Abstract
Cytoplasmic dynein is the only known kinetochore protein capable of driving chromosome movement toward spindle poles. In grasshopper spermatocytes, dynein immunofluorescence staining is bright at prometaphase kinetochores and dimmer at metaphase kinetochores. We have determined that these differences in staining intensity reflect differences in amounts of dynein associated with the kinetochore. Metaphase kinetochores regain bright dynein staining if they are detached from spindle microtubules by micromanipulation and kept detached for 10 min. We show that this increase in dynein staining is not caused by the retraction or unmasking of dynein upon detachment. Thus, dynein genuinely is a transient component of spermatocyte kinetochores.
We further show that microtubule attachment, not tension, regulates dynein localization at kinetochores. Dynein binding is extremely sensitive to the presence of microtubules: fewer than half the normal number of kinetochore microtubules leads to the loss of most kinetochoric dynein. As a result, the bulk of the dynein leaves the kinetochore very early in mitosis, soon after the kinetochores begin to attach to microtubules. The possible functions of this dynein fraction are therefore limited to the initial attachment and movement of chromosomes and/or to a role in the mitotic checkpoint.
Keywords: cytoplasmic dynein, kinetochores, kinetochore microtubules, micromanipulation, tension
Introduction
Cytoplasmic dynein is a large, multisubunit ATPase that moves along microtubules toward their minus ends (Holzbaur and Vallee 1994). Dynein transports and localizes membranous organelles and is also involved in spindle assembly and maintenance (Vallee and Sheetz 1996).
Dynein and CENP-E are the only known kinetochore proteins with demonstrated motor activity (Pfarr et al. 1990; Steuer et al. 1990; Yen et al. 1992; Starr et al. 1998; for reviews see Rieder and Salmon 1998; Maney et al. 2000). Dynein's presence at kinetochores suggests that it could help capture microtubules and transport chromosomes. The rate at which dynein moves in vitro is similar to the rate that chromosomes move just after they capture microtubules (Rieder and Alexander 1990). However, when antibodies to the motor domain of dynein heavy chain were injected into vertebrate cells, chromosomes attached to and moved along spindle microtubules without any obvious difficulties (Vaisberg et al. 1993). Furthermore, Drosophila chromosomes whose kinetochores either lack dynein or have mutated dynein demonstrate no obvious problems with attachment or movement (Starr et al. 1998; Robinson et al. 1999). On the other hand, there is evidence suggesting that dynein is necessary for chromosome attachment to Tetrahymena micronuclear spindles (Lee et al. 1999). In sum, although the evidence regarding dynein's function at kinetochores is ambiguous, dynein may at least contribute to kinetochore microtubule capture and chromosome movement (for review see Rieder and Salmon 1998).
Dynein immunolocalization studies suggest that prometaphase kinetochores have more dynein than metaphase kinetochores (Pfarr et al. 1990; Steuer et al. 1990; Escheverri et al. 1996), and that metaphase kinetochores regain dynein immunofluorescence after microtubule depolymerization (Escheverri et al. 1996). These results imply that kinetochores lose dynein as a consequence of microtubule attachment, but other explanations are possible. For example, kinetochores that are attached to microtubules would appear to have less dynein if kinetochore microtubules block the dynein antibody from binding to dynein. Or, if dynein were “crawling out” onto kinetochore microtubules, such an event could stretch the outer region of the kinetochore and cause diminished kinetochore staining.
We used micromanipulation and quantitative fluorescence microscopy to test whether the amount of dynein localized at kinetochores changes during cell division. We found that dynein is in fact a transient component of the kinetochore. After kinetochores attach to the spindle, dynein actually leaves the kinetochore—it is neither masked by the kinetochore microtubules nor stretched out onto them. In grasshopper spermatocytes, changes in dynein localization are regulated by microtubule attachment, not tension from mitotic forces.
Materials and Methods
Micromanipulation and Live Cell Observations
Spermatocytes from laboratory colonies of the grasshopper Melanoplus sanguinipes (Fabricius) were cultured as described previously (Nicklas et al. 1979) at 22.5°C–25°C. The spermatocytes were viewed by phase–contrast microscopy and micromanipulated by standard procedures (Nicklas and Ward 1994 and references therein). Before manipulation, microneedles were sequentially dipped in 10% SurfaSil (Pierce Chemical Co.) diluted in xylene (Mallinckrodt Baker, Inc.), xylene alone, and finally methanol (Mallinckrodt Baker, Inc.). This silicon coating prevented the microneedle from sticking to chromosomes in lysed cells. Chromosome behavior before, during, and after manipulation was recorded on an optical disk recorder (model 2021; Panasonic Video Systems).
Reagents
The following reagents were used in this study: PHEM (60 mM Pipes [Sigma-Aldrich], 25 mM Hepes [Sigma-Aldrich], pH 6.95, 10 mM EGTA [Sigma-Aldrich], and 4 mM MgCl2 [Fisher Scientific]); MBS (10 mM Mops [Sigma-Aldrich], pH 7.4, and 150 mM NaCl [EM Industries, Inc.]); MBST (MBS with 0.05% Tween 20 [Sigma-Aldrich]); and BSA/MBS (1% bovine serum albumin [Sigma-Aldrich] in MBS).
Immunoblots
Testes from grasshopper nymphs were dissected and placed into Pipes medium (Nicklas et al. 1979). After the fat surrounding the follicles was removed, bibulous paper (Fisher Scientific) was used to wick excess medium away from the follicles before they were placed in a 1.5-ml microcentrifuge tube (Brinkmann Instruments, Inc.). The tube was then immersed in liquid nitrogen and stored at −75°C. Testes from Drosophila melanogaster, strain Oregon R, adults were dissected in EBR buffer (130 mM NaCl, 4.7 mM KCl, 1.9 mM CaCl2, and 10 mM Hepes, pH 6.9), frozen in liquid nitrogen, and stored at −80°C.
Grasshopper and Drosophila testes were homogenized in Laemmeli sample buffer, and SDS-PAGE and immunoblots were carried out as described by Li et al. 1994.
Lysis, Fixation, and Immunostaining
Cultured cells were lysed by 5% Triton X-100 (Sigma-Aldrich) in PHEM buffer which was micropipetted near them. Between 1 and 2 min after lysis, the cell remains were fixed by “microfixative” that was similarly micropipetted (Nicklas et al. 1979). The microfixative contained 6% formaldehyde (freshly prepared from paraformaldehyde; J.T. Baker) and 0.15% glutaraldehyde (Polysciences, Inc.) in PHEM buffer. This unusually concentrated microfixative is diluted by the culture fluid bathing the cells. To promote their attachment to the glass, chromosomes and spindle remnants were pressed down on the coverslip with the silicon-coated microneedle.
