown), providing further evidence that GrB+ CD8 T cells are a true effector population. To characterize the behavior of GrB+CD8 T cells, including CD62Lhigh cells, during influenza virus infection, PBMC were stimulated with influenza virus for 5 days, washed, and rechallenged with live virus. Virus-stimulated PBMC that were rechallenged were compared to control virus-stimulated PBMC that were not rechallenged, for the cell surface expression of CD107b after a further 4 hours of culture. Compared to the controls, virus rechallenged PBMC showed a further shift in CD107b expression, from CD107b− to CD107+ GrB+ CD8 T cells suggesting that rechallenge results in degranulation in additional virus-specific CD8 T cells. In contrast, GrB− CD8 T cells showed no expression of CD107b on the cell surface in controls or rechallenged PBMC, highlighting the specificity of the response in GrB+ CD8 T cells (Fig. 3A, 3B). In prevaccination samples, virus rechallenge resulted in a decrease in the proportion of CD107b+GrB+ CD8 T cells in both younger (N=17) and older adults, but the difference was significant only in older adults potentially due to the larger numbers in this group (N=109; paired t-test, p<0.01). Vaccination was associated with an increase in CD107b+GrB+ CD8 T cells in both controls and rechallenged PBMC but was not statistically significant (Fig. 3C). In older adults, vaccination was associated with a significant increase in the proportion of GrB+ CD8 T cells that became CD107b+ when virus-stimulated PBMC were rechallenged (Fig. 3D). Trends in the younger adult group may not have achieved statistical significance due the smaller group size and reflecting a Type II error. The results showed that all GrB+ CD8 T cells (including CD62Lhigh and CD62Llow cells) could degranulate and contribute to the response to influenza virus, and that the percentages of GrB+CD107b+ CD8 T cells in the older group were significantly higher compared to the younger group (p<0.01) (Fig. 3C, 3D). These results may be due to the significantly higher proportion of CD8 T cells in PBMC isolates from younger compared to older adults.
Fig. 3.
GrB+ CD8 T cells degranulate in response to influenza virus and the proportion of degranulating GrB+ CD8 T cells is higher in older compared to younger adults. Human PBMC stimulated for 5 days with influenza virus, A/Wyoming/03/2003 [A/H3N2], were rechallenged with the same virus in parallel (+ virus), the controls that were not rechallenged. The proportion of cells with cytolytic activity was detected by CD107b expressed on the cell surface in the process of degranulation. A, The stimulated control (not rechallenged), showed that only GrB+CD8 T cells accumulated CD107b on the cell surface while most of GrB− subset were CD107b−. Numbers indicate the percentages of CD107b+ cells in total CD8 T cells. B, GrB− CD8 T cells remained CD107b− with virus rechallenge while the GrB+ subset continued to accumulate and becomes largely CD107b+. Numbers indicate the percentages of CD107b+ cells in total CD8 T cells. C, In younger adults, virus rechallenge did not significantly change the mean percentage of CD107b+ in the GrB+CD8+ subset and this response did not change following vaccination. There was no significant increase in the mean proportion of CD107b+GrB+CD8 T cells. D, In older adults prior to vaccination, virus rechallenge was associated with a decrease in the mean percentage of CD107b+GrB+CD8+ in the total CD8 T cell subset. However, at 4-weeks post-vaccination in older adults, virus rechallenge resulted in a significant increase in the mean percentage of CD107b+GrB+CD8 subset of total CD8 T cells (N=109, p<0.01). Error bars represent standard error of the mean.
3.4. Older adults lack CD8 T lymphocytes and GrB+ CD62Lhigh CD8 TCM lymphocyte responses
To determine whether there was an age-related change in the CD8 TCM cell response to live virus, younger and older adults were compared for the percentage of GrB+CD62Lhigh CD8 TCM cells in 5-day virus-stimulated PBMC. Fig. 4A shows that there were overall fewer CD8 T cells and a lower proportion of GrB+CD62Lhigh CD8 TCM cells following virus stimulation in older compared to younger adults. Further analysis showed there was approximately a one-third reduction in the proportion of CD8 T cells in fresh PBMC (Fig. 4B) and a similar reduction in the percentage of CD8 T cells that were GrB+CD62Lhigh following virus stimulation in older (N=128) compared to younger adults (N=17; p<0.001) (Fig. 4C). These results suggest that there is an age-related decline in the central memory CTL response to influenza virus stimulation.
