Abstract
Desmogleins are desmosomal cadherins that mediate cell–cell adhesion. In stratified squamous epithelia there are two major isoforms of desmoglein, 1 and 3, with different distributions in epidermis and mucous membrane. Since either desmoglein isoform alone can mediate adhesion, the reason for their differential distribution is not known. To address this issue, we engineered transgenic mice with desmoglein 3 under the control of the involucrin promoter. These mice expressed desmoglein 3 with the same distribution in epidermis as found in normal oral mucous membranes, while expression of other major differentiation molecules was unchanged. Although the nucleated epidermis appeared normal, the epidermal stratum corneum was abnormal with gross scaling, and a lamellar histology resembling that of normal mucous membrane. The mice died shortly after birth with severe dehydration, suggesting excessive transepidermal water loss, which was confirmed by in vitro and in vivo measurement. Ultrastructure of the stratum corneum showed premature loss of cohesion of corneocytes. This dysadhesion of corneocytes and its contribution to increased transepidermal water loss was confirmed by tape stripping. These data demonstrate that differential expression of desmoglein isoforms affects the major function of epidermis, the permeability barrier, by altering the structure of the stratum corneum.
Keywords: cadherins, cell adhesion molecules, desmosome, epidermis, skin
Introduction
Desmogleins and desmocollins are transmembrane glycoproteins of the desmosome, a cell–cell adhesive structure prominent in epithelial tissues (Garrod et al. 1999). Both glycoproteins exist in three isoforms encoded by separate genes.
Several lines of evidence suggest that these glycoproteins are critical for the cell–cell adhesion function of the desmosome. First, desmogleins and desmocollins are members of the cadherin gene superfamily, of which the prototypic members (e.g., E-cadherin) mediate adhesion. Second, in some systems, transfection of desmosomal cadherins with plakoglobin, an intracellular plaque protein of the desmosome, confers adhesive properties to cells (Tselpsis et al. 1998). Finally, antidesmoglein antibodies in pemphigus cause a loss of keratinocyte cell adhesion (Mahoney et al. 1999).
Although both desmogleins and desmocollins may be needed for adequate cell adhesion, why there are different isoforms of each in different layers of the same tissue is not clear. For example, desmoglein (Dsg) 1 is expressed throughout all the nucleated cell layers of the epidermis, but Dsg 3 is found only in the deep epidermis, whereas in oral mucous membrane Dsg 3, like Dsg 1, is found throughout all the nucleated cell layers (Amagai et al. 1996; Shirakata et al. 1998). It is unlikely that this tissue-specific distribution of Dsg isoforms is critical for adhesion in the living layers of the epidermis, at least in skin not under stress, because recent evidence suggests that either Dsg 1 or Dsg 3 alone is adequate for cell–cell adhesion. For instance, Dsg 1 alone provides adequate cell adhesion in the superficial nucleated cell layers of the epidermis, even though it is the only Dsg expressed at that level. Furthermore, since superficial blisters do not occur within the skin or mucous membranes of Dsg 3 knockout mice, Dsg 1 suffices for adhesion in the superficial epithelium (Koch et al. 1997). Finally, we recently showed that Dsg 3 can substitute for loss of Dsg 1–mediated adhesive function induced by passive transfer of anti–Dsg 1 pemphigus foliaceus antibodies (Wu et al. 2000). Therefore, Dsg 1 or Dsg 3 alone can provide adequate cell adhesion in normal epidermis.
One explanation for the variable distribution of desmoglein isoforms in different epithelia might be to provide adhesion that is appropriate for the specific types of stress to which these epithelia are subjected. However, another possibility is that there are functions of desmogleins beyond simple adhesion. Accordingly, we hypothesized that the differential distribution of desmoglein isoforms might influence the differentiation and/or function of these epithelia. To test this hypothesis, we constructed a transgenic mouse in which the distribution of Dsg 3 in epidermis was similar to that in oral mucous membranes. In these mice, the epidermal stratum corneum displayed some properties that resembled those of the stratum corneum in mucosal epithelia, including histological features, decreased cohesion, and increased transepidermal water loss that often resulted in lethality during the first week of life due to dehydration. These results demonstrate that the distribution of desmoglein isoforms in stratified squamous epithelia regulates both the structure and function of these epithelia.
Materials and Methods
Construction and Genotyping of Involucrin-Dsg 3 Transgenic Mouse
The involucrin promoter vector (pH3700-pL2), which also contained the first involucrin intron, an SV40 intron, a β-galactosidase gene, and an SV40 polyadenylation site, was a gift from Dr. Lorne Taichman (State University of New York at Stony Brook, Stony Brook, NY; Carroll and Taichman 1992). Mouse cDNA encoding Dsg 3 was cloned as previously described (Ishikawa et al. 1994, Ishikawa et al. 2000; Wu et al. 2000) (Genbank U86016). PCR of the 3′ end was used to add 27 nucleotides that encode the FLAG octapeptide epitope. The final sequence was confirmed by nucleotide sequencing. The β-galactosidase gene was removed from pH3700-pL2 by digestion with restriction enzyme NotI and replaced with the mouse Dsg 3-FLAG cDNA. The transgene (containing the involucrin promoter and mouse Dsg 3-FLAG) was excised from the involucrin cassette with SalI and microinjected into the male pronuclei of B6SJLF1/J mice zygotes before implantation into pseudopregnant foster CD-1 mice.
Mouse tail DNA, extracted with Puregene Genomic DNA Isolation Kit (Gentra Systems), was used for PCR and Southern blotting to establish genotypes. PCR primers from desmoglein 3 exon 7 (5′-AACTTCCCAGTGCTCAGAGAGTCTC-3′) and exon 8 (5′-TTAACCACCTTCAGGATGCCTTC-3′) were used to identify genomic DNA (671-bp product) or transgenic cDNA (213-bp product) (see Fig. 1 B). Southern blotting was performed with a digoxigenin-dUTP–labeled probe generated by PCR of mouse Dsg 3 cDNA with primer sets starting at nucleotide positions 976 and 1497 (5′-GAAGGCATCCTGAAGGTGGT-3′ and 5′-CTTCTCGAGGACAACTGACG-3′), according to the PCR DIG probe synthesis kit (Boehringer). Genomic DNA was digested with HindIII, electrophoresed, and transferred to MagnaGraph nylon membranes (Osmonics), and then, after hybridization and washing, was incubated with horseradish peroxidase–conjugated antidigoxygenin antibody and developed with a chemiluminescence substrate (CSPD; Boehringer).