After 10 min of microfixation, the oil covering the cells was flushed away with “macrofixative” containing 3% formaldehyde in PHEM. Coverslips were then immersed in macrofixative for 5 min (Nicklas et al. 1979) and finally rinsed with three 5-min immersions in MBST. To prevent nonspecific antibody binding, the spindle remains were treated with BSA/MBS for 30 min. To visualize dynein, the cell remnants were stained overnight at 4°C with the monoclonal P1H4 antibody diluted 1:200 in BSA/MBS. After one brief rinse in MBST and a second 20-min rinse in MBST, cell remains were labeled simultaneously with Cy3-conjugated goat anti–rabbit IgG (Jackson ImmunoResearch Laboratories) diluted 1:50 in BSA/PBS and FITC-conjugated goat anti–mouse IgG (Jackson ImmunoResearch Laboratories) diluted 1:25 for 45 min at room temperature. Coverslips were then dipped in MBST, rinsed for 20 min in fresh MBST, and dipped in distilled water. Coverslips were mounted onto slides with Vectashield (Vector Laboratories) supplemented with 10 mM CaCl2. The edges of the coverslips were sealed with nail polish.
Immunofluorescence Images and Measurements
Immunostained cells were examined with an epifluorescence microscope (Axiovert TV100; Carl Zeiss, Inc.) equipped with a 40×/1.3 numerical aperture (Carl Zeiss, Inc.), ICS Plan-Neofluar phase–contrast objective; an optivar set to 2.5×; and a cooled CCD video camera (Micromax model 1300-Y; Princeton Instruments, Inc.). MetaMorph software (Universal Imaging Corp.) was used to acquire digital images and to measure pixel brightness.
The brightness of kinetochore staining was measured in unprocessed images. A circle was placed around a kinetochore and the total pixel brightness within the circle was recorded. This measurement includes the fluorescence of both the kinetochore and the surrounding background. The contribution from background fluorescence was then subtracted from the total brightness in the circle, as follows. A larger concentric circle was placed around the first circle—the brightness in the annulus between the inner and outer circles was used to determine background brightness: br k = br i − a i [(br o − bri)/(a o − ai)], where br k stands for corrected kinetochore brightness, br i stands for brightness of the inner circle, br o stands for brightness of the outer circle, a i stands for area of the inner circle in pixels, and a o stands for area of the outer circle in pixels. The measurements were made on images at a single focal level with the 1.3 numerical aperture objective. The focal depth of this objective is great enough to include the fluorescence from the full depth of a kinetochore (Nicklas et al. 1998).
For illustrations in this report, fluorescence and phase–contrast images of the cells were processed digitally, using commercial software (Photoshop® by Adobe Systems and PowerPoint® by Microsoft Corp.). Prints were made with an inkjet printer (970C Series; Hewlett-Packard Co.).
Results
General Features of Dynein Antibody Staining in Grasshopper Spermatocytes
The P1H4 antibody is a monoclonal antibody that specifically recognizes Drosophila cytoplasmic dynein heavy chain (McGrail and Hays 1997). When probed against grasshopper testis, the antibody recognizes one band with the same molecular mass as Drosophila dynein heavy chain (Fig. 1). Thus, the P1H4 antibody specifically recognizes dynein heavy chain in grasshopper spermatocytes.
Figure 1.
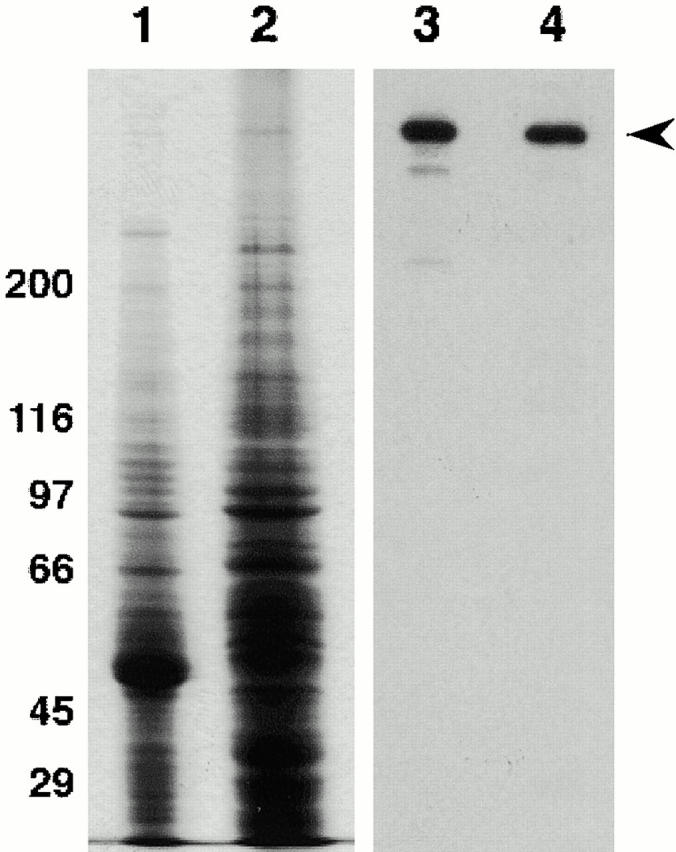
Specificity of the mouse monoclonal antidynein heavy chain antibody P1H4: lanes 1 and 2, Coomassie blue–stained gel of fly testis and grasshopper testis homogenates, respectively; lanes 3 and 4, duplicate gel blotted and probed with the P1H4 monoclonal antibody. The P1H4 specifically detects dynein heavy chain (arrowhead) in both fly and grasshopper testis. Molecular mass values are indicated to the left of lane 1.
In grasshopper spermatocytes, the dynein heavy chain antibody localizes to the spindle, spindle poles, and kinetochores (Fig. 2). The amount of antibody detected at kinetochores depends upon the stage of the spermatocytes: prometaphase kinetochores in meiosis I stain brightly for dynein (Fig. 2A and Fig. B, arrowheads), whereas metaphase kinetochores are more dimly labeled (Fig. 2C and Fig. D, arrowheads).
Figure 2.
