Abstract
The major sperm protein (MSP)-based amoeboid motility of Ascaris suum sperm requires coordinated lamellipodial protrusion and cell body retraction. In these cells, protrusion and retraction are tightly coupled to the assembly and disassembly of the cytoskeleton at opposite ends of the lamellipodium. Although polymerization along the leading edge appears to drive protrusion, the behavior of sperm tethered to the substrate showed that an additional force is required to pull the cell body forward. To examine the mechanism of cell body movement, we used pH to uncouple cytoskeletal polymerization and depolymerization. In sperm treated with pH 6.75 buffer, protrusion of the leading edge slowed dramatically while both cytoskeletal disassembly at the base of the lamellipodium and cell body retraction continued. At pH 6.35, the cytoskeleton pulled away from the leading edge and receded through the lamellipodium as its disassembly at the cell body continued. The cytoskeleton disassembled rapidly and completely in cells treated at pH 5.5, but reformed when the cells were washed with physiological buffer. Cytoskeletal reassembly occurred at the lamellipodial margin and caused membrane protrusion, but the cell body did not move until the cytoskeleton was rebuilt and depolymerization resumed. These results indicate that cell body retraction is mediated by tension in the cytoskeleton, correlated with MSP depolymerization at the base of the lamellipodium.
Keywords: amoeboid motility, retraction, major sperm protein, actin, lamellipodium
Crawling over a substrate is important at some time in the life of many animal cells. This type of movement can take on a variety of forms, ranging from the rapid translocation of macrophages to the slower advance of fibroblasts (Oliver et al. 1993; reviewed in Stossel 1993). In all cases, amoeboid cell motility requires integration of three processes: extension of the leading edge, adhesion to the substratum, and retraction of the cell body (reviewed in Mitchison and Cramer 1996). In most amoeboid cells, locomotion is based on modulation of the actin cytoskeleton, but the exact mechanisms by which force is produced within the cytoskeleton and applied against substrate attachment sites to generate movement are not fully understood.
Protrusion of the leading edge of crawling cells appears to be coupled to polymerization and cross-linking of actin filaments (reviewed in Cooper 1991; Condeelis 1993; Mitchison and Cramer 1996; Zigmond 1996). Evidence from related motile systems, such as comet tail formation by the intracellular bacterial pathogens (reviewed in Cossart and Kochs 1994; Southwick and Purich 1994; Theriot 1995) and the movement of surface-attached beads on Aplysia neuronal growth cones (Forscher et al. 1992; Suter et al. 1998), also suggest that localized polymerization can produce movement. Recently, Oster and colleagues have formulated intriguing hypotheses to explain the physical mechanics of these motions (Peskin et al. 1993; Mogilner and Oster 1996a,Mogilner and Oster 1996b).
In contrast to protrusion, very little is known about how the body or rear of the cell is pulled forward (reviewed in Mitchison and Cramer 1996). In theory, the force for forward movement of the cell body could be generated near the front of the cell. In this model, the force that drives protrusion near the leading edge could also produce tension in the plasma membrane or in its underlying cortical cytoskeleton, causing the cell body to be dragged forward. However, in fish epithelial keratocytes, cell body translocation can occur in the absence of extension of the leading edge (Anderson et al. 1996). Svitkina et al. 1997 showed that myosin II clusters align at the cell body–lamellipodium junction in these cells and proposed that cell body translocation is driven by acto-myosin II contraction. Recent studies revealed that a similar reorganization of the acto-myosin II system is associated with polarization and locomotion of fragments of fish epithelial keratocytes (Verkhovsky et al. 1998). These observations suggest that there may be independent forces for protrusion of the leading edge and retraction of the rear of crawling cells. However, the exact mechanism by which forces are harnessed to generate movement is far from clear.
The amoeboid sperm of nematodes are simple cells that offer distinctive advantages for investigating the forces required for crawling movement. Although nematode sperm contain neither F-actin nor myosin, their locomotion is practically indistinguishable from that of conventional crawling cells (reviewed in Theriot 1996; Roberts and Stewart 1997). Sperm motility is driven by a novel locomotory apparatus based on filaments comprised of the 14-kD major sperm protein (MSP)1 (reviewed in Roberts and Stewart 1995). In sperm from Ascaris suum, the MSP filaments are arranged into dynamic meshworks, called fiber complexes, that extend the entire length of the lamellipodium (Sepsenwol et al. 1989). The fiber complexes are readily visible by light microscopy, so the relationship of cytoskeletal dynamics to cell movement can be studied without disrupting cellular integrity. The MSP filaments polymerize and bundle into fiber complexes at the leading edge in a pattern similar to that observed in actin-based crawling cells (reviewed in Theriot 1996; Roberts and Stewart 1997). This aspect of sperm locomotion has been reconstituted in vitro (Italiano et al. 1996), whereby inside-out vesicles derived from the plasma membrane at the leading edge induce ATP-dependent assembly of discrete filament meshworks, or fibers. Growth of these fibers, which are similar to the fiber complexes observed in crawling sperm, is due to filament polymerization at the membrane vesicle and results in vesicle translocation. Thus, localized polymerization and cross-linking of filaments can be harnessed to produce motion.
The Ascaris sperm in vitro motility system has given insights into the role of MSP polymerization in membrane protrusion, but has left open the question of how the cell body is pulled forward during locomotion of the intact cell. Structural studies have shown that MSP filaments have no overall polarity (Bullock et al. 1998) and so it is unlikely that molecular motor proteins, which depend on the polarity of their associated polymer to operate, contribute to cell body retraction in nematode sperm. In this study, we used different pH buffers to uncouple leading edge protrusion from cell body retraction, and thereby demonstrate that cytoskeletal depolymerization is coupled to forward movement of the cell body. Thus, sperm locomotion depends on the integration of two independent forces associated with cytoskeletal assembly and disassembly at opposite ends of the lamellipodium.
Materials and Methods
Sperm Isolation and Activation
Males of Ascaris suum were collected from the intestines of infected hogs at the Lowell Packing Company. Worms were transported to the laboratory in phosphate-buffered saline containing 10 mM NaHCO3, pH 7, at 37°C, and maintained in this buffer for up to 1 d. Spermatids were isolated by draining the contents of the seminal vesicle into HKB buffer (50 mM Hepes, 65 mM KCl, and 10 mM NaHCO3, pH 7.1). The spherical spermatids were then treated with vas deferens extract, prepared according to Sepsenwol et al. 1986, to initiate lamellipodium formation and completion of development into motile spermatozoa.
