Introduction
The discussion of bacterial morphology has been dominated by questions about how a cell manages to create a rod shape, which, of course, is but one example of the more general question of how a cell constructs any shape. The expectation is that by answering this (deceptively) simple question we may acquire knowledge that will point us to a universal mechanism of shape control. This emphasis is understandable because we are both more familiar with and more comfortable with answering how-type questions. And, indeed, this approach has produced exciting new information, highlighted by other articles in this issue.
What has not been as well explored is why bacteria find it advantageous to exhibit such a prodigious number of different shapes; and so the purpose of this article is to examine some of the reasons that lie behind this variety. I will highlight a few research areas that bear on why bacteria have certain morphologies, but only in a brief and qualitative way. More depth, more examples, and a bit more quantitative treatment can be found in a recent review and the references therein [1]. Portions of this topic have also been discussed by Beveridge [2], Dusenbery [3], Koch [4], and Mitchell [5].
Shape has selective value
The first issue to get settled is that the shape of a bacterium has biological relevance. One argument favoring this assertion is that even though bacteria have a wide variety of shapes, any one genus typically exhibits a limited subset of morphologies, hinting that, with a universe of shapes to choose from, individual bacteria adopt only those that are adaptive. Another clue is that some bacteria can modify their morphology in response to environmental cues or during the course of pathogenesis [e.g., 6], suggesting that shape is important enough to merit regulation.
Two evolutionary arguments also support the utility of bacterial shape. First, shape has a vector through evolutionary time – rod-like organisms having arisen first and coccoid forms being derivatives at the ends of evolutionary lines [7–11]. Progressive development of a trait implies that selective forces are operating. Secondly, prokaryotes with different genealogies may converge morphologically, indicating that a similar shape may confer advantages in certain environments. So, for example, although they have a non-peptidoglycan-based cell wall, the Archaea exhibit a range of morphological forms similar to that of the Bacteria [12]. The simplest conclusion is that morphological adaptation serves an important biological function.
How, then, might morphology contribute to natural selection? Simply put, bacteria with different shapes present different physical features to the outside world, and these features help cells cope with and adapt to external conditions. Even a 0.01% increase in the growth rate of E. coli can impart a fitness advantage of ~10% compared to its unaltered competitors [5], so improvements need not be dramatic to be useful. Consistent with these expectations, shape contributes a measure of survival value in the face of three “Primary” selective pressures: 1) nutrient acquisition, 2) cell division, and 3) predators; and in optimizing five “Secondary” mechanisms: 4) attachment to surfaces, 5) passive dispersal, 6) active motility, and 7) internal or 8) external differentiation [1] (Table 1). The first three are Primary in that they represent fundamental conditions that determine whether cells live or die, because cells must grow and multiply and keep from being killed. The last five are Secondary in that they represent a suite of morphologically associated mechanisms that bacteria use to deal with the Primary forces. Some of the ways these selective forces may affect bacterial morphology are summarized in Table 1. Here, I will discuss only three to give a flavor for how selective pressures impact cell shape.
Table 1.
Selective forces and their potential effects on bacterial shape.
| Shape characteristics potentially affected by selective forces | ||||||||
|---|---|---|---|---|---|---|---|---|
| Selective force | Symmetry | Size | Width | Length | Filaments | Prosthecae | Spirals | Bifids |
| Division | X | X | ||||||
| Nutrients | X | X | X | X | ||||
| Attachment | X | X | X | |||||
| Dispersal | X | X | X | |||||
| Motility | X | X | X | X | X | |||
| Chemotaxis | X | X | ||||||
| Polar Differentiation | X | X | X | |||||
| Predation | X | X | X | X | ||||
| Differentiation and Symbiosis | X | X | X | |||||
Nutrient uptake
A perennial question is why prokaryotic cells are so small, and the typical answer is that they require a large surface-to-volume ratio to support their internal biochemistry. However, Koch estimated how large a cell could be if, like the enormous symbiotic bacterium Epulopiscium fishelsoni [13], it only divided once per day and depended solely on diffusion in a nutrient-rich environment [4]. His answer was that a bacterium could be over 800 μm in diameter! This implies that limitations on the sizes of more typical prokaryotes are not due to the ability to take up nutrients per se but arise from the competition for nutrients, a competition won chiefly by smaller, faster growing cells. The lesson is that although diffusion-limited nutrient access might affect cell size, it does not by itself explain why bacteria are mostly small.
