Abstract
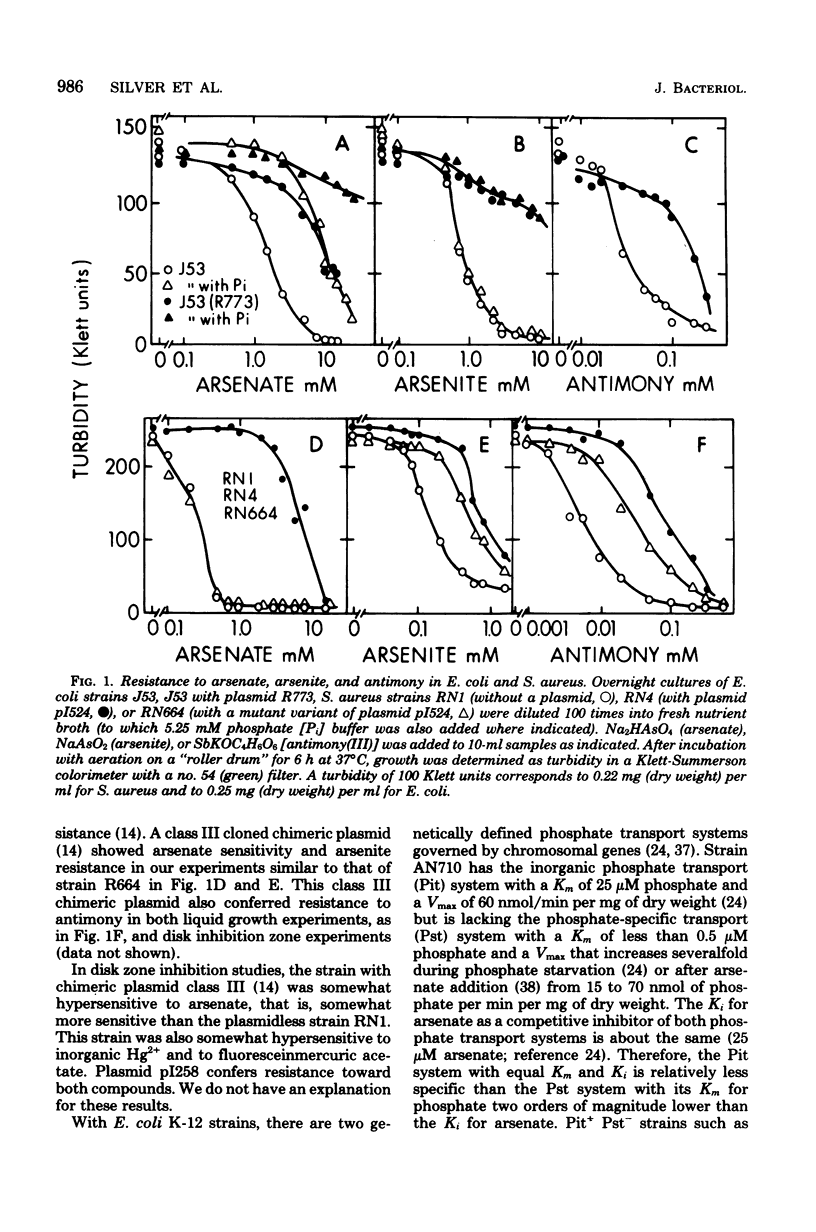
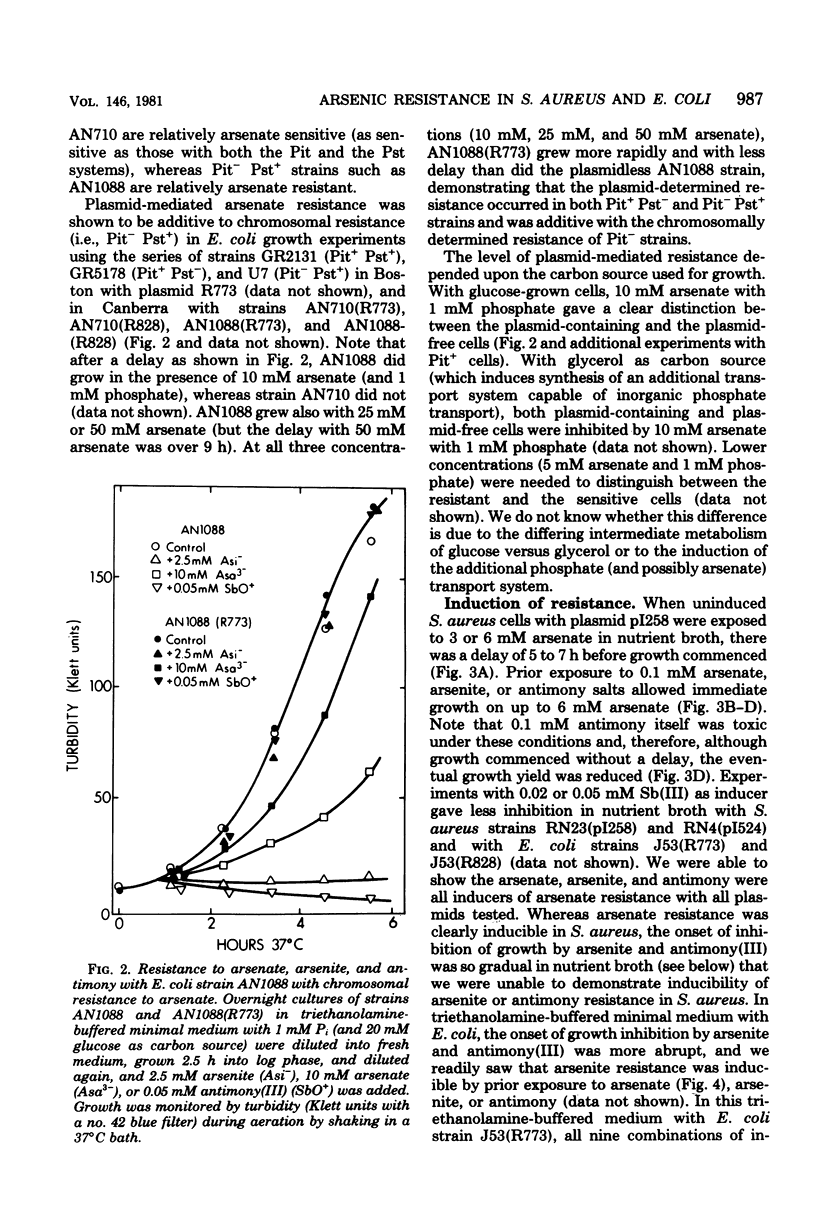
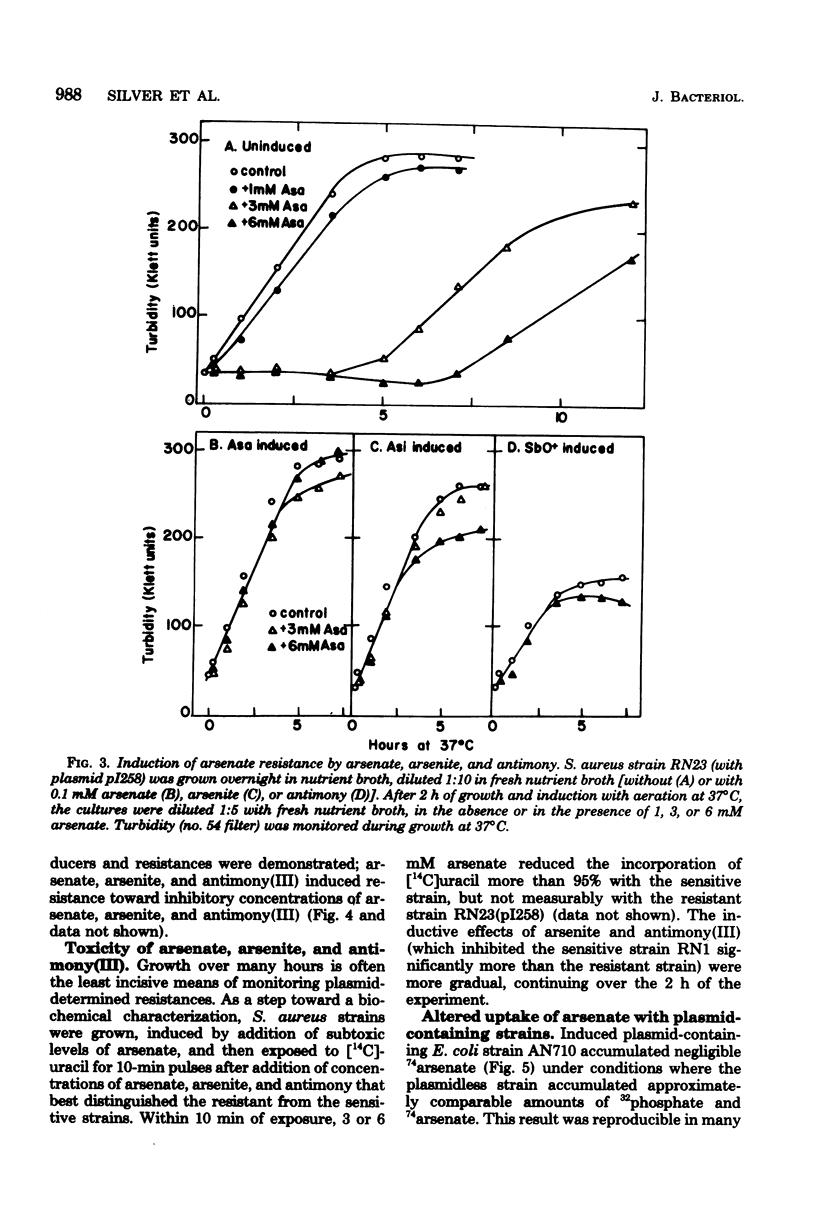
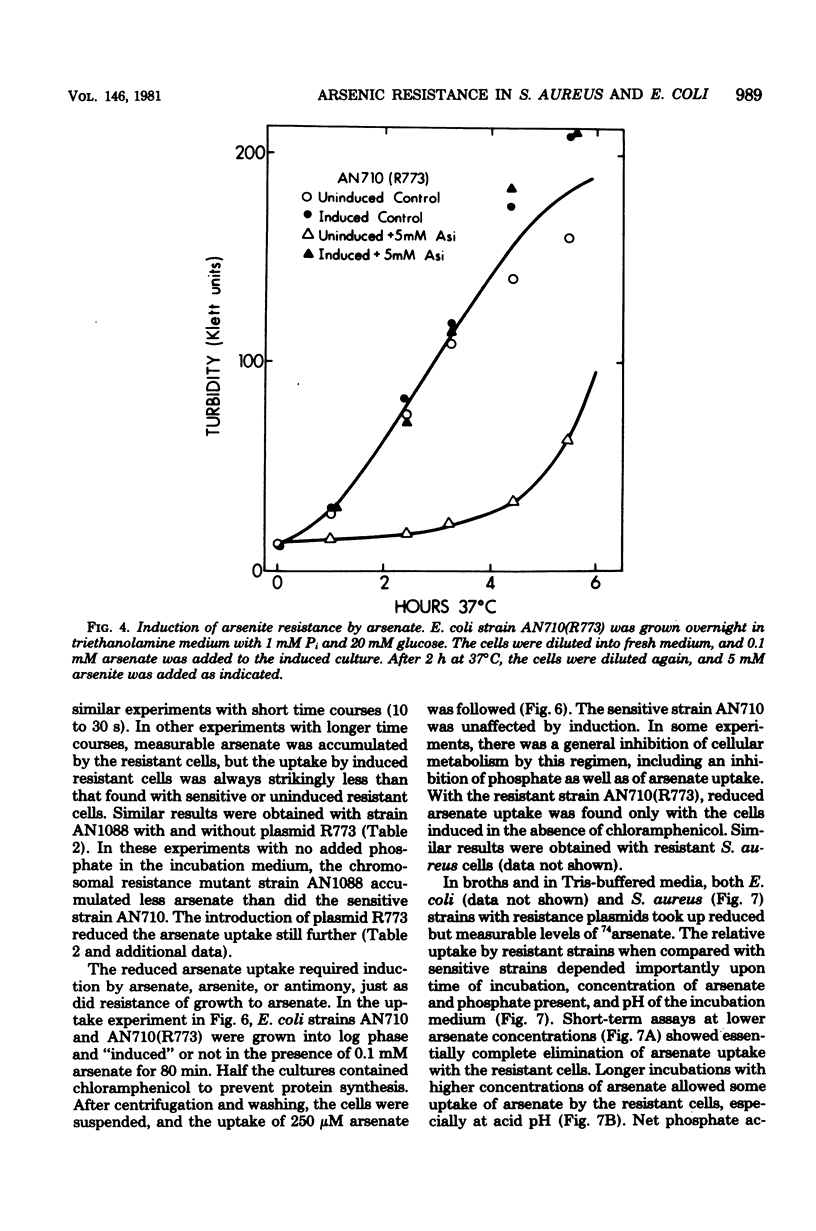
Plasmids in both Escherichia coli and Staphylococcus aureus contain an "operon" that confers resistance to arsenate, arsenite, and antimony(III) salts. The systems were always inducible. All three salts, arsenate, arsenite, and antimony(III), were inducers. Mutants and a cloned deoxyribonucleic acid fragment from plasmid pI258 in S. aureus have lost arsenate resistance but retained resistances to arsenite and antimony, demonstrating that separate genes are involved. Arsenate-resistant arsenite-sensitive S. aureus plasmid mutants were also isolated. In E. coli, plasmid-determined arsenate resistance and reduced uptake were additive to that found with chromosomal arsenate resistance mutants. Arsenate resistance was due to reduced uptake of arsenate by the induced plasmid-containing cells. Under conditions of high arsenate, when some uptake could be demonstrated with the induced resistant cells, the arsenate was rapidly lost by the cells in the absence of extracellular phosphate. Sensitive cells retained arsenate under these conditions. When phosphate was added, phosphate-arsenate exchange occurred. High phosphate in the growth medium protected cells from arsenate, but not from arsenite or antimony(III) toxicity. We do not know the mechanisms of arsenite or antimony resistance. However, arsenite was not oxidized to less toxic arsenate. Since cell-free medium "conditioned" by prior growth to induced resistant cells with toxic levels of arsenite or