Examination of Sperm by Light Microscopy
Activated sperm were pipetted into chambers formed by mounting a 24 × 60-mm glass coverslip onto a glass slide with two strips of hematoseal tube sealant. All glassware was washed in ethanol before use. Sperm were examined under a 100× differential interference contrast (DIC) oil immersion Zeiss plan/neofluar objective (1.3 N.A.) on a Zeiss Axiovert 35 microscope. Preparations were maintained at 37°C using an airstream incubator (Nicholson Precision Instruments). When desired, the solution bathing the cells was changed by pipetting 5–7 chamber volumes (1 chamber volume = 350–400 μl) of the new solution at one side of the chamber, using a tissue wick to draw the fluid into the chamber.
Image Acquisition and Analysis
All images of cells were obtained using a charged-coupled device (CCD) camera (Model TI-24A; Nippon Electronics Corp.), digitized, and processed by background subtraction and contrast enhancement using Image-1AT software and hardware (Universal Imaging), and recorded on a super VHS VCR (JVC Model HR-S5200U). Video images were imported into Adobe Photoshop, processed, and printed on a Codonics printer. Rates of cell movement and related parameters were measured using Image 1AT subroutines.
Results
MSP Cytoskeleton Dynamics in Crawling and Stationary Ascaris Sperm
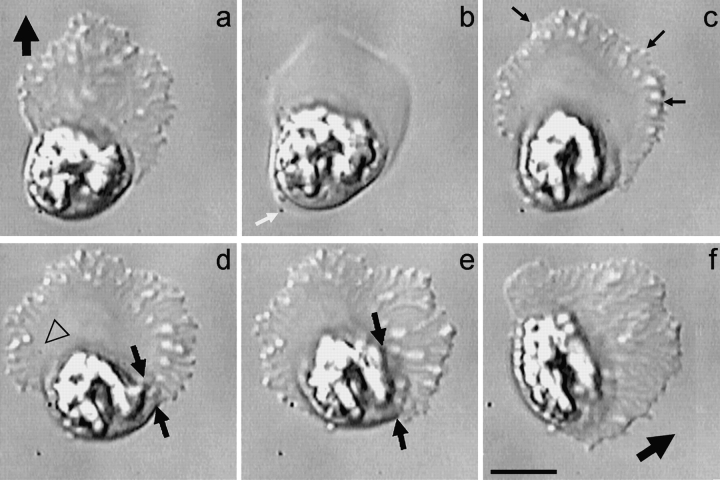
The dynamics of the cytoskeleton of Ascaris sperm can be observed directly in crawling cells using DIC microscopy and indicate that both MSP assembly and disassembly are important in their amoeboid motility. The MSP-based cytoskeleton in these cells form fiber complexes, each comprised of a meshwork of MSP filaments, that can be observed in living cells (Fig. 1; see also Sepsenwol et al. 1989; Roberts and King 1991). Distinctive features, such as branches in the fiber complexes, can be followed and show that the cytoskeleton treadmills as sperm locomote (see Sepsenwol et al. 1989; Roberts and King 1991). When sperm crawl, filaments assemble into fiber complexes along the leading edge and flow retrograde to the base of the lamellipodium, where they disassemble. The rates of cytoskeletal assembly and disassembly are balanced and are coupled to the pace of locomotion. Thus, as illustrated by analysis of morphological markers in the cell shown in Fig. 1, the lamellipodium maintains its shape over time while the cytoskeleton flows rearward with respect to the cell, but does not move, or moves very slowly, with respect to the substrate. This pattern of lamellipodial dynamics has also been measured by computer-assisted microscopy (Royal et al. 1995). For example, difference pictures comparing the shape of the lamellipodium of crawling sperm at two-second intervals showed that the area of the zone of expansion at the leading margin was similar to the area lost due to retraction at the base of the lamellipodium. This study also showed that the average instantaneous velocity of sperm crawling on glass was 30 μm/min, but that the cells surged forward about every 0.35 min, increasing their velocity by an average of 47%. The cells maintained their lamellipodial shape during these surges, indicating that cytoskeletal assembly and disassembly remain balanced even when crawling speed changes (Royal et al. 1995).
Figure 1.
MSP cytoskeletal dynamics in crawling Ascaris sperm. The long branched elements that extend from the leading edge to the base of the lamellipodium are the fiber complexes, each a dense meshwork of MSP filaments. The cytoskeleton flows retrograde as the fiber complexes are assembled at the leading edge and disassembled at the cell body. Because the rates of cytoskeletal flow and locomotion are coupled, morphological markers in the cytoskeleton, such as the branch in the fiber complex indicated by the arrowhead, remain nearly stationary relative to the substrate. The fields of view are identical in each frame and the interval between frames is 10 s. Over the 30-s interval from a–d, both the leading edge and the cell body advanced by 6.5 μm while the lamellipodium maintained a length of 25 μm. This illustrates the balance between the rates of cytoskeletal assembly/leading edge protrusion and cytoskeletal disassembly/cell body retraction during sperm locomotion. Bar, 10 μm.
