Abstract
In the budding yeast Saccharomyces cerevisiae, the mitotic spindle must align along the mother-bud axis to accurately partition the sister chromatids into daughter cells. Previous studies showed that spindle orientation required both astral microtubules and the actin cytoskeleton. We now report that maintenance of correct spindle orientation does not depend on F-actin during G2/M phase of the cell cycle. Depolymerization of F-actin using Latrunculin-A did not perturb spindle orientation after this stage. Even an early step in spindle orientation, the migration of the spindle pole body (SPB), became actin-independent if it was delayed until late in the cell cycle.
Early in the cell cycle, both SPB migration and spindle orientation were very sensitive to perturbation of F-actin. Selective disruption of actin cables using a conditional tropomyosin double-mutant also led to de- fects in spindle orientation, even though cortical actin patches were still polarized. This suggests that actin cables are important for either guiding astral microtubules into the bud or anchoring them in the bud. In addition, F-actin was required early in the cell cycle for the development of the actin-independent spindle orientation capability later in the cell cycle. Finally, neither SPB migration nor the switch from actin-dependent to actin-independent spindle behavior required B-type cyclins.
Keywords: actin, spindle, cell cycle, astral microtubule, Latrunculin-A
In dividing cells, the accurate distribution of genetic information requires spatial coordination between the plane of cytokinesis and the axis of the mitotic spindle. In animal cells, classic experiments moving the mitotic spindle by micromanipulation established that the spindle actively specifies the plane of cytokinesis, such that the cleavage furrow forms perpendicular to the axis of anaphase (Rappaport 1996). In contrast, the site of bud emergence in Saccharomyces cerevisiae specifies both the location and the plane of the subsequent cytokinesis before spindle assembly. Thus, in yeast, the spindle must align perpendicular to a prespecified plane of cytokinesis.
Microtubules in yeast emanate from the spindle pole body (SPB),1 the functional equivalent to the centrosome in animal cells, which is embedded in the nuclear envelope (Byers 1981). The SPB is duplicated at around the time of bud emergence, and later, the duplicated SPBs separate to opposite sides of the nucleus. Astral microtubules extend from the SPBs into the cytoplasm, while intranuclear microtubules extend between the SPBs and from SPBs to kinetochores to form the spindle; as with other fungi, the nuclear envelope does not break down during mitosis (Byers 1981).
Electron microscopic studies demonstrated that cytoplasmic microtubules originating near the duplicated side by side SPBs were oriented towards the bud, even before spindle formation (Byers 1981). Later immunofluorescence studies of fixed cells confirmed these findings (Snyder et al. 1991) and indicated that the spindle is positioned near the mother-bud neck and oriented roughly along the mother-bud axis before anaphase (Adams and Pringle 1984; Jacobs et al. 1988). Both the position and orientation of the spindle were found to be sensitive to selective disruption of cytoplasmic microtubules using specific cold-sensitive tubulin mutants (Palmer et al. 1992; Sullivan and Huffaker 1992). It is now generally accepted that spindle positioning near the neck and orientation along the mother-bud axis is accomplished before anaphase through interactions between cytoplasmic microtubules and polarized cellular determinants, beginning early in the cell cycle.
How do cytoplasmic microtubules accomplish the positioning and orientation of the spindle? An influential study (Palmer et al. 1992) suggested that the actin cytoskeleton played an important role in this process. Assessing the role of actin in spindle orientation is complicated by the fact that actin is essential for constructing the bud towards which the spindle must orient. To circumvent this problem, Palmer and colleagues 1992 first arrested cells following bud construction using hydroxyurea, allowing orientation, but not elongation, of the preanaphase spindle. They then examined the effects of perturbing actin, using the temperature-sensitive act1-4 mutant, on the maintenance of spindle orientation. Shifting these mutants to the restrictive temperature resulted in randomization of spindle orientation, frequently leading to eventual spindle elongation within the mother, rather than along the mother-bud axis. The authors concluded that actin is important for the maintenance of spindle orientation (Palmer et al. 1992), leading to a widely embraced model invoking links between the polarized cortical actin cytoskeleton and astral microtubules that somehow act to orient the spindle.
We have reexamined the role of actin in both early and late stages of spindle orientation. Surprisingly, we found no requirement for F-actin in the maintenance of correct spindle orientation late in the cell cycle. However, we have identified a temporally restricted role for actin cables in the establishment of an oriented spindle early in the cell cycle.
Materials and Methods
Reagents
The yeast pheromone α-factor peptide was synthesized by Research Genetics and stored as a 2 mg/ml stock solution in H2O at −20°C. Hydroxyurea (Sigma Chemical Co.) was added directly to yeast media from solid. Nocodazole (Sigma Chemical Co.) was stored as a 15 mg/ml stock solution in DMSO at −20°C. Rhodamine-conjugated phalloidin (Molecular Probes) was stored as a 200 U/ml stock solution in methanol at −20°C. 4′,6-diamidino-2-phenylindole (DAPI; Sigma Chemical Co.) was stored as a 1 mg/ml stock solution in H2O at −20°C. Latrunculin-A (Lat-A; Molecular Probes) was stored as a 20 mM stock solution in DMSO at −20°C. Monoclonal rat anti–yeast tubulin, YOL1/34 (Accurate Chemical and Scientific Corp.), was used at 1:100 dilution, and goat anti–rat Cy2 secondary antibody (Jackson ImmunoResearch Laboratories) was used at 1:25 dilution.
Yeast Strains and Growth Conditions
The yeast strains used in this study are listed in Table . Strains DLY1, DLY5, and SBY153 (a gift from Steven B. Haase, The Scripps Research Institute, La Jolla, CA) are in the BF264-15DU background (Richardson et al. 1989), and JMY6-10 (John N. McMillan, Duke University Medical Center, Durham, NC) contains the cdc31-1 allele backcrossed six times into the same strain background. Strains 5050 and 5105 were gifts from D. Koshland (Carnegie Institute of Washington, Baltimore, MD) and are described in Palmer et al. 1992. Strain KBY62 was made by transforming the dyn1::LEU2 plasmid described in Li et al. 1993 into W303a. Strains KBY1010 and KBY1011 were made by disrupting KAR9 and BNI1, respectively, in the YEF473 (Bi and Pringle 1996) background. The kar9::LEU2 plasmid described by Miller and Rose 1998 was used to disrupt KAR9, and the entire open reading frame of BNI1 was deleted using the PCR strategy of Baudin et al. 1993 with pRS305 (LEU2) as template and the primers 5′-gatgtttgttttggtattactgttgtcataattttttggtttaatattttatttgaaacttagcctgttacct-gtgctatgcggtatttcacaccg-3′ and 5′-gccatttgtatct-atcttctgtattgaggagaaacattttaactcaagcctag-ttaaattctaaatacacgccgattgtactgagagtgcacc-3′. Strains ABY971 and ABY973 were gifts from D. Pruyne and A. Bretscher (Cornell University, Ithaca, NY) and are described in Pruyne et al. 1998.
Table 1.
Yeast Strains
| Strain | Genotype |
|---|---|
| DLY1 | MATa bar1 ade1 his2 leu2-3,112 trp1-1 a ura3Δns |
| DLY5 | MATa/MATα ade1/ade1 his2/his2 leu2-3,112/leu2-3,112 trp1-1a/trp1-1a ura3Δns/ura3Δns |
| 5050 | MATa/MATα ade2/ADE2 ade3/ADE3 his7/his7 hom3/HOM3 leu2/LEU2 |
| 5105 | MATa/MATα act1-4/act1-4 his3/HIS3 leu2/LEU2 ura3/ura3 |
| JMY6-10 | MATa bar1 cdc31-1 ade1 his2 leu2-3,112 tp1-1a ura3Δns |
| W303a | MATa ade2-1 trp1-1 leu2-3,112 his3-11,15 ura3 can1-100 |
| KBY62 | MATa dyn1::LEU2 ade2-1 trp1-1 leu2-3,112 his3-11,15 ura3 can1-100 |
| YEF473 | MATa his3 leu2 trp1 ura3 lys2 |
| KBY1010 | MATa kar9::LEU2 his3 leu2 trp1 ura3 lys2 |
| KBY1011 | MATa bni1::LEU2 his3 leu2 trp1 ura3 lys2 |
| ABY971 | MATa/MATα tpm1-2::LEU2/tpm1-2::LEU2 tpm2::HIS3/tpm2::HIS3 his3Δ-200/his3Δ-200 |
| leu2-3,112/leu2-3,112 lys2-801/lys2-801 trp1-1/trp1-1 ura3-52/ura3-52 | |
| ABY973 | MATa/MATα tpm2::HIS3/tpm2::HIS3 his3Δ-200/his3Δ-200 leu2-3,112/leu2-3,112 lys2-801/lys2-trp1-1/trp1-1 |
| ura3-52/ura3-52 | |
| SBY153 | MATa clb1::URA3 clb2::LEU2 clb3::TRP1 clb4::HIS2 clb5::ARG4 clb6::ADE1 LEU2::GAL1-CLB |
| his2 trp1-1a ura3Δns arg4 |
Cells were grown in YEPD (1% yeast extract, 2% bacto-peptone, 2% dextrose, 0.01% adenine), YEPS (containing 2% sucrose instead of 2% dextrose), or YEPG (containing 2% galactose instead of 2% dextrose) medium at 30°C, except for the experiments using temperature-sensitive mutants, in which cells were grown at the permissive temperature (23–24°C) and shifted to the indicated restrictive temperatures.