Fig. 4.
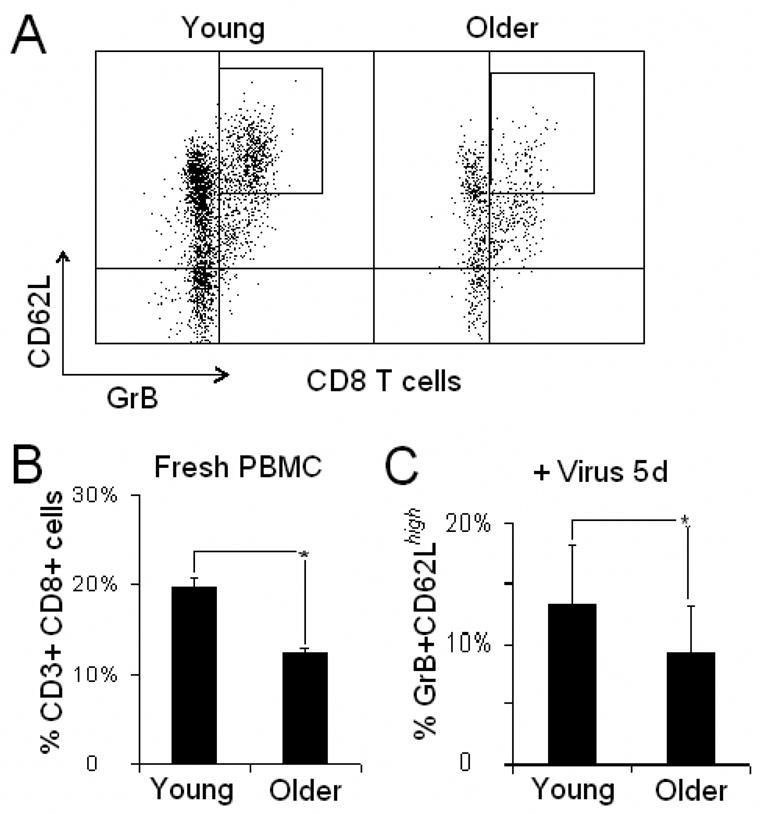
There were lower CD8 T cells within PBMC isolates and a reduced proportion of GrB+CD62Lhigh cells within CD8 T cells following virus stimulation in older adults compared to younger adults. A, Representative dot graphs of CD8 T cells from a younger and an older adult PBMC stimulated with A/Wyoming/03/2003 [A/H3N2] for 5 days showing an overall reduction in the proportion of the GrB+CD62Lhigh CD8 T cell subset in the older adult CD8 T cells. B, The mean percentage of CD8 T cells in fresh PBMC, and C, the mean percentage of the GrB+CD62Lhigh subset of the total CD8 T cell population in virus-stimulated PBMC, was significantly higher in younger (N=17) compared to older adults (N=119, p<0.001 for both comparisons). Error bars represent standard error.
Older adults showed a significant increase from pre- to post-vaccination in the mean percentage of CD8 T cells that were GrB+CD62Lhigh (Fig. 4C). In contrast, there was no significant change in the overall GrB+CD62Lhigh/low subset (18.8% pre-vaccination, 17.9% post-vaccination; data not shown) Younger adults showed a similar trend but the increase did not reach statistical significance for GrB+CD62Lhigh CD8 T cells (Fig. 4C). These results suggest that influenza vaccination enhances GrB+CD62Lhigh CD8 TCM cell response to influenza virus.
