Abstract
Collagen fibrillogenesis is finely regulated during development of tissue-specific extracellular matrices. The role(s) of a leucine-rich repeat protein subfamily in the regulation of fibrillogenesis during tendon development were defined. Lumican-, fibromodulin-, and double-deficient mice demonstrated disruptions in fibrillogenesis. With development, the amount of lumican decreases to barely detectable levels while fibromodulin increases significantly, and these changing patterns may regulate this process. Electron microscopic analysis demonstrated structural abnormalities in the fibrils and alterations in the progression through different assembly steps. In lumican-deficient tendons, alterations were observed early and the mature tendon was nearly normal. Fibromodulin-deficient tendons were comparable with the lumican-null in early developmental periods and acquired a severe phenotype by maturation. The double-deficient mice had a phenotype that was additive early and comparable with the fibromodulin-deficient mice at maturation. Therefore, lumican and fibromodulin both influence initial assembly of intermediates and the entry into fibril growth, while fibromodulin facilitates the progression through growth steps leading to mature fibrils. The observed increased ratio of fibromodulin to lumican and a competition for the same binding site could mediate these transitions. These studies indicate that lumican and fibromodulin have different developmental stage and leucine-rich repeat protein specific functions in the regulation of fibrillogenesis.
Keywords: collagen, proteoglycans, lumican, fibromodulin, tendon
Introduction
The tissue-specific assembly of extracellular matrix results in distinct structural and functional properties. Collagen fibril assembly and growth, major events defining overall extracellular matrix assembly and function, are finely regulated during connective tissue development. The collagen fibrils are heteropolymeric structures that contain more than one fibrillar collagen and macromolecules associated with the fibril surface (Birk and Linsenmayer 1994). Fibril-associated molecules have been implicated in regulating progression of fibril assembly through a series of steps leading to the structurally and mechanically mature fibril.
In tendon, the type I collagen-containing fibril, organized into fibers (fibril bundles), is the major element responsible for structure stabilization and the mechanical attributes of this tissue. The fibril contains collagen molecules assembled into a quarter-staggered array, and this striated fibril has a 67-nm periodicity. Developing tendons have been used extensively for studies of collagen fibrillogenesis in vivo. Fibril development is a multi-step process and, in the avian metatarsal tendons, specific developmental periods are associated with distinct steps in collagen fibril assembly and growth (Birk et al. 1989, Birk et al. 1990, Birk et al. 1995, Birk et al. 1996, Birk et al. 1997; Kadler et al. 1996; Nurminskaya and Birk 1996; Young et al. 2000). During the early period of development, short, small-diameter fibril intermediates are formed and the “molecular assembly” or “fibril assembly” phase predominates. This is followed by fibril growth, where fibrils become both longer and larger in diameter.
Interactions between the fibril-associated members of the leucine-rich repeat family of proteoglycans and fibrillar collagen are obvious candidates for regulating the different tissue-specific steps in collagen fibrillogenesis. Fibromodulin and lumican are members of a subfamily of proteoglycans/glycoproteins with a leucine-rich repeat structural domain (Iozzo 1998, Iozzo 1999). Fibromodulin is a keratan sulfate proteoglycan found in a variety of tissues including tendon (Oldberg et al. 1989). Lumican is a major keratan sulfate proteoglycan of the corneal stroma, but is present in other collagenous extracellular matrices in a glycoprotein form (Funderburgh et al. 1987). Decorin, a chondroitin sulfate proteoglycan, is widely expressed, including during tendon development (Birk et al. 1995). These leucine-rich repeat proteins (LRR proteins) all bind to fibrillar collagens (Vogel et al. 1984; Hedbom and Heinegard 1989; Rada et al. 1993). In vitro, it has been shown that fibromodulin and lumican compete for the same binding site (Svensson et al. 2000) and that this site is separate from the decorin binding site (Hedbom and Heinegard 1993). In addition, fibromodulin, lumican, and decorin all interact with striated fibrils (Scott 1986; Pringle and Dodd 1990; Hedlund et al. 1994; Birk et al. 1995) and can modulate collagen fibril formation and inhibit the lateral growth of fibrils in vitro (Vogel et al. 1984; Hedbom and Heinegard 1993; Rada et al. 1993). Also, the abnormal lateral growth of isolated fibrils was prevented by this class of proteoglycans (Birk et al. 1996).
Gene-targeting studies of lumican, fibromodulin, and decorin indicate that these leucine-rich repeat proteins are involved in the determination of the mature collagen fibril structural phenotype in vivo (Danielson et al. 1997; Chakravarti et al. 1998; Svensson et al. 1999). The absence of lumican affected the mature fibril architecture of connective tissues such as cornea and skin. Null mutations in fibromodulin and decorin produced abnormal fibril structures in tendons and skin. In addition, the decorin and lumican animal models demonstrated reduced dermal tensile strength associated with the mature fibril defects, indicating important functions of these proteins in connective tissue maturation. However, the analysis of the fibromodulin-deficient mouse is complicated by the increased deposition of lumican into the matrix, suggesting a coordinate regulation of these closely related members of the same subfamily. To define the roles of these two closely related molecules in tissue- and development-specific extracellular matrix assembly, the onset of fibrillar abnormalities during development of the LRR protein–deficient mice should be elucidated.
The purpose of the current study is to define the role(s) of a subfamily of leucine-rich repeat proteins, lumican and fibromodulin, in the regulation of specific steps in collagen fibrillogenesis during tendon development. The use of lumican-null, fibromodulin-null, and double-null mice allow the dissection of the regulatory functions of these molecules in the sequential, developmental steps in tendon collagen fibril assembly and growth.
Materials and Methods
Animals
Lumican-null mice (lum tm1sc/lum tm1sc) and fibromodulin-null mice generated by targeted gene disruption have been described (Chakravarti et al. 1998; Svensson et al. 1999). Lumican-null and fibromodulin-null double knockout mice were generated by breeding homozygous lumican-null and fibromodulin-null mice. Mice were genotyped separately for lumican and fibromodulin by PCR. CD-1 out-bred wild-type, and lumican-null, fibromodulin-null, and lumican/fibromodulin-null mice in the same genetic background were housed at Case Western Reserve University and/or Thomas Jefferson University. Only male mice were included in all experiments except for the 4- and 10-d postnatal time point where sex was not determined.