Cell Synchronization and Cytoskeletal Perturbation
Synchronized cell cultures were obtained in a variety of ways. For hydroxyurea-induced synchrony, cell were grown in YEPD to 5 × 106 cells/ml, harvested, and resuspended at 2 × 107 cells/ml in YEPD containing 100–200 mM hydroxyurea, and the cells were incubated for 3 h at 23–30°C, as indicated. For α-factor–induced synchrony, α-factor was added to cell cultures in YEPD at 5 × 106 cells/ml to a final concentration of 25 ng/ml, and the cells were incubated for 3 h at 30°C. Centrifugal elutriation of cells grown in YEPS or YEPG was performed as described (Lew and Reed 1993), except for the temperature in the cdc31-1 experiments, in which the cell cultures and collection flasks were kept in a 37°C water bath, the rotor was prewarmed by spinning the rotor until the chamber warmed up to 37°C, and then using the cooling feature of the centrifuge to keep the temperature constant.
Nocodazole was added to a final concentration of 15 μg/ml. For the cdc31-1 cells, nocodazole was added directly to the collection flasks from the elutriation, whereas in the clb1Δ-6Δ experiment, the cells were first harvested by centrifugation and resuspended in fresh YEPD with nocodazole. Lat-A was added to the indicated concentration from DMSO stock solution, and an equal amount of DMSO was added to the control samples in all experiments (final DMSO concentration did not exceed 1%).
Fluorescence Staining and Microscopy
Cells were fixed in 4.5% formaldehyde for 2 h at 23°C. Immunofluorescence procedures for visualizing tubulin distribution with the YOL1/34 antibody were performed according to Pringle et al. 1991. DNA was visualized by staining with DAPI. F-actin was visualized by staining with 10 U/ml rhodamine-phalloidin added to the secondary antibody incubation for the immunofluorescence procedure. Cells were washed, mounted, and examined using a Zeiss Axioskop microscope equipped with epifluorescence and differential interference contrast (DIC) optics. Images were captured using a Pentamax cooled CCD camera (Princeton Instruments), interfaced with MetaMorph software (Universal Imaging Corp.).
Scoring Spindle Orientation and SPB Migration
Spindle orientation was scored as described by Palmer et al. 1992: a spindle was considered properly oriented if an imaginary line drawn through the long axis of the spindle traversed the mother-bud neck. This scoring criterion contains information on both the position and orientation of the preanaphase spindle. Given that the z-axis was sometimes hard to assess in this scoring, it is possible that some spindles were misscored (in either direction), but this should not affect our conclusions because the misscoring should not be affected by Lat-A treatment.
SPB position was inferred from the tubulin staining on the assumption that the SPB is at the focus of the cytoplasmic microtubule asters: only cells where this focus was obvious were scored. SPB position was classified into one of three categories: neck, indicating a distance of <0.5 μm from the mother-bud neck; mother, or bud, indicating a distance of >0.5 μm from the mother-bud neck on either side. In the experiment of Fig. 5, the bud category is expanded to include all cells with the SPB on the bud side of the neck. Mothers were distinguished from buds by morphological criteria: in Fig. 5, mothers were larger and had a distinct vacuole; in Fig. 6, mothers were shmoo-shaped; and in Fig. 9, buds were elongated. The reliability of these criteria in identifying mother and bud was tested by comparison to the actin-staining pattern in the same cells (actin patches are predominantly in the bud). In all cases, there was excellent agreement between morphological and actin-based criteria.
Figure 5.

Effect of F-actin perturbation on SPB migration and astral microtubule orientation. cdc31-1 cells (JMY6-10) were grown in YEPS at 23°C, shifted to 37°C for 1 h, and G1 cells were isolated by centrifugal elutriation at 37°C. Nocodazole (together with 2% dextrose) was added and the cells were incubated at 37°C until 70% had formed small buds, and then harvested to wash out the nocodazole and resuspended in two aliquots: one received 100 μM Lat-A and the other DMSO as control. After a further 30 min at 37°C, the cells were fixed and processed to visualize F-actin and tubulin. A, Representative pictures of cells with SPBs in the mother, bud, or mother-bud neck are shown, overlaying tubulin staining and DIC images of the same cells. Bar, 5 μm. The position of the SPB was scored as described in Materials and Methods (n = 200 for each sample), and quantitated in the bars shown below the pictures (at time (t) = 0, SPB position was inferred from DAPI-stained cells, since no microtubules were present at the time of nocodazole wash-out). Mothers and buds in this experiment were distinguished by their size (mothers larger than buds) and vacuole morphology (mother cells contained large and distinct vacuoles in DIC images). B, Astral microtubule orientation. Representative pictures of cells are shown, overlaying tubulin staining and DIC images of the same cells. Mother, all microtubules oriented towards mother; bud, all microtubules oriented towards bud; both, microtubules oriented both towards the mother and towards the bud. Bar, 5 μm.
Figure 6.
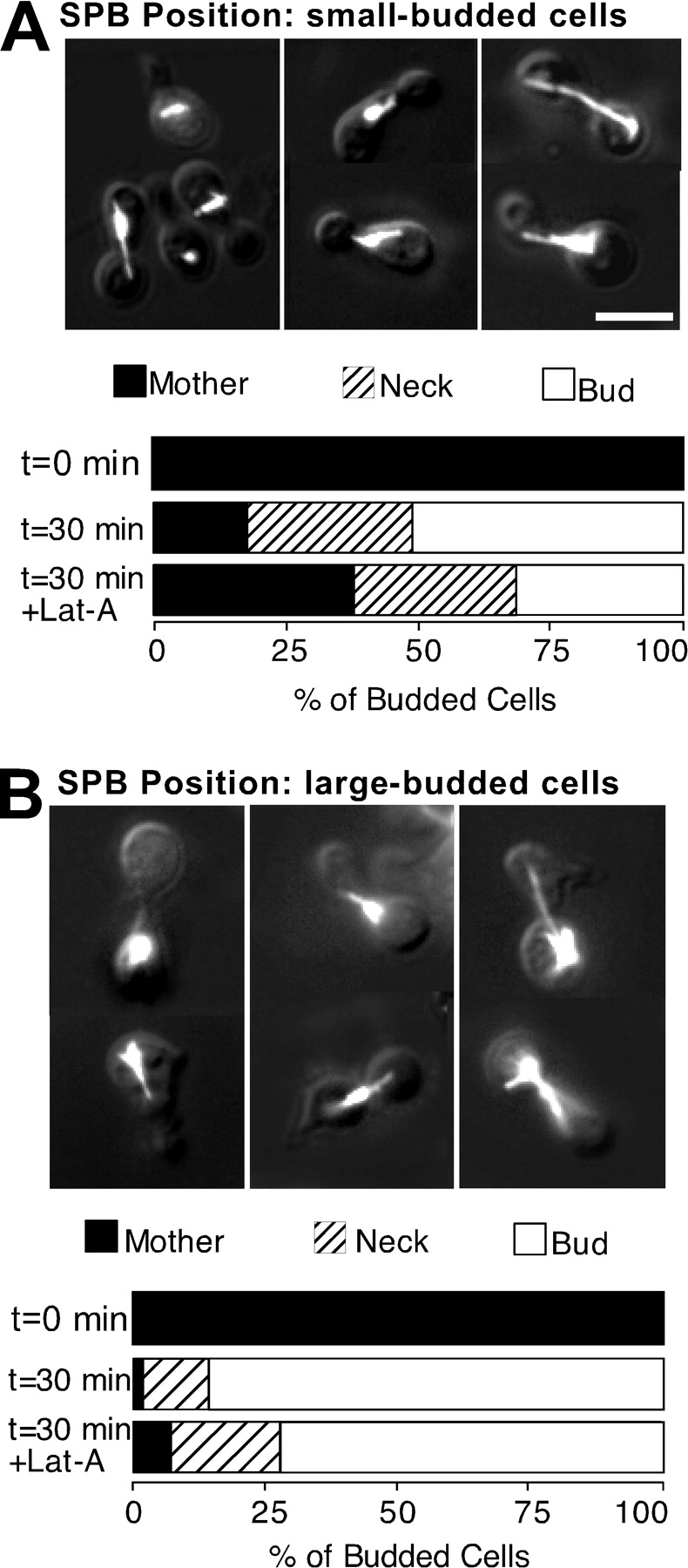
Cell cycle specificity of the effect of F-actin perturbation on SPB migration. Synchronized G1 cells of the cdc31-1 strain (JMY6-10) were isolated as in Fig. 5, and incubated at 37°C in the presence of nocodazole, together with α-factor for 2 h. The resulting shmoo-shaped cells were harvested by centrifugation and resuspended in fresh YEPD (prewarmed to 37°C) with nocodazole, but lacking α-factor, releasing them from G1 arrest and allowing bud formation. These cells were harvested after a further 1 h (small-budded cells, A) or 2 h (large-budded cells, B) and separate aliquots were treated with either 100 μM Lat-A or DMSO (controls). After another 30 min at 37°C, the cells were fixed and processed to visualize F-actin and tubulin. The position of the SPB was scored as described in Materials and Methods (n = 200 for each sample). Mothers and buds in this experiment were distinguished by shmoo-shaped (mother) or round (bud) morphology. Representative pictures of cells are shown, overlaying tubulin staining and DIC images of the same cells. Bar, 5 μm.