3.5. The proportion of CD28null CD8 T cells in fresh PBMC negatively correlated with the proportion of GrB+CD62Lhigh CD8 TCM cells
CD28 plays a critical role in the response to influenza infection. Recent studies have shown that CD28null T cells accumulate with advancing age, particularly in the CD8+ subset (Goronzy et al., 2001). Consistent with these results, the proportion of CD8 T cells that were CD28null in fresh PBMC was higher in the older (37.9%, SE=1.7% of total CD8 T cells) compared to the younger adult group (31.0%. SE=3.8% of total CD8 T cells) (older, N=128; younger, N=19; p<0.05). Due to the important co-stimulatory role of CD28, the relationship between CD28 nullCD8 T cells in fresh PBMC and GrB+CD62Lhigh CD8 T cells in virus-stimulated PBMC in older adults was examined. Prior to vaccination, a significant negative correlation was observed between the percentage of CD8 T cells that were CD28null in fresh PBMC and the proportion that were GrB+ CD62Lhigh in virus-stimulated PBMC (R=−0.22, p<0.001). A similar, but stronger correlation was observed post-vaccination for CD28 null CD8 T cells and GrB+ CD62Lhigh CD8 TCM cells (R=−0.38, p<0.001) (Fig. 5). This relationship was not observed in GrB+CD62Llow CD8 TEM cells or total CD8 T cells. These results suggest that the increased proportion of CD28null CD8 T cells and overall reduction of CD8 T cells with aging contributes to a diminished the reservoir of CTL (CD8+) that can respond to influenza vaccination.
Fig. 5.
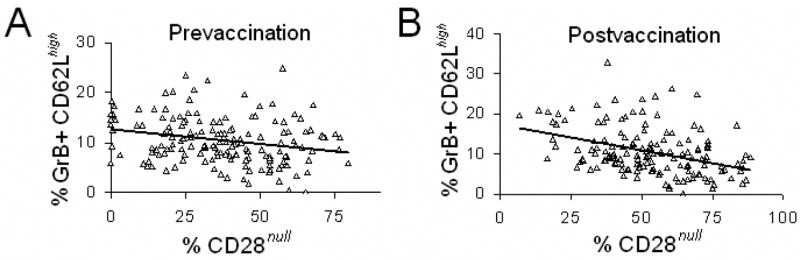
The frequency of CD28null cells in fresh PBMC was negatively correlated with GrB+CD62Lhigh CD8 T cells in virus-stimulated PBMC from older adults. A, Prior to vaccination, there was a negative correlation between the percentage of CD8 T cells that were CD28null in fresh PBMC, and the percentage of CD8 T cells that were GrB+CD62Lhigh in PBMC stimulated with A/Wyoming/03/2003, [A/H3N2] for 5 days (N=149, R=−0.22, p<0.001). B, Four weeks after vaccination, a stronger negative correlation between percentage of CD8 T cells that were CD28null in fresh PBMC and GrB+CD62Lhigh in virus-stimulated PBMC (N= 149, R=−0.38, p<0.001).
4. Discussion
Previous studies have shown that older adults who develop influenza illness in spite of vaccination, have low levels of GrB in virus-stimulated PBMC (McElhaney et al., 2001; 2006). The present study was designed to explore a potential mechanism for the poor response to influenza vaccination in older adults. Initial experiments showed that there was a progressive increase in GrB mRNA and a parallel increase in GrB activity over five days of stimulation with influenza virus. Virtually all GrB+ CTL expressed the activation marker, CD69+, at 20 hours and had an effector phenotype, GrB+CD62Lhigh or GrB+CD62Llow after 5 days in culture. In contrast, Perf mRNA and protein were expressed in both fresh and virus-stimulated PBMC. Thus, GrB was chosen for the subsequent experiments as a more responsive marker to influenza virus stimulation.