Semiquantitative Reverse Transcription–PCR
Semiquantitative reverse transcription (RT)–PCR was performed using standard methods. Flexor digitorum longus tendons were dissected from wild-type mice (CD-1). Embryos at E18 (n = 6) and postnatal mice from 2 (8), 4 (10), 6 (8), 8 (9), 10 (10), 12 (11), 14 (7), 16 (7), and 21 d (3), and 4 (8), 8 (7), and 12 wk (8) were used. Each tendon was collected separately and stored at −80°C. Total RNA was isolated using Trizol reagent (GIBCO BRL). The quantity and quality of isolated total RNA was determined using spectrophotometry and electrophoresis. The RNA yield was 1.5–6 μg per sample in stages before P14 and 6–20 μg in later stages. To generate cDNA, 500 ng of total RNA was reverse transcribed in a 20-μl reaction using oligo-dT primer and M-MLV reverse transcriptase (GIBCO BRL). Each LRR protein and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were amplified simultaneously in the same tube using 0.05 U/μl of Taq DNA polymerase in PCR buffer containing 1.5 mM MgCl2. Each primer was present at 0.2 μM with each dNTP at 200 μM. The PCR program was optimized by adjusting annealing temperature (60°C) and cycle numbers (25 cycles for fibromodulin, 24 cycles for lumican). PCR products were electrophoresed in ethidium bromide–stained agarose gels and analyzed densitometrically using a Gel Doc 2000 (Bio-Rad Laboratories) and Quantity One software. The figures were prepared using Adobe Photoshop. The experiments were repeated at least three times with different batches of RNA. The following primer sets were used for PCR: M-Fbm-635F (22 mer) 5′-TGGAGGGCCTGGAGAACCTCAC-3′, M-Fbm-1052R (23 mer) 5′-GTGCAGAAGCTGCTGATGGAGAA-3′, M-Lum-352F (23 mer) 5′-GTCACAGACCTGCAGTGGCTCAT-3′, M-Lum-1004R (24 mer) 5′-ATCTTGGAGTAAGACAGTGGTCC-3′, M-GAPDH-266F (22 mer) 5′-ATCTTCCAGGAGCGAGACCCCA-3′, and M-GAPDH-555R (24 mer) 5′-TCCACAATGCCAAAGTTGTCATGG-3′.
Western Analysis
Flexor tendons were dissected from the hind limbs of 6–10 mice at 4 and 10 d postnatal and five or six mice at 1 mo postnatal. This was repeated for three independent samples. The tendons were extracted in a fourfold excess (weight/volume) of 4 M guanidine-HCl, 50 mM sodium acetate, pH 5.8, with Complete™ protease inhibitors (Roche Molecular Biochemicals). Protein extracts from tendons were prepared (Larsson et al. 1991) and analyzed by protein transfer blots as described (Svensson et al. 1999).
Immunofluorescence Microscopy
Tendons from 4- and 10-d postnatal mice were fixed with 4% paraformaldehyde in PBS (pH 7.3) for 30 min on ice. The tissues were cryoprotected with 2 M sucrose-PBS, and frozen in OCT (Tissue Tek; Miles Laboratories). Sections (6 μm) were cut and picked up onto poly-l-lysine–coated slides. Sections were treated with sodium borohydride (50 mg/100 ml PBS) for 15 min at room temperature and nonspecific binding sites were blocked by incubation in 5% BSA in phosphate buffered saline overnight at 4°C (Young et al. 2000). Sections were then incubated in rabbit anti–mouse lumican [1:250] or fibromodulin [1:250] prepared against recombinant core proteins (Svensson et al. 1999). This was followed by secondary goat anti–rabbit dichlorotriazynyl amino fluorescein–conjugated antibody [1:150] (Jackson ImmunoResearch Laboratories). Negative controls were incubated identically except the primary antibody was absent. The slides were mounted in Vectashield™ (Vector Laboratories Inc.) and images were captured using an Optronics digital camera. The final figures were prepared using Adobe Photoshop.
Transmission Electron Microscopy
Mice were killed at 4 d, 10 d, 1 mo, and 3 mo after birth. Between three and six mice were used for each of the 16 groups. The exact numbers for each group are indicated in Fig. 6 (below). Hind limbs were fixed in situ in 4% paraformaldehyde, 2.5% glutaraldehyde, 0.1 M sodium cacodylate, pH 7.4, with 8.0 mM CaCl2 for 15 min at room temperature, during which time the flexor digitorium tendons were dissected. This was followed by 100 min at 4°C and processing as previously described (Birk and Trelstad 1986; Birk et al. 1997). In brief, the tendons were post-fixed with 1% osmium tetroxide and enbloc stained with 2% uranyl acetate/50% ethanol. After dehydration in an ethanol series followed by propylene oxide, the tendons were infiltrated and embedded in a mixture of Polybed 812, nadic methyl anhydride, dodecenylsuccinic anhydride, and DMP-30 (Polysciences, Inc.). Thick sections (1 μm) were cut and stained with methylene blue–azur blue for examination and selection of specific regions for EM analysis. Thin sections were prepared using a Reichert UCT ultramicrotome and a diamond knife. Staining was with 2% aqueous uranyl acetate followed by 1% phosphotungstic acid, pH 3.2. Cross sections of midplantar regions of flexor digitorum tendons were analyzed in fibromodulin-, lumican-, fibromodulin/lumican-deficient and wild-type mice using electron microscopy. Sections were examined and photographed at 75 kV using a transmission electron microscope (Hitachi 7000).
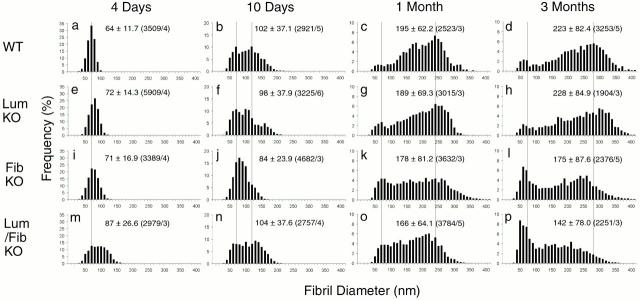
Figure 6.
Collagen fibril diameter distributions during development of wild-type, lumican-null, fibromodulin-null, and double lumican/fibromodulin–null tendons. Collagen fibril diameter distributions are presented as histograms for wild-type (a–d), lumican-deficient (e–h), fibromodulin-deficient (i–l), and double lumican/fibromodulin–deficient (m–p) mouse tendons. Vertical dotted lines indicate peaks observed in wild-type mouse tendons at ∼65, 120, 240, and 280 nm. (a–d) Means, SD, and number of fibrils measured (n)/number of different animals (n a) are presented as mean ± SD (n/n a) in the graphs.