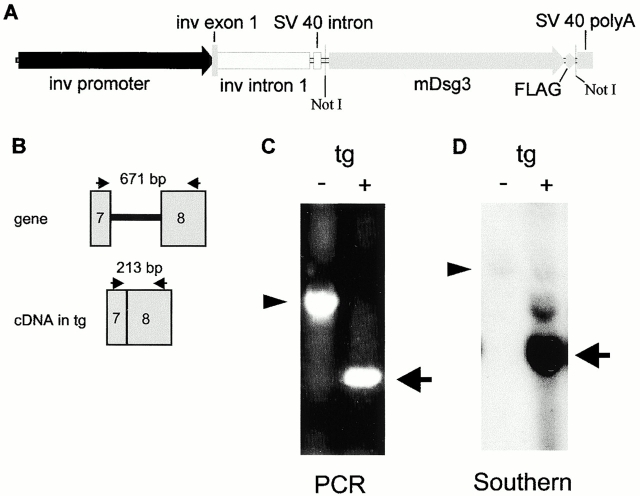
Figure 1.
Genotyping of involucrin-Dsg 3 transgenic mice. (A) Transgenic construct. Inv, involucrin; mDsg3, mouse Dsg 3; FLAG, indicates nucleotides encoding the FLAG peptide epitope; polyA, polyadenylation signal. NotI shows restriction sites used to clone the mDsg 3-FLAG cDNA into the pH3700-pL2 parental vector. (B) PCR strategy to differentiate the transgene from the genomic mouse Dsg 3 DNA. (Arrows) Position of primers, (gray rectangles) exons, (black line) intron. Tg, transgene. (C) PCR analysis of transgenic mouse (+) compared with nontransgenic (−) littermate. (Arrowhead) 671-bp genomic PCR product, (arrow) 213-bp transgene product. (D) Southern blot detects transgene (arrow) and genomic DNA (arrowhead).
Antibodies
A rabbit affinity-purified antibody against extracellular domain 5 of mouse Dsg 3 (Koch et al. 1998) and a rabbit anti–FLAG epitope antibody (Zymed Laboratories) were used for indirect immunofluorescence.
Rabbit antisera against extracellular domain 5 of Dsg 3 and Dsg 1 were used for immunoblotting, as was a mouse monoclonal anti–FLAG antibody (Eastman Kodak Co.). The rabbit anti–mouse Dsg 3 antiserum against extracellular domain 5 of Dsg 3 was prepared as described (Koch et al. 1998). (The same antiserum was used to prepare the affinity-purified antibody described above.) The rabbit antiserum against extracellular domain 5 of Dsg 1 was prepared similarly.
Rabbit antibodies against loricrin, involucrin, filaggrin, and keratin 10 were obtained from Babco.
Immunofluorescence and Immunoblotting
Indirect immunofluorescence of formalin-fixed mouse skin with rabbit antibodies was performed as previously described (Wu et al. 2000). Rabbit IgG was detected with Texas red–conjugated (Molecular Probes) or Cy3-conjugated (Jackson ImmunoResearch Laboratories) goat anti–rabbit IgG.
For immunoblotting, mouse back skin or mucous membrane (tongue) was homogenized on dry ice, and then extracted with Laemmli sample buffer. Samples with equal protein amounts (Protein Assay Kit; Bio-Rad Laboratories), were electrophoresed on 6% Tris-glycine polyacrylamide gels (Novex), and then transferred to nitrocellulose sheets (Trans-Blot; Bio-Rad Laboratories). The sheets were incubated for 1 h in blocking buffer [5% fat-free milk powder in 50 mM Tris-HCl, pH 7.4, 150 mM NaCl (TBS)]. The first antibody, diluted in blocking buffer, was then applied for 1 h at room temperature. After two washes with 0.1% Tween-20 in TBS (anti–FLAG) or 0.5% Triton X-100, 0.5 M NaCl in TBS (anti–Dsg), the sheets were incubated for 2 h with horseradish peroxidase–conjugated anti–rabbit or anti–mouse IgG (Bio-Rad Laboratories) diluted 1:1,000 in blocking buffer. After washing with 0.1% Tween-20 in TBS, the signals were detected with chemiluminescence (ECL; Amersham Pharmacia Biotech). Signals were quantitated by densitometry with ImageQuant software (Molecular Dynamics).
Transepidermal Water-loss Measurement
6-mm punch biopsies, taken from the flank skin of 3–8-d-old transgenic pups and normal littermates, were placed in silicone high vacuum grease (Dow Corning) dermal side down on parafilm, and then weighed every 30 min (Hanley et al. 1996). The grease covered all exposed dermal surfaces so that water loss would only be through the epidermis.
We also used an electrolytic water analyzer (Meeco) to measure transepidermal water loss of 6-d-old pups from three sites (both shoulders and left hip), as previously described (Menon et al. 1985).
Electron Microscopy
Skin samples, fixed in Karnovsky's fixative, were analyzed as previously described (Hou et al. 1991).
Corneocyte Tape Stripping
d-Squame disks (CuDerm Corp.) were used to strip stratum corneum cells as previously described (Dreher et al. 1998); however, instead of the Bio-Rad protein assay, we used the Bradford protein assay (Bradford 1976).
Results
Genetic and Expression Analysis of Involucrin-Dsg 3 Transgenic Mice
To determine whether different distributions of desmoglein isoforms affect the structure or function of epidermis, we engineered a transgenic mouse expressing mouse Dsg 3 in epidermis in the pattern normally found only in mucous membranes. The transgene placed mouse Dsg 3 cDNA under the control of the involucrin promoter (Fig. 1 A). Nucleotides encoding the FLAG peptide sequence, which can be detected with specific antibodies, were added to the 3′ end of the coding sequence. Since involucrin is expressed normally in the suprabasilar layers of epidermis (Rice and Green 1979; Carroll et al. 1993), this transgenic mouse would be expected to express Dsg 3 in both the deep epidermis (from the normal gene) and the more superficial epidermis (from the transgene). Two independent transgenic mouse lines were obtained, and both showed essentially identical findings.
Analysis by PCR with primers in exons 7 and 8 of mouse Dsg 3 DNA identified a germline band of 671 bp, compared with a band from the transgene cDNA of 213 bp, clearly differentiating the transgenic mouse from the nontransgenic littermate (Fig. 1B and Fig. C). This genotyping was confirmed by Southern blotting (Fig. 1 D), which showed a copy number for the transgene of ∼47 and 51, respectively, in the first and second founders' lines.