There is much debate about how CD62Lhigh TCM and CD62Llow TEM subsets relate to each other (Wherry et al., 2003; Roberts et al., 2005; Bouneaud et al., 2005; Kedzierska et al., 2006). Earlier studies suggested that the proliferation capacity of the CTL response resided in the CD62Lhigh TCM subset and with multiple rounds of replication. CD62L expression declined and generated CD62Llow TEM cells with low proliferation capacity (Lanzavecchia and Sallusto, 2002). More recent studies showed that the TCM and TEM are distinct cell lineages (Jackson et al., 2005). However, previous studies did not distinguish true “effectors” from other memory cells. Using GrB intracellular staining as a marker of CTL effectors, GrB+CD62Lhigh CD8 TCM cells accumulated within 20 hours of influenza virus stimulation with virtually no GrB+CD62Llow CD8 TEM cells suggesting that GrB+CD62Lhigh CD8 TCM cells provides the early effector response. By five days in culture, there was considerable expansion of both these GrB+ CD8 T cell subsets and a reduction in GrB−CD62Lhigh and CD62Llow subsets in both stimulated and unstimulated PBMC. These results would support the earlier findings in the mouse, that CD8 TCM cells are the proliferating memory T cell population that gives rise to GrB+CD62Llow CD8 TEM cells. However, the co-expression of GrB and CD107b on both CD62Lhigh CD8 TCM and GrB+CD62Llow CD8 TEM cells provides evidence for degranulation and suggests that both subsets contribute to CTL effector function through the later stages of virus stimulation. While the mean percentage of GrB+ CD8 T cells the express CD107b is relatively low (~2%), it should be highlighted that this in the context of virus-infected targets that vastly outnumber the CD8 effector cells in PBMC cultures. This contrasts with usual cytolytic assays of stimulated human PBMC with effector: target ratios of 25–50: 1 to produce 20–30% mean specific lysis (Powers el al., 1993). Thus, a small but statistically significant increase in the mean percentage of GrB+ CD8 T cells expressing CD107b should translate to clinically meaningful differences in CTL activity. Since the expression of CD62L alone is not sufficient to define distinct CTL functional subpopulations of memory CD8 T cell subsets (Jackson et al., 2005), these findings would support the combined use of intracellular GrB and CD62L as markers for delineating functional subsets of memory CD8 T cells.
It has been shown that CD62Lhigh CD8 TCM cells respond more vigorously to secondary challenge than CD62Llow TEM cells in terms of their expansion and capacity to clear virus (Wherry et al., 2003). In contrast, it was recently reported that CD62Llow TEM response was at least equal to, or greater than, TCM (CD62LhighCCR7+) cells in Sendai virus rechallenge (Roberts et al., 2004). The evidence presented in this paper support the earlier studies showing that GrB+CD62Lhigh CD8 TCM cells were the major responders to influenza stimulation especially in the early phase of infection. Differences in the results in human PBMC reported herein, from the recent published studies in the mouse, may be due to the effects of local draining lymph nodes, the spleen and non-lymphoid peripheral tissues on CTL responses in the mouse. There may also be some inherent differences in how influenza stimulates human PBMC, and how Sendai virus interacts with the immune system in mouse models.
This study also addressed the cellular immune mechanisms that may explain the age-related increase in susceptibility to influenza illness and diminished vaccine efficacy. The results showed that the lower proportion of CD8 T cells in total PBMC and higher proportion of CD28null CD8 cells of CD8 in older compared to younger adults may limit the CTL response to influenza vaccination and protection from influenza illness. Since CD28 is critical for the initial expansion of CD8 T cells during influenza infection (Effros et al., 2005; Goronzy et al., 2001), a reduction in the proliferative response to influenza would be anticipated with increasing numbers of CD28null CD8 T cells. The present study showed that there was a negative correlation between the proportion of CD28null CD8 T cells and the rapidly proliferating GrB+CD62Lhigh CD8 TCM cell subset responding to influenza virus, which suggests that the increased proportion of CD28null CD8 T cells interferes with the CD8 TCM response to virus infection. Also consistent with the hypothesis that CD28null CD8 cells interfere with the memory CTL proliferative response to influenza, there was no similar correlation observed between CD28null CD8 cells and the non-proliferating GrB+CD62Llow CD8 TEM cell subset in virus-stimulated PBMC. The proportion of CD28null cells has previously been associated with poorer antibody responses to vaccination in older adults (Saurwein-Teissl et al., 2002). Interestingly in the current study, an increase in the negative correlation between CD28null CD8 and GrB+CD62Lhigh CD8 TCM subsets was observed following vaccination suggesting that CD28null CD8 T cells may also impact on the response to influenza vaccination. Taken together, these results suggest that CD28null cells have a pervasive effect on the immune response to influenza vaccination, and that high levels of CD28null Cd8 T cells may be a predictor of poor protection from the current split-virus vaccines in older adults. This may be a target for future vaccine development to provide a stronger stimulus to the CTL response and decrease the inhibitory effect of CD28null CD8 T cells.