Figure 9.

Effect of lack of Clb/Cdc28p activity on SPB migration. Clb1Δ-6ΔGAL1::CLB1 cells (SBY153) were grown in YEPG at 30°C, depleted of Clb1p by addition of 2% dextrose for 1 h, and G1 cells were isolated by centrifugal elutriation. The cells were incubated in YEPD in the presence of nocodazole until 70% had formed small buds (A) or until most cells formed large, clearly elongated buds (B). They were then harvested by centrifugation to wash out the nocodazole and resuspended in two aliquots: one received 100 μM Lat-A and the other DMSO as control. After a further 30 min, the cells were fixed and processed to visualize F-actin and tubulin. The position of the SPB was scored as described in Materials and Methods (at least 100 cells were scored for each sample). Mothers and buds in this experiment were distinguished by their size (A, mothers larger than buds) or shape (B, buds are elongated). Pictures show tubulin staining of representative cells overlaid with DIC images of the same cells. Bar, 5 μm.
Statistical Significance
The statistical significance of the differences we observed in the proportion of cells that had correctly oriented spindles or that had undergone SPB migration in the presence or absence of Lat-A was calculated with standard sampling theory for proportions (Spiegel 1972). In brief, the standard error (s) of our estimates was calculated based on the sample size (N) and observed proportion (p), according to the binomial distribution formula: s = √p(1−p)/N. Differences between samples were tested against the null hypothesis that the samples were derived from the same population, using a two-tailed test. In all cases mentioned in the text, the null hypothesis (i.e., no effect of Lat-A) was rejected at a <0.01 significance level.
Results
Effect of Perturbing F-Actin on the Maintenance of Spindle Orientation in Hydroxyurea-arrested Cells
In the course of studies using the actin-depolymerizing drug Lat-A (Ayscough et al. 1997; McMillan et al. 1998), we were surprised to find that Lat-A did not perturb spindle orientation in cells released from hydroxyurea arrest. An example is shown in Fig. 1, where spindle orientation was examined in formaldehyde-fixed cells by indirect immunofluorescence using the monoclonal antitubulin antibody YOL1/34. Following Palmer et al. 1992, we score a spindle as being properly oriented if an imaginary line drawn through the long axis of the spindle traverses the mother-bud neck. This scoring criterion contains information on both the position and orientation of the preanaphase spindle. Wild-type cells were arrested by a three hour incubation in medium containing hydroxyurea, which inhibits DNA replication, but allows bud formation and proper orientation of a preanaphase spindle. After release from the arrest into medium containing Lat-A, the cells did not display significant defects in spindle orientation either before or during anaphase, even though F-actin was completely depolymerized, as judged by rhodamine-phalloidin staining (Fig. 1). In contrast to the prevailing view, we conclude that F-actin is not required for maintenance of spindle orientation.
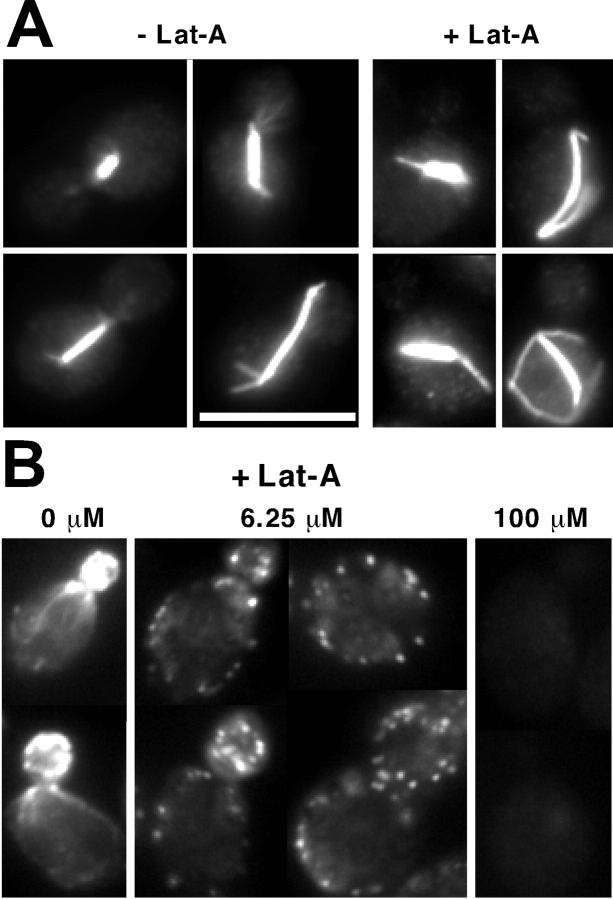
Figure 1.
F-actin is not required for maintenance of spindle orientation. Wild-type cells (DLY5) were grown in YEPD at 30°C, synchronized by addition of 200 mM hydroxyurea for 3 h, harvested, and resuspended in fresh YEPD supplemented with 0 or 100 μM Lat-A. At various times after release from hydroxyurea arrest, the cells were fixed and processed to visualize F-actin, tubulin, and DNA. A, Tubulin (left) and rhodamine-phalloidin (right) staining of cells 30 min after release from hydroxyurea arrest. Bar, 10 μm. B, Kinetics of anaphase. Percent of nuclear division was scored in DAPI-stained cells. C, Spindle orientation was scored as described in Materials and Methods, and reflects counts on both preanaphase and postanaphase spindles. n = 200 for each sample.

Possible reasons for the discrepancy between our results and previous studies include strain differences, different effects of the agents employed to perturb actin (Lat-A versus act1-4), and differences in synchronization or temperature regimes. We have repeated the experiment above in strain 5050 (Palmer et al. 1992), with identical results, indicating that this result is not strain-specific. To address whether Lat-A versus temperature-shift of act1-4 strains produced differing results, we performed an experiment to compare their effects using a single protocol. For consistency with the previous study, we followed the same synchrony and temperature shift protocol reported by Palmer et al. 1992. Compared with the untreated wild-type controls, both Lat-A and temperature-shift of act1-4 caused only mild spindle misorientation in large-budded cells (Fig. 2). We conclude that both actin perturbing agents have similar effects on spindle orientation.
Figure 2.