Fibril Diameter Analyses
For each group, three to five different animals were analyzed. Micrographs (11–34/group) from nonoverlapping regions of the central portion of midplantar flexor digitorum tendons were taken from cross sections at 31,680×. For the purpose of statistical analysis, data from a single photographic negative, containing 100–300 diameter measurements was regarded as a sample and reduced to three sample statistics: first quartile, median, and third quartile. We assumed that different samples were independent. The microscope was calibrated using a line grating. Micrographs were randomly chosen in a masked manner from the different groups, digitized, and diameters were measured using a Bioquant image analysis system (RM Biometrics). Statistical analysis was performed to compare the distributions of fibril diameters in the wild-type group and each of the three deficient groups (lumican −/−, fibromodulin −/−, and double lumican/fibromodulin −/−) at 4 d and 3 mo postnatal. Since the normality assumption was not generally supported by the data, only exact nonparametric methods were employed in this analysis. Left-skewed and sometimes bimodal distributions observed for the 3-mo time points prohibited modeling the variance structure in the samples using a regular mixed model. Therefore, for each of the samples, three statistics, sample median, sample median-to-first quartile distance, and sample median-to-third quartile distance, were analyzed. The median indicates the location of the center of the distribution, while median-to-quartile distances reflect primarily the spread of the data and, secondarily, in the case of nonsymmetric distribution, the degree of skewness and possible bimodality. Distributions of the sample to sample medians and both median-to-quartile distances for each deficient group were compared with the wild-type group using the Kolmogorov-Smirnov test. The null hypothesis of this test was that there is no difference between the two compared distributions. To obtain P values, we used the Bonferoni adjustment (for three multiple comparisons). Computations were done using the StatXact 4 (Cytel Software Co.) and SAS 6.12.
Results
Collagen fibrillogenesis is a multi-step process and, in the avian metatarsal tendons, specific developmental periods are associated with distinct steps in collagen fibril assembly and growth (Birk et al. 1989, Birk et al. 1990, Birk et al. 1995, Birk et al. 1996, Birk et al. 1997; Nurminskaya and Birk 1996; Young et al. 2000). Here, we defined analogous periods of development for the mouse flexor tendons and analyzed temporal expression of lumican and fibromodulin at these stages of tendon development. Lumican-null, fibromodulin-null, and double-null mice were used to determine how tendon fibril formation and growth were affected in the absence of these proteoglycans.
Normal Development of Collagen Fibrils in Mouse Tendons
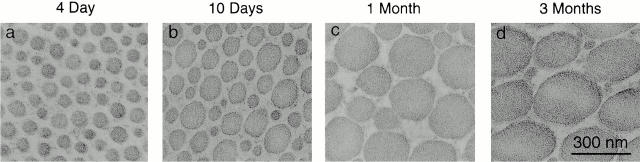
Fibrils were analyzed in cross sections of tendons from 2 d to 7.5 mo in wild-type mice. Relative to specific stages in chicken tendon development, electron microscopic analysis of mouse tendons identified 4 d, 10 d, 1 mo, and 3 mo as representative of different steps in fibrillogenesis (Fig. 1). In the early postnatal period, characterized by the 4-d tendon, the collagen fibrils all had relatively small diameters (64 ± 12 nm, mean ± SD). By 10-d postnatal, the fibril diameters were heterogeneous, with larger diameter fibrils present, in addition to smaller diameter fibrils characteristic of 4 d. By 1 mo, there was a broad distribution of fibril diameters with large diameter fibrils being characteristic of this stage. From 1 to 3 mo, the fibril diameters changed only modestly with the largest diameter fibrils being more prominent in the oldest tendons. The specific stages described were defined as representative of the initial assembly of immature fibril intermediates (4 d), the transition from fibril assembly to growth (10 d), and fibril growth to maturation (1–3 mo). The 3-mo tendon is characteristic of a mature tendon that has completed growth.
Figure 1.
Collagen fibril structure during development in wild-type mouse tendons. Transmission electron micrographs of transverse sections from mouse flexor tendons from normal mice (a–d). Fibril structure was analyzed at different developmental stages, 4 d (a), 10 d (b), 1 mo (c), and 3 mo (d) postnatal. Bar, 300 nm.
Temporal Expression Patterns of Leucine-rich Repeat Proteoglycans
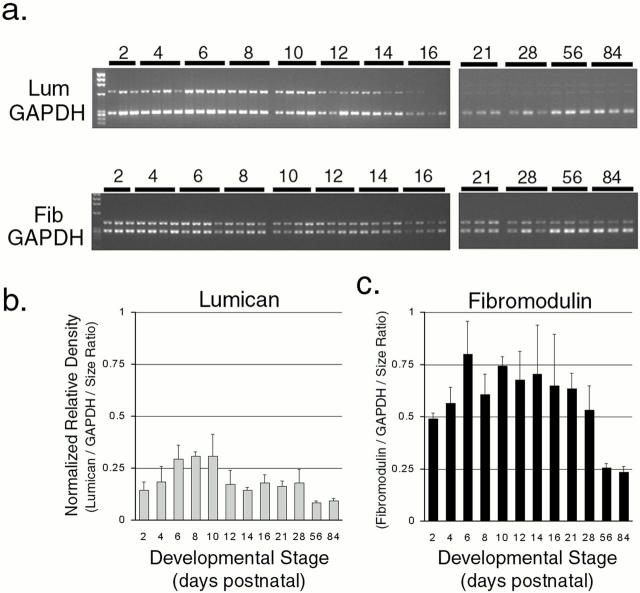
The leucine-rich repeat proteins have been implicated in regulating fibrillogenesis, and we propose that they are involved in regulating the multiple steps observed in tendon fibril formation. Therefore, the temporal expression patterns of lumican and fibromodulin were analyzed during tendon development. Semiquantitative RT-PCR was used to analyze expression patterns for multiple independent samples from 2-d postnatal to adult (Fig. 2). Analysis demonstrated that each proteoglycan had peak expression during early stages from 2 to 14 d. Lumican expression peaked earliest at ∼8 d, and then decreased dramatically. Fibromodulin expression peaked at 14 d and decreased slightly to 1-mo postnatal, followed by a dramatic decrease in the adult expression levels.
Figure 2.
Expression of lumican and fibromodulin during normal tendon development. Lumican and fibromodulin expression were analyzed using semiquantitative RT-PCR. (a) Representative ethidium bromide stained gels showing PCR product bands for lumican, fibromodulin, and coamplified GAPDH bands. Three or four different batches of PCR products generated from independently prepared total RNA between postnatal day 4 and 16 or day 21 and 3 mo were applied to a single gel. (b and c) Band density was determined densitometrically and relative density was obtained by using both band density and the PCR product size ratio, lumican 656 bp, fibromodulin 418 bp, and GAPDH 289 bp. The relative density was normalized using GAPDH and plotted for (b) lumican and (c) fibromodulin.