Expression of the transgene was detected by Western blotting for the FLAG-tagged mouse Dsg 3 of extracted skin from transgenic neonatal mice (Fig. 2 A). Distribution of expression of the transgenic mouse Dsg 3-FLAG was detected by immunofluorescence with anti–FLAG antibodies of mouse skin, which showed cell-surface staining in the suprabasilar layers of the epidermis (Fig. 2 B). The distribution of both transgenic and nontransgenic Dsg 3 in mouse skin was detected by immunofluorescence with an anti–mouse Dsg 3 antibody. Whereas nontransgenic littermates showed Dsg 3 only in the deep epidermis (Fig. 2 C), transgenic animals showed Dsg 3 throughout the entire epidermis (Fig. 2 D), in a pattern identical to that described in oral mucous membrane (Mahoney et al. 1999).
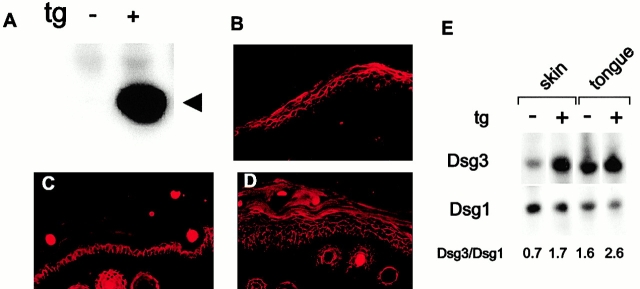
Figure 2.
Analysis of mouse Dsg 3 expression in transgenic and normal mouse skin. (A) Immunoblot with anti–FLAG antibody indicates expression of mouse Dsg 3-FLAG (arrowhead, ∼160 kD) in extract of transgenic (+) mouse skin. Nontransgenic littermate (−) skin serves as a control. (B) Immunofluorescence of transgenic mouse tail skin for FLAG epitope shows expression of transgene in suprabasilar area of epidermis. (C) Immunofluorescence of nontransgenic mouse tail for mouse Dsg 3 shows expression in the basal and immediate suprabasal layer of epidermis. (D) Immunofluorescence of transgenic mouse tail for mouse Dsg 3 shows expression throughout the epidermis. (B–D) Original magnification 100×. (E) Immunoblotting for mouse Dsg 3 and mouse Dsg 1 from extracts of mouse skin and tongue show that transgenic (scaly) epidermis from founder 1 has a Dsg 3/Dsg 1 ratio similar to normal tongue mucous membrane, whereas transgenic mucous membrane from the same founder expresses excess Dsg 3.
To determine the approximate ratios of Dsg 3 to Dsg 1 in transgenic skin compared with normal mucous membrane, we quantitated the band intensities of Western blots (Fig. 2 E). These data showed that the ratio of Dsg 3 to Dsg 1 in transgenic skin of founder 1 was approximately that seen in normal oral mucous membrane (i.e., tongue), but higher than that seen in normal epidermis. The ratio of Dsg 3 to Dsg 1 in the transgenic skin of founder 2 (2.3) was higher than in founder 1 (1.7), although both showed similar scaling phenotypes. These data show that the Dsg 3 to Dsg 1 ratio in transgenic skin more closely resembles that found normal tongue mucosa than that found in normal epidermis.
Involucrin-Dsg 3 Transgenic Mice Show an Abnormal Epidermal Stratum Corneum with Histological Similarities to Oral Mucous Membrane
Although transgenic mice appeared normal at birth, within 2–3 d they developed scaling and were noted to be smaller than control littermates (Fig. 3 A), even though they had visible milk in their stomachs. The degree of scaling of transgenic mice was somewhat variable and correlated positively with the degree of transgene expression, as determined by Western blotting with anti–FLAG antibody. For example, while mice heterozygous for the transgene from the first founder were nonscaling, homozygous mice were scaling and showed at least twice as much expression of Dsg 3-FLAG by Western blotting. Heterozygous mice from the second founder showed scaling at age 2–3 d, and Western blots for Dsg 3-FLAG showed equivalent amounts to that seen in homozygotes from the first founder. Homozygous mice from the first founder died by age 7–10 d. Most heterozygote mice from the second founder died by 10 d, but some survived and those that did were able to breed.
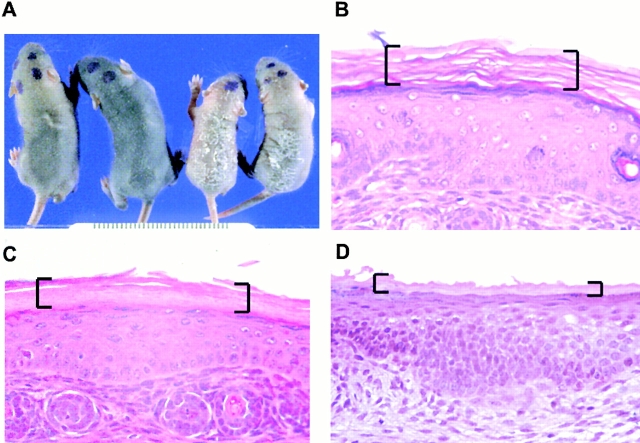
Figure 3.
Involucrin-Dsg 3 transgenic mice have an abnormal stratum corneum. (A) Right two mice are transgenic pups, left two are normal littermates. Scaling of transgenics indicates an abnormal stratum corneum (all pups are 6-d old). (B) Histology (hematoxylin and eosin stain) of nontransgenic tail skin shows typical basket weave stratum corneum (brackets). (C) Histology of transgenic tail skin shows normal living epidermis but compact and lamellar stratum corneum (brackets). (D) Histology of normal palate shows compact and lamellar stratum corneum, similar to that seen in the transgenic epidermis (brackets). (B–D) 4-d-old pups, original magnification 125×.
Scaly, transgenic mice were killed and autopsied at 4–6 d. Other than the skin, the histology of all organs and tissues, including mucous membranes, was normal. The epidermis showed a strikingly altered stratum corneum (Fig. 3 C). Compared with the normal stratum corneum of skin, which typically shows a “basket weave” pattern (Fig. 3 B), the stratum corneum of the transgenic epidermis was thinner with a compact lamellar pattern, similar to that seen in normal oral mucous membrane (Fig. 3 D).
Involucrin-Dsg 3 Transgenic Mice Demonstrate Abnormal Cutaneous Barrier Function
Other than those of the skin, the only abnormalities noted in the transgenic neonatal mice were a significantly increased hematocrit and serum protein concentration, as well as decreased weight. At age 6–7 d, the hematocrit of the scaly transgenic mice was 43 ± 1.2% (mean ± SEM, n = 7), compared with 32 ± 0.7% (n = 7) for normal littermates; the total serum protein of these transgenic mice was 4.1 ± 0.1 g/dl (mean ± SEM), compared with 2.3 ± 0.1 g/dl for normal littermates. The weight of these transgenics was 2.6 ± 0.09 g (mean ± SEM), compared with nontransgenic littermates of the same age that weighed 3.9 ± 0.16 g. This hemoconcentration, along with excess scale and altered histology of the stratum corneum, suggested that the transgenic mice might have a permeability barrier defect, reflected by increased transepidermal water loss leading to dehydration, decreased weight, and eventual death.