Spindle orientation upon actin perturbation of hydroxyurea-arrested cells. Wild-type (WT; 5050) and act1-4 (5105) cells were grown in YEPD at 23°C, synchronized by addition of 100 mM hydroxyurea for 3 h, and shifted to 36°C for 2 h. In addition, one aliquot of the wild-type culture was supplemented with 100 μM Lat-A at the time of the temperature shift. Cells were then fixed and processed to visualize F-actin and tubulin. A, Tubulin staining showing spindle orientation in typical fields of wild-type cells (upper left), cells treated with Lat-A (upper right), large-budded act1-4 cells (lower left), and act1-4 cells of different bud sizes (lower right). Some panels contain medium-budded cells with spindles oriented perpendicular to the plane of the photograph (and perpendicular to the mother-bud axis), which appear as bright dots. Bar, 10 μm. B, Quantification of errors in spindle orientation (scored as described in Materials and Methods) in the indicated cells. n = 200 for each sample.
One difference in the protocol of Fig. 2, compared with that of Fig. 1, lies in the degree of synchrony attained by the hydroxyurea treatment. In Fig. 1, the cells were treated with 200 mM hydroxyurea at 30°C, whereas in Fig. 2, they were treated with 100 mM hydroxyurea at 23°C. This led to significantly better arrest in the experiment of Fig. 1 (89% compared with 67% large-budded cells for the wild-type cultures). In addition, the arrest of the act1-4 mutants was significantly less homogeneous (51% large-budded). In the data reported above, we attempted to compensate for this synchrony difference by scoring only large-budded cells. In fact, examination of the sizable population of smaller budded cells in the act1-4 population revealed a much greater degree (64% compared with 20%) of spindle misorientation (Fig. 2). Thus, the apparent discrepancy between our results and those of Palmer et al. 1992 can be fully accounted for on the assumption that the act1-4 cells scored in that study included a substantial proportion of smaller budded cells.
In sum, these data suggest that whether or not perturbation of actin results in spindle misorientation may depend on when the perturbation occurs during the cell cycle. In the well-arrested cells examined in Fig. 1, no errors occurred upon actin perturbation. In the less well-arrested wild-type cells (or large-budded act1-4 cells) examined in Fig. 2, a moderate degree of spindle misorientation was induced (20%), whereas in the poorly arrested act1-4 population, frequent errors were induced (64%). Thus, cells that are truly arrested by hydroxyurea maintain correct spindle orientation, despite the absence of F-actin, whereas less well-arrested cells apparently do not.
Effect of Perturbing F-Actin on Spindle Orientation in Proliferating Cells
To examine whether cells traversing the cell cycle require actin to establish and/or maintain spindle orientation, a proliferating asynchronous population of wild-type cells was treated for 30 min with Lat-A. This induced a dramatic spindle misorientation in 53% of the cells (Fig. 3). Misaligned preanaphase spindles were observed in small- and medium-budded cells, but at lower frequency in large-budded cells (see below, Fig. 4). In addition, postanaphase spindles in medium-budded cells were often misaligned, probably resulting from elongation of short spindles that had become misoriented soon after Lat-A addition (Fig. 3 A; we return to this point in the Discussion). Thus, spindle orientation is actin-dependent in some fraction of a proliferating cell population, but not in hydroxyurea-arrested cells.
Figure 3.
F-actin dependence of spindle orientation in proliferating cells. Wild-type cells (DLY5) were grown in YEPD at 30°C, treated with the indicated dose of Lat-A (or DMSO for controls) for 30 min, and then fixed and processed to visualize F-actin and tubulin. A, Tubulin staining showing spindle orientation in typical cells treated with 100 μM Lat-A. Bar, 10 μm. B, Rhodamine-phalloidin staining of F-actin in cells treated with the indicated dose of Lat-A. C, Quantification of errors in spindle orientation (scored as described in Materials and Methods) in cells treated with the indicated dose of Lat-A. n = 200 for each sample.
Figure 4.
F-actin dependence of spindle orientation in synchronized cells. Wild-type (DLY1) cells were grown in YEPD at 30°C, synchronized by the addition of 25 ng/ml α-factor for 3 h, harvested, and resuspended in fresh YEPD. At 30, 45, and 60 min after release from α-factor, 6.25 μM Lat-A (or DMSO for controls) was added to aliquots of cells, which were then fixed at 75 min and processed to visualize tubulin. A, Kinetics of bud formation after release from α-factor. B, Quantification of preanaphase spindle orientation (scored as described in Materials and Methods). White bars, control; black bars, Lat-A treated. C, Quantification of postanaphase spindle orientation. White bars, control; black bars, Lat-A treated. D, Quantification of Lat-A induced errors observed in preanaphase (white bars) and postanaphase (black bars) spindles. To derive the proportion of cells displaying Lat-A–induced spindle misorientation, we subtracted the percent of misoriented spindles in control cells from that in Lat-A treated cells from the same sample. At least 100 cells were scored for each sample.
Intriguingly, a similarly dramatic spindle misorientation was induced even by treatment with a low dose of Lat-A, a point we return to below.
Cell Cycle Dependence of the Effect of Perturbing F-Actin on Spindle Orientation
In the experiments above (Fig. 1 and Fig. 2), the conclusion that maintenance of correct spindle orientation was actin-independent relied on perturbation of the cell cycle: hydroxyurea triggers arrest through the DNA replication checkpoint (Elledge 1996). Therefore, it was conceivable that actin independence was somehow induced in response to the checkpoint arrest, rather than constituting a normal part of the unperturbed cell cycle.
To address this issue, we monitored the effect of Lat-A on spindle orientation in wild-type cells progressing through a synchronous cell cycle. Cells were synchronized in G1 with α-factor and then harvested and resuspended in fresh medium. Lat-A (or DMSO for controls) was then added to separate aliquots of cells at 15 min intervals, and the cells were fixed 75 min after release from arrest to examine spindle orientation. Cell cycle synchrony in this experiment was good, with most cells budding between 30 and 45 min after release from pheromone arrest (Fig. 4 A).
When Lat-A was added to small-budded cells early in the cell cycle (30 min sample), preanaphase spindles became misoriented in 35% of the cells (compared with 7% in controls; Fig. 4 B). A similar degree of spindle misorientation was observed after only 15 min in Lat-A (data not shown). In addition, subsequent spindle elongation occurred entirely within the mother in 33% of the cells (compared with 4% in controls; Fig. 4 C). This confirms the requirement for F-actin in spindle orientation early in the cell cycle.
In contrast, when Lat-A was added to large-budded cells late in the cell cycle (60 min sample), preanaphase spindles did not become significantly misoriented (14% misorientation compared with 7% in controls; Fig. 4 B). Furthermore, anaphase spindle elongation was completely unaffected (only 1% incorrect in both Lat-A treated and control samples; Fig. 4 C). This implies that after a certain point (at least 15 min before anaphase) in the normal cell cycle, maintenance of spindle orientation becomes actin-independent.
When Lat-A was added to medium-budded cells at an intermediate time (45 min sample), preanaphase spindles became misoriented in many cells (35% misorientation compared with 10% in controls; Fig. 4 B), but anaphase spindle elongation was not significantly perturbed (8% incorrect compared with 3% in controls; Fig. 4 C). We interpret this to mean that at this intermediate time the population was mixed, with some cells still early enough in the cell cycle to require actin (hence misorienting their spindles in response to Lat-A), and others, later in the cycle, being insensitive to actin perturbation (hence executing a correctly oriented anaphase). The proportion of cells displaying Lat-A induced errors in either preanaphase or postanaphase spindle orientation at different times is summarized in Fig. 4 D. In aggregate, these data strongly support the conclusion that spindle orientation becomes insensitive to actin perturbation at some time during G2/M phase of the cell cycle.
Effect of Perturbing F-Actin on SPB Migration
A simple explanation for the difference in the sensitivity of spindle orientation to actin perturbation at early versus late times in the cell cycle might be that actin is required for the initial establishment, but not for the subsequent maintenance, of spindle orientation. The earliest step in spindle orientation is thought to be the orientation of the SPB (or duplicated side by side SPBs) towards the bud site before spindle assembly (Byers and Goetsch 1975; Snyder et al. 1991). Some studies have suggested that the SPB also undergoes a concerted migration to the bud site or bud neck (DeZwaan et al. 1997; Lee et al. 1999), although this remains controversial (Shaw et al. 1997). Addressing whether these early steps in spindle orientation are actin-dependent is complicated by the fact that actin is also required to construct the bud towards which the SPBs must align. To circumvent this difficulty, we devised a protocol to delay SPB orientation until after a bud had been formed.