Nonetheless, diffusion considerations do explain how bacteria can increase their nutrient harvesting efficiency by altering their gross morphology. Caulobacter crescentus is a curved cell of E. coli-like dimensions that produces a thin, elongated stalk (prostheca) that extends from one pole and affixes the organism to solid surfaces in its aqueous environment [14]. The length of the stalk appears to be regulated by the availability of nutrients because phosphate-poor conditions induce longer stalks in this and similar bacteria [14–16]. Recently, Wagner et al produced strong support for the idea that this simple change in cell shape is a physically useful response [17]. They confirmed the existence of stalk-mediated phosphate uptake and demonstrated mathematically that cells import more phosphate by extruding a long thin stalk than they would if they merely filamented [17]. This is solid experimental evidence that cell shape, in and of itself, affects nutrient acquisition and argues that other nutritional situations may create conditions that favor one bacterial shape over another.
Motility
The correlation between bacterial shape and motility is, by far, the most well examined morphological relationship. Theoretically, all forms of motility place strong physical and energetic demands on cell shape [5]. Most impressively, a change in cell diameter of only 0.2 μm can change the energy required for chemotaxis by a factor of 105 [5]! Energy usage, Brownian forces, and requirements for following chemical gradients force highly motile bacteria into a narrow range of optimal sizes and rod shapes [5,18]. These theoretical considerations are supported by the behavior of filamentous E. coli cells which, though motile and chemotactic, move slowly and cannot tumble to change direction [19]. Different morphological constraints affect cells that move as a group rather than as individuals. Such “swarm cells” are longer than is optimal for single cells because the group aligns itself by extensive side-by-side cell-to-cell contacts [20,21]. Of special note is that certain Proteus mirabilis mutants become non-motile because they produce highly curved swarmer cells that cannot align properly due to the change in shape [22].
Motility has other interesting effects on cell shape, some of them arising from the fact that bacteria swim differently near solid surfaces or through viscous fluids. For example, the curved cells of Vibrio alginolyticus swim forward in a straight line but move in circles when swimming backwards near a flat surface [23,24]. This behavior occurs in a 50–60 μm zone near a surface, while beyond this layer the cells swim in straight lines in either direction [23,24]. In this way, marine microorganisms may increase the time they remain in contact with nutrient-rich surfaces in an otherwise nutrient-poor environment [23]. The phenomenon may be general because, surprisingly, non-tumbling E. coli mutants swim on the right-hand side of thin channel and in clockwise circles when close to a planar surface [25]. The theoretical impact of this “near-surface motility” on cell shape has not been explored in depth, but at least one aspect of bacterial morphology, cell length, changes the dimensions of these circular motions, which may, in turn, affect cell foraging behavior [26]. Finally, cells with spiral morphologies appear to move through viscous fluids much more efficiently than do rod shaped cells with no curvature [27–29], a phenomenon probably dictated by the physical restraints of thin fluid channels in such solutions [30,31].
In short, motility imposes a heavy selective pressure on cell shape. Fast cells are better off as rods with a certain length-to-width ratio, chemotactic cells must adopt shape ratios in line with their environments, and cells that forage near surfaces or navigate viscous environments may do best if they are slightly curved or spiral.
Predation
One of the least widely appreciated evolutionary pressures operating on bacteria is predation by protozoa, also known as protistan grazing or bacterivory. This lack of awareness is surprising since predation is one of the most obvious selective forces affecting larger (i.e., not microbial) organisms. Several reviews have endeavored to rectify this blind spot [32–36]. Whereas nutrient access and division are “bottom-up” pressures that influence cells via fundamental reproductive requirements, protistan grazing is a “top-down” selection where external organisms supply the evolutionary pressure [37]. Bacteria respond to predation by developing means of escape, thereby initiating a familiar arms race between predator and prey that contributes to bacterial diversity [37]. Figure 1 illustrates how cell shape plays a role in three basic defensive strategies: 1) escaping capture, by being too small or too fast; 2) resisting ingestion, by becoming too large or too long; and 3) making themselves inaccessible, by growing in aggregates or biofilms. All of these are affected, directly or indirectly, by one or more aspects of bacterial morphology.
Figure 1.

Defenses against bacterivory. Protists can ingest only those bacteria that are “just right” as far as size and shape are concerned (the “Goldilocks effect”) [1]. Pictured are some of the morphological ways bacteria can protect themselves by becoming “not right.”