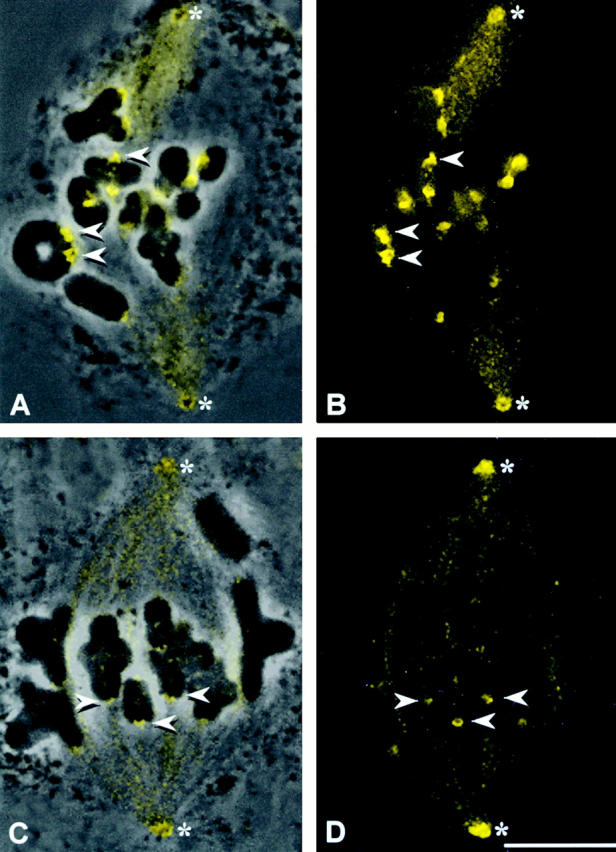
Dynein staining in grasshopper spermatocytes. (A and C) Superimposed phase–contrast and immunofluorescence images. (B and D) Immunofluorescence images. The dynein antibody stains the spindle, spindle poles, and kinetochores (arrowheads) in both prometaphase (A and B) and metaphase (C and D) cells (the absence of a tight metaphase plate in C and D is due to cell lysis before fixation). Dynein staining is far brighter on prometaphase kinetochores (A and B) than at metaphase (C and D). *Spindle poles. Bar, 10 μm.
Dynein Staining at Kinetochores Increases After Kinetochores Are Separated from Spindle Microtubules
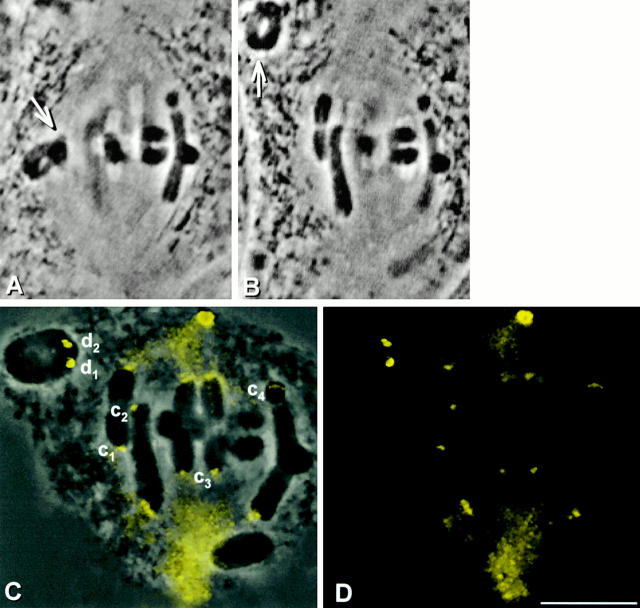
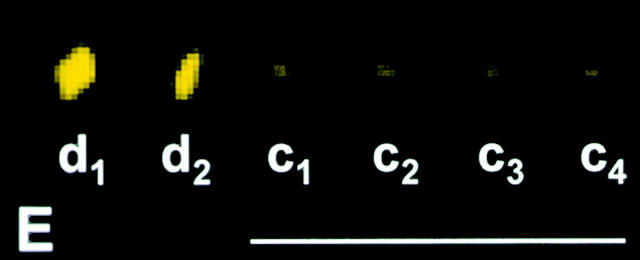
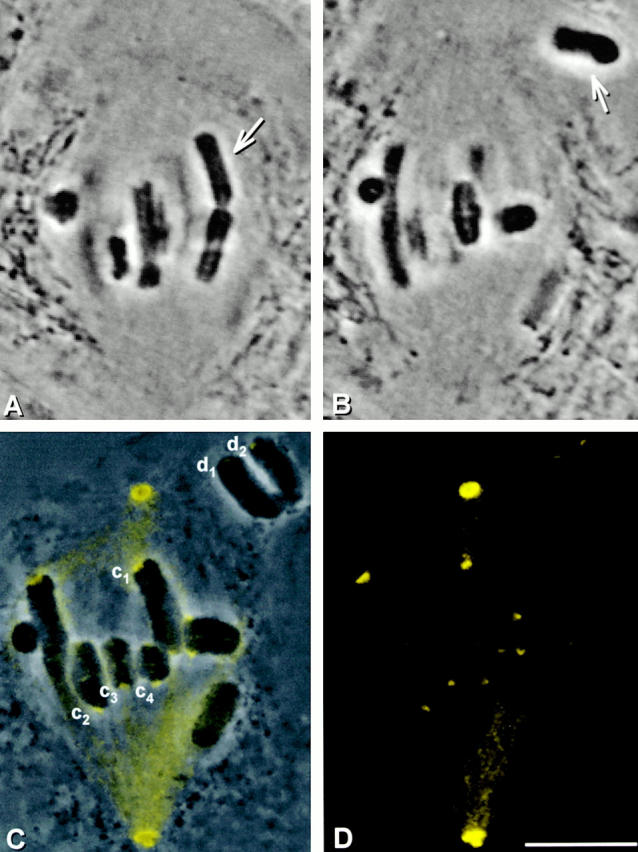
We started with spermatocytes in metaphase of meiosis I and pulled a chromosome (Fig. 3A and Fig. B, arrow) off the spindle with a microneedle, separating the chromosome's kinetochores from spindle microtubules (Nicklas and Kubai 1985). The kinetochores were prevented from reattaching to the spindle for 10 min; during this time the chromosome was frequently nudged with the microneedle to break any attachment to newly captured microtubules. After 10 min, the cell membranes were lysed and the cell remnants were fixed and pressed onto the coverslip with the microneedle. After immunostaining with the P1H4 antibody, kinetochores that had been detached from the spindle were brighter than the attached, unmanipulated kinetochores in the same cell (Fig. 3, C–E; d1, d2, and c1–c4).
Figure 3.
Dynein staining at kinetochores 10 min after they have been detached from the spindle. (A and B) Live cell images. A chromosome (arrow) was detached from a metaphase spindle, placed in the cytoplasm, and held there for 10 min. (C) Superimposed phase–contrast and immunofluorescence images. (D) Immunofluorescence image. (E) Composite showing the kinetochores labeled in C at higher magnification. The detached kinetochores (d1 and d2) stain more brightly than the control kinetochores that remained attached (c1–c4). Bars, 10 μm.