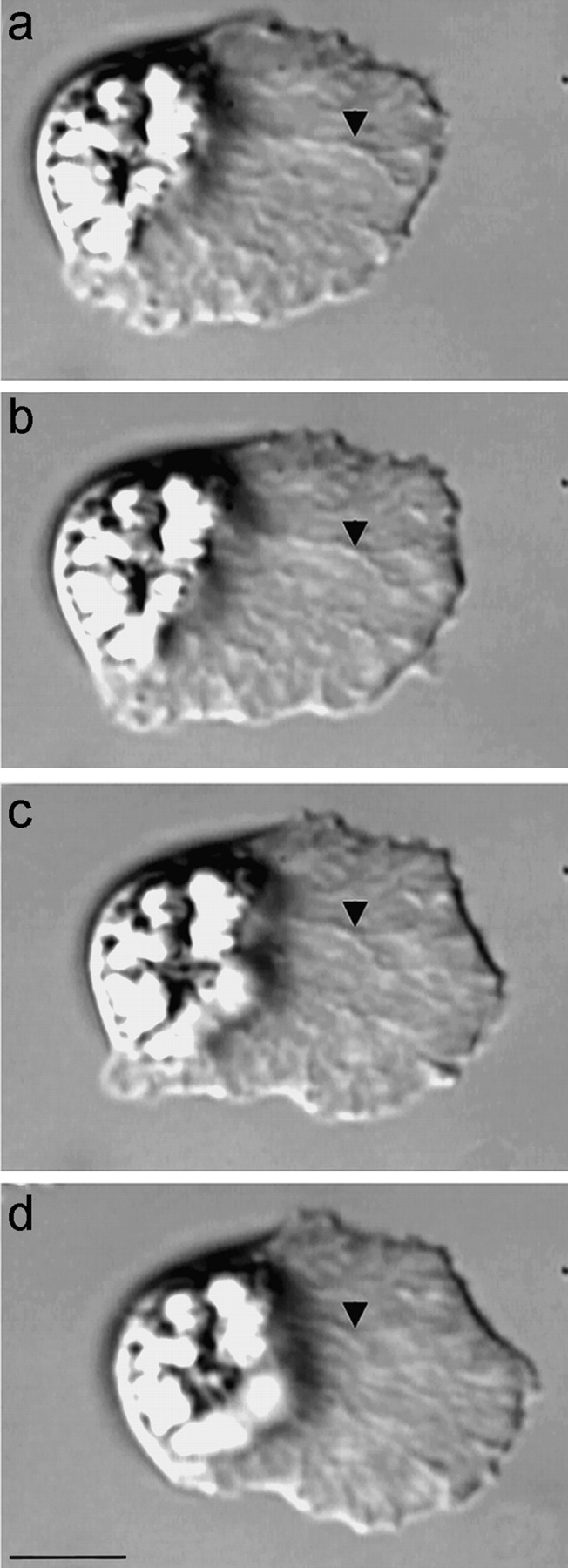
The behavior of crawling sperm that have become tethered to the glass substrate at their cell body (Fig. 2) indicated that lamellipodial extension is not sufficient for cell locomotion. In these cells, the lamellipodium continued to exert force against the cell body sufficient to distort its shape from hemispherical to elongate, but there was no stretching of the lamellipodium (Fig. 2). In tethered cells, the leading edge underwent cycles of extension and retraction with no net advance, and yet cytoskeletal flow continued. In some cells, the lamellipodium lost its attachment and the entire cell was pulled toward the tether site. Others, like the sperm shown in Fig. 2, broke the tether, after which the cell body recoiled toward the lamellipodium and regained its hemispherical shape as protrusion of the leading edge and locomotion resumed. By contrast, there was no shortening of the lamellipodium when the cell body recoiled. These observations show that the cell body does not follow passively behind the motile lamellipodium but, instead, that tension within the cytoskeleton pulls the cell body forward. Moreover, the force pulling against the cell body appears to be generated at the base of the lamellipodium. If that tension was produced at the leading edge by the force that drives protrusion, then the entire cell would stretch when tethered at the rear and recoil when the tether was broken.
Figure 2.
Escape of a tethered sperm. This sequence of images, taken 6 s apart, shows the locomotory behavior of a sperm as it breaks an abnormal attachment that has tethered the trailing margin of its cell body to the glass substrate. When the cell is tethered, force produced in the cytoskeleton causes the cell body to stretch, distorting its shape, but the shape of the lamellipodium is unaffected (a). When this abnormal attachment is broken (b) the cell body recoils to its normal hemispherical shape and the cell resumes locomotion (b–d). The lamellipodium maintained its shape throughout this process and, in fact, lengthened slightly as the cell crawled away. Bar, 10 μm.
Cytoskeleton Polymerization and Depolymerization Can Be Uncoupled by Manipulating Intracellular pH
To probe the mechanism of cell body retraction and its role in sperm locomotion, we used pH to uncouple the cytoskeletal assembly and disassembly that occur at opposite ends of the lamellipodium. The MSP cytoskeleton is sensitive to intracellular pH (Roberts and King 1991; King et al. 1994). We found that treating sperm with HKB buffer containing 20 mM sodium acetate (HKB-acetate) at different pH values caused cytoskeletal assembly and protrusion of the leading edge to either slow dramatically or stop entirely, while cytoskeletal disassembly and retraction of the cell body continued unaltered. The cell shown in Fig. 3, for example, was crawling at 15 μm/min until it was perfused with HKB-acetate, pH 6.75. This treatment caused protrusion of the leading edge to slow to <3 μm/min. However, retraction of the cell body continued at 15 μm/min for a further 30 s, then stopped. This continuing retraction of the cell body appeared to be correlated with localized disassembly of the fiber complexes near the cell body because the distance between the cell body–lamellipodium junction and distinctive morphological features of the fiber complexes decreased as the cell body moved forward. Moreover, the cell body appeared to move forward due to shortening of the lamellipodium rather than rolling forward over the rear of the lamellipodium. If the cell body was rolling, the organelles within it should also roll, but this was not observed. Instead, the organelles maintained their position in the cell body as it retracted (Fig. 3).
Figure 3.
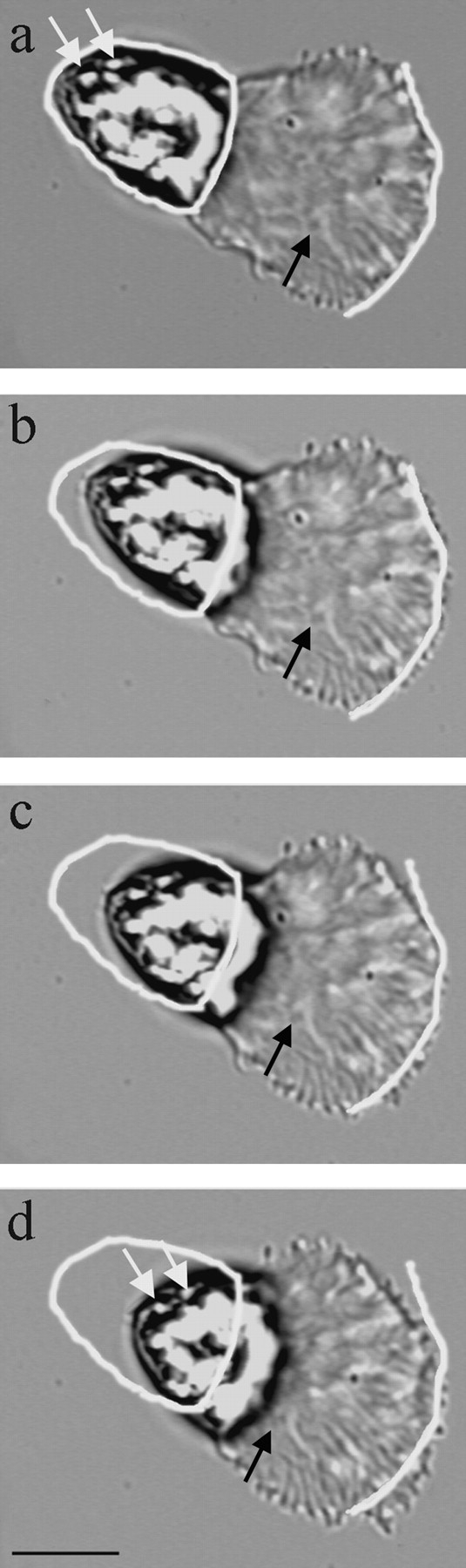
Treatment of sperm with acetate buffer, pH 6.75, uncouples protrusion from retraction. The positions of the cell body and the leading edge, when the cell was perfused with acetate buffer (a), are outlined in white in a–d. During this sequence (elapsed time, 30 s) part of the leading edge protruded slowly (3 μm/min; b–d) while an adjacent portion, toward the top of the frame (c), retracted slightly. Thus, treatment with acetate buffer slowed cytoskeletal assembly dramatically. However, cytoskeletal disassembly was not inhibited and so the lamellipodium and the fiber complexes within shortened and the cell body continued to move forward at 15 μm/min. The black arrow indicates a kink in a fiber complex that moved rearward with respect to the leading edge during the sequence. This indicates that cytoskeletal treadmilling persists even when the rate of protrusion slowed. Note that the distance between the cell body and this kink decreases throughout the sequence, due to continued disassembly at the base of the lamellipodium. The white arrows indicate refringent spots that maintained their position in the cell body during retraction. This shows that the cell body moves forward without rolling. Interval between frames, 10 s. Bar, 10 μm.