Where once there was only speculation, there now exists a large amount of experimental evidence that grazing selects for cells that can alter their size or shape. Protistan feeding pushes bacteria to become very small or very large [36,38,39], to move faster [40], to filament [39,41,42], to produce prosthecae [43], to grow as microcolonies [44,45], to become longer or curved, filamentous or chained [46], or to increase their diameter [47]. Among other intriguing morphological alterations is that of the cyanobacterium Arthrospira, which grows as helical trichomes. A ciliate feeding on this organism rotates on its long axis to ingest up to six full coils [48] (A. Belay, pers. commun.). However, Arthrospira can change its helical handedness from right- to left-handed or by altering the pitch of its spirals, either of which reduces predation [48] (A. Belay). In short, in their struggle against being eaten, bacteria have adopted morphological defenses that may have produced the wealth of shapes we now observe.
Complexities and Summary
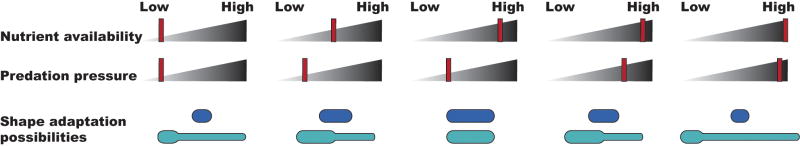
Environmental forces act in concert and elicit complex combinations of responses. This makes biological prediction a chancy effort at best, and it is particulary risky with regard to cell shape because the presence of multiple selective forces may drive morphological change in unexpected directions. For example, Figure 2 illustrates how bacteria might employ one type of shape change to respond to two simultaneous selective pressures. For a rod shaped cell without prosthecae (dark blue), becoming small and coccoid conserves energy during nutritional scarcity and prevents capture by predators. For a Caulobacter-like cell (light blue), the stalk helps harvest nutrients during scarcity and prevents ingestion when predators are numerous. In this case the cells alter their shapes in one or two generations, but other organisms or conditions might require acclimatization over evolutionary time. It is easy to see that adding more selective pressures and considering additional morphological responses would produce a wide variety of shape optima for coping with different conditions.
Figure 2.
Example of simple shape adaptations triggered by selective pressures. The upper two rows of “slider bars” represent: 1) the quantity of available nutrients (from Low to High), and 2) the numbers of nearby predators (from Low to High). As these two environmental conditions change, bacteria may respond with morphological adaptations, two of which are illustrated beneath the sliders. As described in the text, one cell (dark blue) elongates or becomes smaller, while the other (light blue) modifies the length of its prostheca. Intermediate conditions may evoke intermediate responses.
Although a few basic trends stand out (e.g., that motile cells are usually rods), we know exceedingly few morphological rules. This means that, except for the simplest cases, it is difficult or impossible to answer the question, “Why does a bacterium have a particular shape?” Consider, for example, the bacterium Pelagibacter ubique, which constitutes ~25% of all ocean microorganisms and is possibly the most successful, most numerous single prokaryote on earth [49]. Even for such a plain bacterium in a relatively uncomplicated environment, we have no clue as to why it is a tiny curved rod instead of a small straight rod; and beyond this, everything else is even more uncertain. In short, at our present level of understanding, when given an organism’s environment we cannot predict its shape, nor when given its shape can we confidently infer the characteristics of its environment [5].