We used the antimicrotubule drug nocodazole to prevent polymerization of microtubules, and hence SPB orientation or migration, until a bud had been formed. In addition, we used cdc31-1 mutant cells to prevent SPB duplication, so that spindle formation would be blocked and we could examine SPB position directly. cdc31-1 is a temperature-sensitive mutation that prevents SPB duplication at the restrictive temperature (Pringle and Hartwell 1981). cdc31-1 cells were grown at 23°C and shifted to 37°C for 1 h to inactivate Cdc31p. G1 daughter cells were then isolated by centrifugal elutriation and incubated in the presence of 15 μg/ml nocodazole to prevent SPB orientation during bud formation. After a majority of cells had formed small- or medium-sized buds, cells were harvested by centrifugation and resuspended in medium lacking nocodazole (all steps including the elutriation were carried out at 37°C; see Materials and Methods). At this point, the nuclei were distributed randomly within the mother portion of the cell (Fig. 5 A, t = 0). Within 30 min of nocodazole wash-out, the SPB had moved to the vicinity of the mother-bud neck in 76% of the cells (Fig. 5 A). This indicates that the cytoplasmic microtubules emanating from the unduplicated SPB were able to recognize asymmetric cues and promote appropriate SPB migration.
To address whether this SPB migration was actin-dependent, cells were synchronized as above, and Lat-A was added to the culture immediately after washing out the nocodazole. Staining with rhodamine-phalloidin confirmed that the Lat-A caused complete depolymerization of F-actin (data not shown). As shown in Fig. 5 A, this led to a partial disruption of SPB migration to the neck (56% compared with 76% SPB migration in controls). The fact that many SPBs did migrate to the neck suggests that the migration was not actin-dependent in all cells (see also below), though the effect of Lat-A was statistically significant (see Materials and Methods for evaluation of statistical significance). We conclude that SPB migration, an early step in spindle orientation, requires F-actin in at least a fraction of the cells.
Cell Cycle Dependence of the Effect of Perturbing F-Actin on SPB Migration
One model compatible with the data presented thus far would be that spindle orientation involves a single two-step process in which the first step (monitored in the SPB migration experiment of Fig. 5) requires F-actin, and the second step (monitored in the orientation maintenance experiment of Fig. 1) does not. Alternatively, the data might reflect the acquisition of a novel actin-independent mechanism for spindle orientation late in the cell cycle. In this latter model, all steps in spindle orientation could be accomplished in an actin-independent manner late in the cell cycle.
To distinguish between these models, we examined whether the SPB migration step was differentially sensitive to perturbation of actin at early versus late times in the cell cycle (Fig. 6). We isolated cdc31-1 cells synchronized in G1, as before, and incubated them in the presence of nocodazole for different times before wash-out to allow them to reach different stages of the cell cycle. To distinguish the buds from the mother cells at later stages, we marked the mother cells by inducing them to form shmoo projections during a brief exposure to α-factor in G1. Curiously, this pretreatment slightly altered the behavior of the SPB after nocodazole wash-out, so that in most cells, the SPB migrated all the way into the bud, rather than stopping near the neck. The basis for this effect of α-factor is unclear.
When nocodazole was washed out 60 min after α-factor treatment (an early stage in the cell cycle when most cells were small-budded; Fig. 6 A), the SPB migrated to the neck or into the bud. Addition of Lat-A partially disrupted this migration (62% SPB migration in Lat-A treated cells versus 82% in controls). In contrast, Lat-A had little effect when nocodazole was washed out at 120 min after α-factor treatment (a late stage in the cell cycle when most cells were large-budded, Fig. 6 B): the SPB migrated to the neck or into the bud in all cases (93% SPB migration in Lat-A treated cells versus 98% SPB migration in controls). Staining with rhodamine-phalloidin confirmed that the Lat-A caused complete depolymerization of F-actin in all cases (data not shown). Thus, even this early SPB migration step becomes actin-independent late in the cell cycle.
At earlier times, Lat-A had statistically significant effects on SPB migration and spindle orientation, but the effect of Lat-A was not complete (20%–35% increase in errors; Fig. 4 Fig. 5 Fig. 6). This may indicate that, even at early times, some cells can orient their spindles in an actin-independent manner. However, it is probable that the imperfect synchrony of the cell populations assayed led to the inclusion of some G2 cells in our early samples, reducing the apparent effect of actin perturbation. Similarly, random microtubule-powered movements might lead to a seemingly correct orientation in some cells, further reducing the apparent effect of actin perturbation. For these reasons, we do not know whether the requirement for actin at early times is absolute. Regardless, these experiments demonstrate that both SPB migration and maintenance of spindle orientation can occur in an actin-independent manner late in the cell cycle.
Spindle Orientation in Hydroxyurea-arrested Cells Lacking Kar9p, Bni1p, or Dyn1p
Previous studies have implicated a number of proteins, including the putative microtubule capturing protein Kar9p (Miller and Rose 1998), the cortical protein Bni1p (Zahner et al. 1996; Lee et al. 1999; Miller et al. 1999; Yang et al., manuscript in preparation), and the microtubule-based motor protein dynein (heavy chain Dyn1p; Li et al. 1993) in the process of spindle orientation. To address whether these proteins were important for spindle orientation late in the cell cycle, we arrested kar9Δ, bni1Δ, or dyn1Δ mutant strains with hydroxyurea. As shown in Fig. 7, none of these mutants differed from isogenic wild-type strains in terms of their ability to orient spindles. The wild-type W303 cells were not as proficient in this regard, as the wild-type YEF473 cells, presumably reflecting strain background effects. The fact that spindle orientation did (eventually) occur in these strains suggests that, given sufficient time, both the establishment and maintenance of spindle orientation can occur in the absence of these proteins.
Figure 7.
Spindle orientation in hydroxyurea-arrested cells lacking Kar9p, Bni1p, or dynein. Isogenic wild-type (W303a) and dyn1Δ (KBY62) cells, and isogenic wild-type (YEF473), kar9Δ (KBY1010), and bni1Δ (KBY1011) cells were grown in YEPD at 30°C, synchronized by addition of 200 mM hydroxyurea (HU) for 3 h (YEF473 strains) or 4 h (W303a strains), and supplemented with 0 or 100 μM Lat-A for a further 15 min, as indicated. Cells were fixed and processed to visualize F-actin and tubulin, and spindle orientation was scored as described in Materials and Methods. n = 200 for each sample.
To ask whether these proteins might play a redundant role with F-actin–dependent processes to orient the spindle late in the cell cycle, we treated the hydroxyurea-arrested mutant cells with Lat-A. However, no errors were induced by Lat-A in any of the mutants (Fig. 7).
Specific Role for Actin Cables in Spindle Orientation
In the SPB migration experiments, the movement of the SPB to the mother-bud neck (or bud) in a majority of the cells implied that cytoplasmic microtubules successfully interacted with asymmetric determinants to orient and move the SPB. Indeed, in the experiment of Fig. 5, 81% of the cells contained cytoplasmic microtubules extending from the SPB into the bud (Fig. 5 B). Surprisingly, however, in almost half of these cells the SPBs were also associated with microtubules reaching back from the neck into the mother (Fig. 5 B). Thus, SPB migration was associated with orientation of some, but not necessarily all, microtubules towards the bud. Treatment of cells with Lat-A caused a significant reduction (47% compared with 81%) in the proportion of cells displaying cytoplasmic microtubules extending into the bud (Fig. 5 B). This result suggests a role for F-actin in either guiding cytoplasmic microtubules into the bud or in keeping them there.
In proliferating cells, Lat-A induced very rapid spindle misorientation, with a maximal effect by 5 min of treatment (Fig. 8). In addition, even low doses of Lat-A were capable of inducing maximal levels of spindle misorientation (Fig. 3). Unlike cells treated with 100 μM Lat-A, which lacked detectable F-actin as judged by rhodamine-phalloidin staining, cells treated with 6.25 μM Lat-A lacked detectable actin cables, but retained many cortical actin patches, which were sometimes polarized to the bud tip in small-budded cells (Fig. 3 B). However, these cells displayed similar degrees of spindle misorientation (Fig. 3). This suggests that actin cables, rather than actin patches, may be important for spindle orientation.
Figure 8.
