Abstract
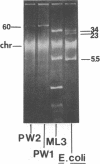
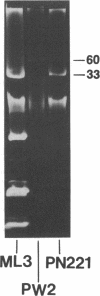
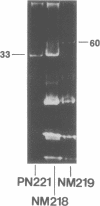
Lactose-positive (Lac+) transconjugants resulting from matings between Streptococcus lactic ML3 and S. lactis LM2301 possess a single plasmid of approximately 60 megadaltons (Mdal) which is nearly twice the size of the lactose plasmid of the donor. The majority of these Lac+ transconjugants aggregated in broth and were able to transfer lactose-fermenting ability at a frequency higher than 10(-1) per donor on milk agar plates or in broth. Lac+ transconjugants which did not clump conjugated at a much lower frequency. Lactose-negative derivatives of Lac+ clumping transconjugants did not aggregate in broth and were missing the 60-Mdal plasmid. The ability to aggregates in broth was very unstable. Strains could lose the ability to clump but retain lactose-fermenting ability. The majority of these Lac+ nonclumping derivatives of clumping transconjugants contained a plasmid of approximately 33 Mdal, the size of the lactose plasmid of the original donor ML3. These strains transferred lactose-fermenting ability at a frequency of approximately 10(-6) per donor, resulting in both Lac+ clumping transconjugants which contained a 60-Mdal plasmid and Lac+ nonclumping transconjugants which possessed a 33-Mdal plasmid. Our results suggest that the genes responsible for cell aggregation and high-frequency conjugation are on the segment of deoxyribonucleic acid which recombined with the 33-Mdal lactose plasmid in S. lactis ML3.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clark A. J., Warren G. J. Conjugal transmission of plasmids. Annu Rev Genet. 1979;13:99–125. doi: 10.1146/annurev.ge.13.120179.000531. [DOI] [PubMed] [Google Scholar]
- Cords B. R., McKay L. L., Guerry P. Extrachromosomal elements in group N streptococci. J Bacteriol. 1974 Mar;117(3):1149–1152. doi: 10.1128/jb.117.3.1149-1152.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G. M., Brown B. L., Clewell D. B. Induced cell aggregation and mating in Streptococcus faecalis: evidence for a bacterial sex pheromone. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3479–3483. doi: 10.1073/pnas.75.7.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G. M., Craig R. A., Carron R. L., Clewell D. B. Plasmid transfer in Streptococcus faecalis: production of multiple sex pheromones by recipients. Plasmid. 1979 Jul;2(3):454–465. doi: 10.1016/0147-619x(79)90029-5. [DOI] [PubMed] [Google Scholar]
- Gasson M. J., Davies F. L. High-frequency conjugation associated with Streptococcus lactis donor cell aggregation. J Bacteriol. 1980 Sep;143(3):1260–1264. doi: 10.1128/jb.143.3.1260-1264.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempler G. M., McKay L. L. Genetic Evidence for Plasmid-Linked Lactose Metabolism in Streptococcus lactis subsp. diacetylactis. Appl Environ Microbiol. 1979 May;37(5):1041–1043. doi: 10.1128/aem.37.5.1041-1043.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaenhammer T. R., McKay L. L., Baldwin K. A. Improved lysis of group N streptococci for isolation and rapid characterization of plasmid deoxyribonucleic acid. Appl Environ Microbiol. 1978 Mar;35(3):592–600. doi: 10.1128/aem.35.3.592-600.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogstad D. J., Smith R. M., Moellering R. C., Jr, Parquette A. R. Visualization of cell-cell contact during conjugation in Streptococcus faecalis. J Bacteriol. 1980 Feb;141(2):963–967. doi: 10.1128/jb.141.2.963-967.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl S. A., Larsen L. D., McKay L. L. Plasmid Profiles of Lactose-Negative and Proteinase-Deficient Mutants of Streptococcus lactis C10, ML(3), and M18. Appl Environ Microbiol. 1979 Jun;37(6):1193–1195. doi: 10.1128/aem.37.6.1193-1195.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A., Efstathiou J. D. Transductional evidence for plasmid linkage of lactose metabolism in streptococcus lactis C2. Appl Environ Microbiol. 1976 Jul;32(1):45–52. doi: 10.1128/aem.32.1.45-52.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A., Walsh P. M. Conjugal transfer of genetic information in group N streptococci. Appl Environ Microbiol. 1980 Jul;40(1):84–89. doi: 10.1128/aem.40.1.84-91.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A., Zottola E. A. Loss of lactose metabolism in lactic streptococci. Appl Microbiol. 1972 Jun;23(6):1090–1096. doi: 10.1128/am.23.6.1090-1096.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzaghi B. E., Sandine W. E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975 Jun;29(6):807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]