Summary
Shape isn’t everything. The point, though, is that morphology is a significant selectable trait in many circumstances and that the subject can be approached experimentally like any other. As we understand more about the mechanisms that regulate cell shape we may soon be able to manipulate bacterial morphology with enough confidence to ask how morphological changes affect survival in different conditions. And as evidence accumulates for the utility of cell shape, we can hope that investigators will be motivated to ask these types of questions more directly.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ••1.Young KD. The selective value of bacterial shape. Microbiol Mol Biol Rev. 2006;70:660–703. doi: 10.1128/MMBR.00001-06. A comprehensive recent attempt to compile, describe and classify the ways in which bacteria may utilize morphology to cope with a variety of evolutionary pressures. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beveridge TJ. The bacterial surface: general considerations towards design and function. Can J Microbiol. 1988;34:363–372. doi: 10.1139/m88-067. [DOI] [PubMed] [Google Scholar]
- •3.Dusenbery DB. Fitness landscapes for effects of shape on chemotaxis and other behaviors of bacteria. J Bacteriol. 1998;180:5978–5983. doi: 10.1128/jb.180.22.5978-5983.1998. An early review addressing the selective pressures that define bacterial shape, especially with regard to motility. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koch AL. What size should a bacterium be? A question of scale. Annu Rev Microbiol. 1996;50:317–348. doi: 10.1146/annurev.micro.50.1.317. [DOI] [PubMed] [Google Scholar]
- ••5.Mitchell JG. The energetics and scaling of search strategies in bacteria. American Naturalist. 2002;160:727–740. doi: 10.1086/343874. An exquisitely detailed, far-ranging discussion of how the physical requirements imposed by motility influence and constrain bacterial shape. [DOI] [PubMed] [Google Scholar]
- •6.Justice SS, Hung C, Theriot JA, Fletcher DA, Anderson GG, Footer MJ, Hultgren SJ. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc Natl Acad Sci U S A. 2004;101:1333–1338. doi: 10.1073/pnas.0308125100. The authors observed pathogenesis-associated differentiation, in which E. coli adopts different morphologies during infection of mouse bladder epithelial cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stackebrandt E, Woese CR. A phylogenetic dissection of the family Micrococcaceae. Curr Microbiol. 1979;2:317–322. [Google Scholar]
- 8.Woese CR, Blanz P, Hespell RB, Hahn CM. Phylogenetic relationships among various helical bacteria. Curr Microbiol. 1982;7:119–124. [Google Scholar]
- 9.Siefert JL, Fox GE. Phylogenetic mapping of bacterial morphology. Microbiol. 1998;144:2803–2808. doi: 10.1099/00221287-144-10-2803. [DOI] [PubMed] [Google Scholar]
- 10.Tamames J, Gonzalez-Moreno M, Mingorance J, Valencia A, Vicente M. Bringing gene order into bacterial shape. Trends Genet. 2001;17:124–126. doi: 10.1016/s0168-9525(00)02212-5. [DOI] [PubMed] [Google Scholar]
- 11.Gupta RS. The phylogeny of proteobacteria: relationships to other eubacterial phyla and eukaryotes. FEMS Microbiol Rev. 2000;24:367–402. doi: 10.1111/j.1574-6976.2000.tb00547.x. [DOI] [PubMed] [Google Scholar]
- 12.Stetter KO. Size limits of very small microorganisms: Proceedings of a Workshop. Space·Studies·Board: National Academic Press; 1999. Smallest cell sizes within hyperthermophilic archaea (“Archaebacteria”) pp. 68–73. [Google Scholar]
- 13.Schulz HN, Jorgensen BB. Big bacteria. Annu Rev Microbiol. 2001;55:105–137. doi: 10.1146/annurev.micro.55.1.105. [DOI] [PubMed] [Google Scholar]
- 14.Brun YV, Janakiraman R. The dimorphic life cycle of Caulobacter and stalked bacteria. In: Brun YV, Shimkets LJ, editors. Prokaryotic Development. American Society for Microbiology; 2000. pp. 297–317. [Google Scholar]
- 15.Poindexter JS. The role of calcium in stalk development and in phosphate acquisition in Caulobacter crescentus. Arch Microbiol. 1984;138:140–152. doi: 10.1007/BF00413014. [DOI] [PubMed] [Google Scholar]
- 16.Poindexter JS. Role of prostheca development in oligotrophic aquatic bacteria. In: Klug MJ, Reddy CA, editors. Current perspectives in microbial ecology. ASM Press; 1984. pp. 33–40. [Google Scholar]