Perfusing moving cells with HKB-acetate buffer at pH 6.35 for 5–10 s stopped both lamellipodial protrusion and cell body retraction and also arrested filament assembly at the leading edge (Fig. 4). However, in these cells the fiber complexes of the cytoskeleton continued to disassemble at the base of the lamellipodium. Remarkably, the tips of the fiber complexes pulled away from the plasma membrane at the leading edge of the lamellipodium and the entire array of fiber complexes then progressed retrograde in concert towards the cell body. As this process continued, the fiber complexes became progressively shorter and the gap between their tips and the leading margin of the lamellipodium widened, so that after 30–60 s, the cytoskeleton was disassembled completely. The rate at which fiber complexes moved rearward ranged from 10–28 μm/min (mean, 18 ± 6 μm/min; n = 11). In general, the rate at which the fiber complexes receded was similar to the rate of forward movement of the cell body before acid treatment. Inspection of the morphology of the cytoskeleton during this retrograde recession allowed us to establish the site of fiber complex disassembly during HKB-acetate buffer treatment. Thus, if cytoskeletal disassembly was due primarily to filament depolymerization throughout the fiber complexes, we would have expected to observe changes in the morphology and optical density of fiber complexes. However, neither property changed markedly. Instead, characteristic features such as branches in the receding fiber complexes moved rearward and remained visible until they reached the site of disassembly at the base of the lamellipodium. Moreover, the optical density of the fiber complexes remained essentially constant as they receded.
Figure 4.
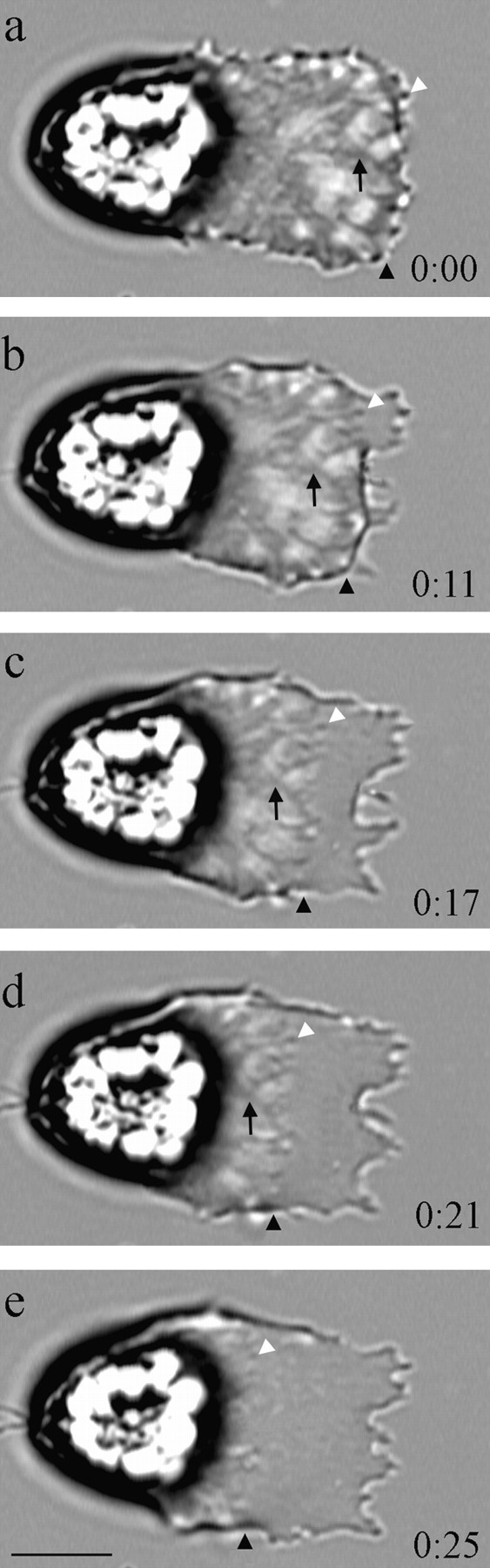
Treatment of sperm with acetate buffer, pH 6.35, causes the cytoskeleton to release from the membrane at the leading edge and recede as disassembly continues at the base of the lamellipodium. Numerals indicate elapsed time in seconds after perfusion with acetate buffer. The black arrowhead indicates the forward margin of the cytoskeleton in each frame. Disassembly occurred primarily at the base of the lamellipodium so that the tips of the fiber complexes (white arrowhead), initially in the surface protrusions along the leading edge, remained clearly visible as the cytoskeleton receded and the gap between the cytoskeleton and the leading edge widened (b–e). The black arrow indicates a branch in a fiber complex that moved progressively rearward toward the site of cytoskeletal disassembly (a–d) until it reached the base of the lamellipodium and disappeared as this part of the cytoskeleton depolymerized. The way in which the cytoskeleton moved, with the fiber complexes maintaining their shape as they shortened, showed that the rearward movement was due to local depolymerization of MSP near the cell body, rather than general depolymerization along the length of the cytoskeleton. Bar, 10 μm.