To test this hypothesis, we first isolated 7-d-old transgenic and nontransgenic littermates from their mothers and weighed them for 5 h (Fig. 4 A). In all cases, transgenic mice lost weight faster than nontransgenic mice. Presumably this accelerated weight loss of transgenic mice could be ascribed to increased transepidermal water loss. However, it is possible that other fluid loss (e.g., urine) could have accounted for these differences, although no other evidence of fluid loss was apparent.
Figure 4.
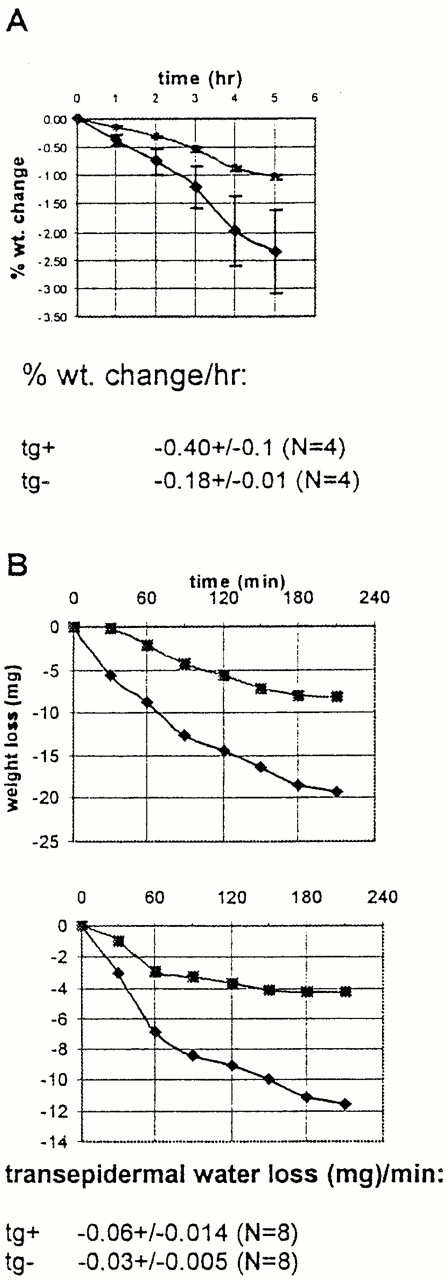
Involucrin-Dsg 3 transgenic mice show increased transepidermal water loss. (A) Neonatal mice were separated from their mothers and weighed over time. Transgenic mice (♦) lose weight faster than nontransgenic littermates (•). Error bars indicate SEM for four mice in each group. Rate of weight loss, mean ± SEM is shown. (B) Weight loss of isolated skin from transepidermal water loss through the epidermis. Graphs show paired comparison of transgenic mouse skin (♦) and nontransgenic littermate skin (▪). Rate of transepidermal water loss, mean ± SEM for eight samples in each group is shown.
To examine epidermal barrier function directly, we measured the rate of water loss from the surface of 6-mm punch biopsies from the skin of transgenic and nontransgenic 3–8-d-old mice. We performed paired comparisons for weight change, as a measure of surface water loss, over time of a transgenic and nontransgenic skin sample for each experiment. In each paired comparison (n = 8), the transgenic skin displayed higher rates of transcutaneous water loss than did the nontransgenic skin (Fig. 4 B shows two-paired comparison and mean ± SEM of all data). We also measured rates of transepidermal water loss in vivo at the skin surface of 6-d-old pups with an electrolytic water analyzer. Transgenic mice displayed significantly higher rates of water loss, 43 ± 9 × 10−2 mg/cm2 per h (mean ± SEM, n = 4), in comparison with nontransgenic littermates, 18 ± 2 × 10−2 mg/cm2 per h (mean ± SEM, n = 3).
Analysis of Epidermal Ultrastructure of Involucrin-Dsg 3 Mice
The epidermal nucleated cell layers of the living epidermis showed comparable normal ultrastructure, including desmosomal substructure, in transgenic and nontransgenic littermates. However, the epidermal stratum corneum of transgenic animals differed strikingly from that of nontransgenic littermates. Normally, with the transition from the stratum granulosum to the stratum corneum, and then, again, in the superficial stratum corneum, the desmosome changes structure (Menon and Elias 1997; Fartasch et al. 1993). In the nucleated layer, it is seen as two opposing dense plaques within the cell with an electron lucent center. In the stratum corneum, it abruptly becomes an electron dense intercellular structure (Fig. 5 B, arrows). In the normal stratum corneum, these desmosomal structures are well maintained with closely opposed cells until near the surface of the skin when the desmosomal structures degenerate and the corneocytes separate (Odland 1991). In contrast to this normal structure, at the stratum granulosum–stratum corneum interface and extending to the lowermost stratum corneum in transgenic mice, we noted premature dissolution of desmosomes with early cleft formation between adjacent corneocytes (compare Fig. 5A with B). This finding in the transgenic epidermal stratum corneum was similar to the early dissolution of desmosomes that occurs in stratum corneum of normal oral mucous membrane (Fig. 5 C). Premature dissolution of desmosomes with cleft formation was even more obvious in ruthenium tetroxide postfixed tissues, where the relationship of the clefts to the extracellular lamellar matrix could be appreciated (Fig. 6). These findings are consistent with loss of barrier function from early loss of corneocyte adhesion in these transgenic animals.
Figure 5.