antimony(III) retained the ability to inhibit the growth of sensitive cells, the mechanism of arsenite and antimony resistance does not involve conversion of AsO2- or SbO+ to less toxic forms or binding by soluble thiols excreted by resistant cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amyes S. G., Smith J. T. The purification and properties of the trimethoprim-resistant dihydrofolate reductase mediated by the R-factor, R388. Eur J Biochem. 1976 Jan 15;61(2):597–603. doi: 10.1111/j.1432-1033.1976.tb10055.x. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett R. L., Malamy M. H. Arsenate resistant mutants of Escherichia coli and phosphate transport. Biochem Biophys Res Commun. 1970 Jul 27;40(2):496–503. doi: 10.1016/0006-291x(70)91036-3. [DOI] [PubMed] [Google Scholar]
- Chopra I., Lacey R. W., Connolly J. Biochemical and genetic basis of tetracycline resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1974 Oct;6(4):397–404. doi: 10.1128/aac.6.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Smith D. I. Plasmid-determined resistance to antimicrobial agents. Annu Rev Microbiol. 1978;32:469–518. doi: 10.1146/annurev.mi.32.100178.002345. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Interaction of arsenate with phosphate-transport systems in wild- type and mutant Streptococcus faecalis. J Bacteriol. 1966 Jun;91(6):2257–2262. doi: 10.1128/jb.91.6.2257-2262.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Spitz E. Accumulation of arsenate, phosphate, and aspartate by Sreptococcus faecalis. J Bacteriol. 1975 Apr;122(1):266–277. doi: 10.1128/jb.122.1.266-277.