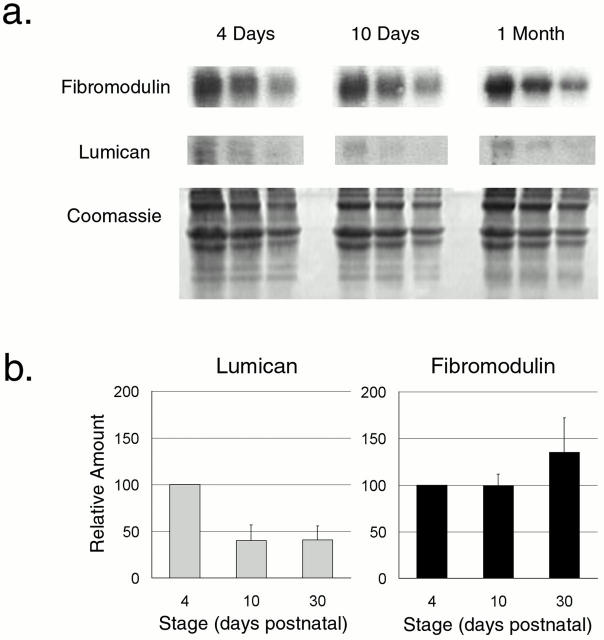
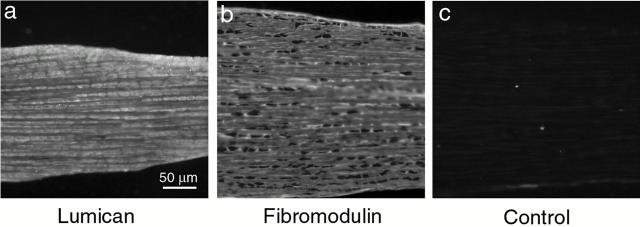
The amount of lumican and fibromodulin core protein present during normal development was analyzed using semiquantitative Western analysis. Fibromodulin core protein increased by ∼30–40% from 4 d to 1 mo, while lumican decreased by ∼60–70% during the same period (Fig. 3). The spatial distribution of the core proteins was analyzed by immunofluorescence histochemistry during normal tendon development. Reactivity for lumican and fibromodulin was present throughout the tendon matrix at 10-d postnatal (Fig. 4). Comparable results were observed in the 4-d and 1-mo tendon (data not shown); however, lumican reactivity was decreased in the 1-mo matrix, consistent with the core protein content. This localization to the region of tendon fibril formation rather than a spatially restricted pattern, for example to the tendon sheath, demonstrates that regulatory interactions are possible. The controls without primary antibody or with an irrelevant antibody were negative. Taken together, these data suggest that lumican functions during early stages in fibrillogenesis, while fibromodulin would function throughout this period with a more prominent role in regulation of the later stages. The codistribution of lumican and fibromodulin with collagen fibrils is consistent with the regulation of fibrillogenesis.
Figure 3.
Lumican and fibromodulin content during normal tendon development. (a) A representative semiquantitative Western analysis of lumican and fibromodulin during development in the normal mouse tendon is presented. Tendons were extracted in 4 M guanidine-HCl at 4 d, 10 d, and 1 mo. 80, 40, and 20 μg of total protein from each time point were loaded onto the gel. The core proteins were transferred, reacted with antilumican or antifibromodulin antisera followed by radiolabeled goat anti–rabbit IgG, and quantitated using phosphoimaging. A duplicate gel stained with Coomassie shows similar amounts of type I collagen in the extracts. (b) The relative lumican and fibromodulin content in the tendon at 4 d, 10 d, and 1 mo postnatal were derived from three independent experiments. The mean values for both lumican and fibromodulin were set to 100 at 4 d and the results were plotted as a function of development (bars indicate SD).
Figure 4.
Localization of lumican and fibromodulin core proteins in normal tendon development. Immunofluorescence microscopy showing the localization of lumican (a) and fibromodulin (b) core proteins in longitudinal sections of normal mouse tendons at 10 d postnatal. Control sections (c) were treated identically without the primary antibody. Bar, 50 μm.
Collagen Fibril Structure during Development in Lumican-, Fibromodulin- and Double Lumican/Fibromodulin–deficient Mice
The formation of collagen fibrils during tendon development in mice deficient in lumican, fibromodulin, or both was analyzed. The fibrils from lumican-, fibromodulin-, and double lumican/fibromodulin–deficient mice showed abnormalities during development relative to the wild-type controls (Fig. 5). Structurally, three distinct abnormalities were observed. First was the premature presence of fibril diameter heterogeneity in the 4-d double-deficient tendon relative to the wild-type controls (Fig. 5, a and m). Secondly, there was an abnormally large number of small diameter fibrils present in the later stages of development, best seen at 3 mo, in the fibromodulin- and double-deficient mice (Fig. 5l and Fig. p). Finally, all three deficient conditions had fibrils with irregular profiles, indicative of abnormal lateral association or a defect in molecular rearrangement after fusion. The 1–3-mo fibromodulin- and double-deficient tendons had large numbers of very abnormal “cauliflower” fibrils, with the double-deficient tendons showing the most severe phenotype (Fig. 5k, Fig. l, Fig. o, and Fig. p). In contrast, the lumican-deficient tendons contained fibrils only slightly irregular in profile at 1–3 mo (Fig. 5g and Fig. h).
Figure 5.
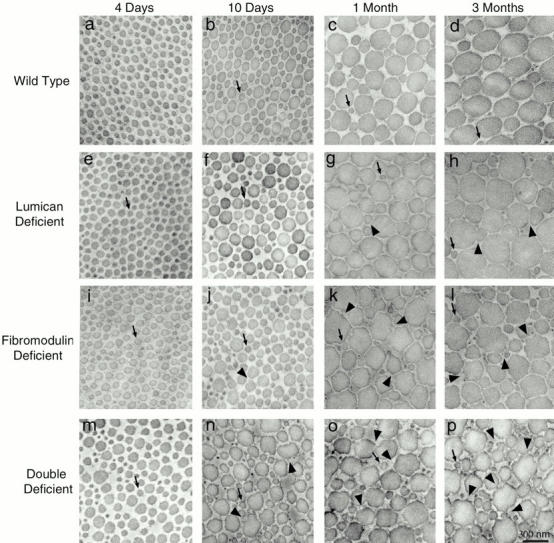
Collagen fibril structure during development in normal wild-type, lumican-, fibromodulin-, and double lumican/fibromodulin–deficient mice. Transmission electron micrographs of transverse sections from mouse flexor tendons from normal mice (a–d) and mutant mice (e–p). Fibril structure was analyzed at different developmental stages between 4 d and 3 mo postnatal, 4 d (a, e, i, and m), 10 d (b, f, j, and n), 1 mo (c, g, k, and o), and 3 mo (d, h, l, and p) postnatal. Arrows indicate fibrils with diameters of ∼64 nm, the diameter seen in normal 4-d postnatal tendons. Arrowheads indicate irregular fibril profiles. Bar, 300 nm.