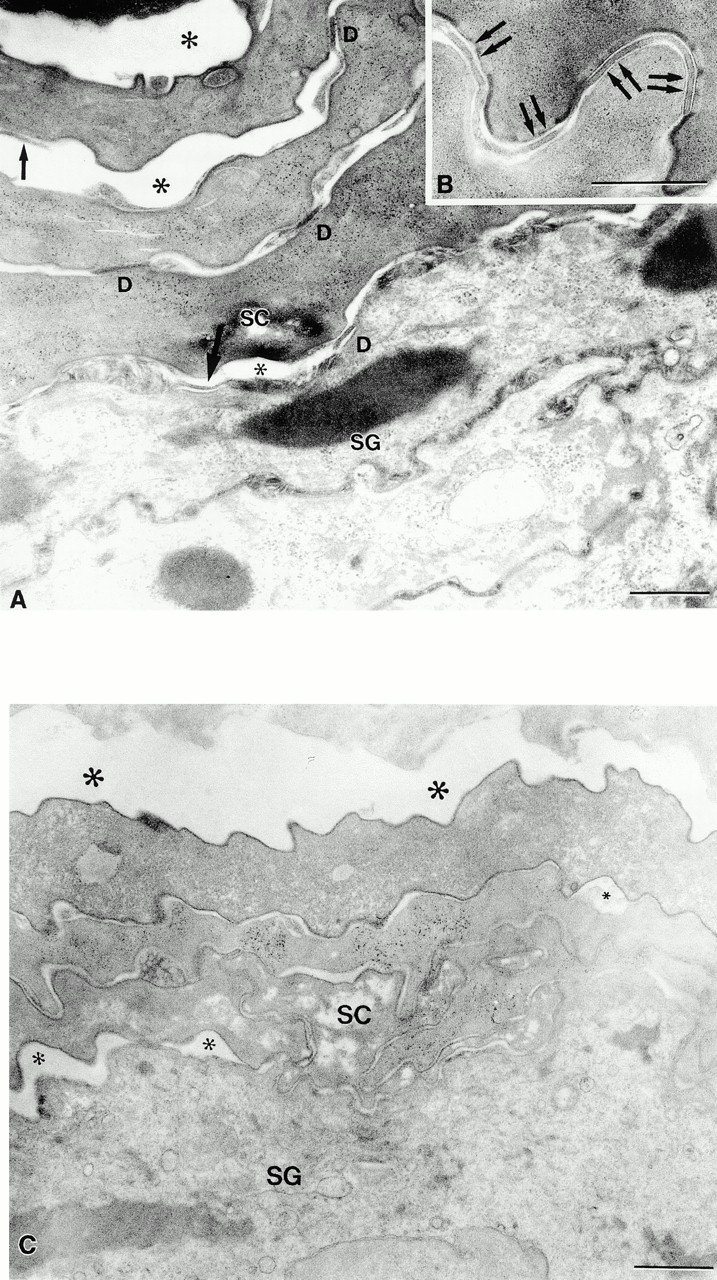
Dsg 3 in the superficial epidermis of transgenic mice results in premature dissolution of desmosomes and cleft formation in the stratum corneum. (A) Dissolution of desmosomes begins at the stratum granulosum (SG)–stratum corneum (SC) interface. Desmosomal detachment (single arrows) results in cleft formation (*) at all levels of the stratum corneum. D, normal-appearing desmosomes. (B) Normal-appearing desmosomes in control stratum corneum. (C) Normal mucous membrane (lip) also reveals early cleft formation. Note small (small *) clefts at SG–SC interface and larger clefts (large *) at higher levels. Bar: 0.5 μm (A and B), 1 μm (C). Osmium tetroxide postfixation.
Figure 6.
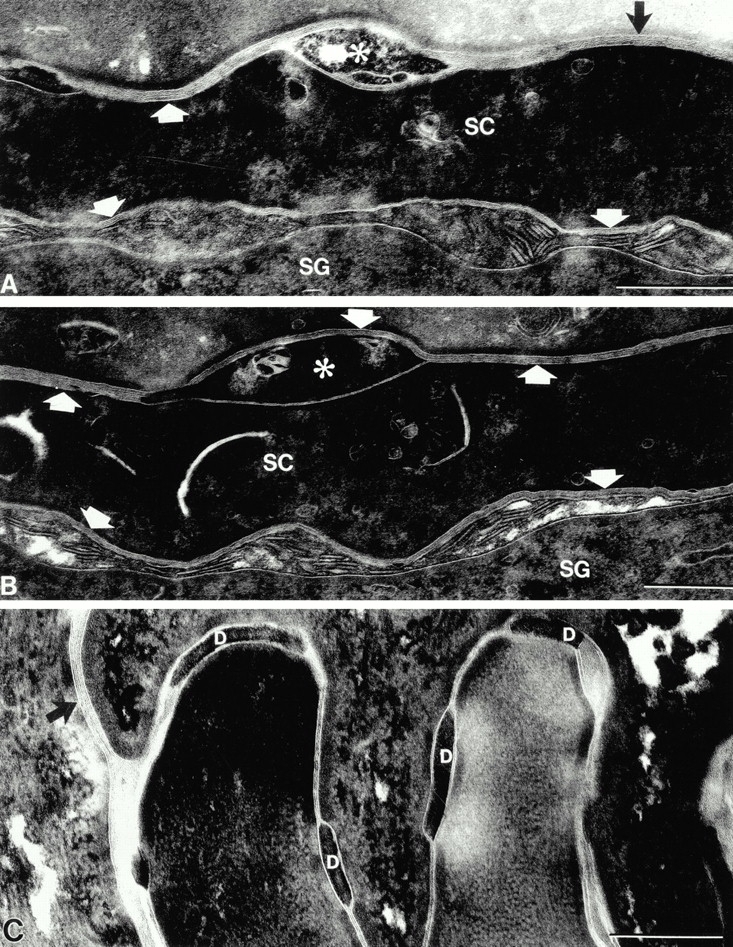
Desmosomal dissolution results in lacunae within extracellular lamellar matrix in the stratum corneum. (A and B) Transformation of secreted lamellar body contents into lamellar membrane bilayers (arrows) begins at the SG–SC interface. Premature desmosome dissolution results in clefts (*) within the lamellar membranes. (C) Control stratum corneum shows normal-appearing desmosomes (D) within the lamellar bilayer system (arrow). Bar: 0.25 μm. Ruthenium tetroxide postfixation.
Tape Stripping of Epidermis Results in Increased Rate of Transepidermal Water Loss in Involucrin-Dsg 3 Mice
For further evidence of decreased corneocyte adhesion, we measured protein obtained with serial tape stripping of the stratum corneum in transgenic mice and normal littermates, a standard measure that correlates with corneocyte adhesion (Reed et al. 1995; Dreher et al. 1998). For transgenic mice, the rate of total protein obtained per tape stripping was markedly increased up to six strippings, compared with normal mice, where rate of total protein obtained was increased for up to eight strippings (Fig. 7 A). In these same animals, the increased rate of protein loss from the stratum corneum of transgenics, compared with normals, correlated with an accelerated rate of increase of transepidermal water loss (Fig. 7 B). These findings are consistent with the ultrastructural observations that show decreased corneocyte adhesion. Taken together, the results suggest that expression of Dsg 3 throughout the epidermis is associated with decreased adhesion of corneocytes, resulting in increased transepidermal water loss from an impaired epidermal barrier.
Figure 7.