Effect of selective disruption of actin cables on spindle orientation. TPM1 tpm2Δ (ABY973) and tpm1-2 tpm2Δ (ABY971) cells were grown in YEPD at 24°C, shifted to 34.5°C for 5 min, fixed, and processed to visualize F-actin and tubulin. Lat-A (100 μM) was added to one aliquot of the TPM1 tpm2Δ culture at the time of shift. A, Control TPM1 tpm2Δ cells before (a and b) and after (c and d) the temperature shift, or after Lat-A treatment (e and f), and tpm1-2 tpm2Δ cells before (g and h) and after (i and j) the temperature shift. Pictures show tubulin staining of representative cells overlaid with DIC images of the same cells (a, c, e, g, and i). Rhodamine-phalloidin staining of F-actin (b, d, f, h, and j) in the same cells shows that all cells before the temperature shift (b and h), and TPM1 tpm2Δ cells after the temperature shift (d), contained actin cables, polarized actin patches, and properly oriented spindles. However, at 34.5°C the tpm1-2 tpm2Δ mutant lacks actin cables while retaining polarized patches (j), and Lat-A treated cells lack all F-actin (f). Bar, 10 μm. B, Quantification of misoriented preanaphase spindles (scored as described in Materials and Methods) in these cells before (0 min) and after (5 min) the temperature shift. The average of two independent experiments is presented. n = 200 for each sample in each experiment.

To examine this issue further, we took advantage of the tpm1-2 tpm2Δ strain described recently by Pruyne et al. 1998. Shift of this strain to the restrictive temperature of 34.5°C results in the complete disappearance of actin cables within 1 min, while cortical actin patches remain polarized for over 15 min (Pruyne et al. 1998; Fig. 8 A). We compared the effects of a 5 min Lat-A treatment (eliminating all F-actin) to a 5 min tpm1-2 tpm2Δ temperature shift (eliminating only actin cables) with regard to spindle orientation. As shown in Fig. 8, both treatments produced comparable degrees of spindle misorientation (the slightly higher basal level of spindle misorientation seen in these strains compared with previous figures may be due to the tpm2Δ mutation). This suggests that actin cables, rather than cortical actin patches, are important for spindle orientation.
Effect of Loss of Clb/Cdc28p Activity on SPB Migration
Many cell cycle events are thought to be triggered by a master cell cycle clock centered around a set of cyclins and cyclin-dependent kinases (Morgan 1997). In S. cerevisiae, events in the post-G1 cell cycle are governed by the B-type cyclins Clb1p-6p, together with the cyclin-dependent kinase Cdc28p (Lew et al. 1997). Thus, a plausible hypothesis would be that Clb1p-6p/Cdc28p complexes are responsible for triggering the observed switch between actin-dependent and actin-independent behavior in terms of spindle orientation. To test this hypothesis we used a strain in which CLB1-6 had been deleted, containing CLB1 driven from the regulatable GAL1 promoter. On galactose medium, Clb1p is overexpressed and the strain is viable. Upon addition of dextrose, the GAL1 promoter is repressed and the cells arrest lacking Clb1p-6p. As previously described (Schwob et al. 1994), the arrested cells initiate bud formation and duplicate the SPB, but cannot replicate DNA or separate the SPBs to form a spindle. In addition, they cannot perform the apical-to-isotropic switch in bud growth: actin remains highly polarized to the bud tip and the buds become elongated (Lew and Reed 1993).
To address whether Clb1p-6p function was required to trigger the switch from actin-dependent to actin-independent spindle orientation, we employed a protocol similar to that described above for cdc31-1 mutants. Dextrose was added to a proliferating culture of clb1Δ-6Δ GAL1:CLB1 cells to shut off Clb1p synthesis, and 1 h later newborn G1 cells were isolated by centrifugal elutriation. Since Clb1p is degraded as cells exit mitosis (Ghiara et al. 1991; Amon et al. 1994), these G1 cells should uniformly lack Clb1p-6p, as indeed was apparent from the ensuing elongated-bud arrest morphology (Fig. 9). After elutriation, nocodazole was added to prevent SPB migration during bud formation. At different times, the nocodazole was washed out and cells were resuspended in media containing Lat-A (or DMSO for controls). 30 min later, the cells were fixed and scored for SPB migration (without Clb1p-6p function, the duplicated SPBs remain side by side and move as a unit).
As shown in Fig. 9, a majority of the SPBs migrated either to the vicinity of the neck or all the way into the bud, and most cells displayed cytoplasmic microtubules extending from the duplicated SPBs into the bud. Note that this result implies that Clb1p-6p function is not required for SPB migration. About half of the cells contained multiple microtubules pointing in different directions, extending both into the bud and back towards the mother. Thus, as was also evident in the cdc31-1 experiments, SPB migration was associated with orientation of some, but not necessarily all, microtubules towards the bud.
When nocodazole was washed out early in the cell cycle (predominantly small-budded cells), the SPBs migrated to the neck or into the bud. As expected, addition of Lat-A significantly perturbed this migration (56% SPB migration in Lat-A treated cells versus 89% in controls; Fig. 9 A). In contrast, Lat-A had little effect when nocodazole was washed out later (predominantly large-budded cells): the SPBs migrated to the neck or into the bud in all cases (76% SPB migration in Lat-A treated cells versus 84% in controls; Fig. 9 B). Thus, a switch to actin-independent SPB migration was observed even in cells lacking Clb1p-6p. We conclude that this switch is not triggered by the cyclin/Cdc28p clock and occurs regardless of DNA replication or other Clb1p-6p–dependent events.
The behavior of the SPB was very similar in the cells lacking Clb1p-6p compared with cdc31-1 mutants. However, these cells differed in the overall lengths of the cytoplasmic microtubules: in particular, only 55% of cdc31-1 cells contained microtubules extending all the way to the cortex, compared with 83% for the clb1Δ-6Δ cells. A subset (18%) of the clb1Δ-6Δ cells also displayed extra-long microtubules that reached the cortex and were bent around the surface of the cell (Fig. 9).
Discussion
Spindle Orientation Late in the Cell Cycle
We report that, contrary to the prevailing view, maintenance of spindle orientation does not require F-actin in budding yeast. The apparent discrepancy between our results and those of Palmer et al. 1992 can be fully explained by differences in the synchrony of the hydroxyurea-treated cultures analyzed. In principle, actin-independent maintenance of spindle orientation could be due to a spindle docking process, whereby spindles that have already been correctly positioned and aligned through an actin-dependent process are immobilized at the mother-bud neck. However, even events that normally occur early in spindle orientation, such as SPB orientation and migration to the neck, occurred in an actin-independent manner if they were delayed until later in the cell cycle. This argues against the docking model and supports the hypothesis that, late in the cell cycle, all steps in spindle orientation can be carried out in the absence of F-actin.
A further surprise was that deletion of KAR9, BNI1, or DYN1, which are important for spindle orientation at other times in the cell cycle, did not prevent spindle orientation in hydroxyurea-arrested cells. Furthermore, simultaneous loss of F-actin (upon Lat-A treatment) and any one of Kar9p, Bni1p, or Dyn1p still did not perturb spindle orientation. In a previous study (Cottingham and Hoyt 1997), inactivation of a temperature-sensitive kip3 allele was similarly ineffective in misorienting the spindle in hydroxyurea-arrested cells, in contrast to its effect earlier in the cell cycle (Cottingham and Hoyt 1997; Miller et al. 1998). These findings suggest that the mechanism responsible for orienting the spindle late in the cell cycle is quite distinct from that occurring earlier in the cell cycle.
Acquisition of Actin Independence during the Cell Cycle
Intriguingly, actin-independent spindle orientation was not observed until G2/M phase of the cell cycle. Thus, there was effectively a switch between actin-dependent and actin-independent behavior on the part of the spindle. This switch did not require spindle formation, because we observed a similar switch to actin-independent behavior on the part of the unduplicated SPB in cdc31-1 mutants. Furthermore, the switch did not require continued cell cycle progression, since cells lacking Clb1p-6p still displayed a switch to actin-independent SPB behavior. Thus, actin independence seems to develop with time, rather than being switched on by a cell cycle cue.
This conclusion stems from experiments in which actin was perturbed using Lat-A after a large bud had been formed. In experiments where Lat-A was added to small-budded cells early in the cell cycle, spindles in many cells failed to orient along the mother-bud axis. If an actin-independent orientation capability were to develop with time under these conditions, we would expect that the initial spindle misorientation would be corrected after a suitable time interval. However, we found that spindle misorientation persisted, and that eventual anaphase frequently occurred entirely within the mother cell under these circumstances. Thus, actin independence did not develop in cells that had been treated with Lat-A early on. This suggests that F-actin is itself required, early in the cell cycle, for the development of a spindle orientation mechanism that is then insensitive to perturbation of F-actin. It is unclear whether this requirement is related to the F-actin–dependent spindle orientation process observed early in the cell cycle or involves a distinct role for F-actin. Indeed, since these experiments required >30 min treatments with Lat-A, it remains possible that the failure to develop actin independence was a secondary consequence of prolonged loss of F-actin. Nevertheless, one simple explanation for these observations would be that, during early stages of bud formation, actin is required for the delivery of cortical determinants into the bud: these determinants could later interact with astral microtubules in an actin-independent fashion to promote spindle orientation.