Collagen Fibril Diameter Distributions during Development of Lumican-Null, Fibromodulin-Null, and Double Lumican/Fibromodulin-Null Tendons
The structural analysis of tendon fibrils (Fig. 5) indicated that alterations in lumican and fibromodulin expression disrupted the regulation of fibrillogenesis, leading to alterations in fibril structure. To assess the function of these proteoglycans, we analyzed the fibril diameters quantitatively during development in the mutant mice.
To characterize the diameter distributions, all the fibril diameters measured (n = 1904–5909) were plotted in single histogram for each group at each developmental point (Fig. 6). To make statistical comparisons possible, the diameter distribution from each photographic negative was separately treated as single sample, and parameters defining the diameter distributions were compared for each deficient group with the wild-type group (Table ). The median was used to indicate the center of the distribution, while median-to-quartile distances reflect the spread of the data and, secondarily, in the case of nonsymmetric distribution, the degree of skewness and possible bimodality.
Table 1.
Analysis of Fibril Diameter Distributions
| Median | Median-Q1 | Median-Q3 | ||||||
|---|---|---|---|---|---|---|---|---|
| No. | Means ± SD | P | Means ± SD | P | Means ± SD | P | ||
| 4 d | ||||||||
| Wild type | 11 | 65.0 ± 4.5 | — | 7.9 ± 1.6 | — | 7.5 ± 1.4 | — | |
| Lumican deficient | 21 | 74.1 ± 7.6 | 0.005 | 10.1 ± 2.1 | 0.037 | 8.3 ± 1.7 | NS | |
| Fibromodulin deficient | 12 | 72.1 ± 6.7 | 0.019 | 12.0 ± 3.5 | 0.012 | 10.8 ± 3.2 | 0.047 | |
| Double deficient | 15 | 88.5 ± 9.4 | <0.001 | 20.6 ± 6.1 | <0.001 | 18.5 ± 5.2 | <0.001 | |
| 3 mo | ||||||||
| Wild type | 34 | 241.0 ± 35.9 | — | 61.0 ± 18.9 | — | 47.1 ± 10.3 | — | |
| Lumican deficient | 21 | 250.0 ± 34.6 | NS | 75.9 ± 31.3 | NS | 46.9 ± 12.8 | NS | |
| Fibromodulin deficient | 20 | 181.0 ± 32.8 | <0.001 | 79.0 ± 22.3 | <0.001 | 71.4 ± 28.4 | 0.001 | |
| Double deficient | 15 | 138.0 ± 26.4 | <0.001 | 63.7 ± 18.2 | NS | 74.2 ± 25.4 | <0.001 | |
To define differences in fibril diameter distributions, the sample medians, sample median-to-first quartile (Q1) distance, and sample median-to-third quartile (Q3) distance were analyzed. The values are presented as means ± SD of the individual samples in each group. The median indicates the location of the center of the distribution, while median-to-quartile distances reflect primarily the spread of the data and secondarily, in the case of nonsymmetric distribution, the degree of skewness and possible bimodality. Values are in nanometers.
At 4-d postnatal, the lumican- and fibromodulin-deficient tendons had fibril diameter distributions that were similar to that observed from wild-type tendon with a mean diameter of 64 nm (Fig. 6, a, e, and i). However, the diameter distributions for lumican- and fibromodulin-deficient mice were shifted to larger diameters; i.e., 72 and 71 nm, respectively. The double-deficient tendons had a broader diameter distribution and there was a shift to even larger diameter fibrils; i.e., 87 nm (Fig. 6 m). Significant differences between the wild-type distribution and the different deficient distributions were shown using a Kolmogorov-Smirnov test (Table ). This demonstrated that the effect of the single mutations was additive during this period of fibrillogenesis and that this class of leucine-rich repeat proteins influenced the assembly of immature fibril intermediates.
At 10-d postnatal, both the lumican- and fibromodulin-deficient tendons had a decrease in the number of the largest diameter fibrils, with a peak at ∼130 nm, compared with the normal tendon. The fibrils with diameters <65 nm were similar to the wild-type tendon. Both deficient tendons demonstrated an accumulation of fibrils with intermediate diameters relative to the wild-type tendon (Fig. 6f and Fig. j). The lumican-deficient tendons had relatively equal numbers of fibrils in all intervals between 65 and 140 nm, while the fibromodulin-deficient tendon demonstrated a clustering at the small diameter end of this range. In spite of the structurally abnormal, irregular fibril profiles (Fig. 5 n), the double lumican/fibromodulin-deficient tendon fibrils had a relatively normal distribution with no apparent preference for different diameter ranges (Fig. 6 n). This distribution is considered to be a transition from the abnormal early phenotype to the later severe phenotype.
At 1–3-mo postnatal, the lumican-deficient tendon had a fibril diameter distribution comparable with the wild-type tendon (Fig. 6c and Fig. d vs. g and h). In contrast, the fibromodulin- and double-deficient tendon fibrils had diameter distributions distinctly different from the normal tendon where preferred fibril diameters were evidenced by a multimodal peak (Fig. 6k, Fig. l, Fig. o, and Fig. p). At 3 mo, these mutant tendons demonstrated a substantial increase in the number of small diameter fibrils; i.e., 65 nm. The double- and fibromodulin-deficient distributions were quite similar. Significant differences between the distribution of fibril diameter in wild-type tendons and both the fibromodulin- and double lumican/fibromodulin-deficient groups were indicated by statistical analysis (Table ). Fibromodulin expression appeared to be required for the progression through fibril growth associated with the later stages of tendon development.
This analysis indicated that, at postnatal day 4, the distribution of fibril diameters in the wild-type tendons was significantly different from the distribution of fibril diameters in each of the three deficient tendons. After 3 mo of development, the distribution of fibril diameters in the wild-type tendon was significantly different from the distribution of fibril diameters in the fibromodulin- and double lumican/fibromodulin-deficient tendons. No significant difference between the wild-type and lumican-deficient tendons after 3 mo was found (Table ).
Discussion
Collagen fibrillogenesis during normal tendon development involves multiple steps. First, a population of short, small-diameter fibril intermediates are assembled. The regulation of the molecular assembly of immature fibril intermediates involves the interaction of type-I collagen with a quantitatively minor fibrillar collagen, such as type-III collagen (Birk and Linsenmayer 1994; Liu et al. 1997). In the tendon, collagen type I/III interactions have been implicated in the regulation of intermediate assembly during this early developmental period (Fleischmajer et al. 1990; Birk and Mayne 1997). We have proposed that this intermediate is stabilized by interactions with fibril-associated molecules. These interactions would have temporal changes during development that would stabilize and destabilize the fibril intermediate. This would regulate the progression through the fibril growth steps and results in the mature, tissue-specific fibril. In this work, we demonstrate that a subfamily of the leucine-rich repeat proteins/proteoglycans (i.e., lumican and fibromodulin) are important regulators of the multiple steps in tendon fibrillogenesis.