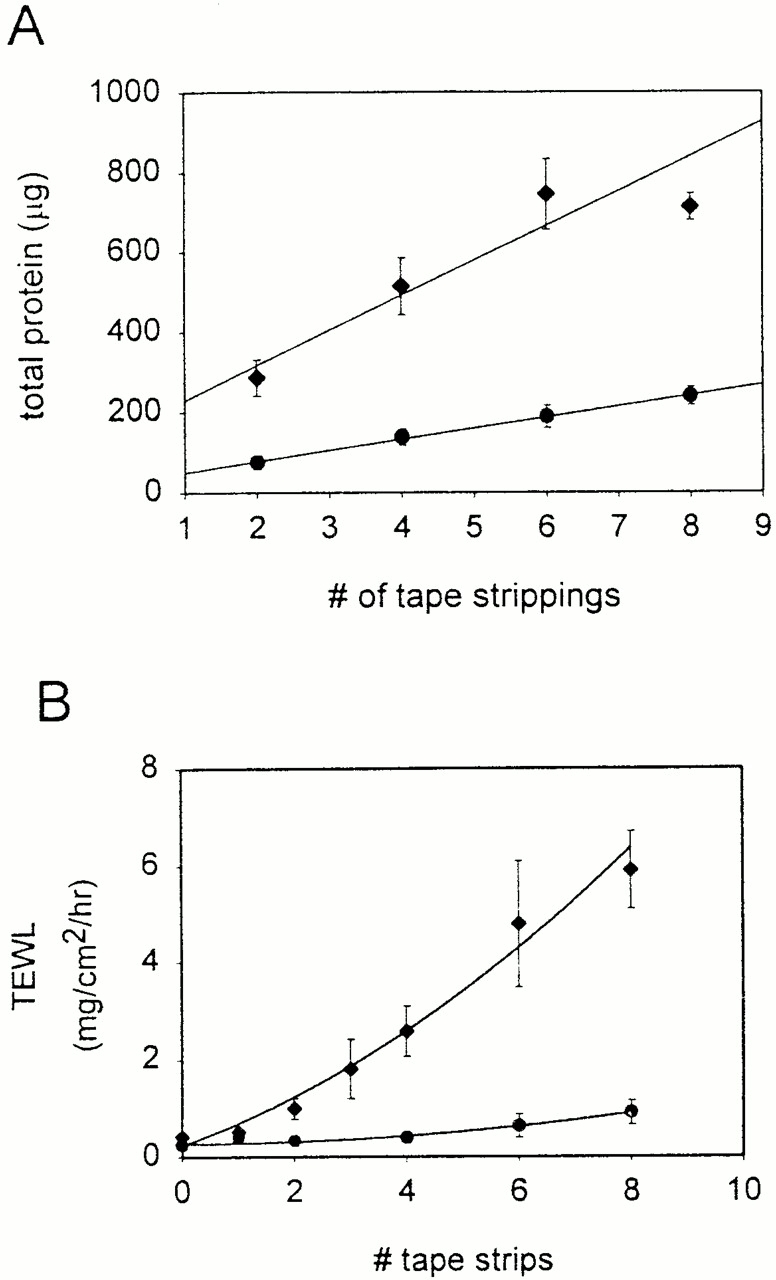
Increased stratum corneum loss with tape stripping correlates with increased transepidermal water loss in involucrin-Dsg 3 mice. (A) Total protein as a function of number of tape strippings of stratum corneum. (B) Transepidermal water loss (TEWL) as a function of number of tape strippings. Transgenic mice show higher rates of protein loss and higher acceleration of TEWL than control mice with stratum corneum stripping.
Expression of Dsg 3 in the Superficial Epidermis Does Not Affect Expression of Major Differentiation Markers
To rule out the possibility that the expression of Dsg 3 in the superficial epidermis might have major effects on multiple pathways of differentiation in the epidermis, we determined expression of loricrin, involucrin, filaggrin, and K10 in the transgenic and normal epidermis by Western blotting (Fig. 8). There were no major changes seen in the expression of these differentiation molecules. These data show that the expression of Dsg 3 in the superficial epidermis does not cause a generalized perturbation in the overall differentiation of epidermis, although we cannot rule out more subtle changes.
Figure 8.
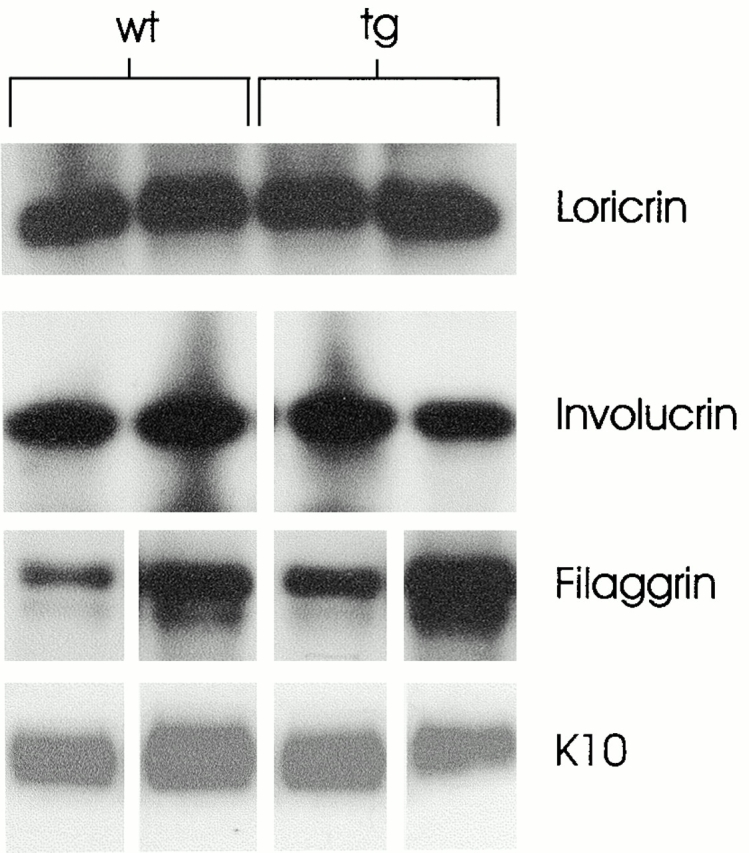
Western blot of major differentiation markers from two different involucrin-Dsg 3 mice (tg) and two control littermates (wt) show no consistent differences in expression. Filaggrin expression was variable, probably due to different amounts of retained stratum corneum or different efficiencies of extraction.