- ••17.Wagner JK, Setayeshgar S, Sharon LA, Reilly JP, Brun YV. Proc Natl Acad Sci U S A. Vol. 103. 2006. A nutrient uptake role for bacterial cell envelope extensions; pp. 11772–11777. A seminal experimental and mathematical demonstration of how Caulocbacter crescentus responds to nutrient availability by elaborating long thin prosthecae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper S, Denny MW. A conjecture on the relationship of bacterial shape to motility in rod-shaped bacteria. FEMS Microbiology Letters. 1997;148:227–231. [Google Scholar]
- 19.Maki N, Gestwicki JE, Lake EM, Kiessling LL, Adler J. Motility and chemotaxis of filamentous cells of Escherichia coli. J Bacteriol. 2000;182:4337–4342. doi: 10.1128/jb.182.15.4337-4342.2000. A key experiment examining why motile cells have certain lengths by examining motility and chemotaxis in filamentous E. coli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kearns DB, Losick R. Swarming motility in undomesticated Bacillus subtilis. Mol Microbiol. 2003;49:581–590. doi: 10.1046/j.1365-2958.2003.03584.x. [DOI] [PubMed] [Google Scholar]
- •21.Julkowska D, Obuchowski M, Holland IB, Seror SJ. Branched swarming patterns on a synthetic medium formed by wild-type Bacillus subtilis strain 3610: detection of different cellular morphologies and constellations of cells as the complex architecture develops. Microbiology. 2004;150:1839–1849. doi: 10.1099/mic.0.27061-0. An interesting observation of how one bacterium adopts different morphologies depending on how each cell is positioned within a multi-cell conglomerate. [DOI] [PubMed] [Google Scholar]
- 22.Hay NA, Tipper DJ, Gygi D, Hughes C. A novel membrane protein influencing cell shape and multicellular swarming of Proteus mirabilis. J Bacteriol. 1999;181:2008–2016. doi: 10.1128/jb.181.7.2008-2016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kudo S, Imai N, Nishitoba M, Sugiyama S, Magariyama Y. Asymmetric swimming pattern of Vibrio alginolyticus cells with single polar flagella. FEMS Microbiol Lett. 2005;242:221–225. doi: 10.1016/j.femsle.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Magariyama Y, Ichiba M, Nakata K, Baba K, Ohtani T, Kudo S, Goto T. Difference in bacterial motion between forward and backward swimming caused by the wall effect. Biophys J. 2005;88:3648–3658. doi: 10.1529/biophysj.104.054049. An experimental demonstration of how “near surface” properties change the pattern of motility in Vibrio alginolyticus, focusing on this organism’s peculiar curved motion when swimming backwards. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••25.DiLuzio WR, Turner L, Mayer M, Garstecki P, Weibel DB, Berg HC, Whitesides GM. Escherichia coli swim on the right-hand side. Nature. 2005;435:1271–1274. doi: 10.1038/nature03660. A captivating experimental achievement, showing that non-tumbling E. coli veer rightward when swimming near the planar surface of a thin channel. [DOI] [PubMed] [Google Scholar]
- •26.Lauga E, Diluzio WR, Whitesides GM, Stone HA. Swimming in circles: motion of bacteria near solid boundaries. Biophys J. 2006;90:400–412. doi: 10.1529/biophysj.105.069401. A computational model describing the effects of bacterial shape on the motility of cells near a planar surface, showing, among other things, that cell length affects the size of the circular path that bacteria traverse in these environments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robertson BR, O’Rourke JL, Neilan BA, Vandamme P, On SL, Fox JG, Lee A. Mucispirillum schaedleri gen. nov., sp nov., a spiral-shaped bacterium colonizing the mucus layer of the gastrointestinal tract of laboratory rodents. Int J Syst Evol Microbiol. 2005;55:1199–1204. doi: 10.1099/ijs.0.63472-0. [DOI] [PubMed] [Google Scholar]
- 28.Atsumi T, Maekawa Y, Yamada T, Kawagishi I, Imae Y, Homma M. Effect of viscosity on swimming by the lateral and polar flagella of Vibrio alginolyticus. J Bacteriol. 1996;178:5024–5026. doi: 10.1128/jb.178.16.5024-5026.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shigematsu M, Umeda A, Fujimoto S, Amako K. Spirochaete-like swimming mode of Campylobacter jejuni in a viscous environment. J Med Microbiol. 1998;47:521–526. doi: 10.1099/00222615-47-6-521. [DOI] [PubMed] [Google Scholar]
- •30.Berg HC, Turner L. Movement of microorganisms in viscous environments. Nature. 1979;278:349–351. doi: 10.1038/278349a0. Classic treatment of the theoretical reasons that spiral bacteria move efficiently through viscous fluids. [DOI] [PubMed] [Google Scholar]