The Role of Actin in Spindle Orientation Early in the Cell Cycle
Perturbing F-actin early in the cell cycle significantly decreased the proportion of cells displaying astral microtubules extending into the bud, and similarly decreased the proportion of cells displaying SPB migration to the mother-bud neck. This suggests that F-actin is important for guiding astral microtubules into the bud, or perhaps for anchoring of astral microtubules within the bud. F-actin displays a polarized distribution that makes it well suited to these tasks: the mother cell contains a cortical basket of actin cables converging on the bud neck, whereas cortical actin-rich patches are largely restricted to the bud (Adams and Pringle 1984; Amberg 1998).
Selective disruption of actin cables in tpm1-2 tpm2Δ mutants (Pruyne et al. 1998) led to spindle misorientation without affecting the polarized distribution of cortical actin patches. This suggests that it is the actin cables, rather than the actin patches, that are important for spindle orientation (although it remains possible that actin patches were affected in some manner in the tpm1-2 tpm2Δ mutants).
One hypothesis consistent with these findings would be that astral microtubules interact via cross-linking proteins with actin cables in the mother cell to be guided into the bud. An attractive candidate for such a cross-linking protein might be the recently described coronin Crn1p, which binds to microtubules and actin filaments (and whose microtubule-binding is enhanced in the presence of F-actin; Heil-Chapdelaine et al. 1998; Goode et al. 1999). However, Crn1p was predominantly localized in cortical actin patches rather than cables, and mutants lacking Crn1p did not display major spindle misorientation, indicating that other proteins must be capable of orienting astral microtubules in its absence. Perhaps the most attractive candidate for such a protein currently known is Kar9p (Miller and Rose 1998; Miller et al. 1999), although it is not known whether Kar9p interacts with microtubules or actin filaments directly. Astral microtubules in kar9Δ cells frequently fail to extend into the bud (Miller and Rose 1998), a phenotype similar to that displayed upon actin perturbation of cells early in the cell cycle (Fig. 5 B and Maddox et al., unpublished observations). Thus, one role for Kar9p might be to promote interactions between astral microtubules and actin cables, leading to frequent penetration of the microtubules into the bud.
Kar9p is predominantly localized to a single spot on the bud cortex, where it has been proposed to anchor cytoplasmic microtubules (Miller and Rose 1998; Miller et al. 1999). The formin Bni1p is localized at the cell cortex, is required for localization of Kar9p in a cortical dot, and is important for proper spindle orientation (Lee et al. 1999; Miller et al. 1999; Yang et al., manuscript in preparation). However, Kar9p retains a reduced ability to promote correct spindle orientation in bni1Δ cells, suggesting that Kar9p localization may not be essential for its function (Miller et al. 1999). Conceivably Kar9p functions (independent of Bni1p?) to guide astral microtubules along actin cables into the bud, and also (together with Bni1p?) to anchor them within the bud.
In summary, spindle orientation is very sensitive to perturbation of actin cables early in the cell cycle, and at this stage the actin cables play a role in either guiding astral microtubules into the bud, anchoring them within the bud, or both. In addition, F-actin is required early in the cell cycle for the development of actin independence later in the cell cycle.
Behavior of Astral Microtubules and the Role of Dynein
Although SPB migration occurred even in cells lacking Clb1p-Clb6p, the astral microtubules were longer in these cells, suggesting that astral microtubule behavior may be regulated by B-type cyclins. Indeed, a recent study proposed a role for Clb5p in regulating astral microtubule behavior (Segal et al. 1998), based on the observation that cells with a partial loss of Clb/Cdc28p activity exhibited spindle migration all the way into the bud, rather than stopping at the neck.
An unexpected finding from our SPB migration assays was that in ∼50% of the cells, SPBs were associated with astral microtubules extending back into the mother cell, as well as into the bud. This suggests that the net movement of SPBs to the mother-bud neck or into the bud was not simply due to asymmetric orientation of the attached microtubules. Rather, there may be some asymmetry in force production between bud-directed and mother-directed microtubules. Several microtubule motor proteins have been shown to affect astral microtubule behavior and spindle orientation in yeast (Eshel et al. 1993; Li et al. 1993; Yeh et al. 1995; Carminati and Stearns 1997; Cottingham and Hoyt 1997; DeZwaan et al. 1997; Saunders et al. 1997; Shaw et al. 1997; Miller et al. 1998). In particular, dynein (and dynactin) mutants frequently undergo anaphase with the spindle entirely within the mother portion of the cell (Li et al. 1993; Yeh et al. 1995), even after release from hydroxyurea arrest (Muhua et al. 1998), suggesting that dynein is important for spindle orientation late in the cell cycle. However, we found that dynein was not required for spindle orientation in hydroxyurea-arrested cells. One hypothesis that reconciles these observations is that dynein is not required for spindle orientation per se, but is important for pulling one pole of the spindle through the neck during anaphase. This would be consistent with the observation that initial spindle elongation during anaphase generally occurs along the mother-bud axis in dynein mutants, albeit within the mother cell (Li et al. 1993; Yeh et al. 1995).
We have found that the mitotic spindle is able to orient along the mother-bud axis in an F-actin–independent manner late in the cell cycle of budding yeast. We suggest that actin plays a role in two aspects of spindle orientation early in the cell cycle. First, actin cables are required to guide astral microtubules into the bud or possibly to anchor them within the bud, allowing SPB positioning near the mother/bud neck. Second, F-actin is required for the development of a cortical asymmetry that subsequently maintains correct spindle orientation in an actin-independent manner late in the cell cycle.
Acknowledgments
We thank Doug Koshland, David Pruyne, and Tony Bretscher for strains, and Phil Crews for a gift of Lat-A. We thank Sally Kornbluth and anonymous reviewers for critical reading of the manuscript, and the members of the Lew, Bloom, and Pringle labs for stimulating interactions.
This work was supported by the United States Public Health Service grant GM53050 and by the American Cancer Society grant RPG-98-046-01-CCG to D.J. Lew.
Footnotes
1.used in this paper: DAPI, 4′,6-diamidino-2-phenylindole; DIC, differential interference contrast; Lat-A, Latrunculin-A; SPB, spindle pole body
Chandra L. Theesfeld and Javier E. Irazoqui contributed equally to this work.