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Baumberg S. Resistance to arsenic compounds conferred by a plasmid transmissible between strains of Escherichia coli. J Bacteriol. 1973 Jul;115(1):459–460. doi: 10.1128/jb.115.1.459-460.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löfdahl S., Sjöström J. E., Philipson L. A vector for recombinant DNA in Staphylococcus aureus. Gene. 1978 Apr;3(2):161–172. doi: 10.1016/0378-1119(78)90059-8. [DOI] [PubMed] [Google Scholar]
- McMurry L., Petrucci R. E., Jr, Levy S. B. Active efflux of tetracycline encoded by four genetically different tetracycline resistance determinants in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3974–3977. doi: 10.1073/pnas.77.7.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara H., Ishikawa T., Sarai Y., Kondo I. Distribution of resistances to metals and antibiotics of staphylococcal strains in Japan. Zentralbl Bakteriol Orig A. 1977 Apr;237(4):470–476. [PubMed] [Google Scholar]
- Nakahara H., Ishikawa T., Sarai Y., Kondo I. Frequency of heavy-metal resistance in bacteria from inpatients in Japan. Nature. 1977 Mar 10;266(5598):165–167. doi: 10.1038/266165a0. [DOI] [PubMed] [Google Scholar]
- Nakahara H., Ishikawa T., Sarai Y., Kondo I., Kozukue H., Silver S. Linkage of mercury, cadmium, and arsenate and drug resistance in clinical isolates of Pseudomonas aeruginosa. Appl Environ Microbiol. 1977 Apr;33(4):975–976. doi: 10.1128/aem.33.4.975-976.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Clowes R. C., Cohen S. N., Curtiss R., 3rd, Datta N., Falkow S. Uniform nomenclature for bacterial plasmids: a proposal. Bacteriol Rev. 1976 Mar;40(1):168–189. doi: 10.1128/br.40.1.168-189.