This experiment was done in five cells, yielding a total of 10 kinetochores that had each been detached for 10 min. We then measured the brightness of these detached kinetochores and of 10–15 unmanipulated, attached control kinetochores in each cell. 9 of the 10 detached kinetochores were brighter than the control kinetochores measured in the same cells. In the exceptional case, the detached kinetochore was just 2% dimmer than the brightest of the kinetochores that had remained attached. On average, detached kinetochores were 9.4 times as bright as attached kinetochores: the difference is statistically significant (Table ). These results suggest that when kinetochores are detached from spindle microtubules, they accumulate dynein.
Table 1.
Kinetochore Brightness Comparisons
| Kinetochore | 10 min after detachment | 0 min after detachment | Relaxed | Reattached |
|---|---|---|---|---|
| 1 | 3.5 | 1.2 | 0.9 | 0.5 |
| 2 | 3.3 | 1.7 | 0.5 | 0.6 |
| 3 | 9.6 | 0.7 | 1.6 | 0.2 |
| 4 | 9.1 | 0.9 | 1.5 | 0.5 |
| 5 | 1.9 | 0.9 | 0.9 | 0.6 |
| 6 | 3.4 | 0.8 | 0.6 | 0.4 |
| 7 | 11.0 | 1.1 | 0.7 | 0.1 |
| 8 | 19.9 | 1.5 | 1.4 | 0.4 |
| 9 | 22.8 | 0.7 | 0.4 | |
| 10 | 9.7 | 0.7 | ||
| Average | 9.4 ± 4.4 | 1.0 ± 0.3 | 1.0 ± 0.4 | 0.4 ± 0.1 |
| P | 0.0005 | 1.00 | 1.00 | <0.00005 |
Dynein Is a Transient Kinetochore Component
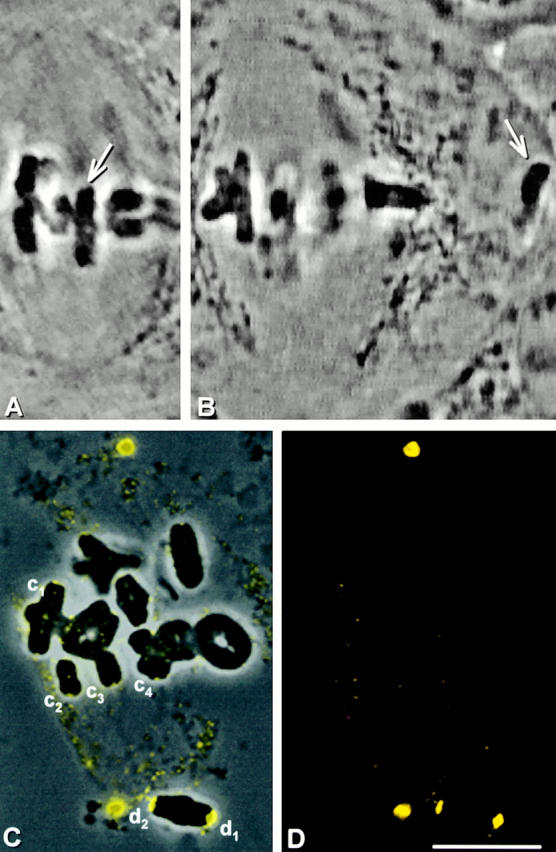
The increase in dynein labeling at detached kinetochores may not be caused by increased amounts of dynein. For example, upon detachment dynein may be unmasked and rendered recognizable by the antibody. Or, if dynein on attached kinetochores crawls out onto microtubules, detachment would cause this dynein to “snap back” into kinetochores, making it more apparent. If either of these alternatives is true, an increase in dynein labeling should be evident immediately after kinetochores are detached from the spindle. We detached one chromosome (Fig. 4A and Fig. B, arrow) from a metaphase I spindle and immediately lysed the cell. After fixation and dynein immunostaining, the kinetochores that had been detached from the spindle were no brighter than those that had remained on the spindle (Fig. 4, C–E; d1, d2, and c1–c4).
Figure 4.
Dynein staining at kinetochores immediately after they have been detached from the spindle. (A and B) Live cell images. A chromosome (arrow) was detached from a metaphase spindle, placed in the cytoplasm, and the cell was then immediately lysed. (C) Superimposed phase–contrast and immunofluorescence images. (D) Immunofluorescence image. (E) Composite showing the kinetochores labeled in C at higher magnification. The detached kinetochores (d1 and d2) have no brighter staining than the control kinetochores that remained attached (c1–c4). Bars, 10 μm.
In all, 10 kinetochores from five cells were similarly detached. We then measured the brightness of the detached kinetochores and an average of 16 attached kinetochores per cell. For all of the detached kinetochores, the brightness value was within the range of that measured for attached kinetochores. On average, the brightness of detached kinetochores relative to attached kinetochores was 1.0, a value statistically consistent with the null hypothesis (see Table ). Therefore, immediately after a kinetochore is detached from the spindle, it has the same amount of dynein as kinetochores that remained on the spindle.