Fibril Phenotypes Observed in Mutant Mouse Tendons Indicate Different Functions for Lumican and Fibromodulin
An ultrastructural analysis of fibrils from different developmental periods in lumican-, fibromodulin-, and double lumican/fibromodulin-deficient tendons demonstrated that alterations in amounts of lumican and fibromodulin resulted in abnormal fibril phenotypes. We classified these into the following four types: (a) irregular fibril profiles, (b) increased diameters of fibril intermediates observed at 4 d postnatal, (c) abnormally large number of small-diameter (64-nm) fibrils in later stages of development, and (d) a shift in the diameter distributions to smaller-diameter fibrils relative to wild-type tendon, to a differing extent, in all deficient mouse tendons.
An obvious structural phenotype is the formation of fibrils with irregular fibril profiles that contrast strongly with the normal circular profiles seen in wild-type tendons. The structural defects were expected based on previous work in mature tissues (Chakravarti et al. 1998, Chakravarti et al. 2000; Svensson et al. 1999). However, the temporal specificity in appearance of these irregular fibrils could not have been predicted, nor could the effect of lumican versus fibromodulin deficiency. In the current study, this specific fibril phenotype was shown to be present to varying degrees in all three mutant tendons by 3 mo with the lumican-null phenotype very mild, the double-null phenotype very severe, and the fibromodulin-null phenotype intermediate in severity at this stage of development. Irregular fibril contours were observed at different developmental stages depending on the mutation; i.e., at 10 d to 3 mo with progressing severity in the double-deficient mice, at 1 and 3 mo with mild severity in the lumican-null mice and at 1–3 mo with intermediate severity in the fibromodulin-null mice. Even more novel than the developmental pattern in abnormality of fibril contours were the leucine-rich repeat protein–specific alterations in assembly and progression through fibril growth steps reported here.
A second structural phenotype was the larger diameter fibrils assembled in all the mutant mice during early development. At 4 d of development, there was a shift in the relatively homogeneous fibril diameter distributions to larger diameters. There was a significant increase in mean diameter of the small diameter population (i.e., −64 nm population from wild-type tendons) without abnormal fibril profiles. We regard these fibrils (i.e., 64 nm), observed in the wild-type tendons, as the initial fibril intermediate originally called fibril segments (Birk et al. 1989). The increased diameter indicates an alteration in initial intermediate assembly. The interaction between lumican and/or fibromodulin and collagen fibrils could stabilize the intermediate blocking accretion of collagen to the fibril. There also is evidence that the LRR proteins can interact with collagen molecules before fibril assembly (Scott et al. 1990; Weber et al. 1996; Keene et al. 2000). An interaction at this level could directly influence molecular assembly and alter fibril diameter. Associated with this phenotype was the premature generation of a heterogeneous population in the double-deficient tendons at 4 d postnatal. We interpret this as an additive effect on assembly due to both lumican and fibromodulin deficiency. An alternative explanation is that there is an alteration in initial assembly with a premature entry into the growth phase. The lack of both lumican and fibromodulin would generate intermediates that prematurely enter the growth phase in the absence of the normal regulatory block.
The third structural phenotype was the presence of an abnormally large number of small diameter fibrils (64 nm population) in the later stages of development in the fibromodulin- and double-deficient mice. This was observed beginning at 10 d, but is obvious at 3 mo. This, coupled with the relative lack of the large diameter fibrils in both the fibromodulin-null and double-null mutant mice at 3 mo, indicates a defect in progression into and through the fibril growth phase.
A fourth phenotype was the shift of the upper end of the diameter distribution toward smaller fibrils. At 10 d, the higher ratio of smaller to larger diameter fibrils was seen in all deficient phenotypes. In the lumican-deficient tendon at 1–3 mo, the distribution became comparable with the wild-type tendons. This indicated that the defect in the early steps, due to loss of regulation by lumican, could be overcome by a normal regulation that is fibromodulin dependent in the later stages. At 1–3 mo, the alteration in diameter distribution seen in the fibromodulin- and double-deficient mice (i.e., decrease in numbers of largest diameter fibrils) suggests that fibromodulin facilitates the growth to mature fibrils. This phenotype was associated with a relative increase in the numbers of fibrils found in the midrange of the diameter distribution. This was consistent with the defect being one of progression through intermediates in addition to entry into the growth phase. At this stage, the double-deficient fibril diameter distribution most likely reflects the effects of fibromodulin since, at this time in normal development, lumican expression is low relative to fibromodulin. The lack of preference for large diameter fibrils in the fibromodulin and double-deficient tendon suggests a loss of regulation of the transition through the later growth stages during the formation of mature fibrils.
Multi-Step Model for Regulation of Fibrillogenesis by Lumican and Fibromodulin
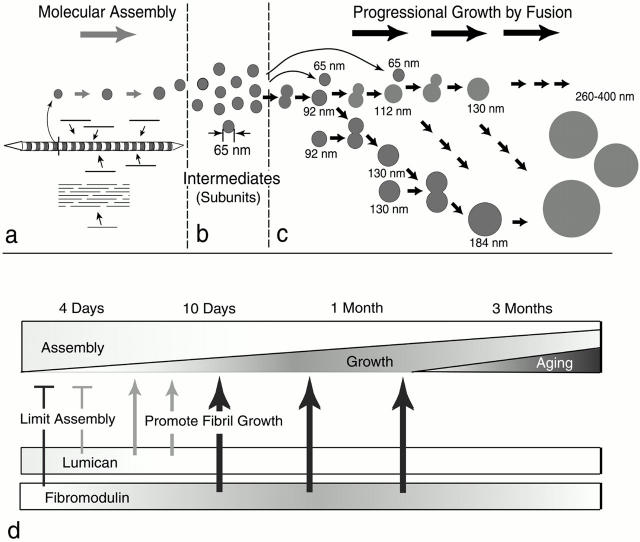
Our model of fibril growth in the tendon and its regulation by lumican and fibromodulin is presented in Fig. 7. The first step in fibrillogenesis is the molecular assembly of collagen monomers into fibril intermediates. Previous studies have shown that growth at this stage is by accretion of collagen and occurs within extracellular compartments, where the microenvironment is under cellular control (Birk and Trelstad 1986). This initial assembly step is regulated, at least in part, by interactions between two fibrillar collagens. In the tendon, these are types I and III (Fleischmajer et al. 1990; Birk and Mayne 1997). In addition, our data indicate that both lumican and fibromodulin influence this step, as evidenced by the larger diameter fibrils seen at 4 d. Recent work indicates that decorin, a related leucine-rich repeat protein, may influence molecular interactions at these early stages or perhaps within secretory vesicles (Weber et al. 1996; Keene et al. 2000). These fibrils have regular profiles that are inconsistent with premature entry into growth, although a rapid molecular rearrangement of the immature fibril intermediates cannot be ruled out. Fibril intermediates ∼64 nm in mouse tendons are stabilized presumably through their interactions with leucine-rich repeat proteoglycans. Our data indicate that both lumican and fibromodulin are involved in this stabilization and mediating the entry into fibril growth. The fibril intermediates are the basic units used in the growth of fibrils (Birk et al. 1989, Birk et al. 1995, Birk et al. 1997; Graham et al. 2000). Fusion of the fibril intermediates generates the mature fibril in a multi-step manner. Progression through this growth process could be both by additive-fusion (i.e., the 64-nm diameter intermediate adds to its product) and by like-fusion (i.e., products from different steps can only fuse with like products of existing fibrils). We suggest that changing patterns of lumican and fibromodulin expression during tendon development are responsible for the regulation of this process. As fibrillogenesis progresses, lumican decreases to barely detectable levels while fibromodulin increases significantly. In addition, lumican and fibromodulin compete for the same binding sites on the fibril and fibromodulin has the higher affinity (Svensson et al. 2000). This suggests that the transitions in growth could be mediated by the fibromodulin-displacing lumican from the fibril surface during a period critical for intermediate fusion. Our data also indicate that fibromodulin facilitates the formation of the mature, large-diameter fibrils seen at 3-mo postnatal.