Discussion
Desmogleins play a major role in stabilizing cell–cell adhesion in the living layers of the epidermis. This function is perhaps best illustrated in the disease pemphigus in which autoantibodies against these transmembrane glycoproteins of the desmosome cause blisters due to loss of keratinocyte adhesion. Although desmogleins provide this critical function, it is not clear why different desmoglein isoforms are expressed at different levels in various stratified squamous epithelia. To address this issue, we constructed transgenic mice that expressed Dsg 3 throughout the entire epidermis, as occurs in normal oral mucous membrane, in contrast to its normal expression only in the deep epidermis. These mice displayed a dramatic phenotype, characterized by scaling shortly after birth, dehydration (usually leading to lethality within the first 10 d of life), a histologically abnormal epidermal stratum corneum, decreased adhesion of corneocytes, and increased transepidermal water loss.
This phenotype was unlikely to be due to toxicity of transgene overexpression, because the ratio of Dsg 3 to Dsg 1 in transgenic epidermis approximated that in normal oral mucous membrane (i.e., tongue). Although this ratio was somewhat higher than in tongue, at least in founder 2, the transgenic tongue had Dsg 3 to Dsg 1 ratios even higher than those of normal mucous membrane and transgenic epidermis, yet did not show any gross abnormalities indicative of toxicity. In addition, the function and processing of the transgenic Dsg 3 seems normal. For example, we know that the transgenic Dsg 3 is expressed on the cell surface, does not accumulate in the cytoplasm (as it might in severe overexpression), and provides physiologic functional adhesion, because it compensates for loss of Dsg 1 adhesion in pemphigus foliaceus (Wu et al. 2000). Furthermore, there is no histologic or ultrastructural evidence of any dominant-negative effect on normal desmosome formation in the nucleated epidermis, which might be expected if there were toxicity from overexpression. Finally, the transgenic phenotype is not due to some toxic general perturbation of epidermal differentiation because there were no changes in expression of major keratinocyte differentiation antigens in the transgenic mice.
Interestingly, changing the Dsg 3 to Dsg 1 ratio in transgenic epidermis to more closely approximate that seen in mucous membranes results in an epidermal stratum corneum that histologically and ultrastructurally resembles the stratum corneum of mucosal epithelia. Furthermore, the transgenic epidermal stratum corneum, like mucous membrane (Lesch et al. 1989), shows increased water permeability compared with normal epidermal stratum corneum.
These data demonstrate that the distribution of desmosomal isoforms within epidermis affects the structure and function of the stratum corneum. How this occurs is suggested by the ultrastructural studies of transgenic skin. In normal stratum corneum, there is an orderly processing of the desmosome, with gradual dissolution beginning about two to three cell layers above the stratum granulosum–stratum corneum interface and continuing to the surface of the skin (Fartasch et al. 1993). These degraded desmosomes form lacunae (clefts) within the extracellular lamellar lipid bilayers between corneocytes in the mid to superficial stratum corneum (Menon and Elias 1997). Under certain circumstances (e.g., occlusion of the stratum corneum), these lacunae can form an interconnected network of hydrophilic pores through which water and other molecules can diffuse. In transgenic stratum corneum, there is a premature dissolution of the desmosomes, with resultant lacunar cleft formation and corneocyte separation in the deepest layers of the stratum corneum. It is reasonable to assume that these changes disrupt the barrier. Consistent with this explanation was our finding that tape stripping of the stratum corneum of these transgenic mice, compared with normal mice, demonstrated increased protein loss, presumably reflecting decreased corneocyte adhesion, associated with increasing rates of transepidermal water loss.
Although it may first appear paradoxical that the histology of transgenic epidermis, which shows premature dissolution of desmosomes, shows a compact lamellar stratum corneum compared with the typical normal basket weave pattern, there is no correlation between the latter pattern and desmosome function or lack thereof. The histology of stratum corneum is an artifact of processing (probably due to loss of lipids and inability to rehydrate those areas with routine processing), because fully rehydrated stratum corneum shows closely opposed cells without gaps (Odland 1991). However, what is clear is that keratinized mucous membrane and transgenic epidermis typically show remarkably similar histology (as well as ultrastructure) of their stratum corneum, which is distinct from the pattern of normal epidermal stratum corneum.
Together, these observations suggest that the ratio of desmosome isoforms in the stratum corneum affects physiologic desmosomal processing, which in turn regulates the degree of corneocyte adhesion and barrier function. Exactly how the expression of Dsg 3 in the stratum corneum might affect desmosomal processing is not obvious, in part because the mechanisms of desquamation in the stratum corneum have not been well characterized. Perhaps the phenotype we observe is simply a consequence of inherently different adhesive functions of Dsg 3 and Dsg 1, such that incorporation of both does not lend mechanical stability to the junctions in the stratum corneum. However, how this instability would lead to degradation of the desmosome is not clear. There are proteolytic and hydrolytic enzymes that are likely involved in regulating desquamation in the stratum corneum, and at least some of these enzymes may concentrate around corneodesmosomes (Elias et al. 1988; Ekholm et al. 2000) (also discussed in Fartasch et al. 1993). It may be that the composition of the corneodesmosome could affect the clustering or activation of some of these enzymes, thereby modifying rates of desmosomal destruction. Another possibility is that Dsg 3 may be more susceptible to proteolytic degradation, and once degraded may have a dominant-negative effect on the desmosome (Allen et al. 1996). However, it will require that we know more about the biochemical pathways of corneodesmosome processing before we understand how Dsg 3 accelerates it.
In summary, these studies not only demonstrate that the distribution of isoforms of a desmosomal cadherin can have dramatic effects on the major physiologic function of epidermis (i.e., permeability barrier formation), but also underscore the importance of desmosomes in regulating that function.
Acknowledgments
We thank Drs. Leena Pulkinen and Fushan Wang for providing the cDNA constructs used in these studies, and Michael Shapiro and Dr. Peter Koch for helping to produce and characterize antidesmoglein antibodies.
Footnotes
Abbreviation used in this paper: Dsg, desmoglein.