In summary, GrB was shown to be an accurate marker of virus-specific stimulation in human CTL and that GrB+ CD62Lhigh CD8 TCM cells were identified as the early effector population responding to influenza virus. In older adults, the response to influenza virus in this CD8 TCM subset declined with an increasing proportion of CD8 T cells with a senescent CD28null phenotype.
Acknowledgments
This work was funded by the National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases, R01 AI68265. The study was conducted through the Lowell P. Weicker, Jr. General Clinical Research Center funded by the NIH, National Center for Research Resources (Grant Number MO1 RR06192) at the University of Connecticut Health Center (UCHC), and in collaboration with the UConn Center on Aging.
We thank Gloria Borders and the stuff of the Lowell P. Weicker, Jr. General Clinical Research Center for coordination of the study, Lisa Kenyon-Pesce in the UConn Center on Aging for subject recruitment and Alison Kleppinger for data management. We also thank Dr. Cheryl Lynn Beseler of Biostatistics Institute, Columbia University for her kind comments and suggestions.
Footnotes
Disclosures
The authors have no financial conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8 T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281(1–2):65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- Bittner V, Weiner DH, Yusuf S, Rogers WJ, McIntyre KM, Bangdiwala SI, Kronenberg MW, Kostis JB, Kohn RM, Guillotte M, et al. Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction: SOLVD investigators. JAMA. 1993;270:1702–1707. [PubMed] [Google Scholar]
- Bouneaud C, Garcia Z, Kourilsky P, Pannetier C. Lineage relationships, homeostasis, and recall capacities of central– and effector–memory CD8 T cells in vivo. J Exp Med. 2005;201:579–590. doi: 10.1084/jem.20040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros RB, Dagarag M, Spaulding C, Man J. The role of CD8 T cell replicative senescence in human aging. Immunol Rev. 2005;205:147–157. doi: 10.1111/j.0105-2896.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- Ewen C, Kane KP, Shostak I, Griebel PJ, Bertram EM, Watts TH, Bleackley RC, McElhaney JE. A novel cytotoxicity assay to evaluate antigen-specific CTL responses using a colorimetric substrate for GrB. J Immunol Methods. 2003;276:89–101. doi: 10.1016/s0022-1759(03)00073-5. [DOI] [PubMed] [Google Scholar]
- Glezen WP, Greenberg SB, Atmar RL, Piedra PA, Couch RB. Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA. 2000;283:499–505. doi: 10.1001/jama.283.4.499. [DOI] [PubMed] [Google Scholar]
- Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O’Fallon WM, Weyand CM. Value of immunological markers in predicting responsiveness to influenza vaccination in olderly individuals. J Virol. 2001;75:12182–12187. doi: 10.1128/JVI.75.24.12182-12187.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SS, Schmitz EJ, Kuroda JM, McKay FP, Sumida MS, Yu F, Lifton AM, Gorgone AD, Letvin LN. Evaluation of CD62L expression as a marker for vaccine-elicited memory cytotoxic T lymphocytes. Immunology. 2005;116:443–453. doi: 10.1111/j.1365-2567.2005.02243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedzierska K, Venturi V, Field K, Davenport PM, Turner JS, Doherty CP. Early establishment of diverse T cell receptor profiles for influenza-specific CD8+ CD62Lhi memory T cells. PNAS. 2006;103:9184–9189. doi: 10.1073/pnas.0603289103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A, Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nat Rev Immunol. 2002;2:982–987. doi: 10.1038/nri959. [DOI] [PubMed] [Google Scholar]
- Lefrancois L, Masopust D. T cell immunity in lymphoid and non-lymphoid tissues. Curr Opin Immunol. 2002;14:503–508. doi: 10.1016/s0952-7915(02)00360-6. [DOI] [PubMed] [Google Scholar]