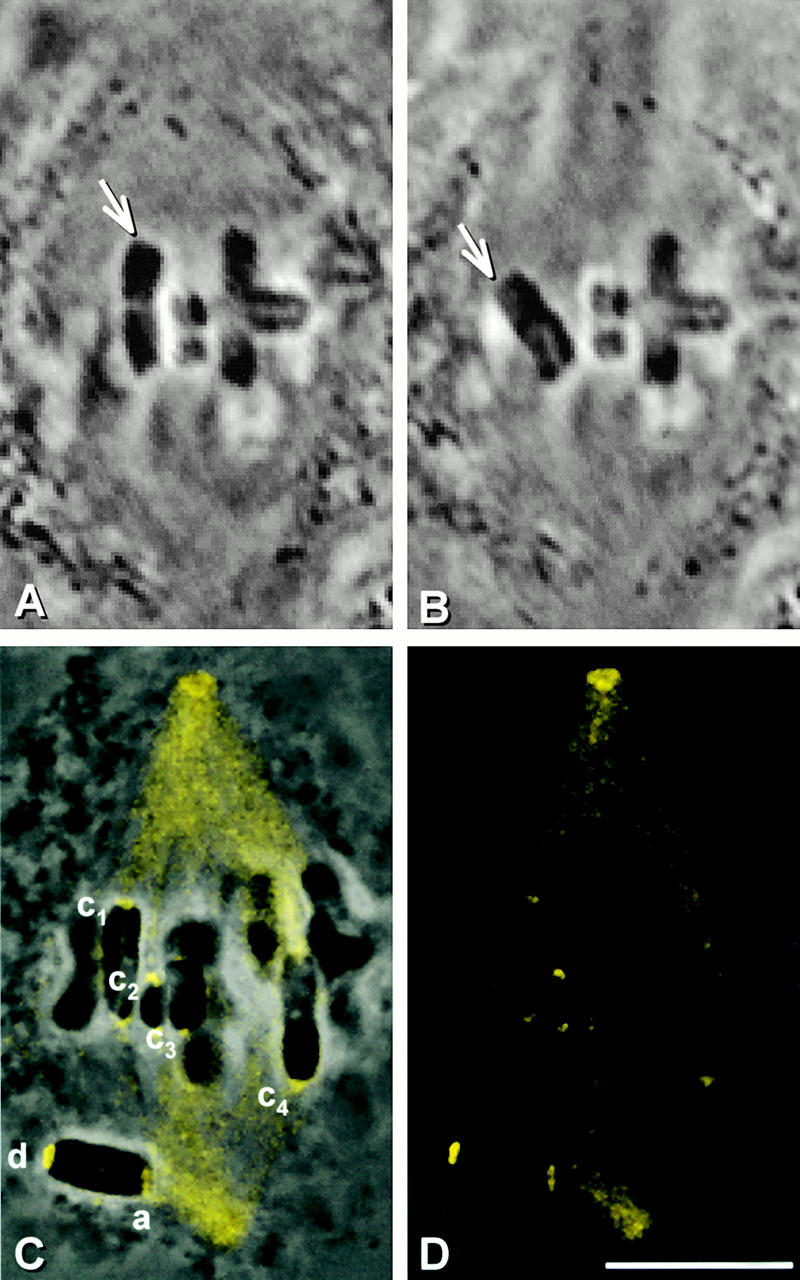
We also studied dynein at kinetochores just after loss of kinetochore microtubules by another means: we depolymerized spindle microtubules during cell lysis. A chromosome from a metaphase I cell (Fig. 5A and Fig. B, arrow) was detached from the spindle and kept free of microtubules in the cytoplasm for 10 min. After 10 min, the cell was lysed in the presence of 10 mM Ca2+, a treatment which depolymerized spindle microtubules (as verified by tubulin immunostaining, data not shown). The chromosomes and spindle remnants were then fixed and stained for dynein. Spindle staining was almost absent—compare Fig. 5 D with Fig. 3 D. The kinetochores that had been detached from spindle microtubules for 10 min stained brightly for dynein, whereas those that remained attached to the spindle until calcium treatment were dim (Fig. 5, C–E; d1, d2, and c1–c4). This observation was consistent in three cells. In each of the cells, we measured the brightness of the two kinetochores from the chromosome that had been detached for 10 min and of 10–12 other kinetochores whose microtubules were depolymerized by calcium treatment just before fixation. On average, the kinetochores that had been detached for 10 min were 12 times as bright as kinetochores that remained attached until calcium treatment. These results demonstrate that the loss of dynein immunofluorescence at kinetochores with attached microtubules is not due to masking or redistribution of dynein by the microtubules.
Figure 5.
Dynein staining at kinetochores after microtubule disassembly. (A and B) Live cell images. A chromosome (arrow) was detached from a metaphase spindle, placed in the cytoplasm, and held there for 10 min. The cell was then lysed in the presence of a calcium-containing microtubule disassembly medium. (C) Superimposed phase–contrast and immunofluorescence images. (D) Immunofluorescence image. (E) Composite showing the kinetochores labeled in C at higher magnification. The kinetochores that had been detached for 10 min (d1 and d2) are brighter than the kinetochores that remained attached until calcium treatment (c1–c4). Bars, 10 μm.
Kinetochore Microtubules, Not Tension, Prevent Dynein Loading at Kinetochores
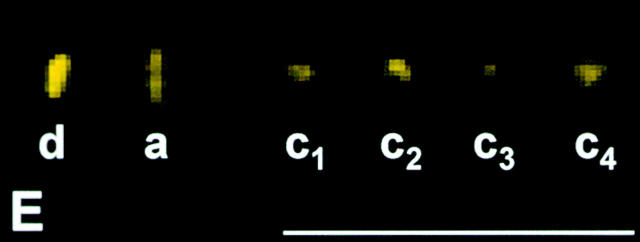
Dynein accumulates at kinetochores that are separated from spindle microtubules. We wanted to know whether the increase is caused by loss of kinetochore microtubules or by loss of tension. Partner kinetochores are under tension from forces directed toward opposite poles when a chromosome is properly attached to the spindle. Tension influences certain aspects of kinetochore chemistry (for review see Nicklas 1997), and there is evidence that it affects dynein localization in Drosophila cells (Starr et al. 1998). We looked at whether dynein accumulates at kinetochores that are released from tension but retain microtubule attachment. Starting with cells in metaphase of meiosis I, a kinetochore of one chromosome (Fig. 6A and Fig. B, arrow) was detached from the spindle with a microneedle while its partner kinetochore remained attached to the spindle. This operation relaxed the tension on the chromosome, as seen by its immediate shortening. The chromosome was maintained in a relaxed state for 10 min. Detached kinetochores that face a spindle pole will reattach in 1.6 min on average (Nicklas and Ward 1994). Therefore, to keep the detached kinetochore from reforming a stable attachment to the spindle during the 10-min interval, the detached kinetochore was frequently nudged with the microneedle. In the absence of tension, the attached kinetochore moved closer to its spindle pole (Fig. 6 C). After the cell was lysed, fixed, and stained for dynein, the detached kinetochore exhibited bright dynein labeling (Fig. 6, C–E; d), as expected. The attached, relaxed partner (Fig. 6, C–E; a) had the same amount of dynein labeling as kinetochores that remained under tension (Fig. 6, C–E; c1–c4).
Figure 6.
The consequence of releasing tension, but not microtubule attachment, at kinetochores. (A and B) Live cell images. One kinetochore (arrow) of a chromosome was detached from a metaphase spindle and kept detached for 10 min. (C) Superimposed phase–contrast and immunofluorescence images. (D) Immunofluorescence image. (E) Composite showing the kinetochores labeled in C at higher magnification. The detached kinetochore (d) stained brightly for dynein, whereas the kinetochore that was relaxed, but still attached (a) is only as bright as kinetochores that remained under tension (c1–c4). Bars, 10 μm.
For each of eight such experiments, we measured the brightness of the relaxed but attached kinetochore and of 10–12 kinetochores that remained attached and under tension. On average the attached, relaxed kinetochores were 1.0 times as bright as attached, tense kinetochores, and there is no statistical difference between these groups (see Table ). Therefore, loss of tension alone does not lead to dynein accumulation at kinetochores; this occurs only after complete loss of microtubules.