Cytoskeletal Polymerization and Depolymerization Are both Required for Locomotion
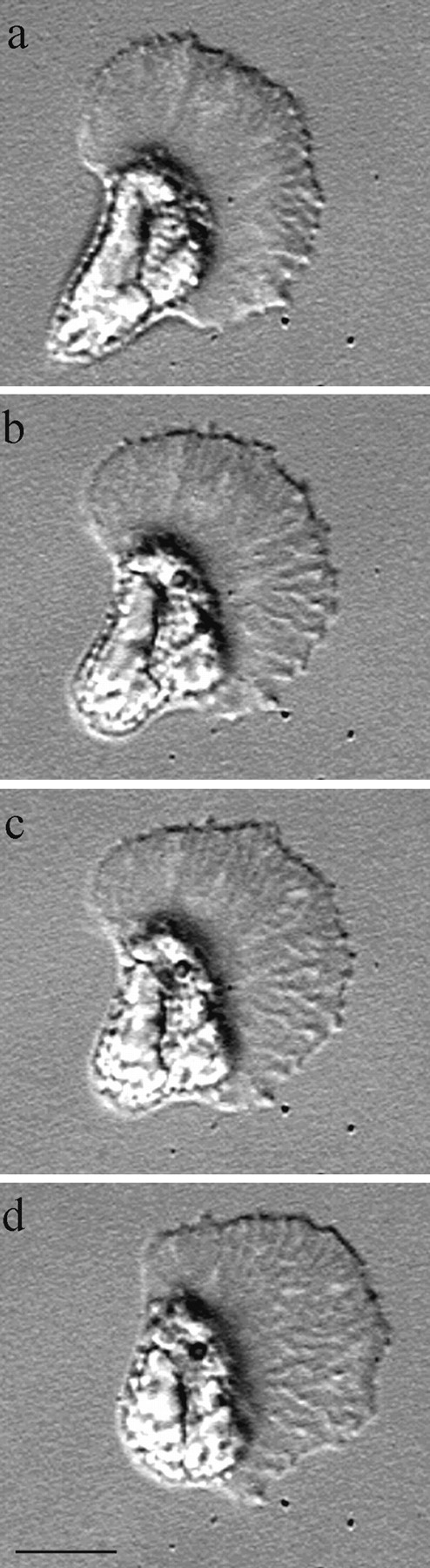
Treatment of sperm with HKB-acetate at pH 5.5 caused the entire MSP cytoskeleton to disassemble rapidly and the lamellipodium to round up (Fig. 5; see also Roberts and King 1991). This effect was completely reversible. The pattern by which the cytoskeleton was rebuilt and locomotion resumed showed that cytoskeletal assembly and disassembly produce independent forces and that both are required for locomotion. When cells treated with pH 5.5 buffer (Fig. 5, a and b) were washed with HKB buffer without acetate, fiber complexes began to form around the periphery of the lamellipodium (Fig. 5 c). This localized assembly resulted in formation of protrusions from the cell surface, but the cell body remained stationary. As these new fiber complexes continued to elongate, those emanating from the side of the lamellipodium reached the cell body and began depolymerization and treadmilling. In the cell shown in Fig. 5, the fiber complexes on the right side of the lamellipodium reached the cell body before those from the other side. When these fiber complexes began to treadmill, the cell body was pulled to that side (Fig. 5 d). Soon, additional fiber complexes growing from the right side of the lamellipodium reached the cell body and the entire cell began to move to the right before the fiber complexes from the other side were completely rebuilt (Fig. 5 e). This asymmetry in cytoskeletal reconstruction, with treadmilling resuming earlier on one side of the lamellipodium than the other, resulted in a 60° change in the direction of locomotion, compared with that observed before acid treatment. The behavior of this unusual cell emphasized that movement, first of the cell body and then the whole cell, was determined by the location where cytoskeletal depolymerization resumed along the cell body–lamellipodium junction. In most cells recovering from this acid treatment, cytoskeletal reconstruction was symmetric and the directions of movement before and after treatment were similar. In each, however, movement of the cell body did not occur until the onset of depolymerization of the reconstructed fiber complexes.
Figure 5.
The pattern of recovery of locomotion after treatment with HKB-acetate, pH 5.5, shows that cytoskeletal depolymerization at the base of the lamellipodium is required for movement of the cell body. In this sequence, a cell, moving in the direction indicated by the black arrow in a, was perfused with pH 5.5 buffer to cause its cytoskeleton to disassemble (b). The white arrow indicates a stationary mark on the substrate. When the cell was washed with HKB buffer, MSP fiber complexes began to reassemble along the periphery of the lamellipodium, causing membrane protrusion (c, arrows). Within 15 s (d) fiber complexes regrowing from the right side of the lamellipodium reached the cell body and started to treadmill (solid black arrows); as indicated by the change in position relative to the stationary mark, the cell body moved in the direction of these treadmilling fiber complexes. At this point, there was still a gap between the fiber complexes growing from the left side and the cell body (open arrow). 15 s later (e), more of the fiber complexes on the right side were fully rebuilt (solid arrows), and treadmilling and movement of the cell body toward that side continued. By 60 s after washing, the cell locomotion resumed (f) in a new direction (bold black arrow) corresponding to the direction of recovery of cell body movement. Note that due to the movement of the cell, the position of the frame in f differs from that in a–e. Bar, 10 μm.
Phenylarsine Oxide Inhibits both Polymerization and Depolymerization and Blocks Locomotion
Manipulation of intracellular pH allowed us to uncouple cytoskeletal assembly from disassembly and thereby examine the separate roles of these processes in locomotion. We sought to determine if other factors are involved in locomotion by identifying a method for keeping the cytoskeleton intact, but blocking both polymerization and depolymerization. Previously, we had shown that antiphosphotyrosine antibodies stained the leading edge of the pseudopod preferentially (Italiano et al. 1996) and so we treated sperm with 30 μM phenylarsine oxide (PAO), a protein tyrosine phosphatase inhibitor. In PAO-treated cells, the fiber complexes remained clearly visible, but we were unable to detect either cytoskeletal flow or locomotion (Fig. 6). However, the effect of the drug was completely reversible; cytoskeletal treadmilling and locomotion resumed within 10 s after washing the cells with a PAO antagonist, dimercaptopropanol, at 5 mM in HKB buffer. Thus, without the forces associated with cytoskeletal polymerization and depolymerization, sperm exhibit no detectable motility.
Figure 6.