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Edelman I., Schwesinger M. D., Gruss A. D., Swanson E. C., Pattee P. A. Genetic translocation in Staphylococcus aureus. Proc Natl Acad Sci U S A. 1979 Jan;76(1):400–404. doi: 10.1073/pnas.76.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Roth C. Plasmid-linked resistance to inorganic salts in Staphylococcus aureus. J Bacteriol. 1968 Apr;95(4):1335–1342. doi: 10.1128/jb.95.4.1335-1342.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg H., Gerdes R. G., Chegwidden K. Two systems for the uptake of phosphate in Escherichia coli. J Bacteriol. 1977 Aug;131(2):505–511. doi: 10.1128/jb.131.2.505-511.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schottel J., Mandal A., Clark D., Silver S., Hedges R. W. Volatilisation of mercury and organomercurials determined by inducible R-factor systems in enteric bacteria. Nature. 1974 Sep 27;251(5473):335–337. doi: 10.1038/251335a0. [DOI] [PubMed] [Google Scholar]
- Shalita Z., Murphy E., Novick R. P. Penicillinase plasmids of Staphylococcus aureus: structural and evolutionary relationships. Plasmid. 1980 May;3(3):291–311. doi: 10.1016/0147-619x(80)90042-6. [DOI] [PubMed] [Google Scholar]
- Sköld O. R-factor-mediated resistance to sulfonamides by a plasmid-borne, drug-resistant dihydropteroate synthase. Antimicrob Agents Chemother. 1976 Jan;9(1):49–54. doi: 10.1128/aac.9.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W. Arsenic resistance in enterobacteria: its transmission by conjugation and by phage. J Gen Microbiol. 1978 Nov;109(1):49–56. doi: 10.1099/00221287-109-1-49. [DOI] [PubMed] [Google Scholar]