Figure 7.
Model for regulation of fibril growth by lumican and fibromodulin. The steps in collagen fibrillogenesis during tendon development are presented (a–c). (a) In the early steps of fibril formation, the molecular assembly of collagen monomers into fibril intermediates occurs in the pericellular space. Collagen molecules (bars) assemble into quarter-staggered arrays forming fibril intermediates, seen here as striated structures with tapered ends in longitudinal section and in cross section as circular profiles. Growth in length and diameter is by accretion of collagen at this stage. (b) Fibril intermediates ∼65 nm in mouse tendons are stabilized, presumably through their interactions with fibril-associated macromolecules. (c) The fibril intermediates are the basic units used in the growth of fibrils. Fusion of the fibril intermediates generates the mature fibril in a multistep manner. Progression through this growth process could be both by additive fusion (i.e., the 64-nm diameter intermediate adds to its product, indicated by horizontal arrows) and by like-fusion (i.e., products from different steps can only fuse with like products, indicated by arrows in the oblique direction). (d, top) The proportion of the assembly and growth steps occurring during development is illustrated. At early stages, assembly is the main event and its proportion gradually decreases to the minimum degree necessary for maintenance at maturation. The proportion of progressional growth increases gradually to ∼1 mo and decreases to maturation. (bottom) The expression data for lumican and fibromodluin is illustrated. The phenotypes observed in mutant mice indicate stage-specific regulatory mechanisms. At 4 d, both lumican and fibromodulin limit the assembly of the collagen monomers (bars). Characteristic of 10 d, progressional growth begins, and changes in both lumican and fibromodulin promote the transition from assembly to fibril growth by fusion (thin arrows). At later stages, only fibromodulin promotes the progressional growth steps (thick arrows).
Our data demonstrate that lumican and fibromodulin have differential expression patterns during normal tendon development. The studies of the lumican-, fibromodulin-, and double-deficient mice indicate that these two leucine-rich repeat proteins/proteoglycans have different developmental stage-specific functions in the regulation of fibrillogenesis. We propose that these developmental stage-specific functions are mediated by the differential expression patterns of lumican and fibromodulin.
Acknowledgments
We thank Marguarita Schmid, Jessie Feng, and Jennifer Paul for expert technical assistance. We thank Robert Trelstad for critically reading the manuscript and Walter Hauck for his input on the statistical analyses.
Supported by grants from the National Institutes of Health–National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR44745 to D.E. Birk), National Eye Institute (EY11654 to S. Chakravarti), and The Swedish Medical Research Council (MFR K99-03X-07478-14A to A. Oldberg).
Footnotes
Abbreviations used in this paper: GADPH, glyceraldehyde-3-phosphate dehydrogenase; LRR proteins, leucine-rich repeat proteins; RT-PCR, reverse transcription–PCR.
References
- Birk D.E., Hahn R.A., Linsenmayer C.Y., Zycband E.I. Characterization of fibril segments from chicken embryo cornea, dermis and tendon. Matrix Biol. 1996;15:111–118. doi: 10.1016/s0945-053x(96)90152-3. [DOI] [PubMed] [Google Scholar]
- Birk D.E., Linsenmayer T.F. Collagen fibril assembly, depostion, and organization into tissue-specific matrices. In: Yurchenco P.D., Birk D.E., Mecham R.P., editors. Extracellular Matrix Assembly and Structure. Academic Press, Inc; New York, NY: 1994. pp. 91–128. [Google Scholar]
- Birk D.E., Mayne R. Localization of collagen types I, III and V during tendon development. Changes in collagen types I and III are correlated with changes in fibril diameter. Eur. J. Cell Biol. 1997;72:352–361. [PubMed] [Google Scholar]
- Birk D.E., Nurminskaya M.V., Zycband E.I. Collagen fibrillogenesis in situfibril segments undergo post-depositional modifications resulting in linear and lateral growth during matrix development. Dev. Dyn. 1995;202:229–243. doi: 10.1002/aja.1002020303. [DOI] [PubMed] [Google Scholar]
- Birk D.E., Trelstad R.L. Extracellular compartments in tendon morphogenesiscollagen fibril, bundle, and macroaggregate formation. J. Cell Biol. 1986;103:231–240. doi: 10.1083/jcb.103.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk D.E., Zycband E.I., Winkelmann D.A., Trelstad R.L. Collagen fibrillogenesis in situfibril segments are intermediates in matrix assembly. Proc. Natl. Acad. Sci. USA. 1989;86:4549–4553. doi: 10.1073/pnas.86.12.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk D.E., Zycband E.I., Winkelmann D.A., Trelstad R.L. Collagen fibrillogenesis in situ. Discontinuous segmental assembly in extracellular compartments. Ann. NY Acad. Sci. 1990;580:176–194. doi: 10.1111/j.1749-6632.1990.tb17928.x. [DOI] [PubMed] [Google Scholar]
- Birk D.E., Zycband E.I., Woodruff S., Winkelmann D.A., Trelstad R.L. Collagen fibrillogenesis in situfibril segments become long fibrils as the developing tendon matures. Dev. Dyn. 1997;208:291–298. doi: 10.1002/(SICI)1097-0177(199703)208:3<291::AID-AJA1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Chakravarti S., Magnuson T., Lass J.H., Jepsen K.J., LaMantia C., Carroll H. Lumican regulates collagen fibril assemblyskin fragility and corneal opacity in the absence of lumican. J. Cell Biol. 1998;141:1277–1286. doi: 10.1083/jcb.141.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarti S., Petroll M., Hassell J., Jester J., Lass J.H., Paul J., Birk D.E. Corneal opacity in lumican-deficient mice due to collagen fibril structure and packing defects in the posterior stroma. IOVS (Invest. Ophthalmol. Vis. Sci.). 2000;41:3365–3373. [PMC free article] [PubMed] [Google Scholar]