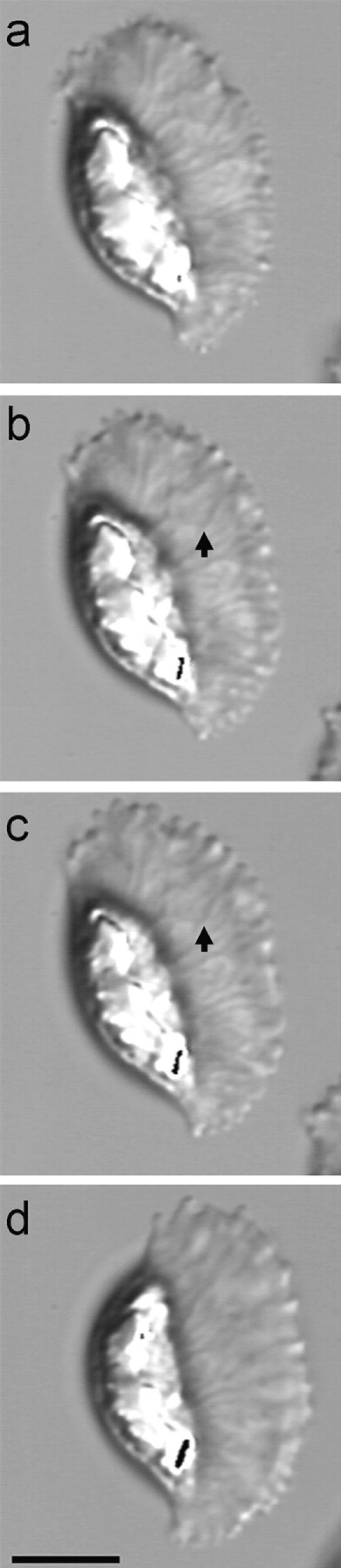
Inhibition of polymerization and depolymerization with PAO completely stops motility. a and b, A crawling sperm before and 15 s after treatment with 30 μM PAO. After 3 min in PAO (c), there was no movement of the cell or treadmilling of its cytoskeleton. For example, the arrows in b and c indicate a bend in a fiber complex that did not change position while the cell was incubated in PAO. However, within 15 s after washing the cell with HKB containing 5 mM dimercaptopropanol (a PAO antagonist), locomotion and cytoskeletal treadmilling resumed (d). Bar, 10 μm.
Discussion
Ascaris sperm offer a powerful experimental system for assessing the roles of cytoskeletal polymerization and depolymerization in amoeboid cell motility because their cytoskeletal dynamics can be observed directly using DIC microscopy. When the cells crawl, MSP polymerization and depolymerization take place simultaneously and at the same rate, but at separate locations: polymerization is localized primarily to the leading edge of the lamellipodium, whereas depolymerization takes place primarily at its base adjacent to the cell body. Previously, we demonstrated that local polymerization and bundling of MSP filaments can move membranes (Italiano et al. 1996). In sperm, as in actin-based cells (reviewed in Mitchison and Cramer 1996), this polymerization-derived force appears to mediate protrusion of the leading edge. By using pH to uncouple lamellipodial extension from cell body retraction, we have demonstrated here that tension associated with cytoskeletal disassembly pulls the cell body forward and that this force is required for locomotion.
The deformation of sperm that become tethered to the substrate during locomotion (Fig. 2) indicated that the cell body was pulled forward by tension generated within the cytoskeleton itself. Polymerization of MSP at the leading edge is unlikely to be the source of this force directly because by pushing against the membrane, the fiber complexes would be placed under compression rather than tension. In principle, pushing the elongating fiber complexes against the leading edge could generate sufficient tension in the plasma membrane or cortical cytoskeleton to drag the cell body forward. However, if this were the case, the tension should cause the entire cell to stretch, not just the cell body. We also would have expected to observe movement of the cell body in cells recovering from treatment at pH 5.5 when polymerization was occurring, but depolymerization was not. Crucially, neither membrane nor cortical cytoskeletal tension can account for the rearward movement of the detached fiber complexes observed at pH 6.35 (Fig. 4). Therefore, our results indicate that cell body retraction is mediated directly by tension in the cytoskeleton rather than by an indirect mechanism, such as release of tension in the cortical cytoskeleton or the plasma membrane at a rate controlled by fiber complex depolymerization. The results obtained by varying pH showed that this cytoskeletal tension was still produced when polymerization, and thus protrusion, was inhibited, but cytoskeletal depolymerization was not. Thus, the force that generates the tension in the cytoskeleton to pull the cell body forward must be produced by another mechanism than that used to push the leading edge forward. It may seem paradoxical to propose that the cytoskeleton is simultaneously under tension and compression, with polymerization-induced compression in the cytoskeleton pushing the leading edge forward while tension in the distal portion pulls the cell body. However, these two processes are separated spatially and, because the fiber complexes are coupled to the substrate through adhesions under the lamellipodium, they are also separated mechanically. Thus, these contacts can adsorb the opposing forces, thereby allowing the extension of the leading edge and retraction of the cell body to occur simultaneously.
Our observations indicate that tension in the sperm cytoskeleton is generated locally at the base of the lamellipodium next to the cell body. The behavior of tethered sperm is consistent with this interpretation, as is the difference in the effects of treatment of sperm with pH 6.75 and 6.35 buffers. At the higher pH, polymerization and lamellipodial extension were greatly reduced, but depolymerization at the base of the lamellipodium continued and the cell body was pulled forward as the fiber complexes shortened (Fig. 3). At pH 6.35, the cytoskeleton detached from the plasma membrane and under these conditions, rather than the cell body moving forward, the cytoskeleton was pulled rearward (Fig. 4). Thus, in both cases, movement was toward the base of the lamellipodium, indicating that this is where tension is generated.
The motile behavior of cells recovering from treatment at pH 5.5 shows that the cytoskeletal tension associated with depolymerization is required for sperm locomotion. During recovery, polymerization and depolymerization were uncoupled until the newly assembled fiber complexes reached the cell body. During this stage, when polymerization was active but depolymerization was inactive, the cell was capable only of protrusion. However, when depolymerization began at the base of the lamellipodium the cell body started to move. As shown in Fig. 5, this movement required depolymerization of only a few fiber complexes and their position determined the direction of cell body movement.
The involvement of a force specific for retraction has also been demonstrated in actin-based crawling cells, although, in these cells, there is not the direct link between retrograde flow and retraction seen in Ascaris sperm. For example, in Aplysia neuronal growth cones, treatment with cytochalasin D stops actin assembly along the leading margin, but the existing actin cytoskeleton continues to flow rearward as it is disassembled at the base of the growth cone (Forscher and Smith 1988). The behavior of the actin cytoskeleton in growth cones treated in this way parallels the recession of the MSP cytoskeleton in sperm incubated in pH 6.35 buffer. In fish epithelial keratocytes, forward movement of the cell body can be uncoupled from protrusion of the leading edge by treatment with cytochalasin B, demonstrating that forward movement of the cell body is not directly dependent on polymerization at the leading edge (Anderson et al. 1996). Thus, these cells exhibit the same pattern of cell body retraction as Ascaris sperm treated with pH 6.75 buffer.