References
- Allen E., Yu Q.C., Fuchs E. Mice expressing a mutant desmosomal cadherin exhibit abnormalities in desmosomes, proliferation, and epidermal differentiation. J. Cell Biol. 1996;133:1367–1382. doi: 10.1083/jcb.133.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amagai M., Koch P.J., Nishikawa T., Stanley J.R. Pemphigus vulgaris antigen (Desmoglein 3) is localized in the lower epidermis, the site of blister formation in patients. J. Invest. Dermatol. 1996;106:351–355. doi: 10.1111/1523-1747.ep12343081. [DOI] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carroll J.M., Albers K.M., Garlick J.A., Harrington R., Taichman L.B. Tissue- and stratum-specific expression of the human involucrin promoter in transgenic mice. Proc. Natl. Acad. Sci. USA. 1993;90:10270–10274. doi: 10.1073/pnas.90.21.10270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll J.M., Taichman L.B. Characterization of the human involucrin promoter using a transient beta-galactosidase assay. J. Cell Sci. 1992;103:925–930. doi: 10.1242/jcs.103.4.925. [DOI] [PubMed] [Google Scholar]
- Dreher F., Arens A., Hostynek J.J., Mudumba S., Ademola J., Maibach H.I. Colorimetric method for quantifying human stratum corneum removed by adhesive-tape stripping. Acta Derm. Venereol. 1998;78:186–189. doi: 10.1080/000155598441495. [DOI] [PubMed] [Google Scholar]
- Ekholm I.E., Brattsand M., Egelrud T. Stratum corneum tryptic enzyme in normal epidermisa missing link in the desquamative process? J. Invest. Dermatol. 2000;114:56–63. doi: 10.1046/j.1523-1747.2000.00820.x. [DOI] [PubMed] [Google Scholar]
- Elias P.M., Menon G.K., Grayson S., Brown B.E. Membrane structural alterations in murine stratum corneumrelationship to the localization of polar lipids and phospholipases. J. Invest. Dermatol. 1988;91:3–10. doi: 10.1111/1523-1747.ep12463279. [DOI] [PubMed] [Google Scholar]
- Fartasch M., Bassukas I.D., Diepgen T.L. Structural relationship between epidermal lipid lamellae, lamellar bodies and desmosomes in human epidermisan ultrastructural study. Br. J. Dermatol. 1993;128:1–9. doi: 10.1111/j.1365-2133.1993.tb00138.x. [DOI] [PubMed] [Google Scholar]
- Garrod D.R., Tselepis C., Runswick S.K., North A.J., Wallis S.R., Chidgey M.A.J. Desmosomal adhesion. Adv. Mol. Cell. Biol. 1999;28:165–202. [Google Scholar]
- Hanley K., Rassner U., Elias P.M., Williams M.L., Feingold K.R. Epidermal barrier ontogenesismaturation in serum-free media and acceleration by glucocorticoids and thyroid hormone but not selected growth factors. J. Invest. Dermatol. 1996;106:404–411. doi: 10.1111/1523-1747.ep12343405. [DOI] [PubMed] [Google Scholar]
- Hou S.Y., Mitra A.K., White S.H., Menon G.K., Ghadially R., Elias P.M. Membrane structures in normal and essential fatty acid–deficient stratum corneumcharacterization by ruthenium tetroxide staining and x-ray diffraction. J. Invest. Dermatol. 1991;96:215–223. doi: 10.1111/1523-1747.ep12461361. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Li K., Sawamura D., Uitto J. Cloning of the mouse desmoglein 3 gene (Dsg3)interspecies conservation within the cadherin superfamily. Exp. Dermatol. 2000;9:229–239. doi: 10.1034/j.1600-0625.2000.009004229.x. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Silos S.A., Tamai K., Copeland N.G., Gilbert D.J., Jenkins N.A., Uitto J. cDNA cloning and chromosomal assignment of the mouse gene for desmoglein 3 (Dsg3), the pemphigus vulgaris antigen. Mamm. Genome. 1994;5:803–804. doi: 10.1007/BF00292018. [DOI] [PubMed] [Google Scholar]
- Koch P.J., Mahoney M.G., Cotsarelis G., Rothenberger K., Lavker R.M., Stanley J.R. Desmoglein 3 anchors telogen hair in the follicle. J. Cell Sci. 1998;111:2529–2537. doi: 10.1242/jcs.111.17.2529. [DOI] [PubMed] [Google Scholar]
- Koch P.J., Mahoney M.G., Ishikawa H., Pulkkinen L., Uitto J., Shultz L., Murphy G.F., Whitaker-Menezes D., Stanley J.R. Targeted disruption of the pemphigus vulgaris antigen (desmoglein 3) gene in mice causes loss of keratinocyte cell adhesion with a phenotype similar to pemphigus vulgaris. J. Cell Biol. 1997;137:1091–1102. doi: 10.1083/jcb.137.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch C.A., Squier C.A., Cruchley A., Williams D.M., Speight P. The permeability of human oral mucosa and skin to water. J. Dent. Res. 1989;68:1345–1349. doi: 10.1177/00220345890680091101. [DOI] [PubMed] [Google Scholar]
- Mahoney M.G., Wang Z., Rothenberger K., Koch P.J., Amagai M., Stanley J.R. Explanation for the clinical and microscopic localization of lesions in pemphigus foliaceus and vulgaris. J. Clin. Invest. 1999;103:461–468. doi: 10.1172/JCI5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon G.K., Elias P.M. Morphologic basis for a pore-pathway in mammalian stratum corneum. Skin Pharmacol. 1997;10:235–246. doi: 10.1159/000211511. [DOI] [PubMed] [Google Scholar]
- Menon G.K., Feingold K.R., Moser A.H., Elias P.M. De novo sterologenesis in the skin. II. Regulation by cutaneous barrier requirements. J. Lipid Res. 1985;26:418–427. [PubMed] [Google Scholar]
- Odland G.F. Structure of the skin. In: Goldsmith L.A., editor. Physiology, Biochemistry, and Molecular Biology of the Skin. Oxford University Press; New York, NY: 1991. pp. 3–62. [Google Scholar]
- Reed J.T., Ghadially R., Elias P.M. Skin type, but neither race nor gender, influence epidermal permeability barrier function. Arch. Dermatol. 1995;131:1134–1138. [PubMed] [Google Scholar]
- Rice R.H., Green H. Presence in human epidermal cells of a soluble protein precursor of the cross-linked envelopeactivation of the cross-linking by calcium ions. Cell. 1979;18:681–694. doi: 10.1016/0092-8674(79)90123-5. [DOI] [PubMed] [Google Scholar]
- Shirakata Y., Amagai M., Hanakawa Y., Nishikawa T., Hashimoto K. Lack of mucosal involvement in pemphigus foliaceus may be due to low expression of desmoglein 1. J. Invest. Dermatol. 1998;110:76–78. doi: 10.1046/j.1523-1747.1998.00085.x. [DOI] [PubMed] [Google Scholar]
- Tselpsis C., Chidgey M., North A., Garrod D. Desmosomal adhesion inhibits invasive behavior. Proc. Natl. Acad. Sci. USA. 1998;95:8064–8069. doi: 10.1073/pnas.95.14.8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Wang Z.H., Yan A., Lyle S., Fakharzadeh S., Wahl J.K., Wheelock M.J., Ishikawa H., Uitto J., Amagai M., Stanley J.R. Protection of neonates against pemphigus foliaceus by desmoglein 3. N. Engl. J. Med. 2000;343:31–35. doi: 10.1056/NEJM200007063430105. [DOI] [PubMed] [Google Scholar]