Kinetochore Microtubules Cause Dynein Unloading at Kinetochores
We next examined regulation of the converse change, the loss of dynein from kinetochores after microtubule attachment. Two chromosomes (Fig. 7A and Fig. B, arrows) from a metaphase I spindle were detached and kept detached for 10 min to cause dynein loading at the kinetochores. Then one of the detached chromosomes was brought back into the spindle and positioned so that one of its kinetochores faced a spindle pole, in a good position to capture microtubules. Once the kinetochore captured microtubules, as judged by its movement toward the pole, it was allowed to continue capturing microtubules for 5 min. The partner kinetochore either remained detached or reattached for brief intervals before it was again detached by micromanipulation. The second detached chromosome was kept from reattaching. After the kinetochore captured microtubules for 5 min, the cell was lysed and then fixed. Immunostaining revealed that the reattached kinetochore (Fig. 7, C–E; r) is dimmer than the detached kinetochores (Fig. 7, C–E; d), about as dim as kinetochores that remained attached to the spindle for the course of the experiment (Fig. 7, C–E; c1–c4).
Figure 7.
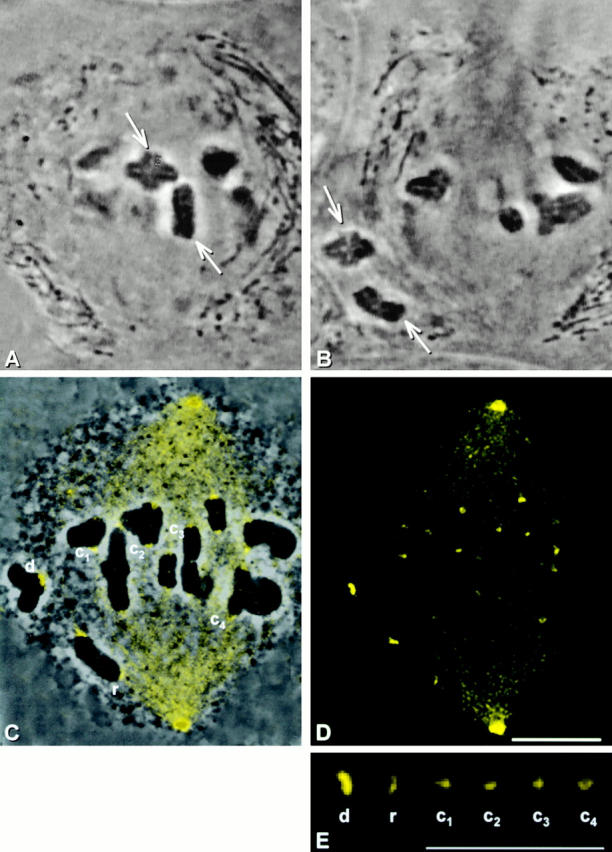
The consequence of microtubule attachment at kinetochores. (A and B) Live cell images. Two chromosomes (arrows) were detached from a metaphase spindle and kept detached for 10 min. After 10 min, one kinetochore of one of the chromosomes reattached to the spindle and was allowed to capture microtubules for 8 min. (C) Superimposed phase–contrast and immunofluorescence images. (D) Immunofluorescence image. (E) Composite showing the kinetochores labeled in C at higher magnification. The detached kinetochores, one of which is labeled d, stained brightly for dynein. Yet the kinetochore that reattached (r) is only as bright as kinetochores that were not manipulated (c1–c4). Bars, 10 μm.
This experiment was done in a total of nine cells ranging in stage from early prometaphase to late metaphase. On average, the reattached kinetochores were allowed to capture microtubules for 6.5 min (range 5–8 min). The reattached kinetochores were on average 40% as bright as the detached kinetochores, a difference that is statistically significant (see Table ). This shows that as kinetochores bind microtubules, they lose dynein.
We wanted to know if microtubule attachment alone caused dynein levels to drop back down to levels characteristic of properly attached, unmanipulated kinetochores. Four of the experimental cells were in early to mid-metaphase of meiosis I; in all of these, the brightness values for reattached kinetochores were in the range of those for kinetochores that had remained attached. In contrast, the detached kinetochores were always the brightest in their cells—on average they were five times as bright as the kinetochores that remained attached. One of the experimental cells was in late metaphase; its reattached kinetochore was brighter than all attached kinetochores, but still dimmer than the detached kinetochores. This suggests that until late metaphase, microtubule attachment alone can cause a fivefold drop in dynein at kinetochores and can account for the difference in dynein between unattached and attached kinetochores.
Discussion
Previously it was shown that the pattern of dynein immunostaining at kinetochores changes as a consequence of microtubule attachment (Escheverri et al. 1996), but whether the observed changes reflected true changes in amounts of dynein was not established. We have now obtained evidence that in grasshopper spermatocytes dynein is in fact a transient component of the kinetochore. We have also demonstrated that changes in dynein accumulation at kinetochores are regulated by microtubule attachment, not tension.
Microtubule Capture and Dynein Loss
Normally, chromosome attachment to the spindle is quickly followed by tension from mitotic forces toward opposite poles, so that the effects of tension and of attachment itself, the accumulation of kinetochore microtubules, cannot be distinguished. By micromanipulation, we can allow attachment but prevent tension and we find that attachment by itself both prevents dynein accumulation (see Fig. 6) and promotes dynein loss (see Fig. 7). In fact, mere attachment reduces the amount of dynein to that seen in control kinetochores that are attached and under tension (see Fig. 7). From other experiments, we know that relatively few kinetochore microtubules are present at kinetochores that are attached to the spindle but are not under tension (King and Nicklas 2000). For instance, a sample counted by electron microscopy showed that attached but relaxed kinetochores had an average of only 13 kinetochore microtubules, whereas kinetochores that were attached and under tension had an average of 32 kinetochore microtubules (King and Nicklas 2000). How can so few microtubules have so dramatic an effect on the binding and loss of dynein over the whole kinetochore? A likely possibility is that dynein loss is determined not by the extent of kinetochore microtubule accumulation, but rather by the rate of kinetochore microtubule capture. As kinetochores attach to the spindle, they repeatedly capture and lose microtubules (Zhai et al. 1995). We do not know how long these newly captured microtubules persist, but presumably they persist long enough to chase dynein off kinetochores. Suppose that dynein and microtubules compete for the same binding sites within kinetochores. Even if the microtubules leave the binding sites, they probably are not filled immediately with dynein. Dynein is sluggish in binding to kinetochores; it takes minutes for it to accumulate noticeably at completely detached kinetochores. If microtubules often beat dynein to open sites, they would not need to persist to chase dynein off and prevent it from rebinding. This would explain why kinetochores that are released from tension yet remain attached do not accumulate dynein (see Fig. 6), even though they have lost over half of their microtubules (King and Nicklas 2000).