- Danielson K.G., Baribault H., Holmes D.F., Graham H., Kadler K.E., Iozzo R.V. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J. Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmajer R., Perlish J.S., Burgeson R.E., Shaikh-Bahai F., Timpl R. Type I and Type III collagen interactions during fibrillogenesis. Ann. NY Acad. Sci. 1990;580:161–175. doi: 10.1111/j.1749-6632.1990.tb17927.x. [DOI] [PubMed] [Google Scholar]
- Funderburgh J.L., Caterson B., Conrad G.W. Distribution of proteoglycans antigenically related to corneal keratan sulfate proteoglycan. J. Biol. Chem. 1987;262:11634–11640. [PubMed] [Google Scholar]
- Graham H.K., Holmes D.F., Watson R.B., Kadler K.E. Identification of collagen fibril fusion during vertebrate tendon morphogenesis. The process relies on unipolar fibrils and is regulated by collagen-proteoglycan interaction. J. Mol. Biol. 2000;295:891–902. doi: 10.1006/jmbi.1999.3384. [DOI] [PubMed] [Google Scholar]
- Hedbom E., Heinegard D. Interaction of a 59-kDa connective tissue matrix protein with collagen I and collagen II. J. Biol. Chem. 1989;264:6898–6905. [PubMed] [Google Scholar]
- Hedbom E., Heinegard D. Binding of fibromodulin and decorin to separate sites on fibrillar collagens. J. Biol. Chem. 1993;268:27307–27312. [PubMed] [Google Scholar]
- Hedlund H., Mengarelli-Widholm S., Heinegard D., Reinholt F.P., Svensson O. Fibromodulin distribution and association with collagen. Matrix Biol. 1994;14:227–232. doi: 10.1016/0945-053x(94)90186-4. [DOI] [PubMed] [Google Scholar]
- Iozzo R.V. Matrix proteoglycansfrom molecular design to cellular function. Annu. Rev. Biochem. 1998;67:609–652. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- Iozzo R.V. The biology of the small leucine-rich proteoglycans. Functional network of interactive proteins. J. Biol. Chem. 1999;274:18843–18846. doi: 10.1074/jbc.274.27.18843. [DOI] [PubMed] [Google Scholar]
- Kadler K.E., Holmes D.F., Trotter J.A., Chapman J.A. Collagen fibril formation. Biochem. J. 1996;316:1–11. doi: 10.1042/bj3160001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene D.R., San Antonio J.D., Mayne R., McQuillan D.J., Sarris G., Santoro S.A., Iozzo R.V. Decorin binds near the C-terminus of Type I collagen. J. Biol. Chem. 2000;275:21801–21804. doi: 10.1074/jbc.C000278200. [DOI] [PubMed] [Google Scholar]
- Larsson T., Sommarin Y., Paulsson M., Antonsson P., Hedbom E., Wendel M., Heinegard D. Cartilage matrix proteins. A basic 36-kDa protein with a restricted distribution to cartilage and bone. J. Biol. Chem. 1991;266:20428–20433. [PubMed] [Google Scholar]
- Liu X., Wu H., Byrne M., Krane S., Jaenisch R. Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proc. Natl. Acad. Sci. USA. 1997;94:1852–1856. doi: 10.1073/pnas.94.5.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurminskaya M.V., Birk D.E. Differential expression of fibromodulin mRNA associated with tendon fibril growthisolation and characterization of a chicken fibromodulin cDNA. Biochem. J. 1996;317:785–789. doi: 10.1042/bj3170785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldberg A., Antonsson P., Lindblom K., Heinegard D. A collagen-binding 59-kd protein (fibromodulin) is structurally related to the small interstitial proteoglycans PG-S1 and PG-S2 (decorin) EMBO (Eur. Mol. Biol. Organ.) J. 1989;8:2601–2604. doi: 10.1002/j.1460-2075.1989.tb08399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle G.A., Dodd C.M. Immunoelectron microscopic localization of the core protein of decorin near the d and e bands of tendon collagen fibrils by use of monoclonal antibodies. J. Histochem. Cytochem. 1990;38:1405–1411. doi: 10.1177/38.10.1698203. [DOI] [PubMed] [Google Scholar]
- Rada J.A., Schrecengost P.K., Hassell J.R. Regulation of corneal collagen fibrillogenesis in vitro by corneal proteoglycan (lumican and decorin) core protein. Exp. Eye Res. 1993;56:635–648. doi: 10.1006/exer.1993.1081. [DOI] [PubMed] [Google Scholar]
- Scott J.E. Proteoglycan-collagen interactions. Ciba. Found. Symp. 1986;124:104–124. doi: 10.1002/9780470513385.ch7. [DOI] [PubMed] [Google Scholar]
- Scott J.E., Cummings C., Greiling H., Stuhlsatz H.W., Gregory J.D., Damle S.P. Examination of corneal proteoglycans and glycosaminoglycans by rotary shadowing and electron microscopy. Int. J. Biol. Macromol. 1990;12:180–184. doi: 10.1016/0141-8130(90)90029-a. [DOI] [PubMed] [Google Scholar]
- Svensson L., Aszodi A., Reinholt F.P., Fassler R., Heinegård D., Oldberg Å. Fibromodulin-null mice have abnormal collagen fibrils, tissue organization, and altered lumican deposition in tendon. J. Biol. Chem. 1999;274:9636–9647. doi: 10.1074/jbc.274.14.9636. [DOI] [PubMed] [Google Scholar]
- Svensson L., Narlid I., Oldberg A. Fibromodulin and lumican bind to the same region on collagen type I fibrils. FEBS Lett. 2000;470:178–182. doi: 10.1016/s0014-5793(00)01314-4. [DOI] [PubMed] [Google Scholar]
- Vogel K.G., Paulsson M., Heinegard D. Specific inhibition of type I and type II collagen fibrillogenesis by small proteoglycan of tendon. Biochem. J. 1984;223:587–597. doi: 10.1042/bj2230587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber I.T., Harrison R.W., Iozzo R.V. Model structure of decorin and implications for collagen fibrillogenesis. J. Biol. Chem. 1996;271:31767–31770. doi: 10.1074/jbc.271.50.31767. [DOI] [PubMed] [Google Scholar]
- Young B.B., Gordon M.K., Birk D.E. Expression of type XIV collagen in developing chicken tendonsassociation with assembly and growth of collagen fibrils. Dev. Dyn. 2000;217:430–439. doi: 10.1002/(SICI)1097-0177(200004)217:4<430::AID-DVDY10>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]