References
- Adams A.E.M., Pringle J.R. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae . J. Cell Biol. 1984;98:934–945. doi: 10.1083/jcb.98.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amberg D.C. Three-dimensional imaging of the yeast actin cytoskeleton through the budding cell cycle. Mol. Biol. Cell. 1998;9:3259–3262. doi: 10.1091/mbc.9.12.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amon A., Irniger S., Nasmyth K. Closing the cell cycle circle in yeastG2 cyclin proteolysis initiated at mitosis persists until the activation of G1 cyclins in the next cycle. Cell. 1994;77:1037–1050. doi: 10.1016/0092-8674(94)90443-x. [DOI] [PubMed] [Google Scholar]
- Ayscough K.R., Stryker J., Pokala N., Sanders M., Crews P., Drubin D.G. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor Latrunculin-A. J. Cell Biol. 1997;137:399–416. doi: 10.1083/jcb.137.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudin A., Ozier-Kalogeropoulos O., Denouel A., Lacroute F., Cullin C. A simple and efficient method for direct gene deletion in Saccharomyces cerevisiae . Nucleic Acids Res. 1993;21:3329–3330. doi: 10.1093/nar/21.14.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi E., Pringle J.R. ZDS1 and ZDS2, genes whose products may regulate Cdc42p in Saccharomyces cerevisiae . Mol. Cell. Biol. 1996;16:5264–5275. doi: 10.1128/mcb.16.10.5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers B. Cytology of the yeast life cycle. In: Strathern J.N., Jones E.W., Broach J.R., editors. The Molecular Biology of the Yeast SaccharomycesLife Cycle and Inheritance. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 1981. pp. 59–96. [Google Scholar]
- Byers B., Goetsch L. Behaviour of spindles and spindle plaques in the cell cycle and conjugation of Saccharomyces cerevisiae . J. Bacteriol. 1975;124:511–523. doi: 10.1128/jb.124.1.511-523.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carminati J., Stearns T. Microtubules orient the mitotic spindle in yeast through dynein-dependent interactions with the cell cortex. J. Cell Biol. 1997;138:629–641. doi: 10.1083/jcb.138.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottingham F.R., Hoyt M.A. Mitotic spindle positioning in Saccharomyces cerevisiae is accomplished by antagonistically acting microtubule motor proteins. J. Cell Biol. 1997;138:1041–1053. doi: 10.1083/jcb.138.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeZwaan T.M., Ellingson E., Pellman D., Roof D.M. Kinesin-related KIP3 of Saccharomyces cerevisiae is required for a distinct step in nuclear migration. J. Cell Biol. 1997;138:1023–1040. doi: 10.1083/jcb.138.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elledge S.J. Cell cycle checkpointspreventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- Eshel D., Urrestarazu L.A., Vissers S., Jauniaux J.C., van Vliet-Reedijk J.C., Planta R.J., Gibbons I.R. Cytoplasmic dynein is required for normal nuclear segregation in yeast. Proc. Natl. Acad. Sci. USA. 1993;90:11172–11176. doi: 10.1073/pnas.90.23.11172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiara J.B., Richardson H.E., Sugimoto K., Henze M., Lew D.J., Wittenberg C., Reed S.I. A cyclin B homolog in S. cerevisiaechronic activation of the Cdc28 protein kinase by cyclin prevents exit from mitosis. Cell. 1991;65:163–174. doi: 10.1016/0092-8674(91)90417-w. [DOI] [PubMed] [Google Scholar]
- Goode B.L., Wong J.J., Butty A.C., Peter M., McCormack A.L., Yates J.R., Drubin D.G., Barnes G. Coronin promotes the rapid assembly and cross-linking of actin filaments and may link the actin and microtubule cytoskeletons in yeast. J. Cell Biol. 1999;144:83–98. doi: 10.1083/jcb.144.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil-Chapdelaine R.A., Tran N.K., Cooper J.A. The role of Saccharomyces cerevisiae coronin in the actin and microtubule cytoskeletons. Curr. Biol. 1998;8:1281–1284. doi: 10.1016/s0960-9822(07)00539-8. [DOI] [PubMed] [Google Scholar]
- Jacobs C.W., Adams A.E.M., Szaniszlo P.J., Pringle J.R. Functions of microtubules in the Saccharomyces cerevisiae cell cycle. J. Cell Biol. 1988;107:1409–1426. doi: 10.1083/jcb.107.4.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L., Klee S.K., Evangelista M., Boone C., Pellman D. Control of mitotic spindle position by the Saccharomyces cerevisiae formin Bni1p. J. Cell Biol. 1999;144:947–961. doi: 10.1083/jcb.144.5.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew D.J., Reed S.I. Morphogenesis in the yeast cell cycleregulation by Cdc28 and cyclins. J. Cell Biol. 1993;120:1305–1320. doi: 10.1083/jcb.120.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew D.J., Weinert T., Pringle J.R. Cell cycle control in Saccharomyces cerevisiae . In: Pringle J.R., Broach J., Jones E., editors. The Molecular and Cellular Biology of the Yeast Saccharomyces. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 1997. pp. 607–695. [Google Scholar]
- Li Y.Y., Yeh E., Hays T., Bloom K. Disruption of mitotic spindle orientation in a yeast dynein mutant. Proc. Natl. Acad. Sci. USA. 1993;90:10096–10100. doi: 10.1073/pnas.90.21.10096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan J.N., Sia R.A.L., Lew D.J. A morphogenesis checkpoint monitors the actin cytoskeleton in yeast. J. Cell Biol. 1998;142:1487–1499. doi: 10.1083/jcb.142.6.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R.K., Rose M.D. Kar9p is a novel cortical protein required for cytoplasmic microtubule orientation in yeast. J. Cell Biol. 1998;140:377–390. doi: 10.1083/jcb.140.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R.K., Heller K.K., Frisen L., Wallack D.L., Loayza D., Gammie A.E., Rose M.D. The kinesin-related proteins, Kip2p and Kip3p, function differently in nuclear migration in yeast. Mol. Biol. Cell. 1998;9:2051–2068. doi: 10.1091/mbc.9.8.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R.K., Matheos D., Rose M.D. The cortical localization of the microtubule orientation protein, Kar9p, is dependent upon actin and proteins required for polarization. J. Cell Biol. 1999;144:963–975. doi: 10.1083/jcb.144.5.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D.O. Cyclin-dependent kinasesengines, clocks, and microprocessors. Annu. Rev. Cell Dev. Biol. 1997;13:261–291. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- Muhua L., Adames N.R., Murphy M.D., Shields C.R., Cooper J.A. A cytokinesis checkpoint requiring the yeast homologue of an APC-binding protein. Nature. 1998;393:487–91. doi: 10.1038/31014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R.E., Sullivan D.S., Huffaker T., Koshland D. Role of astral microtubules and actin in spindle orientation and migration in the budding yeast, Saccharomyces cerevisiae . J. Cell Biol. 1992;119:583–593. doi: 10.1083/jcb.119.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle J.R., Hartwell L.H. The Saccharomyces cerevisiae cell cycle. In: Strathern J.D., Jones E.W., Broach J.R., editors. The Molecular Biology of the Yeast Saccharomyces. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 1981. pp. 97–142. [Google Scholar]
- Pringle J.R., Adams A.E.M., Drubin D.G., Haarer B.K. Immunofluorescence methods for yeast. Methods Enzymol. 1991;194:565–602. doi: 10.1016/0076-6879(91)94043-c. [DOI] [PubMed] [Google Scholar]
- Pruyne D.W., Schott D.H., Bretscher A. Tropomyosin-containing actin cables direct the Myo2p-dependent polarized delivery of secretory vesicles in budding yeast. J. Cell Biol. 1998;143:1931–1945. doi: 10.1083/jcb.143.7.1931. [DOI] [PubMed] [Google Scholar]
- Rappaport R. Cytokinesis in Animal Cells. Cambridge University Press; Cambridge, UK: 1996. [Google Scholar]
- Richardson H.E., Wittenberg C., Cross F.R., Reed S.I. An essential G1 function for cyclin-like proteins in yeast. Cell. 1989;59:1127–1133. doi: 10.1016/0092-8674(89)90768-x. [DOI] [PubMed] [Google Scholar]
- Saunders W., Hornack D., Lengyel V., Deng C. The Saccharomyces cerevisiae kinesin-related motor Kar3p acts at preanaphase spindle poles to limit the number and length of cytoplasmic microtubules. J. Cell Biol. 1997;137:417–431. doi: 10.1083/jcb.137.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwob E., Bohm T., Mendenhall M.D., Nasmyth K. The B-type cyclin kinase inhibitor p40SIC1 controls the G1 to S transition in S. cerevisiae . Cell. 1994;79:233–244. doi: 10.1016/0092-8674(94)90193-7. [DOI] [PubMed] [Google Scholar]
- Segal M., Clarke D.J., Reed S.I. Clb5-associated kinase activity is required early in the spindle pathway for correct preanaphase nuclear positioning in Saccharomyces cerevisiae . J. Cell Biol. 1998;143:135–145. doi: 10.1083/jcb.143.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw S.L., Yeh E., Maddox P., Salmon E.D., Bloom K. Astral microtubule dynamics in yeasta microtubule-based searching mechanism for spindle orientation and nuclear migration into the bud. J. Cell Biol. 1997;139:985–994. doi: 10.1083/jcb.139.4.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M., Gehrung S., Page B.D. Studies concerning the temporal and genetic control of cell polarity in Saccharomyces cerevisiae . J. Cell Biol. 1991;114:515–532. doi: 10.1083/jcb.114.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel M.R. Statistics. McGraw-Hill Publishing Co; NY: 1972. [Google Scholar]
- Sullivan D.S., Huffaker T.C. Astral microtubules are not required for anaphase B in Saccharomyces cerevisiae . J. Cell Biol. 1992;119:379–388. doi: 10.1083/jcb.119.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh E., Skibbens R.V., Cheng J.W., Salmon E.D., Bloom K. Spindle dynamics and cell cycle regulation of dynein in the budding yeast, Saccharomyces cerevisiae . J. Cell Biol. 1995;130:687–700. doi: 10.1083/jcb.130.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahner J.E., Harkins H.A., Pringle J.R. Genetic analysis of the bipolar pattern of bud site selection in the yeast Saccharomyces cerevisiae . Mol. Cell. Biol. 1996;16:1857–1870. doi: 10.1128/mcb.16.4.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]