- Summers A. O., Jacoby G. A. Plasmid-determined resistance to tellurium compounds. J Bacteriol. 1977 Jan;129(1):276–281. doi: 10.1128/jb.129.1.276-281.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers A. O., Silver S. Microbial transformations of metals. Annu Rev Microbiol. 1978;32:637–672. doi: 10.1146/annurev.mi.32.100178.003225. [DOI] [PubMed] [Google Scholar]
- Tadlock C. H., Aposhian H. V. Protection of mice against the lethal effects of sodium arsenite by 2,3 dimercapto-1-propane-sulfonic acid and dimercaptosuccinic acid. Biochem Biophys Res Commun. 1980 May 30;94(2):501–507. doi: 10.1016/0006-291x(80)91259-0. [DOI] [PubMed] [Google Scholar]
- Tynecka Z., Zajac J., Goś Z. Plasmid dependent impermeability barrier to cadmium ions in Staphylococcus aureus. Acta Microbiol Pol A. 1975;7(1):11–20. [PubMed] [Google Scholar]
- Weiss A. A., Murphy S. D., Silver S. Mercury and organomercurial resistances determined by plasmids in Staphylococcus aureus. J Bacteriol. 1977 Oct;132(1):197–208. doi: 10.1128/jb.132.1.197-208.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willsky G. R., Malamy M. H. Effect of arsenate on inorganic phosphate transport in Escherichia coli. J Bacteriol. 1980 Oct;144(1):366–374. doi: 10.1128/jb.144.1.366-374.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise E. M., Jr, Abou-Donia M. M. Sulfonamide resistance mechanism in Escherichia coli: R plasmids can determine sulfonamide-resistant dihydropteroate synthases. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2621–2625. doi: 10.1073/pnas.72.7.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]