Kinetochores lose most, but not all, of their dynein within 10 min after their attachment to spindle microtubules. Thereafter dynein loss slows and some dynein remains associated with metaphase kinetochores even after they have been attached for hours. Loss of residual dynein may be determined in part by the number of sites available for microtubule binding. The number of microtubule binding sites at a kinetochore probably increases throughout the period from prophase to anaphase onset (King and Nicklas 2000). In the early stages of cell division, kinetochores may have sites that can bind dynein but not microtubules. As these sites become competent to bind microtubules, they would lose dynein.
There is evidence that tension is necessary for dynein loss at Drosophila kinetochores (Starr et al. 1998). Attached univalents, which are not under tension, have more dynein labeling at their kinetochores than attached bivalents, which are under tension. In the early stages of cell division, chromosome attachment in Drosophila is more transient than it is in grasshopper spermatocytes (Church and Lin 1985). Drosophila chromosomes may not remain near a spindle pole long enough to capture sufficient microtubules for substantial dynein loss. Alternatively, the speed of dynein binding at Drosophila kinetochores may be faster than it is at grasshopper kinetochores; i.e., it may closely match the speed of microtubule binding.
Clearly, dynein leaves kinetochores after microtubules attach, but we do not know the mechanics of this process nor the fate of the dissociated dynein. Conceivably, dynein binds to captured kinetochore microtubules and then leaves the kinetochore by moving along the microtubules toward the spindle pole. Dynein's presence along spindles (see Fig. 3, Fig. 4, Fig. 6, and Fig. 7) is consistent with this idea. Dynein probably leaves the kinetochore at the same time as dynactin does because they share a similar kinetochore staining pattern (Escheverri et al. 1996). Dynactin is believed to bind to ZW-10 at kinetochores (Starr et al. 1998). It is possible that dynein leaves the kinetochore as part of a complex and that another protein in this hypothetical complex, such as ZW-10, loses interaction with the kinetochore after microtubules attach. For example, dynein could crawl out onto a microtubule with sufficient force to break a labile linkage between an anchor protein and the kinetochore. It is possible that dynein then transports dynactin to the spindle pole, where the dynactin appears to be necessary to maintain microtubule focus (Escheverri et al. 1996; Quintyne et al. 1999).
Dynein's Role at Kinetochores
If dynein's abundance at the kinetochore serves a purpose, it is not an obvious one. It is possible that under normal circumstances an abundance of dynein at kinetochores aids in capturing microtubules and driving chromosome movement (Rieder and Salmon 1998). Dynein is an attractive candidate for driving initial movements: dynein's rate of movement in vitro, ranging from 30 to 90 μm·min−1 (Paschal et al. 1987), is similar to the rate of chromosome movement immediately after nuclear envelope breakdown in insect spermatocytes (Rickards 1975). And dynein is the only known kinetochore protein that can generate fast minus-end–directed motility.
However, kinetochores do not require an abundance of dynein to capture and move along microtubules. It takes 5–10 min for dynein to reload at a detached kinetochore. In contrast, it only takes a minute or two on average for a detached kinetochore to reattach, if it is in a favorable position to capture microtubules (Nicklas and Ward 1994). When kinetochores are in a less favorable position, capture takes longer; kinetochores take on average 8 min to reattach to a pole when they face away from it. This situation arises when a chromosome is misattached and its partner kinetochores face the same spindle pole, a situation that would lead to improper anaphase chromosome segregation if not corrected. Correction requires that one of the kinetochores captures microtubules from the opposite spindle pole. In situations where capture is improbable, an abundance of dynein may facilitate kinetochore capture of microtubules and correction of errors.
Changes in dynein localization resemble changes in spindle checkpoint signals at kinetochores. The spindle checkpoint delays progression into anaphase in the presence of unattached or improperly attached chromosomes (for reviews see Nicklas 1997; Rieder and Salmon 1998). There is at least one protein, Mad2, that relays information about microtubule attachment at kinetochores to the checkpoint machinery (for review see Maney et al. 2000). Yet how Mad2 receives information regarding the attachment state is not known. Several earlier observations prompt the idea that dynein and/or CENP-E might be capable of signaling to the checkpoint that kinetochores are attached to spindle microtubules: (a) both dynein and CENP-E are located at the kinetochore and can bind to microtubules; (b) CENP-E binds in vitro to the protein hBUBR1, a possible checkpoint protein (Cahil et al. 1998; Chan et al. 1999); and (c) cells that cannot localize dynein to kinetochores have higher than normal rates of precocious sister chromatid separation, a failure of normal checkpoint operation (Smith et al. 1985; Starr et al. 1998). Our new results showing that motor protein localization at kinetochores is sensitive to microtubule attachment are certainly consistent with the idea that dynein may have a role in monitoring the attachment state of kinetochores.
In conclusion, dynein is a genuinely transitory component of spermatocyte kinetochores, and its association with the kinetochore is remarkably sensitive to the presence of microtubules. It is not obvious how a few microtubules, turning over fairly rapidly, can affect dynein binding throughout the kinetochore. The bulk of dynein leaves the kinetochores so early in mitosis that its only obvious possible functions are in the initial attachment and movement of chromosomes and perhaps in checkpoint function.
Acknowledgments
We thank Suzanne Ward and Madeline Serr for technical support. Dr. Donna Maroni provided helpful comments on the manuscript.
This work was supported in part by grants GM13745 to R.B. Nicklas and GM44757 to T.S. Hays from the Institute of General Medical Sciences, National Institutes of Health.