The force that generates tension in the MSP cytoskeleton and the forward movement of the cell body could, in principle, be produced at the cell body either by a motor-driven contraction followed by depolymerization or alternatively by the depolymerization of the fiber complexes themselves. In actin-based cells, the force mediating retraction has been thought to involve primarily an actomyosin-based contraction (Lin and Forscher, 1996; Lin et al. 1996; Svitkina et al. 1997; Verkhovsky et al. 1998; Oliver et al. 1999; reviewed in Cramer 1997). Although the morphological data we have presented here do not allow us to exclude completely the possibility that a contractile mechanism produces cell body retraction in Ascaris sperm, no MSP-based motor protein has been identified and we argue, based on several lines of evidence, that the tension in the MSP cytoskeleton that drags the cell body forward is more likely generated by local depolymerization than by molecular motors. In addition to the coupling of cell body retraction to localized cytoskeletal disassembly that occurs in crawling sperm, retraction (or its equivalent) is correlated both spatially and temporally with depolymerization under several different conditions, including selective stretching of the cell body in tethered sperm (Fig. 2), retraction of the cell body in concert with the depolymerizing fiber complexes in cells treated with pH 6.75 buffer (Fig. 3), recession of the entire cytoskeleton toward the site of depolymerization in pH 6.35 treated sperm (Fig. 4), and simultaneous resumption of retraction and fiber complex disassembly in cells recovering from HKB-acetate at pH 5.5 (Fig. 5). Conversely, when cytoskeletal depolymerization was blocked by PAO treatment (Fig. 6), retraction was also inhibited, although the cytoskeleton remained intact to support any contractile activity. Moreover, in addition to the failure to identify any MSP-based motor proteins, structural studies (Bullock et al. 1998) have shown that the helices from which the filaments of the MSP cytoskeleton are constructed lack the structural polarity, characteristic of actin filaments and microtubules, that allows motor proteins to function.
Depolymerization-associated tension in the MSP cytoskeleton may be analogous to the tension generated by microtubule depolymerization (Lombillo et al. 1993; Koshland et al. 1988). For example, depolymerization of kinetochore microtubules at the kinetochore is associated with anaphase chromosome movement (Desai and Mitchison 1995; reviewed by Inoue and Salmon 1995), and depolymerization of the plus-end of cytoplasmic microtubules has been shown to be able to move both membranes and vesicles in Xenopus laevis egg extracts (Waterman-Storer et al. 1995). In the case of anaphase chromosome movement, microtubule depolymerization is thought to be coupled mechanically to the kinetochore through the binding of proteins of the kinesin family to the microtubule (Lombillo et al. 1995). However, these proteins are thought to function primarily to hold the microtubule (and so couple its length change mechanically to the kinetochore) rather than move the microtubule directly. Although we have not been able to identify an MSP-binding protein that could couple MSP depolymerization to the cell body in an analogous manner, such a mechanism would not be inconsistent with the apparent lack of polarity of MSP filaments (Bullock et al. 1998) because simply holding, rather than moving, does not require filament polarity a priori. Alternatively, it could be that depolymerization of the MSP-based cytoskeleton gel in the vicinity of the cell body generates contraction (Mogilner and Oster 1996a). Clearly, further work at the molecular level will be needed to distinguish between these possibilities. However, although the results obtained with Ascaris sperm do not rule out a contribution by motor proteins to retraction in actin-based amoeboid motility, they do show that it would, in principle, be possible both to extend the lamellipodium and retract the cell body by modulating the polymerization state of the cytoskeleton alone and certainly raise the possibility that such a mechanism may contribute to locomotion in at least some of these systems.
Acknowledgments
We are grateful to our colleagues in Tallahassee and Cambridge, especially K. Riddle and G. Roberts for expert technical assistance, and to L. LeClaire and M. Seavy for their many helpful comments, criticisms, and suggestions.
This work was supported by National Institutes of Health Grant GM-29994 to T.M. Roberts and M. Stewart and by United States Department of Agriculture National Research Initiative Competitive Grants Program Grant 9702241 to T.M. Roberts.
Footnotes
1.used in this paper: HKB-acetate, HKB buffer containing 20 mM sodium acetate; MSP, major sperm protein; PAO, phenylarsine oxide
Dr. Italiano's current address is Division of Hematology, Brigham and Women's Hospital, Harvard Medical School, 221 Longwood Avenue, Boston, MA 02115.
References
- Anderson K.I., Wang Y.-L., Small J.V. Coordination of protrusion and translocation of the keratocyte involves rolling of the cell body. J. Cell Biol. 1996;134:1209–1218. doi: 10.1083/jcb.134.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock T.L., McCoy A.J., Kent H.M., Roberts T.M., Stewart M. Structural basis for amoeboid motility in nematode sperm. Nature Struct. Biol. 1998;5:184–189. doi: 10.1038/nsb0398-184. [DOI] [PubMed] [Google Scholar]
- Condeelis J. Life at the leading edgethe formation of cell protrusions. Annu. Rev. Cell Biol. 1993;9:411–444. doi: 10.1146/annurev.cb.09.110193.002211. [DOI] [PubMed] [Google Scholar]
- Cooper J.A. The role of actin polymerization in cell motility. Ann. Rev. Physiol. 1991;53:585–605. doi: 10.1146/annurev.ph.53.030191.003101. [DOI] [PubMed] [Google Scholar]
- Cossart P., Kochs C. The actin-based motility of the facultative intracellular pathogen Listeria monocytogenes . Mol. Microbiol. 1994;13:395–402. doi: 10.1111/j.1365-2958.1994.tb00434.x. [DOI] [PubMed] [Google Scholar]
- Cramer L. Molecular mechanism of actin-dependent retrograde flow in lamellipodia of motile cells. Frontiers Biosci. 1997;2:260–270. doi: 10.2741/a189. [DOI] [PubMed] [Google Scholar]