- 31.Magariyama Y, Kudo S. A mathematical explanation of an increase in bacterial swimming speed with viscosity in linear-polymer solutions. Biophys J. 2002;83:733–739. doi: 10.1016/S0006-3495(02)75204-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matz C, Kjelleberg S. Off the hook - how bacteria survive protozoan grazing. Trends Microbiol. 2005;13:302–307. doi: 10.1016/j.tim.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 33.Jürgens K, Matz C. Predation as a shaping force for the phenotypic and genotypic composition of planktonic bacteria. Antonie Van Leeuwenhoek. 2002;81:413–434. doi: 10.1023/a:1020505204959. [DOI] [PubMed] [Google Scholar]
- 34.Hahn MW, Hofle MG. Grazing of protozoa and its effect on populations of aquatic bacteria. FEMS Microbiol Ecol. 2001;35:113–121. doi: 10.1111/j.1574-6941.2001.tb00794.x. [DOI] [PubMed] [Google Scholar]
- 35.Sherr EB, Sherr BF. Significance of predation by protists in aquatic microbial food webs. Antonie Van Leeuwenhoek. 2002;81:293–308. doi: 10.1023/a:1020591307260. [DOI] [PubMed] [Google Scholar]
- ••36.Pernthaler J. Predation on prokaryotes in the water column and its ecological implications. Nat Rev Microbiol. 2005;3:537–546. doi: 10.1038/nrmicro1180. A particularly accessible review on how protozoan predation fuels bacterial evolution, including the development of several morphological adaptations. [DOI] [PubMed] [Google Scholar]
- 37.Boenigk J, Arndt H. Bacterivory by heterotrophic flagellates: community structure and feeding strategies. Antonie Van Leeuwenhoek. 2002;81:465–480. doi: 10.1023/a:1020509305868. [DOI] [PubMed] [Google Scholar]
- 38.Matz C, Bergfeld T, Rice SA, Kjelleberg S. Microcolonies, quorum sensing and cytotoxicity determine the survival of Pseudomonas aeruginosa biofilms exposed to protozoan grazing. Environ Microbiol. 2004;6:218–226. doi: 10.1111/j.1462-2920.2004.00556.x. [DOI] [PubMed] [Google Scholar]
- 39.Posch T, Simek K, Vrba J, Pernthaler S, Nedoma J, Sattler B, Sonntag B, Psenner R. Predator-induced changes of bacterial size-structure and productivity studied on an experimental microbial community. Aquatic Microbial Ecol. 1999;18:235–246. [Google Scholar]
- •40.Matz C, Jurgens K. High motility reduces grazing mortality of planktonic bacteria. Appl Environ Microbiol. 2005;71:921–929. doi: 10.1128/AEM.71.2.921-929.2005. A well-supported confirmation that efficient and rapid motility helps bacteria evade protozoan predation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pernthaler J, Posch T, Simek K, Vrba J, Amann R, Psenner R. Contrasting bacterial strategies to coexist with a flagellate predator in an experimental microbial assemblage. Appl Environ Microbiol. 1997;63:596–601. doi: 10.1128/aem.63.2.596-601.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simek K, Vrba J, Pernthaler J, Posch T, Hartman P, Nedoma J, Psenner R. Morphological and compositional shifts in an experimental bacterial community influenced by protists with contrasting feeding modes. Appl Environ Microbiol. 1997;63:587–595. doi: 10.1128/aem.63.2.587-595.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bianchi M. Unusual bloom of star-like prosthecate bacteria and filaments as a consequence of grazing pressure. Microb Ecol. 1989;17:137–141. doi: 10.1007/BF02011848. [DOI] [PubMed] [Google Scholar]
- 44.DeLeo PC, Baveye P. Factors affecting protozoan predation of bacteria clogging laboratory aquifer microcosms. Geomicrobiology J. 1997;14:127. [Google Scholar]
- 45.Hahn MW, Hofle MG. Flagellate predation on a bacterial model community: interplay of size-selective grazing, specific bacterial cell size, and bacterial community composition. Appl Environ Microbiol. 1999;65:4863–4872. doi: 10.1128/aem.65.11.4863-4872.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jürgens K, Pernthaler J, Schalla S, Amann R. Morphological and compositional changes in a planktonic bacterial community in response to enhanced protozoan grazing. Appl Environ Microbiol. 1999;65:1241–1250. doi: 10.1128/aem.65.3.1241-1250.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Posch T, Jezbera J, Vrba J, Simek K, Pernthaler J, Andreatta S, Sonntag B. Size selective feeding in Cyclidium glaucoma (Ciliophora, Scuticociliatida) and its effects on bacterial community structure: a study from a continuous cultivation system. Microb Ecol. 2001;42:217–227. doi: 10.1007/s002480000114. [DOI] [PubMed] [Google Scholar]
- 48.Mühling M, Harris N, Belay A, Whitton BA. Reversal of helix orientation in the cyanobacterium Arthrospira. J Phycol. 2003;39:360–367. [Google Scholar]
- 49.Giovannoni SJ, Tripp HJ, Givan S, Podar M, Vergin KL, Baptista D, Bibbs L, Eads J, Richardson TH, Noordewier M, et al. Genome streamlining in a cosmopolitan oceanic bacterium. Science. 2005;309:1242–1245. doi: 10.1126/science.1114057. [DOI] [PubMed] [Google Scholar]