References
- Cahil D.P, Lengauer C., Yu J., Riggins G.J., Willson J.K., Markowitz S.D., Kinzler K.W., Vogelstein B. Mutations of mitotic checkpoint genes in human cancers. Nature. 1998;392:300–303. doi: 10.1038/32688. [DOI] [PubMed] [Google Scholar]
- Chan G.K., Jablonski S.A., Sudakin V., Hittle J.C., Yen T.J. Human BUBR1 is a mitotic checkpoint kinase that monitors CENP-E functions at kinetochores and binds the cyclosome/APC. J. Cell Biol. 1999;146:941–954. doi: 10.1083/jcb.146.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church K., Lin H.P. Kinetochore microtubules and chromosome movement during prometaphase in Drosophila melanogaster spermatocytes studied in life and with the electron microscope. Chromosoma. 1985;92:273–282. doi: 10.1007/BF00329810. [DOI] [PubMed] [Google Scholar]
- Escheverri C.J., Paschal B.M., Vaughan K.T., Vallee R.B. Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J. Cell Biol. 1996;132:617–633. doi: 10.1083/jcb.132.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzbaur E.L., Vallee R.B. DYNEINSmolecular structure and cellular function. Annu. Rev. Cell Biol. 1994;10:339–372. doi: 10.1146/annurev.cb.10.110194.002011. [DOI] [PubMed] [Google Scholar]
- King J.M., Nicklas R.B. Tension on chromosomes increases the number of kinetochore microtubules but only within limits. J. Cell Sci. 2000;113:3815–3823. doi: 10.1242/jcs.113.21.3815. [DOI] [PubMed] [Google Scholar]
- Lee S., Wisniewski J.C., Dentler W.L., Asai D.J. Gene knockouts reveal separate functions for two cytoplasmic dyneins in Tetrahymena thermophila . Mol. Biol. Cell. 1999;10:771–784. doi: 10.1091/mbc.10.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., McGrail M., Serr M., Hays T.S. Drosophila cytoplasmic dynein, a microtubule motor that is asymmetrically localized in the oocyte. J. Cell Biol. 1994;126:1475–1494. doi: 10.1083/jcb.126.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maney T., Ginkel L.M., Hunter A.W., Wordeman L. The kinetochore of higher eucaryotesa molecular view. Int. Rev. Cytol. 2000;194:67–131. doi: 10.1016/s0074-7696(08)62395-5. [DOI] [PubMed] [Google Scholar]
- McGrail M., Hays T.S. The microtubule motor cytoplasmic dynein is required for spindle orientation during germline cell divisions and oocyte differentiation in Drosophila . Development. 1997;124:2409–2419. doi: 10.1242/dev.124.12.2409. [DOI] [PubMed] [Google Scholar]
- Nicklas R.B. How cells get the right chromosomes. Science. 1997;275:632–637. doi: 10.1126/science.275.5300.632. [DOI] [PubMed] [Google Scholar]
- Nicklas R.B., Kubai D.F. Microtubules, chromosome movement, and reorientation after chromosomes are detached from the spindle by micromanipulation. Chromosoma. 1985;92:313–324. doi: 10.1007/BF00329815. [DOI] [PubMed] [Google Scholar]
- Nicklas R.B., Ward S.C. Elements of error correction in mitosismicrotubule capture, release, and tension. J. Cell Biol. 1994;126:1241–1253. doi: 10.1083/jcb.126.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas R.B., Brinkley B.R., Pepper D.A., Kubai D.F., Rickards G.K. Electron microscopy of spermatocytes previously studied in lifemethods and some observations on micromanipulated chromosomes. J. Cell Sci. 1979;35:87–104. doi: 10.1242/jcs.35.1.87. [DOI] [PubMed] [Google Scholar]
- Nicklas R.B., Campbell M.S., Ward S.C., Gorbsky G.J. Tension-sensitive kinetochore phosphorylation in vitro. J. Cell Sci. 1998;111:3189–3196. doi: 10.1242/jcs.111.21.3189. [DOI] [PubMed] [Google Scholar]
- Paschal B.M., Shpetner H.S., Vallee R.B. MAP 1C is a microtubule-activated ATPase which translocates microtubules in vitro and has dynein-like properties. J. Cell Biol. 1987;105:1273–1282. doi: 10.1083/jcb.105.3.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfarr C.M., Coue M., Grissom P.M., Hays T.S., Porter M.E., McIntosh J.R. Cytoplasmic dynein is localized to kinetochores during mitosis. Nature. 1990;345:263–265. doi: 10.1038/345263a0. [DOI] [PubMed] [Google Scholar]
- Quintyne N.J., Gill S.R., Eckley D.M., Crego C.L., Compton D.A., Schroer T.A. Dynactin is required for microtubule anchoring at centrosomes. J. Cell Biol. 1999;147:321–334. doi: 10.1083/jcb.147.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickards G.K. Prophase chromosome movements in living house cricket spermatocytes and their relationship to prometaphase, anaphase and granule movements. Chromosoma. 1975;49:407–455. doi: 10.1007/BF00285133. [DOI] [PubMed] [Google Scholar]
- Rieder C.L., Alexander S.P. Kinetochores are transported poleward along a single astral microtubule during chromosome attachment to the spindle in newt lung cells. J. Cell Biol. 1990;110:81–95. doi: 10.1083/jcb.110.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder C.L., Salmon E.D. The vertebrate cell kinetochore and its roles during mitosis. Trends Cell Biol. 1998;8:310–318. doi: 10.1016/s0962-8924(98)01299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J.T., Wojcik E.J., Sanders M.A., McGrail M., Hays T.S. Cytoplasmic dynein is required for the nuclear attachment and migration of centrosomes during mitosis in Drosophila . J. Cell Biol. 1999;146:597–608. doi: 10.1083/jcb.146.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.A., Baker B.S., Gatti M. Mutations in genes encoding essential mitotic functions in Drosophila melanogaster . Genetics. 1985;110:647–670. doi: 10.1093/genetics/110.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr D.A., Williams B.C., Hays T.S., Goldberg M.L. ZW10 helps recruit dynactin and dynein to the kinetochore. J. Cell Biol. 1998;142:763–774. doi: 10.1083/jcb.142.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuer E.R., Wordeman L., Schroer T.A., Sheetz M.P. Localization of cytoplasmic dynein to mitotic spindles and kinetochores. Nature. 1990;345:266–268. doi: 10.1038/345266a0. [DOI] [PubMed] [Google Scholar]
- Vaisberg E.A., Koonce M.P., McIntosh J.R. Cytoplasmic dynein plays a role in mammalian mitotic spindle formation. J. Cell Biol. 1993;123:849–858. doi: 10.1083/jcb.123.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee R.B., Sheetz M.P. Targeting of motor proteins. Science. 1996;271:1539–1544. doi: 10.1126/science.271.5255.1539. [DOI] [PubMed] [Google Scholar]
- Yen T.J., Li G., Schaar B.T., Szilak I., Cleveland D.W. CENP-E is a putative kinetochore motor that accumulates just before mitosis. Nature. 1992;359:536–539. doi: 10.1038/359536a0. [DOI] [PubMed] [Google Scholar]
- Zhai Y., Kronebusch P.J., Borisy G.G. Kinetochore microtubule dynamics and the metaphase–anaphase transition. J. Cell Biol. 1995;131:721–734. doi: 10.1083/jcb.131.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]