- Desai A., Mitchison T.J. A new role for motor proteins as couplers to depolymerizing microtubules. J. Cell Biol. 1995;128:1–4. doi: 10.1083/jcb.128.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forscher P., Smith S.J. Actions of cytochalasins on the organization of actin filaments and microtubules in a neuronal growth cone. J. Cell Biol. 1988;107:1505–1516. doi: 10.1083/jcb.107.4.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forscher P., Lin C.H., Thompson C. Novel form of growth cone motility involving site-directed actin filament assembly. Nature. 1992;357:515–518. doi: 10.1038/357515a0. [DOI] [PubMed] [Google Scholar]
- Inoue S., Salmon E.D. Force generation by microtubule assembly/disassembly in mitosis and related movements. Mol. Biol. Cell. 1995;6:1619–1640. doi: 10.1091/mbc.6.12.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Italiano J.E., Jr., Roberts T.M., Stewart M., Fontana C. Reconstitution in vitro of the motile apparatus from the amoeboid sperm of Ascaris shows that filament assembly and bundling move membranes. Cell. 1996;84:105–114. doi: 10.1016/s0092-8674(00)80997-6. [DOI] [PubMed] [Google Scholar]
- King K.L., Essig J., Roberts T.M., Moerland T.S. Regulation of the Ascaris major sperm protein (MSP) cytoskeleton by intracellular pH. Cell Motil. Cytoskel. 1994;27:193–205. doi: 10.1002/cm.970270302. [DOI] [PubMed] [Google Scholar]
- Koshland D.E., Mitchison T.J., Kirschner M. Polewards chromosome movement driven by microtubule depolymerization in vitro. Nature. 1988;331:499–504. doi: 10.1038/331499a0. [DOI] [PubMed] [Google Scholar]
- Lin C.H., Forscher P. Cytoskeletal remodeling during growth cone-target interactions. J. Cell Biol. 1993;21:1369–1383. doi: 10.1083/jcb.121.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.H., Espreafico E.M., Mooseker M.S., Forscher P. Myosin drives retrograde F-actin flow in neuronal growth cones. Neuron. 1996;16:769–782. doi: 10.1016/s0896-6273(00)80097-5. [DOI] [PubMed] [Google Scholar]
- Lombillo V.A., Coue M., McIntosh J.R. In vitro motility assays using microtubules tethered to Tetrahymena pellicles. Methods Cell Biol. 1993;39:149–165. doi: 10.1016/s0091-679x(08)60168-5. [DOI] [PubMed] [Google Scholar]
- Lombillo V.A., Nislow C., Yen T.J., Gelfand V.I., McIntosh J.R. Antibodies to the kinesin motor domain and CENP-E inhibit microtubule depolymerization-dependent motion of chromosomes in vitro. J. Cell Biol. 1995;128:107–115. doi: 10.1083/jcb.128.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T.J., Cramer L.P. Actin-based cell motility and cell locomotion. Cell. 1996;84:371–379. doi: 10.1016/s0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- Mogilner A., Oster G. The physics of lamellipodial protrusion Eur. Biophys. J. 25 1996. 47 53a [Google Scholar]
- Mogilner A., Oster G. Cell motility driven by actin polymerization Biophys. J. 71 1996. 3030 3045b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peskin C.S., Odell G.M., Oster G. Cellular motions and thermal fluctuationsthe Brownian ratchet. Biophys. J. 1993;65:316–324. doi: 10.1016/S0006-3495(93)81035-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver T., Lee J., Jacobson K. How do cells move along surfaces? Semin. Cell Biol. 1993;5:139–147. doi: 10.1016/0962-8924(93)90084-e. [DOI] [PubMed] [Google Scholar]
- Oliver T., Dembo M., Jacobson K. Separation of propulsive and adhesive traction stresses in locomoting keratocytes. J. Cell Biol. 1999;145:589–604. doi: 10.1083/jcb.145.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts T.M., King K.L. Centripetal flow and directed reassembly of the major sperm protein cytoskeleton in the amoeboid sperm of the nematode, Ascaris suum . Cell Motil. Cytoskel. 1991;20:228–241. doi: 10.1002/cm.970200306. [DOI] [PubMed] [Google Scholar]
- Roberts T.M., Stewart M. Nematode sperm locomotion. Curr. Opin. Cell Biol. 1995;7:13–17. doi: 10.1016/0955-0674(95)80039-5. [DOI] [PubMed] [Google Scholar]
- Roberts T.M., Stewart M. Nematode spermamoeboid movement without actin. Trends Cell Biol. 1997;7:368–373. doi: 10.1016/S0962-8924(97)01113-6. [DOI] [PubMed] [Google Scholar]
- Royal D., Royal M., Italiano J., Roberts T., Soll D.R. In Ascaris sperm pseudopods, MSP fibers move proximally at a constant rate regardless of the forward rate of cellular translocation. Cell Motil. Cytoskel. 1995;31:241–253. doi: 10.1002/cm.970310307. [DOI] [PubMed] [Google Scholar]
- Sepsenwol S., Braun T., Nguyen M. Adenylate cyclase activity is absent in inactive and motile sperm in the nematode parasite, Ascaris suum . J. Parasitol. 1986;72:962–964. [PubMed] [Google Scholar]
- Sepsenwol S., Ris H., Roberts T.M. A unique cytoskeleton associated with crawling in the amoeboid sperm of the nematode Ascaris suum . J. Cell Biol. 1989;108:55–66. doi: 10.1083/jcb.108.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick F.S., Purich D.L. Dynamic remodelling of the actin cytoskeletonlessons learned from Listeria locomotion. Bioessays. 1994;16:885–891. doi: 10.1002/bies.950161206. [DOI] [PubMed] [Google Scholar]
- Stossel T.P. On the crawling of animal cells. Science. 1993;260:1086–1094. doi: 10.1126/science.8493552. [DOI] [PubMed] [Google Scholar]
- Suter D.M., Errante L.D., Belotserkovsky V., Forscher P. The Ig superfamily cell adhesion molecule, apCAM, mediates growth cone steering by substrate–cytoskeletal coupling. J. Cell Biol. 1998;141:227–240. doi: 10.1083/jcb.141.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkina T.M., Verkhovsky A.B., McQuade K.M., Borisy G.G. Analysis of the actin-myosin II system in fish epidermal keratocytesmechanism of cell body translocation. J. Cell Biol. 1997;139:397–415. doi: 10.1083/jcb.139.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theriot J.A. The cell biology of infection by intracellular bacterial pathogens. Annu. Rev. Cell Dev. Biol. 1995;11:213–239. doi: 10.1146/annurev.cb.11.110195.001241. [DOI] [PubMed] [Google Scholar]
- Theriot J.A. Worm sperm and advances in cell locomotion. Cell. 1996;84:1–4. doi: 10.1016/s0092-8674(00)80068-9. [DOI] [PubMed] [Google Scholar]
- Verkhovsky A.B., Svitkina T.M., Borisy G.G. Self-polarization and directional motility of cytoplasm. Curr. Biol. 1998;9:11–20. doi: 10.1016/s0960-9822(99)80042-6. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer C.M., Gregory J., Parsons S., Salmon E.D. Membrane/microtubule tip attachment complexes (TACs) allow the assembly dynamics of plus ends to push and pull membranes into tubulovesicular networks in interphase Xenopus egg extracts. J. Cell Biol. 1995;130:1161–1169. doi: 10.1083/jcb.130.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond S.H. Signal transduction and actin filament organization. Curr. Opin. Cell Biol. 1996;8:66–73. doi: 10.1016/s0955-0674(96)80050-0. [DOI] [PubMed] [Google Scholar]