- Lindemann M, Witzke O, Lutkes P, Fiedler M, Kreuzfolder E, Philipp T, Roggendorf M, Grosse-Wilde H. ELISpot assay as a sensitive tool to detect cellular immunity following influenza vaccination in kidney transplant recipients. Clin Immunol. 2006;120:342–348. doi: 10.1016/j.clim.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Lumsden JM, Roberts JM, Harris NL, Peach RJ, Ronchese F. Differential requirement for CD80 and CD80/86-dependent costimulation in the lung immune response to an influenza virus infection. J Immunol. 2000;164:79–85. doi: 10.4049/jimmunol.164.1.79. [DOI] [PubMed] [Google Scholar]
- Marzo LA, Klonowski DK, Bon LA, Borrow P, Tough FD, Lefrancois L. Initial T cell frequency dictates memory CD8 T cell lineage commitment. Nat Immunol. 2005;6:793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhaney JE, Pinkoski MJ, Upshaw CM, Bleackley RC. The cell-mediated cytotoxic response to influenza vaccination using an assay for GrB activity. J Immunol Meth. 1996;190:11–20. doi: 10.1016/0022-1759(95)00235-9. [DOI] [PubMed] [Google Scholar]
- McElhaney JE, Gravenstein S, Upshaw CM, Hooton JW, Krause P, Drinka P, Bleackley RC. Granzyme B: a marker of risk for influenza in institutionalized older adults. Vaccine. 2001;19:3744–3751. doi: 10.1016/s0264-410x(01)00087-1. [DOI] [PubMed] [Google Scholar]
- McElhaney JE, Xie D, Hager WD, Barry MB, Wang Y, Kleppinger A, Ewen C, Kane KP, Bleackley RC. T cell responses are better correlates of vaccine protection in the olderly. J Immunol. 2006;176:6333–6339. doi: 10.4049/jimmunol.176.10.6333. [DOI] [PubMed] [Google Scholar]
- Powers DC, Belshe RB. Effect of age on cytotoxic T lymphocyte memory as well as serum and local antibody responses elicited by inactivated influenza virus vaccine. J Infect Dis. 1993;167:584–592. doi: 10.1093/infdis/167.3.584. [DOI] [PubMed] [Google Scholar]
- Roberts AD, Woodland DL. Cutting Edge: effector memory CD8 T cdeels play a prominent role in recall responses to secondary viral infection in the lung. J Immunol. 2004;172:6533. doi: 10.4049/jimmunol.172.11.6533. [DOI] [PubMed] [Google Scholar]
- Roberts AD, Ely KH, Woodland DL. Differential contributions of central and effector memory T cells to recall responses. J Exp Med. 2005;202:123–133. doi: 10.1084/jem.20050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong J, Xu X, Ewen C, Bleackley RC, Kane KP. Isolation and characterization of novel single-chain Fv specific for human granzyme B. Hybrid Hybridomics. 2004;23:219–231. doi: 10.1089/1536859041651349. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Saurwein-Teissl M, Lung TL, Marx F, Gschosser C, Asch E, Blasko I, Parson W, Bock G, Schonitzer D, Trannoy E, Grubeck-Loebenstein B. Lack of antibody production following immunization in older age: association with CD8(+)CD28(−) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol. 2002;168:5893–5899. doi: 10.4049/jimmunol.168.11.5893. [DOI] [PubMed] [Google Scholar]
- Schmidt AC, Johnson TR, Openshaw PJ, Braciale TJ, Falsey AR, Anderson LJ, Wertz GW, Groothuis JR, Prince GA, Melero JA, Graham BS. Respiratory syncytial virus and other pneumoviruses: a review of the international symposium--RSV 2003. Virus Res. 2005;106:1–13. doi: 10.1016/j.virusres.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Schwaiger S, Wolf AM, Robatscher P, Jenewein B, Grubeck-Loebenstein B. IL-4-producing CD8 T cells with a CD62L++(bright) phenotype accumulate in a subgroup of older adults and are associated with the maintenance of intact humoral immunity in older age. J Immunol. 2003;170:613–619. doi: 10.4049/jimmunol.170.1.613. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I. The L-selectin adhesion system. Curr Opin Hematol. 1995;2:68–75. doi: 10.1097/00062752-199502010-00010. [DOI] [PubMed] [Google Scholar]
- Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, Fukuda K. Influenza-associated hospitalizations in the United States. Jama. 2004;292:1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, Von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;3:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]